Abstract
Background
Allograft bone is commonly used to augment bone stock. Unfortunately, allograft is prone to bacterial contamination and current antimicrobial therapies are inadequate. Photoactivated porphyrins combat bacterial growth by production of reactive oxygen species (ROS); however, to our knowledge, they have not been tested in the setting of allograft bone.
Questions/purposes
We asked: (1) Does 5,10,15,20-tetrakis-(4-aminophenyl)-porphyrin (TAPP) stably adsorb to morselized, mineralized allograft? (2) Does Staphylococcus aureus acquire TAPP from TAPP-allograft? (3) Is TAPP-allograft antibacterial to S. aureus? (4) Is ROS production critical for antimicrobial activity? (5) Does illuminated TAPP-allograft dislodge biofilm? (6) Could other photoactive dyes (TAPP, TMPyP, TSP, THP, and methylene blue) confer antimicrobial properties to allograft?
Methods
TAPP adsorption to allograft (TAPP-allograft), its localization in S. aureus, and TAPP-allograft long-term stability were determined spectrophotometrically. Antimicrobial activity was measured while activated with light or in the dark during incubation with S. aureus or after allograft biofilm formation. Glutathione was added to illuminated TAPP-allograft to quench ROS and antimicrobial activity was determined. Light-dependent antimicrobial activity of other photoactive dyes (TMPyP, TSP, THP, and methylene blue) adsorbed to allograft was also tested.
Results
We found (1) porphyrins strongly adhere to bone allograft; and (2) the bacteria are not able to sequester TAPP from the TAPP-allograft; (3) when illuminated, TAPP-allograft is resistant to bacterial adherence; (4) the effects of TAPP are inhibited by the radical scavenger glutathione, indicating ROS-dependent antimicrobial activity; (5) illumination of TAPP-allograft disrupts biofilms; and, (6) other photoactive dyes impede biofilm formation on allograft bone in the presence of light.
Conclusions
Porphyrins stably associate with allograft and are inactive until illuminated. Illuminated TAPP-allograft markedly reduces bacterial colonization, which is restored in the presence of radical scavengers. Finally, illuminated TAPP-allograft disrupts biofilms.
Clinical Relevance
The findings of this in vitro study suggest that loading bone allograft with biocompatible porphyrins before surgery might allow increased sterility of the allograft during implantation. Future testing in an animal model will determine if these in vitro activities can be used to prevent allograft-based infection in an establishing osteomyelitis.
Introduction
Infection occurs with bone allograft use with rates of approximately 11% for large allografts and 4% to 6% in hip revisions [9]. Infection becomes more common when bone supplementation is used during revision of an infected site or after ballistic injury. Autologous bone is the gold standard for replacement and may be associated with a lower risk of infection than allograft; however, donor site morbidity and poor or inadequate bone stock limit its availability. Thus, allograft bone remains in wide use.
Allograft-associated infection arises when allograft is seeded with bacteria from a previous osteomyelitis, a traumatic injury, or hematogenous bacterial contamination. The previously planktonic bacteria adhere to the allograft and initiate production of a biofilm slime, effectively decreasing bacterial antibiotic susceptibility [1, 3] and limiting immune surveillance [25]. Although some antibiotics show moderate success in eradicating surface-associated/biofilm-encased bacteria, the concentrations are often toxic for the target tissue. Considerable success has been achieved by adsorption of antibiotics to allograft and by use of controlled-release bone substitutes [11].
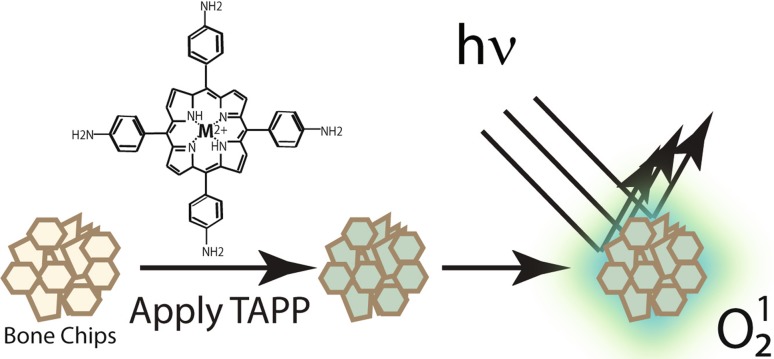
To lessen concerns about fostering antibiotic resistance, photoactivated compounds are being explored to replace and/or augment antibiotic treatments. Phototherapy has been used as an antibacterial treatment in light-accessible sites such as gingival tissue and dental implantation [22]. We reasoned that a similar effect could be achieved by illumination during insertion of allograft into a surgical site. Although the mechanism is not fully understood, the antibacterial effects of porphyrins are mostly attributed to their localization in the bacterial membrane and, when activated by light, generate reactive oxygen species (ROS) [15]. Because ROS is antibacterial, surface-coupled porphyrins can actively sterilize surfaces when illuminated [6, 14]. Our laboratory has been exploring the use of the cationic porphyrin 5,10,15,20-tetrakis-(4-aminophenyl)-porphyrin (TAPP; Fig. 1) as an antibacterial treatment for bone allograft.
Fig. 1.
Photoactivation of TAPP-adsorbed bone allograft. Bone chips are incubated with 5,10,15,20-tetrakis-(4-aminophenyl)-porphyrin (TAPP), structure shown above the arrow to produce TAPP adsorbed to mineralized bone allograft. When illuminated (hν), excitation of the TAPP-adsorbed to allograft can produce ROS (O12) in the immediate vicinity of the surface (green haze); these ROS are known to be antimicrobial.
In this article, we asked: (1) Does TAPP stably adsorb to morselized, mineralized allograft? (2) Does Staphylococcus aureus extract TAPP from the TAPP-allograft? (3) Does TAPP-allograft resist colonization by S. aureus? (4) Is ROS production critical for the antimicrobial activity? (5) Does illuminated TAPP-allograft dislodge biofilm? (6) Could other photoactive dyes (ie, TAPP, TMPyP, TSP, THP, and methylene blue) confer antimicrobial properties to allograft?
Materials and Methods
Study Design
To test Question 1, if a porphyrin would stably associate with allograft bone, TAPP (500 µM) was incubated with allograft overnight, rinsed with phosphate-buffered saline (PBS), and dried (TAPP-allograft). We measured adsorption of TAPP to allograft bone (cortical and cancellous morsels; Musculoskeletal Transplant Foundation, Edison, NJ, USA) by measuring the decrease in absorbance (λ = 435 nm) of 125 μM TAPP solution before and after 1 day incubation with allograft. TAPP elution from TAPP-allograft was measured spectrophotometrically after incubation in distilled water over a 9-week period. To measure the total amount of TAPP adsorbed to the bone, TAPP was eluted by 5-minute incubation in either 0.275 N HCl or 10 μN HCl.
Because the effects of TAPP are attributed to its incorporation into the bacterial membrane, we next asked if TAPP localized in the bacterial membrane when incubated with TAPP-allograft (Question 2). Planktonic S. aureus in PBS were incubated with 200 µM TAPP or with 1 g of TAPP-allograft overnight. Bacteria were then collected, washed, lysed, clarified (4000 rpm, 15 minutes), and TAPP concentration in the lysate was measured spectrophotometrically (TECAN Infinite M1000; Tecan Group, Männedorf, Switzerland).
We then tested Question 3: Does TAPP-allograft resist colonization by S. aureus? A total of 107 CFU/mL S. aureus AH1710 (a green-fluorescent protein-expressing strain [16]) in trypticase soy broth (TSB) was incubated with TAPP-allograft under illumination (light) or in the dark. Bacteria adherent to the TAPP-allograft surface were visualized by confocal laser scanning microscopy or recovered by sonication in 0.3% Tween-20 in PBS followed by serial dilution/plating.
To test Question 4, if ROS production is critical for the antimicrobial activity, illuminated control or TAPP-allograft was incubated with S. aureus AH1710 in the presence of 0 or 20 mM glutathione, a known radical scavenger; adherent bacteria were visualized by confocal scanning microscopy.
We next tested Question 5: does illuminated TAPP-allograft dislodge biofilm? Biofilms of S. aureus AH1710 were formed by incubating control or TAPP-allograft for 48 hours in the dark using TSB containing 1% glucose. Biofilm-coated control or TAPP-allograft was then placed in the dark (no activation) or illuminated (light-activated) for 18 hours in PBS at 37 °C. Biofilm presence was determined by confocal microscopy with three-dimensional reconstruction (Z-stack); alternately, biofilm bacteria were recovered by sonication in 0.3% Tween-20, diluted, plated, and counted.
Finally (Question 6), we asked if other porphyrins (cationic: TAPP and meso-tetra[4-sulfonatophenyl] porphine [TMPyP, 500 µM], anionic: tetrakis[1-methylpyridinium-4-yl]porphyrin p-Toluenesulfonate [TSP, 500 µM], and neutral: tetrakis-pyridyl-tetrahydroporphyrin tosylate [THP, 1 mM]), or the photoactive dye, methylene blue, adsorbed to hydrated, mineralized, bone allograft to render it antimicrobial. After overnight adsorption, the allografts were washed and incubated in the dark for 24 hours at 37 °C with S. aureus AH1710 in TSB containing 1% glucose to allow biofilm formation. Biofilm-coated samples were then placed in the light or dark for 18 hours, rinsed, and adherent bacteria were then visualized with confocal laser scanning microscopy.
Materials
TAPP, TSP, TMPyP, THP, and methylene blue were purchased from Frontier Scientific (Logan, UT, USA). Glutathione (98%) and 0.4% Trypan blue were purchased from Sigma-Aldrich Chemical Company (St Louis, MO, USA). Mineralized bone allograft, cortical and cancellous granules, 0.5 to 5.0 mm in diameter, was the kind gift of the Musculoskeletal Transplant Foundation (Edison, NJ, USA). Vancomycin hydrochloride was obtained from APP Pharmaceuticals (Lake Zurich, IL, USA); TSB and Bacto™ Agar were obtained from Becton, Dickinson, and Company (Franklin Lakes, NJ, USA). All other materials were tissue culture grade or analytical grade and were purchased from Fisher Scientific (Waltham, MA, USA).
Bacterial Culture
S. aureus AH1710, which expresses green fluorescent protein (GFP) under chloramphenicol pressure [8, 16], was grown at 37 °C, 225 rpm, in TSB containing 8 µg/mL of chloramphenicol, 1% glucose. Using a 0.5 McFarland standard (a measure of light dispersion by suspended particles in solution [17] that approximates 1 × 108 colony-forming units [CFU]/mL S. aureus [2]), the culture was brought to 108 CFU/mL and diluted in PBS as indicated.
Illumination Conditions
All samples to be illuminated were placed in a tissue culture plate that was then placed approximately 5 inches below a 100-W, 120-V Sylvania white light (OSRAM, Danvers, MA, USA) in a humidified, transparent chamber; distance from the light was slightly adjusted to maintain the chamber at 37 °C. Controls were incubated in the dark at 37 °C.
Adsorption of Porphyrins to Allograft
Human bone granules (mineralized cancellous and cortical) were hydrated by washing and sonicating in dH2O until washings were clear (referred to as allograft). Two milliliters of 500 µM TAPP, TMPyP, TSP, methylene blue, or 1 mM THP were incubated overnight at room temperature with 2 g allograft. All allografts were rinsed with sterile PBS (three times) before use.
Measurement of Adsorbed TAPP
Allograft (approximately 1 mm3) was added to a 96-well plate containing 125 µM TAPP (100 µL/well), sealed with Parafilm™ (Bemis, Oshkosh, WI, USA), and incubated at room temperature with shaking. At 24 hours, bone morsels were removed, and absorbance (λ = 435 nm [19]) of the solution was measured. TAPP adsorption to allograft was calculated by subtracting peak absorbance before and after allograft incubation and calculating the resulting concentration.
TAPP-allograft Stability
Allograft morsels soaked in 1 mM TAPP overnight were washed three times with distilled, deionized water (ddH2O), and individual morsels were placed into 200 µL ddH2O. Samples were maintained at room temperature in the dark with shaking. Bathing fluid was removed weekly for 9 weeks and stored in the dark, 4 °C until measurement. At the end of the elution period, remaining TAPP adsorbed to the allograft was eluted by incubation in 10 μN HCl or 0.275 N HCl (TAPP is highly soluble at acidic pHs) for 5 minutes. Absorbance of samples was determined spectrophotometrically (λ = 435 nm) and compared with 100 µL of 125 μM TAPP stored under the same conditions.
Recovery of TAPP from S. aureus
Five milliliters of TSB containing 109 CFU/mL S. aureus ATCC® 25923™ were incubated in TSB, in 200 μM TAPP, or with 1 g TAPP-allograft for 18 hours at 37 °C. After vortexing, bacteria were pelleted (1500 rpm, 5 minutes) and washed in water three times. Pelleted bacteria were lysed by sonication (Branson 1510; Branson Ultrasonics, Danbury, CT, USA) in 200 µL of 0.3% Tween-20 for 20 to 30 minutes and the lysate was analyzed spectrophotometrically for porphyrins.
Bacterial Adherence to Bone Allograft
Sterile control or TAPP-allograft morsels were placed in 200 µL PBS containing 107 CFU/mL S. aureus AH1710 and incubated in the light (to activate porphyrin) or dark (control) at 37 °C for 18 hours. After incubation, morsels were rinsed three times with PBS and surface colonization visualized by confocal laser scanning microscopy using three-dimensional reconstruction.
Addition of Glutathione as an Antioxidant
The effect of glutathione on bacterial growth was determined using CLSI M31-A2 and glutathione concentrations of 0 to 80 mM. After overnight incubation at 37 °C, glutathione concentrations above 40 mM showed no bacterial growth. Glutathione (stock: 640 mM GSH in PBS; final concentration, 20 mM) or PBS was added to wells containing 107 CFU bacteria in the presence of control or TAPP-allograft (final volume = 200 μL TSB). After light activation (or no light for control) for 18 hours at 37 °C, samples were washed with PBS and surface colonization visualized using confocal laser scanning microscopy.
Allograft Biofilm
To form a biofilm, approximately 0.2 g of control or TAPP-allograft was incubated with 1 mL of 104 CFU/mL S. aureus AH1710 in TSB containing 1% glucose, 10 µg chloramphenicol (to maintain GFP expression) in the dark under static conditions for 2 days followed by three gentle washes with PBS. Biofilm-containing morsels were then incubated in the dark (control) or light (to activate TAPP) at 37 °C for 18 hours. Adherent bacteria were imaged for 50% of samples using confocal laser scanning microscopy. Adherent bacteria (other 50% of samples) were also recovered, diluted, plated, and counted.
Bacterial Survival Counts
Allograft was gently washed × 3 with PBS followed by sonication in 200 µL 0.3% Tween-20 for 5 minutes to suspend surface-adherent bacteria. Suspended bacteria were diluted in PBS, plated on 3M™ Petrifilms™ (3M, St Paul, MN, USA), incubated for 24 hours at 37 °C, and colonies were counted manually.
Confocal Laser Scanning Microscopy
S. aureus AH1710 was visualized on bone allograft samples using a ×4 lens with 500-μm sections at 25-μm intervals on an Olympus Fluoview 300 confocal microscope (Olympus America Inc, Center Valley, PA, USA). Three-dimensional images were reconstructed and images were assessed for number of bacteria (green colonies) as well as density of colonization and presence of biofilm-like structures.
Statistical Methods
All experiments were repeated a minimum of three times in three independent experiments with data expressed as mean ± SD. One- or two-way analyses of variance, as appropriate, with a Tukey post hoc multiple comparison procedure were used to analyze differences between groups.
Results
Does TAPP Adsorb to Mineralized Allograft?
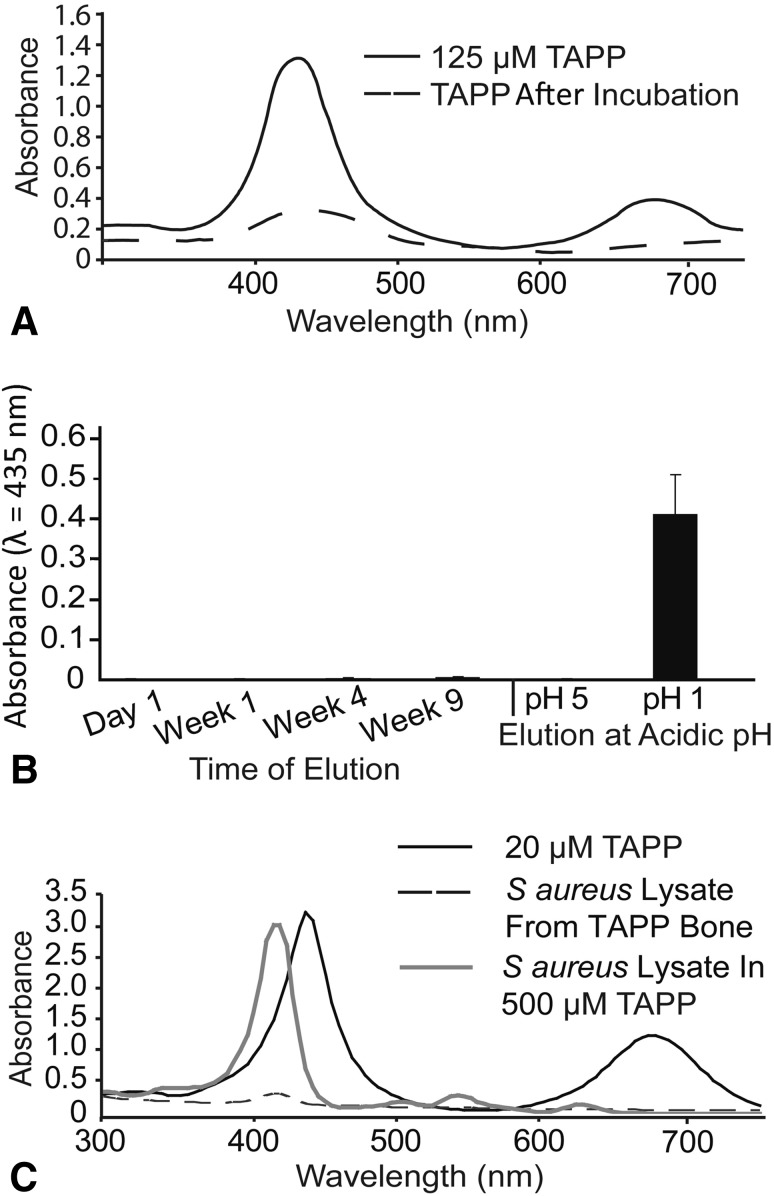
When morselized, mineralized bone (1 mm3 in 200 μL) was incubated overnight with 125-µM TAPP, the amount of TAPP remaining in solution decreased to 8 to 10 µM TAPP (Fig. 2A), suggesting adsorption to bone (TAPP-allograft). Over a 9-week incubation in PBS nor after a 1-hour incubation in pH 5 water, TAPP-allograft showed no detectable TAPP elution (Fig. 2B). When TAPP was incubated in a pH 1 solution to allow ready solubilization, TAPP was completely removed from allograft.
Fig. 2A–C.
TAPP stably adsorbs to mineralized bone allograft. (A) Absorbance spectra of TAPP before (125 μM, solid line) and after (TAPP remaining, dashed line) 24-hour incubation at room temperature with bone morsels. (B) Absorbance of solutions that were collected weekly during elution from TAPP-allograft for up to 9 weeks or after exposure to 10 μN (pH 5) or 0.275 N (pH 1) HCl. Values shown are mean ± SD. The graph is a representative experiment with triplicate samples; the experiment was performed a total of three times with similar results. (C) TAPP absorbance was measured in solution (black line). Absorbance of TAPP localized in planktonic S. aureus was measured after incubation (18 hours), washing, and lysis (gray line); and TAPP localized in S. aureus was measured after incubation (18 hours) with TAPP-allograft followed by washing and lysis (broken line).
Does S. aureus Extract TAPP from the TAPP-allograft?
TAPP, which can localize to bacterial membranes, showed an accumulation of approximately 50 μM in planktonic S. aureus that had been lysed after culturing in 500 μM TAPP for 18 hours (Fig. 2C) with a slight shift in peak absorbance. S. aureus cultured on TAPP-allograft, however, did not appear to sequester TAPP as little absorbance was measured after lysis.
Does TAPP-allograft Resist Colonization by S. aureus?
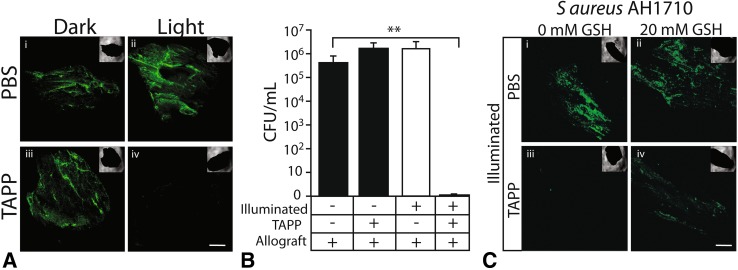
Control allograft was abundantly colonized in both the dark and the light; S. aureus also colonized TAPP-allograft in the dark. In contrast, illuminated TAPP-allograft showed little bacterial colonization (Fig. 3A). When adherent bacteria were recovered and plated, similar numbers of S. aureus (105–106 CFU/mL) were measured on control allograft (light and dark) and dark TAPP-allograft; no viable bacteria were recovered from TAPP-allograft that had been incubated with S. aureus in the light (Fig. 3B).
Fig. 3A–C.
Illuminated TAPP-allograft is antibacterial and its activity is moderated by glutathione. (A) Control allograft (PBS) or TAPP-allograft was incubated (18 hours) with S. aureus in the dark or while illuminated. Adherent bacteria (green) were visualized by confocal laser scanning microscopy. (B) Number of adherent bacteria recovered from the different allograft surfaces as determined by direct counting. The table indicates the absence (−) or presence (+) of light activation (Illumination), TAPP adsorbed to allograft (TAPP), and the presence of allograft bone (Allograft). **p < 0.01 when compared with the dark control as determined by one-way analysis of variance. (C) Control (PBS) or TAPP-allograft was incubated with S. aureus in the light in the presence of 0 or 20 mM glutathione (GSH). Bacterial adherence and density (green) was visualized by confocal laser scanning microscopy. For A and C, magnification: scale bar = 500 µm.
Is ROS Production Critical for Antimicrobial Activity?
In the presence of 0 or 20 mM glutathione, a radical scavenger that will inactivate ROS, S. aureus readily colonized both dark and light control (PBS) allograft. Minimal colonization was present on illuminated TAPP-allograft without glutathione, but in the presence of 20 mM glutathione, some colonization was regained (Fig. 3C).
Does Illuminated TAPP-allograft Dislodge Biofilm?
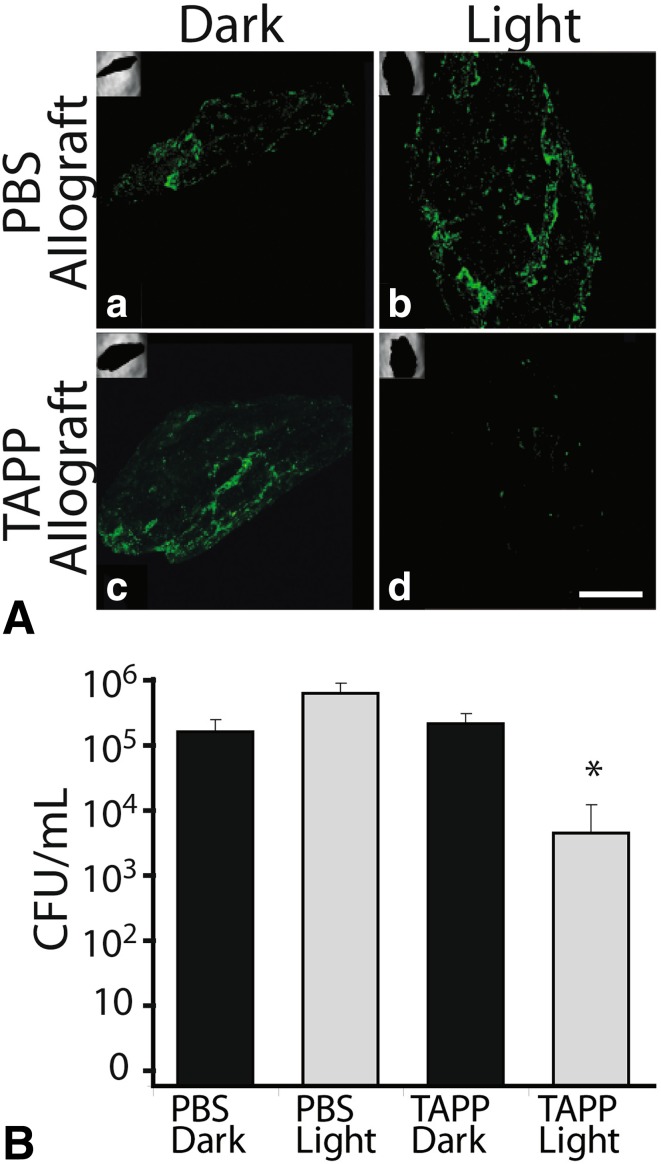
S. aureus biofilms (48 hours, dark) were formed on TAPP and control allograft. Control/PBS allograft remained abundantly colonized after an additional 18 hours in the light or dark as did dark TAPP-allograft. In contrast, biofilm bacteria were not obvious on the illuminated TAPP-allograft samples (Fig. 4A). Numbers of adherent bacteria recovered from PBS allograft (light and dark) and dark TAPP-allograft samples were approximately equivalent; illuminated TAPP-allograft samples showed a 2- to 3-log decrease in adherent bacteria (Fig. 4B).
Fig. 4A–B.
Illuminated TAPP-allograft disperses biofilm. (A) S. aureus AH1710 was allowed to form a biofilm on PBS or TAPP-allograft by incubation for 48 hours in the dark. Bacterial colonization (as a result of expression of GFP) was visualized after another 18 hours in the dark or after illumination (“a” is PBS allograft in the dark or “b” light; “c” is TAPP allograft in the dark or “d” light). Magnification: scale bar = 500 µm. (B) Adherent S. aureus were recovered and counted (shown as CFU/ml) from PBS or TAPP-allograft treated as in Fig. 4A. *p < 0.05 when compared with the other counts as determined by a two-tailed t-test.
Other Photosensitizers on Allograft
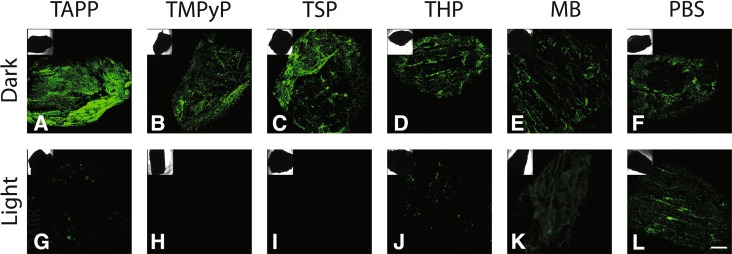
In the dark, S. aureus colonized all allografts with adsorbed photosensitizers (cationic porphyrins TAPP or TMPyP, anionic porphyrin TSP, neutral porphyrin THP, or methylene blue). After illumination, with the exception of methylene blue, all photosensitizer allograft samples showed decreased bacterial colonization. Methylene blue did not remain adsorbed to allograft so was unable to decrease bacterial colonization (Fig. 5).
Fig. 5A–L.
Photoactive compounds adsorbed to bone allograft combat preformed biofilm. Control, TAPP (cationic), TMPyP (cationic), TSP (anionic), THP (neutral), and methylene blue (MB)-adsorbed allograft were allowed to form S. aureus AH1710 biofilms (48 hours, dark) and bacterial colonization was visualized using the innate green fluorescent protein by confocal laser scanning microscopy after an additional 18 hours in the dark (A–F) or light (G–L). Scale bar = 500 µm.
Discussion
Bone allograft is an important material for bone augmentation but, like any other implanted material, is prone to bacterial colonization. We asked if photoactivated compounds adsorbed to these allografts could confer antimicrobial activity to allograft while illuminated. Previous work has shown that photoactivation of porphyrin in solution kills planktonic bacteria [8] and to some extent biofilms [5]. We show here that porphyrins adsorb avidly to mineralized allograft. This adsorption is stable over 9 weeks. Furthermore, S. aureus does not solubilize TAPP from the bone matrix. In the dark, S. aureus readily adheres and grows in the presence of porphyrins. However, light activation results in rapid killing. This killing is presumably the result of the production of ROS as a radical scavenger (glutathione) preventing this effect. Light activation of the TAPP-allograft can cause eradication of biofilms resident on the surface, and these activities are not unique to TAPP because other porphyrins can cause similar effects.
The significance of these findings resides in the assumption that many surgical site infections are initiated during the operation. In the bright environment of the operating room, TAPP-allograft could be illuminated by exposure to intense white light and this illumination would then continue during insertion, ensuring that the implants were sterile. However, these conditions need to be explicitly tested because the work that we describe in this article has been limited to standard bacterial growth media and TAPP-allograft has not been tested in the presence of physiological fluids. Additionally, only morselized allograft, not the widely used large, structural allograft, was tested, although allograft morsels contain both cancellous and cortical bone. Although application to the large allografts may be possible, it needs to be explicitly tested, especially because the geometry of the larger allografts may constrain even illumination. Finally, we used a broad-spectrum lamp rather than a targeted wavelength and we consistently photoactivated for approximately 18 hours. Use of a targeted wavelength and defining the minimum photoactivation time could allow more efficient photoactivation for maintenance of sterility.
We first investigated if TAPP adsorbed to mineralized allograft. Our data showing that porphyrins avidly adsorbed to mineralized bone allograft are consistent with previous descriptions of the accumulation of porphyrins in mineralized tissue and their strong affinity for teeth and calcified plaques in the circulatory system [12]. This adsorption could only be disrupted by strong acidification, as we observed in our studies. Practically, porphyrins accumulate in bone with no clearance until bone/tissue turnover has occurred [10, 20].
Because the antibacterial activity of porphyrins is associated with their localization in cells [4], we next asked if bacteria were able to extract TAPP from TAPP-allograft. Although S. aureus readily acquired TAPP from solution, minimal TAPP was detected in bacteria incubated with TAPP-allograft. These results suggest that bacteria will have to come in contact with the TAPP-allograft for its antibacterial effects.
Next, we addressed if TAPP-allograft resists colonization by S. aureus under illumination. We showed that TAPP-allograft, when illuminated, robustly decreased bacterial colonization. This effect was observed during incubation with planktonic bacteria, a situation that would more nearly recapitulate the perioperative contamination of allograft. This marked (approximately 5–6 logs) decrease is high enough to suggest therapeutic efficacy.
We then asked whether ROS production is critical for the antimicrobial activity. Porphyrins, when internalized in cells, depend on the production of ROS for their antimicrobial activity [8]. Glutathione is a known radical scavenger that can inhibit the activity of TAPP in solution [8]. As observed with solution TAPP, glutathione decreased the activity of photoactivated TAPP-allograft, suggesting that ROS at the surface of the allograft mediates the effect. Furthermore, these data suggest that the antimicrobial effects would be localized to the bone-liquid interface as the mean free path of ROS is on the order of microns.
We next asked if illuminated TAPP-allograft can dislodge biofilm. Our results showed that activation of the TAPP-allograft by illumination causes dispersion of the biofilm bacteria from the surface. Because of the antibiotic recalcitrance of adherent/biofilm-associated bacteria [21], biofilms are often not susceptible to antibiotic levels that are × 100 to × 1000 the minimum inhibitory concentration of planktonic bacteria [3, 7]. Biofilms of Porphyromonas gingivalis have reportedly been treated by light activation of endogenous porphyrin by dentists since 2003 [13, 23]. Therefore, even in the case in which an allograft has been prepopulated by bacteria, an effect such as we describe could effectively sterilize the material before or during the operative procedure.
Finally, we asked if other photoactive dyes, ie, TAPP, TMPyP, TSP, THP, and methylene blue, were able to confer antimicrobial properties to allograft. Based on our data, the ability of the compound to adsorb to a bone allograft is the most important factor. Whereas all porphyrin compounds adsorbed avidly to bone, methylene blue, a strong radical producer, easily eluted from bone because of its solubility. In keeping with this, the porphyrin allografts showed antimicrobial activity on illumination, whereas methylene blue allograft showed very little antimicrobial activity. Despite this strong adsorption to allograft, charged porphyrins (ie, TAPP, TMPyP, and TSP) outperformed the neutral porphyrin THP on illumination. These data are supported by other reports claiming that both cationic and anionic photoporphyrins have greater antimicrobial effects than neutral porphyrins [18, 24]. Taken together, these data suggest that charged porphyrins, whether anionic or cationic, would be excellent candidates for photodynamic antimicrobial therapy. Further studies must be performed to determine the stability, cytotoxicity, and photodegradation/photoactivity profiles for each photoactive dye.
In summary, porphyrins are biologically well tolerated and easily adsorbed to bone, which allows for the option of creating antimicrobial allografts to combat perioperative infections. Sterility of biomaterials in the perioperative period is extremely important to achieve low postoperative infection rates. The most important application may be the sterilization of structural allografts, which will require further development to prove utility. An additional area that may prove to be of great importance is the possibility of increasing the efficacy of traditional antibiotics. In fact, combining the proven efficacy of adsorbed antibiotics with photoactivated TAPP-allograft could prove to be a potent new treatment. We propose that use of a biocompatible, porphyrin-treated bone allograft that may be activated under visible light in the perioperative setting may promote sterility and/or disinfection of the allograft before implantation to ultimately decrease infection rates.
Acknowledgments
We thank the Musculoskeletal Transplant Foundation for supplying bone allograft and Dr Alexander Horswill, University of Iowa, for allowing us to use his S. aureus AH1710 strain.
Footnotes
One of the authors (SSD) was supported by National Institute of Arthritis and Musculoskeletal and Skin Diseases training grant T32-AR-052273 during the study period (USD 10,000 to USD 100,000). One of the authors (IMS) received research funding from National Institutes of Health grant R01 HD061053 during the study period (USD 100,000 to USD 1,000,000). One of the authors (NJH) has received research funding from NIH grants HD06153 and DE019901 (USD 100,000 to USD 1,000,000), Synergy Biomedical, Collegeville, PA, USA (USD 10,000 to USD 100,000), and Zimmer, Warsaw, IN, USA (USD 10,000 to USD 100,000) during the study period. The Musculoskeletal Transplant Foundation provided human bone allograft.
All ICMJE Conflict of Interest Forms for authors and Clinical Orthopaedics and Related Research ® editors and board members are on file with the publication and can be viewed on request.
Clinical Orthopaedics and Related Research ® neither advocates nor endorses the use of any treatment, drug, or device. Readers are encouraged to always seek additional information, including FDA-approval status, of any drug or device prior to clinical use.
References
- 1.Antoci V, Jr, Adams CS, Parvizi J, Davidson HM, Composto RJ, Freeman TA, Wickstrom E, Ducheyne P, Jungkind D, Shapiro IM, Hickok NJ. The inhibition of Staphylococcus epidermidis biofilm formation by vancomycin-modified titanium alloy and implications for the treatment of periprosthetic infection. Biomaterials. 2008;29:4684–4690. doi: 10.1016/j.biomaterials.2008.08.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Antoci V, Jr, Adams CS, Parvizi J, Ducheyne P, Shapiro IM, Hickok NJ. Covalently attached vancomycin provides a nanoscale antibacterial surface. Clin Orthop Relat Res. 2007;461:81–87. doi: 10.1097/BLO.0b013e3181123a50. [DOI] [PubMed] [Google Scholar]
- 3.Antoci V, Jr, King SB, Jose B, Parvizi J, Zeiger AR, Wickstrom E, Freeman TA, Composto RJ, Ducheyne P, Shapiro IM, Hickok NJ, Adams CS. Vancomycin covalently bonded to titanium alloy prevents bacterial colonization. J Orthop Res. 2007;25:858–866. doi: 10.1002/jor.20348. [DOI] [PubMed] [Google Scholar]
- 4.Banfi S, Caruso E, Buccafurni L, Battini V, Zazzaron S, Barbieri P, Orlandi V. Antibacterial activity of tetraaryl-porphyrin photosensitizers: an in vitro study on Gram negative and Gram positive bacteria. J Photochem Photobiol B. 2006;85:28–38. doi: 10.1016/j.jphotobiol.2006.04.003. [DOI] [PubMed] [Google Scholar]
- 5.Beirao S, Fernandes S, Coelho J, Faustino MA, Tome JP, Neves MG, Tome AC, Almeida A, Cunha A. Photodynamic inactivation of bacterial and yeast biofilms with a cationic porphyrin. Photochem Photobiol. 2014 Aug 12 [Epub ahead of print]. [DOI] [PubMed]
- 6.Bonnett R, Krysteva MA, Lalov IG, Artarsky SV. Water disinfection using photosensitizers immobilized on chitosan. Water Res. 2006;40:1269–1275. doi: 10.1016/j.watres.2006.01.014. [DOI] [PubMed] [Google Scholar]
- 7.Dastgheyb S, Parvizi J, Shapiro IM, Hickok NJ, Otto M. Effect of biofilms on recalcitrance of staphylococcal joint infection to antibiotic treatment. J Infect Dis. 2015;211:641–650. doi: 10.1093/infdis/jiu514. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Dastgheyb SS, Eckmann DM, Composto RJ, Hickok NJ. Photo-activated porphyrin in combination with antibiotics: therapies against Staphylococci. J Photochem Photobiol B. 2013;129:27–35. doi: 10.1016/j.jphotobiol.2013.09.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Delloye C, Cornu O, Druez V, Barbier O. Bone allografts: what they can offer and what they cannot. J Bone Joint Surg Br. 2007;89:574–579. doi: 10.1302/0301-620X.89B5.19039. [DOI] [PubMed] [Google Scholar]
- 10.Elder GH. Porphyrias. New York, NY, USA: John Wiley & Sons, Ltd; 2001. [Google Scholar]
- 11.Hickok NJ, Shapiro IM. Immobilized antibiotics to prevent orthopaedic implant infections. Adv Drug Deliv Rev. 2012;64:1165–1176. doi: 10.1016/j.addr.2012.03.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Kessel D, Berguer R. Determinants of porphyrin fluorescence emission spectra of atheromatous plaques. Atherosclerosis. 1988;69:1–4. doi: 10.1016/0021-9150(88)90283-3. [DOI] [PubMed] [Google Scholar]
- 13.Konopka K, Goslinski T. Photodynamic therapy in dentistry. J Dent Res. 2007;86:694–707. doi: 10.1177/154405910708600803. [DOI] [PubMed] [Google Scholar]
- 14.Krouit M, Granet R, Krausz P. Photobactericidal plastic films based on cellulose esterified by chloroacetate and a cationic porphyrin. Bioorg Med Chem. 2008;16:10091–10097. doi: 10.1016/j.bmc.2008.10.010. [DOI] [PubMed] [Google Scholar]
- 15.Maisch T, Spannberger F, Regensburger J, Felgentrager A, Baumler W. Fast and effective: intense pulse light photodynamic inactivation of bacteria. J Ind Microbiol Biotechnol. 2012;39:1013–1021. doi: 10.1007/s10295-012-1103-3. [DOI] [PubMed] [Google Scholar]
- 16.Malone CL, Boles BR, Lauderdale KJ, Thoendel M, Kavanaugh JS, Horswill AR. Fluorescent reporters for Staphylococcus aureus. J Microbiol Methods. 2009;77:251–260. doi: 10.1016/j.mimet.2009.02.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.McFarland J. The nephelometer: an instrument for estimating the number of bacteria in suspensions used for calculating the opsonic index and for vaccines. JAMA. 1907;49:1176–1178. doi: 10.1001/jama.1907.25320140022001f. [DOI] [Google Scholar]
- 18.Merchat M, Spikes JD, Bertoloni G, Jori G. Studies on the mechanism of bacteria photosensitization by meso-substituted cationic porphyrins. J Photochem Photobiol B. 1996;35:149–157. doi: 10.1016/S1011-1344(96)07321-6. [DOI] [PubMed] [Google Scholar]
- 19.Paschenko VZ, Evstigneeva RP, Gorokhov VV, Luzgina VN, Tusov VB, Rubin AB. Photophysical properties of carborane-containing derivatives of 5,10,15,20-tetra(p-aminophenyl)porphyrin. J Photochem Photobiol B. 2000;54:162–167. doi: 10.1016/S1011-1344(00)00011-7. [DOI] [PubMed] [Google Scholar]
- 20.Schmid R, Schwartz S, Watson CJ. Porphyrin content of bone marrow and liver in the various forms of porphyria. AMA Arch Intern Med. 1954;93:167–190. doi: 10.1001/archinte.1954.00240260001001. [DOI] [PubMed] [Google Scholar]
- 21.Stojilkovic I, Evavold BD, Kumar V. Antimicrobial properties of porphyrins. Exp Op Inv Drugs. 2001;10:309–320. doi: 10.1517/13543784.10.2.309. [DOI] [PubMed] [Google Scholar]
- 22.Takasaki AA, Aoki A, Mizutani K, Schwarz F, Sculean A, Wang C-Y, Koshy G, Romanos GE, Ishikawa I, Izumi Y. Application of antimicrobial photodynamic therapy in periodontal and peri-implant diseases. Periodontology 2000. 2009;2009(51):109–140. doi: 10.1111/j.1600-0757.2009.00302.x. [DOI] [PubMed] [Google Scholar]
- 23.Wilson M. Lethal photosensitisation of oral bacteria and its potential application in the photodynamic therapy of oral infections. Photochem Photobiol Sci. 2004;3:412–418. doi: 10.1039/b211266c. [DOI] [PubMed] [Google Scholar]
- 24.Yu KG, Li DH, Zhou CH, Diao JL. Study on the synthesis and antimicrobial activity of novel cationic porphyrins. Chin Chem Lett. 2009;20:411–414. doi: 10.1016/j.cclet.2008.11.030. [DOI] [Google Scholar]
- 25.Zimmerli W, Sendi P. Pathogenesis of implant-associated infection: the role of the host. Semin Immunopathol. 2011;33:295–306. doi: 10.1007/s00281-011-0275-7. [DOI] [PubMed] [Google Scholar]