Abstract
Background
A previous systematic review on prognostic factors for knee osteoarthritis (OA) progression showed associations for generalized OA and hyaluronic acid levels. Knee pain, radiographic severity, sex, quadriceps strength, knee injury, and regular sport activities were not associated. It has been a decade since the literature search of that review and many studies have been performed since then investigating prognostic factors for radiographic knee OA progression.
Questions/purposes
The purpose of this study is to provide an updated systematic review of available evidence regarding prognostic factors for radiographic knee OA progression.
Methods
We searched for observational studies in MEDLINE and EMBASE. Key words were: knee, osteoarthritis (or arthritis, or arthrosis, or degenerative joint disease), progression (or prognosis, or precipitate, or predictive), and case-control (or cohort, or longitudinal, or follow-up). Studies fulfilling the inclusion criteria were assessed for methodologic quality according to established criteria for reviews on prognostic factors in musculoskeletal disorders. Data were extracted and results were pooled if possible or summarized according to a best-evidence synthesis. A total of 1912 additional articles were identified; 43 met our inclusion criteria. The previous review contained 36 articles, thus providing a new total of 79 articles. Seventy-two of the included articles were scored high quality, the remaining seven were low quality.
Results
The pooled odds ratio (OR) of two determinants showed associations with knee OA progression: baseline knee pain (OR, 2.38 [95% CI, 1.74–3.27) and Heberden nodes (OR, 2.66 [95% CI, 1.46–8.84]). Our best-evidence synthesis showed strong evidence that varus alignment, serum hyaluronic acid, and tumor necrosis factor-α are associated with knee OA progression. There is strong evidence that sex, former knee injury, quadriceps strength, smoking, running, and regular performance of sports are not associated with knee OA progression. Evidence for the majority of determined associations, however, was limited, conflicting, or inconclusive.
Conclusions
Baseline knee pain, presence of Heberden nodes, varus alignment, and high levels of serum markers hyaluronic acid and tumor necrosis factor-α predict knee OA progression. Sex, knee injury, and quadriceps strength, among others, did not predict knee OA progression. Large variation remains in definitions of knee OA and knee OA progression. Clinical studies should use more consistent definitions of these factors to facilitate data pooling by future meta-analyses.
Electronic supplementary material
The online version of this article (doi:10.1007/s11999-015-4349-z) contains supplementary material, which is available to authorized users.
Introduction
The prevalence of osteoarthritis of the knee (OA) is increasing worldwide and this burden will continue to increase owing to aging of the general population [95]. Consequent to an increase in incidence is the rise in the number of patients with knee OA who are prone to further deterioration of the knee. It therefore is important to better understand, control, and attempt to prevent further progression of disease in patients with knee OA.
In 2007, Belo et al. [4] published the first systematic review on prognostic factors for progression of knee OA. They found that generalized OA and hyaluronic acid levels were associated with progression of knee OA. Knee pain, baseline radiographic severity, sex, quadriceps strength, knee injury, and regular sport activities were not associated. For the remaining factors the evidence was limited or conflicting. Their literature search had been performed up to December 2003; however, many articles studying radiographic progression of knee OA have been published in the decade since that review. Therefore, we performed an update of the systematic review of observational studies by Belo et al. [4] to determine the currently available evidence on prognostic factors for radiographic progression of knee OA.
Search Strategy and Criteria
Literature Search
In the review by Belo et al. [4], the search of the literature had been performed in MEDLINE and EMBASE for all available observational studies up to December 2003. We searched in MEDLINE and EMBASE from December 2003 up to February 2013. Key words were: knee, osteoarthritis (or arthritis, or arthrosis, or degenerative joint disease), progression (or prognosis, or precipitate, or predictive), and case-control (or cohort, or longitudinal, or follow-up). Articles were reviewed for inclusion independently by two authors (ANB and JNB or JR). The following inclusion criteria were used: 85% or more of participants in the analyses for OA progression had radiographic evidence of knee OA at baseline; the study investigated determinants associated with radiographic knee OA progression; radiographic progression was the outcome measure; the study had a case-control or cohort design with a minimal 1-year followup; full text of the article was available; the study was in English, Dutch, German, or French. Studies that observed the incidence of knee OA were excluded. A detailed description of our search strategy is available online (Appendix 1. Supplemental materials are available with the online version of CORR®). All articles were reviewed for inclusion independently by two authors (ANB and JNB or JR). Studies that used MRI features to define OA progression were excluded. However, studies determining MRI features as prognostic factors were included.
Methodologic Quality
The same methodologic quality assessment criteria as in the original review by Belo et al. [4] were used for this review (Table 1). These criteria were based on established criteria used in systematic reviews of prognostic factors for patients with musculoskeletal disorders and were described by Lievense et al. [49], Scholten-Peeters et al. [69], and Altman [1]. The criteria cover the internal validity and the informativeness of the study. All included articles were scored independently by two authors (ANB and JNB or JR). Cohen’s kappa coefficient (κ) was calculated to indicate the interrater agreement.
Table 1.
Methodologic quality assessment criteria
| Study population |
| Description of source population |
| Valid inclusion criteria |
| Sufficient description of inclusion criteria |
| Followup |
| Followup at least 1 year |
| Prospective or retrospective data collection |
| Loss to followup ≤ 20% |
| Information about loss to followup (selective for age, sex, or severity) |
| Exposure |
| Exposure assessment blinded for the outcome |
| Exposure measured identically in the studied population at baseline and followup |
| Outcome |
| Outcome assessment blinded for exposure |
| Outcome measured identically in the studied population at baseline and followup |
| Analysis |
| Measure of association or measures of variance given |
| Adjusted for age, sex, and severity |
Reprinted with permission of John Wiley and Sons from Belo JN, Berger MY, Reijman M, Koes BW, Bierma-Zeinstra SM. Prognostic factors of progression of osteoarthritis of the knee: a systematic review of observational studies. Arthritis Rheum. 2007;57:13–26.
Data Extraction
Study population characteristics, observed risk factors, definitions of knee OA progression, and measures of association were extracted.
Evidence Synthesis
Odds ratios (ORs), relative risks (RRs), or hazard ratios (HRs) were pooled when there was consistency in definition of study population, measured determinants, and assessed outcome (using Review Manager [RevMan], Version 5.3; Copenhagen, Denmark: The Nordic Cochrane Centre, The Cochrane Collaboration, 2014). We tested for heterogeneity with the chi-square and I-square tests. If heterogeneity was absent, a fixed effects model was applied to calculate pooled OR through the Mantel Haenszel test. In the absence of consistency among definitions for OA, a best-evidence synthesis was used to summarize the data. The level of evidence was based on the updated guidelines by Furlan et al. [34] and was divided into the following levels: (A) strong, ie, consistent (> 75%) findings among two or more high-quality studies; (B) moderate, ie, findings in one high-quality study and consistent findings in two or more low-quality studies; (C) limited, ie, findings in one high-quality study or consistent findings in three or more low-quality studies; and (D) conflicting or inconclusive evidence, ie, less than 75% of the studies reported consistent findings, or the results were based on only one study. High quality was defined as a quality score of 9 or greater (> 65% of the maximal attainable score). When performing the best-evidence synthesis, we only differentiated between high- and low-quality studies.
Studies Included
Of the 1912 articles identified using our search strategy, 43 met the inclusion criteria [2, 5, 7, 11, 13, 19, 20, 25–28, 30, 35, 38–44, 46, 48, 50–52, 55, 57–62, 64–66, 73, 74, 78, 85, 88, 91–93]. Belo et al. reviewed 36 articles [3, 8, 12, 14–16, 18, 21–24, 29, 31, 32, 37, 45, 47, 53, 54, 56, 63, 70–72, 75–77, 79–83, 87, 89, 94, 96]; therefore the total number of included studies was 79, studying 59 different determinants for the progression of knee OA (Table 2). Three reviewers scored 559 items for the methodologic quality assessment of the 43 newly included articles and agreed on 519 items (93%; κ = 0.79). The 53 disagreements were resolved in a single consensus meeting. Seventy-two of the 79 included articles were scored as high quality (score, 9–13), and only one article had the maximum attainable score. The remaining seven were scored as low quality, however no article was scored less than 6. Six different criteria were used for the inclusion of participants with OA and 13 definitions were applied to define radiographic OA progression. Furthermore, there were differences in how the determinants under study were measured, ie, continuous, dichotomous, or categorical with varying cut-off points.
Table 2.
Study characteristics of the reviewed manuscripts (n = 79)
| Study | Number of participants | Followup (months) | Definition of OA for inclusion | Mean age in years ± SD | Women (%) | Quality score |
|---|---|---|---|---|---|---|
| Sharma et al. [78], 2010 | 950 | 30 | K/L | 63.6 ± 7.8 | 62 | 13 |
| Brouwer et al. [13], 2007 | 169 | 72 | K/L | 66.4 ± 6.7 | 59 | 12 |
| Cerejo et al. [16], 2002 | 230 | 18 | K/L | 64 ± 10.8 | 73 | 12 |
| Dieppe et al. [21], 1997 | 415 | 37.6* | K/L | 65.3 | 68 | 12 |
| Felson et al. [29], 2003 | 223 | 15 and 30 | OARSI | 66.2 ± 9.4 | 42 | 12 |
| Madan-Sharma et al. [50], 2008 | 186 | 24 | ACR criteria | 60.2 | 81 | 12 |
| McAlindon et al. [53], 1996 | 556 | 120 | K/L | 70.3 | 63 | 12 |
| Sharma et al. [79], 2001 | 230 | 18 | K/L, JSW | 64.0 ± 11.1 | 75 | 12 |
| Spector et al. [81], 1994 | 58 | 24 | K/L | 56.8 ± 5.9 | 100 | 12 |
| Vilim et al. [87], 2002 | 48 | 36 | K/L, JSW | 62.8 (48–74) | 71 | 12 |
| Bagge et al. [3], 1992 | 74 | 48 | K/L | NR | 57 | 11 |
| Benichou et al. [5], 2010 | 67 | 12 | OARSI | 60 ± 9 | 64 | 11 |
| Botha-Scheepers et al. [11], 2008 | 86 | 24 | ACR criteria | 61 | 80 | 11 |
| Brandt et al. [12], 1999 | 82 | 31.5* | K/L | 70.1 | 70 | 11 |
| Denoble et al. [20], 2011 | 69 | 36 | K/L | 64.5 ± 10.1 | 71 | 11 |
| Dieppe et al. [23], 1993 | 60 | 60 | cOA and rOA | 62.2 ± 1.5 | 65 | 11 |
| Dieppe et al. [22], 2000 | 349 | 96 | K/L | 65.3 | 68 | 11 |
| Ledingham et al. [47], 1995 | 188 | 24 | K/L | 71 (34–91) | 63 | 11 |
| Miyazaki et al. [56], 2002 | 74 | 72 | K/L, JSW | 69.9 ± 7.8 | 81 | 11 |
| Nevitt et al. [59], 2010 | 1754 | 30 | K/L | 63 ± 8 | 63 | 11 |
| Niu et al. [61], 2009 | 2623 | 30 | K/L | 62.4 ± 8.0 | 59 | 11 |
| Sharif et al. [72], 1995 | 75 | 60 | K/L | 64.2 ± 11.6 | 69 | 11 |
| Sharif et al. [75], 1995 | 57 | 60 | JSW | NR | NR | 11 |
| Sharif et al. [76], 2000 | 40 | 60 | K/L | 65.2 ± 9.9 | 61 | 11 |
| Sharif et al. [74], 2004 | 115 | 60 | K/L | 63.6 ± 9.7 | 55 | 11 |
| Sharif et al. [73], 2007 | 115 | 60 | K/L | 63.6 ± 9.7 | 55 | 11 |
| Zhang et al. [96], 1998 | 551 | 96 | K/L | 71 (63–91) | 100 | 11 |
| Zhang et al. [94], 2000 | 473 | 96 | K/L | 71 (63–91) | 100 | 11 |
| Bettica et al. [8], 2002 | 216 | 48 | Osteophytes, JSW | NR | 100 | 10 |
| Cooper et al. [18], 2000 | 354 | 61.2* | K/L | 71.3 | 72 | 10 |
| Dam et al. [19], 2009 | 138 | 21 | ACR criteria | 60 | 48 | 10 |
| Doherty et al. [24], 1996 | 134 | 30 | K/L | 71 (41–88) | 56 | 10 |
| Duncan et al. [25], 2011 | 414 | 36 | K/L | 64.8 ± 8.1 | 51 | 10 |
| Felson et al. [31], 1995 | 869 | 97.2* | K/L | 70.8 ± 5.0 | 64 | 10 |
| Felson et al. [30], 2007 | 715 + 488 | 30 + 120 | NR§, ACR criteria | 53 + 66 | 53 + 40 | 10 |
| Fraenkel et al. [32], 1998 | 423 | 48 | K/L | NR | 67 | 10 |
| Hart et al. [37], 2002 | 830 | 48 | Osteophytes, JSW | 54.1 ± 5.9 | 100 | 10 |
| Kopec et al. [43], 2012 | 259 | 72 | K/L | NR | 65 | 10 |
| Lane et al. [45], 1998 | 55 | 108 | Osteophytes, JSW | 66 | 33 | 10 |
| Larsson et al. [46], 2012 | 74 | 90 | OARSI | 50 (32–73) | 18 | 10 |
| Mazzuca et al. [51], 2006 | 319 | 30 | K/L | 60.0 ± 9.6 | 84 | 10 |
| McAlindon et al. [54], 1996 | 640 | 120 | K/L | 70.3 | 64 | 10 |
| Miyazaki et al. [55], 2012 | 84 | 96 | K/L | 72.3 ± 3.1 | 93 | 10 |
| Muraki et al. [57], 2012 | 1313 | 40 | K/L | 68.7 ± 11.3 | 75 | 10 |
| Nelsonet al. [58], 2010 | 329 | 60 | K/L | 61.9 ± 9.7 | 61 | 10 |
| Pavelka et al. [63], 2000 | 139 | 60 | K/L | 59.1 ± 8.0 | 76 | 10 |
| Reijman et al. [66], 2007 | 532 | 72 | K/L | 68.6 ± 7.0 | 68 | 10 |
| Schouten et al. [70], 1992 | 239 | 146.4* | K/L | 57.2 ± 6.1 | 59 | 10 |
| Sharma et al. [77], 2003 | 171 | 18 | K/L | 64.0 ± 11.1 | 74 | 10 |
| Spector et al. [80], 1992 | 63 | 132 | K/L | 60 and 61 | 72 | 10 |
| Spector et al. [82], 1997 | 845 | 48 | K/L | NR | 100 | 10 |
| Sugiyama et al. [83], 2003 | 110 | 48 | JSW | 50.2 ± 6.0 | 100 | 10 |
| Wilder et al. [88], 2009 | 217 | 67.2* | K/L | 65.9 ± 9.6 | 61 | 10 |
| Yoshimura et al. [91], 2012 | 1296 | 36 | K/L | 63 | 66 | 10 |
| Zhai et al. [93], 2007 | 618 | 84 | NR | 56 | -NR | 10 |
| Attur et al. [2], 2011 | 98 | 24 | K/L | 60.7 | 56 | 9 |
| Bergink et al. [7], 2009 | 1248 | 72 | K/L | 66.2 ± 6.7 | 58 | 9 |
| Bruyere et al. [14], 2003 | 157 | 36 | ACR criteria | 66.0 ± 7.3 | 76 | 9 |
| Bruyere et al. [15], 2003 | 157 | 36 | ACR criteria | 66.0 ± 7.3 | 76 | 9 |
| Felson et al. [27], 2005 | 270 | 30 | K/L | 66.6 ± 9.2 | 40 | 9 |
| Golightly et al. [35], 2010 | 1583 | 72 | K/L | 60.9 ± 10.0 | 64 | 9 |
| Harvey et al. [38], 2010 | 2964 | 30 | K/L | 62 ± 8 | 58 | 9 |
| Haugen et al. [39], 2012 | 267 | 12 | OARSI | 61.0 ± 9.5 | 55 | 9 |
| Kraus et al. [44], 2009 | 138 | 36 | K/L | NR | 74 | 9 |
| Le Graverand et al. [48], 2009 | 141 | 24 | K/L | 56 | 100 | 9 |
| Mazzuca et al. [52], 2004 | 73 | 30 | K/L | 55.2 ± 5.8 | 100 | 9 |
| Nishimura et al. [60], 2010 | 92 | 48 | K/L | 71 ± 4.7 | 61 | 9 |
| Peregoy and Wilder [64], 2011 | 157 | 72 | K/L | 66.5 ± 8.7 | 56 | 9 |
| Reijman et al. [65], 2004 | 237 | 72 | K/L | 69.1 ± 6.9 | 71 | 9 |
| Schouten et al. [71], 1993 | 239 | 146 | K/L | 57.4 ± 6.3 | 59 | 9 |
| Wolfe and Lane [89], 2002 | 583 | 31 + 102 | ACR criteria | 63.4 ± 11.8 | 77 | 9 |
| Yusuf et al. [92], 2011 | 155 | 72 | K/L | 59.6 ± 7.5 | 85 | 9 |
| Fayfman et al. [26], 2009 | 490 | 120 | K/L | 60.5 | 62 | 8 |
| Felson et al. [28], 2004 | 227 | 30 | K/L | 66.4 ± 9.4 | 41 | 8 |
| Hunter et al. [40], 2007 | 595 | 36 | Clinical symptoms | 73.6 ± 2.9 | 60 | 8 |
| Valdes et al. [85], 2004 | 280 | 120 | K/L | 56.9 | 100 | 8 |
| Kerkhof et al. [41], 2010 | 835 | 72 | K/L | 67 | 64 | 6 |
| Kerna et al. [42], 2009 | 141 | 36 | K/L | NR | 70 | 6 |
| Pavelka et al. [62], 2004 | 89 | 24 | ACR criteria | 56.7 ± 7.2 | 66 | 6 |
OA = osteoarthritis; K/L = Kellgren-Lawrence score; OARSI = Osteoarthritis Research Society International atlas; ACR = American College of Rheumatology; JSW = joint space width, cOA = clinical OA; rOA = radiographic OA; NR = not reported; *mean followup in months; §criteria not reported for one of the cohorts.
Study Results
Because of the large number of studied determinants (n = 59), we pragmatically grouped our findings into five different categories: systemic factors (Table 3); disease characteristics (Table 4); intrinsic factors (Table 5); extrinsic factors (Table 6); and markers (Table 7). Some authors presented statistically significant associations to OA progression, but used p values or regression coefficients as measures of association [3, 5, 12, 14, 20, 21, 23, 31, 37, 41, 42, 44, 45, 47, 48, 52, 62, 63, 72, 74, 77, 80, 82, 85, 87, 93]. We chose to present only OR, RR, or HR as measures of associations; however, we have tabulated whether there was a significant association with OA progression in an article.
Table 3.
Systemic factors discussed in the reviewed studies
| Determinant | Study | Instrument of measurement | Definition of knee OA progression | OR/RR/HR (95% CI) | Association with OA progression* |
|---|---|---|---|---|---|
| Age (n = 3690) |
Bagge et al. [3], 1992 | Dichotomous | Increase K/L ≥ 1 (baseline K/L not provided) | Not provided | o |
| Benichou et al. [5], 2010 | < 60 versus ≥ 60 years | Change in JSW (mean difference) | Not provided | o | |
| Dieppe et al. [23], 1993 | JSN ≥ 2 mm | Not provided | o | ||
| Felson et al. [31], 1995 | Increase K/L ≥ 1 (baseline K/L ≥ 2) | Not provided | o | ||
| Mazzuca et al. [51], 2006 | Continuous (years) | Change in JSW (mean difference) | OR 1.13 (0.87–1.48) | o | |
| Miyazaki et al. [56], 2002 | Continuous (years) | JSN > 1 grade on a 4-grade scale | OR 1.22 (1.05–1.41) | + | |
| Muraki et al. [57], 2012 | Per 5-year increase | Increase K/L ≥ 1 (baseline K/L ≥ 2) | OR 1.17 (1.05–1.30) | + | |
| Nishimura et al. [60], 2010 | Continuous (years) | Increase K/L ≥ 1 (baseline K/L ≥ 2) | OR 0.93 (0.83–1.06) | o | |
| Schouten et al. [70], 1992 | Fourth quartile versus first | Change in JSW ≥ 1 on a 9-point scale | OR 3.84 (1.10–13.4) | + | |
| Wolfe and Lane [89], 2002 | Continuous (years) | JSN score = 3 on a 4-point scale | HR 1.00 (0.98–1.02) | o | |
| Female sex (n = 2235) |
Benichou et al. [5], 2010 | Change in JSW (mean difference) | Not provided | o | |
| Dieppe et al. [23], 1993 | JSN ≥ 2 mm | Not provided | o | ||
| Felson et al. [31], 1995 | Increase K/L ≥ 1 (baseline K/L ≥ 2) | RR 1.43 (0.80–2.58) | o | ||
| Ledingham et al. [47], 1995 | Increase K/L or JSW (cutoff not provided) Change in cyst size/number | Not provided OR 2.17 (1.13–4.15) |
o + |
||
| Miyazaki et al. [56], 2002 | JSN > 1 grade on a 4-grade scale | OR 2.14 (0.34–13.5) | o | ||
| Nishimura et al. [60], 2010 | Increase K/L ≥ 1 (baseline K/L ≥ 2) | OR 1.32 (0.22–7.75) | o | ||
| Schouten et al. [70], 1992 | Change in JSW ≥ 1 on a 9-point scale | OR 0.50 (0.22–1.11) | o | ||
| Spector et al. [80], 1992 | Change JSN ≥ 1 (4-grade scale), or ≥ 10% JSW reduction | Not provided | o | ||
| Wolfe and Lane [89], 2002 | JSN score = 3 on a 4-point scale | HR 0.73 (0.44–1.19) | o | ||
| Ethnicity (n = 1091) |
Kopec et al. [43], 2012 | Black versus white | Increase K/L ≥ 1 (baseline K/L ≥ 2) | HR 1.67 (1.05–2.67) | + |
| Low bone density (n = 3057) |
Hart et al. [37], 2002 | Low versus high | Change JSN ≥ 1 grade on a 4-grade scale | Not provided | o |
| Nevitt et al. [59], 2010 | High versus low | Change JSN ≥ 0.5 grade or osteophytes ≥ 1 | OR 1.3 (0.7–2.0) | o | |
| Zhang et al. [94], 2000 | Fourth quartile (high) versus first | Increase K/L ≥ 1 (baseline K/L ≥ 2) | OR 0.1 (0.03–0.3) | − | |
| Osteoporosis (n = 92) |
Nishimura et al. [60], 2010 | Present versus absent | Increase K/L ≥ 1 (baseline K/L ≥ 2) | OR 1.67 (0.44–6.28) | o |
| IGF-1 (n = 662) |
Fraenkel et al. [32], 1998 | Third tertile versus first in women | Increase K/L ≥ 1 (baseline K/L ≥ 2) | OR 0.9 (0.5–1.6) | o |
| Third tertile versus first in men | Increase K/L ≥ 1 (baseline K/L ≥ 2) | OR 0.9 (0.3–3.0) | o | ||
| Schouten et al. [71], 1993 | Third tertile versus first | Change ≥ 2 on a 5-point scale for radiographic OA | OR 2.58 (1.01–6.60) | + | |
| Metabolic syndrome (OW, HT, DL, IGT) (n = 1296) |
Yoshimura et al. [91], 2012 | ≥ 3 components versus none | Increase K/L ≥ 1 (baseline K/L ≥ 2) | OR 2.80 (1.68–4.68) | + |
| Two components versus none | OR 2.29 (1.49–3.54) | + | |||
| One component versus none | OR 1.38 (0.91–2.08) | o | |||
| Estrogen use (n = 551) |
Zhang et al. [96], 1998 | Past use versus never used | Increase K/L ≥ 1 (baseline K/L ≥ 2) | OR 0.9 (0.6–1.4) | o |
| Current use versus never used | Increase K/L ≥ 1 (baseline K/L ≥ 2) | OR 0.4 (0.1–1.5) | o | ||
| Uric acid concentration (n = 239) |
Schouten et al. [70], 1992 | High tertile versus low | Change in JSW ≥ 1 on a 9-point scale | OR 1.36 (0.46–4.02) | o |
| Middle versus low | Change in JSW ≥ 1 on a 9-point scale | OR 1.05 (0.36–3.00) | o | ||
| Plasma homocysteine (n = 490) |
Fayfman et al. [26], 2009 | Third tertile versus first in men Third tertile versus first in women |
Increase K/L ≥ 1 (baseline K/L ≥ 2) | OR 0.6 (0.1–1.1) OR 1.7 (0.8–3.8) |
o o |
| Genetic components (n = 618) |
Zhai et al. [93], 2007 | Hereditability in MZ | Change ≥ 1 in JSN or osteophyte score | Not provided | o |
| Hereditability in DZ | Not provided | + | |||
| SNP (n = 421) |
Kerna et al. [42], 2009 | rs3740199 in women | Increase JSN ≥ 1 or osteophyte grade | OR 2.66 (1.19–5.98) | + |
| rs1871054 | Increase JSN ≥ 1 or osteophyte grade | Not provided | o | ||
| Valdes et al. [85], 2004 | ADAM12_48 | Increase K/L ≥ 1 (baseline K/L not provided) | Not provided | o | |
| CILP_395 | Not provided | + | |||
| TNA_106 | Not provided | o | |||
| Depression/anxiety (n = 583) |
Wolfe and Lane [89], 2002 | Depression, yes versus no | JSN score = 3 | HR 1.09 (0.93–1.28) | o |
| Anxiety, yes versus no | HR 0.95 (0.84–1.08) | o |
* Statistically significant association of the determinant with OA progression: + = positive association, − = negative association, o = no association (adjusted for age and sex if applicable); OA = osteoarthritis; K/L = Kellgren-Lawrence score; JSW = joint space width; JSN = joint space narrowing; IGF-1 = insulin-like growth factor 1; OW = overweight; HT = hypertension; DL = dyslipidemia; IGT = impaired glucose tolerance; MZ = monozygotic; DZ = dizygotic; SNP = single nucleotide polymorphisms; ADAM = A disintegrin and matrix metalloproteinase domain 12; CILP = cartilage intermediate-layer protein, nucleotide pyrophosphohydrolase; TNA = tetranectin (plasminogen-binding protein); OR = odds ratio; RR = relative risk; HR = hazard ratio; n = combined sample size.
Table 4.
Disease characteristics discussed in the reviewed studies
| Determinant | Study | Instrument of measurement | Definition of knee OA progression | OR/RR/HR (95% CI) | Association with OA progression* |
|---|---|---|---|---|---|
| Knee pain (n = 2444) |
Cooper et al. [18], 2000 | Present versus absent | Increase K/L ≥ 1 (baseline K/L ≥ 1) | OR 0.8 (0.4–1.7) | o |
| Increase K/L ≥ 1 (baseline K/L ≥ 2) | OR 2.4 (0.7–8.0) | o | |||
| Dieppe et al. [23], 1993 | Present versus absent | JSN ≥ 2 mm | Not provided | o | |
| Miyazaki et al. [56], 2002 | Present versus absent | Change JSN ≥ 1 grade on a 4-grade scale | OR 0.93 (0.78–1.11) | o | |
| Muraki et al. [57], 2012 | Present versus absent | Increase K/L ≥ 1 (baseline K/L ≥ 2) | OR 2.63 (1.81–3.81 | + | |
| Spector et al. [80], 1992 | Present versus absent | Change JSN ≥ 1 grade on a 4-grade scale, or ≥ 10% JSN | Not provided | o | |
| Wolfe and Lane [89], 2002 | Present versus absent | JSN score = 3 on a 4-point scale | HR 1.55 (1.07–2.24) | + | |
| Severity Radiographic (n = 1874) |
Bruyere et al. [15], 2003 | Severity high versus low | JSN ≥ 0.5 mm | RR 2.39 (0.99–5.79) | o |
| Duncan et al. [25], 2011 | Mild PFJOA versus none† | Increase K/L ≥ 1 (baseline K/L ≥ 2) for TFJOA | OR 4.5 (1.8–11.2) | + | |
| Mild TFJOA versus none† | Increase K/L ≥ 1 (baseline K/L ≥ 2) for PFJOA | OR 1.7 (0.3–9.0) | o | ||
| Ledingham et al. [47], 1995 | Change ≥ 1 rOA feature versus no change | Change in attrition (cutoff not provided) Increase K/L or JSW (cutoff not provided) |
OR 1.72 (1.36–2.19) Not provided |
+ o |
|
| Mazzuca et al. [51], 2006 | JSW high versus low† | Change in JSW (mean difference) | OR 0.67 (0.49–0.91) | + | |
| Patellofemoral OA | Change in JSW (mean difference) | OR 3.01 (1.63–5.57) | + | ||
| Miyazaki et al. [56], 2002 | JSW, > 3 versus < 3 mm | Change JSN ≥ 1 grade on a 4-grade scale | OR 0.74 (0.25–2.19) | o | |
| Pavelka et al. [63], 2000 | JSW (continuous) | Increase K/L ≥ 1 (baseline K/L not provided) | Not provided | o | |
| Wolfe and Lane [89], 2002 | Initial JSN, high versus low | JSN score = 3 on a 4-point scale | HR 2.62 (2.03–3.40) | + | |
| Clinical (n = 1317) |
Dieppe et al. [21], 1997 | Steinbrocker grade | JSN ≥ 2 mm, sclerosis, osteophytes | Not provided | o |
| Mazzuca et al. [51], 2006 | WOMAC-PF† | Change in JSW (mean difference) | OR 1.16 (0.92–1.47) | o | |
| Wolfe and Lane [89], 2002 | Global severity (continuous) | JSN score = 3 on a 4-point scale | HR 1.02 (1.01–1.03) | + | |
| HAQ, high versus low | JSN score = 3 on a 4-point scale | HR 1.34 (0.93–1.93) | o | ||
| Heberden nodes (n = 685) |
Cooper et al. [18], 2000 | Increase K/L ≥ 1 (baseline K/L ≥ 1) | OR 0.7 (0.4–1.6) | o | |
| Increase K/L ≥ 1 (baseline K/L ≥ 2) | OR 2.0 (0.7–5.7) | o | |||
| Nishimura et al. [60], 2010 | Increase K/L ≥ 1 (baseline K/L ≥ 2) | OR 2.01 (0.60–6.76) | o | ||
| Schouten et al. [70], 1992 | Change in JSW ≥ 1 on a 9-point scale | OR 5.97 (1.54–23.1) | + | ||
| Osteoarthritis (n = 694) |
Haugen et al. [39], 2012 | Score hand JSN | Increase K/L ≥ 1 (baseline K/L ≥ 2) | OR 1.00 (0.93–1.08) | o |
| Score hand osteophytes | OR 0.96 (0.87–1.06) | o | |||
| Ledingham et al. [47], 1995 | Multiple joints versus local joint OA | Increase K/L (cutoff not provided) | OR 2.39 (1.16–4.93) | + | |
| Change in attrition | OR 2.42 (1.02–5.77) | + | |||
| Change in JSW or rOA (cutoff not provided) | Not provided | o | |||
| Schouten et al. [70], 1992 | Generalized OA | Change in JSW ≥ 1 on a 9-point scale | OR 3.28 (1.30–8.27) | + | |
| Localized OA | Change in JSW ≥ 1 on a 9-point scale | OR 1.17 (0.51–2.72) | o | ||
| Hand grip strength (muscle strength) (n = 1313) |
Muraki et al. [57], 2012 | Per 1-kg strength increase | Increase K/L ≥ 1 (baseline K/L ≥ 2) | OR 0.99 (0.96–1.01) | o |
| Duration of symptoms (n = 643) |
Dieppe et al. [23], 1993 | Continuous (years) | JSN ≥ 2 mm | Not provided | o |
| Wolfe and Lane [89], 2002 | Continuous (years) | JSN score = 3 on a 4-point scale | HR 1.03 (1.00–1.05) | + |
* Statistically significant association of the determinant with OA progression: + = positive association, − = negative association, o = no association (adjusted for age and sex if applicable); †at baseline; OA = osteoarthritis; K/L = Kellgren-Lawrence score; JSN = joint space narrowing; TFJOA = tibiofemoral joint OA; PFJOA = patellofemoral joint OA; JSW = joint space width; WOMAC-PF = physical function scale of the WOMAC; HAQ = Health Assessment Questionnaire; OR = odds ratio; RR = relative risk; HR = hazard ratio; n = combined sample size; rOA = radiographic OA.
Table 5.
Intrinsic factors discussed in the reviewed studies
| Determinant | Study | Analysis of determinant | Definition of knee OA progression | OR/RR/HR (95% CI) | Association with OA progression* |
|---|---|---|---|---|---|
| Alignment (n = 2642) |
Brouwer et al. [13], 2007 | Varus versus neutral | Increase K/L ≥ 1 (baseline K/L ≥ 2) | OR 2.90 (1.07–7.88) | + |
| Valgus versus neutral | Increase K/L ≥ 1 (baseline K/L ≥ 2) | OR 1.39 (0.48–4.05) | o | ||
| Cerejo et al. [16], 2002 | Varus versus nonvarus (K/L 0–1) | Change JSN > 1 grade on a 4-grade scale | OR 2.50 (0.67–9.39) | + | |
| Varus versus nonvarus (K/L 2) | OR 4.12 (1.92–8.82) | + | |||
| Varus versus nonvarus (K/L 3) | OR 11.0 (3.10–37.8) | + | |||
| Valgus versus nonvalgus (K/L 2) | OR 2.46 (0.95–6.34) | o | |||
| Valgus versus nonvalgus (K/L 3) | OR 10.4 (2.76–39.5) | + | |||
| Hunter et al. [40], 2007 | Patellar tilt, fourth versus first quartile | Medial patellofemoral change JSN ≥ 1 grade on a 4-grade scale | OR 0.19 (0.09–0.43) | − | |
| Sulcus angle, fourth versus first quart | OR 1.49 (0.60–3.73) | o | |||
| Bisect offset, fourth versus first quart | OR 2.23 (1.10–4.50) | + | |||
| Patellar tilt, fourth versus first quartile | Lateral patellofemoral change JSN ≥ 1 grade on a 4-grade scale | OR 1.13 (0.57–2.24) | o | ||
| Sulcus angle, fourth versus first quart | OR 2.09 (0.99–4.41) | o | |||
| Bisect offset, fourth versus first quartile | OR 0.35 (0.15–0.83) | − | |||
| Miyazaki et al. [56], 2002 | Varus versus nonvarus | Change JSN ≥ 1 grade on a 4-grade scale | OR 0.90 (0.66–1.23) | o | |
| Schouten et al. [70], 1992 | Malaligned, present versus absent | Change JSN ≥ 1 grade on a 4-grade scale | OR 5.13 (1.14–23.1) | + | |
| Sharma et al. [79], 2001 | Varus versus nonvarus | Change JSN ≥ 1 grade on a 4-grade scale | OR 4.09 (2.20–7.62) | + | |
| Varus versus mild valgus | OR 2.98 (1.51–5.89) | + | |||
| Valgus versus nonvalgus | OR 4.89 (2.13–11.2) | + | |||
| Valgus versus mild varus | OR 3.42 (1.31–8.96) | + | |||
| Sharma et al. [78], 2010 | Valgus versus neutral | Change medial JSN ≥ 1 grade on a 4-grade scale | OR 0.34 (0.21–0.55) | − | |
| Varus versus neutral | OR 3.59 (2.62–4.92) | + | |||
| Valgus versus neutral | Change lateral JSN ≥ 1 grade on a 4-grade scale | OR 4.85 (3.17–7.42) | + | ||
| Varus versus neutral | OR 0.12 (0.07–0.21) | − | |||
| Yusuf et al. [92], 2011 | Varus (< 182°) versus nonvarus | Change JSN ≥ 1 grade on a 6-grade scale | RR 2.3 (1.4–3.1) | + | |
| Valgus (> 184°) versus nonvalgus | RR 1.7 (0.97–2.6) | o | |||
| Malaligned, BMI > 25 kg/m2 | RR 4.1 (1.8–6.1) | + | |||
| Adduction moment (n = 74) |
Miyazaki et al. [56], 2002 | ≥ 5 versus < 5 (% weight x height) | Change JSN ≥ 1 grade on a 4-grade scale | OR 6.46 (2.40–17.5) | + |
| Knee injury (n = 207) |
Cooper et al. [18], 2000 | Yes versus no | Increase K/L ≥ 1 (baseline K/L ≥ 1) | OR 1.2 (0.5–3.0) | o |
| Increase K/L ≥ 1 (baseline K/L ≥ 2) | OR 1.1 (0.3–4.4) | o | |||
| Schouten et al. [70], 1992 | Knee injury: yes versus no | Change JSN ≥ 1 grade on a 4-grade scale | OR 2.62 (0.93–7.36) | o | |
| Sport injury: yes versus no | Change JSN ≥ 1 grade on a 4-grade scale | OR 0.62 (0.17–2.19) | o | ||
| Bone marrow lesions/edema (n = 186) |
Madan-Sharma et al. [50], 2008 | Present versus absent | JSN > 1 grade on a 4-grade scale | RR 0.9 (0.18–3.0) | o |
| Subchondral bone cysts (MRI) (n = 186) |
Madan-Sharma et al. [50], 2008 | Present versus absent | JSN > 1 grade on a 4-grade scale | RR 1.6 (0.5–4.0) | o |
| Cartilage loss (MRI) (n = 186) |
Madan-Sharma et al. [50], 2008 | Present versus absent | JSN > 1 grade on a 4-grade scale | RR 3.0 (0.5–9.6) | o |
| Joint effusion (n = 186) |
Madan-Sharma [50], 2008 | Present on MRI | JSN > 1 grade on a 4-grade scale | RR 0.6 (0.6–1.8) | o |
| Meniscal damage (n = 186) |
Madan-Sharma et al. [50], 2008 | Present versus absent on MRI | JSN > 1 grade on a 4-grade scale | RR 8.91 (1.1–22.8) | + |
| Meniscectomy (n = 239) |
Schouten et al. [70], 1992 | Yes versus no | Change JSN ≥ 1 grade on a 4-grade scale | OR 2.28 (0.57–9.03) | o |
| Chondrocalcinosis (n = 239) |
Schouten et al. [70], 1992 | Yes versus no | Change JSN ≥ 1 grade on a 4-grade scale | OR 2.01 (0.55–7.42) | o |
| Osteophytes tibiofemoral (n = 337) |
Benichou et al. [5], 2010 | Definite versus not | Change in JSW (mean difference) | Not provided | o |
| Felson et al. [27], 2005 | Ipsilateral score Contralateral score |
Change JSN ≥ 1 grade on a 4-grade scale | OR 1.9 (1.5–2.5) | + | |
| OR 0.6 (0.5–0.8) | − | ||||
| Knee ROM (n = 92) |
Nishimura et al. [60], 2010 | Mean ROM | Increase K/L ≥ 1 (baseline K/L ≥ 2) | OR 0.94 (0.89–0.99) | − |
* Statistically significant association of the determinant with OA progression: + = positive association, − = negative association, o = no association (adjusted for age and sex if applicable); OA = osteoarthritis; K/L = Kellgren-Lawrence score; JSN = joint space narrowing; JSW = joint space width; OR = odds ratio; RR = relative risk; HR = hazard ratio; n = combined sample size.
Table 6.
Extrinsic factors discussed in the reviewed studies
| Determinant | Study | Analysis of determinant | Definition of knee OA progression | OR/RR/HR (95% CI) | Association with OA progression* |
|---|---|---|---|---|---|
| BMI (n = 6791) |
Benichou et al. [5], 2010 | < 30 versus ≥ 30 kg/m2 | Change in JSW (mean difference) | Not provided | + |
| Cooper et al. [18], 2000 | Highest tertile versus lowest | Increase K/L ≥ 1 (baseline K/L ≥ 1) | OR 2.6 (1.0–6.8) | + | |
| Increase K/L ≥ 1 (baseline K/L ≥ 2) | OR 1.3 (0.3–5.0) | o | |||
| Dieppe et al. [23], 1993 | Continuous | JSN ≥ 2 mm or knee surgery | Not provided | o | |
| Felson et al. [28], 2004 | Per 2-unit increase (§) | Change JSN ≥ 1 grade on a 4-grade scale | OR 0.98 (0.8–1.4) | o | |
| As §, with 3°–6° malalignment | OR 1.23 (1.0–1.4) | + | |||
| As §, with ≥ 7° malalignment | OR 0.93 (0.7–1.2) | o | |||
| Ledingham et al. [47], 1995 | Continuous | Change in JSW (cutoff not provided) | OR 1.07(1.02–1.14) | + | |
| Change in osteophytes (cutoff not provided) | OR 1.06 (1.00–1.12) | + | |||
| Change in K/L (cutoff not provided) | Not provided | o | |||
| LeGraverand et al. [48], 2009 | < 30 versus ≥ 30 kg/m2 | Change in JSW (mean difference) | Not provided | o | |
| Miyazaki et al. [56], 2002 | Continuous | JSN ≥ 1 grade on a 4-grade scale | OR 1.21 (0.91–1.61) | o | |
| Muraki et al. [57], 2012 | Per 5-unit increase | Increase K/L ≥ 1 (baseline K/L ≥ 2) | OR 1.43 (1.16–1.77) | + | |
| Nishimura et al. [60], 2010 | Continuous | Increase K/L ≥ 1 (baseline K/L ≥ 2) | OR 0.93 (0.78–1.11) | o | |
| Niu et al. [61], 2009 | < 25 versus ≥ 30 kg/m2 | Increase JSN ≥ 0.5 grade | RR 1.1 (0.9–1.4) | o | |
| Reijman et al. [66], 2007 | ≤ 25 versus > 27.5 kg/m2 | Increase JSN ≥ 1 mm | OR 1.4 (0.8–2.6) | o | |
| Increase K/L ≥ 1 (baseline K/L ≥ 2) | OR 2.1 (1.2–3.7) | + | |||
| Schouten et al. [70], 1992 | Second quartile versus first | Change in JSW ≥ 1 on a 9-point scale | OR 1.77 (0.48–6.50) | o | |
| Third quartile versus first | OR 5.28 (1.54–18.1) | + | |||
| Fourth quartile versus first | OR 11.1 (3.28–37.3) | + | |||
| Spector et al. [81], 1994 | Third tertile versus first | Increase K/L or JSN (cutoff not provided) | RR 4.69 (0.63–34.8) | o | |
| Wolfe and Lane [89], 2002 | Continuous | JSN score = 3 | HR 1.03 (1.00–1.06) | + | |
| Yusuf et al. [92], 2011 | BMI 25–30 versus < 25 | Change JSN ≥ 1 grade on a 6-grade scale | RR 2.4 (1.3–3.6) | + | |
| BMI >30 versus < 25 | Change JSN ≥ 1 grade on a 6-grade scale | RR 2.9 (1.7–4.1) | + | ||
| Quadriceps strength (n = 253) |
Brandt et al. [12], 1999 | Progressive versus nonprogressive group† | Increase K/L ≥ 1 (baseline K/L not provided) | Not provided | o |
| Sharma et al. [77], 2003 | High versus low strength† | Increase JSN ≥ 1 | Not provided | o | |
| Leg length inequality (n = 4547) |
Golightly et al. [35], 2010 | Leg length inequality versus no inequality | Increase K/L ≥ 1 (baseline K/L ≥ 1) | HR 1.22 (0.82–1.80) | o |
| Increase K/L ≥ 1 (baseline K/L ≥ 2) | HR 1.83 (1.10–3.05) | + | |||
| Harvey et al. [38], 2010 | ≥ 1 cm versus no inequality, shorter leg | JSN ≥ 1 grade or knee surgery | OR 1.3 (1.0–1.7) | + | |
| ≥ 2 cm versus no inequality, shorter leg | OR 1.4 (0.5–3.7) | o | |||
| AP knee laxity (n = 84) |
Miyazaki et al. [55], 2012 | Before exercise | Increase K/L ≥ 1 (baseline K/L ≥ 1) or radiographic cartilage loss > 0.2 mm annually | OR 1.29 (0.54–3.08) | o |
| Enhanced laxity resulting from exercise | OR 4.15 (1.12–15.4) | + | |||
| Running (n = 294) |
Lane et al. [45], 1998 | Dichotomous‡ | Increase ≥ 1 on JSW and osteophyte score | Not provided | o |
| Schouten et al. [70], 1992 | Dichotomous† | Change in JSW ≥ 1 on a 9-point scale | OR 0.53 (0.17–1.68) | o | |
| Regular sports (n = 593) |
Cooper et al. [18], 2000 | Dichotomous† | Increase K/L ≥ 1 (baseline K/L ≥ 1) | OR 0.7 (0.4–1.6) | o |
| Increase K/L ≥ 1 (baseline K/L ≥ 2) | OR 0.9 (0.3–2.5) | o | |||
| Schouten et al. [70], 1992 | Physical activity‡ | Change in JSW ≥ 1 on a 9-point scale | OR 0.43 (0.11–1.76) | o | |
| Walking‡ | OR 1.47 (0.36–6.03) | o | |||
| Standing (medium versus low)‡ | OR 3.80 (1.03–14.0) | + | |||
| Standing (high versus low)‡ | OR 2.09 (0.43–10.3) | o | |||
| Nutritional variables (n = 3381) |
Bergink et al. [7], 2009 | Vitamin D intake (low versus high) | Increase K/L ≥ 1 (baseline K/L ≥ 2) | OR 7.7 (1.3–43.5) | − |
| Serum vitamin D (low versus high) | Increase K/L ≥ 1 (baseline K/L ≥ 2) | OR 2.1 (0.6–7.4) | o | ||
| Felson et al. [30], 2007 | Vitamin D serum levels < 20 ng/mL | Change JSN ≥ 1 grade on a 4-grade scale, Framingham | OR 0.83 (0.54–1.27) | o | |
| Vitamin D serum levels < 20 ng/mL | Change JSN ≥ 1 grade on a 4-grade scale, BOKS study | OR 0.63 (0.35–1.14) | o | ||
| McAlindon et al. [53], 1996 | Vitamin D intake (middle versus high) | Increase JSN ≥ 1 | OR 2.99 (1.06–8.49) | − | |
| Serum vitamin D (middle versus high) | Increase JSN ≥ 1 | OR 2.83 (1.02–7.85) | − | ||
| McAlindon et al. [54], 1996 | Vitamin C intake (middle versus low) | Increase K/L ≥ 1 | OR 0.32 (0.14–0.77) | − | |
| β-carotene intake (high versus low) | OR 0.42 (0.19–0.94) | − | |||
| Vitamin E (high versus low) | OR 0.68 (0.28–1.64) | o | |||
| Peregoy and Wilder [64], 2011 | Vitamin C intake | Increase K/L ≥ 1 (baseline K/L ≥ 2) | RR 0.94 (0.79–1.12) | o | |
| Wilder et al. [88], 2009 | Vitamin intake in general | Increase K/L ≥ 1 (baseline K/L ≥ 2) | RR 0.93 (0.87–0.99) | − | |
| Smoking (n = 331) |
Nishimura et al. [60], 2010 | Yes versus no | Increase K/L ≥ 1 (baseline K/L ≥ 2) | OR 0.73 (0.09–6.15) | o |
| Schouten et al. [70], 1992 | Past smoker versus never | Change in JSW ≥ 1 on a 9-point scale | OR 1.07 (0.38–3.04) | o | |
| Current smoker versus never | Change in JSW ≥ 1 on a 9-point scale | OR 0.96 (0.34–2.75) | o |
* Statistically significant association of the determinant with OA progression: + = positive association, − = negative association, o = 1o association (adjusted for age and sex if applicable); †assessed at baseline; ‡assessed at followup; OA = osteoarthritis; JSW = joint space width; K/L = Kellgren-Lawrence score; JSN = joint space narrowing; OR = odds ratio; RR = relative risk; HR = hazard ratio; n = combined sample size.
Table 7.
Markers discussed in the reviewed studies
| Marker | Study | Instrument of measurement | Definition of knee OA progression | OR/RR/HR (95% CI) | Association with OA progression* |
|---|---|---|---|---|---|
| CRP (serum) (n = 1720) |
Kerkhof et al. [41], 2010 | Continuous | Increase K/L ≥ 1 (baseline K/L ≥ 2) or surgery | Not provided | o |
| Sharif et al. [76], 2000 | Continuous | JSN ≥ 2 mm or knee surgery | OR 1.12 (0.81–1.55) | o | |
| Spector et al. [82], 1997 | Continuous | Increase K/L ≥ 1 (baseline K/L not provided) | Not provided | + | |
| IL-1β (serum) (n = 184) |
Attur et al. [2], 2011 | Increased versus normal | Increase K/L ≥ 1 or > 30% JSW reduction | OR 3.2 (1.2–8.7) | + |
| Botha-Scheepers et al. [11], 2008 | Fourth quartile versus first | Change JSN ≥ 1 grade on a 4-grade scale | RR 1.3 (0.5–2.0) | o | |
| IL-10 (serum) (n = 86) |
Botha-Scheepers et al. [11], 2008 | Fourth quartile versus first | Change JSN ≥ 1 grade on a 4-grade scale | RR 4.3 (1.7–6.2) | + |
| IL-1Ra (serum) (n = 86) |
Botha-Scheepers et al. [11], 2008 | Fourth quartile versus first | Change JSN ≥ 1 grade on a 4-grade scale | RR 2.1 (0.7–3.9) | o |
| TNFα (serum) (n = 253) |
Attur et al. [2], 2011 | Increased versus normal | Increase K/L ≥ 1 or > 30% JSW reduction | OR 8.9 (2.6–30.8) | + |
| Botha-Scheepers et al. [11], 2008 | Fourth quartile versus first | Change JSN ≥ 1 grade on a 4-grade scale | RR 6.1 (1.4–9.8) | + | |
| Denoble et al. [20], 2011 | Continuous | Change in osteophyte score | Not provided | + | |
| TGF-β1 (serum) (n = 329) |
Nelson et al. [58], 2010 | Continuous | Increase K/L ≥ 1 (baseline K/L ≥ 1) | HR 1.04 (0.41–2.65) | o |
| Increase K/L ≥ 1 (baseline K/L ≥ 2) | HR 1.10 (0.46–2.63) | o | |||
| Hyaluronic acid (serum) (n = 361) |
Bruyere et al. [14], 2003 | High level versus low | Change in mean JSW (cutoff not provided) | Not provided | + |
| Pavelka et al. [62], 2004 | High level versus low | Change in mean JSW (cutoff not provided) | Not provided | + | |
| Sharif et al. [72], 1995 | High level versus low | JSN ≥ 2 mm or knee surgery | Not provided | + | |
| Sharif et al. [76], 2000 | High level versus low | JSN ≥ 2 mm or knee surgery | OR 2.32 (1.16–4.66) | + | |
| Keratan sulfate (serum) (n = 232) |
Bruyere et al. [14], 2003 | High level versus low | Change in mean JSW (cutoff not provided) | Not provided | + |
| Sharif et al. [72], 1995 | High level versus low | JSN ≥ 2 mm or knee surgery | Not provided | o | |
| COMP (serum) (n = 466) |
Bruyere et al. [14], 2003 | High level versus low | Change in mean JSW (cutoff not provided) | Not provided | o |
| Pavelka et al. [62], 2004 | High level versus low | Change in mean JSW (cutoff not provided) | Not provided | o | |
| Sharif et al. [75], 1995 | High level versus low | JSN ≥ 2 mm or knee surgery | Not provided | + | |
| Sharif et al. [74], 2004 | OA progression versus nonprogession | JSN ≥ 2 mm or knee surgery | Not provided | + | |
| Vilim et al. [87], 2002 | High level versus low | JSN > 0.5 mm | Not provided | + | |
| Pentosidine (serum) (n = 89) |
Pavelka et al. [62], 2004 | High level versus low | Change in mean JSW (cutoff not provided) | Not provided | + |
| YKL-40 (serum) (n = 89) |
Pavelka et al. [62], 2004 | High level versus low | Change in mean JSW (cutoff not provided) | Not provided | o |
| MMP-9 (serum) (n = 89) |
Pavelka et al. [62], 2004 | High level versus low | Change in mean JSW (cutoff not provided) | Not provided | o |
| TIMP-9 (serum) (n = 89) |
Pavelka et al. [62], 2004 | High level versus low | Change in mean JSW (cutoff not provided) | Not provided | o |
| PIIANP (serum) (n = 115) |
Sharif et al. [73], 2007 | Fourth quartile versus first | JSN ≥ 2 mm or knee surgery | RR 3.2 (1.1–9.0) | + |
| CTX-II (urine) (n = 490) |
Dam et al. [19], 2009 | Third tertile versus first | Increase K/L ≥ 1 (disregarding baseline K/L) | OR 2.3 | o |
| Third tertile versus first | JSN > mean JSN of non-OA control group (K/L ≤ 1) | OR 1.8 | o | ||
| Reijman et al. [65], 2004 | Fourth quartile versus first | JSN ≥ 2 mm | OR 6.0 (1.2–30.8) | + | |
| Fourth quartile versus first | JSN ≥ 1.5 mm | OR 1.8 (0.8–4.1) | o | ||
| Fourth quartile versus first | JSN ≥ 1 mm | OR 1.1 (0.7–1.7) | o | ||
| Sharif et al. [73], 2007 | > median versus ≤ median | JSN ≥ 2 mm or knee surgery | RR 3.4 (1.2–9.4) | + | |
| ARGS (synovial) (n = 74) |
Larsson et al. [46], 2012 | Baseline level ARGS > followup level ARGS | ≥ 1-unit increase OARSI score | OR 6.77 (1.38–33.2) | + |
| IL-18 (synovial) (n = 69) |
Denoble et al. [20], 2011 | Continuous | Change in osteophyte score | Not provided | + |
| FSA (radiographic) (n = 138) |
Kraus et al. [44], 2009 | FD progression versus nonprogression | Medial JSN ≥ 1 or osteophyte formation | Not provided | + |
| Bone scintigraphy (n = 73) |
Mazzuca et al. [52], 2004 | 99mTc-MDP uptake | Change in JSW (mean difference) | Not provided | o |
* Statistically significant association of the determinant with OA progression: + = positive association, − = negative association, o = no association (adjusted for age and sex if applicable); OA = osteoarthritis; K/L = Kellgren-Lawrence score; JSN = joint space narrowing; CRP = C-reactive protein; IL = interleukin; TNF = tumor necrosis factor; YKL-40 = chitinase-3-like protein 1; JSW = joint space width; TGF = transforming growth factor; C2C = collagen type II cleavage; COMP = cartilage oligomeric matrix protein; MMP = matrix metalloproteinase; TIMP = tissue inhibitors of metalloproteinase; PIIANP = N-propeptide of type IIA collagen; CTX-II = crosslinked C-telopeptide; ARGS = aggrecan neoepitope amino acid sequence; FSA = fractal signature analysis; FD = fractal dimension (horizontal and vertical); OR = odds ratio; RR = relative risk; HR = hazard ratio; n = combined sample size.
Sensitivity Analysis
For factors in which we were forced to use a best-evidence synthesis, we conducted a sensitivity analysis to check whether differences in sample size could have altered our conclusions. Additionally we checked whether large variances in followup could have led to different conclusions.
Results
Summaries of the results for systemic factors, disease characteristics, intrinsic factors, extrinsic factors, and markers are available (Appendix 2. Supplemental material is available with the online version of CORR®.).
Pooled Results
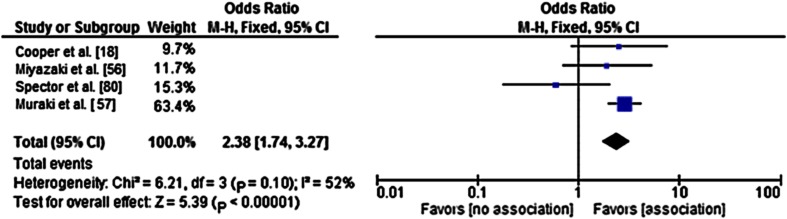
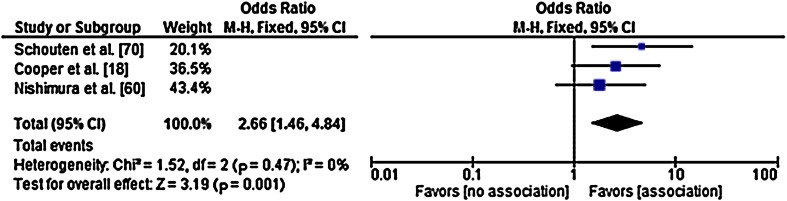
The presence of knee pain at baseline and Heberden nodes were associated with the progression of knee OA. The pooled ORs based on pools of studies with consistency among the definitions for OA inclusion, OA progression, and the determinant under study, were 2.38 for knee pain at baseline (95% CI,1.74–3.27; I2 = 52%) (Fig. 1) and 2.66 for the presence of Heberden nodes (95% CI, 1.46–8.84); I2 = 0%) (Fig. 2). Because of the large number of determinants with only a restricted number of studies per determinant and owing to lack of consistency between the reviewed studies regarding inclusion criteria, outcome measures, and measures of association, statistical pooling was not possible for the majority of the determinants.
Fig. 1.
A forest plot for the pooled odds ratio (OR) shows the association between the presence of knee pain at baseline and radiographic progression of knee osteoarthritis (OA). The OR can deviate from the OR in Table 4 because pooled ORs were obtained through crude ORs, as opposed to the adjusted OR in Table 4. The results from Dieppe and Wolfe for pooling were not available and were not included in this analysis. The results from the chi-square and I2 tests indicate homogeneity between the studies. M–H = Mantel Haenszel test; Fixed = fixed effects model; df = degrees of freedom.
Fig. 2.
A forest plot for the pooled odds ratio (OR) shows the association between the presence of Heberden nodes at baseline and radiographic progression of knee osteoarthritis (OA). The OR can deviate from that in Table 4 because pooled ORs were obtained through crude ORs, as opposed to the adjusted OR in Table 4. The results from the chi-square and I2 tests indicate homogeneity between the studies. M–H = Mantel Haenszel test; Fixed = fixed effects model; df = degrees of freedom.
Best-evidence Synthesis
For the remaining determinants, we applied a best-evidence synthesis, which showed that based on consistent findings in multiple high-quality studies, there seems to be strong evidence that varus alignment, serum TNFα level, and serum hyaluronic acid level are associated with radiographic progression of knee OA. There also is strong evidence that sex (female), former knee injury, quadriceps strength, smoking, running, and regular performance of sports are not associated with progression of knee OA.
There was moderate evidence showing that a higher dietary intake of vitamin D is inversely associated with progression of knee OA. Thus far, there is limited evidence that ethnicity, metabolic syndrome, genetic components adduction moment, meniscal damage, knee ROM, general vitamin and β-carotene intake, serum levels IL-10 and N-propeptide of type II collagen, synovial levels aggrecan neoepitope amino acid sequence and IL-18, and fractal dimension progression on radiographic fractal signature analysis are associated with progression of knee OA. There also is limited evidence that knee OA progression is not associated with osteoporosis; past or present estrogen use; uric acid concentrations; depression or anxiety; hand grip (muscle) strength; bone marrow lesions or edema; meniscectomy; chondrocalcinosis; MRI-detected subchondral bone cysts, cartilage loss, or joint effusion; AP knee laxity; vitamin E intake; serum levels IL-1Ra and transforming growth factor-β1; and 99mTc-MDP uptake on bone scintigraphy.
Conflicting evidence was found for the associations between knee OA progression and age; low bone density; serum insulin growth factor-1 level; baseline radiographic or clinical OA severity; generalized osteoarthritis; duration of symptoms; valgus alignment or malalignment in general; past knee injury; the presence of tibiofemoral osteophytes; BMI; leg length inequality; serum vitamin D level; dietary intake of vitamin C; serum C-reactive protein, IL-1β, keratan sulfate, and serum cartilage oligometric matrix protein levels, and urinary crosslinked C-telopeptide level. Inconclusive evidence was found for the determined associations between knee OA progression and the single nucleotide polymorphisms CILP_395 (cartilage intermediate-layer proteins) and rs3740199, patellofemoral alignment, and serum pentosidine levels. There also was inconclusive evidence for no associations found between knee OA progression and the single nucleotide polymorphisms rs1871054, ADAM12_48 (A disintegrin and matrix metalloproteinase domain 12), and TNA_106 (tetranectin plasminogen-binding protein), and serum levels of YKL-40 (chitinase-3-like protein 1), MMP-9 (matrix metalloproteinase-9); and TIMP-9 (tissue inhibitors of metalloproteinase).
Sensitivity Analysis
In this analysis, we tested whether conclusions from relatively small studies (less than 200) incorrectly influenced conclusions drawn from larger studies with more statistical power studying the same determinant, or that results from studies with a relatively short followup (cutoff 24 months) altered conclusions from studies with a longer followup. Our sensitivity analysis found that our conclusions did not change across the range of clinically plausible differences in followup duration or sample size regarding the strong, moderate, or conflicting evidence we found for the various presented determinants.
Discussion
We performed an updated systematic review of available evidence regarding prognostic factors for radiographic knee OA progression. We found that there is strong evidence that baseline knee pain and Heberden nodes, varus alignment, and high baseline serum levels of hyaluronic acid and TNFα are predictive for knee OA progression. There also seems to be strong evidence that sex (female), former knee injury, quadriceps strength, smoking, running, and regular performance of sports are not predictive for progression of knee OA. For all other studied factors in our review, the evidence is limited, conflicting, or inconclusive. In the best-evidence synthesis, we considered only significant associations as associated prognostic factors. However, several of the included articles had small sample sizes, which consequently can lead to lower statistical power and more often to failure to detect differences that might be present.
A possible limitation to our inclusion criteria was addressed by Zhang et al. [97]. They reported that, unlike randomized trials, observational studies of patients with preexisting disease are subject to various biases that may account for discrepancies found between risk factors for disease incidence and progression. They hypothesized that risk factors actually might exist for progressive knee OA but that flaws in study design and the measure of disease progression may prevent us from detecting risk factors [97]. Having cited their article, it seems reasonable that there is the possibility that we have not determined all risk factors for progression of knee OA, because some factors might not have achieved significance in multivariable analyses in a study and thus were not included in our evidence synthesis. Nonetheless, we believe we have summarized all presently known risk factors of which a possible association with knee OA progression has been studied.
We acknowledge that when applying a best-evidence synthesis, one might unjustly conclude that there may be conflicting or strong evidence for or against an association of the determinant under study with knee OA. We would have preferred to pool the data of all included studies. However, because of large variation in criteria used in the articles for defining disease, or disease progression, pooling of the data generally was not possible. We encountered six different criteria that were used for the inclusion of OA (Table 2). Another approximately 13 different definitions were applied for OA progression (Tables 3–7). Furthermore, there were differences in how the determinants under study were measured, (continuous, dichotomous, or categorical), and varying cutoff points were used. As previously described, we pooled the results for “knee pain” and “Heberden nodes” for which both results showed associations with the progression of knee OA. This is different from the conclusions we would have drawn from a best-evidence synthesis, which would show conflicting evidence for both determinants. In our opinion, it is likely that more of the conflicting associations we presented are attributable to the differences in definitions of knee OA or knee OA progression. For example, the conflicting evidence for BMI probably would be altered if statistical pooling was feasible; given that all 11 significant risk estimates (OR/RR/HR) regarding BMI were positive associations and that six of the 12 nonsignificant associations also were positive associations, it seems likely that if pooled, the combined overall association between BMI and knee OA would be a positive, significant one. In addition, the conflicting evidence for age, seven of the 10 presented analyses (70%) showed no significant association, falling just short for the criteria for ascertaining strong evidence (> 75%) for no association between age and OA progression.
In the original review by Belo et al. [4] and in a review by van Dijk et al. [86], the evidence for association between varus alignment and OA progression was limited. However, a couple studies have been performed since these reviews were published that have determined significant associations with varus alignment, which enabled us to conclude that there is strong evidence for this finding. The latter is in accordance with results published in later systematic reviews by Tanamas et al. [84] and Chapple et al. [17]. Except for the original review by Belo et al., there are to our knowledge no other reviews available that have determined the predictive value of serum hyaluronic acid levels and OA progression [9]. In addition, to our knowledge, no reviews have been published assessing the predictive value of serum level TNFα for knee OA progression.
We found strong evidence that sex was not associated with knee OA progression, as did Belo et al. [4]. This is in contrast to the earlier reviews published by van Dijk et al. [86] and Chapple et al. [17]. van Dijk et al. found limited evidence for the absence of an association with sex, but they included articles that used physical functioning as an outcome measure. Chapple et al. found conflicting evidence; however, their evidence was based on four analyses of three studies, which also are included in our review [21, 47, 70]. Three of the four analyses were consistent (no association); one was conflicting (significant association) [47]. Our evidence synthesis was based on 10 analyses, of which nine analyses were consistent (no association), consequently outweighing the one conflicting finding. van Dijk et al. and Chapple et al. reported limited evidence for the absence of an association between quadriceps strength and knee OA progression. This is consistent with our finding; however, our conclusion is based on more evidence. Consistent results also were found for regular performance of sports, in which van Dijk et al. reported limited and Chapple et al. reported strong evidence for absence of an association. However, in articles by Fransen and McConnell [33] and Bennell and Hinman [6] reviewing the effect of exercise therapy in patients with knee OA, the authors reported that exercise has a short-term benefit in patients with knee OA, although the magnitude of the reported benefit is small. This highlights the importance of the need to understand the working mechanism of exercise therapy.
A topic of considerable interest is the potential association between BMI and knee OA progression. Previous reviewers have established a positive association between BMI and incident knee OA [10, 95]. However, the evidence for an association between BMI and progression of knee OA remains conflicting in our review, which is consistent with the findings by Belo et al. [4] and Chapple et al. [17].
Noteworthy is the lack of overlap in evidence for prognostic factors for hip and knee OA progression. In two large reviews studying prognostic factors for hip OA, Lievense et al. [49] provided strong evidence for an association between hip OA progression with type of hip migration and with atrophic bone response. They also presented strong evidence for the absence of an association with BMI. Wright et al. [90] reported strong evidence for association of hip OA progression with age, joint space width at entry, femoral head migration, femoral osteophytes, bony sclerosis, baseline hip pain, and certain hip OA severity indexes. They also provided strong evidence for the absence of an association with acetabular osteophytes. The discrepancy between the findings for hip and knee OA is unclear but could be attributable to the difference in the number of studies available determining risk factors for progression of hip or knee OA [9].
Future research on the true relationship between prognostic factors for radiographic progression of knee OA is needed, mainly on the factors where conflicting evidence was presented (eg, age, baseline OA severity, BMI). Furthermore, we presented limited, inconclusive, or conflicting evidence on many factors with potential associations with OA progression. It would be important to investigate determinants that can be influenced or modified to reduce the risk of OA progression, perhaps including metabolic syndrome, bone marrow lesions, or osteoporosis. Moreover, there would be obvious advantages to testing the effect of new or existing disease-modifying pharmacologic or surgical interventions in patients with an established increased risk of OA progression.
We found strong evidence that baseline knee pain and Heberden nodes, varus alignment, and high baseline serum levels of hyaluronic acid and TNFα are predictive for knee OA progression. Sex (female), former knee injury, quadriceps strength, smoking, running, and regular performance of sports are not predictive for progression of knee OA. Many studies have been performed and are being performed determining risk factors for knee OA progression, but the variability in how OA and OA progression are defined across the relevant studies remains an impediment to pooling the available evidence. We strongly recommend future researchers use uniform definitions of determinants, disease, and disease progression; it would enable more precise determination of possible risk factors for knee OA progression through meta-analyses. The majority of the included studies used the Kellgren-Lawrence classification as definition of disease and disease progression. This classification has been criticized because the criteria have been described and interpreted differently in various studies [67]. However, the Kellgren-Lawrence criteria provide a reliable classification of knee OA and OA progression, given that the original description of the criteria are applied [67, 68]. We therefore recommend that future researchers use the Kellgren-Lawrence classification to define radiographic OA and OA progression. Furthermore, considering that some MRI scoring systems have been and currently are being developed to define knee OA progression [36], it seems preferable that the same MRI scoring system would be used universally in future studies on prognostic factors for knee OA progression. We would like to call on expert committees, such as the Osteoarthritis Research Society International (OARSI) for OA Imaging to announce their recommendations on this important topic.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Acknowledgments
We thank Louis Volkers MSc, Information Specialist, Medical Library Erasmus MC, University Medical Center Rotterdam, for assistance in the updated literature search for this systematic review.
Footnotes
The Department of General Practice Erasmus MC is partly funded by a program grant of the Dutch Arthritis Foundation for their center of excellence “Osteoarthritis in Primary Care.”
All ICMJE Conflict of Interest Forms for authors and Clinical Orthopaedics and Related Research ® editors and board members are on file with the publication and can be viewed on request.
The majority of this work was performed at Erasmus MC, Rotterdam, The Netherlands. One author (JNB) performed work at Leiden University Medical Center, Leiden, The Netherlands.
References
- 1.Altman DG. Systematic reviews of evaluations of prognostic variables. BMJ. 2001;323:224–228. doi: 10.1136/bmj.323.7306.224. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Attur M, Belitskaya-Levy I, Oh C, Krasnokutsky S, Greenberg J, Samuels J, Smiles S, Lee S, Patel J, Al-Mussawir H, McDaniel G, Kraus VB, Abramson SB. Increased interleukin-1β gene expression in peripheral blood leukocytes is associated with increased pain and predicts risk for progression of symptomatic knee osteoarthritis. Arthritis Rheum. 2011;63:1908–1917. doi: 10.1002/art.30360. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Bagge E, Bjelle A, Svanborg A. Radiographic osteoarthritis in the elderly: a cohort comparison and a longitudinal study of the “70-year old people in Goteborg”. Clin Rheumatol. 1992;11:486–491. doi: 10.1007/BF02283103. [DOI] [PubMed] [Google Scholar]
- 4.Belo JN, Berger MY, Reijman M, Koes BW, Bierma-Zeinstra SM. Prognostic factors of progression of osteoarthritis of the knee: a systematic review of observational studies. Arthritis Rheum. 2007;57:13–26. doi: 10.1002/art.22475. [DOI] [PubMed] [Google Scholar]
- 5.Benichou OD, Hunter DJ, Nelson DR, Guermazi A, Eckstein F, Kwoh K, Myers SL, Wirth W, Duryea J; Osteoarthritis Initiative Investigators. One-year change in radiographic joint space width in patients with unilateral joint space narrowing: data from the Osteoarthritis Initiative. Arthritis Care Res (Hoboken). 2010;62:924–931. [DOI] [PMC free article] [PubMed]
- 6.Bennell KL, Hinman RS. A review of the clinical evidence for exercise in osteoarthritis of the hip and knee. J Sci Med Sport. 2011;14:4–9. doi: 10.1016/j.jsams.2010.08.002. [DOI] [PubMed] [Google Scholar]
- 7.Bergink AP, Uitterlinden AG, Van Leeuwen JP, Buurman CJ, Hofman A, Verhaar JA, Pols HA. Vitamin D status, bone mineral density, and the development of radiographic osteoarthritis of the knee: The Rotterdam Study. J Clin Rheumatol. 2009;15:230–237. doi: 10.1097/RHU.0b013e3181b08f20. [DOI] [PubMed] [Google Scholar]
- 8.Bettica P, Cline G, Hart DJ, Meyer J, Spector TD. Evidence for increased bone resorption in patients with progressive knee osteoarthritis: longitudinal results from the Chingford study. Arthritis Rheum. 2002;46:3178–3184. doi: 10.1002/art.10630. [DOI] [PubMed] [Google Scholar]
- 9.Bierma-Zeinstra SM, Koes BW. Risk factors and prognostic factors of hip and knee osteoarthritis. Nat Clin Pract Rheumatol. 2007;3:78–85. doi: 10.1038/ncprheum0423. [DOI] [PubMed] [Google Scholar]
- 10.Blagojevic M, Jinks C, Jeffery A, Jordan KP. Risk factors for onset of osteoarthritis of the knee in older adults: a systematic review and meta-analysis. Osteoarthritis Cartilage. 2010;18:24–33. doi: 10.1016/j.joca.2009.08.010. [DOI] [PubMed] [Google Scholar]
- 11.Botha-Scheepers S, Watt I, Slagboom E, de Craen AJ, Meulenbelt I, Rosendaal FR, Breedveld FC, Huizinga TW, Kloppenburg M. Innate production of tumour necrosis factor alpha and interleukin 10 is associated with radiological progression of knee osteoarthritis. Ann Rheum Dis. 2008;67:1165–1169. doi: 10.1136/ard.2007.084657. [DOI] [PubMed] [Google Scholar]
- 12.Brandt KD, Heilman DK, Slemenda C, Katz BP, Mazzuca SA, Braunstein EM, Byrd D. Quadriceps strength in women with radiographically progressive osteoarthritis of the knee and those with stable radiographic changes. J Rheumatol. 1999;26:2431–2437. [PubMed] [Google Scholar]
- 13.Brouwer GM, van Tol AW, Bergink AP, Belo JN, Bernsen RM, Reijman M, Pols HA, Bierma-Zeinstra SM. Association between valgus and varus alignment and the development and progression of radiographic osteoarthritis of the knee. Arthritis Rheum. 2007;56:1204–1211. doi: 10.1002/art.22515. [DOI] [PubMed] [Google Scholar]
- 14.Bruyere O, Collette JH, Ethgen O, Rovati LC, Giacovelli G, Henrotin YE, Seidel L, Reginster JY. Biochemical markers of bone and cartilage remodeling in prediction of longterm progression of knee osteoarthritis. J Rheumatol. 2003;30:1043–1050. [PubMed] [Google Scholar]
- 15.Bruyere O, Honore A, Ethgen O, Rovati LC, Giacovelli G, Henrotin YE, Seidel L, Reginster JY. Correlation between radiographic severity of knee osteoarthritis and future disease progression: results from a 3-year prospective, placebo-controlled study evaluating the effect of glucosamine sulfate. Osteoarthritis Cartilage. 2003;11:1–5. doi: 10.1053/joca.2002.0848. [DOI] [PubMed] [Google Scholar]
- 16.Cerejo R, Dunlop DD, Cahue S, Channin D, Song J, Sharma L. The influence of alignment on risk of knee osteoarthritis progression according to baseline stage of disease. Arthritis Rheum. 2002;46:2632–2636. doi: 10.1002/art.10530. [DOI] [PubMed] [Google Scholar]
- 17.Chapple CM, Nicholson H, Baxter GD, Abbott JH. Patient characteristics that predict progression of knee osteoarthritis: a systematic review of prognostic studies. Arthritis Care Res (Hoboken). 2011;63:1115–1125. doi: 10.1002/acr.20492. [DOI] [PubMed] [Google Scholar]
- 18.Cooper C, Snow S, McAlindon TE, Kellingray S, Stuart B, Coggon D, Dieppe PA. Risk factors for the incidence and progression of radiographic knee osteoarthritis. Arthritis Rheum. 2000;43:995–1000. doi: 10.1002/1529-0131(200005)43:5<995::AID-ANR6>3.0.CO;2-1. [DOI] [PubMed] [Google Scholar]
- 19.Dam EB, Byrjalsen I, Karsdal MA, Qvist P, Christiansen C. Increased urinary excretion of C-telopeptides of type II collagen (CTX-II) predicts cartilage loss over 21 months by MRI. Osteoarthritis Cartilage. 2009;17:384–389. doi: 10.1016/j.joca.2008.07.009. [DOI] [PubMed] [Google Scholar]
- 20.Denoble AE, Huffman KM, Stabler TV, Kelly SJ, Hershfield MS, McDaniel GE, Coleman RE, Kraus VB. Uric acid is a danger signal of increasing risk for osteoarthritis through inflammasome activation. Proc Natl Acad Sci U S A. 2011;108:2088–2093. doi: 10.1073/pnas.1012743108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Dieppe PA, Cushnaghan J, Shepstone L. The Bristol ‘OA500’ study: progression of osteoarthritis (OA) over 3 years and the relationship between clinical and radiographic changes at the knee joint. Osteoarthritis Cartilage. 1997;5:87–97. doi: 10.1016/S1063-4584(97)80002-7. [DOI] [PubMed] [Google Scholar]
- 22.Dieppe P, Cushnaghan J, Tucker M, Browning S, Shepstone L. The Bristol ‘OA500 study’: progression and impact of the disease after 8 years. Osteoarthritis Cartilage. 2000;8:63–68. doi: 10.1053/joca.1999.0272. [DOI] [PubMed] [Google Scholar]
- 23.Dieppe P, Cushnaghan J, Young P, Kirwan J. Prediction of the progression of joint space narrowing in osteoarthritis of the knee by bone scintigraphy. Ann Rheum Dis. 1993;52:557–563. doi: 10.1136/ard.52.8.557. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Doherty M, Belcher C, Regan M, Jones A, Ledingham J. Association between synovial fluid levels of inorganic pyrophosphate and short term radiographic outcome of knee osteoarthritis. Ann Rheum Dis. 1996;55:432–436. doi: 10.1136/ard.55.7.432. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Duncan R, Peat G, Thomas E, Hay EM, Croft P. Incidence, progression and sequence of development of radiographic knee osteoarthritis in a symptomatic population. Ann Rheum Dis. 2011;70:1944–1948. doi: 10.1136/ard.2011.151050. [DOI] [PubMed] [Google Scholar]
- 26.Fayfman M, Niu J, Zhang YQ, Felson DT, Sack B, Aliabadi P, Selhub J, Hunter DJ. The relation of plasma homocysteine to radiographic knee osteoarthritis. Osteoarthritis Cartilage. 2009;17:766–771. doi: 10.1016/j.joca.2008.11.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Felson DT, Gale DR, Elon Gale M, Niu J, Hunter DJ, Goggins J, Lavalley MP. Osteophytes and progression of knee osteoarthritis. Rheumatology (Oxford). 2005;44:100–104. [DOI] [PubMed]
- 28.Felson DT, Goggins J, Niu J, Zhang Y, Hunter DJ. The effect of body weight on progression of knee osteoarthritis is dependent on alignment. Arthritis Rheum. 2004;50:3904–3909. doi: 10.1002/art.20726. [DOI] [PubMed] [Google Scholar]
- 29.Felson DT, McLaughlin S, Goggins J, LaValley MP, Gale ME, Totterman S, Li W, Hill C, Gale D. Bone marrow edema and its relation to progression of knee osteoarthritis. Ann Intern Med. 2003;139:330–336. doi: 10.7326/0003-4819-139-5_Part_1-200309020-00008. [DOI] [PubMed] [Google Scholar]
- 30.Felson DT, Niu J, Clancy M, Aliabadi P, Sack B, Guermazi A, Hunter DJ, Amin S, Rogers G, Booth SL. Low levels of vitamin D and worsening of knee osteoarthritis: results of two longitudinal studies. Arthritis Rheum. 2007;56:129–136. doi: 10.1002/art.22292. [DOI] [PubMed] [Google Scholar]
- 31.Felson DT, Zhang Y, Hannan MT, Naimark A, Weissman BN, Aliabadi P, Levy D. The incidence and natural history of knee osteoarthritis in the elderly: The Framingham Osteoarthritis Study. Arthritis Rheum. 1995;38:1500–1505. doi: 10.1002/art.1780381017. [DOI] [PubMed] [Google Scholar]
- 32.Fraenkel L, Zhang Y, Trippel SB, McAlindon TE, LaValley MP, Assif A, Adams KE, Evans SR, Felson DT. Longitudinal analysis of the relationship between serum insulin-like growth factor-I and radiographic knee osteoarthritis. Osteoarthritis Cartilage. 1998;6:362–367. doi: 10.1053/joca.1998.0135. [DOI] [PubMed] [Google Scholar]
- 33.Fransen M, McConnell S. Exercise for osteoarthritis of the knee. Cochrane Database Syst Rev. 2008;4:CD004376. [DOI] [PubMed]
- 34.Furlan AD, Pennick V, Bombardier C, van Tulder M, Editorial Board Cochrane Back Review Group. 2009 updated method guidelines for systematic reviews in the Cochrane Back Review Group. Spine (Phila Pa 1976). 2009;34:1929–1941. [DOI] [PubMed]
- 35.Golightly YM, Allen KD, Helmick CG, Schwartz TA, Renner JB, Jordan JM. Hazard of incident and progressive knee and hip radiographic osteoarthritis and chronic joint symptoms in individuals with and without limb length inequality. J Rheumatol. 2010;37:2133–2140. doi: 10.3899/jrheum.091410. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Guermazi A, Roemer FW, Haugen IK, Crema MD, Hayashi D. MRI-based semiquantitative scoring of joint pathology in osteoarthritis. Nat Rev Rheumatol. 2013;9:236–251. doi: 10.1038/nrrheum.2012.223. [DOI] [PubMed] [Google Scholar]
- 37.Hart DJ, Cronin C, Daniels M, Worthy T, Doyle DV, Spector TD. The relationship of bone density and fracture to incident and progressive radiographic osteoarthritis of the knee: the Chingford Study. Arthritis Rheum. 2002;46:92–99. doi: 10.1002/1529-0131(200201)46:1<92::AID-ART10057>3.0.CO;2-#. [DOI] [PubMed] [Google Scholar]
- 38.Harvey WF, Yang M, Cooke TD, Segal NA, Lane N, Lewis CE, Felson DT. Association of leg-length inequality with knee osteoarthritis: a cohort study. Ann Intern Med. 2010;152:287–295. doi: 10.7326/0003-4819-152-5-201003020-00006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Haugen IK, Cotofana S, Englund M, Kvien TK, Dreher D, Nevitt M, Lane NE. Eckstein F; Osteoarthritis Initiative Investigators. Hand joint space narrowing and osteophytes are associated with magnetic resonance imaging-defined knee cartilage thickness and radiographic knee osteoarthritis: data from the Osteoarthritis Initiative. J Rheumatol. 2012;39:161–166. doi: 10.3899/jrheum.110603. [DOI] [PubMed] [Google Scholar]
- 40.Hunter DJ, Zhang YQ, Niu JB, Felson DT, Kwoh K, Newman A, Kritchevsky S, Harris T, Carbone L, Nevitt M. Patella malalignment, pain and patellofemoral progression: the Health ABC Study. Osteoarthritis Cartilage. 2007;15:1120–1127. doi: 10.1016/j.joca.2007.03.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Kerkhof HJ, Bierma-Zeinstra SM, Castano-Betancourt MC, de Maat MP, Hofman A, Pols HA, Rivadeneira F, Witteman JC, Uitterlinden AG, van Meurs JB. Serum C reactive protein levels and genetic variation in the CRP gene are not associated with the prevalence, incidence or progression of osteoarthritis independent of body mass index. Ann Rheum Dis. 2010;69:1976–1982. doi: 10.1136/ard.2009.125260. [DOI] [PubMed] [Google Scholar]
- 42.Kerna I, Kisand K, Tamm AE, Lintrop M, Veske K, Tamm AO. Missense single nucleotide polymorphism of the ADAM12 gene is associated with radiographic knee osteoarthritis in middle-aged Estonian cohort. Osteoarthritis Cartilage. 2009;17:1093–1098. doi: 10.1016/j.joca.2009.02.006. [DOI] [PubMed] [Google Scholar]
- 43.Kopec JA, Sayre EC, Schwartz TA, Renner JB, Helmick CG, Badley EM, Cibere J, Callahan LF, Jordan JM. Occurrence of radiographic osteoarthritis of the knee and hip among African Americans and whites: a population-based prospective cohort study. Arthritis Care Res (Hoboken). 2013;65:928–935. doi: 10.1002/acr.21924. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Kraus VB, Feng S, Wang S, White S, Ainslie M, Brett A, Holmes A, Charles HC. Trabecular morphometry by fractal signature analysis is a novel marker of osteoarthritis progression. Arthritis Rheum. 2009;60:3711–3722. doi: 10.1002/art.25012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Lane NE, Oehlert JW, Bloch DA, Fries JF. The relationship of running to osteoarthritis of the knee and hip and bone mineral density of the lumbar spine: a 9 year longitudinal study. J Rheumatol. 1998;25:334–341. [PubMed] [Google Scholar]
- 46.Larsson S, Englund M, Struglics A, Lohmander LS. The association between changes in synovial fluid levels of ARGS-aggrecan fragments, progression of radiographic osteoarthritis and self-reported outcomes: a cohort study. Osteoarthritis Cartilage. 2012;20:388–395. doi: 10.1016/j.joca.2012.02.001. [DOI] [PubMed] [Google Scholar]
- 47.Ledingham J, Regan M, Jones A, Doherty M. Factors affecting radiographic progression of knee osteoarthritis. Ann Rheum Dis. 1995;54:53–58. doi: 10.1136/ard.54.1.53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Le Graverand MP, Brandt K, Mazzuca SA, Raunig D, Vignon E. Progressive increase in body mass index is not associated with a progressive increase in joint space narrowing in obese women with osteoarthritis of the knee. Ann Rheum Dis. 2009;68:1734–1738. doi: 10.1136/ard.2007.085530. [DOI] [PubMed] [Google Scholar]
- 49.Lievense AM, Bierma-Zeinstra SM, Verhagen AP, Verhaar JA, Koes BW. Prognostic factors of progress of hip osteoarthritis: a systematic review. Arthritis Rheum. 2002;47:556–562. doi: 10.1002/art.10660. [DOI] [PubMed] [Google Scholar]
- 50.Madan-Sharma R, Kloppenburg M, Kornaat PR, Botha-Scheepers SA, Le Graverand MP, Bloem JL, Watt I. Do MRI features at baseline predict radiographic joint space narrowing in the medial compartment of the osteoarthritic knee 2 years later? Skeletal Radiol. 2008;37:805–811. doi: 10.1007/s00256-008-0508-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Mazzuca SA, Brandt KD, Katz BP, Ding Y, Lane KA, Buckwalter KA. Risk factors for progression of tibiofemoral osteoarthritis: an analysis based on fluoroscopically standardised knee radiography. Ann Rheum Dis. 2006;65:515–519. doi: 10.1136/ard.2005.039115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Mazzuca SA, Brandt KD, Schauwecker DS, Buckwalter KA, Katz BP, Meyer JM, Lane KA. Bone scintigraphy is not a better predictor of progression of knee osteoarthritis than Kellgren and Lawrence grade. J Rheumatol. 2004;31:329–332. [PubMed] [Google Scholar]
- 53.McAlindon TE, Felson DT, Zhang Y, Hannan MT, Aliabadi P, Weissman B, Rush D, Wilson PW, Jacques P. Relation of dietary intake and serum levels of vitamin D to progression of osteoarthritis of the knee among participants in the Framingham Study. Ann Intern Med. 1996;125:353–359. doi: 10.7326/0003-4819-125-5-199609010-00001. [DOI] [PubMed] [Google Scholar]
- 54.McAlindon TE, Jacques P, Zhang Y, Hannan MT, Aliabadi P, Weissman B, Rush D, Levy D, Felson DT. Do antioxidant micronutrients protect against the development and progression of knee osteoarthritis? Arthritis Rheum. 1996;39:648–656. doi: 10.1002/art.1780390417. [DOI] [PubMed] [Google Scholar]
- 55.Miyazaki T, Uchida K, Sato M, Watanabe S, Yoshida A, Wada M, Shimada S, Kuiper JH, Baba H. Knee laxity after staircase exercise predicts radiographic disease progression in medial compartment knee osteoarthritis. Arthritis Rheum. 2012;64:3908–3916. doi: 10.1002/art.34662. [DOI] [PubMed] [Google Scholar]
- 56.Miyazaki T, Wada M, Kawahara H, Sato M, Baba H, Shimada S. Dynamic load at baseline can predict radiographic disease progression in medial compartment knee osteoarthritis. Ann Rheum Dis. 2002;61:617–622. doi: 10.1136/ard.61.7.617. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Muraki S, Akune T, Oka H, Ishimoto Y, Nagata K, Yoshida M, Tokimura F, Nakamura K, Kawaguchi H, Yoshimura N. Incidence and risk factors for radiographic knee osteoarthritis and knee pain in Japanese men and women: a longitudinal population-based cohort study. Arthritis Rheum. 2012;64:1447–1456. doi: 10.1002/art.33508. [DOI] [PubMed] [Google Scholar]
- 58.Nelson AE, Golightly YM, Kraus VB, Stabler T, Renner JB, Helmick CG, Jordan JM. Serum transforming growth factor-beta 1 is not a robust biomarker of incident and progressive radiographic osteoarthritis at the hip and knee: the Johnston County Osteoarthritis Project. Osteoarthritis Cartilage. 2010;18:825–829. doi: 10.1016/j.joca.2010.02.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Nevitt MC, Zhang Y, Javaid MK, Neogi T, Curtis JR, Niu J, McCulloch CE, Segal NA, Felson DT. High systemic bone mineral density increases the risk of incident knee OA and joint space narrowing, but not radiographic progression of existing knee OA: the MOST study. Ann Rheum Dis. 2010;69:163–168. doi: 10.1136/ard.2008.099531. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Nishimura A, Hasegawa M, Kato K, Yamada T, Uchida A, Sudo A. Risk factors for the incidence and progression of radiographic osteoarthritis of the knee among Japanese. Int Orthop. 2011;35:839–843. doi: 10.1007/s00264-010-1073-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Niu J, Zhang YQ, Torner J, Nevitt M, Lewis CE, Aliabadi P, Sack B, Clancy M, Sharma L, Felson DT. Is obesity a risk factor for progressive radiographic knee osteoarthritis? Arthritis Rheum. 2009;61:329–335. doi: 10.1002/art.24337. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Pavelka K, Forejtova S, Olejarova M, Gatterova J, Senolt L, Spacek P, Braun M, Hulejova M, Stovickova J, Pavelkova A. Hyaluronic acid levels may have predictive value for the progression of knee osteoarthritis. Osteoarthritis Cartilage. 2004;12:277–283. doi: 10.1016/j.joca.2004.01.001. [DOI] [PubMed] [Google Scholar]
- 63.Pavelka K, Gatterova J, Altman RD. Radiographic progression of knee osteoarthritis in a Czech cohort. Clin Exp Rheumatol. 2000;18:473–477. [PubMed] [Google Scholar]
- 64.Peregoy J, Wilder FV. The effects of vitamin C supplementation on incident and progressive knee osteoarthritis: a longitudinal study. Public Health Nutr. 2011;14:709–715. doi: 10.1017/S1368980010001783. [DOI] [PubMed] [Google Scholar]
- 65.Reijman M, Hazes JM, Bierma-Zeinstra SM, Koes BW, Christgau S, Christiansen C, Uitterlinden AG, Pols HA. A new marker for osteoarthritis: cross-sectional and longitudinal approach. Arthritis Rheum. 2004;50:2471–2478. doi: 10.1002/art.20332. [DOI] [PubMed] [Google Scholar]
- 66.Reijman M, Pols HA, Bergink AP, Hazes JM, Belo JN, Lievense AM, Bierma-Zeinstra SM. Body mass index associated with onset and progression of osteoarthritis of the knee but not of the hip: the Rotterdam Study. Ann Rheum Dis. 2007;66:158–162. doi: 10.1136/ard.2006.053538. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Schiphof D, de Klerk BM, Kerkhof HJ, Hofman A, Koes BW, Boers M, Bierma-Zeinstra SM. Impact of different descriptions of the Kellgren and Lawrence classification criteria on the diagnosis of knee osteoarthritis. Ann Rheum Dis. 2011;70:1422–1427. doi: 10.1136/ard.2010.147520. [DOI] [PubMed] [Google Scholar]
- 68.Schiphof D, de Klerk BM, Koes BW, Bierma-Zeinstra S. Good reliability, questionable validity of 25 different classification criteria of knee osteoarthritis: a systematic appraisal. J Clin Epidemiol. 2008;61:1205–1215. doi: 10.1016/j.jclinepi.2008.04.003. [DOI] [PubMed] [Google Scholar]
- 69.Scholten-Peeters GG, Verhagen AP, Bekkering GE, van der Windt DA, Barnsley L, Oostendorp RA, Hendriks EJ. Prognostic factors of whiplash-associated disorders: a systematic review of prospective cohort studies. Pain. 2003;104:303–322. doi: 10.1016/S0304-3959(03)00050-2. [DOI] [PubMed] [Google Scholar]
- 70.Schouten JS, van den Ouweland FA, Valkenburg HA. A 12 year follow up study in the general population on prognostic factors of cartilage loss in osteoarthritis of the knee. Ann Rheum Dis. 1992;51:932–937. doi: 10.1136/ard.51.8.932. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Schouten JS, Van den Ouweland FA, Valkenburg HA, Lamberts SW. Insulin-like growth factor-1: a prognostic factor of knee osteoarthritis. Br J Rheumatol. 1993;32:274–280. doi: 10.1093/rheumatology/32.4.274. [DOI] [PubMed] [Google Scholar]
- 72.Sharif M, George E, Shepstone L, Knudson W, Thonar EJ, Cushnaghan J, Dieppe P. Serum hyaluronic acid level as a predictor of disease progression in osteoarthritis of the knee. Arthritis Rheum. 1995;38:760–767. doi: 10.1002/art.1780380608. [DOI] [PubMed] [Google Scholar]
- 73.Sharif M, Kirwan J, Charni N, Sandell LJ, Whittles C, Garnero P. A 5-yr longitudinal study of type IIA collagen synthesis and total type II collagen degradation in patients with knee osteoarthritis: association with disease progression. Rheumatology (Oxford). 2007;46:938–943. doi: 10.1093/rheumatology/kel409. [DOI] [PubMed] [Google Scholar]
- 74.Sharif M, Kirwan JR, Elson CJ, Granell R, Clarke S. Suggestion of nonlinear or phasic progression of knee osteoarthritis based on measurements of serum cartilage oligomeric matrix protein levels over five years. Arthritis Rheum. 2004;50:2479–2488. doi: 10.1002/art.20365. [DOI] [PubMed] [Google Scholar]
- 75.Sharif M, Saxne T, Shepstone L, Kirwan JR, Elson CJ, Heinegard D, Dieppe PA. Relationship between serum cartilage oligomeric matrix protein levels and disease progression in osteoarthritis of the knee joint. Br J Rheumatol. 1995;34:306–310. doi: 10.1093/rheumatology/34.4.306. [DOI] [PubMed] [Google Scholar]
- 76.Sharif M, Shepstone L, Elson CJ, Dieppe PA, Kirwan JR. Increased serum C reactive protein may reflect events that precede radiographic progression in osteoarthritis of the knee. Ann Rheum Dis. 2000;59:71–74. doi: 10.1136/ard.59.1.71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Sharma L, Dunlop DD, Cahue S, Song J, Hayes KW. Quadriceps strength and osteoarthritis progression in malaligned and lax knees. Ann Intern Med. 2003;138:613–619. doi: 10.7326/0003-4819-138-8-200304150-00006. [DOI] [PubMed] [Google Scholar]
- 78.Sharma L, Song J, Dunlop D, Felson D, Lewis CE, Segal N, Torner J, Cooke TD, Hietpas J, Lynch J, Nevitt M. Varus and valgus alignment and incident and progressive knee osteoarthritis. Ann Rheum Dis. 2010;69:1940–1945. doi: 10.1136/ard.2010.129742. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Sharma L, Song J, Felson DT, Cahue S, Shamiyeh E, Dunlop DD. The role of knee alignment in disease progression and functional decline in knee osteoarthritis. JAMA. 2001;286:188–195. doi: 10.1001/jama.286.2.188. [DOI] [PubMed] [Google Scholar]
- 80.Spector TD, Dacre JE, Harris PA, Huskisson EC. Radiological progression of osteoarthritis: an 11 year follow up study of the knee. Ann Rheum Dis. 1992;51:1107–1110. doi: 10.1136/ard.51.10.1107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Spector TD, Hart DJ, Doyle DV. Incidence and progression of osteoarthritis in women with unilateral knee disease in the general population: the effect of obesity. Ann Rheum Dis. 1994;53:565–568. doi: 10.1136/ard.53.9.565. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Spector TD, Hart DJ, Nandra D, Doyle DV, Mackillop N, Gallimore JR, Pepys MB. Low-level increases in serum C-reactive protein are present in early osteoarthritis of the knee and predict progressive disease. Arthritis Rheum. 1997;40:723–727. doi: 10.1002/art.1780400419. [DOI] [PubMed] [Google Scholar]
- 83.Sugiyama S, Itokazu M, Suzuki Y, Shimizu K. Procollagen II C propeptide level in the synovial fluid as a predictor of radiographic progression in early knee osteoarthritis. Ann Rheum Dis. 2003;62:27–32. doi: 10.1136/ard.62.1.27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Tanamas S, Hanna FS, Cicuttini FM, Wluka AE, Berry P, Urquhart DM. Does knee malalignment increase the risk of development and progression of knee osteoarthritis? A systematic review. Arthritis Rheum. 2009;61:459–467. doi: 10.1002/art.24336. [DOI] [PubMed] [Google Scholar]
- 85.Valdes AM, Hart DJ, Jones KA, Surdulescu G, Swarbrick P, Doyle DV, Schafer AJ, Spector TD. Association study of candidate genes for the prevalence and progression of knee osteoarthritis. Arthritis Rheum. 2004;50:2497–2507. doi: 10.1002/art.20443. [DOI] [PubMed] [Google Scholar]
- 86.van Dijk GM, Dekker J, Veenhof C, van den Ende CH, Carpa Study Group Course of functional status and pain in osteoarthritis of the hip or knee: a systematic review of the literature. Arthritis Rheum. 2006;55:779–785. doi: 10.1002/art.22244. [DOI] [PubMed] [Google Scholar]
- 87.Vilim V, Olejarova M, Machacek S, Gatterova J, Kraus VB, Pavelka K. Serum levels of cartilage oligomeric matrix protein (COMP) correlate with radiographic progression of knee osteoarthritis. Osteoarthritis Cartilage. 2002;10:707–713. doi: 10.1053/joca.2002.0819. [DOI] [PubMed] [Google Scholar]
- 88.Wilder FV, Leaverton PE, Rogers MW, Lemrow NB. Vitamin supplements and radiographic knee osteoarthritis: The Clearwater Osteoarthritis Study. J Musculoskelet Res. 2009;12:85–93. doi: 10.1142/S0218957709002274. [DOI] [Google Scholar]
- 89.Wolfe F, Lane NE. The longterm outcome of osteoarthritis: rates and predictors of joint space narrowing in symptomatic patients with knee osteoarthritis. J Rheumatol. 2002;29:139–146. [PubMed] [Google Scholar]
- 90.Wright AA, Cook C, Abbott JH. Variables associated with the progression of hip osteoarthritis: a systematic review. Arthritis Rheum. 2009;61:925–936. doi: 10.1002/art.24641. [DOI] [PubMed] [Google Scholar]
- 91.Yoshimura N, Muraki S, Oka H, Tanaka S, Kawaguchi H, Nakamura K, Akune T. Accumulation of metabolic risk factors such as overweight, hypertension, dyslipidaemia, and impaired glucose tolerance raises the risk of occurrence and progression of knee osteoarthritis: a 3-year follow-up of the ROAD study. Osteoarthritis Cartilage. 2012;20:1217–1226. doi: 10.1016/j.joca.2012.06.006. [DOI] [PubMed] [Google Scholar]
- 92.Yusuf E, Bijsterbosch J, Slagboom PE, Rosendaal FR, Huizinga TW, Kloppenburg M. Body mass index and alignment and their interaction as risk factors for progression of knees with radiographic signs of osteoarthritis. Osteoarthritis Cartilage. 2011;19:1117–1122. doi: 10.1016/j.joca.2011.06.001. [DOI] [PubMed] [Google Scholar]
- 93.Zhai G, Hart DJ, Kato BS, MacGregor A, Spector TD. Genetic influence on the progression of radiographic knee osteoarthritis: a longitudinal twin study. Osteoarthritis Cartilage. 2007;15:222–225. doi: 10.1016/j.joca.2006.09.004. [DOI] [PubMed] [Google Scholar]
- 94.Zhang Y, Hannan MT, Chaisson CE, McAlindon TE, Evans SR, Aliabadi P, Levy D, Felson DT. Bone mineral density and risk of incident and progressive radiographic knee osteoarthritis in women: the Framingham Study. J Rheumatol. 2000;27:1032–1037. [PubMed] [Google Scholar]
- 95.Zhang Y, Jordan JM. Epidemiology of osteoarthritis. Rheum Dis Clin North Am. 2008;34:515–529. doi: 10.1016/j.rdc.2008.05.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Zhang Y, McAlindon TE, Hannan MT, Chaisson CE, Klein R, Wilson PW, Felson DT. Estrogen replacement therapy and worsening of radiographic knee osteoarthritis: the Framingham Study. Arthritis Rheum. 1998;41:1867–1873. doi: 10.1002/1529-0131(199810)41:10<1867::AID-ART20>3.0.CO;2-W. [DOI] [PubMed] [Google Scholar]
- 97.Zhang Y, Niu J, Felson DT, Choi HK, Nevitt M, Neogi T. Methodologic challenges in studying risk factors for progression of knee osteoarthritis. Arthritis Care Res (Hoboken). 2010;62:1527–1532. doi: 10.1002/acr.20287. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.