Abstract
Background
Comorbidity among developmental disorders such as dyslexia, language impairment, attention deficit/hyperactivity disorder and developmental coordination disorder is common. This study explores comorbid weaknesses in preschool children at family risk of dyslexia with and without language impairment and considers the role that comorbidity plays in determining children’s outcomes.
Method
The preschool attention, executive function and motor skills of 112 children at family risk for dyslexia, 29 of whom also met criteria for language impairment, were assessed at ages 3 ½ and 4 ½. The performance of these children was compared to the performance of children with language impairment and typically developing controls.
Results
Weaknesses in attention, executive function and motor skills were associated with language impairment rather than family risk status. Individual differences in language and executive function are strongly related in the preschool period and preschool motor skills predicted unique variance (4%) in early reading skills over and above children’s language ability.
Conclusion
Comorbidity between developmental disorders can be observed in the preschool years: children with language impairment have significant and persistent weaknesses in motor skills and executive function compared to those without language impairment. Children’s early language and motor skills are predictors of children’s later reading skills.
Keywords: Comorbidity, Language disorder, Dyslexia, Motor skills, Executive function
In recent years there has been growing interest in the frequent co-occurrence of developmental disorders; indeed it is now well recognised that pure disorders are rare in development and that ‘co-morbidity’ is common (Hulme & Snowling, 2009; Williams & Lind, 2013).
Evidence suggests that about 40% of school-aged children with one neurodevelopmental disorder (e.g. dyslexia, language impairment, attention deficit/hyperactivity disorder (ADHD) and developmental coordination disorder (DCD)) will also meet diagnostic criteria for another neurodevelopmental disorder (e.g. Kadesjö & Gillberg, 1999; McArthur, Hogben, Edwards, Heath & Mengler, 2000; Rochelle & Talcott, 2006; Willcutt & Pennington, 2000). These findings suggest that etiological factors that adversely influence brain development can have diverse effects (Pennington, 2006). However, less is known about comorbidity between neurodevelopmental disorders during the preschool years when the foundation for later learning is established.
It is well recognized that children with preschool language impairment are at high risk of developing reading difficulties (Bishop & Snowling, 2004) and that children at family risk of dyslexia who go on to have significant reading difficulties are likely to have a history of oral language difficulties (e.g., Scarborough, 1990; Snowling, Gallagher & Frith, 2003).
Attention deficits might also be expected to increase the risk of learning disorders by compromising the acquisition of key academic skills, including behaviour regulation (Barkley, 1997). Indeed, preschool attention problems have been found to predict later reading achievement (Rabiner, Coie & The Conduct Problems Prevention Group, 2000) which may reflect the operation of shared genetic risk factors influencing the development of both reading and attentional difficulties (e.g. Willcutt et al. 2007; McGrath et al., 2010).
Impairments in motor skills are also frequent in children with dyslexia (Kaplan, Wilson, Dewey & Crawford, 1998) although the evidence for an association between motor difficulties and specific reading difficulties in the school years is not strong (Rochelle et al., 2008). The occurrence of motor deficits in children with language impairment, on the other hand, is well documented (Hill, 2001) and Bishop (2002) found shared genetic liability for impairments on speeded motor tasks and tasks requiring speech production, suggesting that the genes that put a child at risk of speech/language difficulties may also affect motor development.
In summary, evidence for comorbidities between neurodevelopmental disorders is strong but a limitation of current evidence is a lack of longitudinal studies. We would argue that understanding how symptoms and cognitive markers of different disorders co-occur in the pre-school years is critical to the development of causal models of learning disorders as it will enable us to understand how comorbidities affect children’s learning. In this regard, a recent study by Aro, Eklund, Nurmi and Poikkeus (2012) showed that children at family risk of dyslexia are at increased risk of poor social outcomes if they have weak language skills and poor behavioural regulation in the pre-school years, ascertained by parental report. In addition, Viholainen, Ahonen, Lyytinen, Cantell, Tolvanen, and Lyytinen, (2006) found that some children at family risk of dyslexia were slower to reach developmental motor milestones in the first two years of life. These children also experienced early language difficulties (they had smaller vocabularies and poorer inflectional skills) at age 3 and turned out to be slower readers at age 7.
Pennington’s (2006) multiple deficit model suggests that a child’s developmental outcome will reflect a complex interplay between multiple risk factors and Hudziak, Achenbach, Althoff and Pine (2007) posit that the manifestation of any given developmental psychopathology will be determined by multiple sources of variance including the degree to which symptoms of comorbid disorders are present. Studying children at family risk of dyslexia gives us the opportunity to investigate cognitive precursors of dyslexia and search for these potential risk markers (endophenotypes).
In this paper we report data from the first two phases of a longitudinal study comparing preschool children who are at family risk of dyslexia with and without language impairment to those who have language impairment-only and TD controls. This design enables us to consider whether two different risk factors for literacy difficulties (familial genetic risk or early language impairment) are differentially associated with comorbid weaknesses in motor skills or executive function (and thus an increased risk of comorbid disorders such as DCD or ADHD). Given that previous studies have shown that only about 50% of children at family risk for dyslexia will go on to have literacy difficulties (Scarborough, 1990; Snowling et al., 2003) this study is also novel in considering the broader cognitive strengths and weaknesses of children at family risk of dyslexia prior to reading instruction and thus enables us to explore the developmental progression of comorbidities whist avoiding the confound of reading failure.
Our primary research question was: Are children at family risk of dyslexia at increased risk for motor difficulties and/or impairments in executive function during the pre-school period? Second, we asked whether these impairments were associated with pre-school language difficulties that were prevalent in the family risk group. Finally we wanted to explore whether markers of comorbid disorders (specifically markers of DCD or ADHD as measured by objective measures of motor skills and executive function) could help predict which children maybe most at risk of later reading difficulties.
Method
Data are from the first two phases of the Wellcome Language and Reading project; children were aged 3–4 years old at T1 and 4–5 years old at T2 approximately 11 months later.
Participants
Families with 3-year-old children were recruited to the study via advertisements placed in local newspapers, nurseries and webpages of support agencies for individuals with dyslexia/language difficulties, and via speech and language therapy services in Yorkshire, UK. None of the 242 children recruited to the study met our exclusionary criteria (MZ twinning, chronic illness, deafness, English as a 2nd language, care provision by local authority and known neurological disorder such as cerebral palsy, epilepsy, ASD). Ethical clearance for the study was granted by the University of York, Department of Psychology’s Ethics Committee and the NHS Research Ethics Committee. Parents provided informed consent for their child to participate. Following recruitment the children were classified into groups using a two-stage process: first, to determine whether or not they were at family risk for dyslexia and second, to determine whether or not they had current language impairment. This 2 (family risk yes/no) × 2 (language impairment yes/no) design yielded 4 groups: family risk only (FR), language impaired only (LI), family risk and language impaired (FRLI) and typically developing (TD).
Family risk.
Previous family risk studies have used either self-report or objective measures to determine risk status. In the current study we obtained self-report (Adult Reading Questionnaire; Snowling, Dawes, Nash & Hulme, 2012) and objectively measured the literacy skills of parents who consented to be assessed. Children were classified as family risk if (1) a parent self-reported as dyslexic, (2) a parent scored below 90 on a literacy composite of nonword reading and spelling (see Snowling et al. 2012 for details), (3) a parent had a discrepancy between nonverbal ability and the literacy composite of 1.5 standard deviations, with a literacy composite standard score of 96 or below, or (4) a sibling had a diagnosis of dyslexia from an educational psychologist or a specialist teacher. According to these criteria, 120/242 children were classified as being at family risk for dyslexia.
Language Impairment.
language impairment status was determined at T1 using three subtests, Basic Concepts, Expressive Vocabulary and Sentence Structure, from the Clinical Evaluation of Language Fundamentals (CELF) – Preschool 2 UK (Semel, Wiig & Secord, 2006) and the screener from the Test of Early Grammatical Impairment (TEGI; Rice & Wexler, 2001) comprising the third person singular /s/ and past tense probes. These tests assess receptive and expressive skills across multiple domains of language (vocabulary, syntax and grammatical inflection) (Tomblin, Records, Buckwalter, Zhang, Smith, & O’Brien, 1997). Children were classified as language impaired if they ‘failed’ 2/4 language tests (a fail being a score of 85 or below on the CELF subtests or failure of the TEGI screener)1.
Based on our research criteria, 35/120 children at family risk of dyslexia were classified as having language impairment as were 31/46 children referred for speech/language difficulties. Children who were referred to the study because of speech/language concerns but who did not meet our criteria for language impairment were excluded from further analyses (N= 15).
Typically Developing group.
Of the 76 children initially referred as typically developing, 5 met criteria for language impairment. These children were considered to be language impaired for the purpose of the study. The remaining 71 children, who were not at family risk of dyslexia, whose parents did not raise concerns about their speech/language development and who did not meet our criteria for language impairment, formed our typically developing group.
There was a small amount of attrition between T1 and T2 therefore data from 2 typically developing children, 2 children at family risk of dyslexia, 4 children with language impairment and 6 children at family risk of dyslexia who also had language impairment are not included here.
Sample characteristics.
Appendix 1 includes information about the age, gender, SES status, Nonverbal IQ (NVIQ) and language ability of the four groups (TD: N= 69, FR: N=83, LI: N=32 and FRLI: N = 29). The groups did not differ in age but there were group differences in SES status and NVIQ. On average the typically developing children were from higher SES backgrounds than children with language impairment (LI, FRLI) and they performed significantly better on the NVIQ tasks than children in the family risk group, who in turn performed better than the children with language impairment. There were relatively more boys than girls classified as language impaired but this difference was not statistically significant. As expected given the diagnostic criteria the children with language impairment (LI, FRLI) performed worse than the typically developing and family risk groups on the language measures (CELF Basic Concepts, CELF Expressive Vocabulary, CELF Sentence Structure and TEGI screener)(see Nash, Hulme, Gooch & Snowling (2013) for further information regarding the speech and language profiles of the groups).
Tests and Procedures
Alongside diagnostic language tests and NVIQ assessment each child completed measures of motor skills and executive function (see Appendix 2 for full details). Parents also completed questionnaires about their child’s attention, behaviour and motor coordination skills. Tests were administered in a single 1.5-hour session at T1 and across two 1-hour sessions at T2. The sessions were conducted in the child’s home and breaks were provided as appropriate.
Motor Skills.
Children completed 3 subtests from the Movement Assessment Battery for Children-2 (Henderson, Sugden, & Barnett, 2007); Posting Coins, Bead Threading and Bicycle Trails to assess fine motor skills (T1, T2).
As part of a structured family interview parents were asked to report the age at which their child started walking and at T2 parents completed the Developmental Coordination Questionnaire (DCDQ’07; Wilson et al. 2009), a screening tool to assist in the identification of developmental coordination difficulties.
Executive Function.
Each child completed a Visual Search task (Apples Task; Breckenridge, 2008) to measure selective attention (T1, T2), a computerised Auditory Continuous Performance Test (ACPT) (e.g. Kerns & Rondeau, 1998; Mahone, Pillion & Hiemenz, 2001) to assess sustained attention (T2), the Dog-Bird Go/No-Go task (a version of the Bear-Dragon Go/No-Go task; Reed, Pien & Rothbart, 1984) (T1) and the Head Toes Knees and Shoulders (HTKS) task (Burrage et al., 2008) (T1, T2) to assess behavioural inhibition/self-regulation plus Block Recall from the Working Memory Test Battery for Children (Pickering & Gathercole, 2001) to measure visuo-spatial memory (T2).
At T2 parents completed the Strengths and Weaknesses of ADHD-symptoms and Normal-behaviour Questionnaire (SWAN; Swanson et al., 2006), a dimensional scale that captures variations in ADHD symptoms related to strengths as well as weaknesses in attention/behaviour (Polderman et al., 2007).
Early literacy.
Early literacy skills were also assessed at T2 with the letter sound knowledge and early word reading tasks from the York Assessment of Reading for Comprehension (YARC; Hulme et al., 2009) and a letter writing task (children had to write 10 letters). Data from the four groups on the measures of early literacy are presented in Appendix 1.
Data analysis
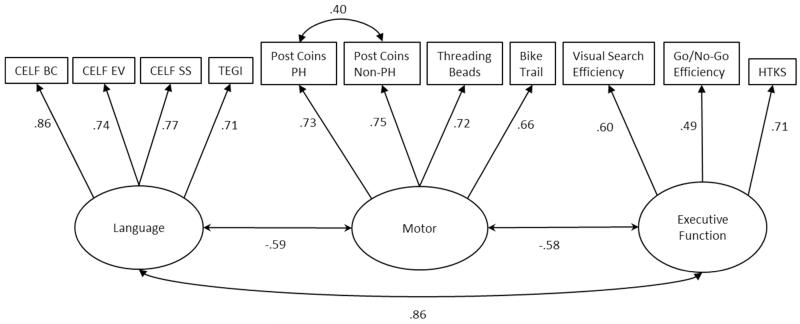
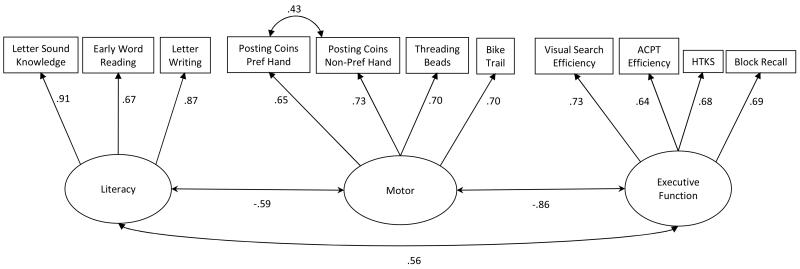
We had multiple measures of language, early literacy, motor skills and executive function so conducted confirmatory factor analysis on measures administered at T1 (Figure 1) and T2 (Figure 2) (analyses were conducted Stata 12.0 using the MLMV estimator to deal with missing values). Subsequently, motor and executive function factor scores from these CFAs were analysed in 2×2, family risk (+/−) × language impairment (+/−), ANOVAs. Interactions in these ANOVAs can be taken as evidence for the non-independence of the two factors (family risk and language impairment).
Figure 1.
Confirmatory factor analysis of the T1 language, motor and executive function variables.
Chi2(40) = 59.891, p = 0.022; RMSEA = 0.048; CFI = 0.974; TLI = 0.964
Figure 2.
Confirmatory Factor Analysis of T2 literacy, motor and executive function variables.
Chi2(40) = 70.881, p = 0.002; RMSEA =0.060; CFI = 0.969; TLI = 0.957
Results
Tables 1 and 2 show means and standard deviations for the four groups on the individual motor skills and executive function measures administered at T1 and T2 together with the motor and executive function factor scores for each group derived from the confirmatory factor analyses presented in Figures 1 and 2. The tables also display results from the ANOVAs along with ηp2 as a measure of effect size and Games Howell post-hoc comparisons.
Table 1.
Means and standard deviations for the four groups on theT1 and T2 motor skills measures. Main effects and interactions from the 2×2 ANOVA are also displayed.
| TD | FR | LI | FRLI | Main effect | Interaction | |||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| FR | LI | FR × LI | ||||||||||||||||
| Measures | N | Mean | SD | N | Mean | SD | N | Mean | SD | N | Mean | SD | F | η p 2 | F | η p 2 | F | η p 2 |
| T1 Tasks | ||||||||||||||||||
| Posting Coins pref (sec) | 69 | 27.77a | 5.24 | 83 | 30.86b | 8.46 | 31 | 46.26c | 29.83 | 29 | 34.48bc | 10.56 | 4.48* | 0.02 | 29.05** | 0.12 | 13.12** | 0.06 |
| Posting Coins non-pref (sec) | 69 | 32.23a | 8.04 | 81 | 35.23ab | 9.37 | 24 | 47.75c | 19.65 | 29 | 39.45bc | 12.47 | 2.2 | 0.01 | 30.52** | 0.13 | 10.02** | 0.05 |
| Threading Beads (sec) | 69 | 55.84a | 17.98 | 81 | 63.41ab | 32.96 | 28 | 84.21b | 44.19 | 27 | 68.56ab | 28.32 | 0.72 | 0.00 | 12.43** | 0.06 | 5.97* | 0.03 |
| Bicycle Trail errors | 66 | 7.68a | 3.99 | 81 | 8.91ab | 3.94 | 29 | 12.17c | 5.84 | 27 | 11.15bc | 5.35 | 0.02 | 0.00 | 22.85** | 0.10 | 2.57 | 0.01 |
| T1 Motor factor score | 69 | −0.45a | 0.43 | 83 | −0.14b | 0.71 | 32 | 0.98c | 1.32 | 29 | 0.38c | 0.67 | 1.53 | 0.01 | 71.20** | 0.25 | 15.36** | 0.07 |
| T2 Tasks | ||||||||||||||||||
| Posting Coins pref (sec) | 69 | 23.14a | 3.60 | 83 | 24.51a | 8.69 | 31 | 30.59b | 10.52 | 28 | 26.29ab | 6.19 | 1.67 | 0.01 | 16.41** | 0.07 | 6.19* | 0.03 |
| Posting Coins non-pref (sec) | 69 | 26.70a | 4.97 | 83 | 27.72ab | 7.08 | 31 | 35.61b | 16.36 | 29 | 29.86ab | 6.85 | 3.29 | 0.02 | 18.00** | 0.08 | 6.77* | 0.03 |
| Threading Beads (sec) | 68 | 36.13a | 10.02 | 83 | 41.49ab | 24.31 | 31 | 54.35b | 24.35 | 29 | 42.83ab | 16.59 | 1.04 | 0.01 | 10.49** | 0.05 | 7.83** | 0.04 |
| Bicycle Trail errors | 69 | 3.72a | 3.31 | 83 | 5.05ab | 4.52 | 30 | 7.13b | 4.88 | 29 | 6.28b | 3.83 | 0.14 | 0.00 | 13.40** | 0.06 | 2.97 | 0.01 |
| T2 Motor factor score | 69 | −0.41a | 0.45 | 83 | −0.09b | 0.91 | 32 | 0.81c | 1.20 | 29 | 0.35c | 0.70 | 0.29 | 0.00 | 44.51** | 0.18 | 9.71** | 0.04 |
| Age of walking (mnths) | 69 | 13.45ab | 2.37 | 81 | 12.56a | 2.33 | 32 | 14.58b | 3.24 | 29 | 13.41ab | 3.38 | 6.43* | 0.03 | 6.00* | 0.03 | 0.12 | 0.00 |
| T2 DCDQ | ||||||||||||||||||
| Dynamic Motor Control (/30) | 62 | 21.87a | 4.33 | 76 | 22.34a | 4.17 | 28 | 19.25a | 6.02 | 24 | 22.29a | 4.93 | 5.40* | 0.03 | 3.12 | 0.02 | 2.89 | 0.02 |
| Fine Motor (/20) | 60 | 15.55a | 3.60 | 76 | 14.95a | 3.67 | 28 | 11.18b | 5.05 | 25 | 13.44ab | 4.01 | 1.69 | 0.01 | 21.28** | 0.10 | 5.05* | 0.03 |
| General Co-ordination (/25) | 62 | 20.06a | 3.81 | 77 | 19.58ab | 3.91 | 29 | 17.14b | 4.70 | 25 | 17.80ab | 4.85 | 0.02 | 0.00 | 12.53** | 0.06 | 0.74 | 0.00 |
| Total (/75) | 60 | 57.50a | 9.33 | 75 | 57.04a | 9.05 | 27 | 47.59b | 14.16 | 24 | 53.08ab | 11.34 | 2.19 | 0.01 | 16.62** | 0.08 | 3.06 | 0.02 |
Note:
p<.05;
p<.01;
means with different subscripts are significantly different (Games Howell post-hoc comparison). Family risk only (FR), language impaired only (LI), family risk and language impaired (FRLI) and typically developing (TD). High Motor factor scores reflect poor performance.
Table 2.
Means and standard deviations for the four groups on the T1 and T2 executive function measures and SWAN Questionnaire ratings. Main effects and interactions from the 2×2 ANOVA are also displayed.
| TD | FR | LI | FRLI | Main effect | Interaction | |||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| FR | LI | FR × LI | ||||||||||||||||
| Measures | N | Mean | SD | N | Mean | SD | N | Mean | SD | N | Mean | SD | F | η p 2 | F | η p 2 | F | η p 2 |
| T1 Tasks | ||||||||||||||||||
| Visual Search efficiency score | 68 | 0.13a | 0.06 | 83 | 0.11a | 0.06 | 25 | 0.05b | 0.08 | 27 | 0.07b | 0.07 | 0.02 | 0.00 | 33.44** | 0.14 | 2.18 | 0.01 |
| Go/No-Go efficiency score | 63 | 0.81a | 0.22 | 69 | 0.75a | 0.23 | 13 | 0.75a | 0.25 | 17 | 0.65a | 0.22 | 2.54 | 0.02 | 3.04 | 0.02 | 0.2 | 0.00 |
| HTKS (/40) | 60 | 13.17a | 12.22 | 67 | 8.63ab | 10.28 | 17 | 1.35c | 3.06 | 13 | 4.23bc | 5.07 | 0.16 | 0.00 | 14.80** | 0.09 | 3.10 | 0.02 |
| T1 Executive Function factor score | 69 | 0.58a | 0.62 | 83 | 0.23b | 0.60 | 32 | −1.08c | 0.65 | 29 | −0.85c | 0.54 | 0.45 | 0.00 | 219.60** | 0.51 | 9.47 | 0.04 |
| T2 Tasks | ||||||||||||||||||
| Visual Search efficiency score | 69 | 0.18a | 0.05 | 82 | 0.16ab | 0.06 | 29 | 0.11c | 0.07 | 28 | 0.13bc | 0.07 | 0.20 | 0.00 | 31.17** | 0.13 | 4.95* | 0.02 |
| ACPT efficiency score | 66 | 0.17a | 0.06 | 80 | 0.14b | 0.10 | 27 | 0.10abc | 0.15 | 25 | 0.05c | 0.14 | 7.59** | 0.04 | 25.89** | 0.12 | 0.25 | 0.00 |
| HTKS (/40) | 68 | 25.76a | 9.93 | 79 | 21.90a | 11.59 | 28 | 9.68b | 11.67 | 27 | 9.70b | 8.26 | 1.29 | 0.01 | 70.17** | 0.26 | 1.22 | 0.01 |
| Block Recall (/ 54) | 69 | 16.86a | 3.36 | 82 | 15.51ab | 4.30 | 26 | 14.23b | 4.02 | 27 | 14.30b | 3.75 | 1.05 | 0.01 | 9.51** | 0.05 | 1.28 | 0.01 |
| T2 Executive Function factor score | 69 | 0.47a | 0.51 | 83 | 0.11b | 0.90 | 32 | −0.81c | 1.04 | 29 | −0.52c | 0.72 | 0.08 | 0.00 | 62.38** | 0.23 | 7.30** | 0.03 |
| T2 SWAN Questionnaire | ||||||||||||||||||
| Inattention (/63) | 63 | 42.19a | 6.49 | 76 | 40.36a | 6.81 | 29 | 33.76b | 10.1 | 25 | 35.32b | 4.98 | 0.01 | 0.00 | 34.68** | 0.16 | 2.21 | 0.01 |
| Hyperactivity (/63) | 63 | 39.71a | 6.87 | 77 | 40.05a | 7.45 | 28 | 35.39ab | 10.2 | 25 | 34.68b | 8.20 | 0.02 | 0.00 | 14.72** | 0.07 | 0.17 | 0.00 |
| Total (/126) | 63 | 81.90a | 12.17 | 76 | 80.41a | 13.08 | 28 | 69.11b | 19.5 | 25 | 70.00b | 12.36 | 0.02 | 0.00 | 26.87** | 0.13 | 0.29 | 0.00 |
Note:
p<.05;
p<.01;
means with different subscripts are significantly different (Games Howell post-hoc comparisons). Family risk only (FR), language impaired only (LI), family risk and language impaired (FRLI) and typically developing (TD).
Motor Skills
At both T1 and T2 children with language impairment (LI, FRLI) performed more poorly than children without language impairment (TD, FR) on the individual motor tasks and the Motor factor score (note high scores on the raw measures indicate poor performance and hence low factor scores reflect good performance). The significant language impairment × family risk interaction on the T1 and T2 Motor factor scores show that the effect of language impairment was significantly greater for children not at family risk of dyslexia (with post-hoc comparisons revealing TD<FR<LI=FRLI). The correlation between T1 and T2 motor factor scores was strong (r=.79, p<0.01) indicating that this factor has good longitudinal stability.
Parental report data supports our findings from objective measures; children with language impairment were reported to have started walking later than children without language impairment. On the DCDQ children with language impairment were rated as having significantly weaker fine-motor and general co-ordination skills compared to children without language impairment. The only exception to the general pattern was that for rated dynamic motor control; children at family risk showed an advantage compared to those not at family risk of dyslexia, although the effect size is small. The relationship between children’s T2 Motor factor score and their total DCDQ rating was moderate (r=−.50, p<0.01) indicating that our behavioural measures of fine motor skills are sensitive to individual difference in symptoms of DCD.
Together the data suggest that in the preschool years children with language impairment have persistent weaknesses in their motor skills compared to children without language impairment.
Executive Functions
At T1 and T2 children with language impairment (LI, FRLI) performed worse than children without language impairment (TD, FR) on the Executive function factor score (a pattern also seen in all the individual measures of executive function2).
The significant language impairment × family risk interaction on the T2 Executive function factor scores show that the effect of language impairment was significantly greater for children not at family risk of dyslexia (with post-hoc comparisons revealing TD>FR>LI=FRLI). The correlation between T1 and T2 Executive function factor scores (which were defined by slightly different measures) was strong (r=.69, p<0.01) indicating that this factor has good longitudinal stability.
Together the data suggest that the children with language impairment have persistent weaknesses across several domains of executive function including selective and sustained attention, complex behavioural inhibition and visuo-spatial STM compared to children without language impairment.
T2 parental report data confirm the pattern of performance on objective measures of attention and behaviour control; children with language impairment were rated as having worse behaviour and attention skills than children without language impairment on the SWAN inattention and hyperactivity scales. The relationship between children’s T2 Executive function factor score and their overall SWAN rating was moderate (r=.44, p<0.01) indicating that our behavioural measures of executive function are sensitive to individual difference in symptoms of ADHD.
Individual differences in motor skills and executive function; relationships with language ability
The data presented so far demonstrate that in the early years the difficulties experienced by children with language impairment are not limited to the verbal domain but are also manifest on measures of motor skills and executive functions.
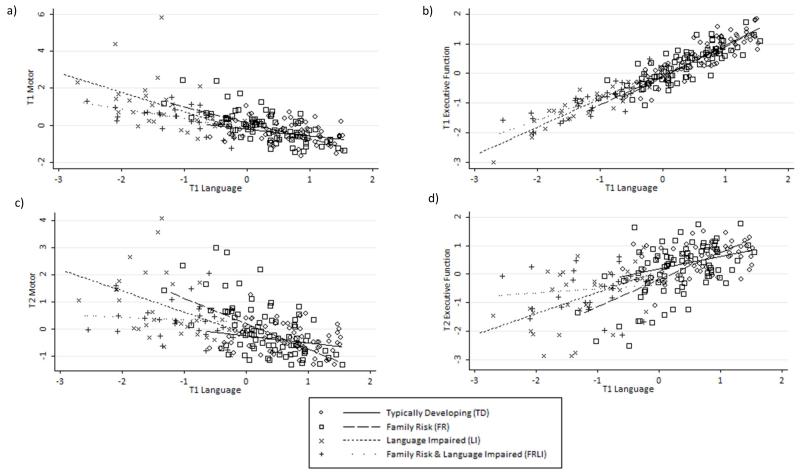
Figures 3 a and c show a moderate relationship between children’s T1 and T2 motor skills and T1 language abilities (r = −.69, p < .001 and r = −.60, p < .001 respectively; r = −.66, p < .001 and r = −.54, p < .001 with age controlled). Figures 3 b and d also show that there is very strong relationship between children’s executive function skills and their language ability at T1 (r = .96, p < .001; r = .95, p < .001 with age controlled) but that this relationship weakens by T2 (r = .63, p < .001; r = .61, p < .001 with age controlled).
Figure 3.
Scatter plots illustrating the relationships between T1 Language and T1 Motor (a), T1 Executive Function (b), T2 Motor (c) and T2 Executive Function (d) factor scores.
There was also a strong relationship between children’s motor and executive function skills at T1 (r = −.70, p < .001; r = −.69, p < .001 with age controlled) and T2 (r = −.95, p < .001; r = −.95, p < .001 with age controlled).
Predicting outcomes of children at family risk of dyslexia
Evidence suggests that individual variations in language skills predict which children at family risk of dyslexia will go onto develop reading difficulties (Snowling et al., 2003; Torppa, Lyytinen, Erskine, Eklund, & Lyytinen, 2010) however it also seems possible that variations in executive or motor skills will also predict variations in reading outcomes for these children. Our CFA (see Figure 1) showed that there were three separable factors assessed at time 1 (Language, Motor, Executive function) but that the Language and Executive function factors were highly correlated (r =.86). A hierarchical regression model with age, and language skills as predictors showed that they accounted for 31% of the variance in literacy skills, and that motor skills then accounted for a further 4% variance (see Table 3). Due to the colinearity between the T1 Language and Executive function factors, executive functions were not a separable predictor after language skills were controlled (1% variance accounted for).
Table 3.
Stepwise regression analysis predicting T2 literacy attainment from individual differences in language and motor skills at T1.
| Step | Variable | ß | t | Unique R2 |
|---|---|---|---|---|
| 1 | Age | 0.07 | 4.12** | 0.15 |
| 2 | Language | 0.23 | 2.97** | 0.16 |
| 3 | Motor | −0.31 | −3.75** | 0.04 |
Note:
= p<.01
Discussion
The comorbidity between language learning disorders (e.g. dyslexia and language impairment) and disorders that are characterised by symptoms beyond the domain of language (e.g. DCD and ADHD) is frequently reported. Despite this relatively little is known about the overlap between these disorders in the preschool years. Understanding the overlap between developmental disorders is important for theory and practice and here we report novel findings from a study that investigated comorbidity in children at family risk of dyslexia before they started school.
Our findings clearly show that between the ages of 3 and 5 years, weaknesses in motor skills and executive functions are associated with preschool language impairment irrespective of the child’s family risk status. In this study, children with language impairment demonstrated significant weaknesses in motor skills and executive functions/attention, as assessed by both objective measures and parental report, compared to children without language impairment. Furthermore, these weaknesses were present at both T1 and T2 suggesting that they are persistent through the pre-school period. Together these findings question the ‘specific’ nature of language impairment and suggest that many children with preschool language difficulties are likely to experience difficulties in other domains. Furthermore, our findings mirror the frequent comorbidity between language disorders, ADHD and DCD observed in school aged children (e.g., Kadesjö & Gillberg, 1999; Willcutt & Pennington, 2000).
Children at family risk of dyslexia who do not have language impairment perform better than those with language impairment (LI and FRLI) on objective measures of motor skills and executive function. This group however still has weaknesses on these objective measures compared to TD children although they are not reflected in parental ratings of motor skills and behaviour/attention. A key question therefore is whether the comorbidity between dyslexia, ADHD and DCD is only apparent (or more apparent) in individuals who have a history of language impairment. The corollary of this would be that individuals with dyslexia who do not have a history of significant language difficulties are less likely to have comorbidities. The current study is longitudinal and will allow a test of this hypothesis once the children reach the age when a reading disorder can be diagnosed.
Given the prevalence of motor and executive function weaknesses in children with language impairment and the correlations between individual difference in language, motor skills and executive functions that were observed, the data lend support to the view that the factors which place an individual at risk for language difficulties also influence the development of attention, behavioural control and motor skills (e.g. Bishop 2002). Furthermore, although we do not yet know which of the children at family risk of dyslexia will go on to have reading difficulties the results from our regression analysis provide some preliminary support for the idea that multiple risk factors (T1 language skills and to a lesser but never-the-less significant extent T1 motor skills) play a role in predicting children’s early literacy outcomes and thus are important in determining which children are most at risk of later literacy difficulties.
Another key finding is the very strong relationship between children’s language skills and executive function in the preschool years. This is particularly important to bear in mind given research which has shown that executive functions, and behavioural control in particular, are important for school readiness (Blair, 2002) and later classroom learning (Blair & Razza, 2007; see Liew, 2012 for a review). Our finding raises the question of whether the proposed role of executive function in studies of school readiness and classroom learning can be separated from the role that individual differences in language skills may play. A future aim of our project is to investigate the role that executive functions play in children’s academic outcomes; in particular we are interested in how these skills impact on children’s literacy development (e.g. the development of reading fluency, reading comprehension and spelling), once formal instruction commences. Indeed our current findings suggest that language may play a crucial role in mediating the relationship between executive function and literacy development. The longitudinal design of this study will allow us to consider whether the pattern of comorbidity between disorders changes over time and/or as a function of individual children’s developmental trajectories and whether children with additional cognitive deficits beyond the language domain are more at risk of developing reading difficulties and thus more in need of early intervention.
Key points.
There is considerable comorbidity between developmental disorders such as Dyslexia, language impairment (LI), Attention Deficit/Hyperactivity Disorder (ADHD) and Developmental Coordination Disorder (DCD).
We investigated comorbid weaknesses in preschool children at family risk of reading difficulties, with and without LI, using measures of executive function and motor skills as markers of ADHD and DCD.
Children with LI had comorbid weaknesses in executive function and motor skills compared to those without LI; Parents also rated these children as having more symptoms of ADHD (inattention and hyperactivity) and DCD (fine and gross motor coordination).
Children at family risk of dyslexia without LI performed better on measures of executive function and motor skills than those with LI (LI and FRLI) but worse than TD controls.
Children’s language and executive function skills are strongly related in the preschool years and individual differences in language skills and motor skills both predict unique variance in children’s early literacy skills.
Future work should aim to investigate how comorbidity effects the manifestation of reading difficulties and how they impact on children’s broader academic attainment and social/emotional development.
Acknowledgements
This study was funded by the Wellcome Trust Programme Grant 082036. We would like to thank Lorna Hamilton and Ruth Leavett for assistance with data collection, Emma Hayiou-Thomas for her support and the children and their families who participated in this study.
Appendix 1
Descriptive characteristics of the TD, FR, LI and FRLI groups including their performance on the diagnostic language tests (T1) and early literacy measures (T2).
| TD |
FR |
LI |
FRLI |
F | p | Group differences | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| N | Mean | SD | N | Mean | SD | N | Mean | SD | N | Mean | SD | ||||
| T1 Age (months) | 69 | 44.59 | 3.12 | 83 | 45.17 | 3.47 | 32 | 43.56 | 2.09 | 29 | 45.07 | 3.24 | 2.15 | ns | n/a |
| T2 Age (months) | 69 | 55.46 | 3.21 | 83 | 56.64 | 3.85 | 32 | 55.19 | 2.58 | 29 | 56.76 | 3.52 | 2.56 | ns | n/a |
| Gender (% males) | 69 | 49 | 83 | 58 | 32 | 69 | 29 | 72 | χ2 6.74 | ns | |||||
| SES (% rank - postcode) | 69 | 71.97 | 27.46 | 83 | 62.19 | 26.67 | 32 | 52.62 | 30.18 | 29 | 50.84 | 30.50 | 5.57 | 0.00 | TD > LI & FRLI |
| NVIQ | 69 | 115.61 | 13.93 | 82 | 107.90 | 14.84 | 29 | 96.07 | 13.10 | 29 | 101.03 | 10.15 | 16.82 | 0.00 | TD > FR > (LI = FRLI) |
| Diagnostic language tasks | |||||||||||||||
| CELF Basic Concepts (SS) | 69 | 12.09 | 2.08 | 83 | 10.96 | 2.38 | 29 | 6.90 | 2.16 | 29 | 6.59 | 2.29 | 65.55 | 0.00 | TD > FR > (LI = FRLI) |
| CELF Expressive Vocab (SS) | 69 | 12.03 | 2.18 | 83 | 11.05 | 2.00 | 28 | 7.32 | 3.31 | 28 | 7.11 | 2.74 | 46.55 | 0.00 | TD > FR > (LI = FRLI) |
| CELF Sentence Structure (SS) | 69 | 11.14 | 2.49 | 83 | 10.42 | 1.94 | 30 | 6.27 | 2.03 | 27 | 6.78 | 2.62 | 51.16 | 0.00 | (TD = FR) > (LI = FRLI) |
| TEGI Screen(% failed) | 63 | 14 | 81 | 33 | 18 | 79 | 21 | 77 | χ2 44.18 | 0.00 | |||||
| T1 Language factor score | 69 | 0.58 | 0.62 | 83 | 0.23 | 0.60 | 32 | −1.08 | 0.65 | 29 | −0.85 | 0.54 | 120.50 | 0.00 | TD > FR > (LI =FRLI) |
| Early literacy tasks | |||||||||||||||
| YARC Letter Sound Knowledge /32 | 69 | 19.55 | 9.44 | 83 | 16.82 | 9.71 | 30 | 11.77 | 9.97 | 29 | 11.83 | 10.41 | 6.82 | 0.00 | TD > (LI = FRLI) |
| YARC Early Word Reading /30 | 69 | 5.09 | 7.12 | 83 | 3.00 | 4.88 | 30 | .77 | 1.61 | 29 | 1.72 | 5.66 | 5.31 | 0.00 | (TD = FR) > LI |
| Letter Writing /10 | 69 | 4.59 | 3.03 | 83 | 3.96 | 3.06 | 30 | 1.97 | 2.46 | 29 | 2.31 | 2.82 | 7.98 | 0.00 | (TD = FR) > (LI = FRLI) |
| T2 Literacy factor score | 69 | 0.36 | 0.87 | 83 | 0.08 | 0.93 | 32 | −0.57 | 0.82 | 29 | −0.44 | 0.91 | 10.54 | 0.00 | (TD = FR) > LI; TD > FRLI |
Note: SES % rank is based on postcode; all lower super output areas (32482) in UK are ranked according to deprivation value. This % value reflects relative rank (ranked value / 32482 = relative rank); lower = more deprived (Source: Department of Communities and Local Government, Indices of Multiple Deprivation, 2007). Nonverbal IQ (NVIQ) is the average standard score of the Block Design and Object Assembly subtests from the Wechsler Preschool and Primary Scale of Intelligence – Third Edition (WPPSI-III; Wechsler, 2003). SS = Scaled scores (mean 10; SD 3). The T1 Language and T2 Literacy factor scores were derived from the CFA shown in Figures 1 and 2 in the main paper.
Appendix 2 Tests and Procedures
Nonverbal IQ
Each child completed Wechsler Preschool and Primary Scale of Intelligence Block Design and Object Assembly subtests (WPPSI-III; Wechsler, 2003) according to the test manual. These standard score were averaged to form the nonverbal IQ (NVIQ) score. The majority of children obtained average NVIQ standard scores within the normal range (i.e. >80), however, 1 TD, 2 FR, 5 LI and 1 FRLI children obtained average standard scores below 80 (all children scored > 70 on at least one of the two NVIQ tests); in addition 1 FR and 3 LI children were unable to complete the tests. These data show that the majority of children with LI met criteria for SLI. All children who met LI criteria, regardless of NVIQ, were included because research suggests that children in the lower range of NVIQ show the same overall profile of Language Impairment as children in the range of 85 and above (Tomblin & Zhang, 1999). Furthermore, proposed revisions to DSM-V do not include NVIQ as part of the selection criteria for language impairment.
Although controlling for NVIQ using techniques such as ANCOVA is common in the SLI literature recent research suggests that this may be inappropriate and Dennis, Francis, Cirino, Schachar, Barnes and Fletcher (2009) outline several reasons why NVIQ should not be used as a covariate in studies of neurodevelopmental disorders. Indeed Conti-Ramsden, St Clair, Pickles and Durkin (2012) found that children with the most severe LI had lower NVIQs than those with less severe LI. Thus, ‘controlling’ for group differences in NVOIQ would at the same time ‘control’ for the variable of interest, language impairment.
Motor skills
Children completed 3 subtests from the Movement Assessment Battery for Children-2 (Henderson, Sugden, & Barnett, 2007):
Posting Coins (T1 and T2).
The child posted 12 coins into a money box as quickly as possible and the time taken with each hand was recorded. This task had good stability between T1 and T2 (r = .69 and .70 for preferred and non-preferred hands respectively).
Bead Threading (T1 and T2).
The child threaded 6 beads onto a piece of string as quickly as possible and the time taken was recorded. This task had good stability between T1 and T2 (r = .67).
Bicycle Trails (T1 and T2).
The child used their preferred hand to draw a single continuous line following a trail without crossing its boundaries and the number of errors was recorded. This task had moderate stability between T1 and T2 (r = .47)
Developmental Coordination Questionnaire 2007 (DCDQ’07; Wilson et al. 2009) (T2).
This questionnaire consists of 15 items tapping three distinct factors. The first factor “Control during Movement” contains items related to dynamic motor control (max score = 30). The second factor is “Fine Motor and Handwriting” (max score = 20) and the third factor relates to “General Coordination” (max score = 25). For each item parents were asked to compare their child’s motor performance to that of his/her peers using a 5 point Likert scale (Not at all like your child = 1, A bit like your child = 2, Moderately like your child = 3, Quite a bit like your child = 4, Extremely like your child = 5). The maximum total DCDQ score is 75; a high score reflects strengths in motor skills.
Executive Function
Each child completed tasks to measure attention, behavioural inhibition and memory.
Visual Search (T1 and T2).
A visual search task (the Apples Task; Breckenridge, 2008), which comprised an array of targets (18 red apples) and distracters (81 red strawberries and 81 white apples), was used to assess selective attention. The child was given 1 minute to search the array and point to targets whilst ignoring distracters. The number of targets identified and the number of commission errors made (pointing to a distracter; false alarms) were recorded. A visual search efficiency score ((Hits: total targets correctly identified – commission errors)/60 seconds) was calculated; this score was used in T1 and T2 the confirmatory factor analyses: a high score reflects better selective attention. This measure had moderate stability between T1 and T2 (r = .53).
Auditory Continuous Performance Test (ACPT) (T2).
An ACPT similar to those developed by Kerns and Rondeau (1998) and Mahone, Pillion and Hiemenz (2001) was used to assess selective attention. The task was designed to not be attention grabbing and thus challenge children’s ability to sustain their attention. Children saw an image of a farm and heard four different animal sounds (3 distracters (cow, duck and frog) and 1 target (dog)). The children were asked to press a response button when the dog barked. Each auditory stimulus was presented 30 times in a pseudorandom order. Each stimulus lasted 2500ms with ITIs of 1750ms. Reaction times as well as omission and commission errors were recorded. An ACPT efficiency score ((Hits: total targets correctly identified – commission errors)/120 trials) was calculated; this score was used in the T2 confirmatory factor analysis and a high score reflects better selective attention.
Dog-Bird Go/No-Go task (T1).
This is a version of the Bear-Dragon Go/No-Go task (Reed, Pien & Rothbart, 1984) and was used to measure behavioural inhibition. Children had to inhibit their natural inclination to follow verbal instructions for motor/hand actions (‘thumbs up’, ‘make a fist’, ‘point’ or ‘wave’) given by two puppets (a dog and a bird). The child was instructed to do what the dog said but to ignore the bird (i.e. inhibit their action). The number of times that the child correctly responded to instructions given by the dog (max 8) or incorrectly followed instructions given by the bird (commission errors; max 8) were recorded. A GoNoGo efficiency score (Hits: number of responses to the dog/total number of responses (responses to dog + bird)) was calculated; this score was used in the confirmatory factor analysis at T1 and a high score reflects better behavioural inhibition.
Head Toes Knees and Shoulders (HTKS) task (Burrage et al., 2008) (T1 and T2).
In this measure of behavioural inhibition the child was first asked to touch their head and then their toes. Once it had been established that they could perform this action they were instructed to do the opposite of what the examiner said (e.g. touch their toes if asked to touch their head and vice versa). The child had four opportunities to practice this, with up to 3 re-explanations of the directions. They then completed a block of 10 test trials with no feedback. If the child was able to successfully inhibit on 5/10 trials they went on to complete a further block of harder trials. For these trials, additional commands to touch their shoulders and knees were added and the child was reminded to do the opposite of what the examiner said (e.g. touch their shoulders if asked to touch their knees and vice versa). After four practice trials the child completed 10 further test trials involving all four commands in a predetermined, pseudorandom order. Each correct response received 2 points, self-corrected responses (partial inhibitions; where the child moved towards the incorrect, intuitive response but demonstrated the correct final response) received 1 point and incorrect responses received 0 points (max score = 40). This task had moderate stability between T1 and T2 (r = .51).
Block Recall (Working Memory Test Battery for Children, Pickering & Gathercole, 2001) (T2).
This task was used to measure visuo-spatial memory. The child saw the examiner tap a sequence of blocks on a board and then recalled the sequence by tapping the blocks in the same order. The number of correct trials was recorded (max 52).
Strengths and Weaknesses of ADHD-symptoms and Normal-behaviour Questionnaire (SWAN; Swanson et al., 2006) (T2).
The items on the SWAN constitute the 18 DSM-IV symptoms of ADHD and include 9 items tapping Inattention and 9 items tapping Hyperactivity/Impulsivity. For each item parents were asked to compare their child’s attention/behavioural skills to those of his/her peers using a 7 point Likert scale (Far Below Average = 1, Below Average = 2, Somewhat Below Average = 3, Average = 4, Somewhat Above Average = 5, Above Average = 6, and Far Above Average = 7). The maximum score on the SWAN is 126 (63 for each of the subscale: Inattention and Hyperactivity/Impulsivity). A low score reflects weaknesses in attention/behavioural skills.
Footnotes
Given the age and low ability of some of the children there were insufficient data from the diagnostic tests to determine language impairment status for 22 cases. For these cases information from the separate TEGI screener subtests and the Preschool Repetition test (Seeff-Gabriel, Chiat & Roy, 2008) at T1 and CELF sentence structure test at T2 was used to come to a clinical judgement about group membership. Seventeen of the 22 cases were considered language impaired.
It is important to note that there was a reasonable amount of data missing for the more complex behavioural inhibition tasks (Go/No-Go and HTKS) particularly in the two groups with language impairment at T1 and thus the means reported here are likely to be an overestimation of the actual ability of these groups.
References
- Aro T, Eklund K, Nurmi J-E, Poikkeus A-M. Early language and behavioural regulation skills as predictors of social outcomes. Journal of Speech, Language and Hearing Research. 2012;55:395–408. doi: 10.1044/1092-4388(2011/10-0245). [DOI] [PubMed] [Google Scholar]
- Barkley RA. Behavioural inhibition, sustained attention, and executive functions: Constructing a unifying theory of ADHD. Psychological Bulletin. 1997;121(1):65–94. doi: 10.1037/0033-2909.121.1.65. [DOI] [PubMed] [Google Scholar]
- Bishop DVM, Snowling MJ. Developmental dyslexia and specific language impairment: Same or different? Psychological Bulletin. 2004;130:858–888. doi: 10.1037/0033-2909.130.6.858. [DOI] [PubMed] [Google Scholar]
- Bishop DVM. Motor immaturity and specific speech and language impairment: evidence for a genetic basis. American Journal of Medical Genetics (Neuropsychiatry Genetics) 2002;114:56–63. doi: 10.1002/ajmg.1630. [DOI] [PubMed] [Google Scholar]
- Blair C. School readiness: Integrating cognition and emotion in a neurobiological conceptualization of children’s functioning at school entry. American Psychologist. 2002;57:111–127. doi: 10.1037//0003-066x.57.2.111. [DOI] [PubMed] [Google Scholar]
- Blair C, Razza RP. Relating effortful control, executive function, and false belief understanding to emerging math and literacy ability in kindergarten. Child Development. 2007;78:647–663. doi: 10.1111/j.1467-8624.2007.01019.x. [DOI] [PubMed] [Google Scholar]
- Breckenridge K. Attention and executive function in Williams syndrome and Down’s syndrome; August, 2008; The University of Oxford, UK: Paper presented at the Development of Executive Functions Workshop; [Google Scholar]
- Burrage MS, Ponitz CC, McCready EA, Shah P, Sims BC, Jewkes AM, Morrison FJ. Age- and schooling-related effects on executive functions in young children: a natural experiment. Child Neuropsychology. 2008;14(6):510–524. doi: 10.1080/09297040701756917. [DOI] [PubMed] [Google Scholar]
- Conti-Ramsden G, St Clair MC, Pickles A, Durkin K. Developmental trajectories of verbal and nonverbal skills in individuals with a history of specific language impairment: from childhood to adolescence. Journal of Speech, Language and Hearing Research. 2012;55(6):1716, 1735. doi: 10.1044/1092-4388(2012/10-0182). [DOI] [PubMed] [Google Scholar]
- Dennis M, Francis DJ, Cirino PT, Schachar R, Barnes MA, Fletcher JM. Why IQ is not a covariate in cognitive studies of neurodevelopmental disorders. Journal of the International Neuropsychological Society. 2009;15:331–343. doi: 10.1017/S1355617709090481. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Henderson SE, Sugden DA, Barnett A. Movement Assessment Battery for Children. Harcourt Assessment; London: 2007. [Google Scholar]
- Hill EL. Non-specific nature of specific language impairment: a review of the literature with regard to concomitant motor impairments. International Journal of Language and Communication Disability. 2001;36:149–171. doi: 10.1080/13682820010019874. [DOI] [PubMed] [Google Scholar]
- Hudziak JJ, Achenbach TM, Althoff RR, Pine DS. A dimensional approach to developmental psychopathology. International Journal of Methods in Psychiatric Research. 2007;16(S1):S16–S23. doi: 10.1002/mpr.217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hulme C, Snowling MJ. Developmental disorders of language learning and co gnition. Wiley-Blackwell; 2009. [Google Scholar]
- Hulme C, Stothard SE, Clarke P, Bowyer-Crane C, Harrington A, Truelove E, Snowling MJ. York Assessment of Reading for Comprehension: Early Reading. GL Assessment; 2009. [Google Scholar]
- Kadesjö B, Gillberg C. De velopmental coordination disorder in Swedish 7-year-old children. Journal of the American Academy of Child and Adolescent Psychiatry. 1999;38(7):820–828. doi: 10.1097/00004583-199907000-00011. [DOI] [PubMed] [Google Scholar]
- Kaplan BJ, Wilson BN, Dewey D, Crawford SG. DCD may not be a discrete disorder. Human Movement Science. 1998;17:471–490. [Google Scholar]
- Kerns K, Rondeau L. Development of a continuous performance test for preschool children. Journal of Attention Disorders. 1998;2:229–238. [Google Scholar]
- Liew J. Effortful control, executive functions, and education: bringing self-regulatory and social-emotional competencies to the table. Child Development Perspectives. 2012;6(2):105–111. [Google Scholar]
- Mahone EM, Pillion JP, Hiemenz JR. Initial development of an auditory continuous performance test for preschoolers. Journal of Attention Disorders. 2001;5:25–38. [Google Scholar]
- McArthur G, Hogben J, Edwards V, Heath S, Mengler E. On the “specifics” of specific reading disability and specific language impairment. Journal of Child Psychology & Psychiatry & Allied Disciplines. 2000;41(7):869–874. [PubMed] [Google Scholar]
- McGrath LM, Pennington BF, Shanahan MA, Santerre-Lemmon LE, Barnard HD, Willcutt EG, Defries JC, Olson RK. A multiple deficit model of reading disability and attention-deficit/hyperactivity disorder: searching for shared cognitive deficits. Journal of Child Psychology and Psychiatry. 2010;52(5):547–57. doi: 10.1111/j.1469-7610.2010.02346.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nash H, Hulme C, Gooch D, Snowling MJ. Preschool Language Profiles of Children at Family Risk of Dyslexia: Continuities with language impairment. Journal of Child Psychology and Psychiatry. 2013 doi: 10.1111/jcpp.12091. DOI: 10.1111/jcpp.12091. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Polderman TJC, Derks EM, Hudziak JJ, Verhulst FC, Posthuma D, Boomsma DI. Across the continuum of attention skills: a twin study of the SWAN ADHD rating scale. Journal of Child Psychology and Psychiatry. 2007;48(11):1080–1078. doi: 10.1111/j.1469-7610.2007.01783.x. [DOI] [PubMed] [Google Scholar]
- Pennington BF. From single to multiple deficit mo dels of developmental disorders. Cognition. 2006;101(2):385–413. doi: 10.1016/j.cognition.2006.04.008. [DOI] [PubMed] [Google Scholar]
- Pickering SJ, Gathercole S. Working Memory Test Battery for Children (WMTB-C) The Psychological corporation Ltd.; London, UK: 2001. [Google Scholar]
- Rabiner DL, Coie JD, Conduct Problems Preven tion Research Group Early attention problems and children’s reading achievement: A longitudinal investigation. Journal of the American Academy of Child and Adolescent Psychiatry. 2000;39:859–867. doi: 10.1097/00004583-200007000-00014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reed M, Pien D, Rothbart M. Inhibitory self-control in preschool children. Merrill-Palmer Quarterly. 1984;30:131–147. [Google Scholar]
- Rice M, Wexler K. Rice/Wexler Test of Early Grammatical impairment. Psychological Corporation; New York: 2001. [Google Scholar]
- Rochelle KSH, Witton C, Talcott JB. Symptoms of hyperactivity and inattention can mediate deficits of postural stability in developmental dyslexia. Experimental Brain Research. 2008;192:627–633. doi: 10.1007/s00221-008-1568-5. [DOI] [PubMed] [Google Scholar]
- Scarborough HS. Very early language deficits in dyslexic children. Child Development. 1990;61:1728–1743. [PubMed] [Google Scholar]
- Seeff-Gabriel B, Chiat S, Roy P. The Early Repetition Battery. Pearson Assessment; London: 2008. [Google Scholar]
- Semel E, Wiig EH, Secord W. Clinical Evaluation of Language Fundamentals - Fourth Edition UK (CELF-4UK) Harcourt Assessment; London: 2006. [Google Scholar]
- Snowling MJ, Dawes P, Nash H, Hulme C. Validity of a protocol for adult self-report of dyslexia and related difficulties. Dyslexia. 2012;18:1–15. doi: 10.1002/dys.1432. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Snowling MJ, Gallagher A, Frith U. Family risk of dyslexia is continuous: Individual differences in the precursors of reading skill. Child Development. 2003;74:358–373. doi: 10.1111/1467-8624.7402003. [DOI] [PubMed] [Google Scholar]
- Swanson JM, Schuck S, Mann M, Carlson C, Hartman K, Sergeant JA, Clevinger W, Wasdell M, McCleary R. Categorical and dimensional definitions and evaluations of symptoms of ADHD: The SNAP and SWAN Rating Scales. 2006 Retrieved May 2006 from http://www.ADHD.net. [PMC free article] [PubMed]
- Tomblin JB, Zhang X. Langu age patterns and etiology in children with specific language impairment. In: Tager-Flusberg H, editor. Neurodevelopmental disorders. MIT Press; Cambridge, MA: 1999. pp. 361–382. [Google Scholar]
- Tomblin JB, Records NL, Buckwalter, Zhang X, Smith E, O’Brien M. Prevalence of specific language impairment in kindergarten children. Journal of Speech and Hearing Research. 1997;40:1245–1260. doi: 10.1044/jslhr.4006.1245. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Torppa M, Lyytinen P, Erskine J, Eklund K, Lyytinen H. Language development, literacy skills, and predictive connections to reading in Finnish children with and without familial risk for dyslexia. Journal of Learning Disabilities. 2010;43(4):308–321. doi: 10.1177/0022219410369096. [DOI] [PubMed] [Google Scholar]
- Viholainen H, Ah onen T, Lyytinen P, Cantell M, Tolvanen A, Lyytinen H. Early motor development and later language and reading skills in children at risk of familial dyslexia. Developmental Medicine & Child Neurology. 2006;48:367–373. doi: 10.1017/S001216220600079X. [DOI] [PubMed] [Google Scholar]
- Wechsler D. Harcourt Assessment, Inc.; San Antonio, TX: 2003. Wechsler Intelligence Scale for Children – Fourth Edition. [Google Scholar]
- Willcutt EG, Pennington BF. Psychiatric comorbidity in children and adolescents with reading disability. Journal of Child Psychology and Psychiatry. 2000;41:1039–1048. [PubMed] [Google Scholar]
- Willcutt EG, Betjeman RS, Wadsworth SJ, Samuelsson S, Corley R, DeFries JC, Byrne B, Pennington BF, Olson RK. Preschool twin study of the relation between attention-deficit/hyperactivity disorder and prereading skills. Reading and Writing: An Interdisciplinary Journal. 2007;20:103–125. [Google Scholar]
- Williams DM, Lind SE. Comorbidity and diagnosis of developmental disorders. In: Marshall CR, editor. Current Issues in Developmental Disorders. Psychology Press; London: 2013. pp. 19–45. [Google Scholar]
- Wilson BN, Crawford SG, Green D, Roberts G, Aylott A, Kaplan BJ. Psychometric properties of the revised developmental coordination disorder questionnaire. Physical & Occupational Therapy in Pediatrics. 2009;29(2):184–204. doi: 10.1080/01942630902784761. [DOI] [PubMed] [Google Scholar]