Abstract
Background
Heterotopic ossification (HO) affects the majority of combat-related lower extremity wounds involving severe fracture and amputation. Defining the timing of early osteogenic-related genes may help identify candidate prophylactic agents and guide the timing of prophylactic therapy after blast and other combat-related extremity injuries.
Questions/purposes
Using a recently developed animal model of combat-related HO, we sought to determine (1) the timing of early chondrogenesis, cartilage formation, and radiographic ectopic bone development; and (2) the early cartilage and bone-related gene and protein patterns in traumatized soft tissue.
Methods
We used an established rat HO model consisting of blast exposure, controlled femur fracture, crush injury, and transfemoral amputation through the zone of injury. Postoperatively, rats were euthanized on Days 3 to 28. We assessed evidence of early ectopic bone formation by micro-CT and histology and performed proteomic and gene expression analysis.
Results
All rats showed radiographic evidence of HO within 28 days. Key chondrogenic (collagen type I alpha 1 [COL1α1], p = 0.016) and osteogenic-related genes (Runt-related transcription factor 2 [RUNX-2], p = 0.029; osteoclacin [OCN], p = 0.032; phosphate-regulating neutral endopeptidase, X-linked [PHEX], p = 0.0290, and POU domain class 5 transcription factor [POU5F], p = 0.016) and proteins (Noggin [NOG], p = 0.04, OCN, p = 0.02, RUNX- 2, p = 0.04, and substance P-1 [SP-1], p = 0.01) in the injured soft tissue, normalized to the contralateral limb and/or sham-treated naïve rats, increased on Days 3 to 14 postinjury. By 14 days, foci of hypertrophic chondrocytes, hyaline cartilage, and woven bone were present in the soft tissue surrounding the amputation site.
Conclusions
We found that genes that regulate early chondrogenic and osteogenic signaling and bone development (COL1α1, RUNX-2, OCN, PHEX, and POU5F1) are induced early during the tissue reparative/healing phase in a rat model simulating a combat-related extremity injury.
Clinical Relevance
The ability to correlate molecular events with histologic and morphologic changes will assist researchers and clinicians to understand HO and hence formulate therapeutic interventions.
Electronic supplementary material
The online version of this article (doi:10.1007/s11999-015-4240-y) contains supplementary material, which is available to authorized users.
Introduction
Heterotopic ossification (HO) refers to the abnormal development of bone in nonosseous tissue, most commonly occurring in the setting of orthopaedic trauma, severe burns, neurotrauma, or major surgery [2, 7, 10, 13]. The prevalence of HO in the residual limbs of returning service members with combat-related amputations is reported to be as high 65% [11, 22]. At least 41% of those patients who develop HO require additional excision procedures. Moreover, delayed healing and wound dehiscence are major problems in severely injured patients recovering from survivable severe battlefield blast-related extremity injuries [12]. Studies from our group have established that acute wound failures and subsequent HO formation are related to multiple complex interrelated systemic and local inflammatory responses to traumatic injury [8, 12]. The optimal treatment strategy for HO has not been defined. Surgical excision is the only definitive management option and treatment of symptomatic HO if physical therapy and prosthesis alteration fail to provide adequate relief [31]. Other prophylaxis strategies include treatment with nonsteroidal antiinflammatory drugs or external-beam radiotherapy, but these options are more confined in the civilian setting and are generally contraindicated in the setting of combat and blast-induced trauma given nonsteroidal anti-inflammatory drugs may delay fracture healing and cause unacceptably high rates of bleeding complications while radiotherapy must be administered within 48 hours of injury (difficult if not impossible in the combat setting) and is known to cause wound- and implant-related complications [11, 22, 23].
A comprehensive assessment of the early histologic and molecular development processes present within blast wounds has not occurred for several reasons. First, clinically relevant animal models have only recently been developed [21, 30]. Second, clinical diagnostic methods are unable to accurately predict the site of HO development [5, 20]. Third, analysis of human tissues obtained early in the débridement process has not been performed. Finally, available human clinical samples, usually collected at the time HO excision, typically contain immature and mature bone. Defining the early development phase of HO in relationship to concurrent wound healing is critical to selection of candidate means of prophylaxis and, importantly, the timing of their administration after high-energy extremity injuries.
We developed a rat model of combat-related HO that incorporates the critical elements associated with combat injury, specifically a blast injury, femur fracture-crush, and transfemoral amputation, through the zone of injury wherein all animals develop radiographic evidence of HO within 2 months postinjury [21]. In this report, we use our model to address (1) the timing of early chondrogenesis, cartilage formation, and radiographic ectopic bone development; and (2) the early cartilage and bone-related gene and protein patterns in traumatized soft tissue subsequent to calcium deposition, tissue mineralization, and ectopic bone formation. It is important to confirm the timing and upregulation of bone-related genes and proteins in our model because some observational clinical studies with soft tissue injury (without fracture or amputation) have shown elevated levels of such genes in a minority of cases [6, 9, 12].
Materials and Methods
Animals
Fifty-four young adult pathogen-free male Sprague-Dawley rats (Rattus norvegicus; 400–500 g) were purchased from Taconic Farms (Germantown, NY, USA). All animals were housed in clean plastic cages and kept on a 12-hour light/dark cycle with unlimited access to food (standard chow) and fresh water ad libitum. The study protocol (12-OUMD-20s) was reviewed and approved by the Walter Reed Army Institute of Research/Naval Medical Research Center Institutional Animal Care and Use Committee in compliance with all applicable Federal regulations governing the protection of animals in research. Postoperatively, rats were monitored at a minimum twice daily for animal activity, signs of pain, wound dehiscence, weight loss, and infection by animal care staff, research staff, and veterinarians. Moribund rats were euthanized.
Rat Heterotopic Ossification Model
Rats were anesthetized with isoflurane (2%–5%) and received full-body blast overpressure (120 ± 7 kPa) exposure, without any shielding to the blast wave, through a pneumatically driven shock tube [1, 4, 29]. Preoperative buprenorphine (0.05 mg/kg) was then administered through intraperitoneal injection and a reproducible comminuted fracture of the right femur was performed using a drop weight apparatus from a height of 88 cm. A crush injury was performed immediately after the fracture by rotating the fracture site between the two support anvils, generating 20 pounds per square inch of pressure for 1 minute [3]. The injured limb was then amputated through the fracture with appropriate hemostasis and débridement of devitalized tissue followed by hamstring and quadriceps myoplasty over the exposed residual femur. Postoperative pain was managed with sustained-release buprenorphine (1.2 mg/kg) administered subcutaneously with repeat dosing after 3 days. Cohorts of four to eight rats per time point were euthanized on postinjury Days 3, 5, 7, 10, 14, 21, and 28 to detect and visualize histologically the early stages of the HO disease process, whereas a cohort of four sham-treated (neither blasted nor injured) naïve rats euthanized on Day 3 served as controls. Two rats were euthanized postoperatively early for self-mutilation of the amputation site and one for consumption and subsequent suffocation as a result of inhalation of the bedding. A surgical team consisting of an orthopaedic surgeon (GJP, EKC or DNH), postdoctorate fellow (ATQ), and two surgical technicians (AMT, DMG) experienced in small animal anesthesia and surgeries conducted the experiments and immediate postoperative care procedures.
Micro-CT for Detection of Ectopic Bone Formation
Postoperatively rats were anesthetized with isoflurane (2%–5%) and the injured leg was imaged using a SkyScan in vivo 1176 high-resolution micro-CT (lCT) x-ray imaging in three dimensions (Bruker-MicroCT, Kontich, Belgium) with the following settings: 89-kV polychromatic x-ray beam, current of 256 μA, and an exposure time of 81 milliseconds for each of 180 rotational steps. The three-dimensional (3-D) images were rendered to reconstruct tomograms with a commercial package (NRecon; Bruker-MicroCT).
Tissue Collection for Chondrogenic, Osteogenic and Angiogenic Gene Expression and Protein Analysis
After euthanasia, skeletal muscle was aseptically collected from the distal quadriceps of the amputated limb as well as from the distal quadriceps muscle of the contralateral limb. Samples were immediately placed in either RNAlater™ (Ambion Inc, Austin, TX, USA) at 4 °C for 48 hours or snap-frozen in dry ice for gene expression and protein analysis, respectively.
Histological Analysis
The residual injured and contralateral femurs with attached associated muscle tissue were surgically removed and placed in 10% formalin. The femurs were decalcified in 5% formic acid, embedded in paraffin, longitudinal sectioned (5 μm), and stained with hematoxylin-eosin (Histoserv, Inc, Germantown, MD, USA). Qualitative observations of wound healing and early ectopic endochondroal bone development (mesenchymal condensation, chondrocyte differentiation, chondrogenesis, hypertrophic vascularized cartilage, hyaline cartilage development, extracellular maturation, and soft tissue mineralization) were performed by a pathologist (CLH) who was blinded to the study.
RNA Isolation and Gene Expression
Total RNA was isolated from skeletal muscle samples as previously described [9]. A custom-made low-density reverse transcription-polymerase chain reaction (RT-PCR) array consisting of 96 primer sets (including respective forward and reverse primers) for 83 rat-specific osteogenic, chondrogenic, adipogenic, and angiogenic genes as well as six housekeeping and seven quality control genes (SABiosciences, Gaithersburg, MD, USA) was used to assess gene expression (genes and their function listed in Supplemental Table 1 [Supplemental materials are available with the online version of CORR®.]). Quantitative RT-PCR and dissociation curve analyses were performed as previously described [9]. Cycle threshold (Ct) measurements per samples were normalized using GAPDH. Relative expression between sham-treated naïve rats and the injured limb muscle samples was determined using the comparative Ct method (2−ΔΔCt) [17]. In comparison to sham-treated naïve rats, genes that were deferentially expressed at least threefold were depicted using a heat map. Assays with Ct values greater than 35 cycles were considered not expressed and are not reported.
Quantification of Protein Expression
A sample (detailed subsequently) of the differentially expressed genes involved in extracellular matrix remodeling, cartilage deposition and vasculogenesis, and mineralization-ossification were verified by enzyme-linked immunosorbent assay (ELISA) [24]. Protein from skeletal muscle samples (30–32 mg) of the injured and contralateral limbs of rats euthanized 3, 5, and 7 days postinjury was isolated using the Total Protein Extraction Kit (EMD Millipore, Billerica, MA, USA) and total concentrations were determined using the BCA Protein Assay Kit (Pierce, Rockford, IL, USA). Quantification of protein levels of rat substance P (SP-1), Neurokinin A, and calcitonin gene-related peptide (CGRP) were assayed by enzyme immunoassay (Phoenix Pharmaceuticals, Inc, Burlingame, CA, USA). In addition, levels of noggin (NOG), osteocalcin (OCN), runt-related transcription factor 2 (RUNX-2), and bone morphogenetic protein 2 (BMP-2) were assayed by ELISA (MyBioSource, San Diego, CA, USA). For each analyte, samples were equally loaded based on the total protein concentration assayed in duplicate. Absolute tissue sample concentrations of each analyte were calculated from a standard curve of known standards and corrected for protein concentration.
Statistical Analysis
Continuous variables (protein expression) were evaluated with Student’s t-test provided the data were normally distributed, whereas noncontinuous data (gene expression) were analyzed with the Mann-Whitney U test. Two-tailed α < 0.05 was considered statistically significant. All data are presented as means ± SD unless otherwise specified.
Results
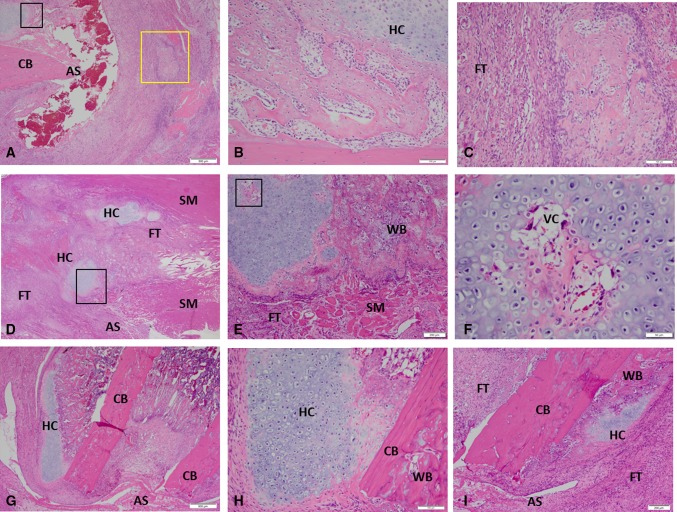
In this physiologic model of combat-related HO, blast exposure in the presence of severe extremity trauma produced μCT radiographic evidence of HO within the soft tissue surrounding the fracture/amputation site in 100% of the animals within 28 days (Fig. 1). We observed no radiographic evidence of neurogenic HO development (around joints and/or in the soft tissue distant from the fracture/amputation site) in our model or in blast only-treated rats. Foci of proliferative/hypertrophic chondrocytes were observed in tissue surrounding the amputation site (Fig. 2A–F) as early as 5 days and certainly by postoperative Day 10. By Day 14, endochondral ossification was evident because the ectopic chondrocyte-rich basophilic hyaline cartilage was replaced with acidophilic bone matrix (osteoid) later followed by the immature woven bone typical of HO arising from the process of endochondral ossification (Fig. 2G–I). None of the contralateral limbs from blast-injured rats or limbs from sham-treated rats developed radiographic or histologic evidence of HO.
Fig. 1A–D.
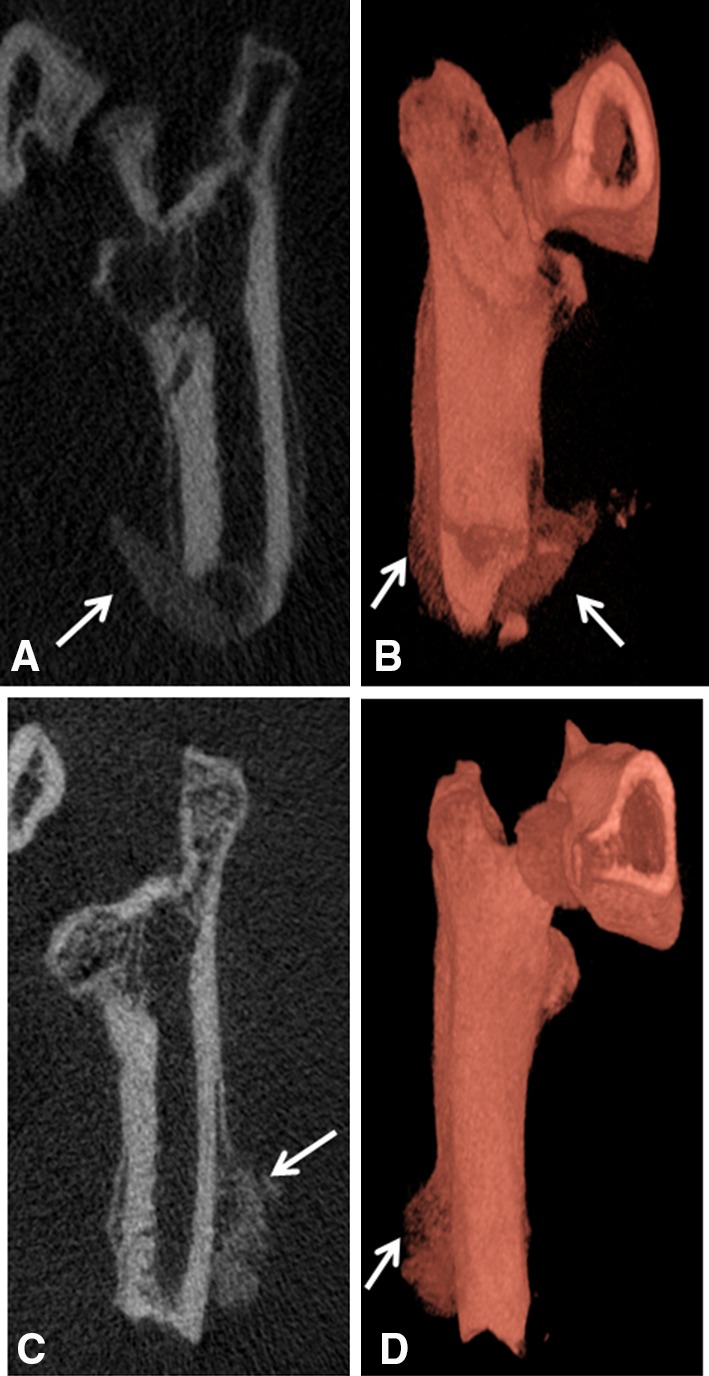
Representative μCT and 3-D reconstructed images of rats euthanized at postinjury Day 21 (A–B) and Day 28 (C–D) are shown. The arrows indicate the formation of ectopic bone.
Fig. 2A–I.
These images show the histological evidence of early HO formation at the site of amputation postinjury Day 7 (A–C), Day 10 (D–F), and Day 14 (G–I) (Stain, hematoxylin and eosin). For detailed evaluation, higher magnification images of five selected regions are shown. (B–C) High-magnification views of the areas are outlined by the black inset box and yellow inset box in A, respectively. (E, F, H) High-magnification views show the areas outlined the black inset box in D, E, and G, respectively. Foci of hyaline and vascularized cartilage with woven bone are observed in the soft tissue surrounding the site of amputation at postinjury Days 10 to 14. AS = amputation site; CB = cortical bone; FT = fibroblastic tissue; HC = hyaline cartilage; SM = skeletal muscle; VC = vascularized cartilage; WB = woven bone.
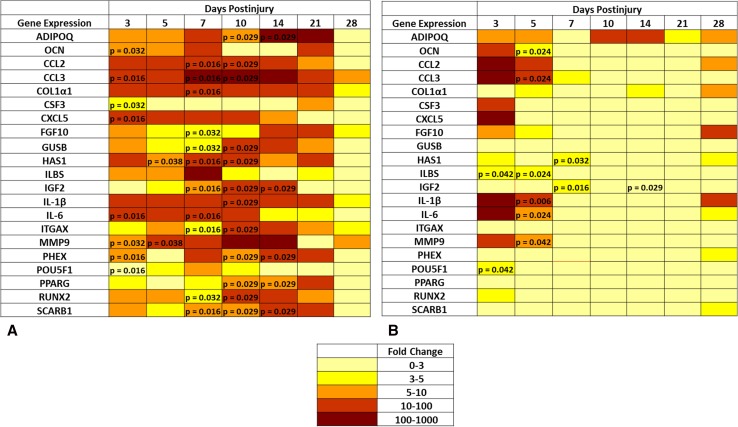
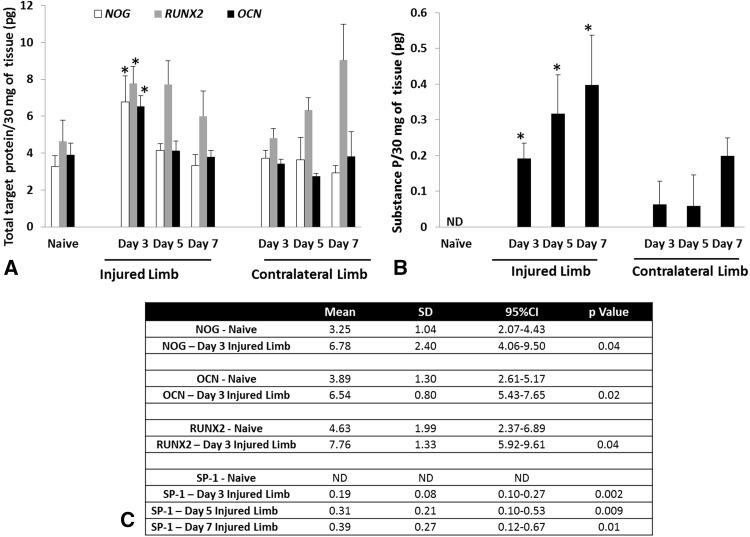
Genes involved in chondrogenesis (COL1α1), osteogenesis (RUNX-2, OCN, PHEX, and POU5F1), wound healing/tissue repair (MMP9, CSF3, FGF-10, and HAS1), and adipogenesis (ADIPOQ and PPARG) were notably overexpressed (greater than threefold) at the amputation site, whereas all angiogenic targets were unchanged (less than threefold) in comparison to quadriceps muscle tissue collected from the contralateral limb and sham-treated naïve rats (Fig. 3). The in vivo tissue production of key osteogenic proteins NOG (6.78 ± 1.38 ng; 95% confidence interval [CI], 4.06–9.50; p = 0.04), OCN (6.54 ± 0.56 ng; 95% CI, 5.43–7.65; p = 0.02), and RUNX-2 (7.76 ± 0.94 ng; 95% CI, 5.92–9.61; p = 0.04) were elevated at 3 days postinjury relative to normal muscle tissue collected from sham-treated 5.92–9.61 naïve controls (Fig. 4A). In addition, we observed that the amount of the peripherally released neurotransmitter SP-1 was higher in the injured limb at 3 to 7 days postinjury (0.2 ± 0.04 ng; 95% CI, 0.10–0.27; 0.32 ± 0.10 ng; 95% CI, 0.10–0.53; and 0.4 ± 0.13 ng; 95% CI, 0.12–0.67; per 30 mg of tissue) when compared with sham-treated naïve control muscle (p = 0.002, p = 0.009, and p = 0.01, respectively) and muscle collected from the contralateral leg, which was subjected only to blast-related trauma (Fig. 4B). There were no differences observed in concentrations of neurokinin A, CGRP, or BMP-2 (data not shown).
Fig. 3A–B.
A transcript heat map depicting the expression level of the subset of the 83 rat chondrogenic, osteogenic, and angiogenicrelated gene targets whose differential expression was greater than threefold compared with the expression level in shamtreated control muscle. (A) Injured leg and (B) contralateral leg and significantly different (p < 0.05; Mann-Whitney U test) compared with sham-treated rats are noted with p values.
Fig. 4A–C.
The amount of NOG, RUNX-2 and OCN (A) and SP-1 (B) protein was quantified from tissue samples harvest at the site of amputation. (C) The levels of NOG, OCN, and RUNX-2 are statistically significant from the sham-treated (naïve) rats at 3 days postinjury, whereas the levels of SP-1 are significantly different from the sham-treated (naïve) rats at 3 to 7 days postinjury (*p < 0.05; Student’s t-test).
Discussion
High-energy blast exposure to the extremities results in extensive soft tissue, muscle, vascular, nerve, and bone destruction often resulting in limb amputations, which collectively pose formidable surgical, postoperative treatment, and rehabilitation challenges. The prevalence of ectopic bone development in the residual limbs of patients with combat-related amputations is reported to be as high 65% [22]. Advances in combat casualty care-related wound healing are limited by an incomplete understanding of fundamental cellular and molecular mechanisms driving normal healing processes versus the dysregulated wound healing responses. In this study, we described the histology and local microenvironment during the early differentiation phase of stem cells/progenitor cells in a rat model of combat-related HO that integrates some of the key injury patterns seen in our blast-wounded service members. Using our established and highly reproducible animal model of trauma-induced HO, we provide definitive evidence that the pathophysiological process of ectopic bone formation is through endochondral ossification, replacement of cartilage by bone. Results from these studies that recapitulate the clinical disease process will be most useful in advancing our understanding of early underlying molecular signaling pathways and cell development stages involved in formation of extraskeletal bone formation and facilitate the identification of targeted novel therapeutic strategies.
Several limitations to our study are noteworthy. The primary limitation is that the work was only conducted on one animal species. It is possible that a large animal model (swine or nonhuman primate), similar in size and physiology to humans, may more closely mimic the trauma-induced local and systemic responses exhibited by combat casualties. In addition, larger animal models may allow for the incorporation of other postoperative surgical and treatment variables such as serial débridement procedures and negative pressure wound therapy, which is difficult to use in a small animal model. Second, factors that influence the development of HO may prove to be the actual biological mechanism accounting for the extent and severity of injury. Unlike on the battlefield, the multifaceted injury patterns, limb amputation, and surgical repair/wound closure in this model were all made in sequence and within hours of injury rather than days to weeks postinjury after serial débridement procedures, as seen in the clinical setting. Third, we assessed the combinatorial effects of the critical elements associated with combat injury, which results in 100% radiographic evidence of ectopic bone formation. In regard to early histological changes and gene expression signaling, it may be worthwhile to evaluate the importance of each injury pattern alone and in various combinations. Lastly, we evaluated the time course of gene expression for a small subset of genes at given times wherein regulation may have occurred earlier than 3 days postinjury and/or expression of some genes may be highly temporal in regulation during early ectopic bone development. Furthermore, it is likely that the same mediators that promote normal wound healing also support ectopic bone development: however, we believe the nature and severity of the injury involving blast exposure in conjunction with a heightened and prolonged local and systemic immune response plays a major role in ectopic bone formation/wound dehiscence versus normal healing in combat-wounded patients.
Micro-CT scans showed an increase in ectopic bone development at 3 to 4 weeks within the soft tissue surrounding the site of amputation. These findings are consistent with those of radiographic studies detecting early evidence of soft tissue mineralization and bone deposition in combat-wounded patients [23]. At Day 7 to 10 postinjury, abundant foci of hypertrophic chondrocytes and vascularized hyaline cartilage were present. By Days 10 to 14, the soft tissue contained abundant hyaline cartilage with hypertrophic chondrocytes with evidence of subsequent mineralization and osteoprogenitor cell migration as denoted by zones of primary woven bone (immature endochondral bone formation. By Day 28, qualitative analysis of ectopic bone formation using 3-D high-resolution μCT showed a peak in the size of radiographically detectable bone in the soft tissue, which was supported by clear histological evidence of woven bone. This is similar to the results observed in another animal model of posttraumatic HO [30].
Gene expression during endochondral bone formation is regulated by a series of chondrogenic and osteogenic inductive cell signaling, proliferation, and differentiation events at the undifferentiated mesenchymal stem/progenitor cell stage involving extracellular matrix remodeling, cartilage deposition and vasculogenesis, mineralization ossification, and subsequent replacement with bone [15, 24]. A cohort of gene transcripts and key osteogenic-related proteins (RUNX-2, OCN, NOG, SP-1) were identified that correlate to the early histological response and development of ectopic bone. RUNX-2 is the early master regulator of osteogenic/osteoblast differentiation [16], and OCN is a prime marker of bone development produced by osteoblasts [18]. SP-1 is a neuroinflammatory peptide that has been reported to promote the mobilization, proliferation, and osteogenic differentiation of mesenchymal stem cells at sensory nerve structures [25, 26]. We showed here that the expression of SP-1 is induced systemically in our trauma-induced model, providing a connection between blast injury and increased formation of ectopic bone. Consistent with previous studies examining endochondral bone development and early ossification, we observed that HO is coupled with an early increase in the expression of transcripts necessary for synthesis of a cartilaginous matrix (COL1α1), bone and osteoblast mineralization (RUNX-2, OCN, PHEX, and POU5F1), tissue remodeling (MMP9, CSF3, FGF-10, and HAS1), and inflammatory cytokines (IL-6, IL1β, CCL2, CCL3, and CXCL5) within the first 14 days postinjury [14].
Recently, Peterson et al. [19] demonstrated in a murine Achilles tenotomy plus partial-thickness dorsum burn injury model that injured mice develop endochondral ectopic bone and functional joint contractures through BMP-mediated canonical small “mothers against” decapentaplegic (SMAD) signaling. Moreover, they report that these orthopaedic disease processes can be attenuated/modulated by targeting adenosine triphosphate (ATP) hydrolysis and SMAD1/5/8 phosphorylation at the burn site using apyrase [19]. Interestingly, it has been reported that focused extracorporeal shockwave therapy (ESWT; low-density shockwaves administered orders of magnitude below blast overpressure conditions used in the study) has been shown to induce osteogenic differentiation of marrow-derived mesenchymal stem cells through ATP release and downstream transcriptional signaling events resulting in activation of P2X7 receptors [28]. Consistent with the finding of Peterson et al., removal of ATP using apyrase inhibited ESWT-induced osteogenic differentiation. ESWT has been used in treatment of bone and soft tissue disorders and shown to stimulate soft tissue expression of osteogenic factors (BMPs, OC, OPN, TGFβ1) but also angiogenic factors (VEGF, FGF) [27, 32]. Therefore, it is not surprising that many of the genes induced in the musculature of the injured limb show parallel, albeit reduced, levels of change in the contralateral limb.
In this study, we defined the histologic time course and pertinent molecular signaling patterns in the early stages of HO development using a rat model of combat-related HO that incorporates the critical elements associated with combat injury. Based on these findings, we propose that the initiation of prophylactic therapy targeted at inhibiting the synthesis of ectopic cartilage should start soon after injury in the rat to avoid any adverse effects on physiologic early wound healing processes such as tissue revascularization and granulation tissue development. The ability to correlate molecular events with histologic and morphologic changes will help researchers and clinicians to understand the HO process. In addition, ascertaining how applicable the findings are to the wound healing process in humans will be important in formulating therapeutic interventions that target early chondrogenic, angiogenic, and osteogenic signaling components of ectopic bone development.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Acknowledgments
We thank Dana M. Golden and Allison M. Tomasino for assistance with animal care, sample collections, and technical expertise; Dr Matthew Wagner for his statistical analysis; and LTC Cary L. Hannold for his detailed histological review of specimens.
Footnotes
This work was supported by CDMRP (W81XWH-14-2-0010; PI-JAF) and BUMED (602115HP.3720.001.A1014; PI-JAF).
Some of the authors are employees of the US Government. This work was prepared as part of their official duties. Title 17 U.S.C. §105 provides that “Copyright protection under this title is not available for any work of the United States Government.” Title 17 U.S.C. §101 defined a US Government work as a work prepared by a military service member or employees of the US Government as part of that person’s official duties. The opinions or assertions contained in this paper are the private views of the authors and are not to be construed as reflecting the views, policy or positions of the Department of the Navy, Department of Defense nor the US Government.
All ICMJE Conflict of Interest Forms for authors and Clinical Orthopaedics and Related Research ® editors and board members are on file with the publication and can be viewed on request.
Clinical Orthopaedics and Related Research ® neither advocates nor endorses the use of any treatment, drug, or device. Readers are encouraged to always seek additional information, including FDA-approval status, of any drug or device prior to clinical use.
Each author certifies that his or her institution approved the animal protocol for this investigation and that all investigations were conducted in conformity with ethical principles of research.
This work performed at the Naval Medical Research Center, Silver Spring, MD, USA.
References
- 1.Ahlers ST, Vasserman-Stokes E, Shaughness MC, Hall AA, Shear DA, Chavko M, McCarron RM, Stone JR. Assessment of the effects of acute and repeated exposure to blast overpressure in rodents: toward a greater understanding of blast and the potential ramifications for injury in humans exposed to blast. Front Neurol. 2012;3:32. doi: 10.3389/fneur.2012.00032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Ahrengart L. Periarticular heterotopic ossification after total hip arthroplasty: risk factors and consequences. Clin Orthop Relat Res. 1991;263:49–58. [PubMed] [Google Scholar]
- 3.Bonnarens F, Einhorn TA. Production of a standard closed fracture in laboratory animal bone. J Orthop Res. 1984;2:97–101. doi: 10.1002/jor.1100020115. [DOI] [PubMed] [Google Scholar]
- 4.Chavko M, Koller WA, Prusaczyk WK, McCarron RM. Measurement of blast wave by a miniature fiber optic pressure transducer in the rat brain. J Neurosci Methods. 2007;159:277–281. doi: 10.1016/j.jneumeth.2006.07.018. [DOI] [PubMed] [Google Scholar]
- 5.Crane NJ, Polfer E, Elster EA, Potter BK, Forsberg JA. Raman spectroscopic analysis of combat-related heterotopic ossification development. Bone. 2013;57:335–342. doi: 10.1016/j.bone.2013.08.026. [DOI] [PubMed] [Google Scholar]
- 6.Davis TA, O’Brien FP, Anam K, Grijalva S, Potter BK, Elster EA. Heterotopic ossification in complex orthopaedic combat wounds: quantification and characterization of osteogenic precursor cell activity in traumatized muscle. J Bone Joint Surg Am. 2011;93:1122–1131. doi: 10.2106/JBJS.J.01417. [DOI] [PubMed] [Google Scholar]
- 7.Evans EB. Heterotopic bone formation in thermal burns. Clin Orthop Relat Res. 1991;263:94–101. [PubMed] [Google Scholar]
- 8.Evans KN, Forsberg JA, Potter BK, Hawksworth JS, Brown TS, Andersen R, Dunne JR, Tadaki D, Elster EA. Inflammatory cytokine and chemokine expression is associated with heterotopic ossification in high-energy penetrating war injuries. J Orthop Trauma. 2012;26:e204–e213. doi: 10.1097/BOT.0b013e31825d60a5. [DOI] [PubMed] [Google Scholar]
- 9.Evans KN, Potter BK, Brown TS, Davis TA, Elster EA, Forsberg JA. Osteogenic gene expression correlates with development of heterotopic ossification in war wounds. Clin Orthop Relat Res. 2014;472:396–404. doi: 10.1007/s11999-013-3325-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Forsberg JA, Davis TA, Elster EA, Gimble JM. Burned to the bone. Sci Transl Med. 2014;6:255fs237. [DOI] [PubMed]
- 11.Forsberg JA, Pepek JM, Wagner S, Wilson K, Flint J, Andersen RC, Tadaki D, Gage FA, Stojadinovic A, Elster EA. Heterotopic ossification in high-energy wartime extremity injuries: prevalence and risk factors. J Bone Joint Surg Am. 2009;91:1084–1091. doi: 10.2106/JBJS.H.00792. [DOI] [PubMed] [Google Scholar]
- 12.Forsberg JA, Potter BK, Polfer EM, Safford SD, Elster EA. Do inflammatory markers portend heterotopic ossification and wound failure in combat wounds? Clin Orthop Relat Res. 2014;472:2845–2854. doi: 10.1007/s11999-014-3694-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Garland DE. A clinical perspective on common forms of acquired heterotopic ossification. Clin Orthop Relat Res. 1991;263:13–29. [PubMed] [Google Scholar]
- 14.James CG, Stanton L-A, Agoston H, Ulici V, Underhill TM, Beier F. Genome-wide analyses of gene expression during mouse endochondral ossification. PLoS One. 2010;5:e8693. doi: 10.1371/journal.pone.0008693. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Kaplan FS, Glaser DL, Hebela N, Shore EM. Heterotopic ossification. J Am Acad Orthop Surg. 2004;12:116–125. doi: 10.5435/00124635-200403000-00007. [DOI] [PubMed] [Google Scholar]
- 16.Kirkham G, Cartmell S. Genes and Proteins Involved in the Regulation of Osteogenesis. London, UK: Hindawi Publishers; 2007. [Google Scholar]
- 17.Livak KJ, Schmittgen TD. Analysis of relative gene expression data using real-time quantitative PCR and the 2(-Delta Delta C(T)) method. Methods. 2001;25:402–408. doi: 10.1006/meth.2001.1262. [DOI] [PubMed] [Google Scholar]
- 18.Nakamura A, Dohi Y, Akahane M, Ohgushi H, Nakajima H, Funaoka H, Takakura Y. Osteocalcin secretion as an early marker of in vitro osteogenic differentiation of rat mesenchymal stem cells. Tissue Eng Part C Methods. 2009;15:169–180. doi: 10.1089/ten.tec.2007.0334. [DOI] [PubMed] [Google Scholar]
- 19.Peterson JR, De La Rosa S, Eboda O, Cilwa KE, Agarwal S, Buchman SR, Cederna PS, Xi C, Morris MD, Herndon DN. Treatment of heterotopic ossification through remote ATP hydrolysis. Sci Transl Med. 2014;6:255ra132. [DOI] [PMC free article] [PubMed]
- 20.Peterson JR, Okagbare PI, De La Rosa S, Cilwa KE, Perosky JE, Eboda ON, Donneys A, Su GL, Buchman SR, Cederna PS, Wang SC, Kozloff KM, Morris MD, Levi B. Early detection of burn induced heterotopic ossification using transcutaneous Raman spectroscopy. Bone. 2013;54:28–34. doi: 10.1016/j.bone.2013.01.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Polfer EM, Hope DH, Elster EA, Qureshi AT, Golden DM, Potter BK, Davis TA, Forsberg JA. Development of a rat model for blast-related post-traumatic heterotopic ossification. Bone Joint J. 2015;97. [DOI] [PubMed]
- 22.Potter BK, Burns TC, Lacap AP, Granville RR, Gajewski DA. Heterotopic ossification following traumatic and combat-related amputations. Prevalence, risk factors, and preliminary results of excision. J Bone Joint Surg Am. 2007;89:476–486. doi: 10.2106/JBJS.F.00412. [DOI] [PubMed] [Google Scholar]
- 23.Potter MBK, Forsberg LJA, Davis TA, Evans CKN, Hawksworth MJS, Tadaki D, Brown TS, Crane NJ, Burns MTC, O’Brien CFP. Heterotopic ossification following combat-related trauma. J Bone Joint Surg Am. 2010;92:74–89. doi: 10.2106/JBJS.J.00776. [DOI] [PubMed] [Google Scholar]
- 24.Provot S, Schipani E. Molecular mechanisms of endochondral bone development. Biochem Biophys Res Commun. 2005;328:658–665. doi: 10.1016/j.bbrc.2004.11.068. [DOI] [PubMed] [Google Scholar]
- 25.Salisbury E, Rodenberg E, Sonnet C, Hipp J, Gannon FH, Vadakkan TJ, Dickinson ME, Olmsted-Davis EA, Davis AR. Sensory nerve induced inflammation contributes to heterotopic ossification. J Cell Biochem. 2011;112:2748–2758. doi: 10.1002/jcb.23225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Salisbury E, Sonnet C, Heggeness M, Davis AR, Olmsted-Davis E. Heterotopic ossification has some nerve. Crit Rev Eukaryot Gene Expr. 2010;20:313–324. doi: 10.1615/CritRevEukarGeneExpr.v20.i4.30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Stojadinovic A, Elster EA, Anam K, Tadaki D, Amare M, Zins S, Davis TA. Angiogenic response to extracorporeal shock wave treatment in murine skin isografts. Angiogenesis. 2008;11:369–380. doi: 10.1007/s10456-008-9120-6. [DOI] [PubMed] [Google Scholar]
- 28.Sun D, Junger WG, Yuan C, Zhang W, Bao Y, Qin D, Wang C, Tan L, Qi B, Zhu D. Shockwaves induce osteogenic differentiation of human mesenchymal stem cells through ATP release and activation of P2X7 receptors. Stem Cells. 2013;31:1170–1180. doi: 10.1002/stem.1356. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Svetlov SI, Prima V, Glushakova O, Svetlov A, Kirk DR, Gutierrez H, Serebruany VL, Curley KC, Wang KK, Hayes RL. Neuro-glial and systemic mechanisms of pathological responses in rat models of primary blast overpressure compared to ‘composite’ blast. Front Neurol. 2012;3:15. doi: 10.3389/fneur.2012.00015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Tannous O, Griffith C, O’Toole RV, Pellegrini VD., Jr Heterotopic ossification after extremity blast amputation in a Sprague-Dawley rat animal model. J Orthop Trauma. 2011;25:506–510. doi: 10.1097/BOT.0b013e31821f6265. [DOI] [PubMed] [Google Scholar]
- 31.Tintle LSM, Baechler LMF, Nanos CGP, Forsberg LJA, Potter MBK. Reoperations following combat-related upper-extremity amputations. J Bone Joint Surg Am. 2012;94:e119.111–116. [DOI] [PubMed]
- 32.Wang C-J. Extracorporeal shockwave therapy in musculoskeletal disorders. J Orthop Surg Res. 2012;7:1–8. doi: 10.1186/1749-799X-7-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.