Abstract
Background
Heterotopic ossification (HO) develops in a majority of combat-related amputations wherein early bacterial colonization has been considered a potential early risk factor. Our group has recently developed a small animal model of trauma-induced HO that incorporates many of the multifaceted injury patterns of combat trauma in the absence of bacterial contamination and subsequent wound colonization.
Questions/purposes
We sought to determine if (1) the presence of bioburden (Acinetobacter baumannii and methicillin-resistant Staphylococcus aureus [MRSA]) increases the magnitude of ectopic bone formation in traumatized muscle after amputation; and (2) what persistent effects bacterial contamination has on late microbial flora within the amputation site.
Methods
Using a blast-related HO model, we exposed 48 rats to blast overpressure, femur fracture, crush injury, and subsequent immediate transfemoral amputation through the zone of injury. Control injured rats (n = 8) were inoculated beneath the myodesis with phosphate-buffered saline not containing bacteria (vehicle) and treatment rats were inoculated with 1 × 106 colony-forming units of A baumannii (n = 20) or MRSA (n = 20). All animals formed HO. Heterotopic ossification was determined by quantitative volumetric measurements of ectopic bone at 12-weeks postinjury using micro-CT and qualitative histomorphometry for assessment of new bone formation in the residual limb. Bone marrow and muscle tissue biopsies were collected from the residual limb at 12 weeks to quantitatively measure the bioburden load and to qualitatively determine the species-level identification of the bacterial flora.
Results
At 12 weeks, we observed a greater volume of HO in rats infected with MRSA (68.9 ± 8.6 mm3; 95% confidence interval [CI], 50.52–85.55) when compared with A baumannii (20.9 ± 3.7 mm3; 95% CI, 13.61–28.14; p < 0.001) or vehicle (16.3 ± 3.2 mm3; 95% CI, 10.06–22.47; p < 0.001). Soft tissue and marrow from the residual limb of rats inoculated with A baumannii tested negative for A baumannii infection but were positive for other strains of bacteria (1.33 × 102 ± 0.89 × 102; 95% CI, −0.42 × 102–3.08 × 102 and 1.25 × 106 ± 0.69 × 106; 95% CI, −0.13 × 106–2.60 × 106 colony-forming units in bone marrow and muscle tissue, respectively), whereas tissue from MRSA-infected rats contained MRSA only (4.84 × 101 ± 3.22 × 101; 95% CI, −1.47 × 101–11.1 × 101 and 2.80 × 107 ± 1.73 × 107; 95% CI, −0.60 × 107–6.20 × 107 in bone marrow and muscle tissue, respectively).
Conclusions
Our findings demonstrate that persistent infection with MRSA results in a greater volume of ectopic bone formation, which may be the result of chronic soft tissue inflammation, and that early wound colonization may be a key risk factor.
Clinical Relevance
Interventions that mitigate wound contamination and inflammation (such as early débridement, systemic and local antibiotics) may also have a beneficial effect with regard to the mitigation of HO formation and should be evaluated with that potential in mind in future preclinical studies.
Introduction
Blast injuries present formidable surgical, treatment, and rehabilitation challenges. The resulting wounds are multifaceted, often resulting in composite tissue loss, comminuted open fractures, and frequent traumatic amputations. Related wound contamination is ubiquitous, often with multidrug-resistant organisms such as Acinetobacter baumannii and methicillin-resistant Staphylococcus aureus (MRSA), often calling for protracted treatment regimens that include serial surgical débridements and broad-spectrum antibiotic therapy [2, 3, 6]. A survey of wound infections from Combat Support Hospitals in Iraq from 2003 to 2004 demonstrated a relatively high frequency of MRSA (26%) followed by Acinetobacter calcoaceticus-baumannii complex (11%), Klebsiella pneumoniae (13%), and Pseudomonas aeruginosa (10%) in combat-related injuries [6]. Wound infection-related complications include wound dehiscence, deep soft tissue infection, biofilm development on orthopaedic implants, and infectious osteomyelitis, often leading to chronic, debilitating infections, further bone and soft tissue destruction, and subsequent limb amputation [2, 20, 22, 23, 33].
Heterotopic ossification (HO) is the formation of mature lamellar bone within soft tissue after severe traumatic injury [10]. It is known to develop in the majority of combat-related amputations, and early bacterial colonization has been considered a potential early risk factor [12, 13]. However, the cellular and early signaling mechanism(s) for combat injury-induced HO formation remain unclear. Recent findings suggest that the heightened and prolonged expression of inflammatory and other reparative mediators may be contribute to HO formation [11, 14]. Moreover, the combat wound appears to provide a unique microenvironment conducive to osteogenesis that promotes the skewed differentiation of endogenous tissue-derived progenitor cells toward ectopic bone development within injured and healing soft tissue [10].
We previously developed a rat model of combat-related HO that incorporates the critical elements associated with combat injury, specifically a systemic blast injury, femur fracture with soft tissue crush, and transfemoral amputation through the zone of injury wherein all animals develop radiographic evidence of HO within 2 months postinjury [25]. Expanding on this model, in this study, we sought to evaluate if (1) the presence of bioburden (A baumannii and MRSA) increases the magnitude of ectopic bone formation in traumatized muscle after amputation; and (2) what persistent effects bacterial contamination has on late microbial flora within the amputation site.
Materials and Methods
Animals
Forty-eight young adult pathogen-free male Sprague-Dawley rats (Rattus norvegicus; 12–14 weeks, 400–500 g) were purchased from Taconic Farms (Germantown, NY, USA). All animals were housed in clean plastic cages and kept on a 12-hour light/dark cycle with unlimited access to food (standard rodent chow) and fresh water ad libitum. The study protocol (12-OUMD-20s) was reviewed and approved by the Walter Reed Army Institute of Research/Naval Medical Research Center Institutional Animal Care and Use Committee in compliance with all applicable Federal regulations governing the protection of animals in research.
Bacteria Culture Conditions
The A baumannii (strain 5075) and MRSA (MRSA strain 107261) organisms used in this study are highly virulent, well-characterized clinical specimens isolated from combat wounds from patients treated at the Walter Reed National Military Medical Center. In brief, frozen (−80 °C) stock cultures were streaked out on a blood agar plate and left to grow overnight at 37 °C and 5% CO2. A single bacterial colony was isolated and suspended in 3 mL of Lysogeny broth/Luria-Bertabi medium (Becton, Dickinson and Co, Sparks, MD, USA) and agitated overnight at 37 °C and 5% CO2. Overnight cultures were diluted 1:50 in 50 mL of fresh prewarmed Luria-Bertabi broth in a 250-mL Erlenmeyer flask and grown to early/midlog phase (OD600 = 0.2–0.5) where cells proliferate in a logarithmic fashion under optimal culture and nutrient conditions resulting in a controlled cell growth rate. Next, 2 mL of the concentrated culture sample was removed. Cells were washed twice using prechilled (4°C) phosphate-buffered saline (PBS), pelleted by centrifugation (5000 rpm for 3 minutes), then resuspended in 1 mL of sterile PBS. The bacterial density was estimated through direct count using a Petroff-Hauser Counting Chamber (Hauser Scientific, Horsham, PA, USA) and confirmed by serial dilution and plating on Luria-Bertabi agar and then diluted to the desired cell concentration, 1 × 107 colony-forming units (CFU)/mL in cold PBS.
Rat Model of Trauma-induced HO and Bacterial Inoculation
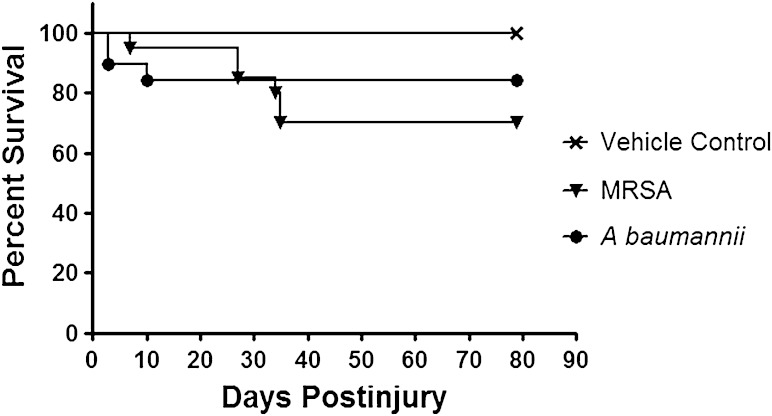
A total of 48 rats were exposed to blast overpressure exposure, femur fracture, soft tissue crush injury, and limb amputation as previously described [25]. After quadriceps myoplasty, three muscle sites immediately surrounding the amputation site were inoculated with: (1) vehicle (100 µL of PBS; n = 8); (2) Abaumannii (100 µL of 1 × 107 CFU; n = 20); or (3) MRSA (100 µL of 1 × 107 CFU; n = 20). Closure of the incision was performed using a 3-0 Vicryl in the deep subcutaneous tissue and a running 4-0 subcuticular Monocryl. Wounds were covered in Vetbond (3M Animal Care Products, St Paul, MN, USA). Postoperatively rats were monitored at least twice daily by animal care staff, research investigators, and veterinarians for animal activity, signs of pain, weight loss, wound dehiscence, or infectious tracts for the duration of the study. Wounds that exhibited signs of infection defined as drainage, progressive marginal erythema, or dehiscence were débrided. Rats were euthanized if they demonstrated signs of infection after a third débridement. We conducted a power analysis based on the effect of a projected 50% increase in ectopic bone volume within the soft tissue surrounding the amputated femur when the injury site was inoculated with either MRSA or A baumannii. Using conservative assumptions and data from our prior studies, the power analysis showed that with eight rats per treatment group and α = 0.05, there is 90% power to detect a 50% increase in ectopic bone volume. Thus, it was anticipated that 20 rats in the infected treatment groups would provide adequate statistical power to detect treatment effects of moderate size on the major outcome variable of ectopic bone volume even with attrition of as many as 12 rats per group. All eight rats in the control group survived until the 12-week micro-CT scan (Fig. 1). Six animals in the MRSA group were euthanized during the fourth and fifth weeks for overwhelming infection. Two of the rats in the A baumannii group died on the day of surgery and were excluded. A low level of mortality after surgery was consistent with findings during model development and represents the devastation of these multifaceted injuries, particularly given that blast overpressure of 120 ± 7 kPa itself is calibrated for 70% to 90% survivability [1, 7, 25]. In addition, two rats infected with A baumannii were euthanized for sustained weight loss greater than 10% during postoperative weeks 2 and 4.
Fig. 1.
Treatment effects on survival outcome of injured rats wherein the traumatized muscle surrounding the amputation site at the time of closure was infected with either MRSA (1 × 106) or A baumannii (1 × 106). Kaplan-Meier survival curves are shown. Animals were euthanized if they demonstrated signs of infection after the third débridement and irrigation of the amputation wound site.
Micro-CT Analysis
Rats anesthetized with isoflurane (2%) were imaged at 12 weeks postinjury using a SkyScan 1176 in vivo high-resolution micro-CT (Bruker-MicroCT, Kontich, Belgium) with the following settings: 89-kV polychromatic xray beam, current of 256 μA, and an exposure time of 81 msec for each of 180 rotational steps. Two investigators (GJP, ATQ) independently reviewed the micro-CT images (170–200 flattened longitudinal micro-CT slices/rat) on a CT-Analyser (Bruker-MicroCT) and calculated the volume of ectopic bone formation using selected regions of interest on every fifth slice encompassing ectopic bone. The binary selected slices were then used to perform three-dimensional image analysis yielding a total volume of HO in the selected area of interest.
Sample Collection and Culture
After the micro-CT scans were assessed for image quality and clarity, scanning efficiency, and reconstructed for volumetric analysis of ectopic bone formation, rats were euthanized with pentobarbital (Fatal Plus; 390 mg/kg intraperitoneally; Patterson Veterinary, Devens, MA, USA). Muscle tissue adjacent to the amputation site and femur was aseptically excised. Femurs were removed and separated from the soft tissues. Bone marrow from the residual femur was extruded from the medullary canal by flushing using a 10-mL syringe fitted with an 18-gauge needle with 10 mL of sterile PBS after proximal and distal osteotomies. Samples were diluted in PBS out to 10−6, plated on a blood agar plate, and incubated overnight at 37° C, 5% CO2. Colonies were counted and screened for differing morphology. Isolates were streaked on a blood agar plate for direct bacterial species identification using the BD Phoenix automated microbiology system in accordance with the manufacturer’s instructions (BD Diagnostics, Sparks, MD, USA).
Histological Analysis
At the time of euthanasia, two rats from each treatment group received an en bloc resection of the residual limb, which was then fixed in 10% formalin, decalcified in 5% formic acid, paraffin-embedded, cut into 5-µm longitudinal sections on a microtome, and stained using hematoxylin and eosin stain (Histoserv, Inc, Germantown, MD, USA). Histologic tissue samples were qualitatively analyzed for evidence of soft tissue cartilage formation, inflammation, lamellar bone formation within the soft tissues, the presence of persistent inflammatory cells, or active bacterial infection. The histopathological analysis was conducted by a veterinary pathologist (CH) blinded to the treatment groups.
Statistical Analysis
Kaplan-Meier modeling was performed to assess the survivability patterns of the control and treatment groups over the duration of the study. Intraclass correlation coefficient (ICC) was calculated to assess the reliability of interobserver measurements of HO formation using the micro-CT analyzing software. Analysis of variance modeling was used to determine whether there was a significant difference in the volume (mm3) of ectopic bone measured among the three groups followed by the Tukey’s honestly significant difference test to determine the mean difference among the three groups. All data, including the bacterial CFU counts, were presented as mean ± SD with 95% confidence interval (CI) unless specified otherwise. Exact p values were stated except when < 0.001. All statistical analysis described previously was performed using the RStudio, Version 0.98.953 (© 2009–2013 RStudio Inc, Boston, MA, USA).
Results
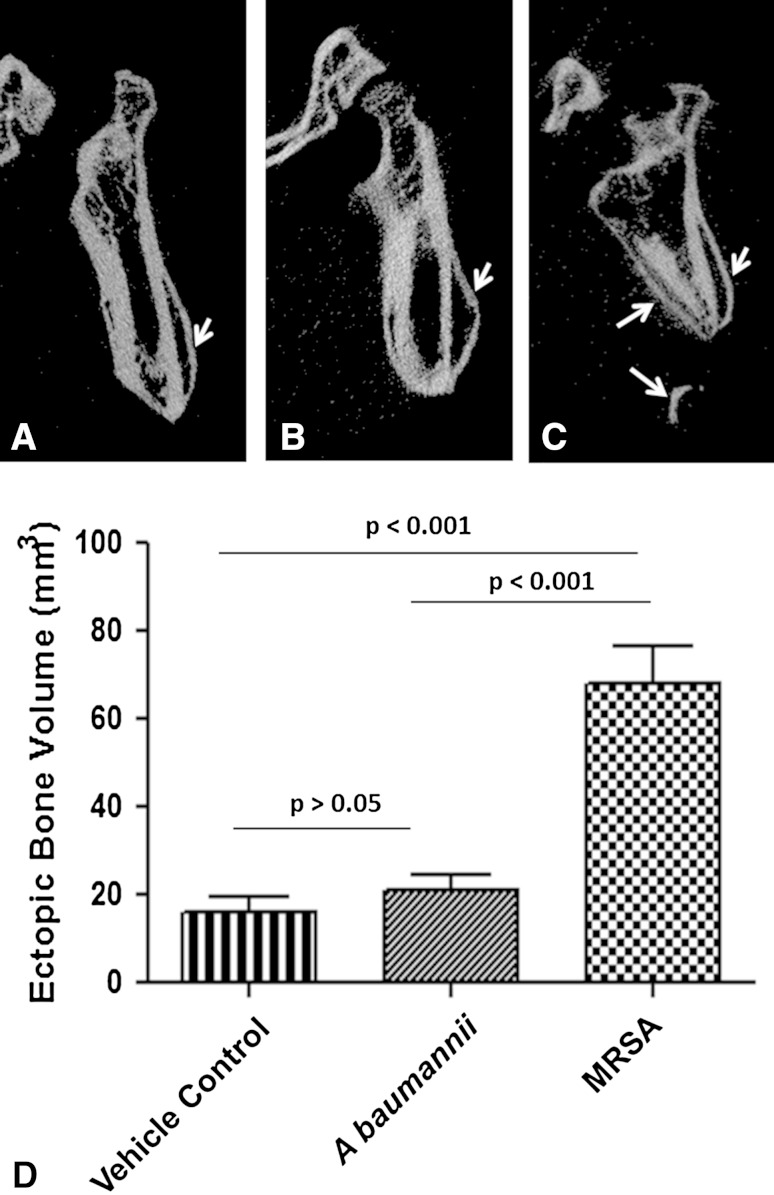
All rats that survived to the end of the study (A baumannii [n = 16], MRSA [n = 14], vehicle [n = 8]) demonstrated the formation of ectopic bone on the 12-week micro-CT scan (Fig. 2A–C). Volumetric measurements of ectopic bone formation (Fig. 2D) were more robust in animals inoculated with MRSA (68.0 ± 8.6 mm3; 95% CI, 50.52–85.55) than A baumannii (20.9 ± 3.7 mm3; 95% CI, 13.61–28.14; p < 0.001) and vehicle control (16.3 ± 3.2 mm3; 95% CI, 10.06–22.47; p < 0.001). Comparison of vehicle control and A baumannii-inoculated groups showed no difference (p = 0.43) with excellent interobserver agreement (ICC = 0.98).
Fig. 2A–D.
MRSA infection increases trauma-induced ectopic bone formation. Representative longitudinal 12-week micro-CT images of the residual femurs of rats inoculated with (A) vehicle control (PBS; noninfected control); (B) A baumannii; and (C) MRSA are shown. The white arrows highlight the areas of ectopic bone formation. (D) The amount of ectopic bone was quantified 12 weeks postinjury from vehicle control (n = 8), A baumannii (n = 16), and MRSA (n = 14) treatment groups. Result expressed are expressed as the mean ± SD.
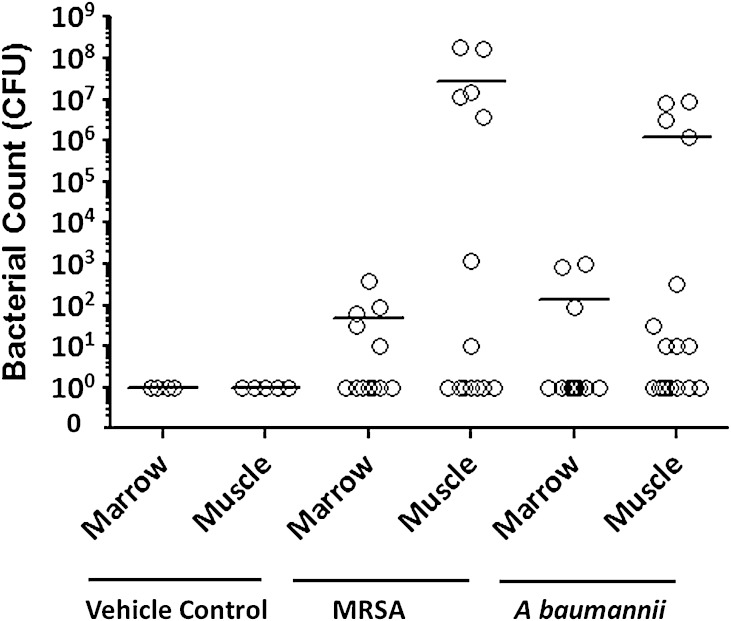
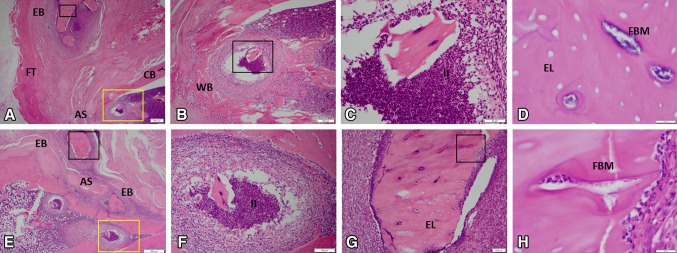
Soft tissue and bone marrow collected from the residual femur from rats inoculated with A baumannii tested negative for A baumannii flora but were positive for other strains of bacteria (1.33 × 102 ± 0.89 × 102; 95% CI, −0.42 × 102–3.08 × 102 and 1.24 × 106 ± 0.69 × 106; 95% CI, −0.13 × 106–2.60 × 106 CFU in bone marrow and muscle tissue, respectively), whereas tissue from MRSA-infected rats contained MRSA only (4.84 × 101 ± 3.22 × 101; 95% CI, −1.47 × 101–11.1 × 102 and 2.80 × 107 ± 1.73 × 107; 95% CI, −0.60 × 107–6.20 × 107) (Table 1). Specifically, bacterial culture results from the surviving MRSA-infected rats showed that in eight of 14 rats the muscle tissue surrounding the amputation site was positive for persistent MRSA infection, whereas five of the 12 bone marrow samples that were available after en bloc resection of two animals were MRSA-positive (Fig. 3). En bloc resection performed on MRSA rats demonstrated evidence of bacterial microcolonies, increased neutrophil infiltration, chronic soft tissue infection, and osteomyelitis (foci of bacterial microcolonies, purulent intramedullary infection, and evidence of bone necrosis indicative of empty osteocytic lacunae with islands of necrotic endochondral bone throughout the skeletal muscle; Fig. 4). All rats inoculated with A baumannii tested negative for the inoculated bacteria in both the soft tissue and bone marrow cultures. However, nine of 16 soft tissue samples and three of the 14 available bone marrow samples had positive cultures at 12 weeks with various bacterial flora species (Fig. 3). En bloc resection of A baumannii residual limbs sent for histology showed inflammatory cells indicative of chronic infection (data not shown); however, representative tissue sections (Fig. 4) failed to capture the periosteal reaction and ectopic bone formation observed on micro-CT (Fig. 2). No bacterial CFUs were detectable in the tissue cultures from vehicle-treated rats.
Table 1.
List of bacteria present in the marrow compartment and soft tissue 12 weeks postinjury
| Tissue | Vehicle control | Acinetobacter baumannii | Methicillin-resistant Staphylococcus aureus |
|---|---|---|---|
| Marrow | Negative | Arcanobacterium haemolyticum; Enterobacter cloacae and Enterococcus faecalis | S aureus |
| Muscle | Negative | Arcanobacterium haemolyticum, Streptococcus porcinus, Staphylococcus cohnii ssp urealyticum, Staphylococcus xylosus, Gardnerella vaginalis, Pasteurella multocida, Enterobacter cloacae, and Enterococcus faecalis | S aureus |
Fig. 3.
Bacterial titers (in CFUs converted to log scale) in the marrow compartment and soft tissue of rats infected with vehicle control (PBS; noninfected control), MRSA, and A baumanni after 12 weeks are shown. Each data point represents the actual CFU value for each animal in each treatment group, whereas the horizontal bar indicates the mean CFU for each treatment group. All rats inoculated with MRSA tested positive for MRSA, whereas rats inoculated with A baumannii tested positive for other microorganisms as detailed in Table 1.
Fig. 4A–H.
The histological features of ectopic bone formation in MRSA-treated rats at 12 weeks are shown in A–H (A, Stain, hematoxylin and eosin; A original magnification, × 1.25; B, original magnification, × 4, yellow boxed region in A; C, original magnification, × 20, black boxed region in B; D, original magnification, × 100, black boxed region in A; E, original magnification, × 2; F, original magnification, × 10, yellow boxed region in E; G, original magnification, × 10, black boxed region in E; H, original magnification, × 10, black boxed region in G). For detailed evaluation, images of six selected regions at higher magnification are shown. In the medullary space and soft tissue, there is evidence of chronic inflammation, neutrophil infiltration, purulent infection, osteomyelitis, and necrotic ectopic bone as indicative of empty osteocytic lacunae containing bacterial microcolonies. AS = amputation site; CB = cortical bone; EL = empty lacunae; EB = ectopic bone; FBM = foci of bacterial microcolonies; FT = fibroblastic tissue; II = intramedullary infection; WB = woven bone.
Discussion
Blast injuries are devastating to the extremities and often include comminuted open fractures, neurovascular injury, soft tissue loss, and traumatic amputations. Wounds often are contaminated with foreign material and microorganisms, including A baumannii and MRSA, which have been proven to exhibit multidrug resistance or relatively high virulence, respectively [2, 6, 16, 21]. Because HO develops within the residual limbs of most blast- and otherwise combat-related amputations [3, 12, 13, 26], considerable efforts are directed toward treating symptoms conservatively; however, surgical resection is ultimately necessary in up to 41% of amputees with HO [30]. As such, considerable focus has been directed toward prevention and mitigation of HO formation; however, understanding factors that exacerbate its development is an important prerequisite. In this effort, we explored, using an established blast-related HO animal model [25], the impact of A baumannii or MRSA colonization on the volume of HO formation and identified the characteristics of chronic infection in each setting.
There are several limitations to our study. First, the rat model is not conducive to many of the surgical modalities used in the treatment of traumatic wounds such as serial débridements with negative pressure wound therapy, which are implicated as putative contributors to HO formation [12]. Second, most war wounds are typically colonized by polymicrobial flora [6]. As such, an inoculum of a specific bacterial pathogen (1 × 106 CFU) does not fully address the synergistic role that polymicrobial infection may have in the persistence and virulence of infection plus it limits our ability to assess differences in HO formation or persistence of infection with varied degrees of infection. Preliminary experiments demonstrated that the bacterial concentration of MRSA used in these studies resulted in established persistent infections with high reproducibility and minimal variation in regard to wound complications. Moreover, it has been reported that approximately 50% of combat wounds become clinically infected (> 1 × 105 CFU) as opposed to merely contaminated [2, 28]. Notably, as a limitation in identifying the presence of all persistent microorganisms, only aerobic wound microflora were cultured from soft tissue and bone marrow. With the expressed intent of describing the impact of microbial bioburden on trauma-induced HO, we acknowledge our limited description of other forms of HO such as genetic and neurogenic. Neurogenic HO has been well described in civilian populations [8, 15], whereas the focus in military research has been predominantly in traumatic HO. In the neurogenic form, neurotransmitters such as glutamate, substance P, and catecholamines act to induce osteoblasts to form ectopic bone within a permissive local environment [5, 18, 27]. Therefore, the induction of progenitor cells with varying osteoinductive factors is common in both traumatic and neurogenic; however, the difference lies in the elevated levels of systemic and local inflammatory cytokines in the former and neurotransmitters in the latter [14]. That having been said, the expression and/or production of inflammatory mediators in this current study was not assessed; therefore, inferences regarding the role of local infection on HO development are based only on histopathological changes noted at study termination.
Contamination of residual limbs with MRSA, but not A baumannii, contributed to the volume of HO that developed in this rat model. This finding is relevant given that MRSA is the predominant organism in 35% to 50% of clinically infected combat wounds [3, 4]. Often, these wounds demand serial débridements to achieve healthy-appearing and/or culture-negative tissue. Despite this, approximately one-fourth of amputations develop late infection after closure of a healthy-appearing wound bed [30]. More débridements, five to seven to be exact, are associated with the development of HO, likely resulting from mechanical trauma to the tissue as well as the systemic inflammatory responses that can result from repeated returns to the operating room [7, 19]. In comparison, our MRSA-inoculated rats developed a greater volume of HO with most (11 of 14 analyzed) doing so in the absence of the described serial surgical interventions, further suggesting MRSA involvement. An unexpected finding of our study is the relative lack of effect of A baumannii on ectopic bone formation despite the selection of the strain based on its clinical ubiquity and relative virulence [2, 29]. This result may be expected given that A baumannii-infected rats cleared their infection. Another explanation involves the signaling of toll-like receptors (TLRs), which are found to be expressed on osteogenic precursor cells [24]. Interestingly, purified lipopolysaccharide, a ligand found on Gram-negative organisms, which has preferential affinity for toll-like receptor 4 (TLR4), demonstrated slow activation of mesenchymal stem cells; however, prolonged exposure to the toxin resulted in decreased expression of TLRs. Alternatively, downregulation of TLRs did not occur with prolonged exposure to the Gram-positive specific cell wall component lipoteichoic acid, perhaps allowing for osteogenic differentiation of mesenchymal stem cells by Gram-positive organisms like MRSA [19, 31]. It may be that infection with Gram-negatives such as A baumannii affects HO development indirectly in clinical practice. This organism is not found in wounds at the time of injury but rather is a nosocomial pathogen found in combat theater hospitals. Infection of wounds during aeromedical evacuation or at combat hospitals may “reinfect” wounds, resulting in serial débridements and negative pressure wound therapy, factors that some infer may contribute to HO development [6, 12, 23].
Chronic infection, like persistently symptomatic HO, can delay or regress the rehabilitation of blast- and otherwise war-related amputees. After combat-related lower extremity amputations, 27% require return to the operating room for wound infection [30] at some point during their hospitalization. In a study of 110 service members with severe orthopaedic wounds that subsequently developed osteomyelitis, a retrospective review showed that A baumannii accounted for 70% of initial admissions to the hospital; however, these responded well to treatment, making up only 6% of recurrences. By comparison, MRSA, presented as the infecting organism in only 8% of initial diagnoses of osteomyelitis, however, was responsible for 31% of readmissions for osteomyelitis with Gram-positives as a whole accounting for 75% of recurrence [33]. In addition, modeling of war wounds in a rabbit demonstrated that monobacterial Gram-negative inoculation at a titer of 1 × 105A baumannii failed to produce osteomyelitis, whereas polymicrobial inoculum and/or those containing MRSA demonstrated active, persistent, infection 8 weeks postinoculation [32]. Our findings also support the persistence of Gram-positive infections with 56% and 21% rates of soft tissue contamination and osteomyelitis in our MRSA cohort, the latter occurring despite the bone not being directly inoculated. Conversely, A baumannii was not present in any 12-week cultures, but rather other antibiotic-resistant ESKAPE pathogens (Enterococcus faecium, Staphylococcus aureus, K pneumoniae, A baumanii, P aeruginosa, and Enterobacter species) such as enteric organisms Enterococcus faecium and Enterobacter cloacae as well as various Gram-positive staphylococci, but not S aureus were isolated at time of culture (Table 1). Negative culture results for A baumannii infection 12 weeks postinoculation are consistent with previous rat studies and may be the result of decreased virulence in bone as compared with other infection sites [9]. Secondary infection with other nosocomial pathogens, particularly Gram-positive organisms, is consistent with clinical findings and suggests that initial infection with A baumannii may produce an environment conducive to secondary infection or overgrowth of other nosocomial organisms [17]. Although the synergism between initial A baumannii infection and subsequent infection still needs to be studied, this result may be informative for clinical treatment plans.
Our study suggests that of the two most common bacterial isolates of combat-related amputations, MRSA infection results in the development of a several-fold increase in the volume of ectopic bone compared with A baumannii and a vehicle control in a rat model. This difference may be related to the microorganisms’ persistent colonization and invocation of chronic infection because this difference was shown in our study minus the surgical treatment already known to influence HO formation. Therefore, in addition to drug therapies that target signaling pathways in bone development and/or proinflammatory osteogenic mediators, we further propose that initiation of prophylactic local and/or systemic Gram-positive antimicrobial therapy at the time of injury and continued treatment of subclinical infection may help mitigate the formation of ectopic bone; further preclinical work, to include assessment of polymicrobial infections, impact of differential TLR signaling, and the evaluation of systemic and/or local antimicrobial interventions, is necessary to further elucidate this effect.
Acknowledgments
We thank Dana Golden, Allison Tomasino, and Erica Crump for assistance with animal care, sample collections, and technical expertise. We also thank Ying Cao for his statistical expertise and assistance. Furthermore, we thank LTC Cary Hannold for his excellent contribution to the histological review of tissue specimens.
Footnotes
This work was supported by USAMRAA BAA #10083004 (Principal Investigator JAF) and USAMRAA PRORP-CDMRP #120056 (Principal Investigator JAF).
All ICMJE Conflict of Interest Forms for authors and Clinical Orthopaedics and Related Research ® editors and board members are on file with the publication and can be viewed on request.
Clinical Orthopaedics and Related Research ® neither advocates nor endorses the use of any treatment, drug, or device. Readers are encouraged to always seek additional information, including FDA-approval status, of any drug or device prior to clinical use.
The authors are employees of the US Government. This work was prepared as part of their official duties. Title 17 USC §105 provides that “Copyright protection under this title is not available for any work of the US Government.” Title 17 USC §101 defined a US Government work as a work prepared by a military service member or employees of the US Government as part of that person’s official duties. The opinions or assertions contained in this paper are the private views of the authors and are not to be construed as reflecting the views, policy or positions of the Departments of the Navy, Department of Defense nor the US Government.
Each author certifies that his or her institution approved the animal protocol for this investigation and that all investigations were conducted in conformity with ethical principles of research.
This work was performed at the Naval Medical Research Center, Silver Spring, MD, USA.
References
- 1.Ahlers ST, Vasserman-Stokes E, Shaughness MC, Hall AA, Shear DA, Chavko M, McCarron RM, Stone JR. Assessment of the effects of acute and repeated exposure to blast overpressure in rodents: toward a greater understanding of blast and the potential ramifications for injury in humans exposed to blast. Front Neurol. 2012;3:32. doi: 10.3389/fneur.2012.00032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Be NA, Allen JE, Brown TS, Gardner SN, McLoughlin KS, Forsberg JA, Kirkup BC, Chromy BA, Luciw PA, Elster EA, Jaing CJ. Microbial profiling of combat wound infection through detection microarray and next-generation sequencing. J Clin Microbiol. 2014;52:2583–2594. doi: 10.1128/JCM.00556-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Brown KV, Dharm-Datta S, Potter BK, Etherington J, Mistlin A, Hsu JR, Clasper JC. Comparison of development of heterotopic ossification in injured US and UK Armed Services personnel with combat-related amputations: preliminary findings and hypotheses regarding causality. J Trauma Inj Infect Crit Care. 2010;69:S116–S122. doi: 10.1097/TA.0b013e3181e44cc7. [DOI] [PubMed] [Google Scholar]
- 4.Burns TC, Stinner DJ, Mack AW, Potter BK, Beer R, Eckel TT, Possley DR, Beltran MJ, Hayda RA, Andersen RC, Keeling JJ, Frisch HM, Murray CK, Wenke JC, Ficke JR, Hsu JR. Microbiology and injury characteristics in severe open tibia fractures from combat. J Trauma Acute Care Surg. 2012;72:1062–1067. doi: 10.1097/TA.0b013e318241f534. [DOI] [PubMed] [Google Scholar]
- 5.Cadosch D, Toffoli AM, Gautschi OP, Frey SP, Zellweger R, Skirving AP, Filgueira L. Serum after traumatic brain injury increases proliferation and supports expression of osteoblast markers in muscle cells. J Bone Joint Surg Am. 2010;92:645–653. doi: 10.2106/JBJS.I.00097. [DOI] [PubMed] [Google Scholar]
- 6.Calhoun JH, Murray CK, Manring M. Multidrug-resistant organisms in military wounds from Iraq and Afghanistan. Clin Orthop Relat Res. 2008;466:1356–1362. doi: 10.1007/s11999-008-0212-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Chavko M, Koller WA, Prusaczyk WK, McCarron RM. Measurement of blast wave by a miniature fiber optic pressure transducer in the rat brain. J Neurosci Methods. 2007;159:277–281. doi: 10.1016/j.jneumeth.2006.07.018. [DOI] [PubMed] [Google Scholar]
- 8.Cipriano CA, Pill SG, Keenan MA. Heterotopic ossification following traumatic brain injury and spinal cord injury. J Am Acad Orthop Surg. 2009;17:689–697. doi: 10.5435/00124635-200911000-00003. [DOI] [PubMed] [Google Scholar]
- 9.Collinet-Adler S, Castro CA, Ledonio CGT, Bechtold JE, Tsukayama DT. Acinetobacter baumannii is not associated with osteomyelitis in a rat model: a pilot study. Clin Orthop Relat Res. 2011;469:274–282. doi: 10.1007/s11999-010-1488-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Davis TA, Lazdun Y, Potter BK, Forsberg JA. Ectopic bone formation in severely combat-injured orthopedic patients—a hematopoietic niche. Bone. 2013;56:119–126. doi: 10.1016/j.bone.2013.05.016. [DOI] [PubMed] [Google Scholar]
- 11.Evans KN, Potter BK, Brown TS, Davis TA, Elster EA, Forsberg JA. Osteogenic gene expression correlates with development of heterotopic ossification in war wounds. Clin Orthop Relat Res. 2014;472:396–404. doi: 10.1007/s11999-013-3325-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Forsberg JA, Pepek JM, Wagner S, Wilson K, Flint J, Andersen RC, Tadaki D, Gage FA, Stojadinovic A, Elster EA. Heterotopic ossification in high-energy wartime extremity injuries: prevalence and risk factors. J Bone Joint Surg Am. 2009;91:1084–1091. doi: 10.2106/JBJS.H.00792. [DOI] [PubMed] [Google Scholar]
- 13.Forsberg JA, Potter BK. Heterotopic ossification in wartime wounds. J Surg Orthop Adv. 2010;19:54–61. [PubMed] [Google Scholar]
- 14.Forsberg JA, Potter BK, Polfer EM, Safford SD, Elster EA. Do inflammatory markers portend heterotopic ossification and wound failure in combat wounds? Clin Orthop Relat Res. 2014;472:1–10. doi: 10.1007/s11999-014-3694-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Garland DE. Clinical observations on fractures and heterotopic ossification in the spinal cord and traumatic brain injured populations. Clin Orthop Relat Res. 1988;233:86–101. [PubMed] [Google Scholar]
- 16.Hospenthal DR, Crouch HK, English JF, Leach F, Pool J, Conger NG, Whitman TJ, Wortmann GW, Robertson JL, Murray CK. Multidrug-resistant bacterial colonization of combat-injured personnel at admission to medical centers after evacuation from Afghanistan and Iraq. J Trauma Inj Infect Crit Care. 2011;71:S52–S57. doi: 10.1097/TA.0b013e31822118fb. [DOI] [PubMed] [Google Scholar]
- 17.Johnson EN, Burns TC, Hayda RA, Hospenthal DR, Murray CK. Infectious complications of open type III tibial fractures among combat casualties. Clin Infect Dis. 2007;45:409–415. doi: 10.1086/520029. [DOI] [PubMed] [Google Scholar]
- 18.Jones KB, Mollano AV, Morcuende JA, Cooper RR, Saltzman CL. Bone and brain: a review of neural, hormonal, and musculoskeletal connections. Iowa Orthop J. 2004;24:123. [PMC free article] [PubMed] [Google Scholar]
- 19.Mo IF, Yip KH, Chan WK, Law HK, Lau YL, Chan GC. Prolonged exposure to bacterial toxins downregulated expression of toll-like receptors in mesenchymal stromal cell-derived osteoprogenitors. BMC Cell Biol. 2008;9:52. doi: 10.1186/1471-2121-9-52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Murray CK, Hinkle MK, Yun HC. History of infections associated with combat-related injuries. J Trauma Inj Infect Crit Care. 2008;64:S221–S231. doi: 10.1097/TA.0b013e318163c40b. [DOI] [PubMed] [Google Scholar]
- 21.Murray CK, Hospenthal DR. Treatment of multidrug resistant Acinetobacter. Curr Opin Infect Dis. 2005;18:502–506. doi: 10.1097/01.qco.0000185985.64759.41. [DOI] [PubMed] [Google Scholar]
- 22.Murray CK, Hsu JR, Solomkin JS, Keeling JJ, Andersen RC, Ficke JR, Calhoun JH. Prevention and management of infections associated with combat-related extremity injuries. J Trauma Inj Infect Crit Care. 2008;64:S239–S251. doi: 10.1097/TA.0b013e318163cd14. [DOI] [PubMed] [Google Scholar]
- 23.Murray CK, Roop SA, Hospenthal DR, Dooley DP, Wenner K, Hammock J, Taufen N, Gourdine E. Bacteriology of war wounds at the time of injury. Mil Med. 2006;171:826–829. doi: 10.7205/MILMED.171.9.826. [DOI] [PubMed] [Google Scholar]
- 24.Pevsner-Fischer M, Morad V, Cohen-Sfady M, Rousso-Noori L, Zanin-Zhorov A, Cohen S, Cohen IR, Zipori D. Toll-like receptors and their ligands control mesenchymal stem cell functions. Blood. 2007;109:1422–1432. doi: 10.1182/blood-2006-06-028704. [DOI] [PubMed] [Google Scholar]
- 25.Polfer EM, Hope DH, Elster EA, Qureshi AT, Golden DM, Potter BK, Davis TA, Forsberg JA. Development of a rat model for blast-related post-traumatic heterotopic ossification. Bone Joint J. 2015;97. [DOI] [PubMed]
- 26.Potter BK, Burns TC, Lacap AP, Granville RR, Gajewski DA. Heterotopic ossification following traumatic and combat-related amputations. Prevalence, risk factors, and preliminary results of excision. J Bone Joint Surg Am. 2007;89:476–486. doi: 10.2106/JBJS.F.00412. [DOI] [PubMed] [Google Scholar]
- 27.Salisbury E, Rodenberg E, Sonnet C, Hipp J, Gannon FH, Vadakkan TJ, Dickinson ME, Olmsted-Davis EA, Davis AR. Sensory nerve induced inflammation contributes to heterotopic ossification. J Cell Biochem. 2011;112:2748–2758. doi: 10.1002/jcb.23225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Sheppard FR, Keiser P, Craft DW, Gage F, Robson M, Brown TS, Petersen K, Sincock S, Kasper M, Hawksworth J. The majority of US combat casualty soft-tissue wounds are not infected or colonized upon arrival or during treatment at a continental US military medical facility. Am J Surg. 2010;200:489–495. doi: 10.1016/j.amjsurg.2010.03.001. [DOI] [PubMed] [Google Scholar]
- 29.Thompson MG, Black CC, Pavlicek RL, Honnold CL, Wise MC, Alamneh YA, Moon JK, Kessler JL, Si Y, Williams R. Validation of a novel murine wound model of Acinetobacter baumannii infection. Antimicrob Agents Chemother. 2014;58:1332–1342. doi: 10.1128/AAC.01944-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Tintle SM, Shawen SB, Forsberg JA, Gajewski DA, Keeling JJ, Andersen RC, Potter BK. Reoperation after combat-related major lower extremity amputations. J Orthop Trauma. 2014;28:232–237. doi: 10.1097/BOT.0b013e3182a53130. [DOI] [PubMed] [Google Scholar]
- 31.Uematsu S, Akira S. Toll-like receptors (TLRs) and their ligands. In: Bauer GH, ed. Toll-like Receptors (TLRs) and Innate Immunity. Handbook of Experimental Pharmacology. Berlin, Heidelberg, Germany: Springer-Verlag; 2008:240. [DOI] [PubMed]
- 32.Yin LY, Manring MM, Calhoun JH. A rabbit osteomyelitis model to simulate multibacterial war wound infections. Mil Med. 2013;178:696–700. doi: 10.7205/MILMED-D-12-00550. [DOI] [PubMed] [Google Scholar]
- 33.Yun HC, Branstetter JG, Murray CK. Osteomyelitis in military personnel wounded in Iraq and Afghanistan. J Trauma Acute Care Surg. 2008;64:S163–S168. doi: 10.1097/TA.0b013e318160868c. [DOI] [PubMed] [Google Scholar]