Abstract
Mammalian cells can express up to 4 different class I PI3K isoforms, each of which is engaged by tyrosine kinases or G-protein coupled receptors (GPCRs) to generate the second messenger signaling molecule PtdIns(3,4,5)P3 (PIP3). The p110α and p110β isoforms are relatively widely expressed, whereas p110γ and p110δ are more highly expressed in cells of the immune system than in other cell types. Nevertheless, each of the 4 class I PI3Ks have been shown to participate in the orchestration of the signaling events that lead to immune cell development, control of gene expression, skewing towards individual cell lineage subsets and proliferation.
Introduction
Because they are constitutively bound by p85-related regulatory subunits p110α, p110β and p110β were thought to be regulated primarily by tyrosine kinases (p85 contains two SH2 domains that bind tyrosine phosphorylated peptides), whereas p110γ which is bound by a Gβγ-binding p101 or p84 regulatory subunit is preferentially activated by G-protein coupled receptors. However, by mechanisms that have yet to be elucidated, p110β and p110δ can also be activated by GPCRs. Over the last few years, experiments combining the use of gene-targeted mice and small molecule inhibitors have identified individual roles for each of the four class I PI3K catalytic subunits in immunity.
Role of p110α and p110δ in B cell development and survival
During early B cell development, p110δ plays a redundant role with p110α to promote further differentiation after the pre-B cells have rearranged their immunoglobulin heavy chain which forms part of the pre-B cell receptor complex1. Remarkably, the expression of a p110α from a single allele is sufficient to allow B cell development beyond this checkpoint in the bone marrow. By contrast, in mature B cells, p110α does not contribute significantly to signaling by the mature B cell receptor which is composed of immunoglobulin heavy and light chains. The molecular basis of the reduced role of p110α as B cells mature is not thought to be due to reduced expression of this isoform, rather, it appears that p110α is less well adapted to respond to acute signaling induced by clustering of the BCR rather than by so-called tonic signaling. Accordingly, B1 and marginal zone B cells, which are thought be auto-reactive, develop in absence of p110α, but not in the absence of p110δ activity1, 2. However, pre-B cell development and mature B cell survival and, which are driven by tonic-signaling by BCR in absence of agonistic antigen, are dependent on both p110α and p110δ.
Role of p110δ and p110γ in T cell development
T cells retain the ability to develop in the thymus and populate the spleen and lymph nodes, despite loss of p110α and p110δ1. However, when p110δ and p110γ were both lost, T cell development was blocked at the developmental stage where the T cell receptor β chain genes have been rearranged and is expressed on the cell surface as part of the pre-TCR complex3. The different requirement for p110δ and p110γ in B cell development versus T cell development could suggest different requirements for receptor engagement. Indeed, T cell development was shown to depend on the engagement of the chemokine receptor CXCR4 which signals via p110γ, thus complementing pre-TCR signaling via p110δ3. A similar scenario was apparent in mature T cells4. B cell development is normal in p110δ-p110γ double knockouts, however this observation does not necessarily rule out a role for GPCR signaling in B cell development, since for unknown reasons, GPCRs tend to signal via p110δ rather than p110γ in B cells5.
p110δ activity in T cells is required for B cell activation in the germinal center
Mature CD4 and CD8 T cell differentiation and effector cytokine production is under tight control by p110δ6, 7. The important role for p110δ in T cell subset differentiation has an initially unforeseen consequence. Based on in vitro proliferation assays it appeared that B cells were much more affected by p110δ deficiency than T cells2. Indeed, primary and secondary T cell-dependent antibody responses are dramatically reduced in p110δ deficient mice1, 2, 4. However, deletion of p110δ in B cells had little effect on such antibody responses whereas deletion of p110δ in T cells mimicked the effect of germline deletion of p110δ8. The latter was correlated with a key role for p110δ in promoting the differentiation of follicular helper T cells, a specialized CD4 T cell subset which is recruited to the germinal centers within the spleen follicles where T cells provide help to B cells to undergo immunoglobulin class switching and affinity maturation8. Thus while in vitro studies predicted that the main defect in p110δ-deficient mice was due to the role of p110δ in B cells, cell-specific gene targeting revealed a more prominent role in T cells during humoral immune responses to protein antigens.
The p110γ, p110δ, and p110β isoforms differentially contribute to ROS production by neutrophils
P110β is not highly expressed in lymphocytes, but plays a significant role in the response by neutrophils to immune complexes composed of IgG and a bound antigen9. Previous work had identified distinct roles for p110γ and p110δ in neutrophils stimulated with a GPCR ligand (fMLP). The initial pulse of PIP3 production is provided only by p110γ followed by more sustained production of PIP3 which is p110δ-dependent (but absent in without the initial activation of p110γ)10. The p110δ-dependent pulse was required to trigger reactive oxygen species (ROS) by the NADPH oxidase. The unique requirement for p110β in the context of the FcγR signaling is explained by the concurrent production of leukotriene B4. Together, FcγR and the leukotriene receptor BLT1 depend on p110β to generate a sustained signal that results in ROS generation, whereas short-term BLT1 signaling is more dependent on p110γ. Dual inhibition of p110β and p110δ revealed some overlap in function between these kinases in this context, but p110β was dominant. These data suggest that p110β may be particularly well adapted to integrate concurrent signals from tyrosine kinase and GPCR linked receptors.
Perspective.
Together, these studies are beginning to delineate unique, and often non-redundant and cell-type specific roles for each of the four class I PI3Ks in immune responses. Inhibition of p110α by itself is unlikely to have a significant effect on immunity. However, dual inhibition of p110δ and p110γ, p110δ and p110α, or indeed p110δ and p110β, can act synergistically to profoundly block T cell development, B cell development, humoral immunity and the activation of neutrophils by immune complexes. These studies may inspire the use of dual specific inhibitors to block diseases and malignancies involving these cell types, but also caution against the unintended effect of pan-specific PI3K inhibitors on immunity.
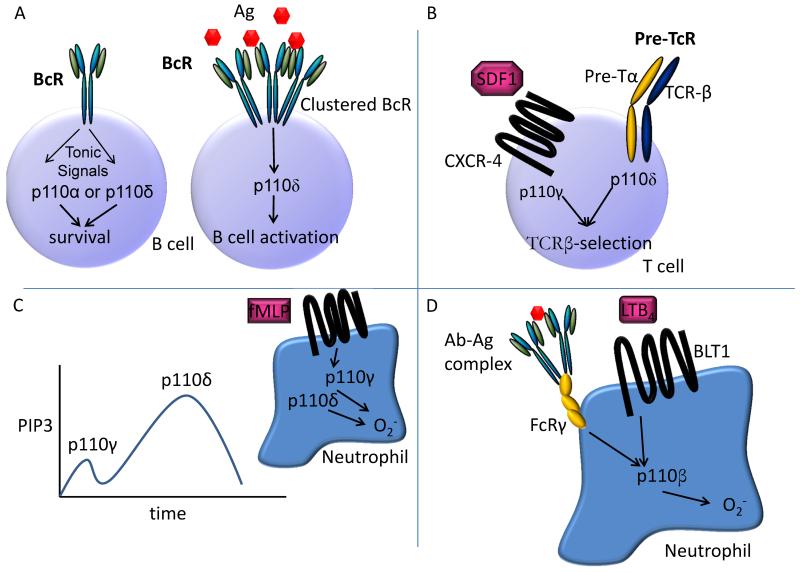
Figure 1.
A. Both p110α and p110δ are engaged by the pre-BCR or mature BCR in absence of acute agonistic stimulation by antigen. By contrast, inhibition of p110δ, but not p110α, blocks Akt-phosphorylation and B cell proliferation in response to clustering of the BCR.
B. In T cells, both the pre-TCR and mature TCR signal primarily via p110δ, whereas chemokines stimulate the activity of p110γ via the activation of GPCRs such as CXCR4. During T cell development, PIP3 generated via either of these pathways is required. In mature T cells, the TCR and chemokine receptors contribute non-redundantly to processes requiring the activation of PI3K.
C. In primed neutrophils stimulated with the bacteria-derived formylated tripeptide fMLP, and initial burst of p110γ-generated PIP3 is followed by a more sustained burst of p110δ-dependent PIP3. It is not clear how p110δ becomes activated after GPCR engagement.
D. The engagement of the FcγR by antibody-antigen complexes stimulates a complex autocrine route that involving LTB4 and the GPCR BLT1. Together, these engage p110β which is uniquely positioned to respond effectively to both tyrosine kinase and GPCR signals.
Acknowledgements
Research in the author’s laboratory is supported by grants from the Biotechnology and Biological Sciences Research Council and the Wellcome Trust.
References
- 1.Ramadani F, et al. The PI3K isoforms p110alpha and p110delta are essential for pre-B cell receptor signaling and B cell development. Sci Signal. 2010;3:ra60. doi: 10.1126/scisignal.2001104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Okkenhaug K, et al. Impaired B and T cell antigen receptor signaling in p110delta PI 3-kinase mutant mice. Science. 2002;297:1031–1034. doi: 10.1126/science.1073560. [DOI] [PubMed] [Google Scholar]
- 3.Janas ML, et al. Thymic development beyond beta-selection requires phosphatidylinositol 3-kinase activation by CXCR4. J Exp Med. 2010;207:247–261. doi: 10.1084/jem.20091430. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Garcon F, et al. CD28 provides T-cell costimulation and enhances PI3K activity at the immune synapse independently of its capacity to interact with the p85/p110 heterodimer. Blood. 2008;111:1464–1471. doi: 10.1182/blood-2007-08-108050. [DOI] [PubMed] [Google Scholar]
- 5.Reif K, et al. Cutting Edge: Differential Roles for Phosphoinositide 3-Kinases, p110γ and p110δ, in Lymphocyte Chemotaxis and Homing. J Immunol. 2004;173:2236–2240. doi: 10.4049/jimmunol.173.4.2236. [DOI] [PubMed] [Google Scholar]
- 6.Okkenhaug K, et al. The p110delta isoform of phosphoinositide 3-kinase controls clonal expansion and differentiation of Th cells. J Immunol. 2006;177:5122–5128. doi: 10.4049/jimmunol.177.8.5122. [DOI] [PubMed] [Google Scholar]
- 7.Soond DR, et al. PI3K p110delta regulates T-cell cytokine production during primary and secondary immune responses in mice and humans. Blood. 2010;115:2203–2213. doi: 10.1182/blood-2009-07-232330. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Rolf J, et al. Phosphoinositide 3-kinase activity in T cells regulates the magnitude of the germinal center reaction. J Immunol. 2010;185:4042–4052. doi: 10.4049/jimmunol.1001730. [DOI] [PubMed] [Google Scholar]
- 9.Kulkarni S, et al. PI3Kbeta Plays a Critical Role in Neutrophil Activation by Immune Complexes. Sci Signal. 2011;4:ra23. doi: 10.1126/scisignal.2001617. [DOI] [PubMed] [Google Scholar]
- 10.Condliffe AM, et al. Sequential activation of class IB and class IA PI3K is important for the primed respiratory burst of human but not murine neutrophils. Blood. 2005;106:1432–1440. doi: 10.1182/blood-2005-03-0944. [DOI] [PubMed] [Google Scholar]