Abstract
Background
Because immunity against Staphylococcus aureus has not been fully elucidated, there is no diagnostic test to gauge how robust a patient’s host response is likely to be. Therefore, we aimed to develop a test for specific antibodies in serum with diagnostic and prognostic potential.
Questions/Purposes
We describe the development and validation of a multiplex immunoassay for characterizing a patient’s immune response against 14 known S aureus antigens, which we then used to answer four questions: (1) Do certain antigens predominate in the immune response against S aureus? (2) Is there a predominant pattern of antigens recognized by patients and mice with infections? (3) Is the immunoglobulin G (IgG) response to any single antigen a useful predictor of ongoing S aureus infection? (4) Does measurement of the combined response against all 14 antigens provide a better predictor of ongoing infection?
Methods
A case-control study was performed. Sera were collected from 35 consecutive patients with S aureus culture-confirmed (methicillin-sensitive S aureus or methicillin-resistant S aureus) musculoskeletal infections (deep implant-associated, osteomyelitis, and cases of established septic arthritis). Patients were excluded only if they did not give informed consent for participation. Twenty-four patients had implant infections after total joint replacements, five had fracture implant infections, four had native knee infections, and two had chronic osteomyelitis without an implant. Control patients were chosen from a group of healthy, medically optimized patients scheduled to undergo elective arthroplasty. Control patients were matched for age (± 3 years), BMI (± 3 kg/m2), and sex as closely as possible to patients with infections. Sera from patients with S aureus infections and murine S aureus tibial implant infections were used to evaluate a multiplex immunoassay for immunoglobulin titers against 14 recombinant S aureus antigens. All patients were treated with organism-targeted antibiotic therapy and appropriate, timely surgery. Treatment response was monitored with clinical examination, erythrocyte sedimentation rate, C-reactive protein, and resampling of the infection site for the pathogen as needed. Elevated inflammatory markers or persistent positive culture results were considered evidence of ongoing infection. Treatment provided was considered standard-of-care therapy in our medical center and all patients were treated jointly with a board-certified infectious disease specialist.
Results
Four antigens elicited more than 65% of the measurable IgG, the most dominant being against iron-regulated surface determinant protein B (IsdB). Patients with infections had different patterns of elevated IgG titers, so that no single titer was elevated in more than 50% of patients with infections (area under the curve [AUC] ≤ 0.80). Multivariate analysis of IgG titers yielded greater predictive power of S aureus infection (AUC = 0.896). Patients with infections who had high titers against IsdB (median of survivors, 7.28 [25%–75% range, 2.22–21.26] vs median of patients with infection-related death, 40.41 [25%–75% range, 23.57–51.37], difference of medians, 33.13; p = 0.043) and iron-regulated surface determinant protein A (IsdA) median of survivors, 2.21 [25%–75% range, 0.79–9.11] vs median of patients with infection-related death, 12.24 [25%–75% range, 8.85–15.95], difference of medians, 10.03; p = 0.043) were more likely to die from infections than those who did not have high titers of IsdB.
Conclusions
Measurement of the host antibody response is a predictor of ongoing infection that may prove to have prognostic value. Future studies will seek to enlarge the patient population with infections to allow us to reduce the number of antigens required to achieve a stronger predictive power.
Clinical Relevance
Measurement of the immune response against S aureus with this diagnostic tool may help guide future studies on prophylaxis and therapy in an era of personalized medicine and pathogen-specific therapies.
Electronic supplementary material
The online version of this article (doi:10.1007/s11999-015-4354-2) contains supplementary material, which is available to authorized users.
Introduction
Deep musculoskeletal infections, including osteomyelitis associated with prosthetic joint infections, are a major clinical problem and are gradually increasing. Approximately 1 million total joint replacements are performed in the United States annually, and the demand is expected to increase to more than 4 million by 2030 [22]. Even though the introduction of improved surgical and patient-care procedures has reduced the number of prosthetic joint infections, the rate of primary prosthetic joint infections remains in the range of 0.5% to 3% [9, 34]. As a result, there are 20,000 new prosthetic joint infections per year, and this number is expected to increase along with the demand for total joint replacements [23]. The most consequential pathogen is Staphylococcus aureus (S aureus), which accounts for approximately 50% of new infections [9, 11]. Methicillin-resistant S aureus strains infect 100,000 patients and contribute to 18,650 deaths annually [21]. This pathogen further complicates a chronic prosthetic joint infection, which has only a 50% success rate in a two-stage revision [29]. As a result, there is renewed interest in vaccines and immunomodulatory approaches to prevent and treat S aureus osteomyelitis. There is also an increasing need for effective diagnostics of infection and the host response.
Although diagnostic criteria for prosthetic joint infections exist [30], rapid and accurate diagnosis remains challenging for many patients with infections [10, 11, 25]. Additionally, while serum diagnostics are available for several microbial pathogens, no host immunity test is available for S aureus infections. To this end, several groups have described the anti-S aureus humoral immune response in physiologic and pathological situations [12, 13, 33, 39, 41, 42, 44]. Gedbjerg et al. [15] described an antiglucosaminidase antibody test to assess infection and prognosis in patients undergoing orthopaedic surgery who have a confirmed S aureus infection. The results showed an interesting trend from this single antigen analysis that warranted development of a multiplex assay to test the hypothesis that measurement of the magnitude and quality of a patient’s antibody response will provide a diagnostic tool for identifying patients who have ongoing infections and a prognostic tool for directing additional intervention for patients at the greatest risk for poor outcomes.
To address this, we described the development and validation of a multiplex immunoassay for characterizing a patient’s immune response against 14 known S aureus antigens, which we then used to answer four questions: (1) Do certain antigens predominate in the immune response against S aureus for mice and humans? (2) Is there a predominant pattern of antigens recognized by patients and mice with infections? (3) Is the immunoglobulin G (IgG) response to any single antigen a useful predictor of ongoing S aureus infection? (4) Does measurement of the combined response against all 14 antigens provide a better predictor of ongoing infection?
Materials and Methods
Study Design
A case-control study was performed. Sera were collected from 35 consecutive patients with S aureus culture-confirmed (methicillin-sensitive S aureus or methicillin-resistant S aureus) deep musculoskeletal infections (deep implant-associated, osteomyelitis, and established septic arthritis cases). Patients were excluded only if they would not or could not give informed consent for participation. Twenty-four patients had implant infections after total joint replacements, five had fracture implant infections, four had native knee infections, and two had chronic osteomyelitis without an implant. Control patients were chosen from a group of healthy, medically optimized patients scheduled to undergo elective arthroplasties. Control patients were matched for age (± 3 years), BMI (± 3 kg/m2), and sex as closely as possible to patients with infections. Diabetes (p = 0.04), C-reactive protein (p < 0.001), and white blood cell counts (p < 0.001) differed between patients with and without infections (Table 1).
Table 1.
Characteristics of the control patients and patients with infections
| Control patients | Patients with S aureus infection | p value | |
|---|---|---|---|
| Characteristic | 40 patients before total joint replacement | 35 patients with deep musculoskeletal infection§ | |
| Gender (female %) | 55% | 57% | 1.00* |
| Age (years) | 64.4 ± 9.3 | 60.1 ±17.7 | 0.34# |
| BMI (kg/m2) | 31.2 ± 6.4 | 32.9 ± 8.1 | 0.53# |
| Tobacco use (%) | 50% | 40% | 0.43* |
| Diabetes type II (%) | 18% | 40% | 0.04* |
| Renal disease (%) | 17% | 15% | 1.00* |
| Cancer (%) | 20% | 14% | 0.55* |
| Autoimmune disorder (%) | 2.5% | 5.7% | 0.59 * |
| WBC (×103/μL) | 7.5 ± 2.6 | 10.5 ± 4.7 | < 0.001# |
| CRP (mg/dL) | 1.75 ± 2.14 | 11.4 ± 9.69 | < 0.001# |
§24 patients with total joint replacement infection, five with fracture infections, four with joint infections, and two with osteomyelitis without implants; *p using Fisher’s exact test; #p using Mann-Whitney test. WBC = white blood cell count; CRP = C-reactive protein.
Blood samples were drawn at the initial meeting with these patients, and no patient was systemically ill when enrolled in the study. To qualify for enrollment in the study, each patient had to have a confirmed diagnosis of S aureus infection. The collection of human sera was approved by the Research Subjects Review Board, and written informed consent to draw blood and for participation was obtained from every subject. Serum was harvested after centrifugation to remove the clot and red blood cells and stored at −80 °C until assayed.
Animal Sera
To determine whether certain antigens predominate in the immune response against S aureus, the murine and human immune responses were studied and compared to answer research Questions 1 and 2. All animal research was performed under protocols approved by the University Committee on Animal Resources. Animal sera were obtained from Balb/c or C57BL/6 mice (Jackson Laboratory, Bar Harbor, ME, USA). Six-to-eight-week-old female mice were inoculated once (Day 0) or twice (Days 0 and 28) by transtibial pin surgery [26] with a vehicle (Tryptic Soy Broth, S aureus, S epidermidis, S lugdunensis, or Escherichia coli), and sera were collected at Day 14 (single challenge) or Day 42 (twice challenged). The challenge was 5 × 105 colony forming units of the bacteria delivered on a stainless steel implant surgically introduced through the tibia of the mouse.
Recombinant Antigens of S aureus
S aureus antigens were selected by the following criteria: (1) expression by the majority of clinical S aureus isolates; (2) high sequence conservation among strains; (3) display on the cell wall or secretion; and (4) function essential for the growth and survival of S aureus in vivo. Using these criteria, 14 S aureus antigens were chosen (Table 2).
Table 2.
Staphylococcus aureus cell surface and secreted antigens examined
| Antigen | Cell wall-modifying proteins | Iron-regulated surface determinants | Cell wall adhesins | Secreted virulence factors |
|---|---|---|---|---|
| Glucosaminidase (Gmd) | ✓ | |||
| Aminidase (Amd) | ✓ | |||
| Sortase A (SrtA) | ✓ | |||
| Iron-regulated surface determinant protein A (IsdA) | ✓ | |||
| Iron-regulated surface determinant protein B (IsdB) | ✓ | |||
| Iron-regulated surface determinant protein H (IsdH) | ✓ | |||
| Clumping factor A (ClfA) | ✓ | |||
| Clumping factor B (ClfB) | ✓ | |||
| Fibronectin binding protein A (FnbpA) | ✓ | |||
| Coagulase | ✓ | |||
| Alpha hemolysin (α-hemolysin) | ✓ | |||
| Staphylococcal complement inhibitor (SCIN) | ✓ | |||
| Chemotaxis inhibitory protein of staphylococcus aureus (CHIPS) | ✓ | |||
| Extracellular fibronectin-binding protein (Efb) | ✓ |
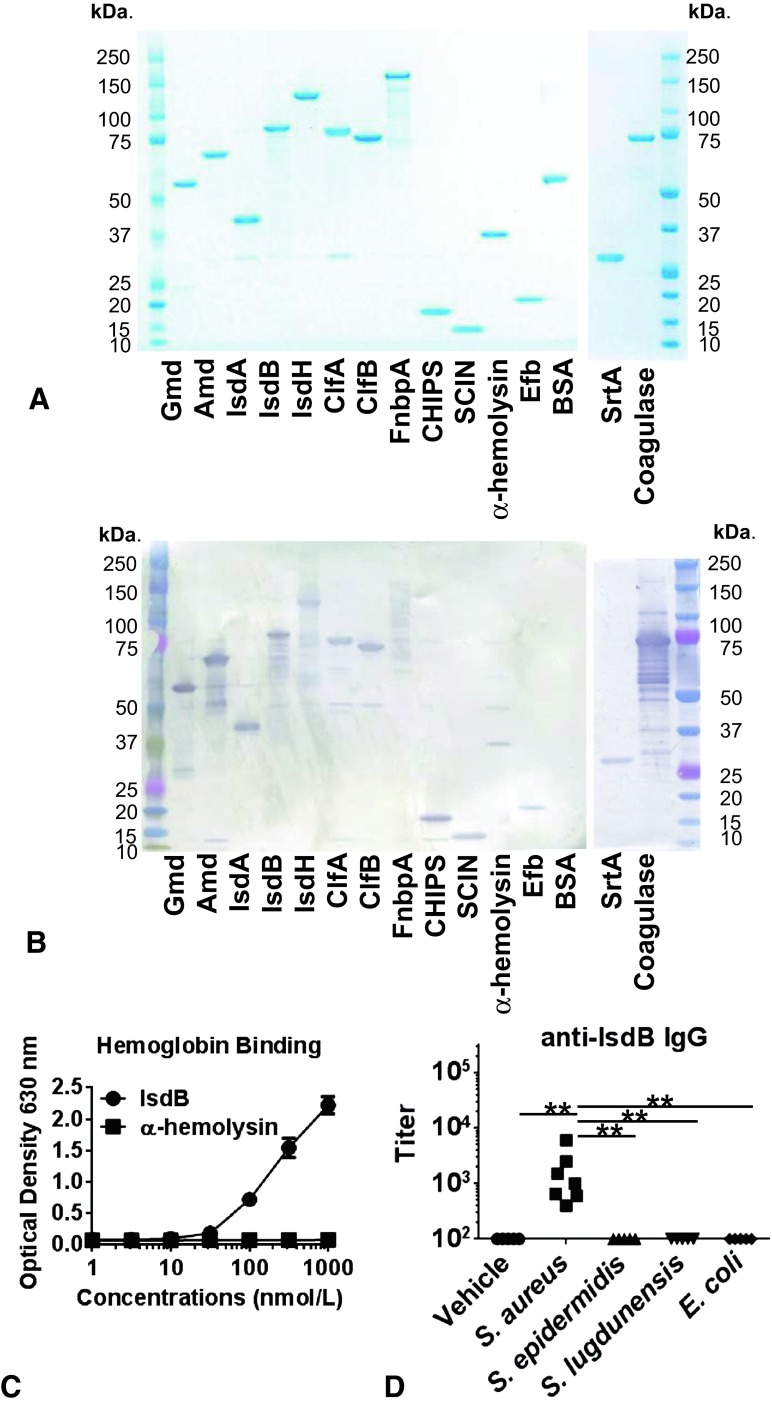
Protein sequences for each antigen available through the National Center for Biotechnology Information (Bethesda, MD, USA) were compiled and consensus protein sequences were determined using Geneious® Pro 5.6.3 (Biomatters Ltd. Auckland, New Zealand). For intracellular expression in E coli, N-terminal signal peptide sequences, and C-terminal anchor domains were removed. N-terminal hexahistidines were added to facilitate purification and C-terminal AvitagTM sequences (Avidity LLC, Aurora, CO, USA) were added to provide unique biotinylation sites to facilitate immobilization on LumAvidin® (Luminex, Austin, TX, USA) microspheres. The corresponding DNA was synthesized de novo, inserted in a puc57 plasmid (GenScript, Piscataway, NJ, USA), and expressed in E coli cotransfected with pBirAcm plasmid (Avidity LLC). Soluble protein was purified by metal chelation chromatography with TALON® resin (Clonetech Laboratories Inc, Mountain View, CA, USA), yielding recombinant protein with a histadine tag on the N-terminus and a single biotin on the C-terminus. For the production of coagulase, a glutathione S-transferase-tag was added on the N-terminus to minimize proteolysis; the glutathione S-transferase-tag was removed after purification [19, 27]. Purity and correct molecular weight were measured by sodium dodecyl sulfate-polyacrylamide gel electrophoresis (SDS-PAGE) (Fig. 1A). The immunoreactivity of each antigen was observed in immunoblots by its reactivity with IgG present in pooled sera from patients with infection (Fig. 1B). Each antigen was more than 90% pure and most ran on SDS-PAGE at the predicted molecular weight; adhesins and iron-regulated surface determinant (Isd) proteins appeared as single bands at higher than predicted molecular weights (Fig. 1A); their identities were confirmed by mass spectrometry, and each antigen reacted with IgG in the pooled positive control sera (Fig. 1B). Antigen authenticity was confirmed using functional assays for each recombinant protein. The functional binding of hemoglobin by IsdB (Fig. 1C) was shown (Appendix 1. Supplemental material is available with the online version of CORR®.) The absence of interspecies crossreactivity of IgG was confirmed for most antigens (IsdA, IsdB, IsdH, clumping factors A and B, fibronectin binding protein A, chemotaxis inhibitory protein of S aureus, staphylococcal complement inhibitor, α-hemolysis, extracellular fibronectin-binding protein, sortase A, and coagulase) by using Day 42 sera from vehicle, S aureus, S epidermidis, S lugdunenesis, or E coli double-challenged Balb/c mice (Fig. 1D). The only exceptions were anti-aminidase and anti-glucosaminidase IgG crossreactivity in some of the sera from S epidermidis and S lugdunensis-challenged mice; this was anticipated based on the high level of amino acid conservation between the autolysins of these three staphylococci. (Appendix 2. Supplemental material is available with the online version of CORR®.)
Fig. 1A–D.
A validation of recombinant S aureus antigens is shown. (A) The 14 recombinant proteins (1μg) and Bovine Serum Albumin (BSA) as a control protein were separated in 4% to 12% gradient sodium dodecyl sulfate-polyacrylamide gel electrophoresis (SDS-PAGE) gels and stained with Coomassie blue, showing that the dominant band in each lane is consistent with the predicted molecular weight of the tagged full-length protein. (B) Identical SDS-PAGE gels were immunoblotted using pooled high-titered human sera as the primary antibody and horseradish peroxidase-conjugated goat-antihuman immunoglobulin G (IgG) as the secondary. The immunoreactivity to the dominant band for each S aureus antigen can be seen. (C) Functional assays specific for each antigen were run to show that each antigen possessed the documented activity of the native protein. A representative example in which the binding of recombinant histadine (His)-IsdB-biotin to immobilized human hemoglobin was detected using a horseradish peroxidase-conjugated streptavidin ELISA is shown. Histadine-α-hemolysis-biotin was used as a negative control. (D) Balb/c mice were challenged with a vehicle, S aureus, S epidermidis, S lugdunensis, or E coli at Days 0 and 28 and sera were collected at Day 42. *p < 0.05, **p < 0.01 with Kruskal-Wallis test. IgG titers against the 14 recombinant antigens were determined by ELISA and data for anti-IsdB titer are presented as a representative example, as infection with other species did not elicit IgG cross-reactive with the S aureus antigens. Gmd = glucosaminidase; Amd = aminidase; IsdA = iron-regulated surface determinant protein A; IsdB = iron-regulated surface determinant protein B; IsdH = iron-regulated surface determinant protein H; ClfA = clumping factor A; ClfB = clumping factor B; FnbpA = fibronectin binding protein A; CHIPS = chemotaxis inhibitory protein of Staphylococcus aureus; SCIN = staphylococcal complement inhibitor; Efb = extracellular fibronectin-binding protein; BSA = bovine serum albumin; SrtA = sortase A.
Multiplex Luminex Assay
Human antibody levels against 14 antigens were determined via multiplex Luminex assay using avidin-coated LumAvidin® microspheres with unique spectral signatures. Spectrally distinct LumAvidin® microspheres were coupled to assigned recombinant proteins and washed. Then the antigen-laden beads were pooled together and incubated with four serial 10-fold dilutions of each serum (1:100 to 1:100,000) in MultiScreen® Filter 96-well plates (Millipore, Billerica, MA, USA) for 2 hours. After washing, the secondary phycoerythrin-conjugated goat anti-human IgG (Southern Biotech, Birmingham, AL, USA) was added and incubated for 1 hour. The fluorescence intensity of the beads and phycoerythrin were measured with a flow cytometer (Bio-Plex® 200; Bio-Rad, Life Sciences Research, Hercules, CA, USA). The accuracy of multiplex antigen measurement was validated by comparison with singleplex measurement using the same serum and also by comparison with the result from conventional ELISA. In every human serum plate, a positive control serum pooled from five patients with infections known to have high titers against histadine-glucosaminidase [1] was included at serial dilutions from 1:100 to 1:316,000. For titer values, each sample was normalized against the mean titer in the 40 control sera which was assigned a value of 1.0; each reported value is presented as a multiple of this value for each antigen, which allows for the broadest distribution in the heat map for all the antigens. For validation of the multiplex assay of 14 antigens, titers from the multiplex analysis were compared with titers from singleplex Luminex assays for each antigen (Fig. 2). The titration curves of the single antigen assay and multiplex assay essentially were identical (intraclass correlation (ICC) = 0.997) (Fig. 2A); similar measurements were made for the other 13 antigens (ICC = 0.994) (Fig. 2B). The titer of specific antibodies against IsdB in the 10 individual human sera (five control patients and five patients with infections) were determined via IsdB ELISA in a 96-well plate and Luminex multiplex assay by two independent investigators (JLD, KN) in blinded manner; mean values were calculated, and the ICC then was calculated. A high level of agreement between the Luminex format and conventional ELISA was observed (ICC = 0.988) (Fig. 2C).
Fig. 2A–C .
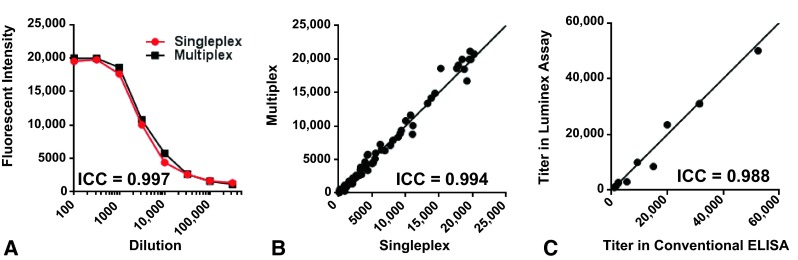
Validation of the multiplex Luminex assays by comparison with single antigen assays is shown. (A) This graph shows a comparison of single-antigen and multiplex Luminex immunoassays. Each of the 14 recombinant proteins was coupled to its assigned LumAvidin™ beads and incubated with a single patient serum at the indicated dilutions either individually (singleplex), or together with the other 13 antigens (multiplex). After a wash step, the secondary antibody, phycoerythrin-conjugated goat-anti-human immunoglobulin (IgG), was added and the fluorescence intensities were measured. Representative data for IsdB are shown. The interclass correlation (ICC) of singleplex versus multiplex assays was measured. (B) Data were generated for the other 13 antigens in single- and multiplex formats, and measured values for all eight dilutions of all 14 antigens were compiled. (C) The ELISA compared with Luminex immunoassay data are shown. The diagonal line in Illustrations B and C represents the formula of y = x.
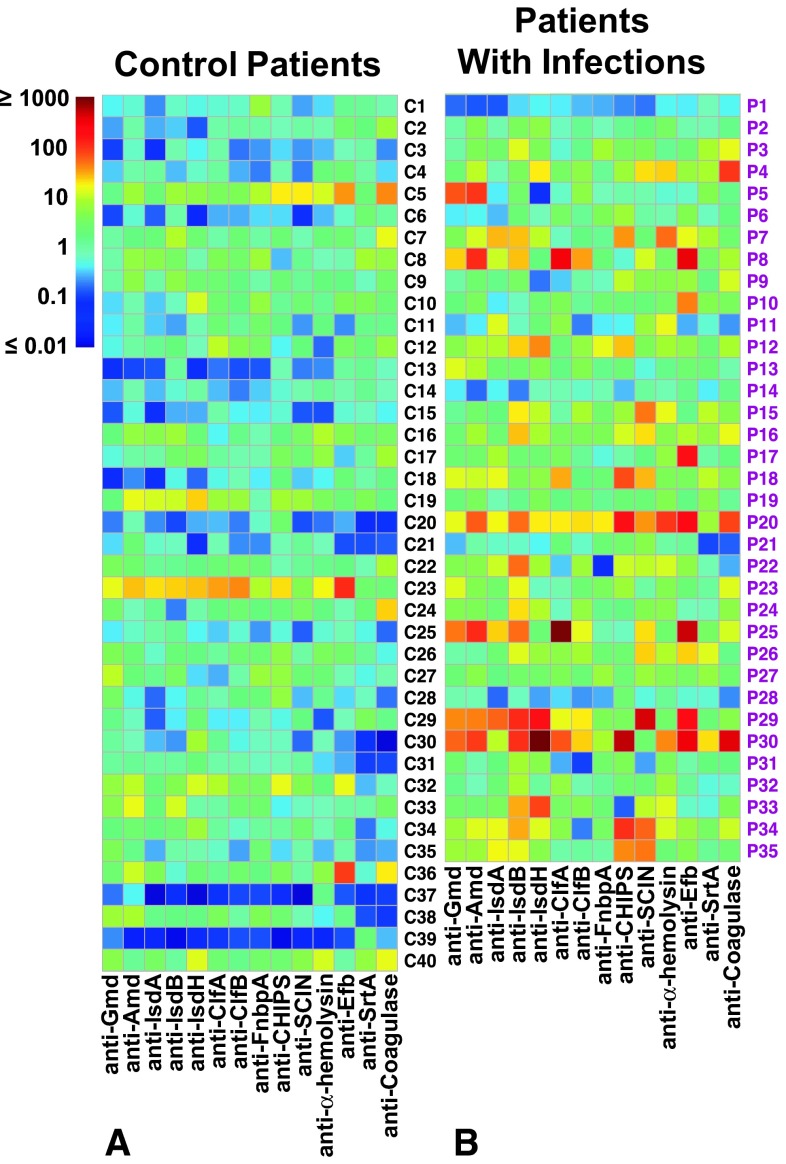
To facilitate the search for patterns in the IgG response among patients, the multiplex IgG titers were compiled into heat maps (Fig. 3). To determine if the IgG titers might have greater predictive power in combination, the results of all 14 antigens were compiled into a single value by multivariable logistic regression analysis to get best separation of control patients and patients with infections.
Fig. 3A–B.
Intraindividual and interindividual variabilities of the increased anti-S aureus immunoglobulin (IgG) response in patients with infection versus healthy control patients are shown. The normalized IgG antibody titers against the 14 antigens in (A) 40 control patients (designated C1 through C40), and (B) 35 patients with S aureus infection sera (designated P1 through P35) were determined by the multiplex Luminex assay, and the data are shown in a heat map format. Although highly variable, global titers were significantly greater in patients with infection versus in control patients (p = 0.00003). Gmd = glucosaminidase; Amd = aminidase; IsdA = iron-regulated surface determinant protein A; IsdB = iron-regulated surface determinant protein B; IsdH = iron-regulated surface determinant protein H; ClfA = clumping factor A; ClfB = clumping factor B; FnbpA = fibronectin binding protein A; CHIPS = chemotaxis inhibitory protein of Staphylococcus aureus; SCIN = staphylococcal complement inhibitor; Efb = extracellular fibronectin-binding protein; SrtA = sortase A.
Clinical Treatments and Definition of Ongoing Infection
All patients with infections were treated with organism-targeted antibiotic therapy, determined in conjunction with a board-certified infectious disease specialist, and surgical intervention consisting of irrigation, débridement, and removal of infected implants with one- or two-stage exchange, as dictated by the specific problem. In the case of native joints, irrigation, débridement, drain insertion, and organism-specific antibiotic therapy were used. Response to treatment was monitored with clinical examination, erythrocyte sedimentation rate, C-reactive protein, and resampling of the infection site for the pathogen as needed. Elevated inflammatory markers, clinically apparent infection, or persistent positive culture results were considered evidence of ongoing infection. Treatment provided was considered standard-of-care therapy in our medical center and all patients were managed jointly with a board-certified infectious disease specialist. Clinical status (alive or dead) also was recorded as the patients were followed for 1 to 3 years. If the patient died, their autopsy report and medical records were examined to identify the cause of death and this was recorded in our data set.
Statistical Analysis
Group comparisons between patients with infections and control patients were performed using the Mann-Whitney U test for ordinal and continuous variables and Fisher’s exact test for categorical variables. The Kruskal-Wallis test with Dunn’s post hoc comparison was used to compare antibody titers among the different bacterial strains in mice. The ICC was determined to validate each Luminex assay. Pearson correlation was used as a measure of linear association of median antibody levels for control patients and patients with infections across antigens. To compare gross antibody levels against all antigens, nonparametric multivariate ANOVA was used. For each antibody in human serum, receiver operating characteristic (ROC) curves were plotted, and area under the curve (AUC) was used as an overall measure of discrimination between control patients and patients with infections. Multivariable logistic regression analysis with 14 antibodies was conducted and the combined diagnostic value of 14 S aureus antibodies was defined as the linear predictor from the logistic model. A p value less than 0.05 was considered significant.
Results
Predominant Pattern of Antigens Recognized by Humans and Mice with Infections
IsdA, IsdB, aminidase, and glucosaminisase are the immunodominant antigens to S aureus in humans and mice. Consistent with S aureus exposure throughout normal life, antibody levels for all 14 antigens were easily detectable in the sera of uninfected human control patients, although the titers varied widely (Fig. 4A). In sera from patients with infections, the median fluorescence intensities were elevated for all antigens except fibronectin-binding protein A compared with those in control patients (p < 0.05), with the greatest elevation observed for IsdB where the median fluorescence intensity increased threefold (control patients: median, 6800 [25%–75% range, 3484–11,963], patients with infections: median, 19,454 [25%–75% range, 10,212–22,833], difference in medians, 12,654; p < 0.001) (Fig. 4B). The relative abundance of IgG for the 14 antigens was similar between patients with infections and control patients (r = 0.95) (Fig. 4C). Overall, the four antigens that elicited the highest mean antibody titers in patients with infection and control patients were IsdB, IsdA, aminidase, and glucosaminidase, which together accounted for more than 65% of the measured IgG. The immunodominance of these same four antigens was observed in S aureus-inoculated mice (Fig. 4D), showing an evolutionarily conserved humoral response against S aureus in mammals (r = 0.89) (Fig. 4E).
Fig. 4A–E.
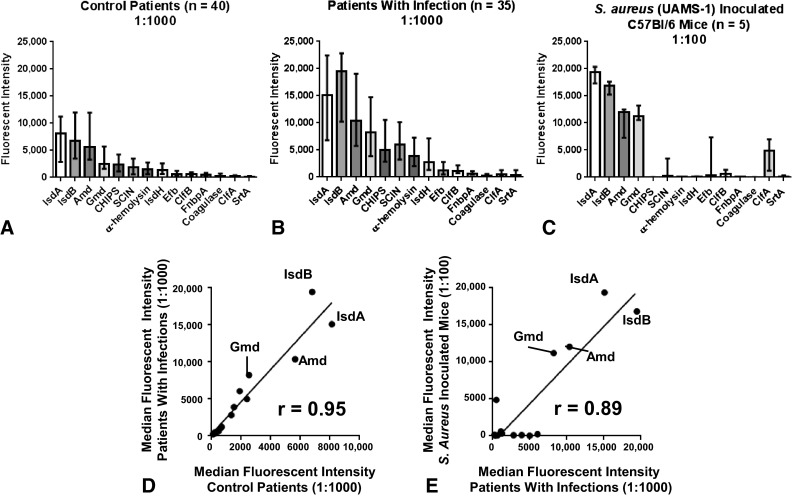
Four of the 14 S aureus antigens (IsdA, IsdB, aminidase, and glucosaminidase) were immunodominant in the sera of infected humans and mice The fluorescent intensity values for each of the 14 antigens were measured in the multiplex Luminex immunoassay using as samples sera from (A) 40 human control patients (diluted 1:1000); (B) 35 patients with S aureus infections (diluted 1:1000); and (C) five C57BL/6 mice which had been challenged once with S aureus (UAMS-1 strain). Median fluorescent intensity values for each antigen are presented along the interquartile range. (D) The immunodominance in control patients and patients with infections is shown. The median fluorescent intensity values of 14 antigens were plotted to determine the correlation between control patients and patients with infections; Pearson R = 0.95 (p < 0.0001). (E) The immunodominance in humans and mice is shown. The median fluorescent intensity values of the 14 antigens were plotted to determine the correlation between titers measured in infected humans and infected mice; Pearson R = 0.89 (p < 0.0001). In each group, more than 2/3 of the measured IgG is specific for just four immunodominant antigens. IsdA = iron-regulated surface determinant protein A; IsdB = iron-regulated surface determinant protein B; Amd = aminidase; Gmd = glucosaminidase; CHIPS = chemotaxis inhibitory protein of Staphylococcus aureus; SCIN = staphylococcal complement inhibitor; IsdH = iron-regulated surface determinant protein H; Efb = extracellular fibronectin-binding protein; ClfB = clumping factor B; FnbpA = fibronectin binding protein A; ClfA = clumping factor A; SrtA = sortase A.
Is the IgG Response to any Antigen a Useful Predictor of Ongoing S aureus Infection?
For individual antigens, we did not identify any antigen titer that was a consistent predictor of S aureus infection, highlighting the great variability in humoral immunity against S aureus.
The data are presented in heat maps (Fig. 3). IgG titers measured in sera from uninfected control patients trended low (dark blue to green), with the exception of titers in four control patients who had significantly elevated levels of IgG for several antigens. In contrast, IgG levels in the sera from patients with infections trended higher, with many yellow and red spots. Three patients with infections had titers below the median of the control patients for more than 10 antigens. Overall, patients with infections had higher anti-S aureus IgG titers than the control patients (p < 0.001) when comparing the 14-dimensional vectors of titers between groups of patients using nonparametric multivariate ANOVA. Looking across the rows (individual patients in the heat map), we did not identify a specific pattern of elevated titers that predominated.
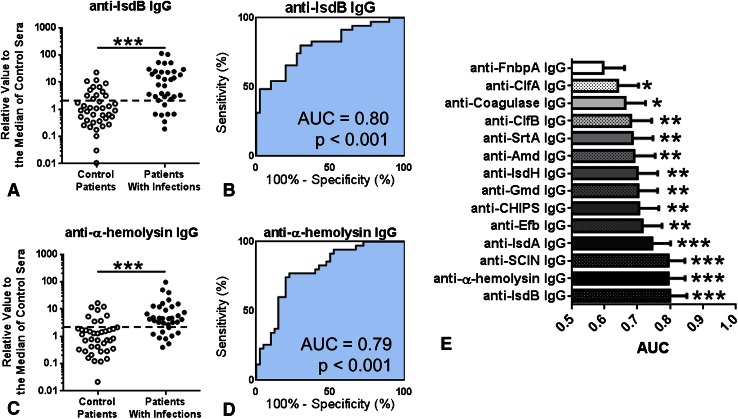
IgG Responses Against IsdB and α-Hemolysin Were the Best Single-antigen Predictors of Ongoing S aureus Infection
Using the calculated AUC in ROC curves derived from the titers, two of the best single antigens were found to be IsdB (control patients: median, 1.00 [25%–75% range, 0.39–3.17], patients with infection: median, 7.90 [25%–75% range, 2.59–3.79], difference in medians, 6.90; p < 0.001) and α-hemolysin (control patients: median, 1.00 [25%–75% range, 0.35–1.90], patients with infection: median, 4.39 [25%–75% range, 2.38–10.46], difference in medians, 3.39; p < 0.001) (Fig. 5A). The range of measured titers in each group spans more than two orders of magnitude, and the overlap between the patients with infections and control patients is more than one order of magnitude. ROC curves with estimated AUC values for the two strongest diagnostic antigens, IsdB (AUC = 0.80 [95% CI, 0.70–0.80], sensitivity 80.0% [95% CI, 63.1%–91.6%], specificity 70.0% [95% CI, 53.5%–83.4%]), and α-hemolysin (AUC = 0.79 [95% CI, 0.69%–0.90%], sensitivity 77.1% [95% CI, 59.9–89.6%], specificity 77.5% [95% CI, 61.6%–89.2%]) revealed that each titer had considerable sensitivity and specificity (Fig. 5A–D). Overall, 13 of 14 antibody titers had an increase in patients with infections and an AUC greater than 0.6 (Fig. 5E); the only exception was fibronectin binding protein A.
Fig. 5A–E.
The diagnostic power of each immunoglobulin G (IgG) antibody titer against the 14 S aureus antigens in the multiplex Luminex assay is shown. (A) Normalized IgG antibody titers for anti-IsdB IgG and were measured in the sera of 40 control patients and 35 patients with infection by the multiplex Luminex assay. The data are plotted as a dot plot in which each dot represents the measured value for one patient. (B) The same data are shown for anti-IsdB area under the curve (AUC). The dashed line in each dot plot is the cutoff value derived from the optimal operating point in the receiver operating characteristic (ROC) curve. The same data are shown for (C) anti-α-hemolysis in the dot plot and the (D) AUC. The clinical predictive measures derived from the ROC curve including the cutoff value, sensitivity (%), specificity (%), and likelihood ratio are shown. (E) The AUC of all 14 antibodies is represented in the order of AUC values. *p < 0.05, **p < 0.01, ***p < 0.001. FnbpA = fibronectin binding protein A; ClfA = clumping factor A; ClfB = clumping factor B; SrtA = sortase A; Amd = aminidase; IsdH = iron-regulated surface determinant protein H; Gmd = glucosaminidase; CHIPS = chemotaxis inhibitory protein of Staphylococcus aureus; Efb = extracellular fibronectin-binding protein; IsdA = iron-regulated surface determinant protein A; SCIN = staphylococcal complement inhibitor; IsdB = iron-regulated surface determinant protein B.
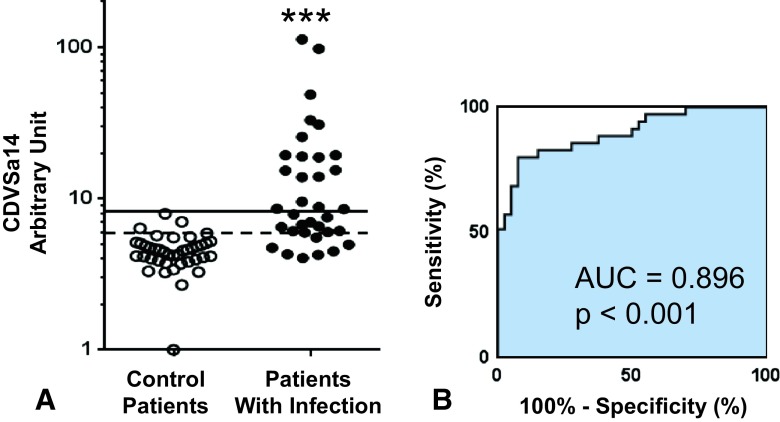
Measurement of the Combined Response Against Al 14 Antigens Provides a Better Predictor of Ongoing Infection
Multivariate analysis using all 14 IgG titers improved prediction of ongoing S aureus infection. The combined diagnostic value of the 14 S aureus antibodies more effectively separated patients from control patients (Fig. 6A), and yielded a ROC curve with an AUC of 0.896 (95% CI, 0.824–0.969; likelihood ratio, 10.7), which was greater than IsdB (p = 0.03), or any other single antigen (Fig. 6B). Sensitivity and specificity improved compared with any single analyte (Fig. 6C). Overall, the sensitivity is 80% (95% CI, 63.06%–91.56%) and specificity is 92.5% (95% CI, 79.61%–98.43%). Using a retrospective cutoff value of 8.23, the combined diagnostic value of the 14 S aureus antibodies achieves 100% (95% CI, 91.19%–100.0%) specificity with more than 50% sensitivity (51.3%; 95% CI, 33.99%–68.62%), meaning that more than ½ of the patients with infections could be diagnosed with an S aureus infection without the risk of treating a patient with a false positive result.
Fig. 6A–B.
The combined diagnostic value of the 14 S aureus antibody titers (CDVSa14) significantly improves the ability to identify patients with infection and control patients. (A) Titer data for the control patients and patients with infection described in Figure 4 were used in a multivariable logistic regression analysis to derive the formula for CDVSa14 = 0.2435 × Gmd – 0.1366 × Amd – 0.1863 × IsdA + 0.2895 × IsdB −0.00240 × IsdH + 0.1062 × ClfA – 0.3982 × ClfB – 0.2856 × FnbpA + 0.0535 × CHIPS + 0.1572 × SCIN + 0.1740 × α-hemolysis + 0.0218 × Efb + 0.4141×SrtA − 0.0688 × Coagulase + 3.952. The CDVSa14 values for each control (open circles) and patient with infection (closed circles) are presented with an optimal operating point cutoff value of 5.93 (dashed line) and 100% specificity cutoff value of 8.23 (solid line); ***p < 0.001 versus control patients. (B) The sensitivity, specificity, and likelihood ratio for the optimal cutoff and 100% specificity cutoff value are shown.
High anti-IsdB and IsdA Titers May be Prognostic Indicators of Poor Outcomes
In a preliminary assessment of the prognostic value of our multiplex assay, we reanalyzed the titers from the four patients with infections who died of infection-related multiple organ failure during the first year after their sera were sampled. The median anti-IsdB and anti-IsdA titers among patients who died were six times greater than those of the survivors. Furthermore, of the 14 antigen titers, only the IgG titers against IsdB (median of survivors, 7.28 [25%–75% range, 2.22–21.26] vs median of patients with infection-related death, 40.41 [25%–75% range, 23.57–51.37], difference of medians, 33.13; p = 0.043) and IsdA (median of survivors, 2.21 [25%–75% range, 0.79–9.11] vs median of patients with infection-related death, 12.24 [25%–75% range, 8.85–15.95], difference of medians, 10.03; p = 0.043) were strong prognostic indicators.
Discussion
Owing to the aging population and the success of total joint replacement as a treatment for end-stage arthritis, the incidence of musculoskeletal infection is gradually increasing, and S aureus is a frequent and often intractable pathogen [9, 22, 23, 34]. Increases in the frequency of multidrug-resistant strains such as methicillin-resistant S aureus and vancomycin-resistant S aureus have reinvigorated research efforts to elucidate host immunity against this pathogen toward species-specific immunologic interventions that ultimately will require improved diagnostics. With the ultimate aim of using the host humoral response as a source of biomarkers for diagnosis and prognosis of deep-seated S aureus infections, we describe a novel immunoassay that measures IgG titers against multiple anti-S aureus antigens in human sera simultaneously.
Our study has several important limitations. First, we selected only a few of the many potentially important S aureus surface or secreted antigens. We chose 14 antigens known to be important, but our selection was not comprehensive; more significant antigens may be discovered in the future. Second, the number of patients with infections and control patients is small, totaling only 75. Our small sample size increases the risk of statistical error—some of our findings could become more or less significant with a larger sample size. Additionally, our population was derived primarily from individuals residing in upstate New York. There may be immunologic differences seen in a geographically diverse patient population. Third, the patient population is heterogeneous. The 35 patients with deep musculoskeletal infections involved in this research mostly had S aureus infections that evolved after total joint replacement, but five patients with native joint infections and two with simple osteomyelitis also were included. Although all patients were treated surgically, the treatments varied from irrigation and débridement and antibiotic therapy to two-stage exchange surgeries for the chronically infected arthroplasties. Some of the patients with infection were considered “referrals of last resort” sent to our quaternary care center for management. This explains the higher than expected mortality rate seen in this case series. In contrast, the control patients were a more homogeneous population scheduled to undergo future total joint replacements. To have access to a larger and more homogeneous population of patients with infected total joint replacements, we have initiated a program to collect patient samples prospectively from sites worldwide. Our findings primarily were associations of antibody responses to patients with infections. Further support for our conclusions comes from the similar responses to S aureus infections seen in mice. This suggests an evolutionary conservation of immune responses to S aureus infection.
Certain Antigens Predominate in the Immune Response Against S aureus
Our multiplex immunoassay focuses on S aureus antigens that are secreted or displayed on the cell wall. The genome of S aureus encodes approximately 2700 proteins including cytoplasmic, membrane bound, cell wall-associated, and secreted proteins [14]. Among these numerous proteins, we focused on cell wall-associated and secreted proteins which are more likely to be accessible to the host immune system. We selected two antigens associated with cell division (glucosaminidase and aminidase), four microbial surface components recognizing adhesive matrix molecules (microbial surface components recognizing adhesive matrix molecules; clumping factor A, clumping factor B, fibronectin binding protein A, coagulase); three iron-regulated surface determinant proteins (IsdA, IsdB, and IsdH); four secreted proteins (α-hemolysis, staphylococcal complement inhibitor, chemotaxis inhibitory protein of S aureus, extracellular fibronectin-binding protein); and one anchor protein (sortase A) that attaches other proteins to the cell wall. These proteins are expressed in most S aureus strains and are highly conserved with pairwise identities between strains ranging from 82.2% (fibronectin binding protein A) to 99.4% (α-hemolysis). The functions of these 14 proteins are very important for S aureus to establish orthopaedic infections. S aureus uses microbial surface components recognizing adhesive matrix molecules and determinant proteins to adhere to host tissue or to the implant surface [1], and they use autolysins (glucosaminidase and aminidase) during cell division [37]. The secreted proteins each interfere with some element of the host immunity and collectively act to thwart the immune response [32]. In addition, some of these 14 proteins are reported to have important roles in building bacterial biofilms to establish chronic infections [4, 6, 18].
We used the Luminex system to measure the multiple antibody levels simultaneously mostly because of its feasibility as a clinical diagnostic tool. The virtue of the Luminex system is its ability to measure multiple antibody titers using a small amount of single serum (less than 1 μL), and it has been used successfully by other investigators [38–42]. We showed that the multiplex Luminex assay is specific for S aureus antigens (Fig. 1D) (Appendix 2. Supplemental material is available with the online version of CORR®), and confirmed that there was no cross-interference in the simultaneous measurement of IgG titers against 14 S aureus antigens and that titers in the multiplex Luminex assay were equivalent to the titers measured with conventional ELISA (Fig. 2). With these validations, we proceeded to measure IgG titers using the sera from our case-control study.
The potential of measurement of host IgG responses for diagnosis and prognosis of patients with deep-seated S aureus infections has not been explored.
With a robust multiplex immunoassay in hand, our next purpose was to determine if measurement of the humoral immune response against S aureus is a plausible diagnostic tool for identification of patients with ongoing S aureus infections. Part of the challenge in measuring the host response is the diversity and abundance of candidate antigens, noted above. Some investigators used whole cells or extracts [16, 20], however, others pointed out the complexity caused by IgG-binding protein A and the potential advantages of using recombinant antigens [16, 17, 20]. Some investigators reported that patients with infection generally had higher antibody levels against purified proteins or recombinant antigens than healthy controls, and observed degrees of overlap between patients with infection and control subjects [8, 13, 33, 44] similar to those reported here. None pursued the IgG responses for use in diagnostic applications. We explored the role of the antigen repertoire in the diagnosis of ongoing S aureus infection.
A Predominant Pattern of Antigens Recognized by Patients or Mice with Infections
Four of the selected antigens were immunodominant in patients with infections, control patients, and mice. In rank order, IgG against IsdA, IsdB, aminidase, and glucosaminidase yielded the highest fluorescence intensity, suggesting that these are immunodominant antigens. In patients with infection, the order of IsdB and IsdA was reversed, but the same four antigens predominated, indicating these four antigens are immunodominant, independent of ongoing infection. Surprisingly, the same four antigens elicited the highest IgG titers in experimentally infected C57BL/6 mice.
There was no predominant pattern of reaction among patients with infection. Examination of the heat map (Fig. 3) reveals several important trends. First, the humoral immune response against S aureus is broad and variable across the antigen repertoire in patients with infection and control patients. No regular pattern was obviously correlated with infection or its absence. Second, patients with infection tended to have higher titers (warmer colors), and the control patients tended to have lower titers (cooler colors), but for any specific antigen the magnitude of the IgG response was overlapping between the two populations. Although patients with infection had higher IgG titers than the control patients, the control patients also had significant IgG titers, some overlapping with the infected population. High IgG titers in noninfected individuals have been observed by others [5, 8, 13, 17, 39–43] and may be related to the high rates of colonization with S aureus, especially among children. Most newborns first contact S aureus just after birth and the colonization rates of S aureus are high in children and adolescents (20%–24]. Even though the colonization rate decreases in adults, most humans are regularly exposed to S aureus possibly with repeated subclinical infections [5]. Moreover, some patients are persistent carriers of S aureus and have higher antibody levels than noncarriers [41, 42]. Therefore in our study, and in contrast to antiviral antibodies [35], control subjects have high IgG titers. With no clear pattern of response among patients with infection and significant antibody levels among control patients, we came to believe that a multiantigen immunoassay would be required to accurately diagnose patients who present with possible orthopaedic infections. Finally, in the IgG heat map, we identified some patients who had low antibody levels for almost all antigens. We considered them to be immunoincompetent. In the absence of other clinical information suggesting being immunocompromised, these patients will be problematic for our immunologic approach.
The IgG Response to Any Single Antigen as a Useful Predictor of Ongoing S aureus Infection
No single IgG response is a useful predictor of ongoing S aureus infection. Although no single IgG titer completely separated control patients and patients with infection, several antigens were better predictors than the others. Notably, anti-IsdB IgG, anti-α-hemolysin IgG, and anti-Staphylococcal complement inhibitor IgG, were the best discriminators with AUC values approximately 0.80. For use as a single antigen immunoassay, we think anti-IsdB IgG is most encouraging because IsdB is expressed in almost all S aureus strains [28], is well conserved among strains, essential for survival in vivo, and one of the most immunodominant antigens.
Measurement of the Combined Response Against all 14 Antigens Provided a Better Predictor of Ongoing Infection
Finally, all 14 IgG titer results were combined as a single ‘composite combined diagnostic value of 14 S aureus antibodies’ value, which is calculated by multivariable logistic regression analysis. The AUC of the single composite combined diagnostic value of 14 S aureus antibodies value was nearly 0.90, which may be clinically useful. The sensitivity (80.0%) and specificity (92.5%) were comparable to those of other diagnostic tests in current clinical use, such as those for anticyclic citrullinated peptide (67% sensitivity and 95% specificity) and rheumatoid factor (69% sensitivity and 85% specificity) [2]. To adapt this method to clinical use, many issues remain to be improved. We used 14 antigens to obtain high AUC, but measuring 14 IgG levels would be cumbersome. Additional efforts to shrink the number of antigens to as few as three or four would be needed. We report the humoral immune response in a musculoskeletal infection with S aureus using sera from a case-control study and show that a multivariate analysis of the IgG response to multiple antigens yielded considerable diagnostic power for ongoing S aureus infections. Additional study is warranted to determine if these measures also have prognostic value. This is suggested by the association of death and measured anti-IsdB IgG levels in our small population.
The primary hypothesis of our study was that certain quantitative and qualitative features of the immune response to S aureus during an ongoing infection can serve as diagnostic and prognostic biomarkers. Currently, the standard assay in most hospitals is bacterial culture. This culture had many limitations despite being familiar, inexpensive, and widely used: (1) the pathogen must be present in the sample so false negatives occur regularly; (2) it takes 24 to 48 hours to yield a definitive result compelling clinicians to use empiric antibiotic therapy; (3) false-positive results can result from contamination during sampling; and (4) false-negative results can occur after ongoing antibiotic therapy. The sampling problem is compounded in chronic implant-associated infections owing to the formation of biofilms that sequester the bacteria. Newer approaches have been introduced to facilitate pathogen recovery in patient specimens including implant sonication [36], and PCR with harvested tissue or joint fluid [3, 4]. To date, these methods are only modest improvements over conventional bacterial culture. Another approach to circumventing the “pathogen-in-the-sample” requirement is diagnosis with circulating bacterial DNA. Diagnosis of sepsis using blood-borne bacterial DNA has been reported [2, 7, 31], with sensitivities greater than 70%. To our knowledge, no reports have addressed deep-seated infections like osteomyelitis or implant-associated infections. A serum-based approach avoids the need for the pathogen in the sample and holds the promise of rapid turnaround times, enabling clinicians to make informed therapeutic interventions sooner. Our study leaves many unanswered questions, including the importance of humoral immunity against S aureus infections, the possibility of pathologic antibodies to S aureus interfering with the clearance of staphylococci, and can any of these responses be modified by external means.
Future directions for our research will focus on analysis of a larger, globally distributed population of patients with implant-associated infections to further refine these serum diagnostic tests. Additionally, further analysis of the human response to the IsdB, and IsdA antigens will be conducted to determine their influence on the murine and human immunity against S aureus. We foresee prognostic value in the measurement of these immune responses and these are foreshadowed by our interest in patients with massively elevated anti-IsdB levels. Clinically, it is our hope to identify, at the earliest possible opportunity, patients who either will or will not require costly and burdensome interventions, ie, to build prognostic and diagnostic tools.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Acknowledgments
We thank Chao Xie MD, PhD, John J. Varrone PhD, Werasak Sutipornpalangkul MD, Sheila N. Bello-Irizarry PhD, Echoe Bouta PhD, Sandeep Soin MD, Rachel LaRosa BS, and Matthew Reader MS (Center for Musculoskeletal Research, University of Rochester Medical Center) for their technical support; Shuichi Matsuda MD, PhD and Hiromu Ito MD, PhD (Department of Orthopaedic Surgery, Graduate School for Medicine, Kyoto University) for their thoughtful discussion; and Sally Quataert Ph|D, Sonia D’Silva PhD and Shelley Secor-Socha BS (Rochester Human Immunology Center, University of Rochester Medical Center) for assistance with Luminex analysis.
Footnotes
The institution of one or more of the authors (EMS, SLK, JLD, CAB) has received, during the study period, funding from the National Institutes of Health, NIAMS (Bethesda, MD, USA) and (EMS, SLK, JLD) the AOTrauma Clinical Priority Program (Davos, Switzerland).
One of the authors certifies that he (JLD) or she or a member of his or her immediate family, has received or may receive payments or benefits, during the study period, an amount of USD 10,000 to 100,000 from Telephus Medical LLC (San Diego, CA, USA) and an amount of less than USD 10,000 from Calorics Pharmaceuticals (Waltham, MA, USA).
One of the authors certifies that he (EMS) or she or a member of his or her immediate family, has received or may receive payments or benefits, during the study period, an amount of USD 10,000 to 100,000 from Telephus Medical LLC (San Diego, CA, USA).
One of the authors certifies that he (SLK) or she or a member of his or her immediate family, has received or may receive payments or benefits, during the study period, an amount of less than USD 10,000 from Surgical Excellence Healthcare Quality Consulting, and an amount of USD 10,000 to 100,000 from Sage Publications (Thousand Oaks, CA, USA) One of the authors certifies that he (CAB) or she or a member of his or her immediate family, has received or may receive payments or benefits, during the study period, an amount of USD 10,000 to 100,000 from the FDA (Silver Spring, MD, USA), and an amount of USD 10,000 to 100,000 from Boston Scientific (Marlborough, MA, USA), an amount of USD 10,000 to 100,000 from Lundbeck Inc (Deerfield, IL, USA), an amount of USD 10,000 to 100,000 from Patient-Centered Outcomes Research Institute (PCORI, Washington, DC, USA), and an amount of less than USD 10,000 from Auspex Pharmaceuticals (La Jolla, CA, USA).
All ICMJE Conflict of Interest Forms for authors and Clinical Orthopaedics and Related Research ® editors and board members are on file with the publication and can be viewed on request.
Clinical Orthopaedics and Related Research ® neither advocates nor endorses the use of any treatment, drug, or device. Readers are encouraged to always seek additional information, including FDA-approval status, of any drug or device prior to clinical use.
Each author certifies that his or her institution approved the human protocol for this investigation that all investigations were conducted in conformity with ethical principles of research, and that informed consent for participation in the study was obtained.
Each author also certifies that his or her institution approved the animal protocol for this investigation and that all investigations were conducted in conformity with ethical principles of research.
References
- 1.Arnold WV, Shirtliff ME, Stoodley P. Bacterial biofilms and periprosthetic infections. J Bone Joint Surg Am. 2013;95:2223–2229. doi: 10.2106/JBJS.2223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Banada PP, Chakravorty S, Shah D, Burday M, Mazzella FM, Alland D. Highly sensitive detection of Staphylococcus aureus directly from patient blood. PLoS One. 2012;7:e31126. doi: 10.1371/journal.pone.0031126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Bogaert D, van Belkum A, Sluijter M, Luijendijk A, de Groot R, Rumke HC, Verbrugh HA, Hermans PW. Colonisation by Streptococcus pneumoniae and Staphylococcus aureus in healthy children. Lancet. 2004;363:1871–1872. doi: 10.1016/S0140-6736(04)16357-5. [DOI] [PubMed] [Google Scholar]
- 4.Bose JL, Lehman MK, Fey PD, Bayles KW. Contribution of the Staphylococcus aureus Atl AM and GL murein hydrolase activities in cell division, autolysis, and biofilm formation. PLoS One. 2012;7:e42244. doi: 10.1371/journal.pone.0042244. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Broker BM, van Belkum A. Immune proteomics of Staphylococcus aureus. Proteomics. 2011;11:3221–3231. doi: 10.1002/pmic.201100010. [DOI] [PubMed] [Google Scholar]
- 6.Caiazza NC, O’Toole GA. Alpha-toxin is required for biofilm formation by Staphylococcus aureus. J Bacteriol. 2003;185:3214–3217. doi: 10.1128/JB.185.10.3214-3217.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Casalta JP, Gouriet F, Roux V, Thuny F, Habib G, Raoult D. Evaluation of the LightCycler SeptiFast test in the rapid etiologic diagnostic of infectious endocarditis. Eur J Clin Microbiol Infect Dis. 2009;28:569–573. doi: 10.1007/s10096-008-0672-6. [DOI] [PubMed] [Google Scholar]
- 8.Clarke SR, Brummell KJ, Horsburgh MJ, McDowell PW, Mohamad SA, Stapleton MR, Acevedo J, Read RC, Day NP, Peacock SJ, Mond JJ, Kokai-Kun JF, Foster SJ. Identification of in vivo-expressed antigens of Staphylococcus aureus and their use in vaccinations for protection against nasal carriage. J Infect Dis. 2006;193:1098–1108. doi: 10.1086/501471. [DOI] [PubMed] [Google Scholar]
- 9.Cram P, Lu X, Kates SL, Singh JA, Li Y, Wolf BR. Total knee arthroplasty volume, utilization, and outcomes among Medicare beneficiaries, 1991–2010. JAMA. 2012;308:1227–1236. doi: 10.1001/2012.jama.11153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Cunha BA, Petelin A, George S. Fever of unknown origin (FUO) in an elderly adult due to Epstein-Barr virus (EBV) presenting as “typhoidal mononucleosis,” mimicking a lymphoma. Heart Lung. 2013;42:79–81. doi: 10.1016/j.hrtlng.2012.06.004. [DOI] [PubMed] [Google Scholar]
- 11.Del Pozo JL, Patel R. Clinical practice: infection associated with prosthetic joints. N Engl J Med. 2009;361:787–794. doi: 10.1056/NEJMcp0905029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.den Reijer PM, Lemmens-den Toom N, Kant S, Snijders SV, Boelens H, Tavakol M, Verkaik NJ, van Belkum A, van Verbrugh HA, Wamel WJ. Characterization of the humoral immune response during Staphylococcus aureus bacteremia and global gene expression by Staphylococcus aureus in human blood. PLoS One. 2013;8:e53391. doi: 10.1371/journal.pone.0053391. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Dryla A, Prustomersky S, Gelbmann D, Hanner M, Bettinger E, Kocsis B, Kustos T, Henics T, Meinke A, Nagy E. Comparison of antibody repertoires against Staphylococcus aureus in healthy individuals and in acutely infected patients. Clin Diagn Lab Immunol. 2005;12:387–398. doi: 10.1128/CDLI.12.3.387-398.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Dunman PM, Murphy E, Haney S, Palacios D, Tucker-Kellogg G, Wu S, Brown EL, Zagursky RJ, Shlaes D, Projan SJ. Transcription profiling-based identification of Staphylococcus aureus genes regulated by the agr and/or sarA loci. J Bacteriol. 2001;183:7341–7353. doi: 10.1128/JB.183.24.7341-7353.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Gedbjerg N, LaRosa R, Hunter JG, Varrone JJ, Kates SL, Schwarz EM, Daiss JL. Anti-glucosaminidase IgG in sera as a biomarker of host immunity against Staphylococcus aureus in orthopaedic surgery patients. J Bone Joint Surg Am. 2013;95:e171. doi: 10.2106/JBJS.L.01654. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Hershman MJ, Appel SH, George CD, Cost KM, Polk HC., Jr The use of a new assay for detecting antibody to Staphylococcus aureus in severely injured patients. J Trauma. 1989;29:75–78. doi: 10.1097/00005373-198901000-00015. [DOI] [PubMed] [Google Scholar]
- 17.Holtfreter S, Kolata J, Broker BM. Towards the immune proteome of Staphylococcus aureus: the anti-S aureus antibody response. Int J Med Microbiol. 2010;300:176–192. doi: 10.1016/j.ijmm.2009.10.002. [DOI] [PubMed] [Google Scholar]
- 18.Houston P, Rowe SE, Pozzi C, Waters EM, O’Gara JP. Essential role for the major autolysin in the fibronectin-binding protein-mediated Staphylococcus aureus biofilm phenotype. Infect Immun. 2011;79:1153–1165. doi: 10.1128/IAI.00364-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Itoh S, Yokoyama R, Kamoshida G, Fujiwara T, Okada H, Takii T, Tsuji T, Fujii S, Hashizume H, Onozaki K. Staphylococcal superantigen-like protein 10 (SSL10) inhibits blood coagulation by binding to prothrombin and factor Xa via their γ-carboxyglutamic acid (Gla) domain. J Biol Chem. 2013;288:21569–21580. doi: 10.1074/jbc.M113.451419. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Jarlov JO, Christensson B, Espersen F, Hertz JB, Hedstrom SA. Antibody response against whole Staphylococcus aureus in patients with staphylococcal septicaemia and endocarditis investigated by ELISA. Acta Pathol Microbiol Immunol Scand B. 1985;93:307–313. doi: 10.1111/j.1699-0463.1985.tb02893.x. [DOI] [PubMed] [Google Scholar]
- 21.Klevens RM, Morrison MA, Nadle J, Petit S, Gershman K, Ray S, Harrison LH, Lynfield R, Dumyati G, Townes JM, Craig AS, Zell ER, Fosheim GE, McDougal LK, Carey RB, Fridkin SK. Active Bacterial Core surveillance (ABCs) MRSA Investigators. Invasive methicillin-resistant Staphylococcus aureus infections in the United States. JAMA. 2007;298:1763–1771. doi: 10.1001/jama.298.15.1763. [DOI] [PubMed] [Google Scholar]
- 22.Kurtz S, Ong K, Lau E, Mowat F, Halpern M. Projections of primary and revision hip and knee arthroplasty in the United States from 2005 to 2030. J Bone Joint Surg Am. 2007;89:780–785. doi: 10.2106/JBJS.F.00222. [DOI] [PubMed] [Google Scholar]
- 23.Kurtz SM, Lau E, Schmier J, Ong KL, Zhao K, Parvizi J. Infection burden for hip and knee arthroplasty in the United States. J Arthroplasty. 2008;23:984–991. doi: 10.1016/j.arth.2007.10.017. [DOI] [PubMed] [Google Scholar]
- 24.Lebon A, Labout JA, Verbrugh HA, Jaddoe VW, Hofman A, van Wamel W, Moll HA, van Belkum A. Dynamics and determinants of Staphylococcus aureus carriage in infancy: the Generation R Study. J Clin Microbiol. 2008;46:3517–3521. doi: 10.1128/JCM.00641-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Lew DP, Waldvogel FA. Osteomyelitis. Lancet. 2004;364:369–379. doi: 10.1016/S0140-6736(04)16727-5. [DOI] [PubMed] [Google Scholar]
- 26.Li D, Gromov K, Soballe K, Puzas JE, O’Keefe RJ, Awad H, Drissi H, Schwarz EM. Quantitative mouse model of implant-associated osteomyelitis and the kinetics of microbial growth, osteolysis, and humoral immunity. J Orthop Res. 2008;26:96–105. doi: 10.1002/jor.20452. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.McAdow M, DeDent AC, Emolo C, Cheng AG, Kreiswirth BN, Missiakas DM, Schneewind O. Coagulases as determinants of protective immune responses against Staphylococcus aureus. Infect Immun. 2012;80:3389–3398. doi: 10.1128/IAI.00562-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.McCarthy AJ, Lindsay JA. Genetic variation in Staphylococcus aureus surface and immune evasion genes is lineage associated: implications for vaccine design and host-pathogen interactions. BMC Microbiol. 2010;10:173. doi: 10.1186/1471-2180-10-173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Parvizi J, Azzam K, Ghanem E, Austin MS, Rothman RH. Periprosthetic infection due to resistant staphylococci: serious problems on the horizon. Clin Orthop Relat Res. 2009;467:1732–1739. doi: 10.1007/s11999-009-0857-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Parvizi J, Zmistowski B, Berbari EF, Bauer TW, Springer BD, Della Valle CJ, Garvin KL, Mont MA, Wongworawat MD, Zalavras CG. New definition for periprosthetic joint infection: from the Workgroup of the Musculoskeletal Infection Society. Clin Orthop Relat Res. 2011;469:2992–2994. doi: 10.1007/s11999-011-2102-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Peters RP, van Agtmael MA, Gierveld S, Danner SA, Groeneveld AB, Vandenbroucke-Grauls CM, Savelkoul PH. Quantitative detection of Staphylococcus aureus and Enterococcus faecalis DNA in blood to diagnose bacteremia in patients in the intensive care unit. J Clin Microbiol. 2007;45:3641–3646. doi: 10.1128/JCM.01056-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Rooijakkers SH, Ruyken M, Roos A, Daha MR, Presanis JS, Sim RB, van Wamel WJ, van Kessel KP, van Strijp JA. Immune evasion by a staphylococcal complement inhibitor that acts on C3 convertases. Nat Immunol. 2005;6:920–927. doi: 10.1038/ni1235. [DOI] [PubMed] [Google Scholar]
- 33.Royan S, Sharp L, Nair SP, Crean S, Henderson B, Poole S, Scott GL, Evans AW. Identification of the secreted macromolecular immunogens of Staphylococcus aureus by analysis of serum. FEMS Immunol Med Microbiol. 2000;29:315–321. doi: 10.1111/j.1574-695X.2000.tb01539.x. [DOI] [PubMed] [Google Scholar]
- 34.Schenker ML, Yannascoli S, Baldwin KD, Ahn J, Mehta S. Does timing to operative debridement affect infectious complications in open long-bone fractures? A systematic review. J Bone Joint Surg Am. 2012;94:1057–1064. doi: 10.2106/JBJS.K.00582. [DOI] [PubMed] [Google Scholar]
- 35.Sobarzo A, Perelman E, Groseth A, Dolnik O, Becker S, Lutwama JJ, Dye JM, Yavelsky V, Lobel L, Marks RS. Profiling the native specific human humoral immune response to Sudan Ebola virus strain Gulu by chemiluminescence enzyme-linked immunosorbent assay. Clin Vaccine Immunol. 2012;19:1844–1852. doi: 10.1128/CVI.00363-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Trampuz A, Piper KE, Jacobson MJ, Hanssen AD, Unni KK, Osmon DR, Mandrekar JN, Cockerill FR, Steckelberg JM, Greenleaf JF, Patel R. Sonication of removed hip and knee prostheses for diagnosis of infection. N Engl J Med. 2007;357:654–663. doi: 10.1056/NEJMoa061588. [DOI] [PubMed] [Google Scholar]
- 37.Varrone JJ, de Mesy Bentley K, Bello-Irizarry SN, Nishitani K, Mack S, Hunter JG, Kates S, Daiss JL, Schwarz E. Passive immunization with anti-glucosaminidase monoclonal antibodies protects mice from implant-associated osteomyelitis by mediating opsonophagocytosis of Staphylococcus aureus megaclusters. J Orthop Res. 2014;32:1389–1396. doi: 10.1002/jor.22672. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Verkaik N, Brouwer E, Hooijkaas H, van Belkum A, van Wamel W. Comparison of carboxylated and Penta-His microspheres for semi-quantitative measurement of antibody responses to His-tagged proteins. J Immunol Methods. 2008;335:121–125. doi: 10.1016/j.jim.2008.02.022. [DOI] [PubMed] [Google Scholar]
- 39.Verkaik NJ, Boelens HA, de Vogel CP, Tavakol M, Bode LG, Verbrugh HA, van Belkum A, van Wamel WJ. Heterogeneity of the humoral immune response following Staphylococcus aureus bacteremia. Eur J Clin Microbiol Infect Dis. 2010;29:509–518. doi: 10.1007/s10096-010-0888-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Verkaik NJ, Dauwalder O, Antri K, Boubekri I, de Vogel CP, Badiou C, Bes M, Vandenesch F, Tazir M, Hooijkaas H, Verbrugh HA, van Belkum A, Etienne J, Lina G, Ramdani-Bouguessa N, van Wamel WJ. Immunogenicity of toxins during Staphylococcus aureus infection. Clin Infect Dis. 2010;50:61–68. doi: 10.1086/648673. [DOI] [PubMed] [Google Scholar]
- 41.Verkaik NJ, de Vogel CP, Boelens HA, Grumann D, Hoogenboezem T, Vink C, Hooijkaas H, Foster TJ, Verbrugh HA, van Belkum A, van Wamel WJ. Anti-staphylococcal humoral immune response in persistent nasal carriers and noncarriers of Staphylococcus aureus. J Infect Dis. 2009;199:625–632. doi: 10.1086/596743. [DOI] [PubMed] [Google Scholar]
- 42.Verkaik NJ, Lebon A, de Vogel CP, Hooijkaas H, Verbrugh HA, Jaddoe VW, Hofman A, Moll HA, van Belkum A, van Wamel WJ. Induction of antibodies by Staphylococcus aureus nasal colonization in young children. Clin Microbiol Infect. 2010;16:1312–1317. doi: 10.1111/j.1469-0691.2009.03073.x. [DOI] [PubMed] [Google Scholar]
- 43.Verkaik NJ, van Wamel WJ, van Belkum A. Immunotherapeutic approaches against Staphylococcus aureus. Immunotherapy. 2011;3:1063–1073. doi: 10.2217/imt.11.84. [DOI] [PubMed] [Google Scholar]
- 44.Wheat J. Diagnostic strategies in osteomyelitis. Am J Med. 1985;78:218–224. doi: 10.1016/0002-9343(85)90388-2. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.