Abstract
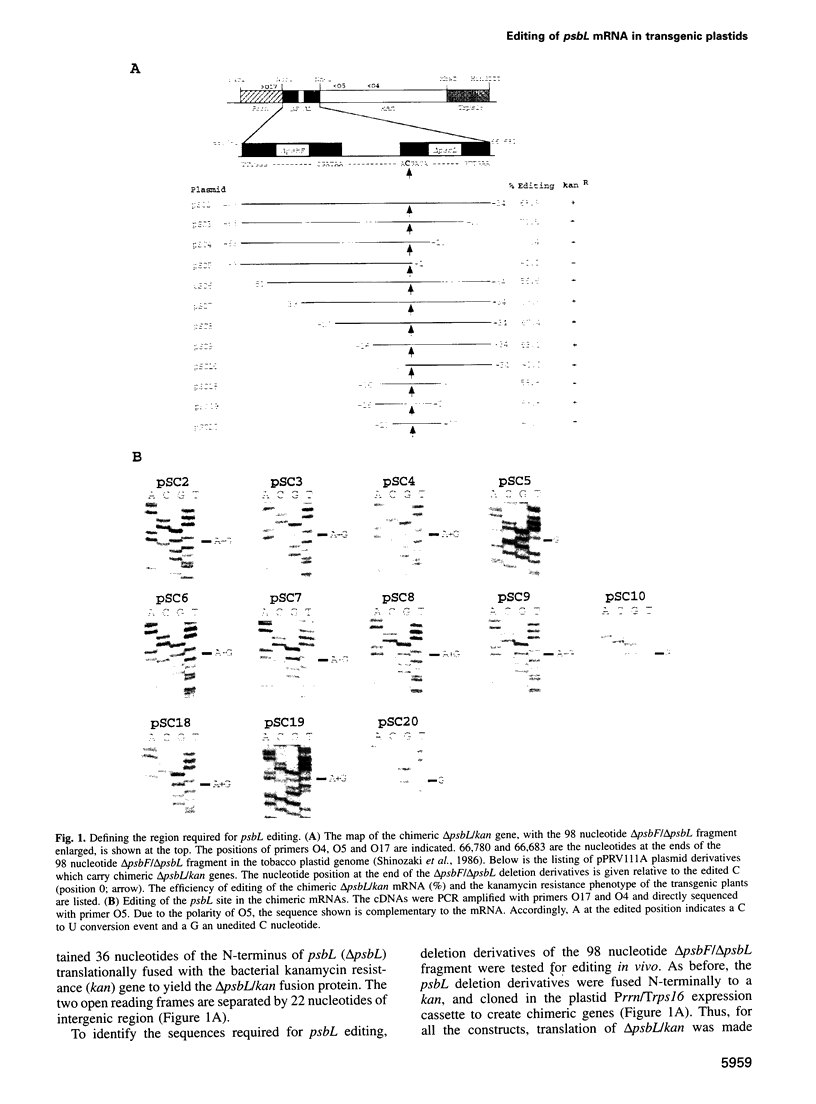
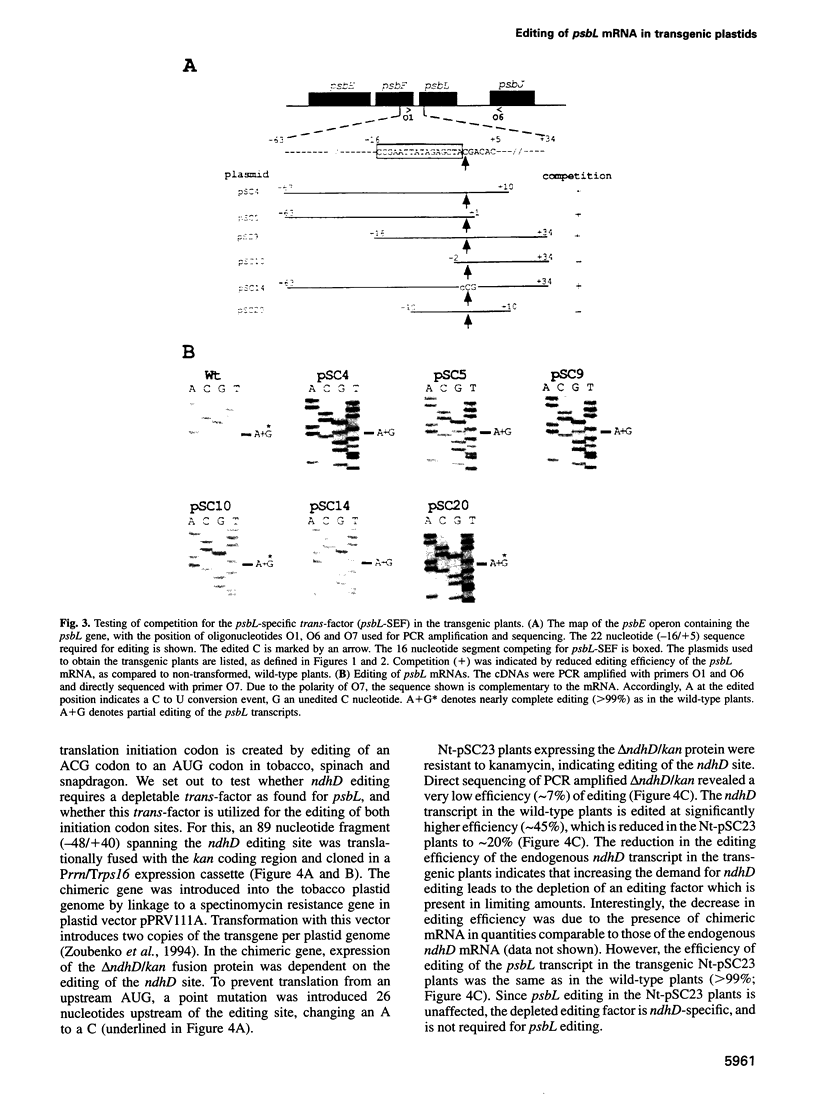
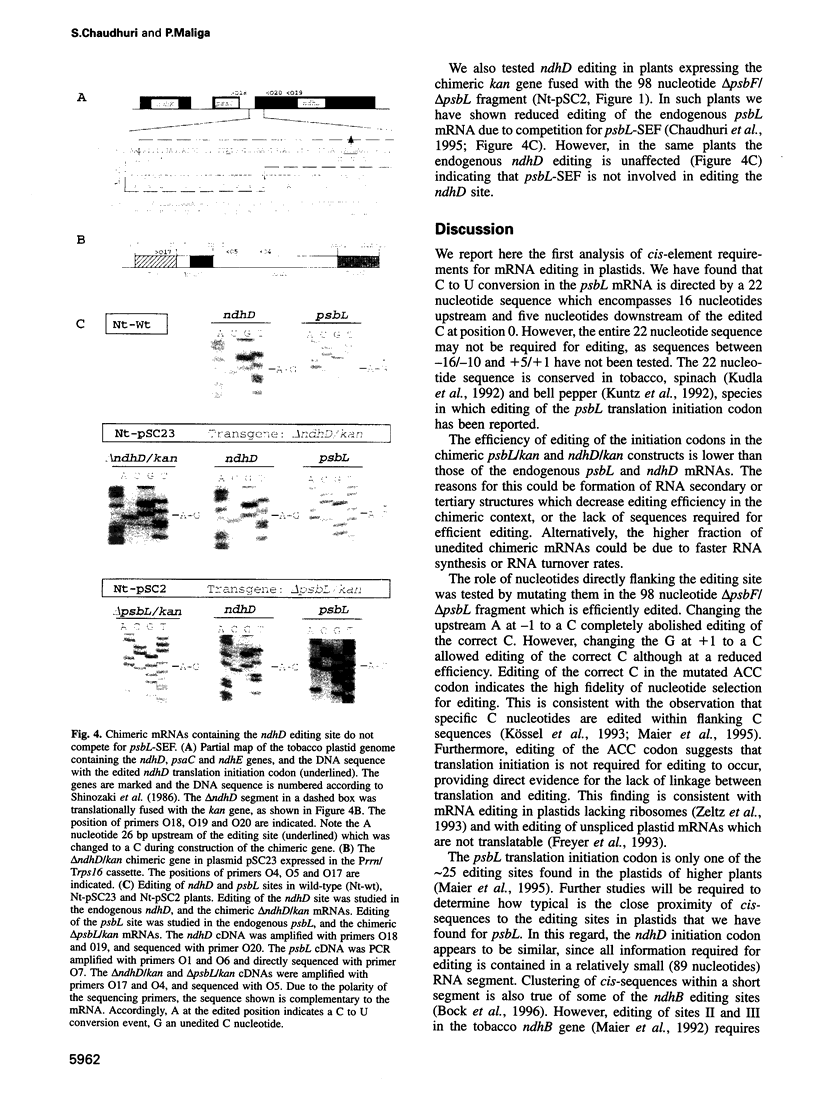
In plastids, editing of an ACG codon to an AUG codon creates the translation initiation codon for the psbL and ndhD transcripts in tobacco. To identify the RNA segment required for psbL editing, chimeric kanamycin resistance genes were constructed containing psbL deletion derivatives, and tested in vivo for editing in transgenic plants. We report here that a 22 nucleotide segment is sufficient to direct efficient psbL editing, including 16 nucleotides upstream and five nucleotides downstream of the editing site. Mutation of the A nucleotide to a C upstream of the editing site completely abolished editing, while mutation of the downstream G to a C only reduced the editing efficiency. Out of the 22 nucleotide editing target sequence, the 16 upstream nucleotides were found to compete with the endogenous psbL transcript for a depletable trans-factor. To test whether editing of initiation codons involves a common trans-factor, a chimeric gene containing the ndhD editing site was expressed in tobacco plastids. As for psbL, editing of the ndhD site requires a depletable trans-factor. However, the ndhD trans-factor is distinct from that required for psbL editing. Distinct cissequences and trans-factor requirements for the psbL and ndhD editing sites indicate an individual recognition mechanism for the editing of plastid initiation codons.
Full text
PDF



Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Araya A., Domec C., Begu D., Litvak S. An in vitro system for the editing of ATP synthase subunit 9 mRNA using wheat mitochondrial extracts. Proc Natl Acad Sci U S A. 1992 Feb 1;89(3):1040–1044. doi: 10.1073/pnas.89.3.1040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Benne R. RNA editing in trypanosomes. Eur J Biochem. 1994 Apr 1;221(1):9–23. doi: 10.1111/j.1432-1033.1994.tb18710.x. [DOI] [PubMed] [Google Scholar]
- Bock R., Hermann M., Kössel H. In vivo dissection of cis-acting determinants for plastid RNA editing. EMBO J. 1996 Sep 16;15(18):5052–5059. [PMC free article] [PubMed] [Google Scholar]
- Bock R., Kössel H., Maliga P. Introduction of a heterologous editing site into the tobacco plastid genome: the lack of RNA editing leads to a mutant phenotype. EMBO J. 1994 Oct 3;13(19):4623–4628. doi: 10.1002/j.1460-2075.1994.tb06784.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bock R., Maliga P. In vivo testing of a tobacco plastid DNA segment for guide RNA function in psbL editing. Mol Gen Genet. 1995 May 20;247(4):439–443. doi: 10.1007/BF00293145. [DOI] [PubMed] [Google Scholar]
- Carrer H., Hockenberry T. N., Svab Z., Maliga P. Kanamycin resistance as a selectable marker for plastid transformation in tobacco. Mol Gen Genet. 1993 Oct;241(1-2):49–56. doi: 10.1007/BF00280200. [DOI] [PubMed] [Google Scholar]
- Carrillo N., Seyer P., Tyagi A., Herrmann R. G. Cytochrome b-559 genes from Oenothera hookeri and Nicotiana tabacum show a remarkably high degree of conservation as compared to spinach. The enigma of cytochrome b-559: highly conserved genes and proteins but no known function. Curr Genet. 1986;10(8):619–624. doi: 10.1007/BF00418129. [DOI] [PubMed] [Google Scholar]
- Chan L. RNA editing: exploring one mode with apolipoprotein B mRNA. Bioessays. 1993 Jan;15(1):33–41. doi: 10.1002/bies.950150106. [DOI] [PubMed] [Google Scholar]
- Chaudhuri S., Carrer H., Maliga P. Site-specific factor involved in the editing of the psbL mRNA in tobacco plastids. EMBO J. 1995 Jun 15;14(12):2951–2957. doi: 10.1002/j.1460-2075.1995.tb07295.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Freyer R., Hoch B., Neckermann K., Maier R. M., Kössel H. RNA editing in maize chloroplasts is a processing step independent of splicing and cleavage to monocistronic mRNAs. Plant J. 1993 Oct;4(4):621–629. doi: 10.1046/j.1365-313x.1993.04040621.x. [DOI] [PubMed] [Google Scholar]
- Gray M. W., Covello P. S. RNA editing in plant mitochondria and chloroplasts. FASEB J. 1993 Jan;7(1):64–71. doi: 10.1096/fasebj.7.1.8422976. [DOI] [PubMed] [Google Scholar]
- Innerarity T. L., Borén J., Yamanaka S., Olofsson S. O. Biosynthesis of apolipoprotein B48-containing lipoproteins. Regulation by novel post-transcriptional mechanisms. J Biol Chem. 1996 Feb 2;271(5):2353–2356. doi: 10.1074/jbc.271.5.2353. [DOI] [PubMed] [Google Scholar]
- Kudla J., Igloi G. L., Metzlaff M., Hagemann R., Kössel H. RNA editing in tobacco chloroplasts leads to the formation of a translatable psbL mRNA by a C to U substitution within the initiation codon. EMBO J. 1992 Mar;11(3):1099–1103. doi: 10.1002/j.1460-2075.1992.tb05149.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kuntz M., Camara B., Weil J. H., Schantz R. The psbL gene from bell pepper (Capsicum annuum): plastid RNA editing also occurs in non-photosynthetic chromoplasts. Plant Mol Biol. 1992 Dec;20(6):1185–1188. doi: 10.1007/BF00028906. [DOI] [PubMed] [Google Scholar]
- Maier R. M., Neckermann K., Hoch B., Akhmedov N. B., Kössel H. Identification of editing positions in the ndhB transcript from maize chloroplasts reveals sequence similarities between editing sites of chloroplasts and plant mitochondria. Nucleic Acids Res. 1992 Dec 11;20(23):6189–6194. doi: 10.1093/nar/20.23.6189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maier R. M., Neckermann K., Igloi G. L., Kössel H. Complete sequence of the maize chloroplast genome: gene content, hotspots of divergence and fine tuning of genetic information by transcript editing. J Mol Biol. 1995 Sep 1;251(5):614–628. doi: 10.1006/jmbi.1995.0460. [DOI] [PubMed] [Google Scholar]
- Neckermann K., Zeltz P., Igloi G. L., Kössel H., Maier R. M. The role of RNA editing in conservation of start codons in chloroplast genomes. Gene. 1994 Sep 2;146(2):177–182. doi: 10.1016/0378-1119(94)90290-9. [DOI] [PubMed] [Google Scholar]
- Sarkar G., Sommer S. S. The "megaprimer" method of site-directed mutagenesis. Biotechniques. 1990 Apr;8(4):404–407. [PubMed] [Google Scholar]
- Scott J. A place in the world for RNA editing. Cell. 1995 Jun 16;81(6):833–836. doi: 10.1016/0092-8674(95)90002-0. [DOI] [PubMed] [Google Scholar]
- Shinozaki K., Ohme M., Tanaka M., Wakasugi T., Hayashida N., Matsubayashi T., Zaita N., Chunwongse J., Obokata J., Yamaguchi-Shinozaki K. The complete nucleotide sequence of the tobacco chloroplast genome: its gene organization and expression. EMBO J. 1986 Sep;5(9):2043–2049. doi: 10.1002/j.1460-2075.1986.tb04464.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Simpson L., Thiemann O. H. Sense from nonsense: RNA editing in mitochondria of kinetoplastid protozoa and slime molds. Cell. 1995 Jun 16;81(6):837–840. doi: 10.1016/0092-8674(95)90003-9. [DOI] [PubMed] [Google Scholar]
- Sutton C. A., Zoubenko O. V., Hanson M. R., Maliga P. A plant mitochondrial sequence transcribed in transgenic tobacco chloroplasts is not edited. Mol Cell Biol. 1995 Mar;15(3):1377–1381. doi: 10.1128/mcb.15.3.1377. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Svab Z., Maliga P. High-frequency plastid transformation in tobacco by selection for a chimeric aadA gene. Proc Natl Acad Sci U S A. 1993 Feb 1;90(3):913–917. doi: 10.1073/pnas.90.3.913. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yu W., Schuster W. Evidence for a site-specific cytidine deamination reaction involved in C to U RNA editing of plant mitochondria. J Biol Chem. 1995 Aug 4;270(31):18227–18233. doi: 10.1074/jbc.270.31.18227. [DOI] [PubMed] [Google Scholar]
- Zeltz P., Hess W. R., Neckermann K., Börner T., Kössel H. Editing of the chloroplast rpoB transcript is independent of chloroplast translation and shows different patterns in barley and maize. EMBO J. 1993 Nov;12(11):4291–4296. doi: 10.1002/j.1460-2075.1993.tb06113.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zoubenko O. V., Allison L. A., Svab Z., Maliga P. Efficient targeting of foreign genes into the tobacco plastid genome. Nucleic Acids Res. 1994 Sep 25;22(19):3819–3824. doi: 10.1093/nar/22.19.3819. [DOI] [PMC free article] [PubMed] [Google Scholar]







