Abstract
It is known that oxidative stress leads to amyloid precursor protein (APP) dysregulation in platelets. Ethanol (EtOH) is a vascular risk factor and induces oxidative stress. The aim of the present study was thus to investigate whether EtOH affects APP processing in rat and human platelets. Platelets were exposed to 50 mM EtOH with and without 2 mM calcium-chloride (CaCl2) for 20 or 180 minutes at 37°C. Platelet aggregation, serotonin release and APP isoforms 130 and 106/110 kDa were analyzed. As a control, 100 mM H2O2 was tested in rat platelets. Our data show that EtOH alone did not affect any of the analyzed parameters, whereas CaCl2 significantly increased aggregation of rat and human platelets. In addition, CaCl2 alone enhanced serotonin release in rat platelets. EtOH counteracted CaCl2-induced aggregation and serotonin release. In the presence of CaCl2, EtOH reduced the 130 kDa APP isoform in rat and human platelets. In conclusion, this study shows that in the presence of CaCl2, EtOH affects the platelet function and APP processing in rat and human platelets.
Keywords: Ethanol, platelets, APP processing, calcium
Introduction
Platelets contain large amounts of amyloid precursor protein (APP) and are the most important source of plasma beta-amyloid (Aβ) [1, 2]. APP is partly expressed as an intact protein on the cell surface and is stored as proteolyzed fragments in α granules in platelets [3–5]. Platelet APP can be processed to Aβ by secretases via the same amyloidogenic and non-amyloidogenic pathways as found in the brain [6]. Deposition of Aβ in vessels (cerebral amyloid angiopathy, CAA) and in the brain (plaques) is an important hallmark of Alzheimer’s disease (AD). However, the causes of Aβ accumulation are still unclear. Dysfunctional Aβ clearance at the blood–brain barrier (BBB) or aberrant APP processing in platelets may be implicated in these processes.
In fact, platelets of AD patients have been found to have an altered ratio of 130 kDa and 106/110 kDa APP isoforms [7]. Various stimuli can activate aberrant APP processing, such as oxidative stress [8], platelet activation [9], calcium dyshomeostasis [10], or high cholesterol levels [11]. As platelets are the primary source of plasma Aβ [12], such a dysregulation involving oxidative stress may result in increased Aβ deposition. It is well known that oxidative stress induced by, e.g. hydrogen peroxide (H2O2) affects APP expression in human platelets [8]. Oxidative stress induces platelet activation and treatment with the antioxidant glutathione partly counteracted altered APP processing in rat platelets [8]. Moreover, it is well established that in platelets Ca2+ is an important second messenger downstream of most signaling pathways [13] and plays an important role in APP processing.
Ethanol (EtOH) can induce oxidative stress in platelets, most likely by generating H2O2 [14]. EtOH easily passes the BBB and can influence neurons as well as blood cells. In blood, EtOH affects platelet function by either dampening platelet activation or decreasing platelet inflammatory response [15]. In addition, EtOH prevents platelet aggregation involving prostacyclin (PGI2), thromboxane A2 (TXA2), plasminogen activator or fibrinogen [16]. It is well established that EtOH is a vascular risk factor and heavy chronic EtOH consumption accounts for cerebrovascular disease [17–19], whereas moderate EtOH exposure reduces the risk for coronary artery disease [20–22]. The cardioprotective effect of EtOH is mediated by antioxidative properties [23], an increase in high-density lipoproteins [24] or vasodilatative and anti-thrombotic activities [23].
In the present study we investigate the effects of EtOH alone and in combination with CaCl2 on platelet function (aggregation and serotonin release) as well as on APP processing in rat and human platelets. We show that EtOH prevents the CaCl2-induced aggregation of platelets and significantly reduces the full-length APP 130 kDa isoform in rat and humans, but only in the presence of CaCl2.
Methods
Platelet isolation and stimulation
Ethylendiamintetraacetate (EDTA) blood (10 ml) was collected and processed within 3 hours as described previously [8]. All experiments in humans and animals were approved by the Austrian Ethics Committee. Adult Sprague–Dawley rats were anaesthetized with a high dose of thiopental (Sandoz, Kundl, Austria), and blood was drawn directly from the heart using a 21-gauge butterfly blood collection system (BD Valu-Set, BD, USA). Human platelets were isolated from blood obtained from healthy volunteers (age = 36 ± 4.7 years, n = 8) Blood was collected in EDTA tubes (S-monovettes, Sarstedt, Nürnbrecht, Germany) and gently mixed. Immediately after collection, anticoagulated blood was centrifuged at 250g for 15 minutes to obtain platelet-rich plasma (PRP). All centrifugation steps were performed at room temperature. PGI2 (500 nM; Sigma, P6188, Austria) was added to prevent platelet activation. Platelets were separated from PRP by centrifuging at 2300g for 10 minutes and washed in Ca2+-free Tyrode’s buffer (136 mM NaCl, 2.7 mM KCl, 12 mM NaHCO3, 0.42 mM NaH2PO4, 1 mM MgSo4, 5 mM glucose, pH 6.5). After further centrifugation at 2300g for 10 minutes, platelets were finally resuspended in 1 ml Tyrode’s buffer (adjusted to pH 7.4). Platelets were counted under the phase-contrast microscope with a counting chamber (Bürker-Türk, Laboroptik, Lancing, UK).
Rat or human platelets (5 × 108 platelets) were then incubated with and without 50 mM EtOH or 2 mM CaCl2 or 100 mM H2O2 (30%, Merck, Darmstadt, Germany) in 120 μl Tyrode’s buffer (pH 7.4) for 20 or 180 minutes at 37°C. Then a small aliquot (10 μl) was pipetted onto gelatin-coated glass slides and processed for immunohistochemistry. The rest was centrifuged for 10 minutes at 2300g, and the supernatants and pellets were frozen at −80°C until use. Supernatants were used for the analysis of serotonin, and pellets were extracted for western blot analysis.
Immunohistochemistry
Immunohistochemistry was performed as recently described [8]. Platelets were spotted onto glass slides, air-dried and then immediately (the same day) postfixed in 4% paraformaldehyde for 30 minutes at 4°C. In order to avoid immunohistochemical artefacts [25], staining was performed on freshly isolated platelets on the same day and additional incubation times during processing or freezing were strictly avoided. Cells were then washed for 30 minutes with 0.1% Triton/phosphate-buffered saline (T-PBS), pretreated with 5% methanol/1% H2O2/PBS. Then the cells were washed twice for 5 minutes with PBS, blocked with 20% horse serum/0.2% BSA/T-PBS. Rat platelets were incubated with the primary antibody mouse anti-CD61 (Serotec, UK; MCA 1773, 1:200) and human platelets with mouse anti-CD41 (Abcam, Cambridge, MA, USA; ab63323, 1:200) in 0.2% BSA/T-PBS at room temperature overnight. Cells were washed and incubated with secondary biotinylated anti-mouse (Vector Laboratories, Burlingame, CA, USA; BA-2001, 1:200) antibody for 1 hour at room temperature. After rinsing twice in PBS, slides were incubated in avidin-biotin complex solution (ABC; Vector Laboratories, USA; Elite Standard PK 6100) for 1 hour, and then washed twice in 50 mM Tris-buffered saline (TBS). The signal was detected using 0.5 mg/ml 3,3′-diaminobenzidine (DAB) in TBS with 0.0003% H2O2 as substrate. Reaction was stopped in TBS, and slides were allowed to dry and were cover-slipped.
Analysis of serotonin
Serotonin release was measured by high-pressure liquid chromatography (HPLC) and electrochemical detection as described by us [8]. The frozen supernatants were thawed and 20 μl was injected onto a reversed-phase C18 Nucleosil column (Bartelt, Graz, Austria) at a flow rate of 1 ml/minute using the following mobile phase: 0.05 M trichloric acid, 0.26 mM EDTA, 1.36 mM NaCl, 1.81 mM heptane sulfonic acid and 15% acetonitril in HPLC water. Detection was performed with an electrochemical detector (Antec-Leyden, Decade II, Biolab, Vienna, Austria) at +0.55 V at 30°C. All unknown samples were correlated to external standards of serotonin (Sigma, Austria; H9523) according to peak heights.
Western blot analysis
To analyze the expression of APP isoforms (130 and 106/110 kDa) western blot analysis was performed as recently described [8]. The frozen pellets were dissolved in 80 μl PBS with a protease inhibitor cocktail (Sigma, P-8340, Austria), and then sonicated on ice with five pulses each for 10 seconds (Branson Sonifier 250, Danbury, USA) and centrifuged at 16,000g for 10 minutes. The total protein in each supernatant (extract) was determined using the Bradford protein determination assay (Bio RAD Protein Assay, Life Science, Vienna, Austria). Bovine serum albumin (BSA; albumin bovine fraction V; Sigma) was used as standard at concentrations of 0, 2, 5, 10, and 20 μg/ml protein. Analysis was performed with the Zenyth microplate reader (Anthos, HVD, Vienna, Austria) at 595 nm. The remaining extracts were frozen at −80°C until use.
For western blot analysis, the extracts were thawed and 10 μg total protein was loaded onto 4–12% Bis-Tris polyacrylamide gel (Invitrogen, Vienna, Austria) and electrophoresed for 45 minutes at 200 V. Samples were electrotransferred to nylon PVDF Immobilon-PSQ membranes (Millipore, Schwalbach, Germany) for 90 minutes at 30 V with 20% methanol blotting buffer (Invitrogen, Vienna, Austria). For detection, the Western Breeze Chemiluminescent System (Invitrogen, Vienna, Austria) was used. Blots were blocked for 30 minutes with blocking buffer, then incubated overnight at 4°C with the primary antibody mouse anti-APP (Millipore-Merck, Schwalbach, Germany; MAB348, 1:2000) or rabbit anti-actin antibody (Sigma, Austria; A2066, 1:1000). Blots were washed and incubated with alkaline phosphatase-conjugated anti-mouse (APP) or anti-rabbit (actin) antibodies for 30 minutes at room temperature. After being washed, blots were incubated in CDP-Star chemiluminescent substrate solution and the signal was visualized with a cooled CCD camera (SearchLight, Aushon Biosystems, Billerica, MA, USA).
Quantification and statistical analysis
Measurement of optical density (OD) in western blots was performed by computer-assisted densitometry (Photoshop). The number of single platelets and aggregates was counted under the microscope (Olympus, BX61) at 40 × magnification. Statistical analysis was performed by one-way analysis of variance (ANOVA) with a subsequent Fisher LSD post-hoc test (KaleidaGraph; Synergy Software). A p value <0.05 was considered statistically significant.
Results
Rat platelet controls
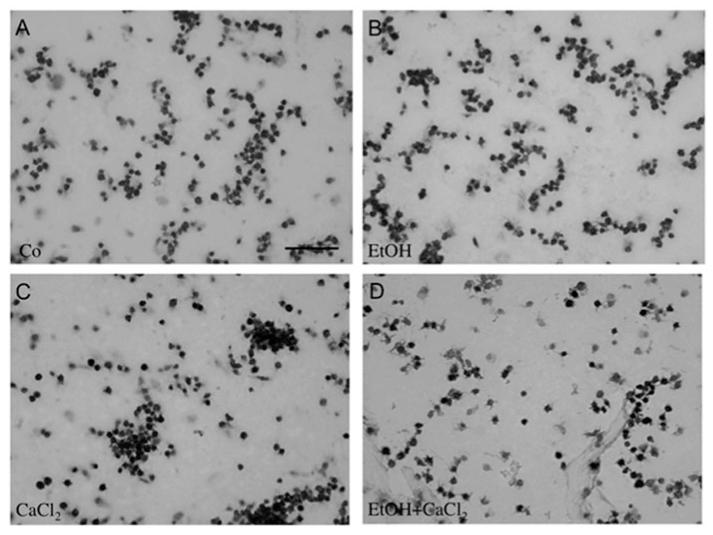
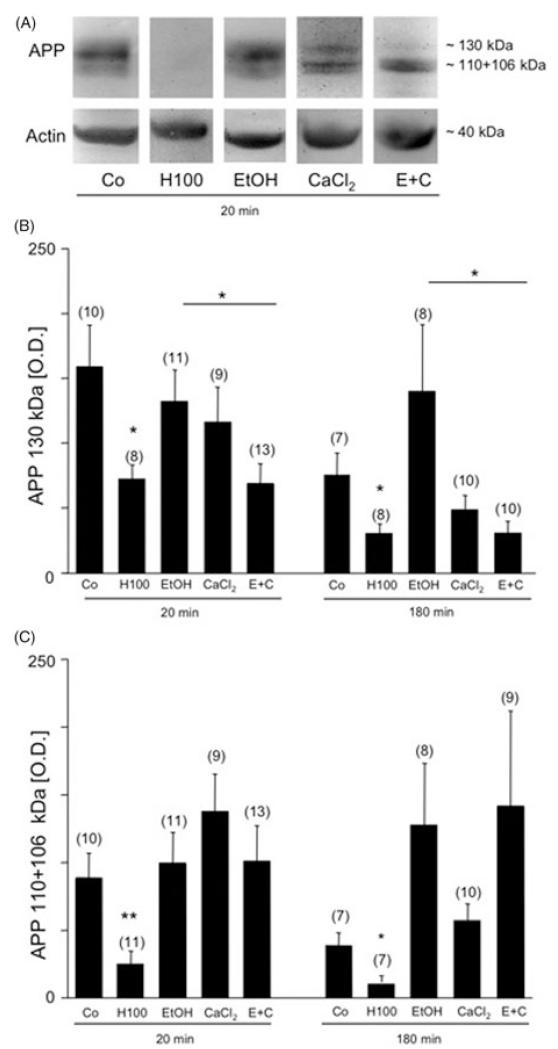
Immunohistochemistry of control platelets revealed approximately 4000 discoid single CD61+cells/mm2 after 20 or 180 minutes (Figure 1A, Table I). In control samples approximately 30 CD61+aggregates/mm2 were seen after 20 minutes, which decreased to approximately 10 CD61+aggregates/mm2 after 180 minutes (Table I). Western blot analysis of control platelets revealed APP fragments at 130 and 106/110 kDa (Figure 2A). Actin was used as a control and was detectable at 40 kDa (Figure 2A). Serotonin release was found to be approximately 150 ng/ml after 20 or 180 minutes (Table II). In order to test maximal stimulation, we exposed rat platelets to 100 mM H2O2, which decreased the number of CD61+cells/mm2 (Table I), enhanced serotonin release (Table II) and decreased APP isoform expression (Figure 2).
Figure 1.
Immunohistochemical staining of rat platelets. Freshly isolated rat platelets (5 × 108) were treated without (Co, A) or with 50 mM EtOH (B), 2 mM CaCl2 (C) or with 50 mM EtOH and 2 mM CaCl2 (D) for 20 minutes; an aliquot (10 μl) was spotted onto gelatin-coated glass slides and immunohistochemically stained for CD61. Note that CaCl2 markedly enhanced the number of CD61+platelet aggregations (C). Scale bar in A ~20 μm.
Table I.
Effects of ethanol and calcium chloride on CD61+cells and aggregates in rat platelets.
| Number of CD61+cells/mm2 |
Number of CD61+aggregates/mm2 |
|||
|---|---|---|---|---|
| Treatment | 20 minutes | 180 minutes | 20 minutes | 180 minutes |
| Control | 4873 ± 353 (9) | 3913 ± 487 (7) | 27 ± 7 (9) | 9 ± 4 (7) |
| H100 | 5020 ± 673 (9) | 27 ± 7 (7)*** | 7 ± 7 (9)* | 0 ± 0 (7)* |
| EtOH | 5913 ± 420 (9) | 4560 ± 653 (7) | 20 ± 7 (9) | 5 ± 3 (7) |
| CaCl2 | 3400 ± 607 (10)* | 2227 ± 253 (8)** | 53 ± 7 (10)** | 24 ± 4 (8)* |
| EtOH+CaCl2 | 4486 ± 433 (13)§ | 2933 ± 353 (7)§ | 27 ± 7 (13)# | 13 ± 5 (7) |
Freshly isolated rat platelets (5 × 108) were treated with 100 mM hydrogen peroxide (H100) or not (control), or with 50 mM ethanol (EtOH) or 2mM calcium chloride (CaCl2), or with 50 mM EtOH and 2 mM CaCl2 (EtOH+CaCl2) for 20 or 180 minutes. An aliquot of the platelets (10 μl) was spotted onto glass slides and immunohistochemically stained for CD61. Single platelets and platelet aggregations were counted under the microscope at 40 × magnification in a 336 × 448 μm field. Values are given as mean ± SEM number of cells/mm2 or aggregations/mm2. Statistical analysis was performed by one-way analysis of variance (ANOVA) with a subsequent Fisher LSD post-hoc test.
Values in parenthesis give the number of experiments.
p<0.001 versus controls
p<0.01 versus controls
p<0.05 versus controls
p<0.05 vs. CaCl2
p<0.05 vs. EtOH
Figure 2.
Western blot analysis of rat platelet extracts. Western blot analysis revealed three isoforms of amyloid precursor protein (APP) at 130 and 106/110 kDa in control (Co) platelets (A). Actin was used for quantitative correlation and revealed a size of approximately 40 kDa (A). Incubation of rat platelets with 100 mM hydrogen peroxide (H100) markedly decreased APP immunoreactivity of the 106/110 kDa fragments after 20 minutes (A, C) and reduced all three APP fragments after 180 minutes (B, C). Exposure of rat platelets to either EtOH alone or CaCl2 alone did not affect the APP isoforms at either time (A, B, C). At both times (A, B) combined treatment with EtOH and CaCl2 markedly decreased the immunoreactivity of the APP 130 kDa fragment as compared to treatment with EtOH alone. Values are given as mean ± SEM OD. Statistical analysis was performed by ANOVA with a subsequent Fisher LSD post-hoc test. Values in parenthesis give the number of subjects; *p < 0.05; **p < 0.01.
Table II.
Effects of ethanol and calcium chloride on serotonin release in rat platelets.
| Treatment | Serotonin [ng/ml × 20 minutes] | Serotonin [ng/ml × 180 minutes] |
|---|---|---|
| Control | 122 ± 20 (11) | 168 ± 42 (7) |
| H100 | 791 ± 163 (11)** | 1343 ± 386 (6)* |
| EtOH | 189 ± 56 (11) | 417 ± 159 (7) |
| CaCl2 | 580 ± 108 (10)*** | 755 ± 160 (9)** |
| EtOH+CaCl2 | 561 ± 106 (13)§ | 325 ± 33 (9) |
Freshly isolated rat platelets (5 × 108) were treated with 100 mM hydrogen peroxide (H100) or not (control), or with 50 mM ethanol (EtOH), or with 2 mM calcium chloride (CaCl2), or with CaCl2 and 50 mM EtOH (EtOH+CaCl2) for 20 or 180 minutes. Platelets were centrifuged and supernatants were evaluated by high-pressure liquid chromatography electrochemical detection (HPLC-EC) to measure serotonin release. Values are given as mean ± SEM ng/ml × 20 or 180 minutes. Statistical analysis was performed by ANOVA with a subsequent Fisher LSD post-hoc test.
Values in parenthesis give the number of experiments.
p<0.001 versus controls
p<0.01 versus controls
p<0.05 versus controls
p<0.05 vs. EtOH
Effects of EtOH on rat platelets
Treatment with 50 mM EtOH for 20 or 180 minutes did not affect any of the tested parameters: CD61+cells/mm2 (Figure 1, Table I), aggregates/mm2 (Figure 1, Table II), serotonin release (Table II), and processing of APP isoforms (Figure 2).
Effects of CaCl2 on rat platelets
When rat platelets were treated with 2 mM CaCl2 for 20 or 180 minutes, the number of CD61+cells/mm2 was reduced (Table I), but the number of aggregates significantly increased (Table II, Figure 1). Serotonin release was significantly enhanced after 20 or 180 minutes (Table II). CaCl2 treatment alone did not affect APP isoform processing (Figure 2).
Effects of EtOH and CaCl2 combination on rat platelets
When rat platelets were treated with combined EtOH and CaCl2, the number of CD61+cells/mm2 was significantly decreased after 20 or 180 minutes as compared to treatment with EtOH alone (Table I). In parallel, the number of CD61+platelet aggregates/mm2 markedly decreased (Table I) and the release of serotonin increased after 20 minutes, but not after 180 minutes. Western blot analysis revealed a significant reduction in the 130 kDa fragment 20 and 180 minutes after combined treatment with EtOH and CaCl2, as compared with treatment with EtOH alone (Figure 2A and B).
Effects of EtOH and CaCl2 on human platelets
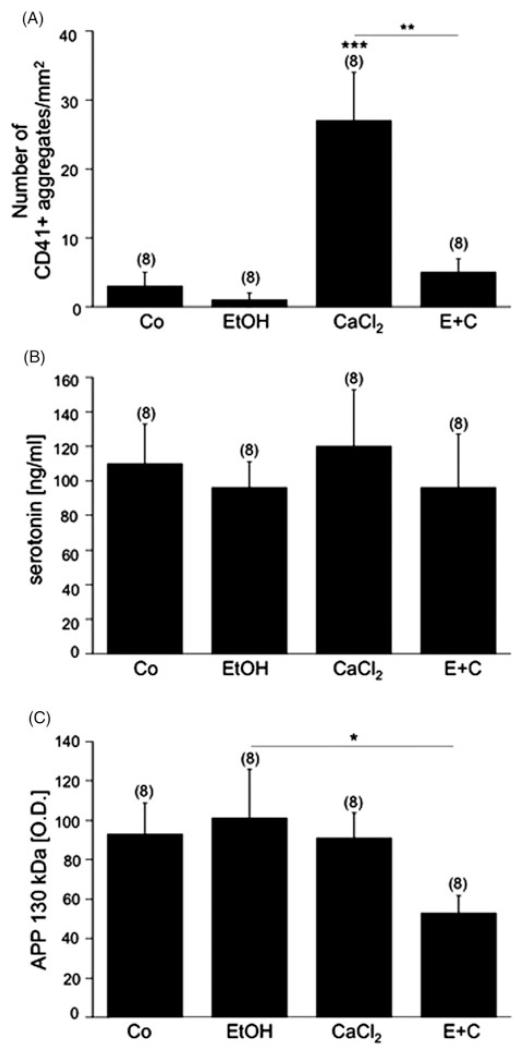
Immunohistochemistry of control human platelets revealed approximately 1000 discoid single CD41+cells/mm2 and approximately 3 CD41+aggregates/mm2 after 20 minutes (Figure 3A). Serotonin release was approximately 110 ng/ml (n = 8, Figure 3B). All three APP isoforms of 130 and 106/110 kDa were detectable by western blot analysis. Exposure of human platelets to 50 mM EtOH for 20 minutes did not affect any of the tested parameters: number of CD41+cells/mm2 or CD41+aggregates/mm2 (Figure 3A), serotonin release (Figure 3B), expression of APP isoforms (Figure 3C). When human platelets were exposed to 2 mM CaCl2 for 20 minutes, CD41+aggregation significantly increased (Figure 3A). However, CaCl2 did not alter the number of CD41+cells/mm2, or change serotonin release (Figure 3B) or APP isoform expression (Figure 3C). Combined EtOH and CaCl2 treatment increased the number of CD41+cells and decreased the CD41+aggregates/mm2 as compared to treatment with EtOH alone (Figure 3A), but did not alter the release of serotonin. Western blot analysis revealed a significant reduction in the APP 130 kDa fragment after EtOH and CaCl2 treatment for 20 minutes as compared to treatment with EtOH alone (Figure 3B).
Figure 3.
Effects of EtOH exposure on human platelets. Freshly isolated rat platelets (5 × 108) were treated without (Co) or with 50 mM EtOH, 2 mM calcium chloride (CaCl2) or with 50 mM EtOH and 2 mM CaCl2 (E+C) for 20 minutes. (A) An aliquot (10 μl) was spotted onto glass slides and immunohistochemically stained for CD41. Note that CaCl2 markedly enhanced the number of CD41+platelet aggregates. (B) Serotonin release was not affected by the various treatments. (C) Combined treatment with EtOH and CaCl2 markedly decreased the immunoreactivity of the APP 130 kDa isoform as compared to treatment with EtOH alone. Values are given as mean ± SEM number of CD41+aggregates/mm2 (A) or ng/ml serotonin (B) or OD (C). Statistical analysis was performed by ANOVA with a subsequent Fisher LSD post-hoc test. Values in parenthesis give the number of subjects; *p < 0.05; **p < 0.01; ***p < 0.001.
Discussion
The present study shows that EtOH counteracts CaCl2-induced platelet aggregation and that combined EtOH and CaCl2 treatment affects APP processing in human and rat platelets.
As previously shown, the mature fully glycosylated 130 kDa APP as well as the smaller 106 and 110 kDa APP isoforms are detectable in rat as well as in human platelets [8, 26]. Platelets contain Aβ peptides that are cleaved from APP by α, β, or γ secretases in the presence of Ca2+ [10]. Aβ, APP as well as soluble fragments of APP (sAPP) are released upon activation from platelet α-granules [26]. In fact, platelets are the major source of plasma Aβ [12], and increased production and accumulation of Aβ may play an important role in the development of CAA [9, 27, 28].
Moderate EtOH consumption protects against the development of cardio- and cerebrovascular disease by increasing high-density lipoprotein levels or by decreasing platelet activation and clotting activity [29–31]. On the other hand, EtOH induces oxidative stress, generates H2O2, and oxidizes sulphydryl groups in human platelets [32]. In the present study rat and human platelets were treated with 50 mM (2.4 g/l) EtOH, which is a well-established experiment concentration [33] that reflects plasma EtOH levels of approximately 2‰. We show that EtOH alone did not affect the total number of platelets, the number of aggregations, serotonin release or APP processing.
In platelets intracellular Ca2+ is basically stored in the dense tubular system [34], and platelet agonists increase intracellular Ca2+ levels and trigger shape change, aggregation, and granule secretion [35, 36]. Our study shows that CaCl2 alone enhanced platelet aggregation as well as serotonin release, reflecting direct activation of platelet granule content. Interestingly, serotonin in human platelets was not altered by CaCl2 treatment. This may point to an improved re-uptake mechanism in human platelets and is in line with previous studies [37]. Furthermore, various Ca2+-related mechanisms in rat and human platelets have been suggested [38]. Thus, rat platelets may be more vulnerable to an imbalance in Ca2+ homeostasis than are human platelets. Moreover, it has been reported that altered Ca2+ homeostasis may play a role in APP processing [10, 39]. However, in the present study CaCl2 alone did not affect APP isoform expression, either in rat or human platelets. This is in line with the findings of others [40] and may suggest that CaCl2 is only a co-factor in aberrant APP processing.
It is well established that EtOH prevents platelet aggregation by influencing Ca2-dependent mechanisms, possibly by preventing transplasmalemmal Ca2 entry [41, 42]. Our present study is in agreement and shows that EtOH counteracts CaCl2-induced platelet aggregation in humans and rats. In parallel, the CaCl2-induced serotonin release of rat platelets was also counteracted by EtOH, clearly pointing to a similar mechanism. After combined EtOH and CaCl2 treatment, the number of CD41+cells was increased as compared to treatment with EtOH alone. This may point to strong anti-aggregatory effects of EtOH in human platelets.
In this study we show for the first time that EtOH affects APP processing by reducing the APP 130 kDa isoform, but only in the presence of CaCl2. In contrast, treatment with neither EtOH nor CaCl2 alone had an effect on the expression of APP isoforms. It is well established that in AD aberrant processing of APP occurs and that the larger 130 kDa isoform is markedly reduced [7, 8, 43, 44]. It is interesting to see that the effect of combined CaCl2 and EtOH on APP isoforms in rat and human platelets resembles the alterations found in platelets of AD patients. However, the mechanism by which the large 130 kDa isoform is reduced is still unclear and controversial. RT-PCR experiments evaluated that the mRNAs of the three major APP transcripts are not altered [4]. In contrast, a significant upregulation of APP TOT, APP KPI, APP 770 and APP 751 has been reported [45]. These findings suggest that no marked alterations in transcript expression occur. Thus, it seems likely that the decline in mature APP in platelets of AD patients is due to abnormalities in processing and not to alternative splicing. Potentiating deleterious effects of high Ca2+ levels and EtOH possibly plays a role in this aberrant APP processing and may be critical for developing alcohol-induced dementia or AD. Indeed, EtOH consumption elevates APP levels and influences the Aβ-producing enzymes β secretase (BACE1), and γ-secretase, presenilin 1 (PS1) and nicastrin in rat brains, thus providing a possible link between altered APP processing and alcohol consumption [46, 47]. In addition, altered Ca2+ homeostasis may be involved in this process, since Ca2+ regulates APP processing [10, 39]. Interestingly, also high cholesterol serum levels reduce expression of the higher molecular weight 130 kDa APP form in human platelets [11]. In fact, cholesterol may play a role in AD pathology by regulating Aβ production and promoting the β-secretase pathway [48]. Thus, the vascular risk factor (e.g. EtOH or cholesterol) as well as altered CaCl2 homeostasis may contribute to APP isoform alterations in platelets and subsequently increased production and accumulation of Aβ, e.g. in vessels.
In conclusion, our data show for the first time a decrease in the 130 kDa isoform induced by EtOH, but only in the presence of CaCl2 in rat and human platelets. The reduction in this isoform is also seen in AD platelets, possibly pointing to similar (oxidative stress) mechanisms. Furthermore, EtOH counteracted CaCl2-mediated platelet aggregation, thus indicating the haemostatic properties of EtOH.
Acknowledgments
We thank Ursula Kirzenberger-Winkler for her excellent technical help.
This study was supported by the Austrian Science Fund (L429-B05 and P24734).
Footnotes
Declaration of interest: The authors report no declarations of interest.
References
- 1.Catricala’ S, Torti M, Ricevuti G. Alzheimer disease and platelets: How’s that relevant. Immun Ageing. 2012;9:20. doi: 10.1186/1742-4933-9-20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Roher AE, Esh CL, Kokjohn TA, Castaño EM, Van Vickle GD, Kalback WM, Patton RL, Luehrs DC, Daugs ID, Kuo YM, et al. Amyloid beta peptides in human plasma and tissues and their significance for Alzheimer’s disease. Alzheimers Dement. 2009;5:18–29. doi: 10.1016/j.jalz.2008.10.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Borroni B, Agosti C, Marcello E, Di Luca M, Padovani A. Blood cell markers in Alzheimer’s disease: Amyloid Precursor form ratio in platelets. Exp Gerontol. 2010;45:53–56. doi: 10.1016/j.exger.2009.08.004. [DOI] [PubMed] [Google Scholar]
- 4.Di Luca M, Colciaghi F, Pastorino L, Borroni B, Padovani A, Cattabeni F. Platelets as a peripheral district where to study pathogenetic mechanisms of Alzheimer disease: The case of amyloid precursor protein. Eur J Pharmacol. 2000;405:277–283. doi: 10.1016/s0014-2999(00)00559-8. [DOI] [PubMed] [Google Scholar]
- 5.Li QX, Berndt MC, Bush AI, Rumble B, Mackenzie I, Friedhuber A, Beyreuther K, Masters CL. Membraneassociated forms of the beta A4 amyloid protein precursor of Alzheimer’s disease in human platelet and brain: Surface expression on the activated human platelet. Blood. 1994;84:133–142. [PubMed] [Google Scholar]
- 6.Tang K, Hynan LS, Baskin F, Rosenberg RN. Platelet amyloid precursor protein processing: A bio-marker for Alzheimer’s disease. J Neurol Sci. 2006;240:53–58. doi: 10.1016/j.jns.2005.09.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Rosenberg RN, Baskin F, Fosmire JA, Risser R, Adams P, Svetlik D, Honig LS, Cullum CM, Weiner MF. Altered amyloid protein processing in platelets of patients with Alzheimer disease. Arch Neurol. 1997;54:139–144. doi: 10.1001/archneur.1997.00550140019007. [DOI] [PubMed] [Google Scholar]
- 8.Ehrlich D, Hochstrasser T, Humpel C. Effects of oxidative stress on amyloid precursor protein processing in rat and human platelets. Platelets. 2013;24:26–36. doi: 10.3109/09537104.2012.661104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Davies TA, Long HJ, Eisenhauer PB, Hastey R, Cribbs DH, Fine RE, Simons ER. Beta amyloid fragments derived from activated platelets deposit in cerebrovascular endothelium: Usage of a novel blood brain barrier endothelial cell model system. Amyloid. 2000;7:153–165. doi: 10.3109/13506120009146830. [DOI] [PubMed] [Google Scholar]
- 10.Buxbaum JD, Ruefli AA, Parker CA, Cypess AM, Greengard P. Calcium regulates processing of the Alzheimer amyloid protein precursor in a protein kinase C-independent manner. Proc Natl Acad Sci USA. 1994;91:4489–4493. doi: 10.1073/pnas.91.10.4489. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Borroni B, Colciaghi F, Lenzi GL, Caimi L, Cattabeni F, Di Luca M, Padovani A. High cholesterol affects platelet APP processing in controls and in AD patients. Neurobiol Age. 2003;24:631–636. doi: 10.1016/s0197-4580(02)00190-2. [DOI] [PubMed] [Google Scholar]
- 12.Chen M, Inestrosa NC, Ross GS, Fernandez HL. Platelets are the principal source of amyloid beta-peptide in human blood. Biochem Biophys Res Commun. 1995;213:96–103. doi: 10.1006/bbrc.1995.2103. [DOI] [PubMed] [Google Scholar]
- 13.Varga-Szabo D, Braun A, Nieswandt B. Calcium signaling in platelets. J Thromb Haemost. 2009;7:1057–1066. doi: 10.1111/j.1538-7836.2009.03455.x. [DOI] [PubMed] [Google Scholar]
- 14.Rosado JA, Nuñez AM, Lopez JJ, Pariente JA, Salido GM. Intracellular Ca2+ homeostasis and aggregation in platelets are impaired by ethanol through the generation of H2O2 and oxidation of sulphydryl groups. Arch Biochem Biophys. 2006;452:9–16. doi: 10.1016/j.abb.2006.05.011. [DOI] [PubMed] [Google Scholar]
- 15.Stach K, Kälsch AI, Weiss C, Elmas E, Borggrefe M, Kälsch T. Effects of ethanol on the properties of platelets and endothelial cells in model experiments. World J Cardiol. 2012;4:201–205. doi: 10.4330/wjc.v4.i6.201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Salem RO, Laposata M. Effects of alcohol on hemostasis. Am J Clin Pathol. 2005;123:96–105. doi: 10.1309/113N8EUFXYUECCNA. [DOI] [PubMed] [Google Scholar]
- 17.Cahill PA, Redmond EM. Alcohol and cardiovascular disease-modulation of vascular cell function. Nutrients. 2012;4:297–318. doi: 10.3390/nu4040297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Mikhailidis DP, Barradas MA, Jeremy JY. The effect of ethanol on platelet function and vascular prostanoids. Alcohol. 1990;7:171–180. doi: 10.1016/0741-8329(90)90080-v. [DOI] [PubMed] [Google Scholar]
- 19.Hillbom M, Kangasaho M, Löwbeer C, Kaste M, Muuronen A, Numminen H. Effects of ethanol on platelet function. Alcohol. 1985;2:429–432. doi: 10.1016/0741-8329(85)90109-0. [DOI] [PubMed] [Google Scholar]
- 20.Ronksley PE, Brien SE, Turner BJ, Mukamal KJ, Ghali WA. Association of alcohol consumption with selected cardiovascular disease outcomes: A systematic review and meta-analysis. BMJ. 2011;342:671. doi: 10.1136/bmj.d671. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Baer DJ, Judd JT, Clevidence BA, Muesing RA, Campbell WS, Brown ED, Taylor PR. Moderate alcohol consumption lowers risk factors for cardiovascular disease in postmenopausal women fed a controlled diet. Am J Clin Nutr. 2002;75:593–599. doi: 10.1093/ajcn/75.3.593. [DOI] [PubMed] [Google Scholar]
- 22.Meister KA, Whelan EM, Kava R. The health effects of moderate alcohol intake in humans: An epidemiologic review. Critic Rev Clin Labor Sci. 2000;37:261–296. doi: 10.1080/10408360091174222. [DOI] [PubMed] [Google Scholar]
- 23.Ruf JC. Alcohol, wine and platelet function. Biol Res. 2004;37:209–215. doi: 10.4067/s0716-97602004000200006. [DOI] [PubMed] [Google Scholar]
- 24.Davies MJ, Baer DJ, Judd JT, Brown ED, Campbell WS, Taylor PR. Effects of moderate alcohol intake on fasting insulin and glucose concentrations and insulin sensitivity in postmenopausal women: A randomized controlled trial. JAMA. 2002;287:2559–2562. doi: 10.1001/jama.287.19.2559. [DOI] [PubMed] [Google Scholar]
- 25.Hermann M, Nussbaumer O, Knöfler R, Hengster P, Nussbaumer W, Streif W. Real-time live confocal fluorescence microscopy as a new tool for assessing platelet vitality. Transfus Med Hemother. 2010;37:299–305. doi: 10.1159/000320368. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Kitazume S, Yoshihisa A, Yamaki T, Oikawa M, Tachida Y, Ogawa K, Imamaki R, Hagiwara Y, Kinoshita N, Takeishi Y, et al. Soluble amyloid precursor protein 770 is released from inflamed endothelial cells and activated platelets: A novel biomarker for acute coronary syndrome. J Biol Chem. 2012;287:40817–40825. doi: 10.1074/jbc.M112.398578. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Roher AE, Esh CL, Kokjohn TA, Castaño EM, Van Vickle GD, Kalback W, Patton RL, Luehrs DC, Daugs ID, Kuo YM, et al. Amyloid beta peptides in human plasma and tissues and their significance for Alzheimer’s disease. Alzheimers Dement. 2009;5:18–29. doi: 10.1016/j.jalz.2008.10.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Deane R, Zlokovic BV. Role of the blood–brain barrier in the pathogenesis of Alzheimer’s disease. Curr Alzheimer Res. 2007;4:191–197. doi: 10.2174/156720507780362245. [DOI] [PubMed] [Google Scholar]
- 29.Panza F, Frisardi V, Seripa D, Logroscino G, Santamato A, Imbimbo BP, Scafato E, Pilotto A, Solfrizzi V. Alcohol consumption in mild cognitive impairment and dementia: Harmful or neuroprotective? Int J Geriatr Psychiatry. 2012;27:1218–1238. doi: 10.1002/gps.3772. [DOI] [PubMed] [Google Scholar]
- 30.Renaud SC, Ruf JC. Effects of alcohol on platelet function. Clin Chim Acta. 1996;246:77–89. doi: 10.1016/0009-8981(96)06228-6. [DOI] [PubMed] [Google Scholar]
- 31.Fraser GE, Anderson JT, Foster N, Goldberg R, Jacobs D, Blackburn H. The effect of alcohol on serum high density lipoprotein (HDL). A controlled experiment. Atherosclerosis. 1983;46:275–286. doi: 10.1016/0021-9150(83)90178-8. [DOI] [PubMed] [Google Scholar]
- 32.Rosado JA, Nunez AM, Parients JA, Salido GM. Alterations in intracellular calcium homeostasis and platelet aggregation induced by ethanol. Biochem Biophys Res Commun. 2006;341:917–924. doi: 10.1016/j.bbrc.2006.01.056. [DOI] [PubMed] [Google Scholar]
- 33.Ehrlich D, Pirchl M, Humpel C. Ethanol transiently suppresses choline-acetyltransferase in basal nucleus of Meynert slices. Brain Research. 2012;1459:35–42. doi: 10.1016/j.brainres.2012.04.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Rink TJ, Sage SO. Calcium signaling in human platelets. Annu Rev Physiol. 1990;52:431–449. doi: 10.1146/annurev.ph.52.030190.002243. [DOI] [PubMed] [Google Scholar]
- 35.Jurk K, Kehrel BE. Platelets: Physiology and biochemistry. Semin Thromb Hemost. 2005;31:381–392. doi: 10.1055/s-2005-916671. [DOI] [PubMed] [Google Scholar]
- 36.Miyamae T, Oshima K, Morikawa T, Hagiwara M. Calcium-induced platelet aggregation in washed platelets from guinea pigs. Pharmacology. 1995;51:180–185. doi: 10.1159/000139333. [DOI] [PubMed] [Google Scholar]
- 37.Hochstrasser T, Ehrlich D, Marksteiner J, Sperner-Unterweger B, Humpel C. Matrix metalloproteinase-2 and epidermal growth factor are decreased in platelets of Alzheimer patients. Curr Alz Res. 2012;9:982–989. doi: 10.2174/156720512803251156. [DOI] [PubMed] [Google Scholar]
- 38.Oshima T, Ishida T, Matsuura H, Ishida M, Ishibashi K, Ozono R, Watanabe M, Kajiyama G, Kanbe M. Lack of effect of ouabain on calcium homeostasis in rat platelets: Comparative study with human platelets. Am J Physiol. 1994;266:651–657. doi: 10.1152/ajpregu.1994.266.3.R651. [DOI] [PubMed] [Google Scholar]
- 39.LaFerla FM. Calcium dyshomeostasis and intracellular signalling in Alzheimer’s disease. Nat Rev Neurosci. 2002;3:862–872. doi: 10.1038/nrn960. [DOI] [PubMed] [Google Scholar]
- 40.Canobbio I, Catricalà S, Balduini C, Torti M. Calmodulin regulates the non-amyloidogenic metabolism of amyloid precursor protein in platelets. Biochim Biophys Acta. 2011;1813:500–506. doi: 10.1016/j.bbamcr.2010.12.002. [DOI] [PubMed] [Google Scholar]
- 41.Marumo M, Wakabayashi I. Diverse effects of ethanol on Ca2+ entry and subsequent aggregation of platelets. Alcohol. 2010;44:343–350. doi: 10.1016/j.alcohol.2010.02.002. [DOI] [PubMed] [Google Scholar]
- 42.Torres Duarte AP, Dong QS, Young J, Abi-Younes S, Myers AK. Inhibition of platelet aggregation in whole blood by alcohol. Thromb Res. 1995;78:107–115. doi: 10.1016/0049-3848(95)00039-9. [DOI] [PubMed] [Google Scholar]
- 43.Hochstrasser T, Ehrlich D, Sperner-Unterweger B, Humpel C. Antidepressants and anti-inflammatory drugs differentially reduce the release of NGF and BDNF from rat platelets. Pharmacopsychiatry. 2012 doi: 10.1055/s-0032-1314843. Epub ahead of print. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Bush AI, Whyte S, Thomas LD, Williamson TG, Van Tiggelen CJ, Currie J, Small DH, Moir RD, Li QX, Rumble B. An abnormality of plasma amyloid protein precursor in Alzheimer’s disease. Ann Neurol. 1992;32:57–65. doi: 10.1002/ana.410320110. [DOI] [PubMed] [Google Scholar]
- 45.Vignini A, Sartini D, Morganti S, Nanetti L, Luzzi S, Provinciali L, Mazzanti L, Emanuelli M. Platelet amyloid precursor protein isoform expression in Alzheimer’s disease: Evidence for peripheral marker. Int J Immunopathol Pharmacol. 2011;24:529–534. doi: 10.1177/039463201102400229. [DOI] [PubMed] [Google Scholar]
- 46.Kim SR, Jeong HY, Yang S, Choi SP, Seo MY, Yun YK, Choi Y, Baik SH, Park JS, Gwon AR, et al. Effects of chronic alcohol consumption on expression levels of APP and Aβ-producing enzymes. BMB Rep. 2011;144:135–139. doi: 10.5483/BMBRep.2011.44.2.135. [DOI] [PubMed] [Google Scholar]
- 47.Lahiri DK, Nall C, Chen D, Zaphiriou M, Morgan C, Nurnberger JI., Sr Developmental expression of the beta-amyloid precursor protein and heat-shock protein 70 in the cerebral hemisphere region of the rat brain. Ann NY Acad Sci. 2002;965:324–333. doi: 10.1111/j.1749-6632.2002.tb04174.x. [DOI] [PubMed] [Google Scholar]
- 48.Refolo LM, Pappolla MA, LaFrancois J, Malester B, Schmidt SD, Thomas-Bryant T, Tint GS, Wang R, Mercken M, Petanceska SS, et al. A cholesterol-lowering drug reduces beta-amyloid pathology in a transgenic mouse model of Alzheimer’s disease. Neurobiol Dis. 2001;8:890–899. doi: 10.1006/nbdi.2001.0422. [DOI] [PubMed] [Google Scholar]