Abstract
The Islets of Langerhans are crucial ‘micro-organs’ embedded in the glandular exocrine pancreas that regulate nutrient metabolism. They not only synthesize, but also secrete endocrine hormones in a modulated fashion in response to physiologic metabolic demand. These highly sophisticated structures with intricate organization of multiple cell types, namely endocrine, vascular, neuronal and mesenchymal cells, have evolved to perform this task to perfection over time. Not surprisingly, islet architecture and function are dissimilar between humans and typically studied model organisms, such as rodents and zebrafish. Further, recent findings also suggest noteworthy differences in human islet development from that in mouse, including delayed appearance and gradual resolution of key differentiation markers, a single-phase of endocrine differentiation, and prenatal association of developing islets with neurovascular milieu. In light of these findings, it is imperative that a systematic study is undertaken to compare islet development between human and mouse. Illuminating inter-species differences in islet development will likely be critical in furthering our pursuit to generate an unlimited supply of truly functional and fully mature β-cells from human pluripotent stem cell (hPSC) sources for therapeutic purposes.
Section I: Introduction
Islets emerge via the aggregation of five discrete endocrine cell types (each producing insulin, glucagon, somatostatin, pancreatic polypeptide or ghrelin in the adult organism) that are intimately associated with endothelial cells and neuronal processes to function together as a single unit. Dysregulation of islet function perturbs glucose homeostasis and eventually leads to diabetes. Efforts are underway to generate insulin-producing β-cells from hPSCs in the hope of treating diabetes. Unfortunately, current differentiation protocols produce β-like cells that possess limited glucose responsiveness, only in static insulin secretion assays, and hence are not fully mature[1]. In particular, these hPSC differentiation protocols have relied heavily on information gleaned from pancreas development in animal models, specially rodents[2]. However, critical differences have been well-established between human and mouse adult β-cells, including the regulation of the insulin promoter and thus insulin gene expression[3], expression of glucose transporters[4, 5], responsiveness to neuropeptides [6, 7], and the repertoire of cell-cycle regulators[8]. Besides these molecular dissimilarities, gross islet cytoarchitecture is also markedly different between the two species [9]. This implies disparities should also exist during development. Consequently, implementing developmental mechanisms elucidated exclusively in animal models in hPSC differentiation may not be sufficient to successfully generate pristine mature human β-cells in vitro. In support of this notion, new insights into human pancreas organogenesis do indeed point to deviation from rodent development. Although limited by histological analysis of cadaveric fetal tissue of different gestational ages or ex vivo organogenesis, an overview of human pancreas development is materializing. In this review, we summarize the emerging differences between human and mouse islet development and morphogenesis, and comment on the implications of such differences on our attempts to generate human β-cells in a dish.
Section II: Early pancreas development: From foregut to endocrine specification
Extensive knowledge of molecular and morphological events that regulate mouse pancreas development has been acquired over the last twenty years through pioneering lineage tracing techniques using sophisticated transgenic mouse models[10]. The pancreas arises from two diametrically juxtaposed anlagen located on the dorsal and ventral portions of the developing foregut endoderm. In mouse and chick, notochord-derived signals promote the exclusion of Sonic Hedgehog (Shh), a member of the Hedgehog family of secreted signaling molecules, in the presumptive pancreatic endoderm prior to dorsal bud formation. The absence of Shh in this area permits expression of Pancreatic and duodenal homeobox factor 1 (Pdx1), a transcription factor essential for pancreas development[11], as early as embryonic day 8.75 (e8.75) in mouse when the notochord is still in contact with the endodermal sheet. While SHH expression is also excluded from the human dorsal foregut epithelium slated to develop into pancreas, PDX1 expression is delayed, and detected only after gut closure and separation of the dorsal aorta and notochord by mesenchyme (29-31 days post conception(dpc)) [12](Fig. 1; Table 1). Other transcription factors, including Ptf1a, Gata4, and Gata6 also mark pancreas specification, and their importance in human pancreas development is evidenced by several reports of pancreatic agenesis and permanent neonatal diabetes mellitus (PNDM) caused by mutations in these genes[13-16]. Unlike the situation in rodents, the expression of GATA4 is delayed during human development, appearing at the same time as PDX1. Also, SOX17, a definitive endoderm marker whose expression is lost in rodent pancreas epithelium, persists in the presumptive human pancreatic endoderm[12]. After specification, pancreatic buds rapidly grow into the surrounding mesenchyme, which produces proliferative signals such as FGF10 and FGF7[17], resulting in the formation of a multipotent pancreatic epithelium (30-33 dpc in humans). This immature epithelium is characterized by the expression of Pdx1, Ptf1a, Gata4, Sox9, Nkx2.2, Hnf1b, Foxa2 and Nkx6.1[18] in mouse. Many of these factors were also expressed in the human counterpart; nevertheless, a striking difference is the absence of NKX2.2 expression, which does not appear until after cells have committed towards the endocrine lineage[12] (Fig. 1).
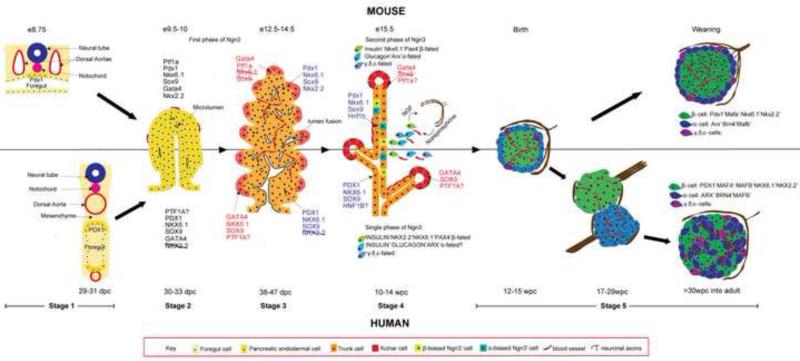
Fig. 1.
Illustration of pancreas morphogenesis in mouse and human. The top half of the figure depicts mouse while the bottom half portrays human development. Stage 1: Pancreas specification: appearance of Pdx1 marks the presumptive pancreatic region in the foregut epithelium. In mouse Pdx1 appears before gut closure when the notochord is in contact with the gut, but in human, PDX1 is delayed and appears only after separation of notochord and aorta from the gut. Stage 2: Formation of pancreatic epithelium: Following Pdx1 expression and Ptf1a activation, pancreatic buds containing MPCs grow into the surrounding mesenchyme. MPCs exhibit similar transcript profile between mouse and human with the exception of NKX2.2. Microlumens are already visible at this stage. Stage 3: Segregation of the tip and trunk domain: MPCs differentiate into pro-acinar tip cells (indicated by light red) and bipotential epithelial cords(light orange) due to opposing functions of Ptf1a and Nkx6.1. This process is dynamic with rampant tubulogenesis. In human, the separation occurs gradually with the tip cells still expressing NKX6.1 and SOX9 at the corresponding age in mice. Stage 4: Endocrine differentiation (also secondary transition in mouse). Terminal differentiation of tips to acinar fate and trunk cells to endocrine/duct fate occur during this period. The second wave of Ngn3 expression coincides with this period in the mouse, whereas in human, this period occurs after embryogenesis and a single phase of Ngn3 expression is observed. The time point indicated denote the peak period of endocrine differentiation in both species. Stage 5: Islet morphogenesis. In mouse, sympathetic axons regulate islet cell clustering by β-adrenergic signaling while a parallel is unknown in human. Moreover, islet formation along with vascularization is completed only at birth in the mouse, whereas in human, islet morphogenesis extends through the second and third trimester but is completed before birth. Initially fetal human islets resemble mouse islets in its architecture. Then, they opens up to give rise to juxtaposed homotypic α/β clusters, and finally acquire the intermingled architecture of adult islets in the last trimester. Innervation of mouse islets is completed at weaning (orange arrow), whereas human islets lose their dense pre-natal neuronal associations. In addition, axons contact vasculature in human adult islets (orange arrowhead). The approximate embryonic/fetal age at which the various stages occur are designated respectively for the two species. Progressively darker shades of the colors representing epithelial and endocrine cells indicate differentiation to more committed fates over time. e-embryonic day; dpc-days post conception; wpc-weeks post conception
Table.1.
Comparison of events and their time of appearance in mouse and human pancreas development.
| Developmental Stage | Mouse | Human | ||
|---|---|---|---|---|
| Events/Processes | Emrbyonic age | Events/Processes | Fetal age | |
| Pancreas specification | Shh exclusion from prospective pancreatic region in the dorsal endoderm (11) | e8.75 | Shh exclusion from prospective pancreatic region in the dorsal endoderm (12) | 25-29 dpc |
| Pdx1 appears in dorsal endoderm before gut closure when the notochord is still in contact with foregut(10) | e8.75 | Pdx1 appears only after gut closure and separation of foregut from notochord and dorsal aorta by the mesenchyme(12) | 29-31 dpc | |
| Gata4 present in ventral endoderm and required for gut closure(68) | e8 | Gata4 present in foregut endoderm only after gut closure (12) | 29-31 dpc | |
| Sox17 absent in dorsal foregut endoderm(69) | e8.5 | Sox17 present in dorsal foregut endoderm(12) | 25-31 dpc | |
| Pancreatic buds/epithelium | MPCs express Pdx1,Ptf1a,Sox9,Gata4,Nkx6.1 and Nkx2.2(10,18) | e9-e11.5 | MPCs express Pdx1,Sox9,Gata4, Nkx6.1 but not Nkx2.2 (12) | 30-35 dpc |
| Microlumens found(20,21) | e10.5 | Microlumens detected (12) | 30-33 dpc | |
| Tip and Trunk domain formation | Nkx6.1 excluded from tip cells whereas Gata4 is restricted in tip cells (19) | e14.5 | Nkx6.1 is still present in tip cells whereas Gata4 is restricted in tip cells (12) | 49-52 dpc |
| Nkx6.1 exclusion from tips complete (12) | 10 wpc | |||
| Mutually exclusive expression of Sox9 and Gata4 expression(70) | e14.5 | Mutually exclusive expression of Sox9 and Gata4 expression (12) | 14 wpc | |
| Trunk domain has Sox9,Hnf1b, Nkx6.1 and Nkx2.2(18,19) | e14.5 | Trunk domain has Sox9,Hnf1b, Nkx6.1 but not Nkx2.2 (12) | 49dpc -14wpc | |
| Endocrine differentiation | Biphasic Ngn3 expression, second wave peaks at e15.5(24) | e8.5-e11 and e12-e18 | Single wave of Ngn3 expression peaking 10-14wpc (25,12) | 8-18 wpc |
| α-cells appear first (30,33) | e8.5 | β-cells appear first (34,35) | 6 wpc | |
| presence of few polyhormonal cells(44) | 20-30% early endocrine cells are polyhoromonal (35,40-43) | <10 wpc | ||
| Nkx2.2 appears after endocrine commitment (12) | ||||
| Islet morphogenesis | Islets have core-mantle structure with β-cells in the center and α,δ,ε and PP cells in the periphery (9,46,49) | Islets have mosaic structure with intermingled β,α,δ,ε and PP cells (9,45-49) | ||
| Islets formed close to birth(10) | >e18 | Islets formation completed in 3rd trimester (42) | >30 dpc | |
| Undergoes a transient mouse-islet like morphology in 2nd trimester(35,40,42,52) | 12-15 wpc | |||
| β-cells outnumber other cell types at birth and form majority of islets cells(24,46) | β-cells equal in number with α,δ-cells at birth and undergo neonatal proliferation to reach baseline mass (35,41,53) | ~2yrs | ||
| Islet Vascularization | Begins during islet cell differentiation(60) | e13.5 | Begins during islet cell differentiation(61,34) | 10 wpc |
| Completed at birth(60) | e20 | Completed in 2nd trimester(61) | 20 wpc | |
| Islet Innervation | Adult Islets are highly innervated with direct contact between axons and endocrine cells(62) | Adult Islets are poorly innervated with contact mostly between axons and smooth muscle cells of the endothelium(62) | ||
| Begins during islet cell clustering (60,64) | e15.5 | Begins during islet cell differentiation(63) | 12 wpc | |
| Completed at weaning(60) | P21 | Highest density of neuroinsular complexes in 3rd trimester and diminishes greatly in adult(63) | 27-38 wpc | |
e-embryonic day; dpc-days post conception; wpc-weeks post conception
The next major event in pancreas development is the segregation of multipotent progenitor cells (MPCs) into spatially distinct ‘tip’ and ‘trunk’ domains. The tip progenitors initially possess the ability to develop into all pancreatic epithelial cell lineages, including acinar, duct, and endocrine cell types. Subsequently, cells that remain at the distal tip of the epithelial structure undergo acinar differentiation, while cells proximal to the tips become bipotent (endocrine/duct) trunk progenitors. The separation of pro-acinar tip cells and trunk cells is thought to be established by an antagonistic relationship between the transcription factors Nkx6.1 and Ptf1a[19]. Ptf1a is progressively restricted to the tip cells, whereas Nkx6.1, Sox9, and Hnf1b are localized exclusively to the trunk domain, also known as the epithelial cords. Although Sox9 is co-expressed with Nkx6.1 in the epithelial cords that give rise to both endocrine and duct cells, it is later confined to the duct lineage in adult. The resolution of the tip and trunk domains is complete by~e14 in mouse when Nkx6.1 is excluded from the tips. In human, however, the process is more gradual with the extended presence of NKX6.1 in the tips until 49-52 dpc (approximately corresponding to e15.5 in mouse). In addition, SOX9 expression is found in human acinar cells until 10-14 weeks post conception(wpc) [12] (Fig. 1). The allocation of progenitors to the two domains is also influenced by tubulogenesis, a morphological event by which the extensive duct network is generated[20]. Tubulogenesis in the pancreas does not follow a stereotypical branching pattern that involves iterative tube extension; instead it occurs through epithelial stratification, acquisition of cell polarity, microlumen formation, microlumen fusion to form a plexus, and finally remodeling into a complex 3D network of tubules[21]. While microlumens are also observed in human pancreatic buds[12], suggestive of similar tissue reorganization, additional work is needed to fully explore the exact nature of this process.
The Sox9+ bipotent trunk domain is poised to generate either endocrine or duct cells contingent on the activation of Neurogenin3 (Ngn3), a pro-endocrine transcription factor. Ngn3 is necessary for commitment to the endocrine fate. As such, Ngn3-deficient mice fail to develop all endocrine cells, and die from diabetes 1-3 days after birth[22]. Bi-allelic mutations in NGN3 were reported to cause PNDM in humans[23], implying a similar role in human islet development. In mouse, a biphasic transient wave of Ngn3 expression [24] is observed, wherein the second wave corresponds to the secondary transition, an extensive terminal differentiation event in the developing pancreas that peaks at e15.5. In stark contrast, only a single-phase of NGN3 expression is observed in human that starts at 8wpc (corresponding to e16 in the mouse) and peaks in the second trimester between 10-14wpc[25] (Fig. 1). During the peak period, only 3-4.5% of SOX9+ trunk cells express NGN3, indicating a rather low efficiency of endocrine cell induction. This small number of endocrine precursors suggests that a prolonged period of endocrine cell specification is required to generate sufficient numbers of islet cells in humans. However, several critical questions still remain. Why do only certain Sox9+ cells activate Ngn3 expression? How does the niche control this differential cell fate? These mysteries are only beginning to be solved. For example, recent studies demonstrated that graded levels of Notch regulate the ductal vs. endocrine lineage choices as well as the tip vs. trunk fates of MPCs. MPCs with active Notch signaling acquire a trunk fate [26], and further within the trunk domain, high Notch signaling activates the expression of a downstream transcription factor Hes1 that promotes duct fate over endocrine commitment [27]. Whether these mechanisms are active in human fetal pancreas is unknown. A recent study examined HES1 expression in 16wpc and 19wpc old human pancreata, stages at which ducts should express Hes1 by extrapolation of mouse findings, but only found significant Hes1 expression in acinar tissue [28]. Investigation of Notch targets in earlier stages of human development are needed to delineate the role of Notch signaling in fate decisions.
Section III: Determination of endocrine cell types: Are Ngn3+ cells pre-committed?
After Ngn3 expression, pro-endocrine cells exit the cell cycle, delaminate into the mesenchyme and trigger downstream endocrine genes in rodents. Concerted activity of several transcription factors (Pdx1, Nkx6.1, Sox9, Nkx2.2, Neurod1, Ngn3, Pax4, Arx, Rfx6, Pax6, and others) orchestrate the formation of the five individual endocrine cell types (α, β, δ, ε and PP) present in adult islets. For instance, opposing functions of Arx and Pax4 determine α vs. β/ δ fate[29]. However, we do not yet fully understand how a given Ngn3+ endocrine-committed cell makes the decision to differentiate into a specific endocrine subtype. The concept that not all Ngn3+ cells are alike in their developmental potential and that their fate is spatio-temporally restricted is gaining consensus [30-32]. Johansson and co-authors proved the existence of competence windows for the generation of different endocrine subpopulations in mouse; temporally controlled activation of Ngn3 in Pdx1+ pancreatic epithelium showed that Ngn3+ cells predominantly formed α-cells [30, 33] at e8.5, β-and PP-cells between e10.5 and e.12.5, and δ-cells from e14.5 [30]. On the contrary, the first endocrine cell type to be detected in human is the β-cell (6 wpc)[34, 35], followed by α-cells(8-9wpc), δ-cells(10wpc), and PP-cells (17wpc), in that order[35]. These findings raise a number of logical questions: does islet cell fate allocation occur prior to or during Ngn3 expression? If Ngn3+cells are pre-biased, what cell-autonomous factors govern the acquisition of one of the various islet cell fates? How would differential expression of such factors be determined? Could it be through the location of cells along the epithelial cords and/or via extrinsic age-dependent signaling from the mesenchyme? These questions are only beginning to be addressed, but there is already some evidence that Nkx6.1 expression is mandatory before Ngn3 activation for commitment to the β-cell lineage [36]. A recent report indicated that repression of NeuroD1 by Nkx2.2 earlier in a Pdx1+ progenitor population, but not in Ngn3+ endocrine precursors, results in acquisition of α-cell fate[32]. In human PDX1+ progenitors, however, only NKX6.1, not NKX2.2, is present prior to NGN3 expression. Thus, the regulatory interactions between NKX2.2 and NEUROD1 are probably not present in human cells, and this may explain why the first endocrine cells to arise in human development are β-cells, and not α-cells. Summarily, these observations insinuate that events preceding Ngn3 expression regulate endocrine cell fate choices. Whether there is any functional significance for the appearance of insulin-producing cells before other cell types in human remains to be investigated. Possibly, these early β-cells participate in paracrine insulin signaling that may be required to maintain the Sox9+-progenitor pool as demonstrated in other organ systems [37-39].
While the majority of endocrine cells in the human fetal pancreas only express a single hormone, polyhormonal cells have been observed by several groups, especially early in development [35, 40-43]. Although the numbers of insulin and glucagon co-expressing cells varied between the different studies, the general trend was progressively decreasing percentages of double-positive cells through development with the highest numbers in the first trimester (20-30% of total insulin+/or glucagon+ cells). These hormone co-expressing cells were always scattered as single cells or found in small aggregates near the epithelial cords, supporting the notion that they likely represent newly generated endocrine cells [35, 41, 42]. Previously, Cre recombinase-mediated lineage tracing in mouse had revealed that cells that activate the insulin promoter at some point in their life do not develop into an adult α-cell and vice versa[44], signifying that insulin-glucagon double-positive cells do not contribute to adult islets. Nonetheless, this finding does not rule out extremely low promoter activity not sufficient to activate Cre recombinase expression. In human, however, the presence of ARX, but not of PDX1, NKX6.1 or MAFA, was demonstrated recently in insulin-glucagon double-positive cells in the developing human pancreas, leading the authors to conclude that the double hormone positive state is a transitionary phase in α-cell development[41]. Additional studies are required to resolve the relevance of polyhormonal expression in developing endocrine cells.
Section IV: Islet cytoarchitecture and Acquisition of form
Despite decades of research, islet morphogenesis remains a poorly understood area as we lack critical information regarding factors that trigger clustering of newly forming endocrine cells into specialized islet structures. Moreover, large inter-species diversity exists in islet architecture and cell composition [9], yet the rodent islet structure with its core-mantle organization is often considered the canonical islet morphology. β-cells constitute the primary component of rodent islets (~80% of cells), whereas human and non-human primate islets have relatively fewer β-cells (~60%), and more α- (~30%) and δ-cells (~10%) [45, 46]. There are two opposing opinions regarding the arrangement of endocrine cells within the human islet; in the first view, human islets have a modified core-mantle structure where smaller groups of β-cells form anatomical subdivisons surrounded by non β-cells [47]. The alternative view dictates that endocrine cells are randomly distributed along islet microcapillaries with no apparent subdivisions[45]. More heterotypic β-α cell interactions were discerned than homotypic β-β or α-α cell interactions in investigations that supported the second premise[45, 46]. A combination of the two theories was described in a trilaminar model proposed by Bosco et al., where a β-cell layer was sandwiched between two non β-cell (mostly α-cell) layers. This trilaminar plate was lined on both sides with blood vessels, and most β-cells extended cytoplasmic extensions through the α-cell layers such that they were in direct contact with endothelial cells[48]. A recent semi-automated analysis of human islets also demonstrated a slight preferential homotypic attachment of β cells amidst increased intermingling with α-cells[49], indicating β- and α-cells are not randomly mixed. Despite the controversy concerning human islet organization, it is apparent that profound differences exist between rodent and human islet architecture. These differences have been implicated in distinct functions. In contrast to mouse islets, human islets do not exhibit synchronized Ca2+ oscillations in response to glucose, but do show a Ca2+ response to low glucose (due to presence of more α-cells)[45]. Additionally, the increased number of heterotypic α-β contacts in human islets suggests a prominent role for α-cells in β-cell function, consistent with prior reports that showed juxtacrine glucagon signaling as well as α-β cellular contacts positively regulate glucose stimulated insulin secretion (GSIS)[50, 51]. Studies that correlate the relative arrangement of endocrine cells with their functional outcome are necessary to illuminate the influence of islet architecture on β-cell function.
Endocrine cells assemble to form islets close to the time of birth in the mouse, whereas in human, islet organization begins as early as 12 wpc. The first sign of islet morphogenesis in humans is preceded by scattered single endocrine cells that arise within the epithelial cords (6-9 wpc). At 10 wpc, α-or/and β-cells group into small clusters, and by 12 wpc they aggregate into a structure resembling the typical core-mantle mouse islet organization with β-cells in the center and α- and the other endocrine cells in the periphery. Soon after, the α-and β-cells expand and re-organize into juxtaposed homotypic clusters (also called bigeminal islets [40]) by 17 wpc. Finally, in the third trimester these homotypic aggregates undergo further remodeling to attain the adult human islet morphology that consists of intermingled endocrine cells [35, 40, 42, 52] (Fig. 1). The cell migration processes involved in the assembly of the mature islet structure are unknown, but one might speculate that signals from endothelial cells guide this event considering the alignment of endocrine cells along intra-islet vasculature in adult islets[45]. Finally at birth, human islets have equal numbers of α-, β- and δ-cells, and the adult composition is established by a neonatal burst in β-cell proliferation concomitant with a decline in δ-cell numbers [53].
Section V: Form follows function: Does islet morphogenesis induce maturation?
Association of diabetes with aberrant islet morphology in mice and human alludes to a correlation between the form and functional status of islets. However, it is not clear whether acquisition of islet structure causes functional maturation of β-cells or vice versa. Maturation of rodent β-cells into functional metabolic regulators has been associated with the appearance of a key transcription factor, MafA[54], and a secreted protein, Ucn3[55]. A switch from MafB (homolog of MafA expressed in immature α and β cells) to MafA occurs perinatally in mouse β-cells, and coincides with islet formation [54, 56]. In contrast, adult human β-cells maintain MAFB expression along with MAFA. MAFA mRNA is also detected in the developing human pancreas albeit at low levels[43], but convincing presence of nuclear MAFA protein has not been demonstrated until 21 wpc[35, 41]. Examination of third trimester fetuses and neonatal islets are required to determine the timing of MAFA appearance in humans. Notably, deletion of MafA in mice significantly disturbed islet morphology and impaired GSIS by affecting genes involved in glucose metabolism, insulin granule docking and insulin synthesis[56]. On a similar note, mutations in SUR1, a K+ATP channel subunit that confers β-cell function and is required for the induction of membrane depolarization, results in disrupted islet architecture[57] and causes NDM in humans[58]. Thus, it appears that factors involved in β-cell function also are necessary for islet structure.
Conversely, islet formation enables cell-cell coupling through Gap-junctions and Connexin-36 signaling, and contributes to functional maturation of β-cells[59]. Aside from endocrine cell coupling, the neurovascular milieu, which forms a significant structural component of islets, has also been implicated in islet maturation. In mouse, islet vascularization begins during islet cell differentiation (e13.5 onwards), and by birth islets are fully vascularized. While the neuronal processes surround the developing endocrine clusters by e15.5, islet innervation, guided by vascular scaffolds, is completed later around the time of weaning [60]. On the other hand, vascularization and innervation of human fetal pancreas is completed long before birth. During human islet development, CD34+ endothelial cells are present in close proximity to small clusters of β/α-cells at 10 wpc. By 12.5 wpc, they penetrate newly forming islets, and by 20 wpc both blood and lymphatic machineries are in place[61]. The human neuronal component, however, differs between adult and fetal stages; adult islets have few nerve fibers, and these are intimately associated with islet microvasculature rather than endocrine cells[62], while fetal islets have a higher density of nerves, ganglions and neuroinsular complexes (NIC). Interestingly, the highest density of NIC was detected in the third trimester (27-38 wpc) when the typical adult mosaic islets form, suggesting a role for neuronal control in islet morphogenesis[63]. Remarkably, norepinephrine-β-adrenergic signaling from sympathetic axons was shown to establish islet cytoarchitecture and β-cell maturation in mice[64].
Maturation of human β-cells is a subject that had not received much attention up until now. Hrvatin and colleagues purified human fetal (14wpc) and adult β-cells, and characterized their transcriptome to unearth genes that are differentially expressed in mature β-cells. They found significant enrichment of genes involved in metabolic processes, such as generation of metabolites, oxidation-reduction, electron transport chain and monosaccharide utilization, and vesicle-mediated secretory processes in adult β-cells. Ucn3, a marker of mature mouse β-cells, however, was not significantly upregulated in the human context[65]. A similar analysis on >27wpc fetal β-cells, when the mosaic islets develop and have maximal neuronal associations, would be interesting, and will shed light on whether islet morphogenesis induces maturation in humans.
Section VII: Conclusions and application to hPSC differentiation
By comparing human and mouse islet organogenesis, we learn that key events are grossly conserved, though differences in timing and developmental factors are evident between species. For example, human pancreas and islet formation is marked by late detection of PDX1 only after separation of the foregut from the dorsal aorta, delayed resolution of tip and trunk progenitors, single-phased transient NGN3 induction, absence of NKX2.2 before endocrine commitment, presence of significant numbers of polyhormonal cells, appearance of β-cells before other cell types, innervation and formation of the unique mosaic islet structure before birth. It has become further apparent that polyhormonal cells represent either early endocrine or α-cell-fated cells during human pancreas development. Polyhormonal cells were the hallmark of β-cell differentiation from hPSCs until recently. The absolute requirement of NKX6.1 for commitment to the β-cell lineage has led to the development of two improved protocols that tweak the early pancreatic progenitor stage. These protocols produce monohormonal β-like cells that have suboptimal functional capacity, responding to glucose only in static GSIS assays [66, 67]. Apart from incorrect lineage specification, the difficulty in generating functional β-cells may be due to the lack of pertinent maturation cues. Thyroid hormone was employed by these two studies to induce MAFA expression [66, 67]. However, using one marker, for instance MAFA, as readout for maturity in hPSC differentiation could be misleading, and a combination of several metabolic processes and transcription factors are necessary to mark mature human β-cells as elucidated in section V.
Other endocrine cell types, especially α-cells form a significant component of human islets and aid β-cell function as described in section IV. Therefore, generating pure β-cell populations in isolation with the intent of fully replicating all regulatory aspects of insulin secretion is counter-intuitive. We can also infer that important roles may be played by neuronal and endothelial cells due to their increased presence in the third trimester of human development during which islet morphogenesis and maturation occurs. Supplementing the hPSC cultures with these cell types or addition of factors derived from these cells, including CTGF and norepinephrine, at appropriate times may enhance the generation of mature β-cells (Fig. 2). Moreover, the focus should now be on inventing conditions to maintain terminally differentiated β-cells in their fully functional state. This may even necessitate the construction of micro-organoids with all the various islet cell types. As described above, immaturity has also been attributed to lack of proper coupling between oxidative phosphorylation and glycolysis, underscoring the magnitude of research that needs to be conducted in hPSC-derived β-cell metabolism. In conclusion, we anticipate that additional insights gained from human development will provide the ultimate blue print to produce bona fide human β-cells in vitro in the coming years.
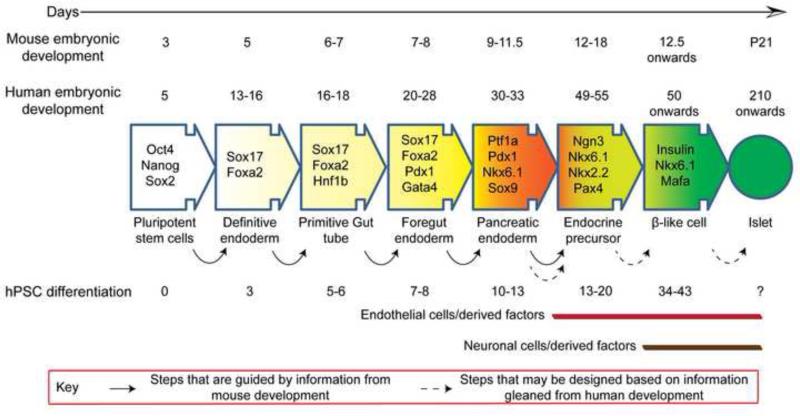
Fig. 2.
Timeline of pancreas development, in vivo, in mouse and human compared with in vitro differentiation of β-cells from hPSCs. hPSC differentiation protocols have been developed largely based on information gained from pancreas development in mice. Current protocols generate sub-par β-like cells that have modest glucose responsiveness but do not resemble mature β-cells of an islet in their metabolic and secretory properties. New data from human pancreas development, such as involvement of neuronal and endothelial cells in later stages of organogenesis when β-cell maturation predominantly occurs, may be used to instruct better differentiation protocols that result in bona fide β-cells. The timeline of hPSC differentiation is based on two recent studies; Pagliuca et al. [66]and Rezania et al.[67]
Acknowledgements
We thank Dr. Jennifer Liu, Dr. Sapna Puri, Dr. Holger Russ and Thomas Hennings for critically reading the manuscript and reviewing the figures. We thank The Leona M. and Harry B. Helmsley Charitable Trust (09PG-T1D018), the Juvenile Diabetes Research Foundation (JDRF 17-2013-380), and the NIH and NCI (R01CA172045) for supporting the work in the laboratory of M.H.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Author Contributions:
G.N and M.H. conceived the general topics of the Review and co-wrote the manuscript.
References
- 1*.Kushner Jake A, MacDonald Patrick E, Atkinson Mark A. Stem Cells to Insulin Secreting Cells: Two Steps Forward and Now a Time to Pause? Cell Stem Cell. 2014;15(5):535–536. doi: 10.1016/j.stem.2014.10.012. [The review discusses the shortcomings in two recent studies that report sucessful generation of insulin secreting cells invitro from human pluripotent stem cells.] [DOI] [PubMed] [Google Scholar]
- 2.Pagliuca FW, Melton DA. How to make a functional β-cell. Development. 2013;140(12):2472–2483. doi: 10.1242/dev.093187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Hay CW, Docherty K. Comparative Analysis of Insulin Gene Promoters: Implications for Diabetes Research. Diabetes. 2006;55(12):3201–3213. doi: 10.2337/db06-0788. [DOI] [PubMed] [Google Scholar]
- 4.McCulloch LJ, van de Bunt M, Braun M, et al. GLUT2 (SLC2A2) is not the principal glucose transporter in human pancreatic beta cells: Implications for understanding genetic association signals at this locus. Molecular Genetics and Metabolism. 2011;104(4):648–653. doi: 10.1016/j.ymgme.2011.08.026. [DOI] [PubMed] [Google Scholar]
- 5.De Vos A, Heimberg H, Quartier E, et al. Human and rat beta cells differ in glucose transporter but not in glucokinase gene expression. The Journal of Clinical Investigation. 1995;96(5):2489–2495. doi: 10.1172/JCI118308. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Peschke E, Bähr I, Mühlbauer E. Melatonin and Pancreatic Islets: Interrelationships between Melatonin, Insulin and Glucagon. International Journal of Molecular Sciences. 2013;14(4):6981–7015. doi: 10.3390/ijms14046981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.McDonald TJ, Tu E, Brenner S, et al. Canine, human, and rat plasma insulin responses to galanin administration: species response differences. American Journal of Physiology - Endocrinology and Metabolism. 1994;266(4):E612–E617. doi: 10.1152/ajpendo.1994.266.4.E612. [DOI] [PubMed] [Google Scholar]
- 8.Fiaschi-Taesch N, Bigatel TA, Sicari B, et al. Survey of the Human Pancreatic β-Cell G1/S Proteome Reveals a Potential Therapeutic Role for Cdk-6 and Cyclin D1 in Enhancing Human β-Cell Replication and Function In Vivo. Diabetes. 2009;58(4):882–893. doi: 10.2337/db08-0631. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9**.Steiner DJ, Kim A, Miller K, et al. Pancreatic islet plasticity: Interspecies comparison of islet architecture and composition. Islets. 2010;2(3):135–145. doi: 10.4161/isl.2.3.11815. [This review delves into the diversity in islet composition and cytoarchitecture among various species and highlights its variation from rodent islets.] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Shih HP, Wang A, Sander M. Pancreas Organogenesis: From Lineage Determination to Morphogenesis. Annual Review of Cell and Developmental Biology. 2013;29(1):81–105. doi: 10.1146/annurev-cellbio-101512-122405. [DOI] [PubMed] [Google Scholar]
- 11.Hebrok M. Hedgehog signaling in pancreas development. Mech Dev. 2003;120(1):45–57. doi: 10.1016/s0925-4773(02)00331-3. [DOI] [PubMed] [Google Scholar]
- 12**.Jennings RE, Berry AA, Kirkwood-Wilson R, et al. Development of the Human Pancreas From Foregut to Endocrine Commitment. Diabetes. 2013;62(10):3514–3522. doi: 10.2337/db12-1479. [This is the first study to examine human pancreas development during embryogenesis until first trimester. The authors observed delay in key developmental events compared to mouse pancreas organogenesis, and absence of primary transition and Nkx2.2 expression prior to endocrine commitment.] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Shaw-Smith C, De Franco E, Allen HL, et al. GATA4 mutations are a cause of neonatal and childhood-onset diabetes. Diabetes. 2014 doi: 10.2337/db14-0061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Eifes S, Chudasama KK, Molnes J, et al. A novel GATA6 mutation in a child with congenital heart malformation and neonatal diabetes. Clinical Case Reports. 2013;1(2):86–90. doi: 10.1002/ccr3.33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Weedon MN, Cebola I, Patch A-M, et al. Recessive mutations in a distal PTF1A enhancer cause isolated pancreatic agenesis. Nat Genet. 2014;46(1):61–64. doi: 10.1038/ng.2826. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.De León DDSC. Permanent Neonatal Diabetes Mellitus. In: Pagon RA, Adam MP, Ardinger HH, et al., editors. GeneReviews® [Internet] University of Washington, Seattle; Seattle (WA): Feb 8, 2008. [2014 Jan 23]. pp. 1993–2014. [PubMed] [Google Scholar]
- 17.Ye F, Duvillié B. Scharfmann R.Fibroblast growth factors 7 and 10 are expressed in the human embryonic pancreatic mesenchyme and promote the proliferation of embryonic pancreatic epithelial cells. Diabetologia. 2005;48(2):277–281. doi: 10.1007/s00125-004-1638-6. [DOI] [PubMed] [Google Scholar]
- 18*.Zhou Q, Law AC, Rajagopal J, et al. A Multipotent Progenitor Domain Guides Pancreatic Organogenesis. Developmental Cell. 2007;13(1):103–114. doi: 10.1016/j.devcel.2007.06.001. [The authors uncovered the existence of a multipotent domain,characterized by the expression of Pdx1,Ptf1a,Cpa1 and cMyc, that gives rise to acinar,endocrine and duct compartments at the tip of branching pancreatic tree. The multipotent tips grow out into the mesechyme leaving behind differentiated progeny in the trunk of the branches.] [DOI] [PubMed] [Google Scholar]
- 19*.Schaffer AE, Freude KK, Nelson SB, et al. Nkx6 Transcription Factors and Ptf1a Function as Antagonistic Lineage Determinants in Multipotent Pancreatic Progenitors. Developmental Cell. 2010;18(6):1022–1029. doi: 10.1016/j.devcel.2010.05.015. [Using pancreas specific gain and loss-of function approaches, the authors determined cross-repressive activity of Nkx6 and Ptf1a in committing multipotent progenitors toward endocrine or acinar fates, respectively.] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Kesavan G, Sand FW, Greiner TU, et al. Cdc42-Mediated Tubulogenesis Controls Cell Specification. Cell. 139(4):791–801. doi: 10.1016/j.cell.2009.08.049. [DOI] [PubMed] [Google Scholar]
- 21.Villasenor A, Chong DC, Henkemeyer M, et al. Epithelial dynamics of pancreatic branching morphogenesis. Development (Cambridge, England) 2010;137(24):4295–4305. doi: 10.1242/dev.052993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Gradwohl G, Dierich A, LeMeur M, et al. neurogenin3 is required for the development of the four endocrine cell lineages of the pancreas. Proceedings of the National Academy of Sciences. 2000;97(4):1607–1611. doi: 10.1073/pnas.97.4.1607. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Rubio-Cabezas O, Jensen JN, Hodgson MI, et al. Permanent Neonatal Diabetes and Enteric Anendocrinosis Associated With Biallelic Mutations in NEUROG3. Diabetes. 2011;60(4):1349–1353. doi: 10.2337/db10-1008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Villasenor A, Chong DC, Cleaver O. Biphasic Ngn3 expression in the developing pancreas. Developmental Dynamics. 2008;237(11):3270–3279. doi: 10.1002/dvdy.21740. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25*.Salisbury RJ, Blaylock J, Berry AA, et al. The window period of NEUROGENIN3 during human gestation. Islets. 2014;6(3):e954436. doi: 10.4161/19382014.2014.954436. [This study examined the appearance of Neurogenin3 during the course of human pancreas development and observed transient expression between 8-18 wpc peaking at 10-14wpc.] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Afelik S, Qu X, Hasrouni E, et al. Notch-mediated patterning and cell fate allocation of pancreatic progenitor cells. Development. 2012;139(10):1744–1753. doi: 10.1242/dev.075804. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Shih HP, Kopp JL, Sandhu M, et al. A Notch-dependent molecular circuitry initiates pancreatic endocrine and ductal cell differentiation. Development. 2012;139(14):2488–2499. doi: 10.1242/dev.078634. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Hu H, Zhou L, Awadallah A, et al. Significance of Notch1-signaling Pathway in Human Pancreatic Development and Carcinogenesis. Applied Immunohistochemistry & Molecular Morphology. 2013;21(3):242–247. doi: 10.1097/PAI.0b013e3182655ab7. 10.1097/PAI.0b013e3182655ab7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Mastracci TL, Sussel L. The endocrine pancreas: insights into development, differentiation, and diabetes. Wiley Interdisciplinary Reviews: Developmental Biology. 2012;1(5):609–628. doi: 10.1002/wdev.44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Johansson KA, Dursun U, Jordan N, et al. Temporal Control of Neurogenin3 Activity in Pancreas Progenitors Reveals Competence Windows for the Generation of Different Endocrine Cell Types. Developmental Cell. 2007;12(3):457–465. doi: 10.1016/j.devcel.2007.02.010. [DOI] [PubMed] [Google Scholar]
- 31.Desgraz R, Herrera PL. Pancreatic neurogenin 3-expressing cells are unipotent islet precursors. Development. 2009;136(21):3567–3574. doi: 10.1242/dev.039214. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32*.Mastracci TL, Anderson KR, Papizan JB, et al. Regulation of <italic>Neurod1</italic> Contributes to the Lineage Potential of Neurogenin3+ Endocrine Precursor Cells in the Pancreas. PLoS Genet. 2013;9(2):e1003278. doi: 10.1371/journal.pgen.1003278. [Using conditional deletion of Neurod1 in a Nkx2.2 null mouse, the authors discovered that Nkx2.2-mediated repression of Neurod1 in a Pdx1+ pancreatic progenitor population is essential for commitment of a subset of Neurogenin3+ endocrine progenitor cells to the alpha cell lineage.] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Schwitzgebel VM, Scheel DW, Conners JR, et al. Expression of neurogenin3 reveals an islet cell precursor population in the pancreas. Development. 2000;127(16):3533–3542. doi: 10.1242/dev.127.16.3533. [DOI] [PubMed] [Google Scholar]
- 34**.Piper K, Brickwood S, Turnpenny L, et al. Beta cell differentiation during early human pancreas development. Journal of Endocrinology. 2004;181(1):11–23. doi: 10.1677/joe.0.1810011. [The authors observed proliferating epithelial structures positive for PDX1 and CK19 early in development . The first endocrine cells to appear were insulin+cells that maintained PDX1 but had diminished CK19.Vascularization was observed as early as 10wpc.] [DOI] [PubMed] [Google Scholar]
- 35**.Jeon J, Correa-Medina M, Ricordi C, et al. Endocrine Cell Clustering During Human Pancreas Development. Journal of Histochemistry & Cytochemistry. 2009;57(9):811–824. doi: 10.1369/jhc.2009.953307. [The authors performed a detailed characterization of islet morphogenesis till mid second trimester. Interestingly, early in development, the human endocrine cells organize into a mouse-like islet structure and they re-arrange into homotypic alpha/beta clusters that are distinct from the mosaic islet architecture found in adult human islets.] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Nelson SB, Schaffer AE, Sander M. The transcription factors Nkx6.1 and Nkx6.2 possess equivalent activities in promoting beta-cell fate specification in Pdx1+ pancreatic progenitor cells. Development. 2007;134(13):2491–2500. doi: 10.1242/dev.002691. [DOI] [PubMed] [Google Scholar]
- 37.McDonald E, Li J, Krishnamurthy M, et al. SOX9 regulates endocrine cell differentiation during human fetal pancreas development. The International Journal of Biochemistry & Cell Biology. 2012;44(1):72–83. doi: 10.1016/j.biocel.2011.09.008. [DOI] [PubMed] [Google Scholar]
- 38.Nef S, Verma-Kurvari S, Merenmies J, et al. Testis determination requires insulin receptor family function in mice. Nature. 2003;426(6964):291–295. doi: 10.1038/nature02059. [DOI] [PubMed] [Google Scholar]
- 39.Insulin Receptor Substrate-1 Deficiency Promotes Apoptosis in the Putative Intestinal Crypt Stem Cell Region, Limits Apcmin/+ Tumors, and Regulates Sox9. Endocrinology. 2008;149(1):261–267. doi: 10.1210/en.2007-0869. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Bocian-Sobkowska J, Zabel M, Wozniak W, et al. Polyhormonal aspect of the endocrine cells of the human fetal pancreas. Histochemistry and Cell Biology. 1999;112(2):147–153. doi: 10.1007/s004180050401. [DOI] [PubMed] [Google Scholar]
- 41**.Riedel MJ, Asadi A, Wang R, et al. Immunohistochemical characterisation of cells co-producing insulin and glucagon in the developing human pancreas. Diabetologia. 2012;55(2):372–381. doi: 10.1007/s00125-011-2344-9. [Considerable numbers of insulin-glucagon double positive cells were found, especially early in human development and these double positive cells surprisingly expressed ARX but not PDX1 or NKX6.1. This lead the authors to conclude that these bihormonal cells potentially represent cells committed toward the alpha cell lineage.] [DOI] [PubMed] [Google Scholar]
- 42.Anderson SJ, Seeberger KL, Ellis CE, et al. Immunohistochemical Characterization of Insulin, Glucagon, PDX1, SOX17 and NGN3 Expression in Human Fetal Pancreatic Development. Journal of Stem Cell Research & Therapy. 2013 [Google Scholar]
- 43.Sarkar SA, Kobberup S, Wong R, et al. Global gene expression profiling and histochemical analysis of the developing human fetal pancreas. Diabetologia. 2008;51(2):285–297. doi: 10.1007/s00125-007-0880-0. [DOI] [PubMed] [Google Scholar]
- 44.Herrera PL. Adult insulin-and glucagon-producing cells differentiate from two independent cell lineages. Development. 2000;127(11):2317–2322. doi: 10.1242/dev.127.11.2317. [DOI] [PubMed] [Google Scholar]
- 45*.Cabrera O, Berman DM, Kenyon NS, et al. The unique cytoarchitecture of human pancreatic islets has implications for islet cell function. Proceedings of the National Academy of Sciences of the United States of America. 2006;103(7):2334–2339. doi: 10.1073/pnas.0510790103. [According to this study, human islets do not show anatomical subdivisions unlike mouse islets. Alpha, beta, delta cells were found scattered throughout the islet randomly. 71% of beta cell interactions were heterotypic suggesting paracrine signalling plays a major role in human islet function.] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Brissova M, Fowler MJ, Nicholson WE, et al. Assessment of Human Pancreatic Islet Architecture and Composition by Laser Scanning Confocal Microscopy. Journal of Histochemistry & Cytochemistry. 2005;53(9):1087–1097. doi: 10.1369/jhc.5C6684.2005. [DOI] [PubMed] [Google Scholar]
- 47.Orci L. The microanatomy of the islets of Langerhans. Metabolism. 1976;25(11):1303–1313. doi: 10.1016/s0026-0495(76)80129-1. [DOI] [PubMed] [Google Scholar]
- 48*.Bosco D, Armanet M, Morel P, et al. Unique Arrangement of α- and β-Cells in Human Islets of Langerhans. Diabetes. 2010;59(5):1202–1210. doi: 10.2337/db09-1177. [This study revealed that islet cells are organized into trilaminar epithelial plates, with beta cell layer sandwiched between two alpha cell layers. The trilaminar plate, in turn, is bordered by vessels on both the sides. Beta cells contact the endothelial cells by cytoplasmic extensions.] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Kilimnik G, Jo J, Periwal V, et al. Quantification of islet size and architecture. Islets. 2012;4(2):167–172. doi: 10.4161/isl.19256. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Wojtusciszyn A, Armanet M, Morel P, et al. Insulin secretion from human beta cells is heterogeneous and dependent on cell-to-cell contacts. Diabetologia. 2008;51(10):1843–1852. doi: 10.1007/s00125-008-1103-z. [DOI] [PubMed] [Google Scholar]
- 51.Huypens P, Ling Z, Pipeleers D, et al. Glucagon receptors on human islet cells contribute to glucose competence of insulin release. Diabetologia. 2000;43(8):1012–1019. doi: 10.1007/s001250051484. [DOI] [PubMed] [Google Scholar]
- 52.Robb P. THE DEVELOPMENT OF THE ISLETS OF LANGERHANS IN THE HUMAN FŒTUS. Experimental Physiology. 1961;46(4):335–343. doi: 10.1113/expphysiol.1961.sp001551. [DOI] [PubMed] [Google Scholar]
- 53**.Gregg B, Moore P, Demozay D, et al. Formation of a human beta-cell population within pancreatic islets is set early in life. J Clin Endocrinol Metab. 2012;97:3197–3206. doi: 10.1210/jc.2012-1206. [Neogenesis was predominant preterm whereas beta-cell proliferation peaked in the first 2 years of life setting the baseline of beta-cell population in an individual. The beta- to alpha-cell ratio doubled neonatally whereas the beta-to delta-cell ratio increased to 7 fold during childhood.] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Hang Y, Stein R. MafA and MafB activity in pancreatic β cells. Trends in Endocrinology & Metabolism. 2011;22(9):364–373. doi: 10.1016/j.tem.2011.05.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Blum B, Hrvatin S, Schuetz C, et al. Functional beta-cell maturation is marked by an increased glucose threshold and by expression of urocortin 3. Nat Biotech. 2012;30(3):261–264. doi: 10.1038/nbt.2141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Hang Y, Yamamoto T, Benninger RKP, et al. The MafA transcription factor becomes essential to islet β-cells soon after birth. Diabetes. 2014 doi: 10.2337/db13-1001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Busiah K, Verkarre V, Cavé H, et al. Human Pancreas Endocrine Cell Populations and Activating <b><i>ABCC8</i></b> Mutations. Hormone Research in Paediatrics. 2014;82(1):59–64. doi: 10.1159/000360004. [DOI] [PubMed] [Google Scholar]
- 58.Polak M, Cave H. Neonatal diabetes mellitus: a disease linked to multiple mechanisms. Orphanet Journal of Rare Diseases. 2007;2(1):12. doi: 10.1186/1750-1172-2-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Carvalho CPF, Barbosa H CL, Britan A, et al. Beta cell coupling and connexin expression change during the functional maturation of rat pancreatic islets. Diabetologia. 2010;53(7):1428–1437. doi: 10.1007/s00125-010-1726-8. [DOI] [PubMed] [Google Scholar]
- 60*.Reinert RB, Cai Q, Hong J-Y, et al. Vascular endothelial growth factor coordinates islet innervation via vascular scaffolding. Development. 2014;141(7):1480–1491. doi: 10.1242/dev.098657. [Using pancreas specific VEGF knockout and beta-cell specific VEGF overexpression, the authors determined that VEGF secreted by endocrine cells is essential for patterning of intra-islet capillaries, which in turn provides scaffolding for islet-innervation.] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61*.Roost M, van Iperen L, de Melo Bernardo A, et al. Lymphangiogenesis and angiogenesis during human fetal pancreas development. Vascular Cell. 2014;6(1):22. doi: 10.1186/2045-824X-6-22. [On examination of human fetal pancreas for blood and lymphatic machinery, the authors found extensive network of blood vessels intruding the islets as early as 12wpc and lymphatic vessels in the periphery of islets from 15-20wpc onwards to support endocrine function.] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Rodriguez-Diaz R, Abdulreda MH, Formoso AL, et al. AUTONOMIC AXONS IN THE HUMAN ENDOCRINE PANCREAS SHOW UNIQUE INNERVATION PATTERNS. Cell metabolism. 2011;14(1):45–54. doi: 10.1016/j.cmet.2011.05.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63*.Proshchina AE, Krivova YS, Barabanov VM, et al. Ontogeny of neuro-insular complexes and islets innervation in the human pancreas. Frontiers in Endocrinology. 2014:5. doi: 10.3389/fendo.2014.00057. [This is the first report to study neuronal association of islets in human fetal pancreas. Islet innervation started from 12wpc, and dense neuro-insular complexes were found during mid and late fetal periods when morphogenesis into mosaic islets, typically found in adults, occurs.] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64**.Borden P, Houtz J, Leach Steven D, et al. Sympathetic Innervation during Development Is Necessary for Pancreatic Islet Architecture and Functional Maturation. Cell Reports. 2013;4(2):287–301. doi: 10.1016/j.celrep.2013.06.019. [The authors discovered that norepinephrine released by sympathetic neurons induces islet cell migration and establishes islet architecture. Ablation of sympathetic innervation by genetic and pharmocological means resulted in disrupted islet architecture and impaired glucose tolerance in mice.] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65**.Hrvatin S, O'Donnell CW, Deng F, et al. Differentiated human stem cells resemble fetal, not adult, β cells. Proceedings of the National Academy of Sciences. 2014;111(8):3038–3043. doi: 10.1073/pnas.1400709111. [Through whole transcriptome analysis, this study identified that different human pluripotent stem cells(hPSCs) sources give rise to similar INS+ cells. However, hPSC derived INS+cells were enriched in progenitor markers like fetal beta-cells, and unlike adult beta cells that were enriched in metabolic and secretory biological processes.] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66**.Pagliuca Felicia W, Millman Jeffrey R, Gürtler M, et al. Generation of Functional Human Pancreatic β Cells In Vitro. Cell. 2014;159(2):428–439. doi: 10.1016/j.cell.2014.09.040. [The authors established a scalable system for generation of glucose-responsive INS+ cells from hPSC sources in vitro. These INS+ cells expressed some markers characteristic of adult beta cells and also showed a Ca2+ flux in response to glucose.] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67**.Rezania A, Bruin JE, Arora P, et al. Reversal of diabetes with insulin-producing cells derived in vitro from human pluripotent stem cells. Nat Biotech. 2014;32(11):1121–1133. doi: 10.1038/nbt.3033. [This was the first study to report in vitro generation of functional insulin-producing cells from hPSC sources. The INS+ cells exhibited similar levels of glucose-responsiveness as human islets in static incubations but showed differences from human islets in dynamic glucose challenges. In addition, these cells reversed hyperglycemia in diabetic mice in 40 days.] [DOI] [PubMed] [Google Scholar]
- 68.Kuo CT, Morrisey EE, Anandappa R, et al. GATA4 transcription factor is required for ventral morphogenesis and heart tube formation. Genes & Development. 1997;11(8):1048–1060. doi: 10.1101/gad.11.8.1048. [DOI] [PubMed] [Google Scholar]
- 69.Kanai-Azuma M, Kanai Y, Gad JM, et al. Depletion of definitive gut endoderm in Sox17-null mutant mice. Development. 2002;129(10):2367–2379. doi: 10.1242/dev.129.10.2367. [DOI] [PubMed] [Google Scholar]
- 70.Rodr xEd, guez S, et al. GATA believe it: new essential regulators of pancreas development. The Journal of Clinical Investigation. 2012;122(10):3469–3471. doi: 10.1172/JCI65751. [DOI] [PMC free article] [PubMed] [Google Scholar]