Abstract
A major gap in our understanding of cell biology is how cells generate and interact with their surrounding extracellular matrix. Studying this problem during development has been particularly fruitful. Recent work on the basement membrane in developmental systems is transforming our view of this matrix from one of a static support structure to that of a dynamic scaffold that is regularly remodeled to actively shape tissues and direct cell behaviors.
Cell biology is an enormously broad discipline that examines cell structure and function, as well as interactions between the cell and its environment. Studying cell biology during development offers one of the most dynamic, process-rich, and physiologically relevant settings for understanding the functions of cells. Thus, many seminal findings on cell signaling, the cell cycle, cell migration, cell polarization, and programmed cell death have been discovered in developmental contexts.
One component of a cell’s environment, the extracellular matrix, is both of the cell and outside the cell, and its relationship with cells is therefore complex. In vitro studies have suggested roles for extracellular matrix in directing cell shape, differentiation, survival, and migration (Hay, 1981; Bernfield and Banerjee, 1982; Hadley et al., 1985; Goodman et al., 1989). Specific functions for extracellular matrix have been difficult to establish in vivo, however, because of the challenge of examining cell–matrix interactions in animals. Many vertebrate tissues encased with matrix are situated deep inside the organism and are inaccessible to light microscopy. Matrix components in most animals have also not yet been functionally tagged with fluorescent molecules to follow their dynamics in situ. Furthermore, genetic loss of many extracellular matrix components in animals leads to a cascade of diverse cell biological defects where the specific mechanism that initiated the perturbation is unclear (Rozario and DeSimone, 2010). Yet, advances in imaging techniques and the ability to perform complex genetic manipulations are helping to make progress in our understanding of the extracellular matrix in vivo. Here I present one example of how we are learning more about the cell biology of extracellular matrix from studies during development.
I am particularly fascinated by the basement membrane, a thin, dense, cell-associated form of extracellular matrix that underlies epithelia and endothelia and surrounds fat, muscle, and Schwann cells (Yurchenco, 2011). The emergence of basement membrane coincided with the appearance of multicellularity in animals, suggesting that basement membranes were a prerequisite for formation of tissues and multicellular life (Ozbek et al., 2010; Hynes, 2012). Basement membranes are highly conserved and are composed of a core set of approximately six proteins or protein assemblies, including laminin, type IV collagen, perlecan, and nidogen. Work from cell culture and developing embryos have indicated that basement membranes are initially built on a polymeric network of secreted laminin molecules, which binds to sulfated glycolipids as well as integrin and dystroglycan receptors on the cell surface (Hohenester and Yurchenco, 2013). This laminin lattice serves as a template for the addition of other basement membrane components, including type IV collagen, which has the unique ability to self-associate with intermolecular covalent bonds that are thought to provide basement membranes with their tensile strength and stability (Khoshnoodi et al., 2008; Fidler et al., 2014).
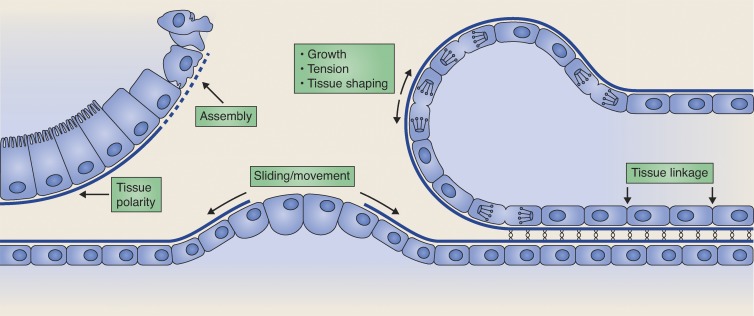
Basement membranes are generally thought of as stationary matrices that protect tissues from mechanical stresses, provide filtration and barrier functions, and act as a reservoir for growth factors (Yurchenco, 2011). Recent studies in visually accessible developmental systems, however, are revealing that basement membranes are dynamic scaffoldings that play instructive roles in tissue morphogenesis. For example, live imaging in Drosophila melanogaster using GFP-tagged type IV collagen has shown that tissue-specific regulation of basement membrane collagen has an important role in shaping numerous organs during development (Haigo and Bilder, 2011; Pastor-Pareja and Xu, 2011). Optical highlighting of laminin and type IV collagen in Caenorhabditis elegans larvae and collagen in cultured mouse salivary gland buds has also revealed that entire sheets of basement membrane move to facilitate tissue attachment and organ growth (Ihara et al., 2011; Harunaga et al., 2014; Matus et al., 2014). Work in developmental contexts has also shown regulated laminin deposition in coordinating polarized tissue formation and localized nidogen and perlecan accumulation in directing axon guidance and dendrite branching (Kim and Wadsworth, 2000; Rasmussen et al., 2012; Liang et al., 2015). Finally, work in my laboratory using tissue shifting and photobleaching of GFP-tagged laminin has identified a new adhesion system (B-LINK) that links neighboring tissues by connecting their adjacent basement membranes (Morrissey et al., 2014). These studies during development have uncovered the dynamic nature of basement membranes and the manner in which their remodeling actively directs specific cell behaviors and tissue formation events (summarized in Fig. 1).
Figure 1.
The basement membrane is a dynamic scaffold. During development, basement membranes assemble, grow, constrict tissues, and are actively remodeled to regulate diverse cellular behaviors and morphogenetic processes, including tissue polarity, tissue shaping, and tissue linkage.
Developmental studies are poised to address many remaining fundamental questions on the cell biology of basement membranes. Basement membrane structure, composition, and assembly are still poorly understood and are primarily inferred from indirect biochemical and reconstitution studies. Developmental models will continue to be invaluable in validating biochemical studies. This is illustrated by recent work confirming the importance of the enzyme peroxidasin in creating sulfilimine cross-links in type IV collagen networks that are critical for basement membrane stability during fly, zebrafish, and C. elegans development (Gotenstein et al., 2010; Bhave et al., 2012; Fidler et al., 2014). In contrast, genetic loss of nidogen in C. elegans and mice, which was thought from biochemical analysis to be critical in bridging type IV collagen and laminin networks, has revealed that nidogen is not essential in connecting these two networks (Kim and Wadsworth, 2000; Hohenester and Yurchenco, 2013). In addition, live visualization of fluorescently tagged basement membrane components in transparent animals such as C. elegans and zebrafish will allow rapid and expanded forward genetic screens to determine mechanisms regulating basement membrane assembly and maintenance. As proteomic and expression profiling studies have revealed >200 matrix or matrix-associated basement membrane proteins, the complexity and regulation of basement membranes is likely vast (Manabe et al., 2008; Uechi et al., 2014).
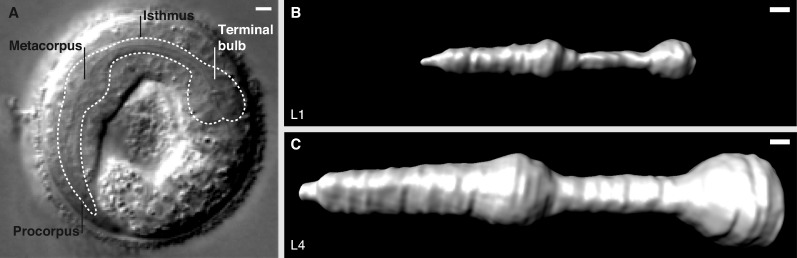
Developmental systems can also be used to address how basement membranes grow. This will be especially relevant with mechanically active tissues such as muscles. One experimentally accessible model is the C. elegans pharynx, a highly contractile organ (which beats ∼200 times/minute) encased in a basement membrane that expands dramatically during development (Fig. 2; Avery and Horvitz, 1989). How basement membranes balance tissue support, type IV collagen cross-linking, and dramatic expansion during development remains an open question.
Figure 2.
The basement membrane encasing the C. elegans foregut (pharynx) grows dramatically during development. (A) A differential interference contrast image of an embryo with the pharynx outlined. The pharynx is a basement membrane-encased contractile feeding organ that grinds and pumps food (bacteria) posteriorly into the intestine. (B and C) 3D-rendered isosurfaces of type IV collagen::mCherry show the approximately threefold increase in basement membrane surface area during pharyngeal growth from the L1 (B) to L4 (C) larval developmental stage. Bars, 5 µm. Images courtesy of R. Jayadev (Duke University, Durham, NC).
An additional important area that requires further investigation is how basement membranes respond to physical forces. Analysis of mutants in genes encoding basement membrane components and a limited number of biophysical studies suggests that basement membranes mechanically support tissues (Pöschl et al., 2004; Candiello et al., 2007; Halfter et al., 2013). With the ability to visualize load across proteins with FRET-based tension sensors (Grashoff et al., 2010; Meng et al., 2011) it should be possible to probe individual basement membrane molecules in optically clear animals to identify components that support load and when these proteins experience mechanical stress (e.g., growth, tissue deformation, contractions).
Live imaging of cell–basement membrane interactions during development will also allow a clearer understanding of the roles basement membrane components, associated growth factors, proteases, and receptors have in regulating diverse cellular behaviors (Hynes, 2009; Yurchenco, 2011). Thus, rather than complex endpoint phenotypes, live cell approaches allow examination of normal and disrupted cellular behaviors as they occur. For example, using live imaging we have shown that the integrin cell–matrix receptor has independent functions in both mediating cell–basement membrane attachment and establishing a specialized cell membrane domain that directs invasion through the basement membrane (Hagedorn et al., 2009; Wang et al., 2014).
Finally, I expect that broad analysis of organismal development and physiology will continue to provide significant findings in cell–matrix biology. This is illustrated by studies showing dramatic changes in basement membrane accumulation during aging, and the findings that enhanced expression of matrix remodeling components increase organismal longevity (Candiello et al., 2010; Ewald et al., 2015). CRISPR/Cas9-mediated genome editing will also allow functional and live-cell analysis of cell–matrix interactions outside of current model systems, especially in basal metazoans such as translucent Cnidarian and Ctenophore embryos (Ikmi et al., 2014). These technical advances, and a wide experimental net, will bring a more comprehensive understanding of the fascinating, fundamental, and ancient interactions of cells and their surrounding extracellular matrix. This will have profound importance to human health, as numerous inherited diseases are caused by mutations in basement membrane components and changes in basement membrane structure are associated with the pathogenesis of diseases such as diabetes, hypertension, Alzheimer’s, and cancer (Tsilibary, 2003; Zlokovic, 2008; Van Agtmael and Bruckner-Tuderman, 2010; Lu et al., 2012; Kelley et al., 2014).
Acknowledgements
I thank Kacy Gordon, Matt Clay, Dan Keeley, Meghan Morrissey, and Ranjay Jayadev for helpful comments. The illustration in Fig. 1 was provided by Neil Smith (www.neilsmithillustration.co.uk).
D.R. Sherwood was supported by the Pew Scholars Program in the Biomedical Sciences and National Institutes of Health grants GM079320 and GM100083.
The author declares no competing financial interest.
References
- Avery L., and Horvitz H.R.. 1989. Pharyngeal pumping continues after laser killing of the pharyngeal nervous system of C. elegans. Neuron. 3:473–485. 10.1016/0896-6273(89)90206-7 [DOI] [PubMed] [Google Scholar]
- Bernfield M., and Banerjee S.D.. 1982. The turnover of basal lamina glycosaminoglycan correlates with epithelial morphogenesis. Dev. Biol. 90:291–305. 10.1016/0012-1606(82)90378-5 [DOI] [PubMed] [Google Scholar]
- Bhave G., Cummings C.F., Vanacore R.M., Kumagai-Cresse C., Ero-Tolliver I.A., Rafi M., Kang J.S., Pedchenko V., Fessler L.I., Fessler J.H., and Hudson B.G.. 2012. Peroxidasin forms sulfilimine chemical bonds using hypohalous acids in tissue genesis. Nat. Chem. Biol. 8:784–790. 10.1038/nchembio.1038 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Candiello J., Balasubramani M., Schreiber E.M., Cole G.J., Mayer U., Halfter W., and Lin H.. 2007. Biomechanical properties of native basement membranes. FEBS J. 274:2897–2908. 10.1111/j.1742-4658.2007.05823.x [DOI] [PubMed] [Google Scholar]
- Candiello J., Cole G.J., and Halfter W.. 2010. Age-dependent changes in the structure, composition and biophysical properties of a human basement membrane. Matrix Biol. 29:402–410. 10.1016/j.matbio.2010.03.004 [DOI] [PubMed] [Google Scholar]
- Ewald C.Y., Landis J.N., Porter Abate J., Murphy C.T., and Blackwell T.K.. 2015. Dauer-independent insulin/IGF-1-signalling implicates collagen remodelling in longevity. Nature. 519:97–101. 10.1038/nature14021 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fidler A.L., Vanacore R.M., Chetyrkin S.V., Pedchenko V.K., Bhave G., Yin V.P., Stothers C.L., Rose K.L., McDonald W.H., Clark T.A., et al. 2014. A unique covalent bond in basement membrane is a primordial innovation for tissue evolution. Proc. Natl. Acad. Sci. USA. 111:331–336. 10.1073/pnas.1318499111 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goodman S.L., Risse G., and von der Mark K.. 1989. The E8 subfragment of laminin promotes locomotion of myoblasts over extracellular matrix. J. Cell Biol. 109:799–809. 10.1083/jcb.109.2.799 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gotenstein J.R., Swale R.E., Fukuda T., Wu Z., Giurumescu C.A., Goncharov A., Jin Y., and Chisholm A.D.. 2010. The C. elegans peroxidasin PXN-2 is essential for embryonic morphogenesis and inhibits adult axon regeneration. Development. 137:3603–3613. 10.1242/dev.049189 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grashoff C., Hoffman B.D., Brenner M.D., Zhou R., Parsons M., Yang M.T., McLean M.A., Sligar S.G., Chen C.S., Ha T., and Schwartz M.A.. 2010. Measuring mechanical tension across vinculin reveals regulation of focal adhesion dynamics. Nature. 466:263–266. 10.1038/nature09198 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hadley M.A., Byers S.W., Suárez-Quian C.A., Kleinman H.K., and Dym M.. 1985. Extracellular matrix regulates Sertoli cell differentiation, testicular cord formation, and germ cell development in vitro. J. Cell Biol. 101:1511–1522. 10.1083/jcb.101.4.1511 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hagedorn E.J., Yashiro H., Ziel J.W., Ihara S., Wang Z., and Sherwood D.R.. 2009. Integrin acts upstream of netrin signaling to regulate formation of the anchor cell’s invasive membrane in C. elegans. Dev. Cell. 17:187–198. 10.1016/j.devcel.2009.06.006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haigo S.L., and Bilder D.. 2011. Global tissue revolutions in a morphogenetic movement controlling elongation. Science. 331:1071–1074. 10.1126/science.1199424 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Halfter W., Candiello J., Hu H., Zhang P., Schreiber E., and Balasubramani M.. 2013. Protein composition and biomechanical properties of in vivo-derived basement membranes. Cell Adhes. Migr. 7:64–71. 10.4161/cam.22479 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harunaga J.S., Doyle A.D., and Yamada K.M.. 2014. Local and global dynamics of the basement membrane during branching morphogenesis require protease activity and actomyosin contractility. Dev. Biol. 394:197–205. 10.1016/j.ydbio.2014.08.014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hay E.D. 1981. Extracellular matrix. J. Cell Biol. 91:205s–223s. 10.1083/jcb.91.3.205s [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hohenester E., and Yurchenco P.D.. 2013. Laminins in basement membrane assembly. Cell Adhes. Migr. 7:56–63. 10.4161/cam.21831 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hynes R.O. 2009. The extracellular matrix: not just pretty fibrils. Science. 326:1216–1219. 10.1126/science.1176009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hynes R.O. 2012. The evolution of metazoan extracellular matrix. J. Cell Biol. 196:671–679. 10.1083/jcb.201109041 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ihara S., Hagedorn E.J., Morrissey M.A., Chi Q., Motegi F., Kramer J.M., and Sherwood D.R.. 2011. Basement membrane sliding and targeted adhesion remodels tissue boundaries during uterine-vulval attachment in Caenorhabditis elegans. Nat. Cell Biol. 13:641–651. 10.1038/ncb2233 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ikmi A., McKinney S.A., Delventhal K.M., and Gibson M.C.. 2014. TALEN and CRISPR/Cas9-mediated genome editing in the early-branching metazoan Nematostella vectensis. Nat. Commun. 5:5486 10.1038/ncomms6486 [DOI] [PubMed] [Google Scholar]
- Kelley L.C., Lohmer L.L., Hagedorn E.J., and Sherwood D.R.. 2014. Traversing the basement membrane in vivo: a diversity of strategies. J. Cell Biol. 204:291–302. 10.1083/jcb.201311112 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Khoshnoodi J., Pedchenko V., and Hudson B.G.. 2008. Mammalian collagen IV. Microsc. Res. Tech. 71:357–370. 10.1002/jemt.20564 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim S., and Wadsworth W.G.. 2000. Positioning of longitudinal nerves in C. elegans by nidogen. Science. 288:150–154. 10.1126/science.288.5463.150 [DOI] [PubMed] [Google Scholar]
- Liang X., Dong X., Moerman D.G., Shen K., and Wang X.. 2015. Sarcomeres pattern proprioceptive sensory dendritic endings through UNC-52/Perlecan in C. elegans. Dev. Cell. 33:388–400. 10.1016/j.devcel.2015.03.010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lu P., Weaver V.M., and Werb Z.. 2012. The extracellular matrix: a dynamic niche in cancer progression. J. Cell Biol. 196:395–406. 10.1083/jcb.201102147 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Manabe R., Tsutsui K., Yamada T., Kimura M., Nakano I., Shimono C., Sanzen N., Furutani Y., Fukuda T., Oguri Y., et al. 2008. Transcriptome-based systematic identification of extracellular matrix proteins. Proc. Natl. Acad. Sci. USA. 105:12849–12854. 10.1073/pnas.0803640105 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matus D.Q., Chang E., Makohon-Moore S.C., Hagedorn M.A., Chi Q., and Sherwood D.R.. 2014. Cell division and targeted cell cycle arrest opens and stabilizes basement membrane gaps. Nat. Commun. 5:4184 10.1038/ncomms5184 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meng F., Suchyna T.M., Lazakovitch E., Gronostajski R.M., and Sachs F.. 2011. Real time FRET based detection of mechanical stress in cytoskeletal and extracellular matrix proteins. Cell. Mol. Bioeng. 4:148–159. 10.1007/s12195-010-0140-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morrissey M.A., Keeley D.P., Hagedorn E.J., McClatchey S.T., Chi Q., Hall D.H., and Sherwood D.R.. 2014. B-LINK: a hemicentin, plakin, and integrin-dependent adhesion system that links tissues by connecting adjacent basement membranes. Dev. Cell. 31:319–331. 10.1016/j.devcel.2014.08.024 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ozbek S., Balasubramanian P.G., Chiquet-Ehrismann R., Tucker R.P., and Adams J.C.. 2010. The evolution of extracellular matrix. Mol. Biol. Cell. 21:4300–4305. 10.1091/mbc.E10-03-0251 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pastor-Pareja J.C., and Xu T.. 2011. Shaping cells and organs in Drosophila by opposing roles of fat body-secreted Collagen IV and perlecan. Dev. Cell. 21:245–256. 10.1016/j.devcel.2011.06.026 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pöschl E., Schlötzer-Schrehardt U., Brachvogel B., Saito K., Ninomiya Y., and Mayer U.. 2004. Collagen IV is essential for basement membrane stability but dispensable for initiation of its assembly during early development. Development. 131:1619–1628. 10.1242/dev.01037 [DOI] [PubMed] [Google Scholar]
- Rasmussen J.P., Reddy S.S., and Priess J.R.. 2012. Laminin is required to orient epithelial polarity in the C. elegans pharynx. Development. 139:2050–2060. 10.1242/dev.078360 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rozario T., and DeSimone D.W.. 2010. The extracellular matrix in development and morphogenesis: a dynamic view. Dev. Biol. 341:126–140. 10.1016/j.ydbio.2009.10.026 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tsilibary E.C. 2003. Microvascular basement membranes in diabetes mellitus. J. Pathol. 200:537–546. 10.1002/path.1439 [DOI] [PubMed] [Google Scholar]
- Uechi G., Sun Z., Schreiber E.M., Halfter W., and Balasubramani M.. 2014. Proteomic view of basement membranes from human retinal blood vessels, inner limiting membranes, and lens capsules. J. Proteome Res. 13:3693–3705. 10.1021/pr5002065 [DOI] [PubMed] [Google Scholar]
- Van Agtmael T., and Bruckner-Tuderman L.. 2010. Basement membranes and human disease. Cell Tissue Res. 339:167–188. 10.1007/s00441-009-0866-y [DOI] [PubMed] [Google Scholar]
- Wang Z., Chi Q., and Sherwood D.R.. 2014. MIG-10 (lamellipodin) has netrin-independent functions and is a FOS-1A transcriptional target during anchor cell invasion in C. elegans. Development. 141:1342–1353. 10.1242/dev.102434 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yurchenco P.D. 2011. Basement membranes: cell scaffoldings and signaling platforms. Cold Spring Harb. Perspect. Biol. 3:a004911 10.1101/cshperspect.a004911 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zlokovic B.V. 2008. The blood-brain barrier in health and chronic neurodegenerative disorders. Neuron. 57:178–201. 10.1016/j.neuron.2008.01.003 [DOI] [PubMed] [Google Scholar]