Abstract
Background
A giant cell tumor of bone is a primary benign but locally aggressive neoplasm. Malignant transformation in a histologically typical giant cell tumor of bone, without radiotherapy exposure, is an uncommon event, occurring in less than 1% of giant cell tumors of bone. Although surgery is the standard initial treatment, denosumab, a monoclonal antibody drug that inhibits receptor activator of nuclear factor-κB ligand (RANKL), has shown considerable activity regarding disease and control of symptoms in patients with recurrence, unresectable, and metastatic giant cell tumors of bone.
Case Description
We report the case of a 20-year-old woman with a recurrent benign, giant cell tumor of bone, who had a bone sarcoma develop while receiving denosumab treatment.
Literature Review
To our knowledge, there have been no reports of infection or malignancy with low-dose denosumab administration for osteoporosis. However, while there are relatively few reported side effects, the safety of denosumab and adverse events seen with higher doses, as used in treatment of giant cell tumors of bone are not well defined.
Clinical Relevance
Denosumab has become a valuable adjunct for treatment of recurrent or unresectable giant cell tumor of bone. It is not clear if our patient’s malignant transformation of a giant cell tumor of bone while receiving denosumab treatment was caused by denosumab, but it is important to be aware of the possibility if more cases occur. Future studies should focus on the safety of high-dose denosumab administration in patients with a benign unresectable giant cell tumor of bone.
Introduction
A giant cell tumor of bone is a primary benign but locally aggressive neoplasm [14]. The tumor has characteristic large multinucleated osteoclast-like giant cells expressing receptor activator of nuclear factor-κB (RANK) and mesenchymal spindle-like stromal cells expressing RANK ligand (RANKL); this cell interaction leads to bone resorption [17, 23]. Although surgery is the standard primary treatment, denosumab, a monoclonal antibody drug that inhibits RANKL, has shown considerable activity regarding disease and symptoms in cases of recurrent and metastatic giant cell tumor of bone [4].
It has been well recognized that malignant transformation of giant cell tumor of bone may occur. However with an incidence ranging from 1.4% to 6.6%, most cases follow radiation therapy or multiple local recurrences [1, 3, 9, 12, 18]. In histologically typical giant cell tumor of bone, without former radiotherapy, sarcomatous change has been reported in less than 1% of patients [24].
We describe the case of a patient with a benign recurrent giant cell tumor of bone who had a secondary malignant giant cell tumor of bone develop during treatment with denosumab.
Case Report
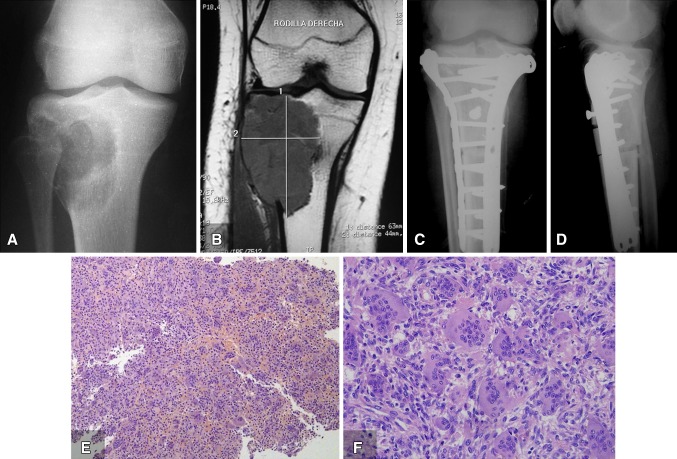
A 15-year-old female presented to another institution with right knee pain in July 2009. Radiographs (Fig. 1A), MRI (Fig. 1B), and CT were performed. After CT, a guided biopsy showed a benign giant cell tumor of bone. Intralesional resection and reconstruction were performed at another institution in September 2009 (Fig. 1C, D). Evaluation of the entire specimen from the curettage confirmed the histologic diagnosis giant cell tumor of bone (Fig. 1E, F).
Fig. 1A–F.
The preoperative (A) AP radiograph and (B) T1-weighted MR image show a lytic mass. Rodilla derecha = right knee. The patient underwent tumor resection and allograft reconstruction at another center, as shown in (C) AP and (D) lateral radiographs. The surgical resection specimen from September 2009 shows a population of mononuclear plump stromal cells with round, ovoid, or spindle nuclei and evenly distributed multinucleated giant cells. (E) The low-power (Stain, hematoxylin & eosin; original magnification, ×10) and (F) high-power views (Stain, hematoxylin & eosin; original magnification, ×40) show sheets of mononuclear cells interspersed with multinucleated giant cells.
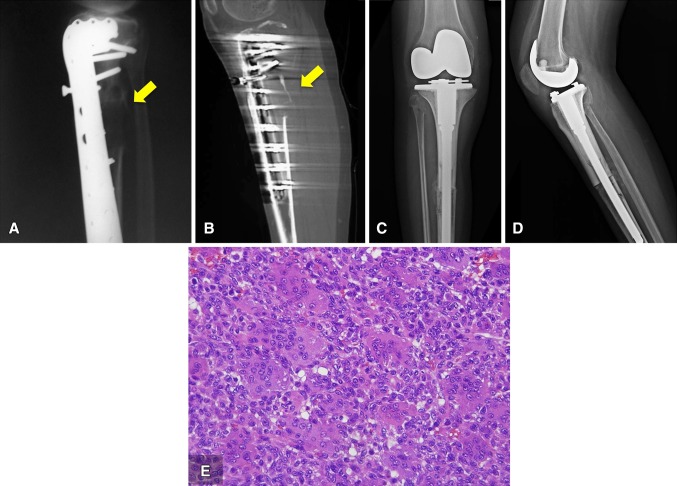
One year after the first procedure, the patient presented at our center with right knee pain. Radiographic and CT studies revealed an osteolytic lesion that destroyed the posterior cortex of the allograft and the tibia (Fig. 2A, B). A CT-guided biopsy showed recurrent giant cell tumor of bone; therefore, a proximal tibal en bloc resection was performed in August 2010 (Fig. 2C, D). The histologic features of the specimen were consistent with a benign giant cell tumor of bone (Fig. 2E). The patient’s postoperative course was uneventful until January 2013, when a followup radiograph and CT showed a new local soft tissue recurrence in the popliteal fossae (Fig. 3A). An intralesional resection was performed in February 2013. The histologic features of the recurrence corresponded to a benign giant cell tumor of bone, just as had the previous specimens (Fig. 3B).
Fig. 2A–E.
The (A) lateral radiograph and (B) CT scan show the osteolytic lesion destroyed the posterior cortex (yellow arrow) in the union of the allograft and the native tibia. (C) AP and (D) lateral view radiographs show en bloc resection and reconstruction with an alloprothesis, performed in August 2010. (E) The high-power view (Stain, hematoxylin & eosin; original magnification, ×40) of the surgical resection specimen shows the two typical cell populations; the nuclei of the mononuclear cells are similar to the nuclei of the giant cells.
Fig. 3A–B.
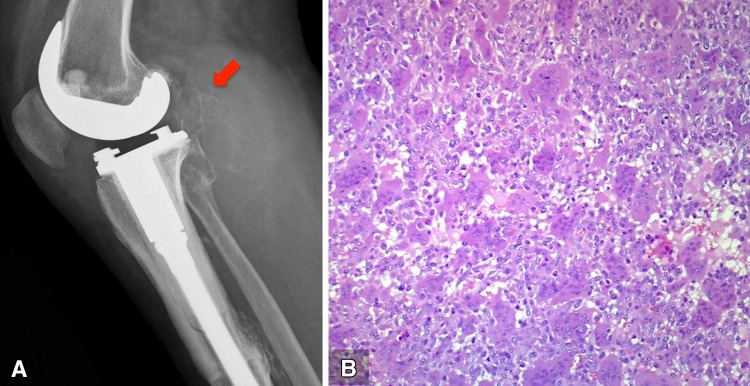
(A) The lateral radiograph obtained in January 2013 shows a lytic mass in the popliteal fossae (red arrow). An intralesional resection was performed in February 2013. (B) The surgical specimen shows diffuse proliferation of mononuclear stromal cells and osteoclast-like nultinuclear giant cells, corresponding to typical findings of a giant cell tumor of bone (Stain, hematoxylin &eosin; original magnification, ×40).
Owing to the local aggressiveness of the tumor and the belief that a radical resection was not feasible, in May 2013 we decided to initiate treatment with denosumab as an adjuvant to avoid future recurrences. The administration began with a 360 mg subcutaneous dose, then 120 mg subcutaneously monthly (every 28 days) according to current FDA-approved protocol [22]. By October 2013, we observed clearly demarcated osteosclerosis of the recurrent mass located in the popliteal region (Fig. 4). Pain around the patient's right knee diminished but another tumor resection was recommended as it recently was reported that although denosumab almost completely eliminates the expression of RANKL and histologic evaluation shows the absence of giant cells, the neoplastic stromal cells component may persist and regrow [10].
Fig. 4.
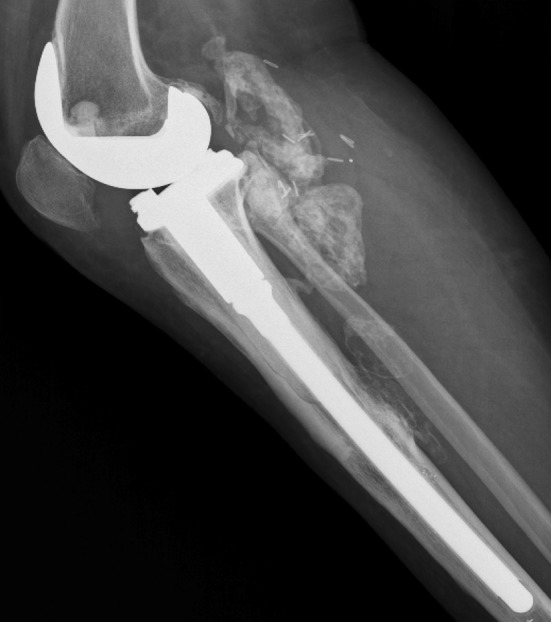
After 5 months of denosumab treatment, a clear demarked sclerotic formation appeared in October 2013.
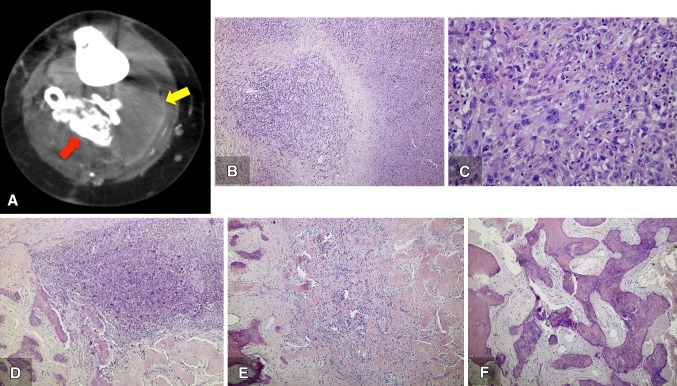
Because of the patient’s clinical improvement, however, she refused surgical resection and continued with denosumab treatment. The clinical improvement persisted until June 2014, when a rapidly growing, painful, palpable mass was detected in the popliteal fossa. A CT scan revealed that the palpable mass involved two different sections with two levels of density: a low-density component and a high-density, calcified component (Fig. 5A). An open biopsy was performed, obtaining samples from both components. When histologically analyzing the specimens, we observed that the low-density component, previously identified with CT scan, corresponded to a high-grade sarcoma (Fig. 5 B, C), while the high-density component corresponded to areas of giant cell tumor of bone with changes related to denosumab treatment (Fig. 5 D-F). Denosumab-related changes comprise depletion or absence of giant cells, fibrosis, and ossification. As no associated regional lymphadenopathy was detected, and chest CT showed no evidence of metastases, an above-knee amputation was performed. The amputation specimen revealed that the low-density components corresponded to a tan–yellow mass measuring 15 cm × 7 cm. Histologic analysis of these components showed a high-grade pleomorphic sarcoma with 15 mitoses per high-power field and extensive necrosis (approximately 40% of the tumor). No neoplastic bone formation was observed. Histologic analysis of the specimen remainder showed absence of giant cells with persistence of mononuclear stromal cells, fibrosis, and ossification as seen after denosumab treatment.
Fig. 5A–F.
In July 2014, (A) a CT scan showed two different levels of density: a low-density component (yellow arrow) and a high density, calcified component (red arrow). An open biopsy was performed. The surgical specimen showed that the areas corresponding to the low-density component revealed (B) a high-grade neoplasm with extensive necrosis and spindle to pleomorphic cells with prominent nucleoli and (C) a high mitotic rate (Stain, hematoxylin & eosin; original magnification, ×40). The high density component showed calcified nodules, (D) fibrous tissue and a partially ossified giant cell tumor, (E) scattered spindled mononuclear cells between osteoid and woven bone, and (F) woven bone and fibrous tissue with no mononuclear or giant cells (Stain, hematoxylin & eosin; original magnification, ×40).
At last followup, 6 months after the amputation, the patient was disease-free.
Discussion
Giant cell tumor of bone is classified as a benign tumor, although recurrence rates range from 0% to 65%, depending on the type of treatment provided and tumor location [8]. Secondary malignant transformation in a previously histologically typical giant cell tumor of bone without any record of previous radiotherapy is exceptionally uncommon (occurring in less than 1% of patients) [24]; therefore, only a few case reports address this event [6, 7, 11, 13, 16, 19].
Although extremely unusual, sarcomas arising in a benign giant cell tumor have been reported [15, 24]. They usually contain areas of benign giant cell tumors and a biopsy may not initially reveal the existence of a malignant tumor [1]. Because our patient had multiple surgical resections with complete histologic analysis of the pathologic specimens, it is improbable that the malignant component would have been present initially and misdiagnosed.
Sarcoma arising in a giant cell tumor of bone may follow radiation therapy or occur in postsurgical recurrences. Some patients may have a giant-cell-rich sarcoma or osteosarcoma at initial diagnosis. Although the sarcoma cannot be distinguished on the basis of radiographic and histologic presentations, it is believed that the etiology differs [1]. Sakkers et al. [19] suggested a theory concerning malignant transformation of giant cell tumor of bone treated by curettage and bone grafting; in this context, reparative proliferative changes that occur at the margins of an area of dead bone could serve as the departure point for development of a malignant tumor.
A giant cell tumor of bone is thought to be biphenotypic, involving osteoclast-like giant cells expressing RANK, and mesenchymal spindle-like neoplastic stromal cells expressing RANKL. The interaction between these cells leads to bone resorption [23]. Denosumab, a monoclonal antibody that binds RANKL and directly inhibits osteoclastogenesis, has shown objective changes in the tumor along with clinical responses in patients with an unresectable tumor or large recurrences who have few other treatment options [25]. It recently was reported that denosumab almost completely eliminates the expression of RANKL, and histologic evaluation shows the absence of giant cells but the persistence of neoplastic stromal cells [10]. We believe that patients treated with denosumab should have a surgical resection after denosumab administration whenever possible to avoid regrowth of the tumor.
To our knowledge, there have been no reports of significant adverse events, such as malignancy or infections with lower doses of denosumab administration in patients with osteoporosis (60 mg subcutaneously every 6 months) in the 3- or 6-year Fracture Reduction Evaluation of Denosumab in Osteoporosis Every 6 Months (FREEDOM) extension trial [2]. However, the safety of denosumab and adverse events with higher doses (beginning with a 360-mg dose and then 120-mg monthly every 28 days), as used in treating patients with giant cell tumor of bone, has not been well defined. The effectiveness of denosumab regarding disease and symptom control in patients with an unresectable or recurrent giant cell tumor of bone has been seen, without severe adverse effects [22]; however, Thomas et al. [22] reported, in a Phase 2 study, that the reason for two of their four patients discontinuing the denosumab regimen before completion of planned treatment was because of disease progression of a malignant giant cell tumor of bone. Additionally although five patients had serious adverse events, none were deemed to be treatment-related. It has been questioned if RANKL inhibitors affect inflammation and immunity, as RANKL is expressed by endothelial cells, T lymphocytes, synovial fibroblasts, and various tumor cells [5]. Nevertheless, no meaningful clinical extraskeletal effects have been reported after denosumab administration for osteoporosis, and the overall rate of infections, cancer, and patient mortality have been similar with denosumab use compared with placebo at doses used for the treatment of patients with osteoporosis [5, 20]. High-dose denosumab therapy, similar to that used in patients with an unresectable giant cell tumor of bone, was recommended for prevention of skeletal-related events in patients with bone metastasis from a solid tumor [21]. In that study, it was difficult to assess long-term side effects related to denosumab therapy as patients had a limited life expectancy owing to the oncologic disease [21].
We present the findings of a patient who had a sarcoma develop in a previously benign, recurrent giant cell tumor of bone while receiving denosumab therapy. We cannot confirm whether denosumab treatment was causative or contributed to the development of the sarcoma or whether this was coincidental. We do believe, however, that this finding should be reported because denosumab is being used more frequently in the treatment of patients with a benign giant cell tumor of bone. Treating physicians should be aware of the possible association and further prospective studies should focus on the safety of denosumab therapy when used in high doses for treatment of patients with a benign unresectable giant cell tumor of bone.
Footnotes
One of the authors certifies that he (LAA-T) has or may receive payments or benefits, during the study period, an amount of USD 10,000-100,000 from Stryker Corporation (Kalamazoo, MI, USA).
All ICMJE Conflict of Interest Forms for authors and Clinical Orthopaedics and Related Research ® editors and board members are on file with the publication and can be viewed on request.
Clinical Orthopaedics and Related Research ® neither advocates nor endorses the use of any treatment, drug, or device. Readers are encouraged to always seek additional information, including FDA-approval status, of any drug or device prior to clinical use.
Each author certifies that his institution has approved the reporting of this study and that all investigations were conducted in conformity with ethical principles of research.
References
- 1.Bertoni F, Bacchini P, Staals EL. Malignancy in giant cell tumor of bone. Cancer. 2003;97:2520–2529. doi: 10.1002/cncr.11359. [DOI] [PubMed] [Google Scholar]
- 2.Bone HG, Chapurlat R, Brandi ML, Brown JP, Czerwinski E, Krieg MA, Mellström D, Radominski SC, Reginster JY, Resch H, Ivorra JA, Roux C, Vittinghoff E, Daizadeh NS, Wang A, Bradley MN, Franchimont N, Geller ML, Wagman RB, Cummings SR, Papapoulos S. The effect of three or six years of denosumab exposure in women with postmenopausal osteoporosis: results from the FREEDOM extension. J Clin Endocrinol Metab. 2013;98:4483–4492. doi: 10.1210/jc.2013-1597. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Campanacci M, Baldini N, Boriani S, Sudanese A. Giant-cell tumor of bone. J Bone Joint Surg Am. 1987;69:106–114. [PubMed] [Google Scholar]
- 4.Chawla S, Henshaw R, Seeger L, Choy E, Blay JY, Ferrari S, Kroep J, Grimer R, Reichardt P, Rutkowski P, Schuetze S, Skubitz K, Staddon A, Thomas D, Qian Y, Jacobs I. Safety and efficacy of denosumab for adults and skeletally mature adolescents with giant cell tumour of bone: interim analysis of an open-label, parallel-group, phase 2 study. Lancet Oncol. 2013;14:901–908. doi: 10.1016/S1470-2045(13)70277-8. [DOI] [PubMed] [Google Scholar]
- 5.Ferrari-Lacraz S, Ferrari S. Do RANKL inhibitors (denosumab) affect inflammation and immunity? Osteoporos. Int. 2011;22:435–446. doi: 10.1007/s00198-010-1326-y. [DOI] [PubMed] [Google Scholar]
- 6.Grote HJ, Braun M, Kalinski T, Pomjanski N, Back W, Bleyl U, Böcking A, Roessner A. Spontaneous malignant transformation of conventional giant cell tumor. Skeletal Radiol. 2004;33:169–175. doi: 10.1007/s00256-003-0682-5. [DOI] [PubMed] [Google Scholar]
- 7.Hefti FL, Gächter A, Remagen W, Nidecker A. Recurrent giant-cell tumor with metaplasia and malignant change, not associated with radiotherapy: a case report. J Bone Joint Surg Am. 1992;74:930–934. [PubMed] [Google Scholar]
- 8.Klenke FM, Wenger DE, Inwards CY, Rose PS, Sim FH. Giant cell tumor of bone: risk factors for recurrence. Clin Orthop Relat Res. 2011;469:591–599. doi: 10.1007/s11999-010-1501-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Li J, Zhu Y, Wei Y. Fibrosarcoma development 15 years after curettage and bone grafting of giant cell tumor of bone. Orthopedics. 2014;37:e512–516. doi: 10.3928/01477447-20140430-66. [DOI] [PubMed] [Google Scholar]
- 10.Mak IW, Evaniew N, Popovic S, Tozer R, Ghert M. A translational study of the neoplastic cells of giant cell tumor of bone following neoadjuvant denosumab. J Bone Joint Surg Am. 2014;96:e127. doi: 10.2106/JBJS.M.01332. [DOI] [PubMed] [Google Scholar]
- 11.Marui T, Yamamoto T, Yoshihara H, Kurosaka M, Mizuno K, Akamatsu T. De novo malignant transformation of giant cell tumor of bone. Skeletal Radiol. 2001;30:104–108. doi: 10.1007/s002560000305. [DOI] [PubMed] [Google Scholar]
- 12.McDonald DJ, Sim FH, McLeod RA, Dahlin DC. Giant-cell tumor of bone. J Bone Joint Surg Am. 1986;68:235–242. [PubMed] [Google Scholar]
- 13.Mori Y, Tuchiya H, Karita M, Nonomura A, Nojima T, Tomita K. Malignant transformation of a giant cell tumor 25 years after initial treatment. Clin Orthop Relat Res. 2000;381:185–191. doi: 10.1097/00003086-200012000-00022. [DOI] [PubMed] [Google Scholar]
- 14.Muscolo DL, Ayerza MA, Aponte-Tinao LA. Giant cell tumours of bone. Curr Orthop. 2001;15:41–50. doi: 10.1054/cuor.2001.0163. [DOI] [Google Scholar]
- 15.Nascimento AG, Huvos AG, Marcove RC. Primary malignant giant cell tumor of bone: a study of eight cases and review of the literature. Cancer. 1979;44:1393–1402. doi: 10.1002/1097-0142(197910)44:4<1393::AID-CNCR2820440433>3.0.CO;2-Z. [DOI] [PubMed] [Google Scholar]
- 16.Ortiz-Cruz EJ, Quinn RH, Fanburg JC, Rosenberg AE, Mankin HJ. Late development of a malignant fibrous histiocytoma at the site of a giant cell tumor. Clin Orthop Relat Res. 1995;318:199–204. [PubMed] [Google Scholar]
- 17.Raskin KA, Schwab JH, Mankin HJ, Springfield DS, Hornicek FJ. Giant cell tumor of bone. J Am Acad Orthop Surg. 2013;21:118–126. doi: 10.5435/JAAOS-21-02-118. [DOI] [PubMed] [Google Scholar]
- 18.Rock MG, Sim FH, Unni KK, Witrak GA, Frassica FJ, Schray MF, Beabout JW, Dahlin DC. Secondary malignant giant-cell tumor of bone: clinicopathological assessment of nineteen patients. J Bone Joint Surg Am. 1986;68:1073–1079. [PubMed] [Google Scholar]
- 19.Sakkers RJ, van der Heul RO, Kroon HM, Taminiau AH, Hogendoorn PC. Late malignant transformation of a benign giant-cell tumor of bone: a case report. J Bone Joint Surg Am. 1997;79:259–262. doi: 10.2106/00004623-199702000-00016. [DOI] [PubMed] [Google Scholar]
- 20.Sinningen K, Tsourdi E, Rauner M, Rachner TD, Hamann C, Hofbauer LC. Skeletal and extraskeletal actions of denosumab. Endocrine. 2012;42:52–62. doi: 10.1007/s12020-012-9696-x. [DOI] [PubMed] [Google Scholar]
- 21.Smith MR, Saad F, Coleman R, Shore N, Fizazi K, Tombal B, Miller K, Sieber P, Karsh L, Damião R, Tammela TL, Egerdie B, Van Poppel H, Chin J, Morote J, Gómez-Veiga F, Borkowski T, Ye Z, Kupic A, Dansey R, Goessl C. Denosumab and bone-metastasis-free survival in men with castration-resistant prostate cancer: results of a phase 3, randomised, placebo-controlled trial. Lancet. 2012;379:39–46. doi: 10.1016/S0140-6736(11)61226-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Thomas D, Henshaw R, Skubitz K, Chawla S, Staddon A, Blay JY, Roudier M, Smith J, Ye Z, Sohn W, Dansey R, Jun S. Denosumab in patients with giant-cell tumour of bone: an open-label, phase 2 study. Lancet Oncol. 2010;11:275–280. doi: 10.1016/S1470-2045(10)70010-3. [DOI] [PubMed] [Google Scholar]
- 23.Thomas DM. RANKL, denosumab, and giant cell tumor of bone. Curr Opin Oncol. 2012;24:397–403. doi: 10.1097/CCO.0b013e328354c129. [DOI] [PubMed] [Google Scholar]
- 24.Unni K. Giant Cell Tumor / Malignancy in Giant Cell Tumor of Bone. Dahlin's Bone Tumors: General Aspects and Data on 11,087 Cases. 5th ed. Philadelphia, PA: Lippincott-Raven; 1996:263–289.
- 25.Xu SF, Adams B, Yu XC, Xu M. Denosumab and giant cell tumour of bone: a review and future management considerations. Curr Oncol. 2013;20:e442–447. doi: 10.3747/co.20.1497. [DOI] [PMC free article] [PubMed] [Google Scholar]