Abstract
Background
Whole-body vibration (WBV) is associated with back and neck pain in military personnel and civilians. However, the role of vibration frequency and the physiological mechanisms involved in pain symptoms are unknown.
Questions/purposes
This study asked the following questions: (1) What is the resonance frequency of the rat spine for WBV along the spinal axis, and how does frequency of WBV alter the extent of spinal compression/extension? (2) Does a single WBV exposure at resonance induce pain that is sustained? (3) Does WBV at resonance alter the protein kinase C epsilon (PKCε) response in the dorsal root ganglia (DRG)? (4) Does WBV at resonance alter expression of calcitonin gene-related peptide (CGRP) in the spinal dorsal horn? (5) Does WBV at resonance alter the spinal neuroimmune responses that regulate pain?
Methods
Resonance of the rat (410 ± 34 g, n = 9) was measured by imposing WBV at frequencies from 3 to 15 Hz. Separate groups (317 ± 20 g, n = 10/treatment) underwent WBV at resonance (8 Hz) or at a nonresonant frequency (15 Hz). Behavioral sensitivity was assessed throughout to measure pain, and PKCε in the DRG was quantified as well as spinal CGRP, glial activation, and cytokine levels at Day 14.
Results
Accelerometer-based thoracic transmissibility peaks at 8 Hz (1.86 ± 0.19) and 9 Hz (1.95 ± 0.19, mean difference [MD] 0.290 ± 0.266, p < 0.03), whereas the video-based thoracic transmissibility peaks at 8 Hz (1.90 ± 0.27), 9 Hz (2.07 ± 0.20), and 10 Hz (1.80 ± 0.25, MD 0.359 ± 0.284, p < 0.01). WBV at 8 Hz produces more cervical extension (0.745 ± 0.582 mm, MD 0.242 ± 0.214, p < 0.03) and compression (0.870 ± 0.676 mm, MD 0.326 ± 0.261, p < 0.02) than 15 Hz (extension, 0.503 ± 0.279 mm; compression, 0.544 ± 0.400 mm). Pain is longer lasting (through Day 14) and more robust (p < 0.01) after WBV at the resonant frequency (8 Hz) compared with 15 Hz WBV. PKCε in the nociceptors of the DRG increases according to the severity of WBV with greatest increases after 8 Hz WBV (p < 0.03). However, spinal CGRP, cytokines, and glial activation are only evident after painful WBV at resonance.
Conclusions
WBV at resonance produces long-lasting pain and widespread activation of a host of nociceptive and neuroimmune responses as compared with WBV at a nonresonance condition. Based on this work, future investigations into the temporal and regional neuroimmune response to resonant WBV in both genders would be useful.
Clinical Relevance
Although WBV is a major issue affecting the military population, there is little insight about its mechanisms of injury and pain. The neuroimmune responses produced by WBV are similar to other pain states, suggesting that pain from WBV may be mediated by similar mechanisms as other neuropathic pain conditions. This mechanistic insight suggests WBV-induced injury and pain may be tempered by antiinflammatory intervention.
Electronic supplementary material
The online version of this article (doi:10.1007/s11999-015-4315-9) contains supplementary material, which is available to authorized users.
Introduction
Spinal pain has a lifetime prevalence of 54% to 80% in the United States [51]. Neck and back pain is a major disability for military personnel and is among the most diagnosed illness/injury for active-duty service members [5–9]. Epidemiological reports implicate whole-body vibration (WBV) as the cause of neck/back pain [13, 14, 48, 55, 84] with those who have served in combat settings at greatest risk. Over half (69%) of military helicopter pilots exposed to WBV report pain with the frequency of symptoms correlated with the length of exposure [24, 59]. Because the greatest injury risk exists at resonance [36], studies have defined the resonant frequency of many species, including humans (approximately 4 Hz) [11, 63], Rhesus monkey (5–14 Hz) [72], and rabbit (4.5 Hz) [80]. Although several rat models have examined pain induction and maintenance after either vibration of isolated limbs or WBV [3, 4, 11, 19, 26, 43], the vibration response, and its relevance to injury, in the rat is unknown.
Pain can result from direct injury to the peripheral and/or central nervous system or through nociceptive cascades that are initiated in the dorsal root ganglia (DRG) and/or spinal cord. Workers using vibrating hand tools exhibit nerve damage, pain, and paraesthesia in the hands [34, 66]. Isolated vibration of the limb or tail in the rat produces damage in myelinated fibers [49, 53]. WBV of the rat induces deformation in the cervical spine, which if repeated daily produces long-lasting behavioral sensitivity [11]. Although vibration may induce neural tissue damage, which may be a mechanism of pain, this has not been investigated for WBV.
The protein kinase C (PKC) pathway and in particular, the PKCε isoform regulate peripheral nociceptor function [41, 77] and contribute to prolonged pain [1, 25, 26, 28, 43]. PKCε is responsible for heat and pain sensitivity in peripheral nociceptors [16, 93] and, through PKC pathway signaling, also modulates spinal calcitonin gene-related peptide (CGRP) activity [69, 74]. After injury, CGRP is released by nociceptive fibers in the superficial dorsal horn [56, 58, 74, 76] and can stimulate spinal immune cell activation [15, 64] and cytokine release [69]. These immune responses not only further exacerbate a host of other inflammatory cytokines and chemokines, but also regulate neuronal responses through modulation at synaptic connections [22, 32, 46, 86].
We have previously shown that WBV at 15 Hz can induce pain in the rat that either resolves or is sustained, depending on how often the exposure is presented, occasionally or daily [11]. Upregulation of neurotrophins in the intervertebral discs is also evident for the case with sustained pain [40]. Although that collection of work supports the notion that WBV causes pain, it does not address whether sustained pain is possible after a single WBV exposure and the relative role of the resonance frequency on pain and injury risk.
Accordingly, we performed two complementary studies in the rat to define the mechanical and physiological outcomes of resonant WBV. First, we asked: (1) What is the resonance frequency of the rat spine for WBV along the spinal axis, and how does frequency of WBV alter the extent of spinal compression/extension? Additional questions derive from those findings: (2) Does a single WBV exposure at resonance induce pain that is sustained? (3) Does WBV at resonance alter the PKCε response in the DRG? (4) Does WBV at resonance alter expression of CGRP in the spinal dorsal horn? (5) Does WBV at resonance alter the spinal neuroimmune responses that regulate pain?
Materials and Methods
Procedures were approved by the Institutional Animal Care and Use Committee and performed according to the Committee for Research and Ethical Issues of the International Association for the Study of Pain [95]. Male Holtzman rats (Harlan, Indianapolis, IN, USA) were used in this study to reflect the majority of the population affected [62]. Rats were maintained with a 12-hour/12-hour light/dark cycle and free access to food and water as recommended by the Association for Assessment and Accreditation of Laboratory Animal Care International. All processing and analysis was performed by observers blinded to treatments.
WBV Transmissibility and Resonance
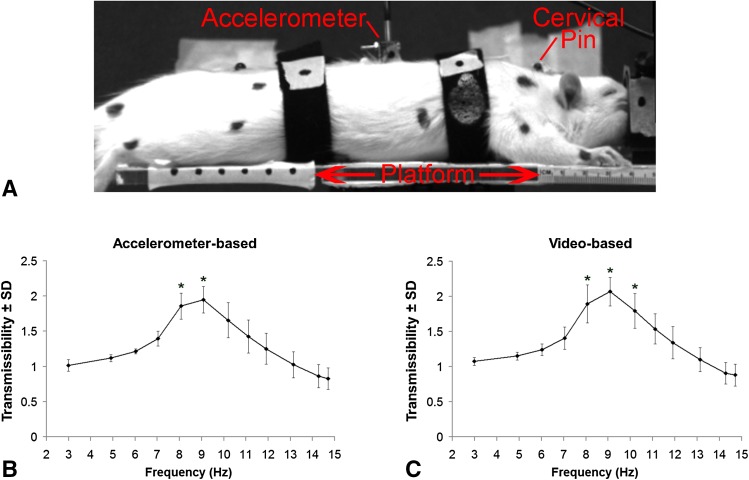
To answer our first question, of determining the resonance frequency, rats were exposed to WBV over a range of frequencies; the maximum energy transferred from a vibrating platform to the rat spine was determined from the transmissibility profile and taken as resonance. To enable rigid fixation of sensors directly to the vertebrae of the spine, the study to determine the resonance frequency of the rat spine was performed using dead rats (n = 9). Rats (410 ± 34 g) were anesthetized by intraperitoneal injection of sodium pentobarbital (65 mg/kg) and transcardially perfused with 200 mL phosphate-buffered saline (PBS) (Life Technologies, Carlsbad, CA, USA) followed by 200 mL 4% paraformaldehyde in PBS (PFA; Sigma, St Louis, MO, USA). Rats were placed in the prone position on a rigid vibrating platform to enable WBV along the spine’s long axis using straps positioned behind the shoulders and above the pelvis (Fig. 1A) [11]. A sinusoidal input (1.5-mm peak-to-peak amplitude) was applied using a shaker (K2007E01; Modal Shop, Cincinnati, OH) at 3 Hz and increased to 15 Hz in 1-Hz increments for 1 minute each. Uniaxial accelerometers (7521A2; 120 Hz; Dytran, Chatsworth, CA, USA) were affixed to the platform and rigidly pinned between the T9 and T10 vertebrae of the rat (Fig. 1A). A high-speed CCD video camera (0.035-mm resolution; 120 Hz; Phantom Miro eX1; Vision Research Inc, Wayne, NJ, USA; 640 × 480 pixels) tracked markers on the thoracic accelerometer and the platform (Fig. 1A) and relative displacements were digitized using ProAnalyst (Xcitex, Woburn, MA, USA) motion tracking software.
Fig. 1A–C.
A representative image (A) showing a rat on the test setup with the platform and the cervical pin and the accelerometer that are both rigidly inserted into the spine. The arrow indicates that the direction of vibration is along the long axis of the rat’s spine. The transmissibility profiles are separately calculated using data derived from the accelerometer (B) and the video imaging system (C); the peak transmissibility is between 8 Hz and 10 Hz, which are greater than all other frequencies (*p < 0.03) but not different from each other.
The video-based displacements and accelerometer data were used separately to compute the transmissibility of the thoracic spine at each input frequency [30, 42, 52, 68]. The accelerometer data were filtered using a fifth-order Butterworth bandwidth filter and the root mean square (RMS) acceleration of the thoracic accelerometer was divided by the platform acceleration to compute accelerometer-based transmissibility at each frequency. The video-based transmissibilities were calculated as the ratio of the RMS displacement of the thoracic spine divided by that of the platform at each frequency [12, 45]. A repeated-measures analysis of variance (ANOVA) was used to compare transmissibilities between frequencies and between acquisition approaches. Resonance was determined as the transmissibility that was greater than all other frequencies (p < 0.04).
Pain and Neuroimmune Regulation After WBV at Resonance
We assessed the behavioral and physiological effects of WBV at resonance to answer questions 2 through 5 on pain and neuroimmune regulation. Rats (317 ± 20 g) were vibrated at resonance (8 Hz, n = 10) and also at a frequency (15 Hz, n = 10) that has been previously characterized [11, 40]. Vibration was imposed under inhalation anesthesia (4% induction, 2% maintenance) in the two separate groups. To control for anesthetic effects, a sham group (n = 10) underwent the same exposure paradigm but was not vibrated. WBV was imposed as described for the resonance study but with the accelerometer strapped to the rat [11, 40]. Vibration was applied for 30 minutes on Days 0 and 7 for all WBV rats used in this study [11]. Accelerations were kept at 0.5 g for each WBV group with peak-to-peak amplitudes of approximately 5 mm (8 Hz) and 1.5 mm (15 Hz).
The transmissibility and spinal deformation were measured during each WBV exposure to define the relative biomechanical severity of the exposures. Accordingly, markers were placed on the platform, the accelerometers, and on the skin at the rostral and caudal ends of the cervical spine and the thoracic spine. Transmissibility was calculated as described previously. Relative extension and compression deformations of the cervical spine were measured by digitizing the distance between the cervical markers and calculating the vector length at the maximum of extension and compression relative to the resting vector length [11]. Biomechanical metrics (transmissibility, extension, and compression) were compared between groups using separate t-tests.
To answer our second question, behavioral sensitivity, a common measurement of pain, was assessed by evaluating the bilateral forepaw withdrawal thresholds to mechanical stimulation. Testing was performed on Day 0 (baseline) immediately before WBV exposure and on Days 1, 7 (before the second WBV exposure), 8, and 14. The withdrawal threshold was defined as the lowest von Frey filament eliciting a response, confirmed by the next higher filament also provoking a response [11, 17, 81]. On each day, three rounds of assessment were performed with at least 10 minutes between rounds. For each day and rat, the average threshold was divided by its baseline response; a repeated-measures ANOVA with Tukey’s correction was used to compare groups.
Cervical DRG and spinal cord tissue (C5-C6) was harvested from a subset of rats (n = 6/group) on Day 14 after behavioral testing for immunohistochemical assessment of neurophysiological and inflammatory responses. Tissue was harvested after procedures described previously for perfusion and postfixed in PFA. Tissue was cryoprotected in 30% sucrose and then embedded in optimal cutting temperature compound. Axial sections (14 μm thick) were thaw-mounted on charged slides and dried at room temperature for 48 hours.
To answer our third question on the effect of WBV on neuronal PKCε levels in the DRG, sections were colabeled for PKCε and microtubule-associated protein-2 (MAP2). Sections were rinsed and blocked in 10% goat serum in PBS with 0.3% Triton-X100 and then incubated overnight in a rabbit primary antibody to PKCε (1:500; Santa Cruz, Dallas, TX, USA) with a mouse primary antibody to MAP2 (1:100; Covance, Conshohocken, PA, USA). Sections were washed and incubated in fluorescent goat secondary antibodies for visualization (1:1000; Life Technologies) and, after coverslipping with antifade medium and drying, were imaged at × 200 using a BX51 microscope (Olympus, Center Valley, PA, USA). Images (three to six per rat) were cropped (200 × 200 pixels) to include at least 10 nonoverlapping neurons in each section, selected spatially to include the maximum number of cell bodies within the DRG. The percent pixels positive for PKCε per MAP2-positive area was calculated [28, 47, 81]. The percent of small-diameter (4–20 µm) or medium-diameter (22–40 µm) neurons that were positive for PKCε in each group was also measured [35, 81]. Separate one-way ANOVAs with Tukey’s correction were used to compare neuronal PKCε for each analysis.
To answer our fourth and fifth questions about the effects of resonant WBV on spinal CGRP and neuroimmune cascades, spinal sections were labeled for the neuropeptide, CGRP, and the glial markers, glial fibrillary acidic protein (GFAP, astrocytic activation), and ionized calcium-binding adapter molecule 1 (Iba1, microglial/macrophage marker). Sections were labeled with rabbit primary antibodies to either CGRP (1:1000; Bachem, San Carlos, CA, USA) or Iba1 (1:1000; Wako, Osaka, Japan) or a mouse primary antibody to GFAP (1:500; Millipore, Billerica, MA, USA) and visualized with fluorescent goat secondary antibodies. Tissue from naïve rats (n = 6), not exposed to any anesthesia, also was included for normalization. Sections (three to six per rat) were imaged and cropped (750 × 200 pixels) to include only the superficial laminae of the dorsal horn. The percent pixels positive for each protein was calculated [29, 91] and separate one-way ANOVAs with Tukey’s correction were used to compare the average expression of each protein.
In separate groups (n = 4/group), as part of our fifth question about the effect of WBV on the spinal neuroimmune response, C6 spinal cord was harvested at Day 14. Tissue was flash-frozen on dry ice and stored at −70 °C until assayed. Tissue from each group was pooled to minimize variation between individual rats. Protein was extracted by homogenization in PBS with protease inhibitors. A commercial proteome profiler array (ARY008; R&D Systems, Minneapolis, MN, USA) was used to detect a panel of cytokines and chemokines, according to the manufacturer’s instructions. The integrated pixel intensity after background subtraction was quantified for each spot. Duplicate spots were averaged and expressed as fold change over sham and were compared between WBV groups using separate t-tests.
Results
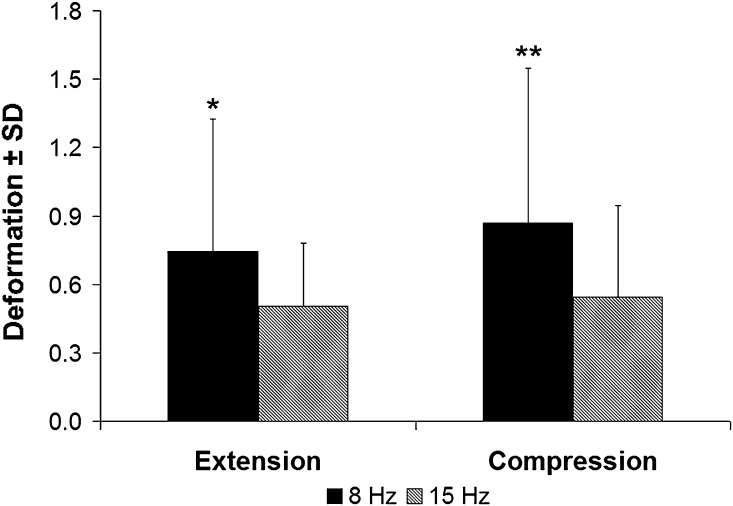
The transmissibility response in the rat peaked between 8 Hz and 10 Hz (Fig. 1). Additionally, the transmissibility profile determined by the accelerometer (Fig. 1B) was not different from that calculated using video data (Fig. 1C). Using accelerometer-based data, the peak thoracic transmissibility is at 8 Hz (1.86 ± 0.19) and 9 Hz (1.95 ± 0.19), which are not different from each other but are higher (mean difference with 95% confidence interval [CI], 0.290 ± 0.266; p < 0.03) than any other frequency. Similarly, the peaks (mean difference with 95% CI, 0.359 ± 0.284; p < 0.01) of the video-based thoracic transmissibility profile are evident at 8 Hz (1.90 ± 0.27), 9 Hz (2.07 ± 0.20), and 10 Hz (1.80 ± 0.25) with no difference among them. These same trends are evident in the physiologic study with thoracic transmissibility at 8 Hz greater than that at 15 Hz regardless of measurement approach (mean difference with 95% CI, 0.370 ± 0.141; p < 0.01) (Fig. S1 [Supplemental materials are available with the online version of CORR®.]). Moreover, WBV at 8 Hz produces more cervical extension (0.745 ± 0.582 mm; mean difference with 95% CI, 0.242 ± 0.214; p < 0.03) and compression (0.870 ± 0.676 mm, mean difference with 95% CI, 0.326 ± 0.261; p < 0.02) than 15 Hz (extension, 0.503 ± 0.279 mm; compression, 0.544 ± 0.400 mm) (Fig. 2).
Fig. 2.
The extent of deformation measured in the cervical spine is greater for an 8-Hz WBV exposure than a 15-Hz WBV exposure. The neck exhibits more cervical extension (*p < 0.03) and compression (**p < 0.02) during an 8-Hz vibration than a 15-Hz one and also exhibits greater variability in that group.
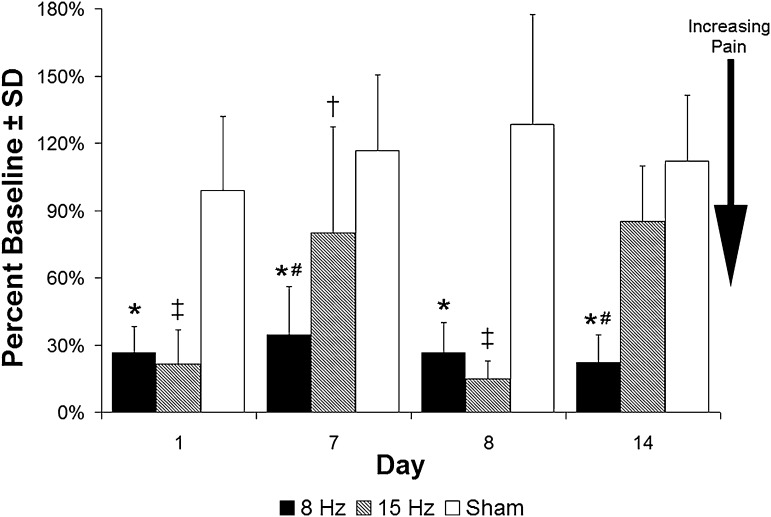
Forepaw sensitivity, a method of measuring behavioral sensitivity (and commonly taken as a measure of pain), was induced at Day 1 after each of the WBV magnitudes (p < 0.01), whereas sham anesthesia exposure did not alter responses from baseline at any day (Fig. 3). On Day 7 after the first exposure, only the 8-Hz group exhibited a reduction in withdrawal threshold from baseline and compared with the sham group (p < 0.01); the response of the 15-Hz group was not different from its corresponding baseline response. After the second WBV exposure, behavioral sensitivity was again produced in both groups on Day 8 and the withdrawal threshold was decreased (indicating increasing pain) compared with both the corresponding baseline (p < 0.01) and sham responses (p < 0.01). By Day 14, sensitivity was resolved in the 15-Hz group but remained in the 8-Hz group compared with both baseline and sham levels (p < 0.01) (Fig. 3).
Fig. 3.
Exposure to either WBV profile on Day 0 and Day 7 induces a robust decrease in the percent of baseline withdrawal thresholds, taken as increased behavioral sensitivity (ie, a pain response) in the forepaw on Days 1 and 8. On all days, the withdrawal threshold for 8 Hz is less than sham and baseline (*p < 0.01), and on Days 7 and 14, it also is lower than the withdrawal threshold for the 15-Hz WBV (#p < 0.01). In contrast, a 15-Hz WBV exposure lowers the withdrawal threshold compared with sham only on Day 7 (†p < 0.03) and lower than both baseline and sham on Days 1 and 8 (‡p < 0.01) after exposure.
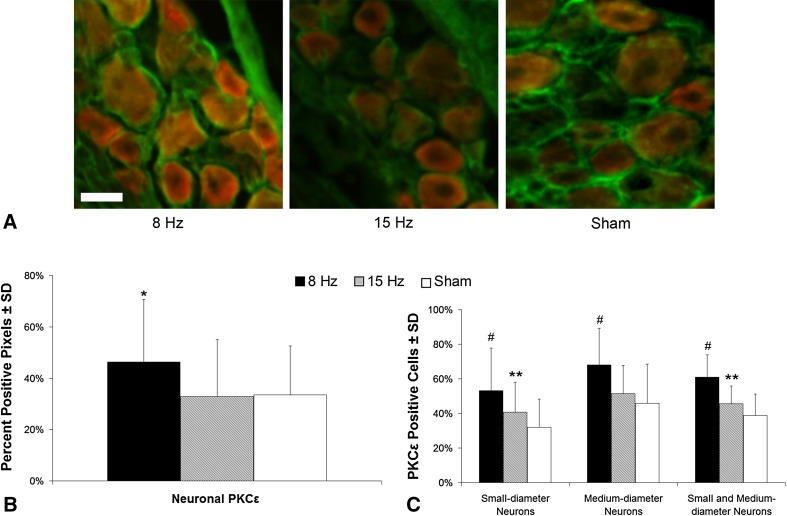
PKCε expression in the DRG at Day 14 followed the behavioral responses (Fig. 4). There was more (p < 0.01) neuronal PKCε in DRGs after 8 Hz WBV than after 15 Hz and sham exposures (Fig. 4B). There were also more small- and medium-diameter neurons that were positive for PKCε after an 8-Hz WBV than after a 15-Hz (p < 0.01) and sham (p < 0.01) exposure (Fig. 4C). There were more PKCε-positive small-diameter neurons after a 15-Hz WBV than sham (p < 0.03) (Fig. 4C).
Fig. 4A–C.
Representative images and quantification of PKCε labeling (red) in cervical DRGs colabeled with MAP2 (green), as a neuronal marker, at Day 14. PKCε is increased most robustly after WBV at the painful resonance frequency (8 Hz). Neuronal PKCε (orange) is evident in all groups (A), but more extensively after an 8-Hz vibration, and when quantified, there is more neuronal PKCε (B) evident after an 8-Hz exposure than after a 15-Hz or sham exposure (*p < 0.01). In addition, there are more small- and medium-diameter neurons that are positive for PKCε (C) in the 8-Hz group than in either the sham or 15-Hz groups (#p < 0.01). There are also more PKCε (C) positive small-diameter neurons in the 15-Hz group than the sham group (**p < 0.03). The scale bar in A is 25 µm and applies to all panels.
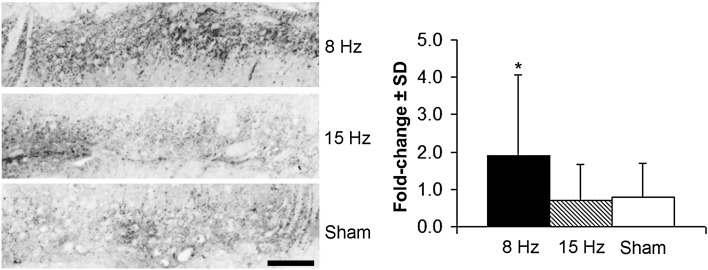
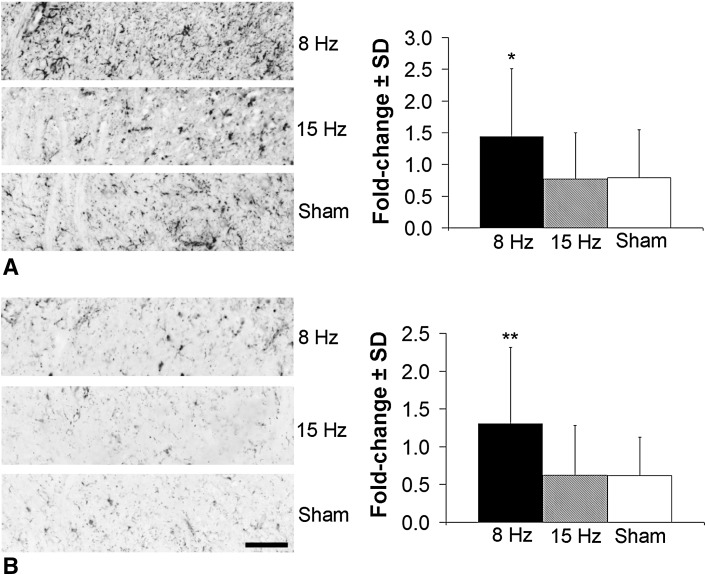
Spinally regulated mediators in the superficial dorsal horn increased only in the 8-Hz WBV group. CGRP was more than doubled after an 8-Hz WBV (p < 0.02) and unchanged from sham levels after 15-Hz WBV (Fig. 5). Similarly, both GFAP (Fig. 6A) and Iba1 (Fig. 6B) exhibited an increase (p < 0.01) only after painful WBV at resonance. WBV at 15 Hz did not alter the extent of either astrocytic or microglial activation, as measured by GFAP and Iba1, respectively, from sham levels (Fig. 6).
Fig. 5.
Representative images showing CGRP immunoreactivity in the cervical superficial dorsal horn and quantification as fold change over normal expression for each group at Day 14. There is more CGRP labeling in the 8-Hz group than in each of the 15-Hz and sham groups (*p < 0.02). The scale bar is 100 µm and applies to all panels.
Fig. 6A–B.
Glial cell activation in the superficial spinal dorsal horn at Day 14 increases only after WBV at the resonance frequency (8 Hz). Representative images show increased GFAP immunoreactivity (A), indicative of activated astrocytes, and quantification for each group expressed as fold change over normal at Day 14. There is more GFAP labeling in the 8-Hz group than in either the 15-Hz or sham groups (*p < 0.01). The same is observed with Iba1 immunoreactivity (B), which is greater in the 8-Hz group than in each of the 15-Hz and sham groups (**p < 0.01). The scale bar in (B) is 100 µm and applies to all panels in both A and B.
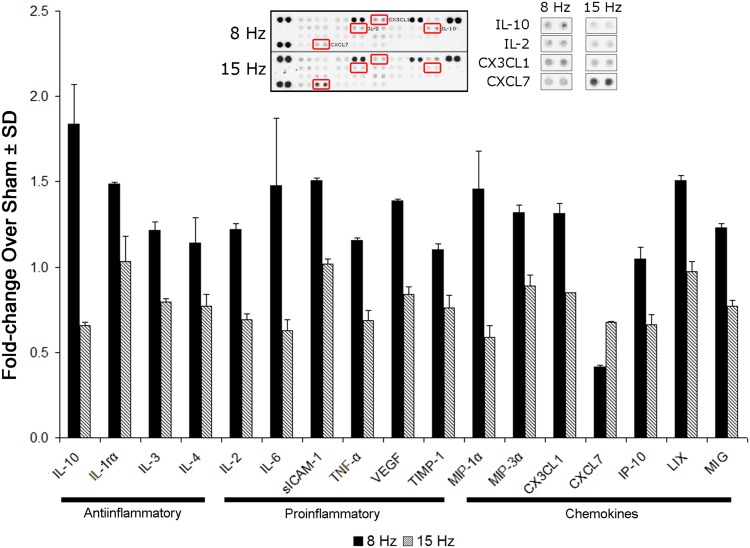
All of the cytokines/chemokines probed were detected in the spinal cord, and many were altered after 8 Hz WBV (Fig. 7). However, many were not different between the WBV groups: cytokine-induced neutrophil chemoattractant (CINC)-1, CINC-2α/β, CINC-3, ciliary neurotrophic factor (CNTF), granulocyte-macrophage colony-stimulating factor (GM-CSF), interferon-γ, interleukin (IL)-1α, IL-1β, IL-13, IL-17, L-selectin, and regulated-on-activation, normal T-cell expressed and secreted (RANTES). Most of the pro- and antiinflammatory cytokines and chemokines increased by between 1.5- to threefold after an 8-Hz WBV compared with a 15-Hz exposure (p < 0.05) (Fig. 7). CXCL7 was the only chemokine probed that decreased in response to WBV at resonance (Fig. 7).
Fig. 7.
Bar chart showing those pro- and antiinflammatory cytokines and chemokines that are altered after resonant 8 Hz WBV compared with vibration at 15 Hz (p < 0.05) (shown as fold change over sham levels). Although 10 cytokines and six chemokines are upregulated (p < 0.05), CXCL7 is lower (p < 0.01) after 8-Hz WBV related to 15-Hz WBV. sICAM-1 = soluble intercellular adhesion molecule 1; TNF-α = tumor necrosis factor alpha; VEGF = vascular endothelial growth factor; TIMP-1 = tissue inhibitors of metalloproteinases metallopeptidase inhibitor-1; MIP = macrophage inflammatory protein; IP-10 = interferon gamma-induced protein 10; LIX = lipopolysaccharide-inducible CXC chemokines; MIG = monokine induced by interferon-gamma.
Discussion
Despite having high incidence and economic burdens, and impacting the quality of life of both military and civilian populations [5–9, 24, 51, 59], vibration-induced pain is not well understood. Although epidemiological studies link WBV and pain [14, 48, 55], they do not provide mechanistic insight. Rodent models are useful to investigate the mechanisms of injury and pain [23, 39, 78, 85, 86]. Vibration of isolated limbs or the tail induces local myelin damage, endothelial cell death, growth factor upregulation, and increased vulnerability to reinjury [3, 21, 26, 49, 53]. More recently, it has been found that WBV induces widespread pain and aberrant nerve fiber growth [11, 40, 71], but the resonance condition has not been investigated. Accordingly, we defined the resonance frequency of the rat spine, because that species is used to study pain and evaluate potential therapeutics [23, 39, 60, 78, 85, 86]. We hypothesized that vibration at resonance produces more robust pain than nonresonant WBV [11, 40] and that the neuroimmune cellular and molecular pathways are more extensively activated for that WBV exposure.
Like with any animal study, ours has limitations. The resonant frequency of the spine was defined using only dead rats to enable instrumenting the spine (Fig. 1A). In both live and deceased studies, 8 Hz resulted in greater biomechanical changes (eg, transmissibility and cervical spine deformation) than 15 Hz (Figs. 1, 2, S1). Cervical transmissibility also peaks at 8–9 Hz (data not shown), supporting the larger cervical spine deformations that are observed here (Fig. 2). Because the rat’s head is free to translate within the nose cone in the test setup, possible contributions of that cannot be fully discounted. However, because those relative movements are the same in both exposure groups (data not shown), this is not likely. To apply a consistent acceleration (0.5 g) to both WBV groups, the displacement amplitude was different, yet pilot studies (unpublished) varying accelerations and displacements produce similar behavioral outcomes, supporting WBV frequency as a potent factor. Behavioral sensitivity as assessed in this study is a commonly accepted measurement of pain in both laboratory animals and humans; however, this metric does not capture spontaneous or affective components of pain. Given that approximately 85% of the active-duty military population is male [62], that sex of rats was used; because sex differences have been reported in pain and cellular cascades [73], additional studies are needed using female rats. Although Day 14 as an endpoint was selected using previous studies [11, 40], tissue responses are only a snapshot in time (Figs. 4–7). Although this study limited assessments to DRG and the spinal cord, other nonneural tissues are susceptible to WBV injury [10, 40]; regardless, the largest modifications are expected after WBV at resonance.
The peak transmissibility of the thoracic spine of the rat for spinally directed WBV is between 8 and 10 Hz with greater cervical deformations at 8 Hz than 15 Hz when acceleration is constant and displacement is varied (Figs. 1, 2, S1). This resonance is consistent in terms of order of magnitude with spinal responses of other species: human (approximately 4 Hz) [10, 64], primate (5–14 Hz) [72], and rabbit (4.5 Hz) [80]. Spinal muscle activity in the human is also maximal at resonance [10]. Although concomitant muscle activation suggests a compensatory mechanism that may stabilize the spine, it also presents a potential for local tissue injury.
WBV at the thoracic resonance induces sustained pain, but 15 Hz WBV produces only transient sensitivity (Fig. 3). This is similar to reports that repeated exposure to near-resonant frequencies causes long-lasting pain, numbness, and loss of dexterity in humans [34, 66, 83]. When WBV at 15 Hz is imposed daily for 1 week in the rat using the same mechanical conditions as the current study, the pain is similar to a single exposure at resonance [11]. The second 15-Hz WBV exposure does not exacerbate the pain response (Fig. 3), whereas a second direct compression injury to the nerve root induces more severe and longer lasting pain [38]. This difference in pain after repeated tissue insults may reflect the difference between direct injury to neural tissue and dampened effects from WBV.
Because the biomechanically more severe injury produces pain that is sustained (Fig. 3), increased PKCε in the nociceptive DRG neurons is expected (Fig. 4). The PKC pathway is important in transducing painful stimuli from those nociceptors, both activating and modulating downstream signaling [44, 77, 94]. PKCε is also upregulated in the DRG after painful joint injury, peripheral inflammation, or heat application [16, 28, 81, 93]. In fact, increased PKCε is evident days after WBV in our current study and also after isolated spinal joint injury [28, 81], suggesting ongoing pain signaling from the periphery even after trauma is removed (Figs. 3, 4). PKCε increases over sham levels in the small-diameter neurons after a 15-Hz WBV, which does not exhibit pain (Figs. 3, 4), suggesting PKCε regulation is sensitive to tissue loading magnitude in the periphery but may not be sufficient to modulate pain responses.
The sensitivity to pain and injury severity is also evident in spinal CGRP expression (Fig. 5), which also increases after a host of painful peripheral traumas and inflammatory stimuli [18, 50, 56, 69, 92]. In contrast, it decreases after a painful root compression [37, 92], most likely from axonal degeneration in the root decreasing trafficking to the dorsal horn. Because CGRP enhances PKC-mediated signaling to increase neuronal hyperexcitability [44, 58, 74] and because only peripheral PKCε is upregulated after nonpainful 15 Hz WBV, it is likely that several responses sustain pain from WBV. CGRP is also proinflammatory in the dorsal horn, recruiting immune cells [15, 69] and modulating cytokine release [69].
Spinal glia are activated only after the acceleration inducing 8 Hz in this study (Fig. 6). Also, a host of inflammatory cytokines and chemokines are altered by 8 Hz WBV compared with 15 Hz (Fig. 7). Neuroinflammation is associated with pain [22, 75, 79], and spinal glial activation occurs after a variety of painful injuries [18, 22, 25, 31, 32, 38, 60, 79, 82, 87, 88, 90]. The proinflammatory cytokines, IL-6 and tumor necrosis factor-α, nearly double after acceleration that was painful and occurred at the resonance of 8 Hz, like painful nerve injury [67, 88, 89]. However, both pro- and antiinflammatory mediators contribute to spinal hyperexcitability and pain [39, 78, 88, 89]. The antiinflammatory cytokines, IL-10 and IL-1ra, also increase only after resonant WBV (Fig. 7). CX3CL1/fractalkine increases after painful 8 Hz WBV (Fig. 7) and provides positive feedback to further stimulate glial cells, which can lead to long-term spinal inflammation and pain [22, 32, 39, 54]. These data suggest similar inflammatory cascades may be initiated after painful WBV like in other neuropathic conditions. Only the chemokine CXCL7 decreases, but little is known about its role in pain; although it has been reported to recruit leukocytes, it also has actions different from prototypical chemokines [33, 70]. Nonetheless, additional investigations are needed to understand its apparent discordant response to painful 8 Hz WBV.
To our knowledge, this is the first study defining the resonant frequency of the rat under WBV and demonstrating that condition produces greater spinal deformations, pain, and widespread cellular neuroimmune activation. Responses persist for days after a single exposure. PKCε expression in small-diameter afferents is the only mediator in this study that increases after each WBV magnitude with greater increases in the 8-Hz group. For all other neuroimmune responses, only 8 Hz WBV exhibits changes compared with control levels. Of note, PKCε is also the only peripheral mediator that was probed. There is strong evidence across a variety of pain models of different tissue injuries that spinal neuroimmune responses are correlated with pain [2, 27, 29, 60, 61, 65, 67, 81, 87–89]. Importantly, similar pain pathways are initiated by WBV, suggesting potential therapeutic targets and/or helping to identify treatments for patients with these symptoms. Our results identify potential questions leading to further investigation of the temporal and anatomical neuroimmune response to resonant WBV. That work would not only help shape potential sites and times for therapeutic interventions, but also help determine if similar effects exist for low back pain. Given the robust inflammatory response after WBV, antiinflammatory and/or nonsteroidal antiinflammatory drug treatment could prove to be potentially effective therapeutic avenues. Findings are important given that spinal pain is one of the most common causes for evacuation from military operation with 86% of those not returning to deployment [20, 57]. Although our study provides important physiologic context for exposure to vibration at resonance and there is anecdotal evidence supporting the assertion that service members are regularly exposed to spinally resonant frequencies [10], there still remains a need for field data relating exposures to pain.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Fig. S1 Transmissibility is greater in live rats undergoing resonant 8 Hz WBV compared with 15 Hz. Regardless of whether data acquisition is by accelerometer or video approaches, the thoracic transmissibility is greatest in the 8-Hz WBV group (*p < 0.01) (DOCX 37 kb)
Acknowledgments
We thank Timothy P. Holsgrove PhD, for help with statistical analysis.
Footnotes
The institution of the authors (BAW) has received funding from the Department of Defense (W81XWH-10-2-0140).
All ICMJE Conflict of Interest Forms for authors and Clinical Orthopaedics and Related Research ® editors and board members are on file with the publication and can be viewed on request.
Clinical Orthopaedics and Related Research ® neither advocates nor endorses the use of any treatment, drug, or device. Readers are encouraged to always seek additional information, including FDA-approval status, of any drug or device prior to clinical use.
Each author certifies that his or her institution approved the animal protocol for this investigation and that all investigations were conducted in conformity with ethical principles of research.
References
- 1.Aley KO, Messing RO, Mochly-Rosen D, Levine JD. Chronic hypersensitivity for inflammatory nociceptor sensitization mediated by the ε isozyme of protein kinase C. J Neurosci. 2000;20:4680–4685. doi: 10.1523/JNEUROSCI.20-12-04680.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Alkhatib B, Rosenzweig DH, Krock E, Roughley PJ, Beckman L, Steffen T, Weber MH, Ouellet JA, Haglund L. Acute mechanical injury of the human intervertebral disc: link to degeneration and pain. Eur Cell Mater. 2014;28:98–111. doi: 10.22203/ecm.v028a08. [DOI] [PubMed] [Google Scholar]
- 3.Alvarez P, Chen X, Bogen O, Green PG, Levine JD. IB4(+) nociceptors mediate persistent muscle pain induced by GDNF. J Neurophysiol. 2012;108:2545–2553. doi: 10.1152/jn.00576.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Alvarez P, Gear RW, Green PG, Levine JD. IB4-saporin attenuates acute and eliminates chronic muscle pain in the rat. Exp Neurol. 2012;233:859–865. doi: 10.1016/j.expneurol.2011.12.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Armed Forces Health Surveillance Center Absolute and relative morbidity burdens attributable to various illnesses and injuries, US Armed Forces, 2009. Medical Surveillance Monthly Report. 2010;17:16–20. [Google Scholar]
- 6.Armed Forces Health Surveillance Center Absolute and relative morbidity burdens attributable to various illnesses and injuries, US Armed Forces, 2010. Medical Surveillance Monthly Report. 2011;18:2–7. [Google Scholar]
- 7.Armed Forces Health Surveillance Center Absolute and relative morbidity burdens attributable to various illnesses and injuries, US Armed Forces, 2011. Medical Surveillance Monthly Report. 2012;19:4–9. [PubMed] [Google Scholar]
- 8.Armed Forces Health Surveillance Center Absolute and relative morbidity burdens attributable to various illnesses and injuries, US Armed Forces, 2012. Medical Surveillance Monthly Report. 2013;20:5–10. [Google Scholar]
- 9.Armed Forces Health Surveillance Center (AFHSC) Absolute and relative morbidity burdens attributable to various illnesses and injuries, US Armed Forces, 2013. Medical Surveillance Monthly Report. 2014;21:2–7. [PubMed] [Google Scholar]
- 10.Baig HA, Dorman DB, Bulka BA, Shivers BL, Chancey VC, Winkelstein BA. Characterization of the frequency and muscle responses of the lumbar and thoracic spines of seated volunteers during sinusoidal whole body vibration. J Biomech Eng. 2014;136:101002. doi: 10.1115/1.4027998. [DOI] [PubMed] [Google Scholar]
- 11.Baig HA, Guarino BB, Lipschutz D, Winkelstein BA. Whole body vibration induces forepaw and hind paw behavioral sensitivity in the rat. J Orthop Res. 2013;31:1739–1744. doi: 10.1002/jor.22432. [DOI] [PubMed] [Google Scholar]
- 12.Boileau PÉ, Rakheja S. Whole-body vertical biodynamic response characteristics of the seated vehicle driver: measurement and model development. Int J Ind Ergonom. 1998;22:449–472. doi: 10.1016/S0169-8141(97)00030-9. [DOI] [Google Scholar]
- 13.Boshuizen HC, Bongers PM, Hulshof CT. Self-reported back pain in fork-lift truck and freight-container tractor drivers exposed to whole-body vibration. Spine. 1991;17:59–65. doi: 10.1097/00007632-199201000-00010. [DOI] [PubMed] [Google Scholar]
- 14.Bovenzi M, Hulshof CTJ. An updated review of epidemiologic studies on the relationship between exposure to whole-body vibration and low back pain (1986–1997) Int Arch Occup Environ Health. 1999;76:351–365. doi: 10.1007/s004200050387. [DOI] [PubMed] [Google Scholar]
- 15.Cady RJ, Glenn JR, Smith KM, Durham PL. Calcitonin gene-related peptide promotes cellular changes in trigeminal neurons and glia implicated in peripheral and central sensitization. Mol Pain. 2011;7:94. doi: 10.1186/1744-8069-7-94. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Cesare P, Dekker LV, Sardini A, Parker PJ, McNaughton PA. Specific involvement of PKCε in sensitization of the neuronal response to painful heat. Neuron. 1999;23:617–624. doi: 10.1016/S0896-6273(00)80813-2. [DOI] [PubMed] [Google Scholar]
- 17.Chaplan SR, Bach FW, Pogrel JW, Chung JM, Yaksh TL. Quantitative assessment of tactile allodynia in the rat paw. J Neurosci Methods. 1994;53:55–63. doi: 10.1016/0165-0270(94)90144-9. [DOI] [PubMed] [Google Scholar]
- 18.Chen TC, Wu JJ, Chang WP, Hsu PN, Hsieh ST, Shyu BC. Spontaneous inflammatory pain model from a mouse line with N-ethyl-N-nitrosourea mutagenesis. J Biomed Sci. 2012;19:55. doi: 10.1186/1423-0127-19-55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Chen X, Green PG, Levine JD. Neuropathic pain-like alterations in muscle nociceptor function associated with vibration-induced muscle pain. Pain. 2010;151:460–466. doi: 10.1016/j.pain.2010.08.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Cohen SP, Brown C, Kurihara C, Plunkett A, Nguyen C, Strassels SA. Diagnoses and factors associated with medical evacuation and return to duty for service members participating in Operation Iraqi Freedom or Operation Enduring Freedom: a prospective cohort study. Lancet. 2010;375:301–309. doi: 10.1016/S0140-6736(09)61797-9. [DOI] [PubMed] [Google Scholar]
- 21.Curry BD, Bain JL, Yan JG, Zhang LL, Yamaguchi M, Matloub HS, Riley DA. Vibration injury damages arterial endothelial cells. Muscle Nerve. 2002;25:527–534. doi: 10.1002/mus.10058. [DOI] [PubMed] [Google Scholar]
- 22.DeLeo JA, Tanga FY, Tawfik VL. Neuroimmune activation and neuroinflammation in chronic pain and opioid tolerance/hyperalgesia. Neuroscientist. 2004;10:40–52. doi: 10.1177/1073858403259950. [DOI] [PubMed] [Google Scholar]
- 23.DeLeo JA, Winkelstein BA. Physiology of chronic spinal pain syndromes: from animal models to biomechanics. Spine. 2002;27:2526–2537. doi: 10.1097/00007632-200211150-00026. [DOI] [PubMed] [Google Scholar]
- 24.De Oliveira CG, Nadal J. Transmissibility of helicopter vibration in the spines of pilots in flight. Aviat Space Environ Med. 2005;76:576–580. [PubMed] [Google Scholar]
- 25.Dina OA, Chen X, Reichling D, Levine JD. Role of protein kinase Cε and protein kinase A in a model of paclitaxel-induced painful peripheral neuropathy in the rat. Neuroscience. 2001;108:507–515. doi: 10.1016/S0306-4522(01)00425-0. [DOI] [PubMed] [Google Scholar]
- 26.Dina OA, Joseph EK, Levine JD, Green PG. Mechanisms mediating vibration-induced chronic musculoskeletal pain analyzed in the rat. J Pain. 2010;11:369–377. doi: 10.1016/j.jpain.2009.08.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Dong L, Crosby ND, Winkelstein BA. Gabapentin alleviates facet-mediated pain in the rat through reduced neuronal hyperexcitability and astrocytic activation in the spinal cord. J Pain. 2013;14:1564–1572. doi: 10.1016/j.jpain.2013.07.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Dong L, Quindlen JC, Lipschutz DE, Winkelstein BA. Whiplash-like facet joint loading initiates glutamatergic responses in the DRG and spinal cord associated with behavioral hypersensitivity. Brain Res. 2012;1461:51–63. doi: 10.1016/j.brainres.2012.04.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Dong L, Smith JR, Winkelstein BA. Ketorolac reduces spinal astrocytic activation and PAR1 expression associated with attenuation of pain after facet joint injury. J Neurotrauma. 2013;30:818–825. doi: 10.1089/neu.2012.2600. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Fritton JC, Rubin CT, Qin YX, McLeod KJ. Whole-body vibration in the skeleton: development of a resonance-based testing device. Ann Biomed Eng. 1997;25:831–839. doi: 10.1007/BF02684167. [DOI] [PubMed] [Google Scholar]
- 31.Fujimoto Y, Yamasaki T, Tanaka N, Mochizuki Y, Kajihara H, Ikuta Y, Ochi M. Differential activation of astrocytes and microglia after spinal cord injury in the fetal rat. Eur Spine J. 2006;15:223–233. doi: 10.1007/s00586-005-0933-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Gao YJ, Ji RR. Chemokines, neuronal-glial interactions, and central processing of neuropathic pain. Pharmacol Ther. 2010;126:56–58. doi: 10.1016/j.pharmthera.2010.01.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Ghasemzadeh M, Kaplan ZS, Alwis I, Schoenwaelder SM, Ashworth KJ, Westein E, Hosseini E, Salem HH, Slattery R, McColl SR, Hickey MJ, Ruggeri ZM, Yuan Y, Jackson SP. The CXCR1/2 ligand NAP-2 promotes directed intravascular leukocyte migration through platelet thrombi. Blood. 2013;121:4555–4566. doi: 10.1182/blood-2012-09-459636. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Goldsmith PC, Molina FA, Bunker CB, Terenghi G, Leslie TA, Fowler CJ, Polak JM, Dowd PM. Cutaneous nerve fibre depletion in vibration white finger. J M Soc Med. 1994;87:377–381. doi: 10.1177/014107689408700703. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Harper AA, Lawson SN. Conduction velocity is related to morphological cell type in rat dorsal root ganglion neurons. J Physiol. 1985;359:31–46. doi: 10.1113/jphysiol.1985.sp015573. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Hill TE, Desmoulin GT, Hunter CJ. Is vibration truly an injurious stimulus in the human spine? J Biomech. 2009;42:2631–2635. doi: 10.1016/j.jbiomech.2009.10.001. [DOI] [PubMed] [Google Scholar]
- 37.Hubbard RD, Chen Z, Winkelstein BA. Transient cervical nerve root compression modulates pain: load thresholds for allodynia and sustained changes in spinal neuropeptide expression. J Biomech. 2008;41:677–685. doi: 10.1016/j.jbiomech.2007.09.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Hunt JL, Winkelstein BA, Rutkowski MD, Weinstein JN, DeLeo JA. Repeated injury to the lumbar nerve roots produces enhanced mechanical allodynia and persistent spinal neuroinflammation. Spine. 2001;26:2073–2079. doi: 10.1097/00007632-200110010-00005. [DOI] [PubMed] [Google Scholar]
- 39.Ji RR, Xu ZZ, Gao YJ. Emerging targets in neuroinflammation-driven chronic pain. Nat Rev Drug Discov. 2014;13:533–548. doi: 10.1038/nrd4334. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Kartha S, Zeeman ME, Baig HA, Guarino BB, Winkelstein BA. Upregulation of BDNF and NGF in cervical intervertebral discs exposed to painful whole-body vibration. Spine. 2014;39:1542–1548. doi: 10.1097/BRS.0000000000000457. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Khasar SG, Lin YH, Martin A, Dadgar J, McMahon T, Wang D, Hundle B, Aley KO, Isenberg W, McCarter G, Green PG, Hodge CW, Levine JD, Messing RO. A novel nociceptor signaling pathway revealed in protein kinase Cε mutant mice. Neuron. 1999;24:253–260. doi: 10.1016/S0896-6273(00)80837-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Kiiski J, Heinonen A, Järvinen TL, Kannus P, Sievänen H. Transmission of vertical whole body vibration to the human body. J Bone Miner Res. 2008;23:1318–1325. doi: 10.1359/jbmr.080315. [DOI] [PubMed] [Google Scholar]
- 43.Kim HK, Schattschneider J, Lee I, Chung K, Baron R, Chung JM. Prolonged maintenance of capsaicin-induced hyperalgesia by brief daily vibration stimuli. Pain. 2007;129:93–101. doi: 10.1016/j.pain.2006.09.036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Kim MJ, Lee SY, Yang KY, Nam SH, Kim HJ, Kim YJ, Bae YC, Ahn DK. Differential regulation of peripheral IL-1β-induced mechanical allodynia and thermal hyperalgesia in rats. Pain. 2014;155:723–732. doi: 10.1016/j.pain.2013.12.030. [DOI] [PubMed] [Google Scholar]
- 45.Kitazaki S, Griffin MJ. A modal analysis of whole-body vertical vibration, using a finite element model of the human body. J Sound Vib. 1997;200:83–103. doi: 10.1006/jsvi.1996.0674. [DOI] [Google Scholar]
- 46.Kras JV, Dong L, Winkelstein BA. Increased interleukin-1α and prostaglandin E2 expression in the spinal cord at 1 day after painful facet joint injury: evidence of early spinal inflammation. Spine. 2014;39:207–212. doi: 10.1097/BRS.0000000000000107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Kras JV, Weisshaar CL, Quindlen J, Winkelstein BA. Brain-derived neurotrophic factor is upregulated in the cervical dorsal root ganglia and spinal cord and contributes to the maintenance of pain from facet joint injury in the rat. J Neurosci Res. 2013;91:1312–1321. doi: 10.1002/jnr.23254. [DOI] [PubMed] [Google Scholar]
- 48.Kumar A, Varghese M, Mohan D, Mahajan P, Gulati P, Kale S. Effect of whole-body vibration on the low back. A study of tractor-driving farmers in north India. Spine. 1999;24:2506–2515. doi: 10.1097/00007632-199912010-00013. [DOI] [PubMed] [Google Scholar]
- 49.Loffredo MA, Yan JG, Kao D, Zhang LL, Matloub HS, Riley DA. Persistent reduction of conduction velocity and myelinated axon damage in vibrated rat tail nerves. Muscle Nerve. 2009;39:770–775. doi: 10.1002/mus.21235. [DOI] [PubMed] [Google Scholar]
- 50.Malon JT, Maddula S, Bell H, Cao L. Involvement of calcitonin gene-related peptide and CCL2 production in CD40-mediated behavioral hypersensitivity in a model of neuropathic pain. Neuron Glia Biol. 2011;7:117–128. doi: 10.1017/S1740925X12000026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Manchikanti L, Singh V, Datta S, Cohen SP, Hirsch JA. Comprehensive review of epidemiology, scope, and impact of spinal pain. Pain Physician. 2009;12:E35–E70. [PubMed] [Google Scholar]
- 52.Mandapuram S, Rakheja S, Boileau PÉ, Maeda S, Shibata N. Apparent mass and seat-to-head transmissibility responses of seated occupants under single and dual axis horizontal vibration. Ind Health. 2010;48:698–714. doi: 10.2486/indhealth.MSWBVI-15. [DOI] [PubMed] [Google Scholar]
- 53.Matloub HS, Yan JG, Kolachalam RB, Zhang LL, Sanger JR, Riley DA. Neuropathological changes in vibration injury: an experimental study. Microsurgery. 2005;25:71–75. doi: 10.1002/micr.20081. [DOI] [PubMed] [Google Scholar]
- 54.Milligan ED, Zapata V, Chacur M, Schoeniger D, Biedenkapp J, O’Connor KA, Verge GM, Chapman G, Green P, Foster AC, Naeve GS, Maier SF, Watkins LR. Evidence that exogenous and endogenous fractalkine can induce spinal nociceptive facilitation in rats. Eur J Neurosci. 2004;20:2294–2302. doi: 10.1111/j.1460-9568.2004.03709.x. [DOI] [PubMed] [Google Scholar]
- 55.Milosavljevic S, Bagheri N, Vasiljev RM, McBride DI, Rehn B. Does daily exposure to whole-body vibration and mechanical shock relate to the prevalence of low back and neck pain in a rural workforce? Ann Occup Hyg. 2011;56:10–17. doi: 10.1093/annhyg/mer068. [DOI] [PubMed] [Google Scholar]
- 56.Morton CR, Hutchison WD. Release of sensory neuropeptides in the spinal cord: studies with calcitonin gene-related peptide and galanin. Neuroscience. 1989;31:807–815. doi: 10.1016/0306-4522(89)90443-0. [DOI] [PubMed] [Google Scholar]
- 57.Mydlarz D. Degenerative disc disease, active component, US Armed Forces, 2001–2011. Medical Surveillance Monthly Report. 2012;19:6–9. [PubMed] [Google Scholar]
- 58.Neugebauer V, Rümenapp P, Schaible HG. Calcitonin gene-related peptide is involved in the spinal processing of mechanosensory input from the rat’s knee joint and in the generation and maintenance of hyperexcitability of dorsal horn-neurons during development of acute inflammation. Neuroscience. 1996;71:1095–1109. doi: 10.1016/0306-4522(95)00473-4. [DOI] [PubMed] [Google Scholar]
- 59.Nevin RL, Means GE. Pain and discomfort in deployed helicopter aviators wearing body armor. Aviat Space Environ Med. 2009;80:807–810. doi: 10.3357/ASEM.2236.2009. [DOI] [PubMed] [Google Scholar]
- 60.Nicholson KJ, Gilliland TM, Winkelstein BA. Upregulation of GLT-1 by treatment with ceftriaxone alleviates radicular pain by reducing spinal astrocyte activation and neuronal hyperexcitability. J Neurosci Res. 2014;92:116–129. doi: 10.1002/jnr.23295. [DOI] [PubMed] [Google Scholar]
- 61.Nomoto J, Kuroki T, Nemoto M, Kondo K, Harada N, Nagao T. Effects of edaravone on a rat model of punch-drunk syndrome. Neurol Med Chir. 2011;51:1–7. doi: 10.2176/nmc.51.1. [DOI] [PubMed] [Google Scholar]
- 62.Office of the Deputy Assistant Secretary of Defense for Military Community & Family Policy. Characteristics of the total Military force. 2013 Demographics—Profile of the Military Community. 2013:6–8.
- 63.Panjabi MM, Andersson GB, Jorneus L, Hult E, Mattsson L. In vivo measurements of spinal column vibrations. J Bone Joint Surg Am. 1986;68:695–702. [PubMed] [Google Scholar]
- 64.Priller J, Haas CA, Reddington M, Kreutzberg GW. Calcitonin gene-related peptide and ATP induce immediate early gene expression in cultured rat microglial cells. Glia. 1995;15:447–457. doi: 10.1002/glia.440150408. [DOI] [PubMed] [Google Scholar]
- 65.Risbud MV, Shapiro IM. Role of cytokines in intervertebral disc degeneration: pain and disc content. Nat Rev Rheumatol. 2014;10:44–56. doi: 10.1038/nrrheum.2013.160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Rolke R, Rolke S, Vogt T, Birklein F, Geber C, Treede RD, Letzel S, Voelter-Mahlknecht S. Hand-arm vibration syndrome: clinical characteristics, conventional electrophysiology and quantitative sensory testing. Clin Neurophysiol. 2013;124:1680–1688. doi: 10.1016/j.clinph.2013.01.025. [DOI] [PubMed] [Google Scholar]
- 67.Rothman SM, Huang Z, Lee KE, Weisshaar CL, Winkelstein BA. Cytokine mRNA expression in painful radiculopathy. J Pain. 2009;10:90–99. doi: 10.1016/j.jpain.2008.07.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Rubin C, Pope M, Fritton JC, Magnusson M, Hansson T, McLeod K. Transmissibility of 15-Hertz to 35-Hertz vibrations to the human hip and lumbar spine: determining the physiologic feasibility of delivering low-level anabolic mechanical stimuli to skeletal regions at greatest risk of fracture because of osteoporosis. Spine. 2003;28:2621–2627. doi: 10.1097/01.BRS.0000102682.61791.C9. [DOI] [PubMed] [Google Scholar]
- 69.Russell FA, King R, Smillie S-J, Kodji X, Brain SD. Calcitonin gene-related peptide: physiology and pathophysiology. Physiol Rev. 2014;94:1099–1142. doi: 10.1152/physrev.00034.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Russo RC, Garcia CC, Teixeira MM, Amaral FA. The CXCL8/IL-8 chemokine family and its receptors in inflammatory diseases. Expert Rev Clin Immunol. 2014;10:593–619. doi: 10.1586/1744666X.2014.894886. [DOI] [PubMed] [Google Scholar]
- 71.Sandhu E, Miles JD, Dahners LE, Keller BV, Weinhold PS. Whole body vibration increases area and stiffness of the flexor carpi ulnaris tendon in the rat. J Biomech. 2011;44:1189–1191. doi: 10.1016/j.jbiomech.2011.02.017. [DOI] [PubMed] [Google Scholar]
- 72.Smith SD, Kazarian LE. The effects of acceleration on the mechanical impedance response of a primate model exposed to sinusoidal vibration. Ann Biomed Eng. 1994;22:78–87. doi: 10.1007/BF02368224. [DOI] [PubMed] [Google Scholar]
- 73.Sorge RE, Martin LJ, Isbester KA, Sotocinal SG, Rosen S, Tuttle AH, Wieskopf JS, Acland EL, Dokova A, Kadoura B, Leger P, Mapplebeck JC, McPhail M, Delaney A, Wigerblad G, Schumann AP, Quinn T, Frasnelli J, Svensson CI, Sternberg WF, Mogil JS. Olfactory exposure to males, including men, causes stress and related analgesia in rodents. Nat Methods. 2014;11:629–632. doi: 10.1038/nmeth.2935. [DOI] [PubMed] [Google Scholar]
- 74.Sun RQ, Tu YJ, Lawand NB, Yan JY, Lin Q, Willis WD. Calcitonin gene-related peptide receptor activation produces PKA- and PKC-dependent mechanical hyperalgesia and central sensitization. J Neurophysiol. 2004;92:2859–2866. doi: 10.1152/jn.00339.2004. [DOI] [PubMed] [Google Scholar]
- 75.Taub DD, Oppenheim JJ. Chemokines, inflammation and the immune system. Ther Immunol. 1994;1:229–246. [PubMed] [Google Scholar]
- 76.Todd AJ. Neuronal circuitry for pain processing in the dorsal horn. Nat Rev Neurosci. 2010;11:823–836. doi: 10.1038/nrn2947. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Velazquez KT, Mohammad H, Sweitzer SM. Protein kinase C in pain: involvement of multiple isoforms. Pharmacol Res. 1997;55:578–589. doi: 10.1016/j.phrs.2007.04.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Verri WA, Jr, Cunha TM, Parada CA, Poole S, Cunha FQ, Ferreira SH. Hypernociceptive role of cytokines and chemokines: targets for analgesic drug development? Pharmacol Ther. 2006;112:116–138. doi: 10.1016/j.pharmthera.2006.04.001. [DOI] [PubMed] [Google Scholar]
- 79.Watkins LR, Milligan ED, Maier SF. Glial activation: a driving force for pathological pain. Trends Neurosci. 2001;24:450–455. doi: 10.1016/S0166-2236(00)01854-3. [DOI] [PubMed] [Google Scholar]
- 80.Weinstein J, Pope M, Schmidt R, Seroussi R. Neuropharmacologic effects of vibration on the dorsal root ganglion, an animal model. Spine. 1987;13:521–525. doi: 10.1097/00007632-198805000-00015. [DOI] [PubMed] [Google Scholar]
- 81.Weisshaar CL, Dong L, Bowman AS, Perez FM, Guarino BB, Sweitzer SM, Winkelstein BA. Metabotropic glutamate receptor-5 and protein kinase C-epsilon increase in dorsal root ganglion neurons and spinal glial activation in an adolescent rat model of painful neck injury. J Neurotrauma. 2010;27:2261–2267. doi: 10.1089/neu.2010.1460. [DOI] [PubMed] [Google Scholar]
- 82.Weisshaar CL, Winkelstein BA. Ablating spinal NK1-bearing neurons eliminates the development of pain and reduces spinal neuronal hyperexcitability and inflammation from mechanical joint injury in the rat. J Pain. 2014;15:378–386. doi: 10.1016/j.jpain.2013.12.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Whitham EM, Griffin MJ. The effects of vibration frequency and direction on the location of areas of discomfort caused by whole-body vibration. Appl Ergon. 1978;9:231–239. doi: 10.1016/0003-6870(78)90084-4. [DOI] [PubMed] [Google Scholar]
- 84.Wilder DG, Pope MH. Epidemiological and aetiological aspects of low back pain in vibration environments—an update. Clin Biomech. 1996;11:61–73. doi: 10.1016/0268-0033(95)00039-9. [DOI] [PubMed] [Google Scholar]
- 85.Winkelstein BA. Mechanisms of central sensitization, neuroimmunology & injury biomechanics in persistent pain: implications for musculoskeletal disorders. J Electromyogr Kinesiol. 2004;14:87–93. doi: 10.1016/j.jelekin.2003.09.017. [DOI] [PubMed] [Google Scholar]
- 86.Winkelstein BA. How can animal models inform on the transition to chronic symptoms in whiplash? Spine. 2011;36:S218–S225. doi: 10.1097/BRS.0b013e3182387f96. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Winkelstein BA, DeLeo JA. Nerve root injury severity differentially modulates spinal glial activation in a rat lumbar radiculopathy model: considerations for persistent pain. Brain Res. 2002;956:294–301. doi: 10.1016/S0006-8993(02)03560-6. [DOI] [PubMed] [Google Scholar]
- 88.Winkelstein BA, Rutkowski MD, Sweitzer SM, Pahl JL, DeLeo JA. Nerve injury proximal or distal to the DRG induces similar spinal glial activation and selective cytokine expression but differential behavioral responses to pharmacologic treatment. J Comp Neurol. 2001;439:127–139. doi: 10.1002/cne.2000. [DOI] [PubMed] [Google Scholar]
- 89.Winkelstein BA, Rutkowski MD, Weinstein JN, DeLeo JA. Quantification of neural tissue injury in a rat radiculopathy model: comparison of local deformation, behavioral outcomes, and spinal cytokine mRNA for two surgeons. J Neurosci Methods. 2001;111:49–57. doi: 10.1016/S0165-0270(01)00445-9. [DOI] [PubMed] [Google Scholar]
- 90.Winkelstein BA, Santos DG. An intact facet capsular ligament modulates behavioral sensitivity and spinal glial activation produced by cervical facet joint tension. Spine. 2008;33:856–862. doi: 10.1097/BRS.0b013e31816b4710. [DOI] [PubMed] [Google Scholar]
- 91.Zhang S, Nicholson KJ, Smith JR, Gilliland TM, Syré PP, Winkelstein BA. The roles of mechanical compression and chemical irritation in regulating spinal neuronal signaling in painful cervical nerve root injury. Stapp Car Crash J. 2013;57:219–242. doi: 10.4271/2013-22-0009. [DOI] [PubMed] [Google Scholar]
- 92.Zheng LF, Wang R, Xu YZ, Yi XN, Zhang JW, Zeng ZC. Calcitonin gene-related peptide dynamics in rat dorsal root ganglia and spinal cord following different sciatic nerve injuries. Brain Res. 2008;1187:20–32. doi: 10.1016/j.brainres.2007.10.044. [DOI] [PubMed] [Google Scholar]
- 93.Zhou Y, Li GD, Zhao ZQ. State-dependent phosphorylation of ε-isozyme of protein kinase C in adult rat dorsal root ganglia after inflammation and nerve injury. J Neurochem. 2003;85:571–580. doi: 10.1046/j.1471-4159.2003.01675.x. [DOI] [PubMed] [Google Scholar]
- 94.Zhou Y, Zhou ZS, Zhao ZQ. PKC regulates capsaicin-induced currents of dorsal root ganglion neurons in rats. Neuropharmacology. 2001;41:601–608. doi: 10.1016/S0028-3908(01)00106-X. [DOI] [PubMed] [Google Scholar]
- 95.Zimmermann M. Ethical guidelines for investigations of experimental pain in conscious animals. Pain. 1983;16:109–110. doi: 10.1016/0304-3959(83)90201-4. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Fig. S1 Transmissibility is greater in live rats undergoing resonant 8 Hz WBV compared with 15 Hz. Regardless of whether data acquisition is by accelerometer or video approaches, the thoracic transmissibility is greatest in the 8-Hz WBV group (*p < 0.01) (DOCX 37 kb)