Abstract
Introduction
The present study was undertaken to assess the caries activity by comparing the pH, buffering capacity, calcium, phosphorous, amylase along with the association of mutans in saliva for caries-free and caries-active children and to find out the interrelationship amongst the two groups.
Methodology
The study sample of 80 children, aged between 4 and 8 years were included in the study. Caries status of each child was recorded using DMFS. They were divided into two groups: (i) caries-free group (40) and (ii) caries-active group (40). After collecting the salivary samples, mutans were determined using Saliva-Check mutans kit and buffering capacity by Saliva-Check Buffer kit. The remaining samples were sent to laboratory for analyzing pH by electrode pH meter, calcium by OCPC(o-cresolphthalein complexone) photometric method, phosphorous by phosphomolybdate/UV method, amylase by CNP-G3(2chloro-4-nitrophenyl-alpha-maltotrioside) method using semi-autoanalyzer.
Results
The results obtained were tabulated and subjected to statistical analysis. The pH, buffering capacity, calcium and phosphorous level were found to be increased with the decrease in the caries activity of the children whereas amylase activity was increased with the increase in caries activity. It was observed that 77.5% children were tested positive and 22.5% were tested negative for mutans in caries-active group whereas 100% children were tested negative for mutans in caries-free group.
Conclusion
The physicochemical properties of saliva, such as pH, buffering capacity, calcium, phosphorous, amylase and Streptococcus mutans has a definite relationship with caries activity.
Keywords: Saliva, Amylase, Phosphorous, Streptococcus mutans, Dental caries
1. Introduction
Oral cavity is constantly exposed to the influence of adverse environmental factors and saliva is the first secretion to come in contact with exogenous substances. This complex biofluid represents a mixture of various fluids and components that helps in maintaining the oral health.1
Dental caries is an infectious, communicable disease resulting in demineralization and destruction of tooth structure by acid-forming bacteria. The number of mutans streptococci in saliva can be used for evaluating the caries risk.2
The study of saliva has always been an interesting aspect as saliva represents all the ions usually present in body fluids. The ability of saliva to buffer acids is essential for maintaining pH values in the oral environment. Bicarbonate ions play a major role in determining the pH and buffering capacity of saliva that can help protect teeth against attack from acids produced by bacteria.
Saliva also plays an important role in maintaining the integrity of dental tissues due to the presence of calcium, phosphorous and other inorganic ions as this environment is known to facilitate remineralization of incipient lesions or demineralized zones of enamel. Thus calcium and phosphorous in saliva forms a natural defense mechanism against dissolution of teeth.3
Amongst the various other salivary enzymes and electrolytes, amylase is the most abundant enzyme found in human saliva and is thought to be causing caries by binding its alpha portion to the bacteria.
Thus the study aims to compare the physicochemical properties of saliva between the caries-free group and caries-active group, to find out the activity of Streptococcus mutans in both the groups and find out the association between salivary constituents, mutans and caries.
2. Material and methods
This study was conducted in the Department of Pedodontics and Preventive Dentistry and Department of Biochemistry, at I.T.S – CDSR, Muradnagar, Ghaziabad (UP). The permission of the Ethical and Research Committee was obtained prior to the study. Eighty children (boys 26 and 54girls) between age group 4–8 years participated in the study. They were divided into two groups: caries-free and caries-active groups. Caries status of each child was scored by using decayed, missing, filled surface index (DMFS) given by Klein, Palmer, Knutson. Forty children were caries-free having DMFS = 0 as a control group and 40 were caries-active having DMFS ≥ 5 as a study group. After explaining the procedures for the study to the patient and their parents, informed consent was obtained. Each child was given a simple explanation as to the nature and reason for the tests following which the unstimulated salivary samples were collected in a graduated beaker and were further subjected to centrifugation for lab analysis. This excludes the chair side tests.
The collected salivary samples were conducted to following tests:
2.1. Chair side tests
-
•
Saliva-check mutans kit (G.C Corporation, Japan) was used to analyze the presence of mutans. The sensitivity of kit is 90.9% and specificity is 97.4%. This kit provides a semi-quantitative evaluation of the level of mutans streptococci in saliva in 15 min by using monoclonal antibodies. (Fig. 1)
-
•
Saliva–check buffer kit (G.C corporation, Japan) was used to determine the buffering capacity (Fig. 2).
Fig. 1.
Saliva-check mutans kit.
Fig. 2.
Saliva-check buffer kit.
2.2. Lab tests
-
•
Systronics electrode pH meter 361(lutron) by systronics India Ltd. used to evaluate the Salivary pH4 (Fig. 3).
-
•
Total calcium and phosphorous were determined colorimetrically on semiautoanalyser by OCPC (o-cresolphthalein complexone) photometric method at 570 nm and phosphomolybdate/UV method as an endpoint reaction at 340 nm respectively5 by commercially available kit from Erba Transasia Biomedical, Ltd.
-
•
Total amylase was determined by CNP-G3(2chloro-4-nitrophenyl-alpha-maltotrioside) method on semiautoanalyser (model no. microlab 200 by Merck Japan) (Fig. 4) by commercially available kit from Erba Transasia Biomedical, Ltd.
Fig. 3.

Systronics electrode pH meter 361.
Fig. 4.

Semiautoanalyser.
The values obtained for each method were documented and further subjected to statistically analysis.
2.3. Statistical analysis
The obtained data were complied and tabulated and then analyzed by SPSS (version 16) software. Mean and standard deviation were calculated for all the variables. Quantitative data such as pH, buffering capacity, calcium, phosphorous, and amylase were expressed by mean and standard deviation. Independent t-test was applied to observe the difference between the means for normally distributed data. Qualitative data such as presence of S. mutans was represented by percentage test. The difference between the percentages was observed by Chi-square test. The correlation of DMFS with pH, buffering capacity, calcium, phosphorous were examined using Pearson correlation coefficient and amylase by Spearman's correlation. Significance for all the statistical tests was determined at a p-value of 0.05 or less.
3. Results
-
•
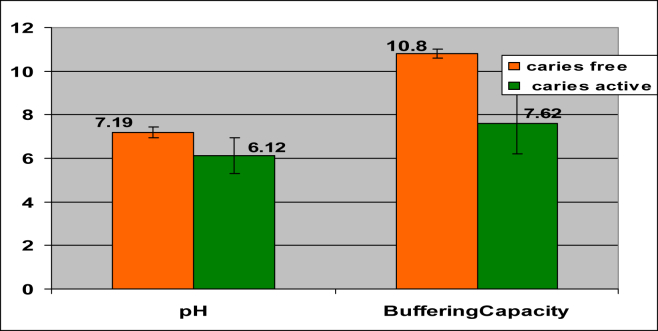
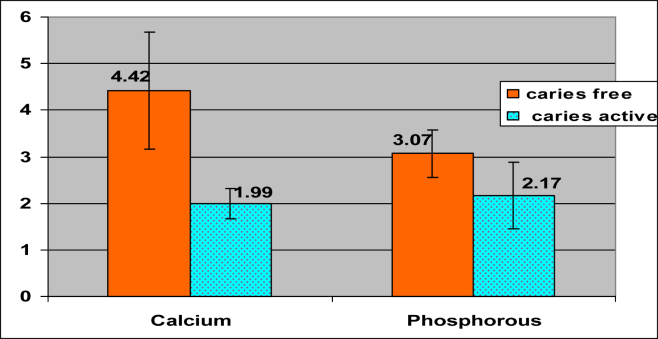
It was observed that the mean values for pH, buffering capacity, calcium and phosphorous were higher in caries-free group that suggests with the increase in the level of tested parameters, caries activity decreases and with the increase in the mean value of α-amylase, caries activity also increases. (Table 1, Figs. 5 and 6)
-
•
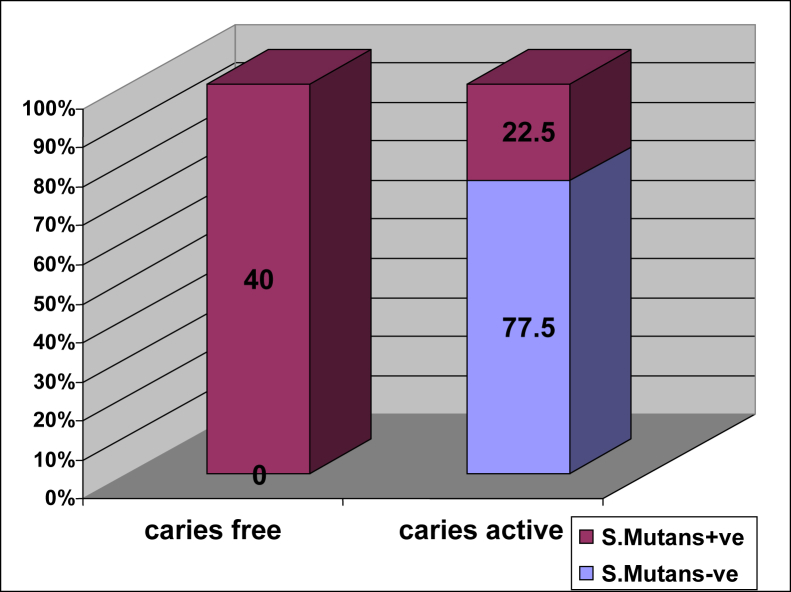
Association of mutans with caries among the two groups was seen. 77.5% samples were tested positive and 22.5% were negative among caries-active group (Table 2, Fig. 7).
-
•
Distribution of children in different DMFS category was done according to age. The comparison of dmfs score less than 0 and more than 0 was compared among various age groups and there was no significant difference was found among the various age groups (Table 3).
-
•
Comparison of DMFS score between boys and girls was done. No statistically significant difference was observed in the mean DMFS among the boys and girls (Table 4).
Table 1.
Mean and standard deviation of DMFS, pH, buffering capacity for caries-free group and caries-active groups.
| Group | N | Mean | Std. deviation | P-value | |
|---|---|---|---|---|---|
| DMFS | Caries-free | 40 | – | – | – |
| Caries-active | 40 | 8.55 | 1.877 | ||
| Ph | Caries-free | 40 | 7.20 | 0.24 | 0.000 |
| Caries-active | 40 | 6.07 | 0.53 | ||
| Buffer capacity | Caries-free | 40 | 10.92 | 0.86 | 0.000 |
| Caries-active | 40 | 7.46 | 1.46 | ||
| Calcium | Caries-free | 40 | 4.84 | 1.01 | 0.000 |
| Caries-active | 40 | 2.04 | 0.53 | ||
| Phosphorous | Caries-free | 40 | 3.07 | 0.35 | 0.000 |
| Caries-active | 40 | 2.11 | 0.67 | ||
| α-amylase | Caries-free | 40 | 7809.10 | 2240.3 | 0.000 |
| Caries-active | 40 | 19793.4 | 14651 |
Fig. 5.
The bar diagram depicts the comparison for pH and buffering capacity between the two groups.
Fig. 6.
The bar diagram depicts the comparison for Mean and standard deviation for Calcium and Phosphorous between the two groups.
Table 2.
Percentage of presence of mutans among Carie-free and Caries-active groups.
| S. mutans | Caries-free no. (%) | Caries-active no. (%) | Total |
|---|---|---|---|
| Negative | 40 (100.0%) | 9 (22.5%) | 49 (61.3%) |
| Positive | 0 (0%) | 31 (77.5%) | 31 (38.8%) |
| Total | 40 (100.0%) | 40 (100.0%) | 80 (100.0%) |
Fig. 7.
Bar diagram shows the percentage of presence of mutans among the two groups.
Table 3.
Distribution of children in different DMFS category according to age.
| Dmfs |
||||
|---|---|---|---|---|
| Mean | Std. deviation | F-value | P-value | |
| 4 years | 7.00 | 4.76 | 0.982 | 0.423# |
| 5 years | 4.76 | 5.10 | ||
| 6 years | 3.17 | 4.78 | ||
| 7 years | 3.35 | 4.17 | ||
| 8 years | 3.17 | 3.54 | ||
One-way ANOVA test.
#Non-significant difference (P-value > 0.05).
Table 4.
Comparison of DMFS score between boys and girls.
| Sex | Dmfs |
|||
|---|---|---|---|---|
| Mean | Std. deviation | P-value | Mean difference | |
| Male | 5.19 | 4.613 | 0.095 | 1.803 |
| Female | 3.39 | 4.410 | ||
Unpaired or Independent Samples Test.
# Non-significant difference (P-value > 0.05).
4. Discussion
Saliva is a complex body fluid that provides a general protective function for exposed oral hard tissues. Thereby role of saliva in protecting caries can be summarized under four aspects: clearance, buffer capacity, balancing demineralization or remineralization and antimicrobial action. The main advantage of saliva as a diagnostic tool is its easy availability along with its positive correlation amongst salivary constituents.
4.1. Salivary pH
In the present study, pH was determined by electrode pH meter and an inverse relationship between DMFS and pH was found. The pH values for caries-free group was in the range of 6.9–7.2 and for caries-active group was 5.8–6.2 which is in accordance with Gopinath6 who measured pH by saliva testing kit by GC Asia and found pH values in the range of 5.2–6.2 in caries active group. Tulunoglu7 also showed that pH values were higher in caries-active group as compare to caries-free group in Turkish children that was also in concurrence with the study.
4.2. Salivary buffering capacity
Salivary buffering system is one of the mechanisms protecting human teeth from environmental hazards. In this study the mean buffering capacity that is 10.92 for caries-free group was higher as compared to caries-active group that is 7.46 which was statistically significant (p < 0.001) and inversely proportional to DMFS. Similar results were found in a study by Tulunoglu et al.7 Muracciole8 found that the subjects immune to caries had a high buffering power and subjects with rampant caries had low buffering power. Gopinath6 also observed that 95% subjects with more than 5 DMFS had a significantly low saliva buffering capacity. Malekipour9 showed that buffering capacity for caries-active group was low as compared to caries-free group. All these studies were in concurrence to the present study.
4.3. Salivary calcium and phosphorous
The balance between demineralization and remineralization depends on the salivary calcium and phosphorous concentration. Calcium and phosphorous should be saturated in saliva to cause an effect on demineralization and remineralization.5 In current study it was found that salivary calcium and salivary phosphorous values were significantly higher in caries-free group than in caries-active group. An inverse relationship between calcium and phosphorous with DMFS was observed which is coinciding with Shaw and Murray10 who observed that mean levels of both calcium and phosphorous for the caries free group were higher than for those children in caries active group. Horton11 concluded very clearly the association between carious teeth and low calcium in saliva. Tulunoglu7 found calcium levels higher for caries-free group than caries-active group. Shannon12 observed that parotid fluid inorganic phosphorous concentration was inversely related to dental caries experience.
4.4. Streptococcus mutans
Dental caries is the most prevalent dental disease affecting human race. Specific types of acid-producing bacteria, especially S. mutans, are strong acid producers and hence cause an acidic environment thereby creating the risk for cavities. In this study SALIVA CHECK MUTANS chair side test kit was used to determine mutans and found that 77.5% of children in caries-active group tested positive for mutans and 22.5% tested were negative, whereas 100% of children in caries-free group tested negative for mutans. Brathall13 in their study observed that low levels of S. mutans were associated with very low caries prevalence and higher levels were associated with high levels of carious lesions. Kristoffersson14 concluded that those children with high salivary S. mutans counts had significantly more DMFS than children with no detectable S. mutans.
4.5. Salivary amylase
Salivary α-amylase is one of the richest components of human saliva. Binding of α-amylase to bacteria and teeth have important implications for dental plaque along with caries formation. The present study determines amylase by CNP-G3 method. The amylase concentration was higher for caries-active group as compared to caries-free. This showed a direct relationship between amylase and DMFS. The p-value for amylase was statistically significant (p < 0.001) which is in accordance with Balekjian15 who showed that parotid saliva samples of caries rampant group had a significantly higher level of iso-amylase than caries-resistant group. Sylvester8 showed that parotid and whole saliva from susceptible rats showed greater amylase activity than the saliva from resistant rats.
4.6. Conclusion
The results of this study indicate that saliva is an important factor in determining the prevalence of dental caries and can predict the possibility for future lesion. These tests can give sufficient and reliable information required to educate and motivate the child as well as parents. Hence the above salivary tests can form a routine diagnostic tool and can be used as an alternative investigation for diagnosing oral conditions as it is readily available and non-invasive when treating the patients with high caries risk. The study has shown a definite relationship of pH, buffering capacity, calcium, phosphorous, amylase and S. mutans with the caries activity.
Conflicts of interest
All authors have none to declare.
References
- 1.Leone W.C., Oppenheim G.F. Physical and chemical aspects of saliva as indicators of risk for dental caries in humans. J Dent Edu. 2001;65:1054–1062. [PubMed] [Google Scholar]
- 2.Kohler B. Intra-familial levels of streptococcus mutans and some of bacterial transmission. Scand J Dent Res. 1978;86:35–42. doi: 10.1111/j.1600-0722.1978.tb00605.x. [DOI] [PubMed] [Google Scholar]
- 3.Newbrun E. 3rd ed. Quintessence Publishing Co Inc; Chicago, Illinois: 1989. Cariology; pp. 29–61. [Google Scholar]
- 4.Sylvester C.J., Rosen S., Hoppert C.A., Hunt H.R. A comparison of certain properties from specific major salivary glands of caries-resistant and caries-susceptible rats. J Dent Res. 1964;43:528–535. doi: 10.1177/00220345640430040701. [DOI] [PubMed] [Google Scholar]
- 5.Shahrabi M., Nikfarjam J., Alikhani A. A comparison of salivary calcium, phosphate, and alkaline phosphatase in children with severe, moderate caries and caries free in Tehran's kindergartens. J Ind Soc Pedo Prev Dent. 2008;26:74–77. doi: 10.4103/0970-4388.41621. [DOI] [PubMed] [Google Scholar]
- 6.Gopinath V.K., Arzreanne A.R. Saliva as a diagnostic tool for assessment of dental caries. Arch Orofac Sci. 2006;1:57–59. [Google Scholar]
- 7.Tulunoglu O., Demirtas S., Tulunoglu I. Total antioxidant levels of saliva in children related to caries, age and gender. Int J Paed Dent. 2006;16:186–191. doi: 10.1111/j.1365-263X.2006.00733.x. [DOI] [PubMed] [Google Scholar]
- 8.Muracciole C.J. Evaluation of caries activity by the buffers of saliva. J Dent Res. 1955;34:387–389. doi: 10.1177/00220345550340031301. [DOI] [PubMed] [Google Scholar]
- 9.Malekipour M.R., Messripour M., Shirani F. Buffering capacity of saliva in patients with active dental caries. Asian J Biochem. 2008;3:280–283. [Google Scholar]
- 10.Shaw L., Murray J.J., Burchell C.K. Calcium and phosphorous content of plaque and saliva in relation to dental caries. Caries Res. 1983;17:543–548. doi: 10.1159/000260715. [DOI] [PubMed] [Google Scholar]
- 11.Horton k, Marrack J., Price I. The relation of calcium in the saliva to dental caries. Biochem J. 1929;23:1075–1078. doi: 10.1042/bj0231075. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Shannon L.I., Isbell M.G., Gibson A.W., O'Leary J.T. Inorganic phosphate concentration in body fluids as related to dental caries status. J Dent Res. 1962;41:1373–1377. doi: 10.1177/00220345620410061401. [DOI] [PubMed] [Google Scholar]
- 13.Bratthall D., Serinirach R., Carlsson P., Lekfuangfu S. Streptococcus mutans and dental caries in urban and rural school children in Thailand. Community Dent Oral Epidemiol. 1986;14:274–276. doi: 10.1111/j.1600-0528.1986.tb01071.x. [DOI] [PubMed] [Google Scholar]
- 14.Kristoffersson K., Axelsson P., Birkhed D., Bratthall D. Caries prevalence, salivary Streptococcus mutans and dietary scores in 13-year-old Swedish school children. Community Dent Oral Epidemiol. 1986;14:202–205. doi: 10.1111/j.1600-0528.1986.tb01535.x. [DOI] [PubMed] [Google Scholar]
- 15.Balekjian A.Y., Meyer T.S., Montague M.E., Longton R.W. Electrophoretic patterns of parotid fluid proteins from caries-resistant and caries-susceptible individuals. J Dent Res. 1975;54:850–856. doi: 10.1177/00220345750540042501. [DOI] [PubMed] [Google Scholar]