Abstract
BACKGROUND/OBJECTIVE
Apolipoprotein A5 gene promoter region T-1131C polymorphism (APOA5 T-1131C) is known to be associated with elevated plasma TG levels, although little is known of the influence of the interaction between APOA5 T-1131C and lifestyle modification on TG levels. To investigate this matter, we studied APOA5 T-1131C and plasma TG levels of subjects participating in a three-month lifestyle modification program.
SUBJECTS/METHODS
A three-month lifestyle modification program was conducted with 297 participants (Age: 57 ± 8 years) in Izumo City, Japan, from 2001-2007. Changes in energy balance (the difference between energy intake and energy expenditure) and BMI were used to evaluate the participants' responses to the lifestyle modification.
RESULTS
Even after adjusting for confounding factors, plasma TG levels were significantly different at baseline among three genotype subgroups: TT, 126 ± 68 mg/dl; TC, 134 ± 74 mg/dl; and CC, 172 ± 101 mg/dl. Lifestyle modification resulted in significant reductions in plasma TG levels in the TT, TC, and CC genotype subgroups: -21.9 ± 61.0 mg/dl, -20.9 ± 51.0 mg/dl, and -42.6 ± 78.5 mg/dl, respectively, with no significant differences between them. In a stepwise regression analysis, age, APOA5 T-1131C, body mass index (BMI), homeostasis model assessment-insulin resistance (HOMA-IR), and the 18:1/18:0 ratio showed independent association with plasma TG levels at baseline. In a general linear model analysis, APOA5 T-1131C C-allele carriers showed significantly greater TG reduction with decreased energy balance than wild type carriers after adjustment for age, gender, and baseline plasma TG levels.
CONCLUSIONS
The genetic effects of APOA5 T-1131C independently affected plasma TG levels. However, lifestyle modification was effective in significantly reducing plasma TG levels despite the APOA5 T-1131C genotype background.
Keywords: Plasma TG, lifestyle modification, APOA5 T-1131C, energy balance
INTRODUCTION
The characteristic lipid abnormality of plasma lipids, such as hypertriglyceridemia (HTG), characterized by high plasma triglyceride (TG) levels, elevated low-density lipoprotein cholesterol (LDL-C) levels, and reduced high density lipoprotein cholesterol (HDL-C) levels, is considered an independent risk factor for development of cardiovascular disease (CAD) [1,2,3]. HTG is a complex disorder with many factors (e.g. genetic and environmental) contributing to its etiology [4]. High hypertriglyceridemic susceptibility in the Japanese population has been reported [5,6]. Japanese showing more favorable lipid profiles may benefit from interactions etween genetic background and environmental factors, such as diet, excise, and lifestyle [7]. Therefore, Japanese with high susceptibility to hypertriglyceridemia should be encouraged to adopt appropriate lifestyles that promote normal levels of plasma TG. In addition, genetic background must be considered as certain genetic polymorphisms have substantial influence on plasma TG levels [8,9]. It has been reported that lifestyle-modification might potentially improve hypertriglyceridemia and overcome genetic influence to a certain degree [10,11]. Clarification of the interaction between genetic background and lifestyle modification may be useful to achieving a reduction in plasma TG levels.
We considered two possible hypotheses in this regard. The first is that genetic factors offset any efforts to reduce plasma TG through lifestyle modification, as has been observed in cases of familial hypertriglyceridemia [12]. Second, both genetic factors and lifestyle modification affect plasma TG levels in a manner similar to that of the relationship between the APOE genotype and serum cholesterol [13].
To examine these two possibilities, we studied the effects of lifestyle modification on plasma TG levels in light of an individual's genetic background [14]. The APOA5 T-1131C polymorphism (rs12286037) is suitable for this purpose for several reasons: It is highly represented in the Japanese population and has been consistently shown, mostly through genome wide association, to confer hypertriglyceridemia susceptibility [8,9,15]. Second, this polymorphism, or another polymorphism in linkage disequilibrium with it, has been shown to affect the ability of the APOA5 gene to encode for APOA5 protein, the latter of which is known to lower plasma TG levels principally through stimulation of lipoprotein lipase (LPL)-mediated triglyceride hydrolysis [16]. Third, a previous study demonstrated that body mass index (BMI) was reduced to a significantly greater degree in APOA5 T-1131C C allele carriers compared with wild type carriers during a short-term fat-restricted diet [17], suggesting that APOA5 T-1131C polymorphism may also modulate response to lifestyle modification.
Two possible outcomes can be anticipated, neither of which may be mutually exclusive: First, that APOA5 T-1131C has a direct effect on changes in TG levels. Second, that it affects change in plasma TG levels through its effect on lifestyle modification.
Therefore, we investigated the interaction between APOA5 T-1131C and lifestyle modification on change in plasma TG levels in Japanese during a three-month lifestyle modification. We used changes in energy balance and BMI to evaluate the participants' responses to the lifestyle modification. Readily available quantitative measurements of outcome, i.e., plasma TG levels and lifestyle modification parameters, are advantageous in an objective study of this interaction.
SUBJECTS AND METHODS
Subjects
From 2001 to 2007, 404 clinically healthy volunteers, recruited through an advertisement in a local newspaper, participated in a three-month program of lifestyle modification in Izumo, Japan, for prevention of obesity. Of the 404 participants, 353 completed the program and the dataset. Participants taking medication for hyperlipidemia, cardiovascular diseases and type 2 diabetes were excluded from the analysis as these treatments might impact lipid metabolism. Ultimately, 293 participants (age: 56.7 ± 8.4 years, plasma TG levels: 143 ± 104 mg/dl) were analyzed in this study. Participants underwent a program for three months, including recommendation of dietary recipes, promotion of exercise and support through group therapy as described elsewhere [18]. Data on demographic factors and lifestyles were obtained using a self-reported questionnaire. Participants were encouraged to record pedometer measurements daily (HJ-002, Omron Co. Ltd, Tokyo, Japan), and their body weight weekly. The Shimane University School of Medicine ethics committee approved the study protocol (approval number 37 and 290) and all participants provided written informed consent.
Diet and Physical activity
Information on the participant's daily diet was obtained using an established self-administered quantitative food frequency questionnaire before and after the three-month lifestyle modification. Trained nutritionists asked the participants to report their weekly consumption of food, and the average amount and frequency of food intake for one month were estimated regarding "the 30 kinds of food groups" based on a report from the Working Committee for Health Guideline of the Japanese Ministry of Health and Welfare for Japanese people. Average daily nutrient intake (kcal/day) for one month was calculated using the standard food composition tables for Japanese [19]. Habitual physical activity was assessed before and after the lifestyle modification using the questionnaire, which evaluated physical activity including physical exercise during leisure time as well as walking. Participants were asked to submit the pedometer measurements for the one week before as well as during the lifestyle modification program. We calculated daily energy expenditure (kcal/day) using metabolic equivalent units (MET) by the following formula: calories of physical activity = body weight (kg) × metabolic equivalent (MET) × time (h). The MET for sitting/resting was 1 and that of normal walking was 3. We used MET values for a variety of physical activities, based on estimations set out in the Exercise and Physical Activity Guide from the Ministry of Health, Labor and Welfare of Japan [20]. Energy balance was calculated as the difference between energy intake and energy expenditure.
Anthropometric measurements
Before and after the lifestyle modification, and following an overnight fast, the participants underwent anthropometric evaluations. The subject's body weight with very light clothing was measured to an accuracy of ± 0.2 kg, and height was measured to an accuracy of ± 0.5 cm using a height bar. Body mass index (BMI) was computed as weight (kg) divided by squared height (m2). Resting energy expenditure (REE) was measured using an indirect calorimeter. Following a 15 min rest period, expired gas was collected through a mouthpiece while holding the nose closed for a 3-minute period. Oxygen concentration was determined by nondispersive infrared analysis (VMB-002N, VINE, Tokyo, Japan). REE was calculated from the consumed oxygen (L) using a conversion rate of 4.825 kcal/L oxygen [21].
Blood samples
Venous blood was collected from the antecubital vein after a 12-h overnight fast. Concentrations of plasma triglycerides and glucose were determined using an enzymatic assay kit (Kyowa Chemical, Japan) placed on an Autoanalyzer 7350 (Hitachi, Japan). Insulin concentration was measured using a human ELISA kit (Mercodia AB, Sweden). HOMA-IR was calculated using the following formula: fasting plasma insulin (µU/ml) × fasting plasma glucose (mg/dl) / 405. This index was used to evaluate insulin resistance. Fatty acid composition was measured as described elsewhere [5]. The total plasma 18:1/18:0 ratio was calculated by dividing the molecular percentage of oleic acid by the molecular percentage of stearic acid as a plasma marker of stearoyl-CoA desaturase activity.
DNA analysis
Genomic DNA was prepared from leukocytes using an Illustra blood genomicPrep Mini Spin Kit (GE Healthcare, Uppsala Sweden). APOA5 T-1131C polymorphism was genotyped by PCR-RFLP using Mse I restriction endonuclease under previously described conditions [22] and TaqMan genotyping assay by 7900HT Fast Real Time PCR system (Life technologies, CA, USA).
Statistical analysis
Because the data for plasma triglycerides, insulin, and HOMA-IR were significantly skewed, they were transformed logarithmically before performing a statistical analysis. ANOVA was used to assess differences in parameters between the three genotype groups of APOA5 T-1131C. Bonferroni test was performed for post hoc analyses using the TT group as a reference category. Pearson's chi square test was used to assess the difference in the sex ratio between the genotype groups. The results of the association between APOA5 T-1131C polymorphism and plasma TG levels were adjusted for age, gender, BMI and 18:1/18:0 ratio, since these variables were known to show strong correlation with plasma TG levels [21,22]. A repeated measures ANOVA was employed to test differences in the lifestyle modification-induced change in factors between genotype subgroups. We used stepwise regression analysis to determine independent factors that affect plasma TG levels at baseline and lifestyle modification-induced change in TG levels. Gender, age, APOA5 T-1131C, and baseline/change in BMI, HOMA-IR and 18:1/18:0 ratio were selected as potent independent factors. A generalized linear model (GLM) was used to study the interaction between APOA5 T-1131C and energy balance effect on changes in plasma TG level after adjustment for gender, age, and baseline plasma TG levels. The analysis of data was performed using SPSS statistical analysis software (Version 16, SPSS Japan Inc., Tokyo, Japan).
RESULTS
The frequency of the APOA5 T-1131C allele was 42.9%, and the frequency of heterozygotes and CC homozygote carriers of the mutation was 55.6% and 14.7%, respectively. It is worth noting that the C allele was more frequent (78%) in hypertriglyceridemic (≧150 mg/dl) subjects, who constituted 34% of all participants. At baseline, average plasma TG value was 143 ± 104 mg/dl. Plasma TG levels were significantly higher in males than females (177 ± 159 mg/dl vs. 128 ± 66 mg/dl).
Plasma TG levels differed significantly among genotype subgroups at baseline (TT 126 ± 68 mg/dl, TC 134 ± 74 mg/dl and CC 172 ± 101 mg/dl) and after the lifestyle modification (TT 104 ± 48 mg/dl, TC 113 ± 74 mg/dl and CC 130 ± 63 mg/dl) (Table 1). This significance remained even after controlling for confounding factors, including age, gender, BMI, and 18:1/18:0 ratio (Table 2). Significant differences in the 18:1/18:0 ratio were also observed among the genotype subgroups at baseline, but this significance disappeared after adjusting for age, gender, BMI, and plasma TG levels. No significant differences in other variables were observed among genotype subgroups, either at baseline or after the lifestyle modification.
Table 1. Characteristics of subjects in baseline and after lifestyle modification by APOA5 T-1131C.
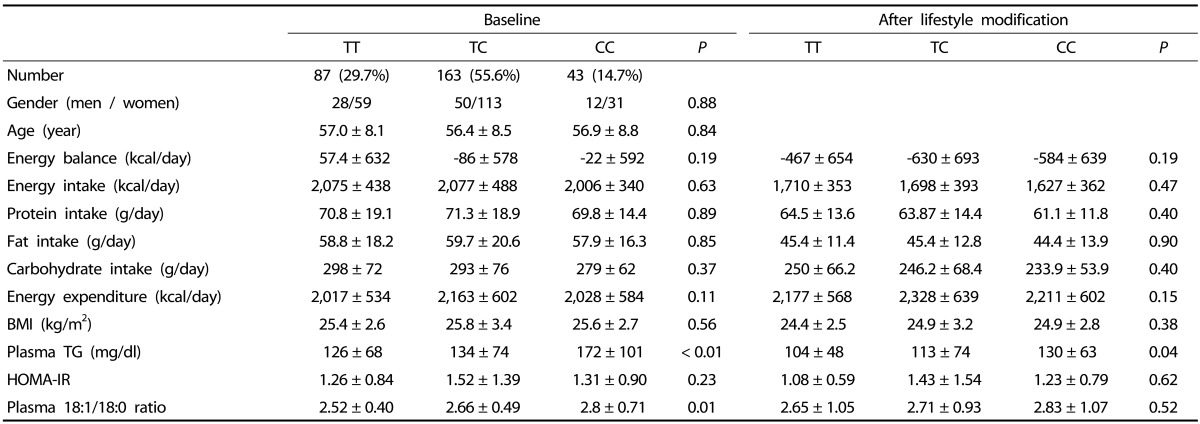
Data are expressed as mean ± SD. Differences between genotype subgroups were estimated by ANOVA except for gender where Pearson's chi square was used. P < 0.05 was significant. BMI, body mass index; TG, triglyceride; HOMA-IR, homeostasis model assessment of insulin resistance. Plasma 18:1/18:0 ratio was calculated by dividing the molecular percentage of oleic acid by the molecular percentage of stearic acid Energy balance was calculated by energy in intake minus energy in expenditure. Triglyceride and HOMA-IR were transformed logarithmically before performing a statistical analysis.
Table 2. Estimated average of plasma triglyceride (mg/dl) by APOA5 T-1131C after adjustment for confounding factors.

Data are expressed as mean ± SE and calculated by GLM adjusted for age, gender, BMI and plasma 18:1/18:0 ratio. P < 0.05 was significant.
Triglyceride was transformed logarithmically before performing a statistical analysis.
Table 3 shows the response of the genotype subgroups to the lifestyle modification. Lower plasma TG levels were observed relative to baseline in all three genotype subgroups: -21.9 ± 61.0 mg/dl, -20.9 ± 50.1 mg/dl, and -42.6 ± 78.5 mg/dl, respectively, for the TT, TC, and CC genotype subgroups. The lifestyle modification led to a significant decrease of all macronutrient (protein, carbohydrate, and fat) intakes and a significant increase in energy expenditure for all genotype subgroups. As a result of a significant decrease in energy balance (-515 ± 399 kcal/day, -512 ± 429 kcal/day and -546 ± 397 kcal/day, respectively, for the TT, TC, and CC genotype subgroups), the TT, TC, and CC genotype subgroups experienced significant weight loss of 3.1 ± 3.4%, 2.7 ± 3.2%, and 2.6 ± 2.7%, respectively. In contrast, the lifestyle modification had no significant impact on HOMA-IR and changes in 18:1/18:0 ratio in any of the genotype subgroups. No difference regarding change in any of the considered variables was found among genotype subgroups.
Table 3. Response of APOA5 T-1131C subgroups by lifestyle modification.

Data are expressed as mean ± SD.
ANOVA was used to assess differences in parameters by three groups of APOA5 genetic polymorphism (Pd). Pa-c were response to lifestyle modification in each genotype subgroup; a: TT subgroup; b: TC subgroup; c: CC subgroup. P < 0.05 was significant.BMI, body mass index; TG, triglyceride; HOMA-IR, homeostasis model assessment of insulin resistance. Plasma 18:1/18:0 ratio was calculated by dividing the molecular percentage of oleic acid by the molecular percentage of stearic acid Energy balance was calculated by energy in intake minus energy in expenditure. Triglyceride and HOMA-IR were transformed logarithmically before performing a statistical analysis.
We then performed stepwise regression analysis to examine the independent effects of factors influencing plasma TG levels at baseline and lifestyle modification-induced change in plasma TG levels. Of the selected variables, age, APOA5 T-1131C, BMI, HOMA-IR, and 18:1/18:0 ratio showed independent association with plasma TG levels at baseline, whereas change in BMI was the unique independent predictor of change in plasma TG levels (Table 4).
Table 4. Stepwise regression analysis predicting change of plasma triglyceride level between baseline and response to lifestyle modification.
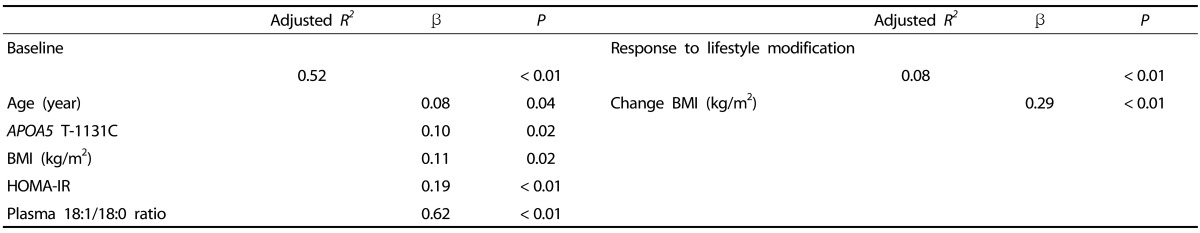
Data are expressed as mean ± SD.
Selected as potent independent variables: age, gender, APOA5 T-1131C, BMI, energy balance, homeostasis model assessment of insulin resistance (HOMA-IR), 18:1/18:0 ratio for baseline, and change in BMI for response to lifestyle modification.
Finally, using GLM, we found a significant association between APOA5 T-1131C (TT vs TC/CC) and energy balance after correcting for age, gender, and baseline plasma TG levels (P = 0.03). A significantly greater decrease in plasma TG levels was observed in risk C allele carriers with decreasing energy balance than in wild type carriers.
DISCUSSION
The main finding of the current study is that APOA5 T-1131C is independently associated with plasma TG levels at baseline but has no significant effect on changes in plasma TG levels brought about by lifestyle modification. In the current study, the lifestyle modification program, as represented by the strong effect of changes in BMI on plasma TG-levels, was effective in reducing plasma TG levels regardless of the APOA5 T-1131C genotype background. This finding is in accordance with those of two previous studies which also found that APOA5 T-1131C polymorphism had no effect on lifestyle modification-induced change in plasma TG levels [17,23], although APOA5 T-1131C polymorphism did affect total and high density lipoprotein cholesterol (TC and HDL-c) changes produced by lifestyle modification [24]. Therefore it appears that, despite their baseline hypertriglyceridemia susceptibility, C allele carriers benefit from lifestyle modification as do wild type carriers. This study highlights the beneficial effect of lifestyle modification on CVD risk factors. In this regard, it has been previously reported that moderate and even low-intensity lifestyle counseling lifestyle modification targeting improvement in physical activity and nutritional behavior and modest weight loss are practical and effective methods for improving a range of metabolic risk factors including dyslipidemia [25,26].
We also demonstrated that plasma TG levels were reduced to a greater degree in APOA5 T-1131C C allele carriers with a decreased energy balance than in wild type carriers with a decreased energy balance. However, this finding raises a question with regard to APOA5 T-1131C polymorphism hypertriglyceridemic susceptibility. We would normally expect APOA5 T-1131C C allele carriers with decreased energy balance to show smaller reductions in plasma TG levels as this polymorphism (or another one with which it is in linkage disequilibrium) is known to affect APOA5 protein synthesis and therefore LPL activation [16]. One likely explanation is that, as LPL activation is multifactorial and the effect of APOA5 on LPL is limited [27,28], it is conceivable that a reduced effect of APOA5 on LPL may be the result of other relevant factors controlling LPL activity. Of these relevant factors, physical activity, a major component of energy balance, was found to activate LPL either through an increase in plasma catecholamines and insulin levels or by a direct increase of pre-heparin LPL activity [29,30]. We therefore surmise that, before the lifestyle modification, when participants engaged in less physical activity, APOA5 T-1131C was effective in limiting LPL activation, thereby provoking elevation of plasma TG levels. After lifestyle modification and despite higher baseline plasma TG levels, plasma TG levels were reduced in proportionally greater amounts in at-risk C allele carriers with reduced energy balance. We thus concluded that the disadvantage in risk C allele carriers can be significantly offset by lifestyle modification, especially by changes in dietary content.
The hallmark of this study is the inclusion of the energy balance factor, a primary influencer of plasma TG levels. However, this study has some limitations. The study is not exempt from error in measurement of lifestyle factors, given the difficulty of measuring complex factors such as diet and physical activity, especially when self-reported. In addition, the relatively limited size of our sample must be considered.
In conclusion, we found that, despite their baseline hypertriglyceridemia susceptibility, plasma TG levels were lowered in proportionally greater amounts during the lifestyle modification in APOA5 T-1131C C allele carriers with reduced energy balance. Therefore we recommend this type of lifestyle modification program, particularly for risk C allele carriers, to lower and maintain plasma TG levels within normal ranges.
Footnotes
This study was supported in part by Grants-in-Aid for Scientific Research from the Japanese Ministry of Education, Culture, Sports, Science and Technology (21790553 and 24790586 to MY) and by Shimane University Medical Education and Research Foundation (MY).
References
- 1.Ho JS, Cannaday JJ, Barlow CE, Mitchell TL, Cooper KH, FitzGerald SJ. Relation of the number of metabolic syndrome risk factors with all-cause and cardiovascular mortality. Am J Cardiol. 2008;102:689–692. doi: 10.1016/j.amjcard.2008.05.010. [DOI] [PubMed] [Google Scholar]
- 2.Patel A, Barzi F, Jamrozik K, Lam TH, Ueshima H, Whitlock G, Woodward M Asia Pacific Cohort Studies Collaboration. Serum triglycerides as a risk factor for cardiovascular diseases in the Asia-Pacific region. Circulation. 2004;110:2678–2686. doi: 10.1161/01.CIR.0000145615.33955.83. [DOI] [PubMed] [Google Scholar]
- 3.Sarwar N, Danesh J, Eiriksdottir G, Sigurdsson G, Wareham N, Bingham S, Boekholdt SM, Khaw KT, Gudnason V. Triglycerides and the risk of coronary heart disease: 10,158 incident cases among 262,525 participants in 29 Western prospective studies. Circulation. 2007;115:450–458. doi: 10.1161/CIRCULATIONAHA.106.637793. [DOI] [PubMed] [Google Scholar]
- 4.Yuan G, Al-Shali KZ, Hegele RA. Hypertriglyceridemia: its etiology, effects and treatment. CMAJ. 2007;176:1113–1120. doi: 10.1503/cmaj.060963. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Shiwaku K, Hashimoto M, Kitajima K, Nogi A, Anuurad E, Enkhmaa B, Kim JM, Kim IS, Lee SK, Oyunsuren T, Shido O, Yamane Y. Triglyceride levels are ethnic-specifically associated with an index of stearoyl-CoA desaturase activity and n-3 PUFA levels in Asians. J Lipid Res. 2004;45:914–922. doi: 10.1194/jlr.M300483-JLR200. [DOI] [PubMed] [Google Scholar]
- 6.Parzianello L, Parzianello NA, Coelho JC. Increased triglyceride levels in a Japanese population living in southern Brazil. Arch Med Res. 2005;36:59–64. doi: 10.1016/j.arcmed.2004.11.004. [DOI] [PubMed] [Google Scholar]
- 7.Ordovas JM. Gene-diet interaction and plasma lipid responses to dietary intervention. Biochem Soc Trans. 2002;30:68–73. doi: 10.1042/. [DOI] [PubMed] [Google Scholar]
- 8.Willer CJ, Sanna S, Jackson AU, Scuteri A, Bonnycastle LL, Clarke R, Heath SC, Timpson NJ, Najjar SS, Stringham HM, Strait J, Duren WL, Maschio A, Busonero F, Mulas A, Albai G, Swift AJ, Morken MA, Narisu N, Bennett D, Parish S, Shen H, Galan P, Meneton P, Hercberg S, Zelenika D, Chen WM, Li Y, Scott LJ, Scheet PA, Sundvall J, Watanabe RM, Nagaraja R, Ebrahim S, Lawlor DA, Ben-Shlomo Y, Davey-Smith G, Shuldiner AR, Collins R, Bergman RN, Uda M, Tuomilehto J, Cao A, Collins FS, Lakatta E, Lathrop GM, Boehnke M, Schlessinger D, Mohlke KL, Abecasis GR. Newly identified loci that influence lipid concentrations and risk of coronary artery disease. Nat Genet. 2008;40:161–169. doi: 10.1038/ng.76. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Kathiresan S, Melander O, Guiducci C, Surti A, Burtt NP, Rieder MJ, Cooper GM, Roos C, Voight BF, Havulinna AS, Wahlstrand B, Hedner T, Corella D, Tai ES, Ordovas JM, Berglund G, Vartiainen E, Jousilahti P, Hedblad B, Taskinen MR, Newton-Cheh C, Salomaa V, Peltonen L, Groop L, Altshuler DM, Orho-Melander M. Six new loci associated with blood low-density lipoprotein cholesterol, high-density lipoprotein cholesterol or triglycerides in humans. Nat Genet. 2008;40:189–197. doi: 10.1038/ng.75. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Maraki M, Sidossis LS. Effects of energy balance on postprandial triacylglycerol metabolism. Curr Opin Clin Nutr Metab Care. 2010;13:608–617. doi: 10.1097/MCO.0b013e32833f1aae. [DOI] [PubMed] [Google Scholar]
- 11.Perez-Martinez P, Lopez-Miranda J, Perez-Jimenez F, Ordovas JM. Influence of genetic factors in the modulation of postprandial lipemia. Atheroscler Suppl. 2008;9:49–55. doi: 10.1016/j.atherosclerosissup.2008.05.005. [DOI] [PubMed] [Google Scholar]
- 12.Kolovou GD, Kostakou PM, Anagnostopoulou KK. Familial hypercholesterolemia and triglyceride metabolism. Int J Cardiol. 2011;147:349–358. doi: 10.1016/j.ijcard.2010.08.009. [DOI] [PubMed] [Google Scholar]
- 13.Tamasawa N, Murakami H, Yamato K, Matsui J, Tanabe J, Suda T. Influence of apolipoprotein E genotype on the response to caloric restriction in type 2 diabetic patients with hyperlipidaemia. Diabetes Obes Metab. 2003;5:345–348. doi: 10.1046/j.1463-1326.2003.00286.x. [DOI] [PubMed] [Google Scholar]
- 14.Ordovas JM, Tai ES. Why study gene-environment interactions? Curr Opin Lipidol. 2008;19:158–167. doi: 10.1097/MOL.0b013e3282f6a809. [DOI] [PubMed] [Google Scholar]
- 15.Nabika T, Nasreen S, Kobayashi S, Masuda J. The genetic effect of the apoprotein AV gene on the serum triglyceride level in Japanese. Atherosclerosis. 2002;165:201–204. doi: 10.1016/s0021-9150(02)00252-6. [DOI] [PubMed] [Google Scholar]
- 16.Wong K, Ryan RO. Characterization of apolipoprotein A-V structure and mode of plasma triacylglycerol regulation. Curr Opin Lipidol. 2007;18:319–324. doi: 10.1097/MOL.0b013e328133856c. [DOI] [PubMed] [Google Scholar]
- 17.Aberle J, Evans D, Beil FU, Seedorf U. A polymorphism in the apolipoprotein A5 gene is associated with weight loss after short-term diet. Clin Genet. 2005;68:152–154. doi: 10.1111/j.1399-0004.2005.00463.x. [DOI] [PubMed] [Google Scholar]
- 18.McCormack G, Giles-Corti B, Lange A, Smith T, Martin K, Pikora TJ. An update of recent evidence of the relationship between objective and self-report measures of the physical environment and physical activity behaviours. J Sci Med Sport. 2004;7:81–92. doi: 10.1016/s1440-2440(04)80282-2. [DOI] [PubMed] [Google Scholar]
- 19.Resources Council of the Science and Technology Agency of Japan. Standard Tables of Food Composition in Japan. 5th rev. Tokyo: Resources Council of the Science and Technology Agency of Japan; 2000. [Google Scholar]
- 20.Ministry of Health, Labour and Welfare (JP) Exercise and Physical Activity Guide for Health Promotion 2006: To Prevent Lifestyle-Related Diseases. Tokyo: Ministry of Health, Labour and Welfare; 2006. [Google Scholar]
- 21.VINE Corp. (KR) The Instruction Manual of Non-Dispersive Infrared Analysis. Anyang: VINE Corp.; 2000. [Google Scholar]
- 22.Pennacchio LA, Olivier M, Hubacek JA, Cohen JC, Cox DR, Fruchart JC, Krauss RM, Rubin EM. An apolipoprotein influencing triglycerides in humans and mice revealed by comparative sequencing. Science. 2001;294:169–173. doi: 10.1126/science.1064852. [DOI] [PubMed] [Google Scholar]
- 23.Suchanek P, Lorenzova A, Poledne R, Hubacek JA. Changes of plasma lipids during weight reduction in females depends on APOA5 variants. Ann Nutr Metab. 2008;53:104–108. doi: 10.1159/000165358. [DOI] [PubMed] [Google Scholar]
- 24.Zhang X, Qi Q, Bray GA, Hu FB, Sacks FM, Qi L. APOA5 genotype modulates 2-y changes in lipid profile in response to weight-loss diet intervention: the Pounds Lost Trial. Am J Clin Nutr. 2012;96:917–922. doi: 10.3945/ajcn.112.040907. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.St George A, Bauman A, Johnston A, Farrell G, Chey T, George J. Effect of a lifestyle intervention in patients with abnormal liver enzymes and metabolic risk factors. J Gastroenterol Hepatol. 2009;24:399–407. doi: 10.1111/j.1440-1746.2008.05694.x. [DOI] [PubMed] [Google Scholar]
- 26.Stone NJ. Successful control of dyslipidemia in patients with metabolic syndrome: focus on lifestyle changes. Clin Cornerstone. 2006;8(Suppl 1):S15–S20. doi: 10.1016/s1098-3597(06)80004-9. [DOI] [PubMed] [Google Scholar]
- 27.Wang H, Eckel RH. Lipoprotein lipase: from gene to obesity. Am J Physiol Endocrinol Metab. 2009;297:E271–E288. doi: 10.1152/ajpendo.90920.2008. [DOI] [PubMed] [Google Scholar]
- 28.Merkel M, Loeffler B, Kluger M, Fabig N, Geppert G, Pennacchio LA, Laatsch A, Heeren J. Apolipoprotein AV accelerates plasma hydrolysis of triglyceride-rich lipoproteins by interaction with proteoglycan-bound lipoprotein lipase. J Biol Chem. 2005;280:21553–21560. doi: 10.1074/jbc.M411412200. [DOI] [PubMed] [Google Scholar]
- 29.Seip RL, Mair K, Cole TG, Semenkovich CF. Induction of human skeletal muscle lipoprotein lipase gene expression by short-term exercise is transient. Am J Physiol. 1997;272:E255–E261. doi: 10.1152/ajpendo.1997.272.2.E255. [DOI] [PubMed] [Google Scholar]
- 30.Miyashita M, Eto M, Sasai H, Tsujimoto T, Nomata Y, Tanaka K. Twelve-week jogging training increases pre-heparin serum lipoprotein lipase concentrations in overweight/obese middle-aged men. J Atheroscler Thromb. 2010;17:21–29. doi: 10.5551/jat.2337. [DOI] [PubMed] [Google Scholar]


