Abstract
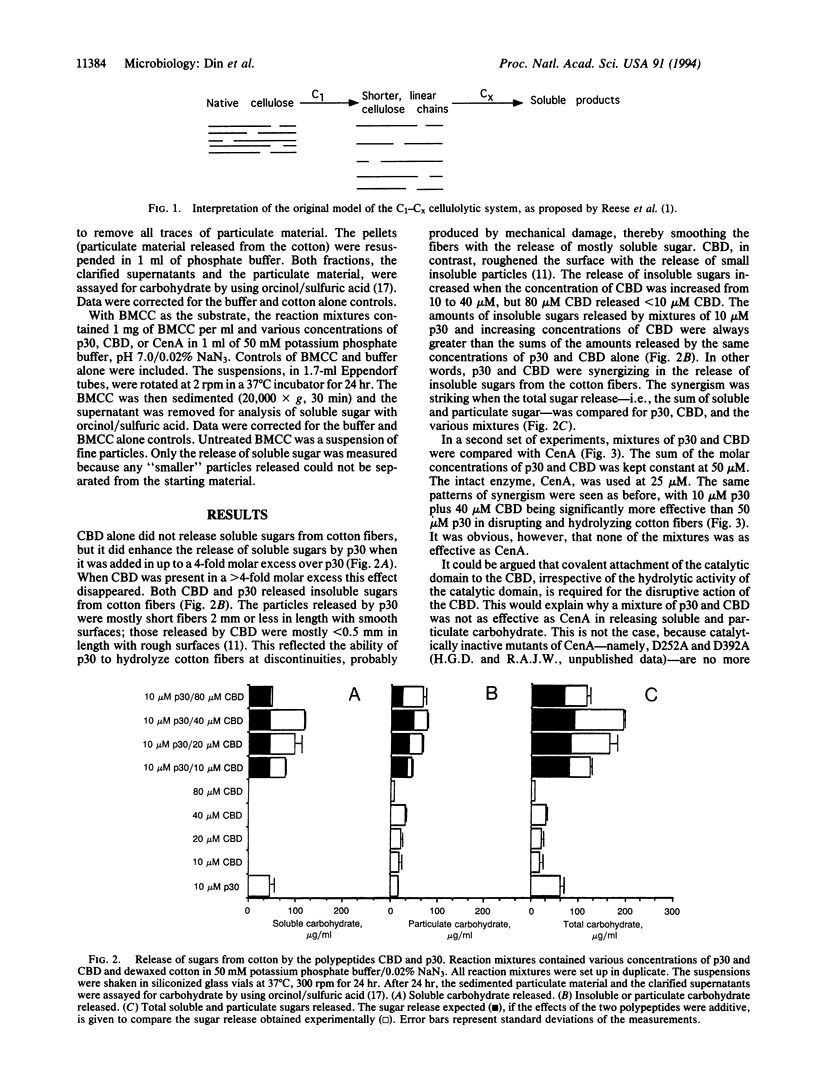
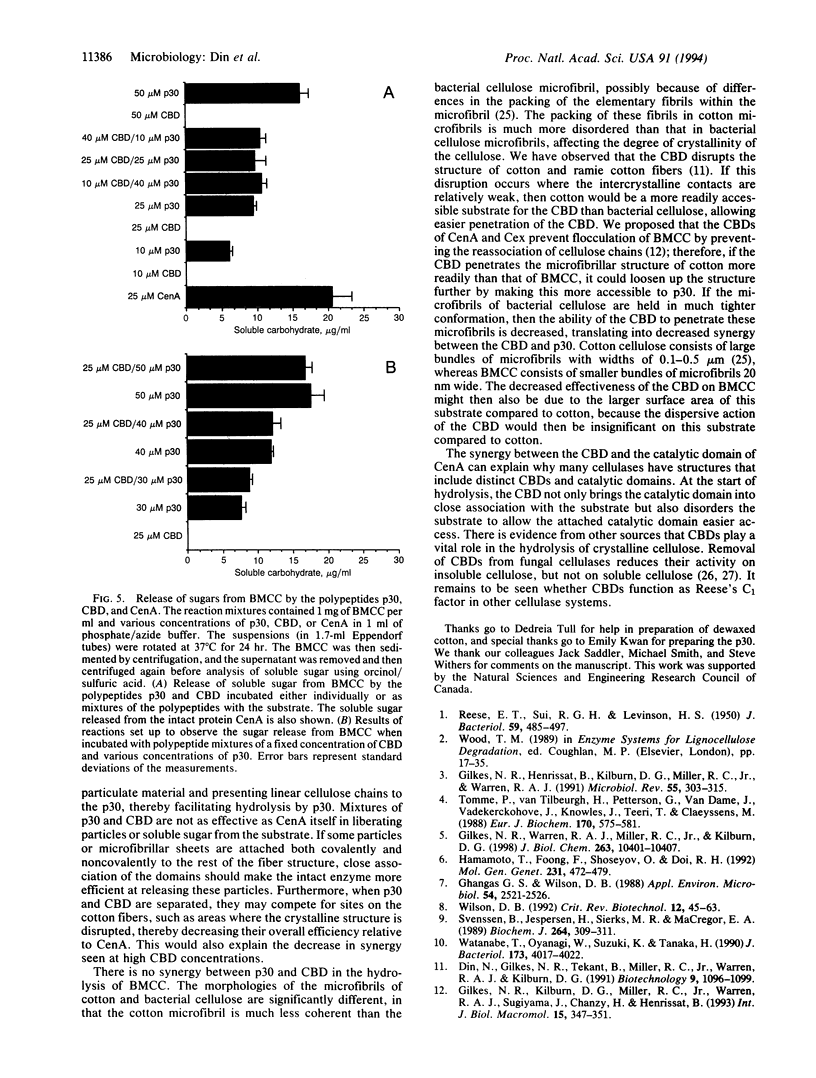
Endoglucanase A (CenA) from the bacterium Cellulomonas fimi is composed of a catalytic domain and a nonhydrolytic cellulose-binding domain that can function independently. The individual domains interact synergistically in the disruption and hydrolysis of cellulose fibers. This intramolecular synergism is distinct from the well-known intermolecular synergism between individual cellulases. The catalytic domain corresponds to the hydrolytic Cx system and the cellulose-binding domain corresponds to the nonhydrolytic C1 system postulated by Reese et al. [Reese, E. T., Sui, R. G. H. & Levinson, H. S. (1950) J. Bacteriol. 59, 485-497] to be required for the hydrolysis of cellulose.
Full text
PDF


Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Din N., Forsythe I. J., Burtnick L. D., Gilkes N. R., Miller R. C., Jr, Warren R. A., Kilburn D. G. The cellulose-binding domain of endoglucanase A (CenA) from Cellulomonas fimi: evidence for the involvement of tryptophan residues in binding. Mol Microbiol. 1994 Feb;11(4):747–755. doi: 10.1111/j.1365-2958.1994.tb00352.x. [DOI] [PubMed] [Google Scholar]
- Ghangas G. S., Wilson D. B. Cloning of the Thermomonospora fusca Endoglucanase E2 Gene in Streptomyces lividans: Affinity Purification and Functional Domains of the Cloned Gene Product. Appl Environ Microbiol. 1988 Oct;54(10):2521–2526. doi: 10.1128/aem.54.10.2521-2526.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gilkes N. R., Henrissat B., Kilburn D. G., Miller R. C., Jr, Warren R. A. Domains in microbial beta-1, 4-glycanases: sequence conservation, function, and enzyme families. Microbiol Rev. 1991 Jun;55(2):303–315. doi: 10.1128/mr.55.2.303-315.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gilkes N. R., Jervis E., Henrissat B., Tekant B., Miller R. C., Jr, Warren R. A., Kilburn D. G. The adsorption of a bacterial cellulase and its two isolated domains to crystalline cellulose. J Biol Chem. 1992 Apr 5;267(10):6743–6749. [PubMed] [Google Scholar]
- Gilkes N. R., Kilburn D. G., Miller R. C., Jr, Warren R. A., Sugiyama J., Chanzy H., Henrissat B. Visualization of the adsorption of a bacterial endo-beta-1,4-glucanase and its isolated cellulose-binding domain to crystalline cellulose. Int J Biol Macromol. 1993 Dec;15(6):347–351. doi: 10.1016/0141-8130(93)90052-n. [DOI] [PubMed] [Google Scholar]
- Gilkes N. R., Warren R. A., Miller R. C., Jr, Kilburn D. G. Precise excision of the cellulose binding domains from two Cellulomonas fimi cellulases by a homologous protease and the effect on catalysis. J Biol Chem. 1988 Jul 25;263(21):10401–10407. [PubMed] [Google Scholar]
- Hamamoto T., Foong F., Shoseyov O., Doi R. H. Analysis of functional domains of endoglucanases from Clostridium cellulovorans by gene cloning, nucleotide sequencing and chimeric protein construction. Mol Gen Genet. 1992 Feb;231(3):472–479. doi: 10.1007/BF00292718. [DOI] [PubMed] [Google Scholar]
- REESE E. T., SIU R. G. H., LEVINSON H. S. The biological degradation of soluble cellulose derivatives and its relationship to the mechanism of cellulose hydrolysis. J Bacteriol. 1950 Apr;59(4):485–497. doi: 10.1128/jb.59.4.485-497.1950. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scopes R. K. Measurement of protein by spectrophotometry at 205 nm. Anal Biochem. 1974 May;59(1):277–282. doi: 10.1016/0003-2697(74)90034-7. [DOI] [PubMed] [Google Scholar]
- Svensson B., Jespersen H., Sierks M. R., MacGregor E. A. Sequence homology between putative raw-starch binding domains from different starch-degrading enzymes. Biochem J. 1989 Nov 15;264(1):309–311. doi: 10.1042/bj2640309. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tomme P., Van Tilbeurgh H., Pettersson G., Van Damme J., Vandekerckhove J., Knowles J., Teeri T., Claeyssens M. Studies of the cellulolytic system of Trichoderma reesei QM 9414. Analysis of domain function in two cellobiohydrolases by limited proteolysis. Eur J Biochem. 1988 Jan 4;170(3):575–581. doi: 10.1111/j.1432-1033.1988.tb13736.x. [DOI] [PubMed] [Google Scholar]
- Tomme P., Van Tilbeurgh H., Pettersson G., Van Damme J., Vandekerckhove J., Knowles J., Teeri T., Claeyssens M. Studies of the cellulolytic system of Trichoderma reesei QM 9414. Analysis of domain function in two cellobiohydrolases by limited proteolysis. Eur J Biochem. 1988 Jan 4;170(3):575–581. doi: 10.1111/j.1432-1033.1988.tb13736.x. [DOI] [PubMed] [Google Scholar]
- Watanabe T., Oyanagi W., Suzuki K., Tanaka H. Chitinase system of Bacillus circulans WL-12 and importance of chitinase A1 in chitin degradation. J Bacteriol. 1990 Jul;172(7):4017–4022. doi: 10.1128/jb.172.7.4017-4022.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wilson D. B. Biochemistry and genetics of actinomycete cellulases. Crit Rev Biotechnol. 1992;12(1-2):45–63. doi: 10.3109/07388559209069187. [DOI] [PubMed] [Google Scholar]
- Wood T. M., McCrae S. I., Bhat K. M. The mechanism of fungal cellulase action. Synergism between enzyme components of Penicillium pinophilum cellulase in solubilizing hydrogen bond-ordered cellulose. Biochem J. 1989 May 15;260(1):37–43. doi: 10.1042/bj2600037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wood T. M., McCrae S. I. The purification and properties of the C 1 component of Trichoderma koningii cellulase. Biochem J. 1972 Aug;128(5):1183–1192. doi: 10.1042/bj1281183. [DOI] [PMC free article] [PubMed] [Google Scholar]



