Abstract
Background
Heterotopic ossification (HO) is common after combat-related amputations and surgical excision remains the only definitive treatment for persistently symptomatic HO. There is no consensus in the literature regarding the timing of surgery, and recurrence frequency, reexcision, and complications have not been reported in large numbers of patients.
Questions/purposes
(1) What are the rates of symptomatic recurrence resulting in reexcision and other complications resulting in reoperation in patients with HO? (2) Is either radiographic or symptomatic recurrence dependent on timing and type of initial surgery, the experience of the surgeon in performing the procedure, the severity of preexcision HO, the presence of concomitant neurologic injury, or the use of postoperative HO prophylaxis?
Methods
Between March 2005 and March 2013 our institution treated 994 patients with 1377 combat-related major extremity amputations; of those, 172 amputations underwent subsequent excision of symptomatic HO. The mechanism of injury resulting in nearly all amputations (n = 168) was blast-related trauma. We reviewed medical records and radiographs to collect initial grade of HO, radiographic recurrence, complete compared with partial excision, concomitant neurologic injury, timing to initial surgery, surgeon experience, and use of postexcision prophylaxis with our primary study outcome being a return to the operating room (OR) for repeat excision of symptomatic HO. All 172 combat-related amputations were considered for this study irrespective of followup, which was noted to be robust, with 157 (91%) amputations having at least 6 months clinical followup by an orthopaedic surgeon or physiatrist (median, 20 months; range, 0–88 months).
Results
Eleven of 172 patients (6.5%) underwent reexcision of HO, and 67 complications resulting in return to the OR occurred in 53 patients (31%) of patients. Multivariate analysis of our primary outcome measure showed more frequent symptomatic recurrences requiring reexcision when initial excision was performed as a partial excision (p = 0.03; odds ratio [OR], 5.0; 95% confidence interval [CI], 1.2–29.6) or when the initial excision was performed within 180 days of injury (p = 0.047; OR, 4.1; 95% CI, 1.02–16.6). There was no association between symptomatic recurrence and HO grade, central nervous system injury, experience of the attending surgeon, or postoperative prophylaxis. Radiographic recurrence was observed when partial excisions (eight of 30 [27%]) were done compared with complete excisions (five of 77 [7%]; p = 0.008).
Conclusions
HO is common after combat-related amputations, and patients undergoing surgical excision of HO for this indication often have complications that result in repeat surgical procedures. Partial excisions of immature lesions more often resulted in both symptomatic and radiographic recurrence. The likelihood of a patient undergoing reexcision can be minimized by performing a complete excision at least 180 days from injury to surgery with no evidence of a reduced risk of reexcision by waiting longer than 270 days.
Level of Evidence
Level III, therapeutic study.
Introduction
Heterotopic ossification (HO) is the formation of mature lamellar bone in nonosseous tissue such as muscle. It is known to occur after blunt trauma elbow and acetabular fractures, burns, traumatic brain injury, and spinal cord injury. Recently, interest in HO has increased as a result of its high prevalence in combat-related extremity injuries and amputations [9, 10, 14, 15, 17]. Studies have reported the prevalence of HO to be as high as 65% in combat-related amputations, and HO can result in pain, wound breakdown, difficulty with prosthesis wear, entrapment of neurovascular structures, and limited ROM [2, 4, 6, 16, 18, 20, 22, 23, 26, 29, 31, 32, 35]. Before operative excision, nonsurgical modalities such as pain control and serial prosthetic socket adjustments should be exhausted; however, up to 41% of affected patients who have undergone amputation will choose to have surgical excision to treat persistent symptoms and failed nonsurgical treatments [7, 8, 24, 27, 28].
Although substantive literature exists on the surgical excision of HO, techniques for removal are variably described and there remains no clear consensus on appropriate timing of resection with regard to recurrence [2, 3, 11, 26, 30, 36]. Given the potential morbidity associated with surgical excision such as significant blood loss, high wound complication rates, difficult immediate postoperative pain control, and delays in rehabilitation, an appropriate focus on preventing symptomatic recurrence is critical. Garland and Orwin stated that normal bone scans, alkaline phosphatase blood serum levels, and mature roentgenographic appearance of HO—classic landmarks of HO maturity—were unreliable in predicting recurrence [13]. Although symptomatic HO recurrences have been described, very little has been reported on HO recurrence after excision in patients with trauma-related amputations.
The purpose of our study was to evaluate a population of military persons who underwent or sustained amputations resulting from combat-related injuries to determine: (1) What are the rates of symptomatic recurrence resulting in reexcision and other complications resulting in reoperation in amputees with HO? (2) Is either radiographic or symptomatic recurrence dependent on timing and completeness of excision, surgeon experience, the severity of preexcision HO, the presence of concomitant neurologic injury, or the use of postoperative HO prophylaxis?
Patients and Methods
After receiving approval from our institutional review board, the Surgical Scheduling System (S3) at our institution was retrospectively reviewed to identify all patients who underwent HO excision at Walter Reed Army Medical Center and, more recently, at the integrated Walter Reed National Military Medical Center between March 2005 and March 2013. The patients were then crossreferenced with our internal amputee database to determine whether the HO excision was performed in a combat-related amputation. Followup on these patients was assessed through the Armed Forces Health Longitudinal Technology Application, the outpatient medical record for the Military Health System. Combat-related amputations were defined as any major extremity amputation (at or proximal to the wrist or ankle) performed or completed as a result of injuries sustained in combat, including so-called late or delayed amputations.
We identified 994 patients who underwent amputation during the study period (March 2005 to February 2013). Of the 994 patients with 1377 residual limbs, 172 residual limbs in 133 patients underwent HO excision during the study period (Fig. 1). All 172 combat-related amputations that underwent HO excision were considered for our study given the high likelihood that clinically symptomatic recurrences would return to receive re-excision; if necessary, at our institution. Median followup done by an orthopaedic surgeon or physiatrist for study patients was 924 days (range, 210–3166 days) from the date of injury and 590 days (range, 7–954 days) from the date of initial HO excision. With 2 months considered by physicians as a minimum followup for the recurrence of HO, 169 of 172 (98%) residual limbs met these criteria (Fig. 1). All patients were men. Average patient age was 26 years (range, 19–44 years; Table 1).
Fig. 1.
This flow chart shows the breakdown of our study population. Residual limbs were included in the study if an HO excision was performed at the level of amputation in combat traumatic amputation.
Table 1.
Patient demographics and surgical procedures
| Demographic (IQR) N = 172 patients |
Number (%) or median |
|---|---|
| Age at the time of injury (years) | 25 (22, 26) |
| Age at the time of HO excision (years) | 26 (22, 27) |
| Sex, number (%) | |
| Men | 172 (100) |
| Women | 0 |
| Mechanism of injury, number (%) | |
| Blast | 168 (98) |
| Gunshot wound | 2 (1.2) |
| Vehicle rollover | 2 (1.2) |
| Neurologic injury (+TBI/SCI diagnosis), number (%) | 132 (77) |
| No neurologic injury (−TBI/SCI diagnosis) | 40 (23) |
| Amputation level, number (%) | – |
| Lower extremity | 155 (90) |
| Hemipelvectomy | 3 (1.7) |
| Hip disarticulation | 11 (6) |
| Transfemoral | 100 (58) |
| Knee disarticulation | 3 (1.7) |
| Transtibial | 37 (22) |
| Tibiotalar | 1 (0.6) |
| Upper extremity | 17 (10) |
| Transhumeral | 8 (5) |
| Transradial | 8 (5) |
| Wrist disarticulation | 1 (0.6) |
| Post-resection prophylaxis | 101 (59) |
| NSAIDs | 90 (53) |
| XRT | 9 (5) |
| No prophylaxis | 73 (42) |
| Procedures performed at time of initial excision, N = 294 procedures | |
| HO excision | 172 |
| Amputation revision | 73 |
| Débridement | 16 |
| Neurectomy | 10 |
| Hardware removal | 9 |
| Scar revision | 4 |
| STSG | 3 |
| Arthroscopy | 2 |
| Knee ligament reconstruction | 2 |
| Intramedullary nail | 1 |
| External fixation revision | 1 |
| Vascular repair | 1 |
| Returns to the OR, N = 67 | |
| Débridement—concern for wound infection | 25 |
| Amputation revision | 14 |
| Reexcision | 11 |
| Soft tissue/scar revision | 7 |
| Neurectomy | 5 |
| Hardware removal | 3 |
| Quadricepsplasty | 1 |
| Hematoma evacuation | 1 |
IQR = interquartile range; TBI = traumatic brain injury; SCI = spinal cord injury; NSAIDs = nonsteroidal antiinflammatory drugs; XRT = external beam radiation therapy; HO = heterotopic ossification; STSG = split-thickness skin graft; OR = operating room.
We collected patient demographics, level of amputation, date of initial injury, mechanism of injury, presence of concomitant neurologic injury, date of HO excision or reexcision, HO prophylaxis when used, clinical indications for excision (Fig. 2), and concurrent surgical procedures as well as complications and late problems resulting in return to the operating room (OR). The preoperative, immediate postoperative, and latest followup radiographs were reviewed by the primary author (GJP) and HO was graded using the Walter Reed HO classification [27]. In assessing predictors of HO recurrence, we focused on surgeon-dependent factors such as surgeon experience, timing of excision, and completeness of excision as well as factors considered to be associated with HO development such as concomitant neurologic injury and the use of prophylaxis.
Fig. 2.
The bar graph shows the primary indications for HO excision in our study. WR = Walter Reed; I&D = irrigation and débridement.
With regard to mechanism of injury, we found there to be a fairly homogenous cohort of blast-related traumatic amputations. Blast-related injury was considered if the result of an improvised explosive device, mortar round, or grenade. Of the 172 combat-related amputations in this study, 168 were the result of blast-related trauma. Other mechanisms of injury included two high-velocity gunshot wounds and two crush injuries from vehicle rollover.
Surgeries to excise HO from amputations were performed by a total of 17 military staff orthopaedic surgeons at our institution (two of whom, BKP and JAF, are authors), including three surgeons who performed more than 10 HO excisions during the study period and were therefore considered more experienced. Three surgeons (BKP) were considered experienced, performing 138 total primary excisions, and 14 surgeons were less experienced, performing 34 cumulative primary excisions. Clinical judgment correlating radiographic and cross-sectional imaging with patient symptoms was done by the individual treating surgeon to determine the extent of HO excision needed to accomplish treatment goals. The decision to return to the OR after initial excision was made by the attending orthopaedic surgeon based on clinical acumen if the residual limb had persistent or recurrent symptomatic HO, there was concern for infection, or an amputation required revision for soft tissue failure or symptomatic neuromata. If there was concern for infection as opposed to sterile wound dehiscence, intraoperative deep cultures were obtained with swabs and/or tissue samples.
Both inpatient and outpatient rehabilitative care was carried out by experienced physiotherapists skilled in the treatment of patients who had sustained amputation. The use of postexcision HO recurrence prophylaxis, including nonsteroidal antiinflammatory drugs (NSAIDs) or external radiation therapy (XRT), was not standardized and was determined by the treating surgeon. Most frequently, we used a celecoxib for 2 to 4 weeks postoperatively at either 100 mg twice daily or 200 mg daily beginning on the day after surgery for HO recurrence prophylaxis. In the nine patients to whom XRT was administered, a 7-Gy dose was delivered on the first postoperative day.
Our primary study outcome was return to the OR for reexcision of symptomatic recurrent or residual HO. We compared the frequency of reexcision based on the presence of concomitant neurologic injury, preoperative grade of HO, the timing and extent of initial resection, the relative experience of the treating surgeon, and the use of postexcision NSAIDs or XRT as recurrence prophylaxis. In addition to identifying factors associated with reexcision of symptomatic HO, we analyzed the radiographs in both symptomatic and asymptomatic patients at latest followup for radiographic recurrence after complete excision or progression in partially resected lesions. Partial resection was defined by radiographic evidence of residual HO when comparing images before and after the resection procedure. Finally, we determined which complications and late problems resulting in revision surgery after HO excision. Because our electronic medical record is universal among military treatment facilities across the US Department of Defense, loss to followup occurred only when patients were retired from military service and sought treatment at either Veterans Affairs medical facilities or civilian treatment facilities; however, it would be unlikely that patients who had symptomatic recurrence of HO after an excision at our institution would have a reexcision elsewhere or at least without our consultation.
Data analysis was performed using RStudio (Version 0.98.953; RStudio, Inc, Boston, MA, USA). Counts (percentage) were reported for each subgroup of interest for both clinical/surgical and radiographic features. Fisher’s exact test was used to determine potential differences in proportions between groups. The method of Benjamini–Hochberg’s false discovery rate adjustment was used on the p values when multiple comparisons were made. We defined statistical significance as α < 0.05. We used Firth’s method of logistic modeling to isolate potential confounding variables that may influence our primary outcome measure. Two models were used with each incorporating one of the time dependent variables (ie, > 180 days or < 180 days and > 270 days or < 270 days). We calculated the odds ratios (ORs) and 95% confidence intervals (CIs) for each variable that reached statistical significance (α < 0.05).
Results
Overall, 11 (6%) of the 172 residual limbs underwent reexcision of symptomatic HO. Median time to initial HO excision was 250 days (interquartile range, 195, 271). Median time from injury to initial excision for the 11 limbs ultimately undergoing reexcision was 186 days (range, 51–278 days). In addition to the 11 limbs that underwent reexcision of HO, there were 56 (33%) other postoperative complications that resulted in a return to the OR. Twenty-five (45%) of the complications were débrided as a result of concern for infection with infection verified by positive culture in 22 patients (Table 1). Univariate subgroup analysis demonstrated that excisions performed before 270 days and excisions performed in residual limbs in which the patient had a diagnosed neurologic injury were associated with increased frequency of OR returns for reasons other than reexcision (Table 2).
Table 2.
Summary of HO excision clinical and radiographic outcomes
| Surgical factors of residual limb HO excisions | Clinical outcomes | Radiographic outcomes | ||||
|---|---|---|---|---|---|---|
| Return to OR for early or late complication (n = 67) | Infection (n = 22) | Return to OR for reexcision of HO (n = 11) | Radiographic progression of partial excisions (n = 30) | Radiographic recurrence of complete excision (n = 77) | Sum of available radiographs—recurrence or progression (n = 107) | |
| Complete versus partial excisions, number (%) (N = 167) | ||||||
| Partial excisions, number (%) (n = 56) | 21 (38) | 5 (9) | 11 (20) | 8/30 (27) | NA | 8/30 (27) |
| Complete excisions, number (%) (n = 111) | 46 (41) | 17 (15) | 2 (1.8) | NA | 5/77 (7) | 5/77 (7) |
| p-value | 0.86 | 0.33 | < 0.001 | NA | NA | 0.008 |
| Walter Reed HO grade, number (%) (N = 172) | ||||||
| 1 (n = 49) | 17 (34.7) | 6 (12) | 2 (4) | 0/3 (0) | 2/29 (7) | 2/32 (6) |
| 2 (n = 56) | 23 (41.1) | 10 (16) | 2 (4) | 5/11 (46) | 1/19 (5) | 6/30 (20) |
| 3 (n = 67) | 27 (40.2) | 6 (13) | 7 (10) | 3/16 (19) | 2/29 (7) | 5/45 (11) |
| p value | 1 versus 2 NS 1 versus 3 NS 2 versus 3 NS |
NS | NS | NS | NS | NS |
| Experienced (> 10 cases) versus less experienced surgeon (N = 172) | ||||||
| Experienced, number (%) (n = 134) | 47 (35) | 19 (14) | 5 (4) | 6/21 (29) | 3/64 (5) | 9/85 (11) |
| Inexperienced, number (%) (n = 38) | 20 (42) | 17 (13) | 6 (16) | 2/9 (22) | 2/13 (15) | 4/22 (18) |
| p value | 0.63 | 0.41 | 0.02 | ~1.0 | 0.20 | 0.16 |
| Timing of initial surgery (days), number (%) (N = 172) | ||||||
| ≤ 180 (n = 36) | 15 (56) | 5 (14) | 6 (17) | 2/7 (29) | 3/17 (18) | 5/24 (21) |
| > 180 (n = 136) | 52 (35) | 17 (13) | 5 (4) | 6/23 (26) | 2/60 (3) | 8/83 (10) |
| p value | 0.7 | 0.78 | 0.01 | ~1.0 | 0.07 | 0.16 |
| Timing of initial surgery (days),, number (%) (N = 172) | ||||||
| ≤ 270 (n = 36) | 48 (46) | 15 (14) | 9 (9) | 7/23 (30) | 3/47 | 10/70 (14) |
| > 270 (n = 136) | 19 (28) | 7 (10) | 2 (3) | 1/7 (14) | 2/30 | 3/37 (8) |
| p value | 0.025 | 0.64 | 0.21 | 0.64 | NS | 0.54 |
| Neurologic injury, number (%) (N = 172) | ||||||
| Yes (n = 132) | 60 (45) | 17 (13) | 11 (8) | 5/22 (23) | 3/60 | 8/82 (10) |
| No (n = 40) | 7 (12) | 5 (13) | 0 (0) | 3/8 (38) | 2/17 | 5/25 (20) |
| p value | 0.002 | 1.0 | 0.07 | 0.64 | 0.3 | 0.18 |
| HO prophylaxis, number (%) (N = 172) | ||||||
| None (n = 73) | 32 (44) | 9 (12) | 7 (10) | 1/12 (8) | 4/35 | 5/47 (11) |
| NSAIDs (n = 90) | 34 (38) | 12 (13) | 4 (4) | 7/17 (41) | 1/38 | 8/55 (15) |
| XRT (n = 9) | 1 (11) | 1 (11) | 0 (0) | 0/1 (0) | 0/4 | 0/5 (0) |
| p value | NS | NS | NS | NS | NS | None versus NSAID NS None versus XRT p = 0.05 NSAIDs versus XRT p = 0.03 |
HO = heterotopic ossification; OR = operating room; NSAIDs = nonsteroidal antiinflammatory drugs; XRT = external beam radiation therapy; NS = nonsignificant; NA = not applicable.
We found that partial excisions and excisions done before 180 days after injury were associated with an increased likelihood of a patient undergoing reexcision. Residual limbs undergoing a partial initial excision were more likely to return to the OR for reexcision (11 of 56 [20%]) compared with complete incision (two of 111 [2%]; p < 0.001). Logistic modeling showed that partial excisions were more than five times likely to return to the OR for reexcision (p = 0.03; OR, 5.0; 95% CI, 1.17–29.6), Furthermore, surgical excisions of HO done before 180 days after injury were more likely to recur (six of 36 [17%]) symptomatically than those done after 180 days (five of 136 [4%]; p = 0.01) and conferred an increased risk of reexcision (p = 0.047; OR, 4.07; 95% CI, 1.02–16.6) (Table 3). In contrast, when all initial excisions done before 270 days were considered, there was no increased risk for reexcision on multivariate analysis (Tables 2, 4). Surgeons who performed fewer than 10 excisions did not have any increased risk of recurrence when multivariate analysis was performed to control for confounding bias. There was also no difference in reexcision frequency or increased risk of reexcision when considering concomitant neurologic injury, use of postexcision HO prophylaxis, or severity (preexcision grade) of HO (Tables 2, 3, 4). Radiographic progression was seen in eight of 30 limbs (27%) that had partial excision, whereas radiographic recurrence of initially complete excisions was seen in five of 77 (7%). Radiographic recurrence was observed when partial excisions (eight of 30 [27%]) were done compared with complete excisions (five of 77 [7%]; p = 0.008). There were no differences in symptomatic recurrence risk with regard to HO prophylaxis or prophylactic modality.
Table 3.
Logistic modeling of factors associated with HO reexcision with included timing of excision occurring before or after 180 days after injury
| Variable | Coefficient | SE | OR (95% CI) | p value |
|---|---|---|---|---|
| CNS injury | 1.89 | 1.39 | 6.6 (0.72–883) | 0.11 |
| Complete versus partial excision | 1.61 | 0.74 | 5.0 (1.17–29.6) | 0.03* |
| HO severity (WR classification) | 0.09 | 0.45 | 1.10 (0.43–2.9) | 0.83 |
| Excision done < 180 days | 1.40 | 0.68 | 4.07 (1.02–16.6) | 0.047* |
| Surgeon experience | 1.06 | 0.69 | 2.88 (0.69–11.8) | 0.14 |
| Postexcision prophylaxis (NSAIDs) | −1.72 | 2.10 | 0.17 (0.003–4.4) | 0.32 |
| Postexcision prophylaxis (XRT) | 0.68 | 0.67 | 1.96 (0.51–8.8) | 0.33 |
* Statistically significant difference; HO = heterotopic ossification; OR = odds ratio; CI = confidence interval; CNS = central nervous system; WR = Walter Reed; NSAIDs = nonsteroidal antiinflammatory drugs; XRT = external beam radiation.
Table 4.
Logistic modeling of factors associated with HO reexcision with included timing of excision occurring before or after 270 days after injury
| Variable | Coefficient | SE | OR (95% CI) | p value |
|---|---|---|---|---|
| CNS injury | 1.71 | 1.33 | 5.57 (0.64–734.1) | 0.14 |
| Complete versus partial excision | 1.57 | 0.72 | 4.84 (1.15–28.3) | 0.03* |
| HO severity (WR classification) | 0.18 | 0.43 | 1.19 (0.49–3.13) | 0.7 |
| Excision done < 270 days | 0.39 | 0.74 | 1.48 (0.35–8.5) | 0.6 |
| Surgeon experience | 1.27 | 0.66 | 3.6 (0.94–14.0) | 0.06 |
| Postexcision prophylaxis (NSAIDs) | −0.99 | 2.01 | 0.36 (0.002–7.9) | 0.56 |
| Postexcision prophylaxis (XRT) | 0.52 | 0.64 | 1.69 (0.46–7.0) | 0.43 |
* Statistically significant difference; HO = heterotopic ossification; OR = odds ratio; CI = confidence interval; CNS = central nervous system; WR = Walter Reed; NSAIDs = nonsteroidal antiinflammatory drugs; XRT = external beam radiation.
Discussion
HO is a troublesome and frequent complication in the care of patients with amputations. Prior research has demonstrated that HO forms in approximately 65% of combat-related amputations with 20% to 41% of patients requiring surgical excision [7, 8, 24, 27, 28]. However, risk factors for HO formation remain poorly understood. Our study demonstrated that the frequency of recurrence in this difficult-to-treat patient population is relatively low at 6% (11 of 172); however, complications after excision are relatively common, occurring in nearly one-third of the patients thus treated. When removing HO from residual limbs, our study showed that definitive treatment is most effectively obtained when a complete excision of HO is performed at least 180 days after the initial injury.
Limitations of our study include its retrospective nature whereby variable treatment approaches by 17 different surgeons are sometimes difficult to determine from the medical record. Although almost all of the amputations we studied resulted from traumatic blast injury in a young population, there remains heterogeneity in our patient population with regard to severity of initial extremity injury, wide spectra of concomitant trauma as well as variable success of treating HO symptoms without surgery that may be influenced by other patient factors. Patient followup in our study was robust overall; however, three patients were included who were not seen by our orthopaedic department beyond 2 months of surgery. Although direct surgeon-patient followup is desired to assess surgical outcomes, we are confident those patients did not have early recurrence for two reasons: (1) active-duty military members are, by standard practice, not allowed to leave military service within an minimum of 4 months after surgery, and only when all active health concerns are addressed that surgery sufficiently addressed their complaint; and (2) reported followup in our study considered only physician-patient encounters; however, our patients have ongoing clinical assessments and visits by physiotherapists and prosthetists in our institution and those throughout the Department of Defense/Veterans’ Administration consortium who are well adept in assessing HO symptoms. Furthermore, we did not have a protocol in place for timing of initial excision, because often the wounds, associated injuries, symptoms, related activity levels, and patient or surgeon preference dictated surgical timing. Many studies now support surgical excision earlier than the 12 to 18 months historically proposed as the requisite delay [9–11, 14]; however, those series represent excisions about the elbow [25, 32] or were performed in conjunction with radiotherapy protocols.
Our study, the largest single study reporting HO excision results in any patient population, validates the potential success of earlier excision (ie, at or after 6 months from injury), even in the absence of postoperative radiation, which was performed after only nine of 172 excisions. Overall, our reexcision rate was slightly less (7% versus 12%) than that seen in a systematic review of 384 HO excisions from the elbow, the only larger study, albeit aggregated, that reported recurrence rates [21]. That study, in particular, was classified HO as a result of trauma, burn, or neurologic injury; however, in our patient population, combat-related trauma was the cause of amputation and neurologic injury, and therefore a distinction between the two is difficult given our logistic modeling findings. We observed a slightly lower incidence of excision with 172 excisions in a cohort of 1377 amputations during the study period; however, this does not account for the large number of patients who did not develop HO as well as a few who were treated at our hospital but may have received an HO excision at another military treatment facility, by a civilian surgeon, or outside of the study period. The purpose of our study was not to report either the prevalence of HO or HO excisions, but rather the results of HO excision from residual limbs. Furthermore, although there was no consistent postoperative HO prophylaxis after initial excision in our cohort, we did not find a higher reexcision frequency based on prophylaxis status in our series.
Surgical excision of HO can be a very morbid operation with high risks of patient blood loss and transfusion, infection, wound problems, and damage to surrounding neurovascular structures [19, 26]. Many times, subsequent returns to the OR are necessary because patient limbs after traumatic amputations are at considerable risk of complications, in general, and are subject to ongoing reconstructions to achieve a stable limb that will tolerate regular, and ideally aggressive, prosthetic wear. Based on our experience, complete excision is suggested when practical; partial excisions may be performed in select circumstances when symptoms are extremely focal or complete excision would result in the sacrifice of too much tissue and/or would result in a larger, more complicated excision such as having to take down an otherwise stable myodesis or revising to a substantially shorter residual limb (Fig. 3). Taking out only the symptomatic areas can thus provide relief without incurring the increased morbidity of a more invasive surgery and can be used to more quickly return patients to rehabilitation and ambulation in well-selected patients; conversely, our findings suggest that this approach may increase the risk of reexcision (Fig. 4). We believe the complexity of surgical excision and numerous concurrent procedures explain our initial findings that excisions performed by experienced surgeons resulted in fewer return trips to the OR. Despite failure to show an increased symptomatic recurrence risk on multivariate analysis, we believe that complex or severe HO excisions should be done in consultation with surgeons experienced with HO resection, particularly given that residual limb HO resection in our most complex cases was done by our experienced surgeons and thus may have contributed to the statistical equivalence in our study.
Fig. 3A–B.

Radiograph (A) shows profound proximal thigh HO with symptomatic region encircled that was causing recurrent skin ulcerations. Radiograph (B) demonstrates interval focal excision of symptomatic area of HO. This patient, a bilateral transfemoral amputee, was ambulating with prosthetics at latest followup.
Fig. 4A–D.

(A) Radiographs showing HO resulting in patient inability to wear myoelectric prosthesis. (B) The initial excision is slightly obscured by plaster; however, a near-complete excision can be seen. (C) This radiograph demonstrates interval recurrence of HO with a symptomatic spike distally again resulting in the patient’s inability to wear a prosthesis. (D) This is a postoperative radiograph showing surgical reexcision with synostosis maintained for radioulnar stability.
The potential for symptomatic recurrence remains the primary concern when patients undergo surgical excision of symptomatic HO. Our results demonstrated a significant decrease in symptomatic recurrence if initial excision was performed later than 6 months (180 days) from initial injury. Prior studies have stated that before excision, HO should be mature, assessed by bone scan, and patient alkaline phosphatase blood levels should fall within normal limits [2, 17, 30]. Other studies have suggested waiting a specified amount of time or until the HO has a mature, stable neocortex [25, 30, 33, 34]. Still other studies have found that these factors were not protective against recurrence, and increased rates of recurrence in patients with central nervous system injuries have been consistently reported [1, 12, 13, 30]. Although there is no clear consensus on when to perform excision, we have demonstrated that waiting 6 months decreases the rate of recurrence in our patient population and, if recurrence does occur, it is most often radiographic only and not symptomatic (Fig. 5). Additionally, we found that there was no apparent value added in terms of recurrence risk reduction by waiting beyond 270 days, thus providing a window of 6 to 9 months for HO excision in symptomatic patients. However, if there is a progressive neurologic deficit or persistent wound healing complications resulting from HO, early excision is sometimes required, accepting the potential for increased rates of recurrence or reoperation [2, 5, 19, 26]. Dolomisiewicz et al. [5] recently characterized severely symptomatic early fulminant HO in a small series of amputees in which expectant management would not have been clinically prudent.
Fig. 5A–C.
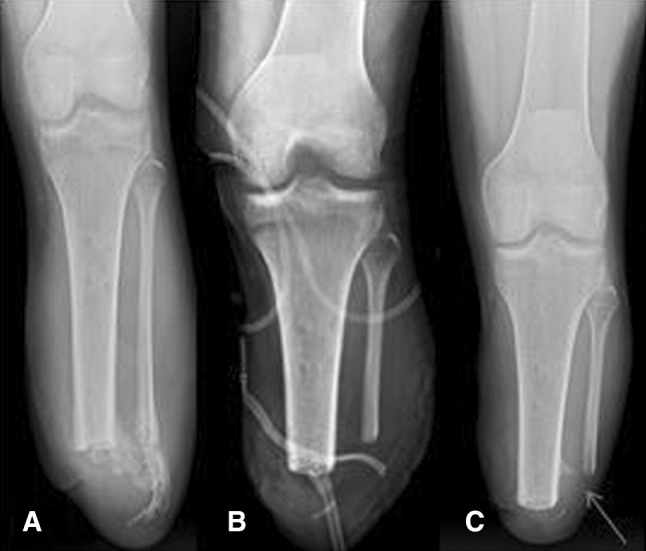
Here shown is a series of radiographs showing symptomatic, Grade 3 HO (A) before surgery; (B) immediately after complete excision; and (C) with asymptomatic radiographic recurrence, indicated by arrow, 6 months after resection.
Symptomatic HO can be detrimental to patient function and quality of life for those with trauma-related amputations. In our study, we found that complete surgical removal of HO performed beyond 180 days postinjury minimized recurrence risk. Therefore, we believe that patients can be counseled that functional limitations and pain from HO within residual limbs can be definitively and successfully treated between 6 and 9 months after injury with surgical excision, albeit with a relatively high risk of complications that may result in reoperation. Future studies may further qualify and quantify functional status and pain mitigation after surgical excision for HO in patients with amputations, and additional mechanistic and prophylaxis studies are currently underway.
Acknowledgments
We thank Ying Cao MS, and Matthew Wagner PhD, of the Naval Medical Research Center for their assistance with statistical analysis.
Footnotes
Each author certifies that he or she, or a member of his or her immediate family, has no funding or commercial associations (eg, consultancies, stock ownership, equity interest, patent/licensing arrangements, etc) that might pose a conflict of interest in connection with the submitted article.
All ICMJE Conflict of Interest Forms for authors and Clinical Orthopaedics and Related Research ® editors and board members are on file with the publication and can be viewed on request.
All of the authors are employees of the US Government and this work was prepared as part of their official duties. As such, there is no copyright to transfer. The views expressed in this article are those of the authors and do not necessarily reflect the official policy or position of the Department of the Navy, Department of the Army, Department of Defense, nor the US Government. Nothing in the presentation implies any Federal/Department of Defense/Department of the Navy endorsement.
Each author certifies that his or her institution approved the human protocol for this investigation, that all investigations were conducted in conformity with ethical principles of research, and that informed consent for participation in the study was obtained.
This work was performed at Walter Reed National Military Medical Center, Bethesda, MD, USA.
References
- 1.Baldwin K, Hosalkar HS, Donegan DJ, Rendon N, Ramsey M, Keenan MA. Surgical resection of heterotopic bone about the elbow: an institutional experience with traumatic and neurologic etiologies. J Hand Surg Am. 2011;36:798–803. doi: 10.1016/j.jhsa.2011.01.015. [DOI] [PubMed] [Google Scholar]
- 2.Brooke MM, Heard DL, de Lateur BJ, Moeller DA, Alquist AD. Heterotopic ossification and peripheral nerve entrapment: early diagnosis and excision. Arch Phys Med Rehabil. 1991;72:425–429. [PubMed] [Google Scholar]
- 3.Denormandie P, Viguie G, Denys P, Dizien O, Carlier R. Results of excision of heterotopic new bone around the elbow in patients with head injuries. A series of 25 cases. Chir Main. 1999;18:99–107. doi: 10.1016/s0753-9053(99)80062-3. [DOI] [PubMed] [Google Scholar]
- 4.Derian PS, Bibighaus AJ. Sciatic nerve entrapment by ectopic bone after posterior fracture-dislocation of the hip. South Med J. 1974;67:209–210. doi: 10.1097/00007611-197402000-00020. [DOI] [PubMed] [Google Scholar]
- 5.Dolomisiewicz EA, Miller ME, Potter BK. Fulminant heterotopic ossification after combat-related amputation: a report of 2 cases. PM R. 2014;6:279–283. doi: 10.1016/j.pmrj.2013.10.004. [DOI] [PubMed] [Google Scholar]
- 6.Fikry T, Saidi H, Madhar M, Latifi M, Essadki B. [Cubital tunnel syndrome and heterotopic ossification. Eight case reports] [in French] Chir Main. 2004;23:109–113. doi: 10.1016/j.main.2004.02.008. [DOI] [PubMed] [Google Scholar]
- 7.Forsberg JA, Pepek JM, Wagner S, Wilson K, Flint J, Andersen RC, Tadaki D, Gage FA, Stojadinovic A, Elster EA. Heterotopic ossification in high-energy wartime extremity injuries: prevalence and risk factors. J Bone Joint Surg Am. 2009;91:1084–1091. doi: 10.2106/JBJS.H.00792. [DOI] [PubMed] [Google Scholar]
- 8.Forsberg JA, Potter BK. Heterotopic ossification in wartime wounds. J Surg Orthop Adv. 2010;19:54–61. [PubMed] [Google Scholar]
- 9.Garland DE. Clinical observations on fractures and heterotopic ossification in the spinal cord and traumatic brain injured populations. Clin Orthop Relat Res. 1988;233:86–101. [PubMed] [Google Scholar]
- 10.Garland DE. A clinical perspective on common forms of acquired heterotopic ossification. Clin Orthop Relat Res. 1991;263:13–29. [PubMed] [Google Scholar]
- 11.Garland DE. Surgical approaches for resection of heterotopic ossification in traumatic brain-injured adults. Clin Orthop Relat Res. 1991;263:59–70. [PubMed] [Google Scholar]
- 12.Garland DE, Hanscom DA, Keenan MA, Smith C, Moore T. Resection of heterotopic ossification in the adult with head trauma. J Bone Joint Surg Am. 1985;67:1261–1269. [PubMed] [Google Scholar]
- 13.Garland DE, Orwin JF. Resection of heterotopic ossification in patients with spinal cord injuries. Clin Orthop Relat Res. 1989;242:169–176. [PubMed] [Google Scholar]
- 14.Garland DE, Razza BE, Waters RL. Forceful joint manipulation in head-injured adults with heterotopic ossification. Clin Orthop Relat Res. 1982;169:133–138. [PubMed] [Google Scholar]
- 15.Genet F, Jourdan C, Schnitzler A, Lautridou C, Guillemot D, Judet T, Poiraudeau S, Denormandie P. Troublesome heterotopic ossification after central nervous system damage: a survey of 570 surgeries. PLoS One. 2011;6:e16632. doi: 10.1371/journal.pone.0016632. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Jones BV, Ward MW. Myositis ossificans in the biceps femoris muscles causing sciatic nerve palsy. A case report. J Bone Joint Surg Br. 1980;62:506–507. doi: 10.1302/0301-620X.62B4.7430235. [DOI] [PubMed] [Google Scholar]
- 17.Kaplan FS, Glaser DL, Hebela N, Shore EM. Heterotopic ossification. J Am Acad Orthop Surg. 2004;12:116–125. doi: 10.5435/00124635-200403000-00007. [DOI] [PubMed] [Google Scholar]
- 18.Kleiman SG, Stevens J, Kolb L, Pankovich A. Late sciatic-nerve palsy following posterior fracture-dislocation of the hip. A case report. J Bone Joint Surg Am. 1971;53:781–782. [PubMed] [Google Scholar]
- 19.Koulouvaris P, Tsailas P, Tsiavos K, Soucacos PN. Clinical observations on surgical details of resection of heterotopic ossification at the hip in traumatic brain-injured adult. J Surg Orthop Adv. 2010;19:177–180. [PubMed] [Google Scholar]
- 20.Laborde A, Hermier M, Cotton F. Clinical Vignette. Sciatic nerve entrapment secondary to heterotopic ossification: imaging findings and potential effect of selective cox-2 inhibitors. Rheumatology (Oxford). 2005;44:110. [DOI] [PubMed]
- 21.Lee EK, Namdari S, Hosalkar HS, Keenan MA, Baldwin KD. Clinical results of the excision of heterotopic bone around the elbow: a systematic review. J Shoulder Elbow Surg. 2013;22:716–722. doi: 10.1016/j.jse.2012.11.020. [DOI] [PubMed] [Google Scholar]
- 22.Lippin Y, Shvoron A, Yaffe B, Zwas ST, Tsur H. Postburn peroneal nerve palsy—a report of two consecutive cases. Burns. 1993;19:246–248. doi: 10.1016/0305-4179(93)90161-Z. [DOI] [PubMed] [Google Scholar]
- 23.Manidakis N, Kanakaris NK, Nikolaou VS, Giannoudis PV. Early palsy of the sciatic nerve due to heterotopic ossification after surgery for fracture of the posterior wall of the acetabulum. J Bone Joint Surg Br. 2009;91:253–257. doi: 10.1302/0301-620X.91B2.21183. [DOI] [PubMed] [Google Scholar]
- 24.Matsumoto ME, Khan M, Jayabalan P, Ziebarth J, Munin MC. Heterotopic ossification in civilians with lower limb amputations. Arch Phys Med Rehabil. 2014;95:1710–1713. doi: 10.1016/j.apmr.2014.03.010. [DOI] [PubMed] [Google Scholar]
- 25.McAuliffe JA, Wolfson AH. Early excision of heterotopic ossification about the elbow followed by radiation therapy. J Bone Joint Surg Am. 1997;79:749–755. doi: 10.2106/00004623-199705000-00015. [DOI] [PubMed] [Google Scholar]
- 26.Polfer EM, Forsberg JA, Fleming ME, Potter BK. Neurovascular entrapment due to combat-related heterotopic ossification in the lower extremity. J Bone Joint Surg Am. 2013;95:e195(1–6). [DOI] [PubMed]
- 27.Potter BK, Burns TC, Lacap AP, Granville RR, Gajewski DA. Heterotopic ossification following traumatic and combat-related amputations. Prevalence, risk factors, and preliminary results of excision. J Bone Joint Surg Am. 2007;89:476–486. doi: 10.2106/JBJS.F.00412. [DOI] [PubMed] [Google Scholar]
- 28.Potter BK, Forsberg JA, Davis TA, Evans KN, Hawksworth JS, Tadaki D, Brown TS, Crane NJ, Burns TC, O’Brien FP, Elster EA. Heterotopic ossification following combat-related trauma. J Bone Joint Surg Am. 2010;92(Suppl 2):74–89. doi: 10.2106/JBJS.J.00776. [DOI] [PubMed] [Google Scholar]
- 29.Safaz I, Alaca R, Bozlar U, Yasar E. Bilateral sciatic nerve entrapment due to heterotopic ossification in a traumatic brain-injured patient. Am J Phys Med Rehabil. 2008;87:65–67. doi: 10.1097/PHM.0b013e31815b5b4d. [DOI] [PubMed] [Google Scholar]
- 30.Stover SL, Niemann KM, Tulloss JR. Experience with surgical resection of heterotopic bone in spinal cord injury patients. Clin Orthop Relat Res. 1991;263:71–77. [PubMed] [Google Scholar]
- 31.Thakkar DH, Porter RW. Heterotopic ossification enveloping the sciatic nerve following posterior fracture-dislocation of the hip: a case report. Injury. 1981;13:207–209. doi: 10.1016/0020-1383(81)90240-0. [DOI] [PubMed] [Google Scholar]
- 32.Tsionos I, Leclercq C, Rochet JM. Heterotopic ossification of the elbow in patients with burns. Results after early excision. J Bone Joint Surg Br. 2004;86:396–403. doi: 10.1302/0301-620X.86B3.14480. [DOI] [PubMed] [Google Scholar]
- 33.Viola RW, Hanel DP. Early ‘simple’ release of posttraumatic elbow contracture associated with heterotopic ossification. J Hand Surg Am. 1999;24:370–380. doi: 10.1053/jhsu.1999.0370. [DOI] [PubMed] [Google Scholar]
- 34.Viola RW, Hastings H., 2nd Treatment of ectopic ossification about the elbow. Clin Orthop Relat Res. 2000;370:65–86. doi: 10.1097/00003086-200001000-00008. [DOI] [PubMed] [Google Scholar]
- 35.Vorenkamp SE, Nelson TL. Ulnar nerve entrapment due to heterotopic bone formation after a severe burn. J Hand Surg Am. 1987;12:378–380. doi: 10.1016/S0363-5023(87)80009-6. [DOI] [PubMed] [Google Scholar]
- 36.Wick M, Muller EJ, Hahn MP, Muhr G. Surgical excision of heterotopic bone after hip surgery followed by oral indomethacin application: is there a clinical benefit for the patient? Arch Orthop Trauma Surg. 1999;119:151–155. doi: 10.1007/s004020050379. [DOI] [PubMed] [Google Scholar]




