Abstract
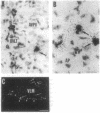
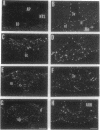
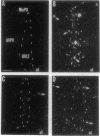
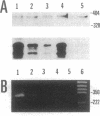
Tumor necrosis factor alpha (TNF-alpha) is a cytokine that is responsible, in part, for several aspects of the acute-phase response to inflammation, including the generation of fever. TNF-alpha has direct effects on central nervous system neurons deep within the hypothalamus that are involved in producing the febrile response, but the blood-brain barrier prevents circulating TNF-alpha from having access to these sites. We therefore have hypothesized that TNF-alpha may be produced in the brain and used as a mediator in the cerebral components of the acute-phase response. We used in situ hybridization to determine the distribution of production of TNF-alpha mRNA in the mouse brain after systemic administration of lipopolysaccharide. During the initial phase of fever, hybridization was observed in perivascular cells and neurons in circumventricular organs, including the vascular organ of the lamina terminalis, median eminence, and area postrema, as well as along the ventral surface of the medulla; hybridization was also prominent over many cell in the meninges. During the late phase of the response, hybridization was observed over neurons in the pericircumventricular nuclei such as the anteroventral periventricular and arcuate nuclei of the hypothalamus and the nucleus of the solitary tract. TNF-alpha produced by a cascade of neurons within the brain may participate in the complex autonomic, neuroendocrine, metabolic, and behavioral responses to infection and inflammation.
Full text
PDF



Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Beutler B. A., Milsark I. W., Cerami A. Cachectin/tumor necrosis factor: production, distribution, and metabolic fate in vivo. J Immunol. 1985 Dec;135(6):3972–3977. [PubMed] [Google Scholar]
- Beutler B., Cerami A. The biology of cachectin/TNF--a primary mediator of the host response. Annu Rev Immunol. 1989;7:625–655. doi: 10.1146/annurev.iy.07.040189.003205. [DOI] [PubMed] [Google Scholar]
- Bieger D., Hopkins D. A. Viscerotopic representation of the upper alimentary tract in the medulla oblongata in the rat: the nucleus ambiguus. J Comp Neurol. 1987 Aug 22;262(4):546–562. doi: 10.1002/cne.902620408. [DOI] [PubMed] [Google Scholar]
- Blick M., Sherwin S. A., Rosenblum M., Gutterman J. Phase I study of recombinant tumor necrosis factor in cancer patients. Cancer Res. 1987 Jun 1;47(11):2986–2989. [PubMed] [Google Scholar]
- Breder C. D., Tsujimoto M., Terano Y., Scott D. W., Saper C. B. Distribution and characterization of tumor necrosis factor-alpha-like immunoreactivity in the murine central nervous system. J Comp Neurol. 1993 Nov 22;337(4):543–567. doi: 10.1002/cne.903370403. [DOI] [PubMed] [Google Scholar]
- Breder C. D., Yamada Y., Yasuda K., Seino S., Saper C. B., Bell G. I. Differential expression of somatostatin receptor subtypes in brain. J Neurosci. 1992 Oct;12(10):3920–3934. doi: 10.1523/JNEUROSCI.12-10-03920.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brizzee K. R., Klara P. M. The structure of the mammalian area postrema. Fed Proc. 1984 Dec;43(15):2944–2948. [PubMed] [Google Scholar]
- Dinarello C. A., Cannon J. G., Wolff S. M., Bernheim H. A., Beutler B., Cerami A., Figari I. S., Palladino M. A., Jr, O'Connor J. V. Tumor necrosis factor (cachectin) is an endogenous pyrogen and induces production of interleukin 1. J Exp Med. 1986 Jun 1;163(6):1433–1450. doi: 10.1084/jem.163.6.1433. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dinarello C. A. Endogenous pyrogens. Methods Enzymol. 1988;163:495–510. doi: 10.1016/0076-6879(88)63046-1. [DOI] [PubMed] [Google Scholar]
- Gutierrez E. G., Banks W. A., Kastin A. J. Murine tumor necrosis factor alpha is transported from blood to brain in the mouse. J Neuroimmunol. 1993 Sep;47(2):169–176. doi: 10.1016/0165-5728(93)90027-v. [DOI] [PubMed] [Google Scholar]
- Herbert H., Moga M. M., Saper C. B. Connections of the parabrachial nucleus with the nucleus of the solitary tract and the medullary reticular formation in the rat. J Comp Neurol. 1990 Mar 22;293(4):540–580. doi: 10.1002/cne.902930404. [DOI] [PubMed] [Google Scholar]
- Hickey W. F., Kimura H. Perivascular microglial cells of the CNS are bone marrow-derived and present antigen in vivo. Science. 1988 Jan 15;239(4837):290–292. doi: 10.1126/science.3276004. [DOI] [PubMed] [Google Scholar]
- Hickey W. F., Vass K., Lassmann H. Bone marrow-derived elements in the central nervous system: an immunohistochemical and ultrastructural survey of rat chimeras. J Neuropathol Exp Neurol. 1992 May;51(3):246–256. doi: 10.1097/00005072-199205000-00002. [DOI] [PubMed] [Google Scholar]
- Jones S. B., Westfall M. V., Sayeed M. M. Plasma catecholamines during E. coli bacteremia in conscious rats. Am J Physiol. 1988 Mar;254(3 Pt 2):R470–R477. doi: 10.1152/ajpregu.1988.254.3.R470. [DOI] [PubMed] [Google Scholar]
- Kawakami M., Cerami A. Studies of endotoxin-induced decrease in lipoprotein lipase activity. J Exp Med. 1981 Sep 1;154(3):631–639. doi: 10.1084/jem.154.3.631. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Krueger J. M., Obal F., Jr, Opp M., Toth L., Johannsen L., Cady A. B. Somnogenic cytokines and models concerning their effects on sleep. Yale J Biol Med. 1990 Mar-Apr;63(2):157–172. [PMC free article] [PubMed] [Google Scholar]
- Leitman D. C., Ribeiro R. C., Mackow E. R., Baxter J. D., West B. L. Identification of a tumor necrosis factor-responsive element in the tumor necrosis factor alpha gene. J Biol Chem. 1991 May 25;266(15):9343–9346. [PubMed] [Google Scholar]
- Mathison J. C., Wolfson E., Ulevitch R. J. Participation of tumor necrosis factor in the mediation of gram negative bacterial lipopolysaccharide-induced injury in rabbits. J Clin Invest. 1988 Jun;81(6):1925–1937. doi: 10.1172/JCI113540. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Michie H. R., Manogue K. R., Spriggs D. R., Revhaug A., O'Dwyer S., Dinarello C. A., Cerami A., Wolff S. M., Wilmore D. W. Detection of circulating tumor necrosis factor after endotoxin administration. N Engl J Med. 1988 Jun 9;318(23):1481–1486. doi: 10.1056/NEJM198806093182301. [DOI] [PubMed] [Google Scholar]
- Miller C. L., Feldhaus A. L., Rooney J. W., Rhodes L. D., Sibley C. H., Singh H. Regulation and a possible stage-specific function of Oct-2 during pre-B-cell differentiation. Mol Cell Biol. 1991 Oct;11(10):4885–4894. doi: 10.1128/mcb.11.10.4885. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pennica D., Hayflick J. S., Bringman T. S., Palladino M. A., Goeddel D. V. Cloning and expression in Escherichia coli of the cDNA for murine tumor necrosis factor. Proc Natl Acad Sci U S A. 1985 Sep;82(18):6060–6064. doi: 10.1073/pnas.82.18.6060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rettori V., Milenkovic L., Beutler B. A., McCann S. M. Hypothalamic action of cachectin to alter pituitary hormone release. Brain Res Bull. 1989 Dec;23(6):471–475. doi: 10.1016/0361-9230(89)90192-5. [DOI] [PubMed] [Google Scholar]
- Saigusa T. Participation of interleukin-1 and tumor necrosis factor in the responses of the sympathetic nervous system during lipopolysaccharide-induced fever. Pflugers Arch. 1990 May;416(3):225–229. doi: 10.1007/BF00392057. [DOI] [PubMed] [Google Scholar]
- Shoham S., Davenne D., Cady A. B., Dinarello C. A., Krueger J. M. Recombinant tumor necrosis factor and interleukin 1 enhance slow-wave sleep. Am J Physiol. 1987 Jul;253(1 Pt 2):R142–R149. doi: 10.1152/ajpregu.1987.253.1.R142. [DOI] [PubMed] [Google Scholar]
- Socher S. H., Friedman A., Martinez D. Recombinant human tumor necrosis factor induces acute reductions in food intake and body weight in mice. J Exp Med. 1988 Jun 1;167(6):1957–1962. doi: 10.1084/jem.167.6.1957. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tracey K. J., Morgello S., Koplin B., Fahey T. J., 3rd, Fox J., Aledo A., Manogue K. R., Cerami A. Metabolic effects of cachectin/tumor necrosis factor are modified by site of production. Cachectin/tumor necrosis factor-secreting tumor in skeletal muscle induces chronic cachexia, while implantation in brain induces predominantly acute anorexia. J Clin Invest. 1990 Dec;86(6):2014–2024. doi: 10.1172/JCI114937. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ulich T. R., Guo K. Z., Irwin B., Remick D. G., Davatelis G. N. Endotoxin-induced cytokine gene expression in vivo. II. Regulation of tumor necrosis factor and interleukin-1 alpha/beta expression and suppression. Am J Pathol. 1990 Nov;137(5):1173–1185. [PMC free article] [PubMed] [Google Scholar]
- Waage A., Halstensen A., Shalaby R., Brandtzaeg P., Kierulf P., Espevik T. Local production of tumor necrosis factor alpha, interleukin 1, and interleukin 6 in meningococcal meningitis. Relation to the inflammatory response. J Exp Med. 1989 Dec 1;170(6):1859–1867. doi: 10.1084/jem.170.6.1859. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xie W. L., Chipman J. G., Robertson D. L., Erikson R. L., Simmons D. L. Expression of a mitogen-responsive gene encoding prostaglandin synthase is regulated by mRNA splicing. Proc Natl Acad Sci U S A. 1991 Apr 1;88(7):2692–2696. doi: 10.1073/pnas.88.7.2692. [DOI] [PMC free article] [PubMed] [Google Scholar]