Abstract
Background
Short-term muscle atrophy induced by botulinum toxin A (BTxA) has been observed to impair osteogenesis in a rat closed femur fracture model. However, it is unclear whether the underlying mechanism is a direct effect of BTxA on muscle-bone interactions or an indirect effect that is driven by skeletal unloading. Because skeletal trauma in the closed fracture model also leads to disuse atrophy, we sought to mitigate this confounding variable by examining BTxA effects on muscle-bone interactions in two complementary in vivo models in which osteogenesis is induced in the absence of skeletal unloading. The overall aim of this study was to identify a potential strategy to inhibit pathological bone formation and heterotopic ossification (HO).
Questions/purposes
(1) Does muscle paralysis inhibit periosteal osteogenesis induced by a transcortical defect? (2) Does muscle paralysis inhibit heterotopic bone formation stimulated by intramuscular bone morphogenetic protein (BMP) injection?
Methods
Focal osteogenesis was induced in the right hindlimb of mice through surgical initiation of a small transcortical defect in the tibia (fracture callus; n = 7/group) or intramuscular injection of BMP-2 (HO lesion; n = 6/group), both in the presence/absence of adjacent calf paralysis. High-resolution micro-CT images were obtained in all experimental groups 21 days postinduction and total volume (ie, perimeter of periosteal callus or HO lesion) and bone volume (calcified tissue within the total volume) were quantified as primary outcome measures. Finally, these outcome measures were compared to determine the effect of muscle paralysis on inhibition of local osteogenesis in both studies.
Results
After a transcortical defect, BTxA-treated mice showed profound inhibition of osteogenesis in the periosteal fracture callus 21 days postsurgery compared with saline-treated mice (total volume: 0.08 ± 0.06 versus 0.42 ± 0.11 mm3, p < 0.001; bone volume: 0.07 ± 0.05 versus 0.32 ± 0.07 mm3, p < 0.001). Similarly, BMP-2-induced HO formation was inhibited by adjacent muscle paralysis at the same time point (total volume: 1.42 ± 0.31 versus 3.42 ± 2.11 mm3, p = 0.034; bone volume: 0.68 ± 0.18 versus 1.36 ± 0.79 mm3, p = 0.045).
Conclusions
Our data indicate that BTxA-induced neuromuscular inhibition mitigated osteogenesis associated with both a transcortical defect and BMP-2-induced HO.
Clinical Relevance
Focal neuromuscular inhibition represents a promising new approach that may lead to a new clinical intervention to mitigate trauma-induced HO, a healthcare challenge that is severely debilitating for civilian and war-wounded populations, is costly to both the patient and the healthcare system, and currently lacks effective treatments.
Introduction
Heterotopic ossification (HO) is a frequent complication of musculoskeletal trauma after joint arthroplasty, hip and elbow fractures, and amputation [9, 11, 24, 31]. Because traumatic brain injury is an additional risk factor for HO [7, 14], high-energy wartime extremity injuries place war-wounded patients at an increased risk of developing debilitating heterotopic lesions [10]. Although the pathophysiology of HO is not completely clarified, traumatic insults impacting the neuromuscular system are known to initiate an inflammatory cascade leading to heterotopic bone formation. Indeed, recent data demonstrate that dysregulation of bone morphogenetic protein 4 (BMP-4) at the neuromuscular junction is a precursor of HO [8, 16]. This pathway therefore implicates a causal link between neuromuscular dysfunction and the development of HO.
Others have recently shown that transient paralysis of the quadriceps induced by botulinum toxin A (BTxA) inhibits periosteal osteogenesis and callus formation in a rat femur fracture model [3, 13]. Given the known mechanism of action of BTxA (inhibition of neurotransmitter release), we therefore speculated that the blockade of neuromuscular signaling by BTxA inhibits neuromuscular interactions integral to the osteogenic response elicited by musculoskeletal trauma. Furthermore, because fracture healing and HO share common initiating events (including inflammation and BMP signaling) [17, 27, 30, 32], we also speculated that BTxA-induced muscle paralysis would prevent the formation of heterotopic bone. However, interpreting the precise mechanism by which muscle paralysis inhibits osteogenesis in the rat fracture model is confounded by the heterogeneity of the tissue response, soft tissue injury, and modified gait kinematics, all of which can alter the observed cellular responses.
To explore the interactions between neuromuscular signaling and osteogenesis, we superimposed BTxA-induced calf paralysis on two established in vivo models of osteogenesis. The mouse transcortical defect model produces a morphologically reproducible periosteal callus in the tibia with less soft tissue trauma and without the mechanical deficits observed in closed fracture models [25]. Complementing this model, we implemented a model of HO in which an ectopic bone lesion is induced by intramuscular injection of BMP [20].
Therefore, the purpose of this study was to determine if the inhibition of neuromuscular signaling by BTxA is sufficient to prevent the osteogenic response to skeletal injury and/or BMP-induced bone formation. By superimposing transient muscle paralysis on two distinct models of focal osteogenesis, we sought to address whether (1) muscle paralysis inhibits periosteal osteogenesis induced by a transcortical defect; and (2) if muscle paralysis inhibits heterotopic bone formation stimulated by intramuscular BMP injection.
Materials and Methods
All animal experiments were performed using protocols and procedures approved by the Institutional Animal Care and Use Committee of the University of Washington. Female C57Bl/6 mice were obtained from Jackson Laboratories (Sacramento, CA, USA) with studies initiated in skeletally mature adult mice (ages 4–5 months at the start of the experiment). In all studies, animals were allowed free cage activity and food and water ad libitum throughout the experiment.
The first study determined the effect of adjacent muscle paralysis on periosteal osteogenesis after a transcortical defect of the mouse tibia. In this experiment, female C57 mice were randomized to (1) transcortical defect + saline; and (2) transcortical defect + BTxA (n = 7 per group). On Day 0 of the experiment, mice were anesthetized with isoflurane and an incision was made over the anteromedial surface of the right tibial diaphysis. The periosteal surface was exposed and a 0.6-mm penetrating hole was created in the medial cortex 1 mm distal from the termination of the tibial tuberosity (Fig. 1). While still anesthetized, the gastrocnemius of mice was either injected with 20 µL saline or BTxA (2.0 U/100 g body weight) in a volume of 20 µL [33]. All mice were observed postoperatively to ensure return to normal ambulation and pain was managed prophylactically through a single subcutaneous injection of Buprenex (0.05 mg/kg; Reckitt Benckiser Pharmaceuticals Inc, Richmond, VA, USA). One mouse was removed from the BTxA-injected group as a result of a fracture that occurred during postsurgical handling. Calf muscle paralysis was confirmed 24 hours postinjection by visual confirmation of reduced toe extension and ankle plantar flexion in the affected limb [33]. Immediately after surgery and injections, mice underwent micro-CT scans (Scanco vivaCT 40; 10.5 µm voxel resolution; Scanco Medical, Bruttisellen, Switzerland) to confirm the location of the transcortical defect. A second ex vivo micro-CT scan was obtained after animal euthanasia 21 days postsurgery for quantification of fracture callus formation.
Fig. 1.

Whole bone micro-CT scan shows the location of the transcortical defect. The surgically induced defect was 0.6 mm in diameter and was drilled approximately 1 mm proximal to the tibiofibular junction.
The second study determined the effect of transient muscle paralysis on BMP-2-induced HO formation. In this experiment, female C57 mice were randomized to (1) BMP-2 + saline; and (2) BMP-2 + BTxA (n = 6 per group). To remove any potential confounding effects of injection interactions, BTxA and BMP-2 were dosed on consecutive days. Twenty-four hours before the BMP-2 implantation (Day -1), anesthetized mice received intramuscular injections in their right gastrocnemius of either 20 µL saline or BTxA (2.0 U/100 g body weight) in a volume of 20 µL. On Day 0 of the experiment, implants were prepared by adding recombinant human BMP-2 (Syd Labs Inc, Boston, MA, USA) to liquid Cultrex basement membrane extract, PathClear (BME; Trevigen Inc, Gaithersburg, MD, USA) at 4 °C. All mice were anesthetized and their gastrocnemii were injected with a dose of BMP-2/BME solution shown in pilot studies to produce a consistent HO lesion in the calf muscle groups (2.5 µg BMP-2/20 µL of BME). On Day 21, micro-CT topogram (or “micro-CT scout”) images were obtained to determine the location of the heterotopic lesion within the affected muscle. Based on topogram images, mice underwent micro-CT scans (Scanco vivaCT 40; 21-μm voxel resolution) originating from the tibiofibular junction and continued proximally sufficient to image the entire heterotopic lesion. Before data analysis, one mouse was removed from the BTxA injection group as a result of its heterotopic lesion fusing with the fibula, which precluded accurate quantification of HO morphology using a user-invariant quantification algorithm described subsequently.
All high-resolution micro-CT images were acquired using a Scanco vivaCT 40 (55 kVP, 145 μA). Bone was segmented within the scan volume using standard image thresholding techniques [29] and threshold values specific for both callus and HO were identified as that which provided the best correspondence between two-dimensional binarized images and the original gray-scale images [4]. In the transcortical defect experiment, ex vivo scans were obtained by placing the dissected tibiae into a customized apparatus and a 2-mm long image volume centered about the defect was obtained. Semiautomated contours were created throughout the scan volume that separated the periosteal fracture callus from the periosteal bone surface. Once isolated, a threshold of 615.9 mg HA/ccm was used to identify bone within the periosteal fracture callus. In the HO experiment, mice were induced and maintained under isoflurane anesthesia and in vivo scans were obtained by securing the right hindlimb with a custom apparatus to maximize leg stability during the scan process. Once scans were obtained, HO volumes were isolated using an automated contouring algorithm. A threshold of 480.7 mg HA/ccm was used to identify calcified tissue within the heterotopic nodule. Standard bone morphologic parameters (described subsequently) were obtained in both the transcortical defect and HO experiments.
Two primary outcome measures were quantified in each experiment: (1) total volume (perimeter of periosteal callus or HO lesion); and (2) bone volume (calcified tissue within the total volume). Homoscedastic one-tailed t-tests were used to determine the effect of muscle paralysis on inhibition of local osteogenesis in both studies. Based on our previous pilot data suggesting a 50% reduction in osteogenic potential in both models and an alpha level of 0.05, the group sizes in each experiment provided sufficient statistical power to test the studies’ questions (power = 0.97 in transcortical defect experiment; power = 0.95 in HO experiment).
Results
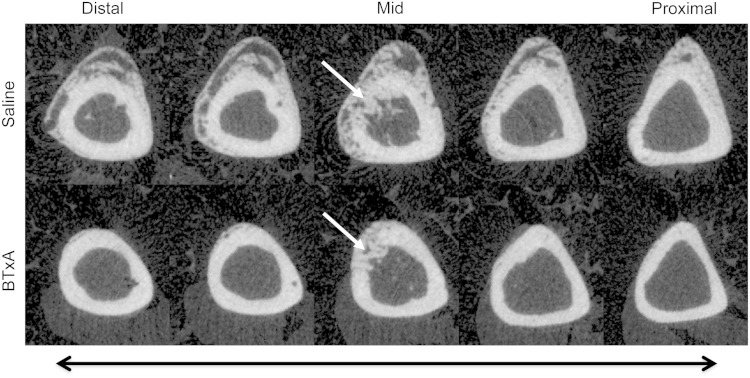
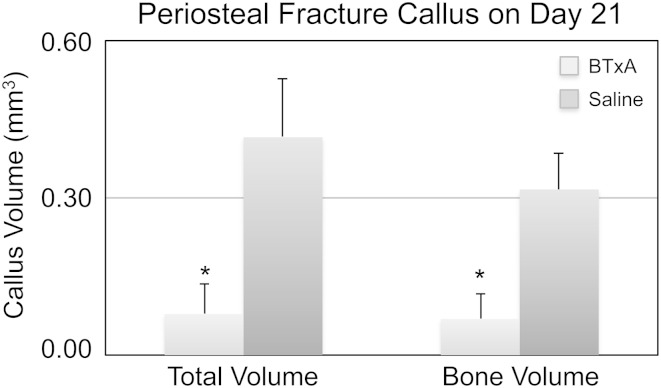
Transient muscle paralysis inhibited periosteal osteogenesis induced by a transcortical defect. Micro-CT of saline-injected mice showed profound bone formation on the periosteal surface, which appeared morphologically identical to bone formation observed after bone fracture (Fig. 2). In contrast, periosteal osteogenesis proximal and distal to the transcortical defect in BTxA-treated mice was profoundly inhibited (Fig. 2). When compared with saline-treated animals, transient paralysis of the calf muscles diminished total callus volume (0.08 ± 0.06 versus 0.42 ± 0.11 mm3, mean ± SD, p < 0.001; Fig. 3) and bone volume within the callus volume (0.07 ± 0.05 versus 0.32 ± 0.07 mm3, p < 0.001; Fig. 3).
Fig. 2.
Serial micro-CT images along a 2-mm region of the tibial diaphysis in a saline-treated mouse (“saline,” top) demonstrates exuberant osteogenesis both distal and proximal to the surgically created bone defect. Furthermore, it can be seen that the cortical defect is being repaired by calcifying tissues (white arrow in the middiaphyseal image). In contrast, transient paralysis of the calf muscle induced by BTX (“BTxA,” bottom) inhibited osteogenesis along the entire diaphyseal length without affecting calcifying tissues adjacent to or within the injury itself.
Fig. 3.
Total fracture callus (mean ± SD) and callus bone volume 21 days after a transcortical defect was significantly inhibited by BTxA-induced paralysis in the adjacent calf muscle group as compared with the saline group (*p < 0.001).
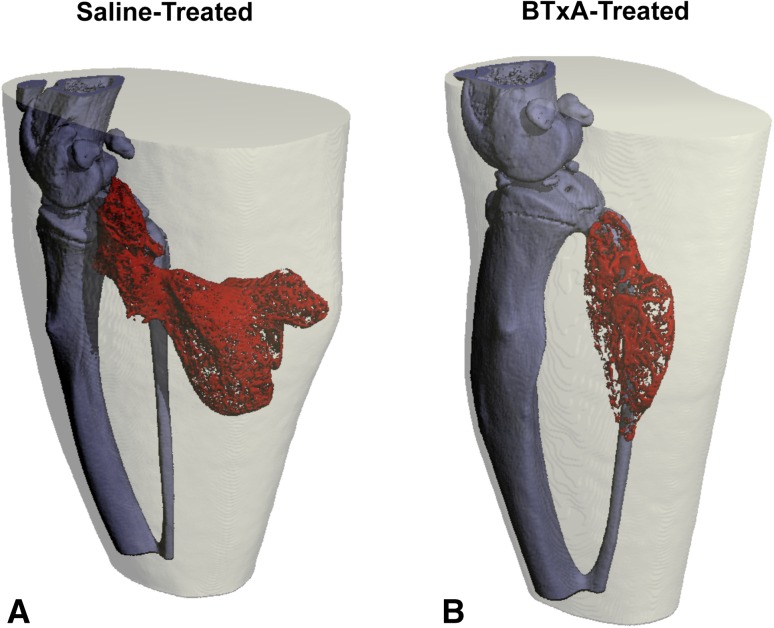
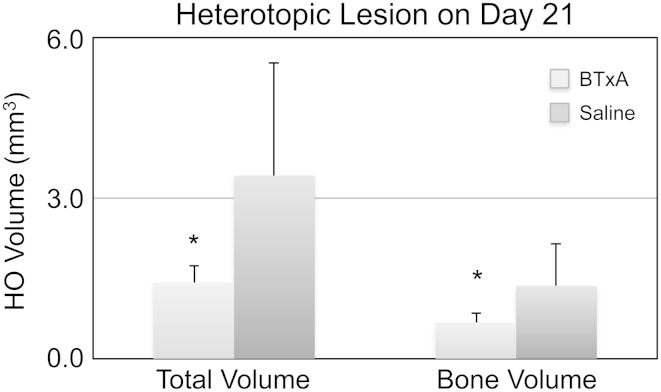
Transient muscle paralysis also inhibited heterotopic bone formation induced by BMP-2 implantation. Three-dimensional micro-CT reconstructions demonstrated that BMP-2/BME implanted into the gastrocnemius induced ectopic bone in saline-injected mice (Fig. 4A; saline). Although ectopic bone was still observed in BTxA-treated mice, bone nodules were diminished (Fig. 4B; BTxA). When compared with saline-treated animals, transient paralysis of the calf muscles reduced the total volume of the HO nodule (1.42 ± 0.31 versus 3.42 ± 2.11 mm3, p = 0.034; Fig. 5) and the bone volume within the nodule (0.68 ± 0.18 versus 1.36 ± 0.79 mm3, p = 0.045; Fig. 5).
Fig. 4A–B.
Three-dimensional volumetric reconstructions are shown of heterotopic bone nodules (red) 21 days after BMP implantation in the calf (A; saline). Transient muscle paralysis dramatically inhibits HO formation (B; BTxA) when compared with saline-injected controls.
Fig. 5.
Total volume (mean ± SD) and bone volume of the heterotopic lesion 21 days after BMP-2/BME implantation were significantly inhibited by prophylactic BTxA-induced paralysis in the adjacent calf muscle group as compared with the saline group (*p = 0.034 and 0.045 for total volume and bone volume, respectively).
Discussion
The osteogenic cascade initiated in fracture healing (inflammation, angiogenesis, chondrogenesis, and osteogenesis) is recapitulated during heterotopic bone formation. On traumatic insult, neuroinflammatory cytokines trigger BMP signaling pathways that lead to bone formation. Given the role of neuronal signaling pathways in both fracture repair and heterotopic bone formation, we speculated that an intervention transiently inhibiting neuromuscular signaling would also mitigate heterotopic bone formation. We implemented two complementary in vivo models of induced osteogenesis that begin to isolate the role of neuromuscular function in mediation of focal soft tissue osteogenesis. Our data indicated that BTxA-induced neuromuscular inhibition mitigated osteogenesis associated with both transcortical defect and BMP-2-induced HO.
These observations must be considered in the context of the limitations of our approach. In testing the role of neuromuscular inhibition on induced osteogenesis, BTxA was used to paralyze muscle adjacent to the induced osteogenesis. Given the toxicity of BTxA, it is possible that the inhibition of osteogenesis was a direct effect of BTxA on bone cells rather than a result of neuromuscular dysfunction. We have previously reported that BTxA-induced calf paralysis does not diminish baseline periosteal osteoblast function [33]. We have additionally conducted a followup pilot control study in which BTxA was injected directly into the marrow cavity through the transcortical defect (n = 4). This intervention did not alter calf muscle volume, had a minimal effect on periosteal osteogenesis, and did not alter endocortical osteogenesis or healing of the cortical defect itself (data not shown). Although these data do not rule out a direct effect of BTxA on bone cells, they do provide evidence to mitigate this limitation. A second limitation of the study was associated with the single imaging end point (Day 21 for both studies). The 21-day time point was chosen based on preliminary data indicating that defect healing (transcortical defect model) and ectopic bone formation (HO model) both plateaued by this time. However, it is possible that the muscle paralysis delayed rather than inhibited osteogenesis. We have since evaluated additional time points in both models (Day 12 in the transcortical defect model; Days 12 and 28 in the HO model) and the inhibitory effects of muscle paralysis on osteogenesis appear to be consistent across time points and models. These observations strongly imply that neuromuscular inhibition truly diminishes osteogenesis and does not simply alter the temporal dynamics of the osteogenic process.
Neuromuscular inhibition clearly inhibited periosteal osteogenesis after a transcortical defect. The simplicity of this model compared with a closed or open fracture model (eg, smaller and more confined callus formation, lack of gait defect) supports a direct role for muscle/bone crosstalk in fracture healing [12, 19, 21, 28]. Previous studies using closed and open fracture models have reported conflicting findings over the potential benefit (or lack thereof) of muscle paralysis on fracture healing [3, 13]. It has been postulated that variations in vascularity and soft tissue damage at the fracture site and the extent of neuromuscular inhibition have contributed to this variability [1, 22]. We believe that the simplicity of the transcortical defect model overcomes these confounding variables and holds potential to clarify how BTxA-induced blockade of neuromuscular signaling alters osteogenic fracture healing pathways as well as insight into the angiogenic and neuronal pathways integral to the response to trauma.
We were able to demonstrate that prophylactic neuromuscular inhibition mitigates focal and predictable HO formation after BMP-2 implantation in a mouse. However, BMP-2 is one of many genes known to be upregulated during heterotopic bone formation. We have recently performed experiments showing muscle paralysis inhibits BMP-4-induced HO in the same mouse model (data not shown). Although we have not specifically confirmed that muscle paralysis would inhibit HO induced primarily by other known osteogenic cytokines such as BMP-1, BMP-9 or transforming growth factor-β [15, 18], our findings that paralysis inhibits pathologic bone formation during fracture, an osteogenic response induced by a complex signaling cascade, further suggests that this inhibitory therapy may be used regardless of the specific underlying signaling impetus. Additionally, the ability to inhibit HO formation in the BMP-2 model is primarily based on a priori knowledge of where and when HO formation will occur, similar to the clinical conditions after THA or acetabular fracture repair. However, other pathologies such as spinal cord injury and traumatic brain injury are known to cause systemic HO formation whose timing and location are much more difficult to predict. If neuromuscular inhibition was to be applied as a therapy for these conditions, further research needs to be done to determine if muscle paralysis is still an amenable therapy once heterotopic ossification has begun to mature.
Our BMP-2-induced HO findings are consistent with a central role of local neuromuscular function in HO pathogenesis. Although this is not the first report implicating neuronal dysfunction in HO pathogenesis, our results demonstrate that local modulation of this signaling, in addition to systemic alterations of the nervous system, impact HO formation. For example, mice lacking sensory neurons have been shown to have an attenuated response to BMP-2-induced HO formation [27]. More recently, inhibition of a neural patterning gene in the Hedgehog pathway has been shown to diminish a hereditary predilection toward formation of HO [26]. Given that current HO treatments such as radiation and nonsteroidal antiinflammatory drugs have potentially severe side effects [5, 6, 24], transient muscle paralysis and/or focal inhibition of neuromuscular signaling presents an alternative intervention with potential for preventing heterotopic bone after orthopaedic trauma. However, translation of this approach to the clinic will require a better understanding of the cellular mechanisms controlling muscle/bone/nerve interactions so that optimal inhibition of HO can be achieved while the potentially adverse effects of transient neuromuscular inhibition are minimized [2, 23].
In summary, transient muscle paralysis after a single injection of BTxA in mice inhibited periosteal osteogenesis induced by a transcortical defect and the formation of HO induced by intramuscular implantation of BMP-2. These observations further support a central role of neuromuscular signaling pathways in normal bone repair and pathological bone formation. We believe that these findings identify a promising new approach that may lead to an effective clinical intervention for a healthcare challenge that is severely debilitating for civilian and war-wounded populations, is costly to both the patient and the healthcare system, and currently lacks effective treatments.
Footnotes
This work was supported, in part, by a grant from NIAMS (AR60304; TSG) and Synthes USA (West Chester, PA; SDB) and funding from the Zimmer FX Biology Professorship (SDB) and the Sigvard T. Hansen, Jr Endowed Chair (TSG).
All ICMJE Conflict of Interest Forms for authors and Clinical Orthopaedics and Related Research ® editors and board members are on file with the publication and can be viewed on request.
Clinical Orthopaedics and Related Research ® neither advocates nor endorses the use of any treatment, drug, or device. Readers are encouraged to always seek additional information, including FDA-approval status, of any drug or device prior to clinical use.
Each author certifies that his or her institution approved the animal protocol for this investigation and that all investigations were conducted in conformity with ethical principles of research.
References
- 1.Atmaca H, Memisoglu K. The relation between botulinum toxin-A and fracture healing. comment on Hao et al.: short-term muscle atrophy caused by botulinum toxin-A local injection impairs fracture healing in the rat femur. J Orthop Res. 2013;31:510. doi: 10.1002/jor.22232. [DOI] [PubMed] [Google Scholar]
- 2.Ausk BJ, Huber P, Srinivasan S, Bain SD, Kwon RY, McNamara EA, Poliachik SL, Sybrowsky CL, Gross TS. Metaphyseal and diaphyseal bone loss in the tibia following transient muscle paralysis are spatiotemporally distinct resorption events. Bone. 2013;57:413–422. doi: 10.1016/j.bone.2013.09.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Aydin A, Memisoglu K, Cengiz A, Atmaca H, Muezzinoglu B, Muezzinoglu US. Effects of botulinum toxin A on fracture healing in rats: an experimental study. J Orthop Sci. 2012;17:796–801. doi: 10.1007/s00776-012-0269-x. [DOI] [PubMed] [Google Scholar]
- 4.Bouxsein ML, Boyd SK, Christiansen BA, Guldberg RE, Jepsen KJ, Muller R. Guidelines for assessment of bone microstructure in rodents using micro-computed tomography. J Bone Miner Res. 2010;25:1468–1486. doi: 10.1002/jbmr.141. [DOI] [PubMed] [Google Scholar]
- 5.Burd TA, Hughes MS, Anglen JO. Heterotopic ossification prophylaxis with indomethacin increases the risk of long-bone nonunion. J Bone Joint Surg Br. 2003;85:700–705. [PubMed] [Google Scholar]
- 6.Chao ST, Joyce MJ, Suh JH. Treatment of heterotopic ossification. Orthopedics. 2007;30:457–464; quiz 465–466. [DOI] [PubMed]
- 7.Cipriano CA, Pill SG, Keenan MA. Heterotopic ossification following traumatic brain injury and spinal cord injury. J Am Acad Orthop Surg. 2009;17:689–697. doi: 10.5435/00124635-200911000-00003. [DOI] [PubMed] [Google Scholar]
- 8.Feldman GJ, Billings PC, Patel RV, Caron RJ, Guenther C, Kingsley DM, Kaplan FS, Shore EM. Over-expression of BMP4 and BMP5 in a child with axial skeletal malformations and heterotopic ossification: a new syndrome. Am J Med Genet A. 2007;143:699–706. doi: 10.1002/ajmg.a.31649. [DOI] [PubMed] [Google Scholar]
- 9.Firoozabadi R, O’Mara TJ, Swenson A, Agel J, Beck JD, Routt M. Risk factors for the development of heterotopic ossification after acetabular fracture fixation. Clin Orthop Relat Res. 2014;472:3383–3388. doi: 10.1007/s11999-014-3719-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Forsberg JA, Pepek JM, Wagner S, Wilson K, Flint J, Andersen RC, Tadaki D, Gage FA, Stojadinovic A, Elster EA. Heterotopic ossification in high-energy wartime extremity injuries: prevalence and risk factors. J Bone Joint Surg Am. 2009;91:1084–1091. doi: 10.2106/JBJS.H.00792. [DOI] [PubMed] [Google Scholar]
- 11.Foruria AM, Augustin S, Morrey BF, Sanchez-Sotelo J. Heterotopic ossification after surgery for fractures and fracture-dislocations involving the proximal aspect of the radius or ulna. J Bone Joint Surg Am. 2013;95:e66. doi: 10.2106/JBJS.K.01533. [DOI] [PubMed] [Google Scholar]
- 12.Glass GE, Chan JK, Freidin A, Feldmann M, Horwood NJ, Nanchahal J. TNF-alpha promotes fracture repair by augmenting the recruitment and differentiation of muscle-derived stromal cells. Proc Natl Acad Sci U S A. 2011;108:1585–1590. doi: 10.1073/pnas.1018501108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Hao Y, Ma Y, Wang X, Jin F, Ge S. Short-term muscle atrophy caused by botulinum toxin-A local injection impairs fracture healing in the rat femur. J Orthop Res. 2012;30:574–580. doi: 10.1002/jor.21553. [DOI] [PubMed] [Google Scholar]
- 14.Hendricks HT, Geurts AC, van Ginneken BC, Heeren AJ, Vos PE. Brain injury severity and autonomic dysregulation accurately predict heterotopic ossification in patients with traumatic brain injury. Clin Rehabil. 2007;21:545–553. doi: 10.1177/0269215507075260. [DOI] [PubMed] [Google Scholar]
- 15.Jackson WM, Aragon AB, Onodera J, Koehler SM, Ji Y, Bulken-Hoover JD, Vogler JA, Tuan RS, Nesti LJ. Cytokine expression in muscle following traumatic injury. J Orthop Res. 2011;29:1613–1620. doi: 10.1002/jor.21354. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Kan L, Hu M, Gomes WA, Kessler JA. Transgenic mice overexpressing BMP4 develop a fibrodysplasia ossificans progressiva (FOP)-like phenotype. Am J Pathol. 2004;165:1107–1115. doi: 10.1016/S0002-9440(10)63372-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Kaplan FS, Glaser DL, Hebela N, Shore EM. Heterotopic ossification. J Am Acad Orthop Surg. 2004;12:116–125. doi: 10.5435/00124635-200403000-00007. [DOI] [PubMed] [Google Scholar]
- 18.Leblanc E, Trensz F, Haroun S, Drouin G, Bergeron E, Penton CM, Montanaro F, Roux S, Faucheux N, Grenier G. BMP-9-induced muscle heterotopic ossification requires changes to the skeletal muscle microenvironment. J Bone Miner Res. 2011;26:1166–1177. doi: 10.1002/jbmr.311. [DOI] [PubMed] [Google Scholar]
- 19.Liu R, Schindeler A, Little DG. The potential role of muscle in bone repair. J Musculoskelet Neuronal Interact. 2010;10:71–76. [PubMed] [Google Scholar]
- 20.Lounev VY, Ramachandran R, Wosczyna MN, Yamamoto M, Maidment AD, Shore EM, Glaser DL, Goldhamer DJ, Kaplan FS. Identification of progenitor cells that contribute to heterotopic skeletogenesis. J Bone Joint Surg Am. 2009;91:652–663. doi: 10.2106/JBJS.H.01177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Papakostidis C, Kanakaris NK, Pretel J, Faour O, Morell DJ, Giannoudis PV. Prevalence of complications of open tibial shaft fractures stratified as per the Gustilo-Anderson classification. Injury. 2011;42:1408–1415. doi: 10.1016/j.injury.2011.10.015. [DOI] [PubMed] [Google Scholar]
- 22.Park TH. Letter regarding ‘Effects of botulinum toxin A on fracture healing in rats: an experimental study.’ J Orthop Sci. 2012;17:828; author reply 829. [DOI] [PubMed]
- 23.Poliachik SL, Bain SD, Threet D, Huber P, Gross TS. Transient muscle paralysis disrupts bone homeostasis by rapid degradation of bone morphology. Bone. 2010;46:18–23. doi: 10.1016/j.bone.2009.10.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Potter BK, Burns TC, Lacap AP, Granville RR, Gajewski DA. Heterotopic ossification following traumatic and combat-related amputations. Prevalence, risk factors, and preliminary results of excision. J Bone Joint Surg Am. 2007;89:476–486. doi: 10.2106/JBJS.F.00412. [DOI] [PubMed] [Google Scholar]
- 25.Prasad J, Wiater BP, Nork SE, Bain SD, Gross TS. Characterizing gait induced normal strains in a murine tibia cortical bone defect model. J Biomech. 2010;43:2765–2770. doi: 10.1016/j.jbiomech.2010.06.030. [DOI] [PubMed] [Google Scholar]
- 26.Regard JB, Malhotra D, Gvozdenovic-Jeremic J, Josey M, Chen M, Weinstein LS, Lu J, Shore EM, Kaplan FS, Yang Y. Activation of Hedgehog signaling by loss of GNAS causes heterotopic ossification. Nat Med. 2013;19:1505–1512. doi: 10.1038/nm.3314. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Salisbury E, Rodenberg E, Sonnet C, Hipp J, Gannon FH, Vadakkan TJ, Dickinson ME, Olmsted-Davis EA, Davis AR. Sensory nerve induced inflammation contributes to heterotopic ossification. J Cell Biochem. 2011;112:2748–2758. doi: 10.1002/jcb.23225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Shah K, Majeed Z, Jonason J, O’Keefe RJ. The role of muscle in bone repair: the cells, signals, and tissue responses to injury. Curr Osteoporos Rep. 2013;11:130–135. doi: 10.1007/s11914-013-0146-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Stauber M, Muller R. Micro-computed tomography: a method for the non-destructive evaluation of the three-dimensional structure of biological specimens. Methods Mol Biol. 2008;455:273–292. doi: 10.1007/978-1-59745-104-8_19. [DOI] [PubMed] [Google Scholar]
- 30.Suda RK, Billings PC, Egan KP, Kim JH, McCarrick-Walmsley R, Glaser DL, Porter DL, Shore EM, Pignolo RJ. Circulating osteogenic precursor cells in heterotopic bone formation. Stem Cells. 2009;27:2209–2219. doi: 10.1002/stem.150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Tippets DM, Zaryanov AV, Burke WV, Patel PD, Suarez JC, Ely EE, Figueroa NM. Incidence of heterotopic ossification in direct anterior total hip arthroplasty: a retrospective radiographic review. J Arthroplasty. 2014;29:1835–1838. doi: 10.1016/j.arth.2014.04.027. [DOI] [PubMed] [Google Scholar]
- 32.Tsuji K, Bandyopadhyay A, Harfe BD, Cox K, Kakar S, Gerstenfeld L, Einhorn T, Tabin CJ, Rosen V. BMP2 activity, although dispensable for bone formation, is required for the initiation of fracture healing. Nat Genet. 2006;38:1424–1429. doi: 10.1038/ng1916. [DOI] [PubMed] [Google Scholar]
- 33.Warner SE, Sanford DA, Becker BA, Bain SD, Srinivasan S, Gross TS. Botox induced muscle paralysis rapidly degrades bone. Bone. 2006;38:257–264. doi: 10.1016/j.bone.2005.08.009. [DOI] [PMC free article] [PubMed] [Google Scholar]