Abstract
Chronic hamstring origin avulsions and ischial tunnel syndrome are common causes of posterior hip pain. Although physical therapy has shown benefits in some cases, recent evidence has reported better outcomes with surgical treatment in appropriately selected patients. The full-open approach has been the classic procedure to address this problem. However, the complications related to extensive tissue exposure and the proximity of the incision to the perianal zone have led to the description of full-endoscopic techniques. Achieving an accurate hamstring repair could be technically demanding with a full-endoscopic procedure. Accurate reattachment is crucial in hamstring repair because of the functional demand of the muscles crossing of 2 major joints (hip and knee). This surgical note describes a mixed technique including a mini-open approach, neuromonitoring, and dry endoscopic-assisted repair of the hamstring origin as an alternative for treating patients with chronic hamstring avulsions and ischial tunnel syndrome that remain symptomatic despite nonoperative treatment.
Ischial tunnel syndrome (also known as hamstring syndrome) is described as pain in the lower buttock region radiating down the posterior thigh to the popliteal fossa and is often associated with hamstring weakness.1,2 In a cadaveric study by Miller and Webb,3 the sciatic nerve was found to have an intimate relation with the ischium and the hamstring origin. The sciatic nerve was located an average of 1.2 ± 0.2 cm from the most lateral aspect of the ischial tuberosity (Fig 1).3 Because of the intimate relation, hamstring avulsions can lead to ischial tunnel syndrome involving the sciatic nerve due to scarring around the nerve or the formation of tight fibrotic bands, or reduction in total space for sciatic nerve.4,5
Fig 1.
Dissection of the subgluteal space in a left hip in a cadaver in the prone position through a posterior approach. The gluteus maximus was removed to see the subgluteal space. One should observe the intimate relation between the hamstring origin attachment and the sciatic nerve. (1, conjoined tendon origin [semitendinosus and biceps femoris]; 2, semimembranosus origin [lateral to conjoined tendon; yellow arrow]; 3, sciatic nerve; 4, sacrotuberous ligament; 5, sacrospinous ligament; 6, inferior gluteal artery; 7, piriformis muscle; 8, superior gemellus muscle; 9, obturator internus muscle; 10, inferior gemellus muscle; 11, quadratus femoris muscle [inferior half was removed to see lesser trochanter]; 12, lesser trochanter; 13, vastus lateralis muscle; 14, greater trochanter; 15, gluteus medius muscle.)
In patients with chronic hamstring avulsions, common complaints include pain with sitting, with stretching, and with exercise (sprinting and acceleration).1,2 Palpable tenderness is located around the ischial tuberosity, and hamstring muscle strength shows marked weakness and pain at 30° of knee flexion, although strength is normal and pain is improved at 90° of knee flexion (Fig 2).2
Fig 2.
(A) Re-creation of patient symptoms of sciatic irritation by contraction of hamstring against resistance with patient in seated position at 30° of knee flexion and (B) alleviation at 90° of knee flexion. The white lines show the knee flexion angle when performing the test, and the red arrows indicate the vector of force of the patient's leg.
Radiographs usually yield negative findings unless bony avulsion is present.6 On magnetic resonance imaging, the following findings associated with hamstring tendinopathy have been described: increased tendon size, pretendinous T2 signal with a distal feathery appearance, and ischial tuberosity and/or sciatic nerve edema (Fig 3).7
Fig 3.

Axial magnetic resonance image of hip (T2 sequence). One should observe the left ischial tuberosity with increased hamstring tendon size and unattached appearance of the semimembranosus origin next to the sciatic nerve (red arrow) and compare it with the contralateral side (green arrow). (F, femur; GM, gluteus maximus; SN, sciatic nerve.)
Most muscle or myotendinous hamstring injuries improve with a variety of nonsurgical modalities and rest.6 However, treatment of hamstring origin avulsions is still controversial, and surgery has been recommended in the literature for osseous avulsions with more than 2 cm of retraction, complete avulsions in all 3 tendons with or without retraction, and partial avulsions that remain symptomatic despite nonoperative treatment.5,6,8 Although good results have been reported with open surgery,9-12 Dierckman and Guanche,13 as well as other authors,8,14 have been successfully leading the implementation of new endoscopic techniques to avoid complications due to extensive open approaches.
Full-endoscopic techniques have been very helpful for addressing hamstring avulsions and decrease the risk of nerve injury by avoiding nerve retraction during the surgical procedure.13 However, achieving an accurate reattachment of the tendons over the anatomic footprint can be a technically demanding procedure for surgeons who are not trained in hip arthroscopy. A mini-open surgical technique assisted by dry endoscopy is a reasonable alternative to address chronic hamstring avulsions and ischial tunnel syndrome.
Technique
The procedure is usually performed on an outpatient basis with the patient under general anesthesia. Local anesthesia with 30 mL of 0.25% bupivacaine hydrochloride may be used for postoperative pain control. The patient is placed in the prone position on the operating table with the contralateral leg draped (Fig 4).
Fig 4.

Patient in prone position with left hip and leg draped free. The superolateral mark (X) was made before surgery for corroborating the symptomatic side. The inferomedial mark (dot) was drawn over the most painful spot as described by the patient (at the tip of the ischial tuberosity).
Intraoperative Neurophysiological Monitoring
Neuromonitoring has been suggested as one means of reducing the incidence of intraoperative nerve insult.15-18 The primary emphasis of intraoperative neuromonitoring in this technique is to protect the sciatic nerve function. Transcranial motor evoked potentials and spontaneous electromyographic activity are recorded from the gluteus medius, biceps femoris, vastus lateralis, tibialis anterior, and gastrocnemius muscles innervated by the superior gluteal nerve, tibial nerve, femoral nerve, deep peroneal branches of the sciatic nerve, and tibial branches of the sciatic nerve, respectively.
Somatosensory evoked potentials to stimulate the superficial and deep peroneal nerves and the posterior tibial nerve are performed. The same muscles monitored for electromyographic activity are used to monitor motor potentials. Stimulation of both the left and right sides is performed (Fig 5).
Fig 5.
Neuromonitoring setup. The patient is placed in the prone position. Transcranial motor evoked potentials and spontaneous electromyographic activity are recorded from the gluteus medius, biceps femoris, vastus lateralis, tibialis anterior, and gastrocnemius muscles innervated by the superior gluteal nerve, tibial nerve, femoral nerve, deep peroneal branches of the sciatic nerve, and tibial branches of the sciatic nerve, respectively. Somatosensory evoked potentials to stimulate the superficial and deep peroneal nerves and the posterior tibial nerve are performed. The same muscles monitored for electromyographic activity are used to monitor motor potentials. (A) Stimulation of both the left and right sides is performed. (B) Intraoperative neuromonitoring data.
Mini-Open Approach
With the patient in the prone position, the locations of the ischial tuberosity and the ischiofemoral space are identified through fluoroscopy. An 8-cm transverse line is drawn superolaterally to inferomedially over the fluoroscopic mark, superior to the gluteal crease (Fig 6).
Fig 6.

Fluoroscopic-guided open approach over the lateral aspect of the ischial tuberosity and the ischiofemoral space on the left side with the patient in the prone position. A radiopaque tool is used to orient the mark on the skin. The incision's orientation (dashed line) travels superolaterally to inferomedially to allow an adequate angle of attack for anchor placement. (IT, ischial tuberosity; LT, lesser trochanter.)
After sterile draping and preparation, a subcutaneous plane is developed. The fascia is opened along the longitudinal aspect of the gluteus maximus (Fig 7). The gluteus maximus is dissected carefully, close to the lateral border of the ischium, approximately at the junction of the proximal two-thirds and distal one-third of the muscle. Special attention should be paid to sciatic nerve and vein manipulation and retraction because of their predominant location in this area.
Fig 7.
Direction of muscle fibers versus incision on the left side with the patient in the prone position through a posterior approach. The green dashed line indicates the muscle fibers of the gluteus maximus after subcutaneous dissection. The direction of these fibers differs from the direction of the incision (yellow dashed line). This is an important factor to consider when performing dissection of the muscular fibers to access the subgluteal space.
Dry Endoscopic-Assisted Tendon Evaluation and Reattachment
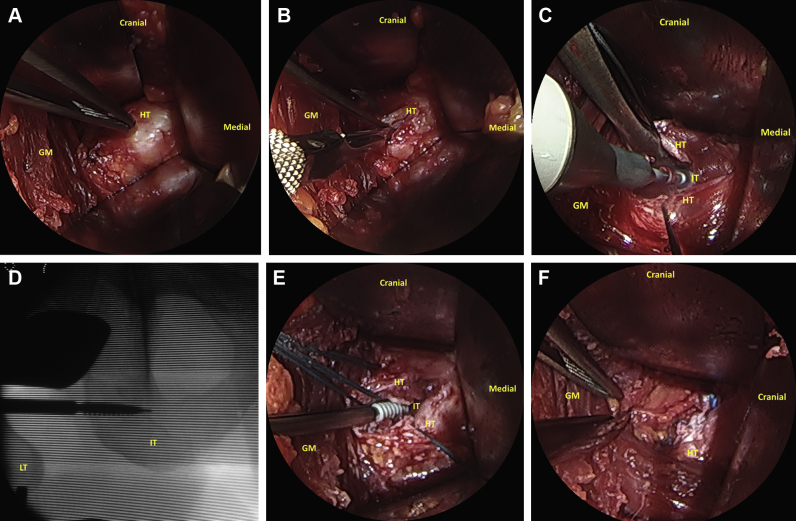
A 30° or 70° dry endoscope (Smith & Nephew, London, England) is inserted through the incision and advanced to evaluate the origin of the hamstring under magnification (Fig 8A). At this point, the surgeon can accurately confirm the size and type of injury, as well as the tendons involved, under magnification. While the surgeon is visualizing with the endoscope, the midsubstance of the tendon is opened longitudinally with a blade and inspected (Fig 8B). The degenerated tendon fibers and the bursa are removed before repair, with care taken to preserve the healthy tendon. The footprint of the torn tendon is decorticated with a 4.0-mm burr (Abrader; Smith & Nephew) to make the bone more parallel to improve the healing process by producing a bleeding bed (Fig 8C). Once the parallel orientation is obtained, the tendon is reattached to the anatomic footprint with a 4.5-mm suture (Healicol; Smith & Nephew) under direct and fluoroscopic visualization (Fig 8 D and E). These steps are then repeated for the next anchors as needed (Fig 8F).
Fig 8.
Sequence of the mini-open approach for repairing a semimembranosus avulsion under dry endoscopic assistance on the left side with the patient in the prone position through a posterior approach: (A) hamstring origin evaluation, (B) longitudinal cut of the hamstring origin in the middle of the damaged zone, (C) decortication using a burr, (D) footprint drilling under fluoroscopic control, (E) anchor placement at the footprint, and (F) reattachment of the tendon using sutures and final aspect after repair and stability verification. (GM, gluteus maximus; HT, hamstring tendons; IT, ischial tuberosity; LT, lesser trochanter.)
At the end of the procedure, the sciatic nerve should be inspected through direct visualization and intraoperative neural monitoring before and after the hamstring origin repair. The wound dressing should be hermetic to decrease the infection risk due to the proximity to the perianal zone. The described technique is demonstrated in Video 1.
Postoperative Rehabilitation
Physical therapy is initiated within 2 to 3 days after surgery. A hinged knee brace is indicated immediately after surgery on the operative side to prevent strain of the hamstring repair. The brace will allow knee flexion and avoid knee extension beyond an established amount (usually 30° to 45° of flexion). At the fourth week postoperatively, a gradual increase in the amount of extension allowed by the brace is recommended. Most patients will discontinue the knee brace at 6 to 8 weeks postoperatively. Use of crutches is recommended for 6 to 8 weeks, with partial weight bearing on the operative side (foot flat as tolerated) during the first 2 weeks and a gradual increase in weight bearing thereafter.
Discussion
Surgical treatment of hamstring avulsions and ischial tunnel syndrome is a challenging problem in hip surgery. These injuries require a delicate exploration and an accurate repair because of the functional demand of the hamstring muscles crossing 2 major joints (hip and knee).
Most hamstring injuries are strains of the muscle or myotendinous junction that can be treated successfully with physical therapy. However, chronic hamstring origin avulsions and secondary ischial tunnel syndrome respond to physical therapy less frequently than muscle or myotendinous strains without sciatic nerve involvement.
Harris et al.19 performed a systematic review comparing clinical outcomes after open surgery versus nonsurgical treatment for hamstring proximal injuries. They included 18 studies (298 patients) and concluded that the open surgical repair resulted in significantly better subjective outcomes, a greater rate of return to patients' preinjury level of sport, and greater strength/endurance than nonsurgical management. The results were apparent in both patients with acute tears and those with chronic tears.
Considering that retraction of the hamstring muscle produces traction of the sciatic nerve and subsequent symptoms, Young et al.2 reported a study showing improvement of the sciatic symptoms after surgical release of the hamstring tendons. However, this approach has not been compared with reattachment in controlled studies in terms of strength and return to physical and sport activities.
Despite the results with open approaches, several complications have been reported, including sciatic nerve injuries, retears, and postoperative infection due to the proximity of the incision to the perianal zone.19 The risk of infection and other complications related to extensive open approaches has recently led to the successful development of full-endoscopic techniques for addressing hamstring origin tears.8,13,14
Thus far, there have been no studies reporting the results or complications of full-endoscopic techniques. As shown at meetings and in technical notes, the hamstring origin repair can be achieved by experienced surgeons trained in hip arthroscopy and extra-articular endoscopy. However, in the experience of the senior author (H.D.M.), achieving an accurate hamstring repair is technically demanding with a full-endoscopic procedure.
This report presents a mixed technique as an alternative to perform hamstring origin repair using guidance with dry endoscopy and a smaller incision than the open traditional approaches. Neurophysiological monitoring is performed routinely to decrease the risk of iatrogenic sciatic nerve damage.
The theoretical advantages when compared with a full-endoscopic repair could include a less technically demanding procedure for achieving an accurate repair, no risk of fluid extravasation, and a similar risk of wound infections. The preliminary results of an ongoing project after a short-term follow-up in patients who underwent dry endoscopic-assisted mini-open repair have shown comparable outcomes to full-open surgery, with no reports of infections or retears to date.
A potential limitation of the endoscopic-assisted mini-open technique as compared with full-open approaches could be the inability to mobilize large and retracted chronic hamstring tears. A summary of the described technique, including advantages, disadvantages, tips, pitfalls, and risks, is shown in Table 1.
Table 1.
Key Points for Dry Endoscopic-Assisted Mini-Open Hamstring Repair
| Indications |
| Chronic hamstring origin tears |
| Ischial tunnel syndrome |
| Advantages |
| The surgeon can view the pathologic area under magnification. |
| The technique is less technically demanding than full-endoscopic approaches. |
| Accurate anchor placement can be achieved. |
| A lesser trochanterplasty for concomitant ischiofemoral impingement can be performed. |
| The risk of wound infection is theoretically lower than that with full-open procedures and comparable with full-endoscopic techniques. |
| The sciatic nerve can be protected under direct visualization. |
| Sciatic nerve decompression can be achieved. |
| There is no risk of unrecognized fluid extravasation. |
| Disadvantages |
| Large retracted tears might not be able to be addressed. |
| Theoretical risks |
| Wound infection |
| Retear |
| Nerve damage |
| Vascular damage |
| Nonaesthetic scar |
| Tips and pearls |
| Accurate diagnosis of hamstring origin tears should be made through a comprehensive history, physical examination, and radiologic evaluation. |
| The surgeon should ensure that the injury can be addressed with the mini-open technique. |
| A concomitant diagnosis of ischiofemoral impingement should be defined before surgery. |
| Neurophysiological monitoring should be used. |
| The incision should be guided by fluoroscopy. |
| The incision should travel superolaterally to inferomedially to have a better attack angle for anchor placement. |
| A hermetic dressing should be used because the incision is close to the perianal zone. |
| Pitfalls |
| Failure to diagnose concomitant ischiofemoral impingement |
| Failure to accurately identify hamstring proximal attachment site |
| Under-tensioning of repair |
| Use of technique for large tears including those of myotendinous junction |
Dry endoscopic-assisted mini-open hamstring origin repair with neuromonitoring is a reproducible procedure and could be effective and safe for addressing chronic hamstring origin avulsions (especially semimembranosus tears) and ischial tunnel syndrome. Further studies reporting and comparing outcomes of full-open, full-endoscopic, and endoscopic-assisted surgical techniques are needed.
Footnotes
The authors report the following potential conflict of interest or source of funding: H.D.M. is a consultant for Smith & Nephew.
Supplementary Data
Hamstring origin repair with a 4.5-mm suture anchor through a mini-open posterior approach after cortical decortication of the region performed on a 47-year-old female patient (prone position, left side). The procedure was assisted using a dry 70° endoscope and neuromonitoring. This approach allows surgical treatment for ischiofemoral impingement by performing a concomitant lesser trochanterplasty.
References
- 1.Puranen J., Orava S. The hamstring syndrome: A new diagnosis of gluteal sciatic pain. Am J Sports Med. 1988;16:517–521. doi: 10.1177/036354658801600515. [DOI] [PubMed] [Google Scholar]
- 2.Young I.J., van Riet R.P., Bell S.N. Surgical release for proximal hamstring syndrome. Am J Sports Med. 2008;36:2372–2378. doi: 10.1177/0363546508322905. [DOI] [PubMed] [Google Scholar]
- 3.Miller S.L., Webb G.R. The proximal origin of the hamstrings and surrounding anatomy encountered during repair. Surgical technique. J Bone Joint Surg Am. 2008;90:108–116. doi: 10.2106/JBJS.G.01281. (suppl 2) [DOI] [PubMed] [Google Scholar]
- 4.Martin H.D., Shears S.A., Johnson J.C., Smathers A.M., Palmer I.J. The endoscopic treatment of sciatic nerve entrapment/deep gluteal syndrome. Arthroscopy. 2011;27:172–181. doi: 10.1016/j.arthro.2010.07.008. [DOI] [PubMed] [Google Scholar]
- 5.Wood D.G., Packham I., Trikha S.P., Linklater J. Avulsion of the proximal hamstring origin. J Bone Joint Surg Am. 2008;90:2365–2374. doi: 10.2106/JBJS.G.00685. [DOI] [PubMed] [Google Scholar]
- 6.Cohen S., Bradley J. Acute proximal hamstring rupture. J Am Acad Orthop Surg. 2007;15:350–355. doi: 10.5435/00124635-200706000-00004. [DOI] [PubMed] [Google Scholar]
- 7.De Smet A.A., Blankenbaker D.G., Alsheik N.H., Lindstrom M.J. MRI appearance of the proximal hamstring tendons in patients with and without symptomatic proximal hamstring tendinopathy. AJR Am J Roentgenol. 2012;198:418–422. doi: 10.2214/AJR.11.6590. [DOI] [PubMed] [Google Scholar]
- 8.Domb B.G., Linder D., Sharp K.G., Sadik A., Gerhardt M.B. Endoscopic repair of proximal hamstring avulsion. Arthrosc Tech. 2013;2:e35–e39. doi: 10.1016/j.eats.2012.10.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Cohen S.B., Rangavajjula A., Vyas D., Bradley J.P. Functional results and outcomes after repair of proximal hamstring avulsions. Am J Sports Med. 2012;40:2092–2098. doi: 10.1177/0363546512456012. [DOI] [PubMed] [Google Scholar]
- 10.Lempainen L., Sarimo J., Heikkilä J., Mattila K., Orava S. Surgical treatment of partial tears of the proximal origin of the hamstring muscles. Br J Sports Med. 2006;40:688–691. doi: 10.1136/bjsm.2006.028191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Rust D.A., Giveans M.R., Stone R.M., Samuelson K.M., Larson C.M. Functional outcomes and return to sports after acute repair, chronic repair, and allograft reconstruction for proximal hamstring ruptures. Am J Sports Med. 2014;42:1377–1383. doi: 10.1177/0363546514528788. [DOI] [PubMed] [Google Scholar]
- 12.Sarimo J., Lempainen L., Mattila K., Orava S. Complete proximal hamstring avulsions: A series of 41 patients with operative treatment. Am J Sports Med. 2008;36:1110–1115. doi: 10.1177/0363546508314427. [DOI] [PubMed] [Google Scholar]
- 13.Dierckman B.D., Guanche C.A. Endoscopic proximal hamstring repair and ischial bursectomy. Arthrosc Tech. 2012;1:e201–e207. doi: 10.1016/j.eats.2012.07.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Jackson T.J., Trenga A., Lindner D., El-Bitar Y., Domb B.G. Endoscopic transtendinous repair for partial-thickness proximal hamstring tendon tears. Arthrosc Tech. 2014;3:e127–e130. doi: 10.1016/j.eats.2013.09.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Porat M., Orozco F., Goyal N., Post Z., Ong A. Neurophysiologic monitoring can predict iatrogenic injury during acetabular and pelvic fracture fixation. HSS J. 2013;9:218–222. doi: 10.1007/s11420-013-9347-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Baumgaertner M.R., Wegner D., Booke J. SSEP monitoring during pelvic and acetabular fracture surgery. J Orthop Trauma. 1994;8:127–133. doi: 10.1097/00005131-199404000-00009. [DOI] [PubMed] [Google Scholar]
- 17.Calder H.B., Mast J., Johnstone C. Intraoperative evoked potential monitoring in acetabular surgery. Clin Orthop Relat Res. 1994;305:160–167. [PubMed] [Google Scholar]
- 18.Sutherland C.J., Miller D.H., Owen J.H. Use of spontaneous electromyography during revision and complex total hip arthroplasty. J Arthroplasty. 1996;11:206–209. doi: 10.1016/s0883-5403(05)80020-8. [DOI] [PubMed] [Google Scholar]
- 19.Harris J.D., Griesser M.J., Best T.M., Ellis T.J. Treatment of proximal hamstring ruptures—A systematic review. Int J Sports Med. 2011;32:490–495. doi: 10.1055/s-0031-1273753. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Hamstring origin repair with a 4.5-mm suture anchor through a mini-open posterior approach after cortical decortication of the region performed on a 47-year-old female patient (prone position, left side). The procedure was assisted using a dry 70° endoscope and neuromonitoring. This approach allows surgical treatment for ischiofemoral impingement by performing a concomitant lesser trochanterplasty.