Abstract
[Purpose]
The purpose of this study was to investigate the effect of regular treadmill exercise on the mRNA expressions of myokines and angiogenesis factors in the skeletal muscle of obese rats.
[Methods]
Thirty two male Sprague-Dawley rats (4weeks old) were divided into the CO (control) and HF (high fat diet) groups. Obesity was induced in the HF group by consumption of 45% high-fat diet for 15 weeks. These groups were further subdivided into training groups (COT and HFT); the training groups conducted moderate intensity treadmill training for 8 weeks. Soleus muscles were excised and analyzed by real-time quantitative PCR.
[Results]
mRNA expression of myokines, such as PGC-1α, IL-6, and IL-15, in the COT and HFT groups (which conducted regular exercise), were higher as compared with the CO and HF groups (p < 0.05). Also, the levels in the HF group were significantly lower when compared with CO group (p < 0.05). Expression of angiogenesis mRNA, namely mTOR, VEGF, and FLT1, were significantly lower in the HF group, as compared to the CO group (p < 0.05). In addition, COT group had a higher expression of mTORC1, mTORC2, VEGF and FLT mRNA, than the CO group (p < 0.05); the HFT group also had higher expressions of mTOR, VEGF and FLT1 mRNA than the HF group (p < 0.05).
[Conclusion]
These results indicate that mRNA expression of myokines was increased through the activity of muscle contraction, and it also promoted the mRNA expression of angiogenesis due to activation of mTOR. Thus, we conclude that not only under normal health conditions, but in obesity and excess nutritional circumstances also, regular exercise seems to act positively on the glycemic control and insulin sensitivity through the angiogenesis signaling pathway.
Keywords: Regular exercise, obesity, myokine, angiogenesis, mRNA expression
INTRODUCTION
Excess energy is mostly stored as adipocyte in the adipose tissue, resulting in obesity. It is well known that obesity is a major source of cytokines and other factors being secreted from adipocytes [1,2], which causes adversity in the body. However, recent studies have reported that adipose tissues secrete large amount of hormones that regulate appetite and energy homeostasis, and thus interest has been refocused on these tissues as being the key factor to perform important endocrine functions [1,3]. The factors defined as an inflammatory cytokine in adipose tissue are called myokines in the muscles, and it has been proven that the physiological actions of myokines is different than the cytokines [4].
IL-6, a typical inflammatory cytokine, is secreted from adipose tissue, and has been associated with tissue damage and in adult diseases [5]. However, IL-6 has been newly defined with respect to the muscle contractions in skeletal muscles [4]. IL-6 is mainly generated in the skeletal muscles and is released into the blood during exercise [6], whereas 10-35% of serum IL-6 is secreted from adipose tissue at rest, and acts as a cytokine [7]. Production and secretion of IL-6 in the skeletal muscles is facilitated by muscle contraction and the degradation of the muscle glycogen concentration [8], resulting in increase of glucose production and lipolysis by inducing glucose uptake and fat oxidation [9]. Also, IL-15 acts as an anabolic factor on muscle protein metabolism [10]. IL-15 inhibits muscle wasting rates related with cachexia in the cultured cells, and it is well known that IL-15 plays an important role in both muscle generation and degeneration [11]. In addition, Carbó et al. confirmed that IL-15 has a role of inducing the growth of muscles and reducing the adipose tissue; their studies reported that white adipose tissue was reduced by 33% when IL-15 was administered to adults rats for 7 days [12]. Peroxisome proliferator-activated receptor Gamma Coactivator 1 α (PGC-1α), a transcriptional coactivator, serves to increase the generation of ATP within mitochondria [13], and there is fiber-type switching towards being more oxidative and high endurance [14]. It has been shown that PGC-1α increases because of various physical activities, both regular aerobic and resistance exercises [15].
On the other hand, the activation of the mammalian target of rapamycin (mTOR) induced by exercise, plays a role as a restrictive mediator in mTOR complex 1 and complex 2 (mTORC1 and C2). In other words, the excessive intake of nutrition stimulates the activity of mTORC1 through the activation of mTOR and regulates the activity of insulin negatively through inhibition of insulin receptor substrate 1 (IRS-1) activity [16]. However, although effects of exercise are not yet clear, it is thought that the activation of mTORC2 mediates the insulin resistance in skeletal muscle by inhibition of ribosomal S6 kinase 1 (S6K1) activation [17].
In general, it is believed that the activation of mTOR complex due to excess nutrition negatively effects the action of insulin in skeletal muscles, and this in turn is due to the negative effect of protein kinase B (PKB), also known as AKT. However, evidence shows that long-term and continuous exercise is the key factor to enable both positive aspects of insulin action and effective aspects of the mTOR complex [18]. Takahashi et al. also reported that increasing angiogenesis occurred through the synthesis of vascular endothelial growth factor (VEGF), muscle fiber hypertrophy and angiogenesis factor, regulated by AKT signals generated in the muscle tissues. Thus, this study showed that muscle fiber hypertrophy occurs by an increase of exercise-induced angiogenesis [18]. Moreover, in situations of hypoxia caused by exercise, VEGF is upregulated when the activity of hypoxia-inducible factor 1α (HIF-1α), increases [20]. Considering all the results, we see that exercising has positive effects on angiogenesis.
As known from previous studies, the skeletal muscle contraction induced by regular exercise may be able to give positive changes in the mRNA expressions of myokines and angiogenesis. Therefore, it was important to confirm the changes of mRNA expression in skeletal muscle of obese rats through physical exercise. The purpose of this study was to investigate the effect of regular treadmill exercise on the mRNA expression of myokines and angiogenesis factors in skeletal muscle of high-fat diet induced obese rats.
METHODS
Experimental animals
Thirty two male Sprague-Dawley rats (4weeks old) were obtained from Dahan Biolink (Eumseong, Korea) and maintained in the D University College of Medicine Animal Laboratory. The animals were housed 3~4 mice per cage and acclimatized for a week. Laboratory was under a controlled condition of light (12 h of light, 12 h of dark), temperature (23 ± 2℃), and humidity (55~60%). All mice were given free access to food (commercial standard chow) and tap water, and their parameters were recorded daily. Animal experiments were approved by Dong-A University Medical School Institutional Animal Care and Use Committee, and all procedures were conducted in accordance with committee guidelines.
Dietary treatment and induction of obesity
One week after their arrival, all animals were randomly assigned to a Control (CO, n = 16) or High-fat diet group (HF, n = 16). HF group was fed 45% fat chow (20% carbohydrate, 45% lipid, and 14% protein) to induce obesity, whereas CO group was fed standard chow for 15 weeks. All animals had free access to tap water, and dietary intakes were recorded every morning (09:00); body weight was also measured every week at the same time (09:30).
Exercise treatment
After the period of inducing obesity, CO group was randomly divided to CO (n = 8) or CO + Training group (COT, n = 8); HF group was similarly randomized into HF (n = 8) or HF + Training group (HFT, n = 8). Rats of both the training groups underwent exercise training on a motor-driven animal treadmill 5 times/week for 8 weeks, with HFT group maintaining their high fat diet. The exercise protocol used was a modification conducted in a previous study [21]. Exercise intensity consisted of 5 m/min for 5 minutes, 12m/min for 5 minutes and 18m/min for 20 minutes at 0% slope for the first 4 weeks of training session(low intensity). For last 4 weeks, exercise intensity was increased to 10 m/min for 5 minutes, 16m/min for 5 minutes, and 22m/min for 30 minutes at the same slope (moderate intensity) [21,22].
Blood and tissue samplings
To exclude the temporary effects of treadmill exercise, sacrifice was conducted 48 h after the last exercise session. All animals were sacrificed between 09:00 and 12:00 AM. Food was removed from the animals’ cage 12 h before sacrifice. After complete anaesthesia (ethyl ether), the blood samples (5 ml) were drawn from the abdominal vena cava. Thereafter, the blood was centrifuged at 3,000 rpm for 10 min, and the supernatant was collected. Subsequently, 10g of soleus muscle was excised; the samples were weighed and immediately frozen in liquid nitrogen, and stored at -80℃.
Lipid profiles
Plasma total cholesterol (TC), triglyceride (TG), and high-density-lipoprotein-cholesterol (HDL-C) concentrations were measured on Sunrise automatic biochemistry analyzer (TEKAN, Switzerland) by the enzymatic colorimetric method using commercially available radioimmunoassay kit (Mercodia, Sweden). The low-density-lipoprotein-cholesterol (LDL-C) was calculated by the formula described by Friedwald, Levy & Fredrickson [23]:
LDL-C = TC - (HDL-C + TG/5)
The serum glucose level was estimated using a GlucoDr glucometer (Allmedicus, Korea). Plasma insulin level was determined spectrophotometrically with a rat insulin ELISA kit (Mercodia, Sweden) according to the manufacturer's instructions. Insulin resistance index (IRI) was assessed by homeostasis model assessment estimate of insulin resistance (HOMA-IR) as follows:
IRI = Fasting insulin(µIU/mL) × Fasting glucose(mg/dL) / 405
Real-time quantitative PCR
Total soleus muscle RNA was extracted using QIAzol lysis reagent (QIAGEN, MD, USA), according to the manufacturer’s protocol. Muscle samples (0.1g) were homogenized in 1mL QIAZOL lysis reagent and then mixed with 200 μl chloroform, shaken vigorously, and kept at room temperature for 15 minutes. The mixtures were then centrifuged at 13,000 rpm for 15min at 4℃. After transferring the supernatant to a new eppendorf tube, 2-Propanol (SIGMA Aldrich, MO, USA) was added; samples were left at room temperature for 10 min, after which they were centrifuged at 12,000rpm for 10 min at 4℃. After confirming pelleting, the RNA pellet was washed with 1mL 75% ethanol and then centrifuged at 7,500rpm for 5min at 4℃. After removing the ethanol, sample was dried completely, and the resulting RNA was dissolved in 50 μl RNase-free water. The purity of extracted RNA was measured by the ratio of the absorbance at 260 nm to that at 280 nm. cDNA was synthesized from 2 μg of the total RNA, and addition of oligo dt, RNase-free water and a mixture which was containing 10X M-MLV- RT buffer, reverse-transcriptase, 2mM dNTP mixture and Rnase inhibitor.
Real-time quantitative PCR was carried out on StepOne Real-Time PCR System (Applied Biosystems, CA, USA). Aliquots of 2 μl of the cDNA samples were mixed with 1 μl each forward/reverse primer, 6 μl of Rnase-free water and 10 μl of SYBR green Master mix (Applied biosystems, CA, USA). The reaction parameters were as follows: 95℃ for 10min for 45 cycles; each cycle was performed at 95℃ for 15s and at 60℃ for 1min. Fold difference was employed to calculate the amount of the target gene with the endogenous control gene (β-actin). The sequence of primers used in the present study was designed by Bioneer (Daejeon, Korea), as shown in Table 1.
Table 1.
The primers information for quantitative real-time PCR
| Target mRNA | Forward primer | Revers primers |
|---|---|---|
| PGC-1α | 5'-ACAGAAACAGCAGCAGAGACA-3' | 5'-TGGGGTCAGAGGAAGAGATAA-3' |
| IL-6 | 5‘-TCTTGGGACTGATGTTGTTG-3’ | 5‘-TAAGCCTCCGACTTGTGAA-3’ |
| IL-15 | 5'-ACTACCTGTGTTTCCTTCTCAAC-3' | 5'-TTGGCCTCTGTTTTAGGG-3' |
| mTOR | 5'-TGAGAGAGGAGATGGAGGAA-3' | 5'-TTCAGAGCGGAGAAAGCA-3' |
| mTORC1 | 5'-TGACTTACCGAGAGCACACA-3' | 5'-ACATTCACAGACTCAGGCATC-3' |
| mTORC2 | 5'-GAAGGTGCTAAAACTGAAGGTG-3' | 5'-CAGAACTCGGAAACAAGGAA-3' |
| VEGF | 5'-TTCAGAGCGGAGAAAGCA-3' | 5'-CATCTGAAGTACGTTCGTTTA-3' |
| FLT1 | 5'-CCCTGGATGAGCAGTGTG-3' | 5'-AAATGCCGAAGCCTGAAC-3' |
| B-actin | 5'-GCCTCACTGTCCACCTTCCA-3' | 5'-GGGCCGGACTCATCGTACT-3' |
Statistical analysis
All calculations were performed using the Statistical Package for Social Sciences version 22.0 (SPSS Inc., Chicago, IL, USA) and are presented as means ± standard error. To compare difference between the groups analysis of variance (ANOVA) test Pairwise comparisons were performed with Duncan's test. A significant level was set at p < 0.05 for statistical analysis.
RESULTS
Changes in body weight
Changes of the body weight after 8 weeks of training are presented in Fig. 1.
Fig. 1. Changes of body weight after 8 weeks of exercise.
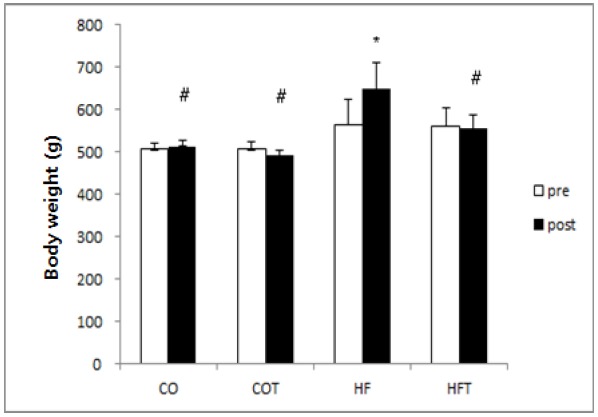
Presented Mean ± SE, * significantly different before exercise, p < 0.05, # : significantly different compared to HF group, p < 0.05
The body weight of HF group was significantly increased due to the continuous intake of high-fat diet (p < 0.05), but the weight of COT and HFT group were not significantly different, even though the body weight decreased after 8 weeks of training. Considering the results of the difference between groups after 8 weeks of training, the weight of HF group was significantly higher than all other groups (p < 0.05).
Changes in the lipid profiles
Changes in lipid profiles after 8 weeks of training are presented in Table 2. The HF group (high-fat dietary intake) is significantly higher in TG, glucose, insulin, and HOMA-IR (p < 0.05). Although TC and LDL-C is higher in HF group than CO group, the difference was not significant. However, lipid profiles in COT and HFT group, who participated in regular exercise, changed positively. TC, TG, insulin and HOMA-IR in COT and HFT group were significantly lower than in the HF group (p < 0.05); the CO group (intake of normal diet) was also significantly different than the HF group (p < 0.05).
Table 2.
Changes of lipid profiles after 8 weeks of exercise.
| Variable | CO | COT | HF | HFT |
|---|---|---|---|---|
| TC (mg/dl) | 191.55 ± 2.85 | 167.01 ± 5.91* | 201.35 ± 11.16 | 163.80 ± 15.92* |
| TG (mg/dl) | 76.25 ± 3.89* | 73.16 ± 4.57* | 115.52 ± 14.20 | 68.09 ± 7.82* |
| HDL-c (mg/dl) | 33.37 ± 1.63 | 36.84 ± 2.30* | 26.93 ± 2.05 | 33.26 ± 2.54 |
| LDL-c (mg/dl) | 142.93 ± 3.43 | 115.54 ± 6.58 | 151.42 ± 11.24 | 116.93 ± 18.02 |
| Glucose (mg/dl) | 139.00 ± 3.09* | 135.38 ± 2.34* | 170.00 ± 10.80 | 147.27 ± 6.43 |
| Insulin (uIU/ml) | 31.04 ± 6.38* | 18.93 ± 2.65* | 69.97 ± 14.57 | 40.42 ± 8.30* |
| HOMA-IR | 10.70 ± 2.30* | 6.31 ± 0.85* | 28.39 ± 5.12 | 14.66 ± 2.95* |
Presented Mean ± SE,
vs HF p < 0.05
Changes in mRNA expressions of myokines and angiogenesis factors
Changes in the mRNA expression of myokines after 8 weeks of training are presented in Fig. 2. mRNA expression of all myokine factors in the HF group were reduced, as compared to the CO group (p < 0.05). Expression of PGC-1α mRNA in COT and HFT groups was significantly higher compared to the CO and HF groups (p < 0.05). Also, expressions of IL-6 and IL-15 mRNA in COT and HFT group were significantly higher compared with CO and HF groups (p < 0.05).
Fig. 2. Changes of mRNA expression of myokines.
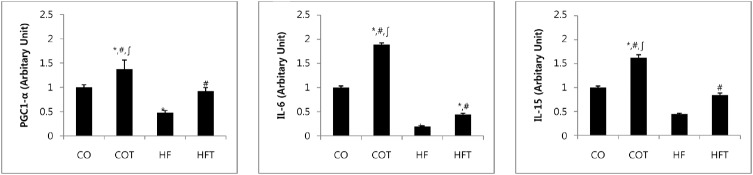
Presented Mean ± SE, * vs CO group, p < 0.05, # vs HF group, p < 0.05, ∫ vs HFT group, p < 0.05
Changes in mRNA expression of angiogenesis factors after 8 weeks of training are presented in Fig. 3. Expression of mTOR mRNA in HF group was significantly lower compared with all other groups (p < 0.05). Expression of mTOR-C1 mRNA in COT group was significantly higher compared with HF and HFT groups (p < 0.05); the mTOR-C2 mRNA in COT group was significantly higher compared with all other groups (p < 0.05). Moreover, expression of VEGF mRNA in the COT and HFT groups, which conducted regular exercise was significantly higher when compared with CO and HF group (p < 0.05). Expression of FLT1 mRNA in HF group was lower than CO group, and COT and HFT groups were also significantly higher compared with CO and HF group respectively (p < 0.05).
Fig. 3. Changes of mRNA expression of angiogenesis factors.
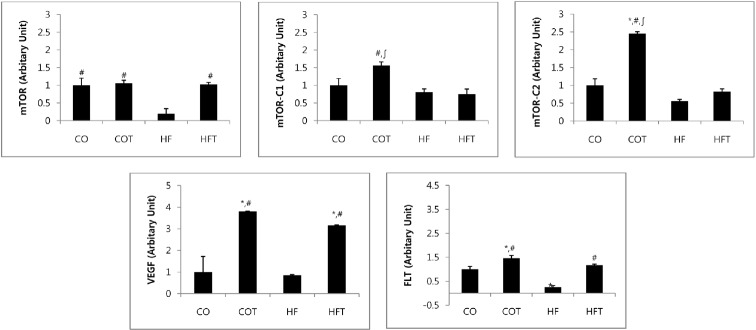
Presented Mean ± SE, * vs CO group, p < 0.05, # vs HF group, p < 0.05, ∫vs HFT group, p < 0.05
DISCUSSION
This study was conducted to analyze the effect of regular treadmill exercise on the mRNA expression of myokines and angiogenesis factors in skeletal muscle in obese rats, induced by high-fat diet for 15 weeks. The results in this study revealed that obesity, resulting from the continuous intake of high-fat diet, increases the body weight and reduces the mRNA expression of myokines and angiogenesis factors in the skeletal muscle. However, regular exercise positively improves the body weight and lipid profile, and increases the mRNA expression of myokines and angiogenesis factors through the activity of muscle contractions.
It is known that obesity resulting from a high-fat intake induces insulin resistance [24] and leptin resistance [21] in peripheral tissues. However, exercise decreases the insulin and leptin resistance, through enhanced insulin mediated glucose metabolism. Hence, both endurance and resistance exercises have been suggested to be effective in training adaptation and improvement of insulin sensitivity in skeletal muscles [25].
In this study, we also found that regular exercise reduced the body weight and improved the insulin resistance by reducing the amount of glucose and insulin.
In previous studies of myokines induced by exercise, blood concentration of myokines was known to increase with moderate and high-intensity exercise which does not induce muscle damage, and thereafter rapidly decreasing soon after exercise [26,27]. Moreover, the study conducted by Nieman et al. reported that blood concentration of myokines was increased after treadmill running for 3 hours at the 70% VO2max intensity, in 16 marathon runners [28]. However, moderate intensity exercise using cycle ergometer [29] and rowing [30] does not induce an increase in blood concentration. These results reflected that an increase of myokine concentration in blood is proportional to the type of exercise, the duration, intensity, and muscle mass [26,27].
As reported above many studies have described the relationship between blood myokines and exercise. Nieman et al. reported that myokine mRNA level does not changed after 3 h running [31]. Nielsen et al. reported that at the recovery 48 h after eccentric contractions of skeletal muscle, blood concentration of myokine does not change, whereas the IL-15 mRNA expression in muscle tissue increases [32]. In this study, we confirmed that regular exercise increase the mRNA expression of all myokine factors. This result is considered to be an outcome of continuous muscle contractions through regular exercise, contradictory to the acute exercise conducted in previous study.
mTORC1 negatively regulates the insulin activity through the inhibition of IRS-1; however, it is not yet clear how mTORC2 prevents the activity of S6K1 [17]. Moreover, mTORC2 is an enzyme that acts to regulate the activation and phosphorylation of AKT, and the lack of mTORC2 in skeletal muscle induces the reduction of insulin mediated glucose uptake [33]. Thus, mTORC2 and AKT seems to be effective factors, and it could be essential for the understanding of glucose homeostasis. Obesity due to the excess nutrition has an adverse effect on the mTOR activity, whereas exercise improved the insulin resistance without reducing the mTOR/S6K1 in obese rats. Therefore, it may be involved in other aspects of glucose metabolism [34]. The positive activation of mTOR induced by physical activity can be confirmed a few hours after exercise, both in activities involving various aerobic exercise and high-intensity resistance exercise [35,36]. Moreover, the mTOR activation mechanism due to exercise was also analyzed through the PI3K activation in insulin mediated IRS1 pathway, in experiments conducted by Kirwan et al. [37]. The study indicated that PI3K activation has an important role as a regulator of glucose metabolism via potential of GLUT4 in skeletal muscle, through an increasing activity of PI3K in the exercise group [37]. As mentioned above, the results of our study showed that obesity due to the excess nutrition inhibits the mTOR complex activity. Also, in case of obesity induced by high-fat diet, mRNA expression of mTOR complex does not change positively via exercise. However, regular exercise increases the mRNA expression of mTOR C1 and C2, especially a significant increase of mTOR2 mRNA expression induced activation of AKT, which in turn acts positively on the glucose metabolism.
Excess nutrition is negative to insulin activity and suppresses the activity of AKT and mTOR that exist in the sub-paths of insulin [18]. However, the AKT serine-threonine protein kinase, activated by PI3K pathway through exercise in the form of external stimulation [38] regulates the size of tissue and cell hypertrophy [39]. In this sense, angiogenesis was increased through synthesis of VEGF, which is the factor that induces muscle fiber hypertrophy and angiogenesis by AKT signal generated from skeletal muscles [19]; it has previously been demonstrated that the cause of muscle hypertrophy is influenced by enhancement of angiogenesis due to the implementation of regular exercise. Moreover, the states of hypoxia by aerobic exercise upregulates the production of VEGF, resulting from an increasing activity of hypoxia-inducible factor 1α (HIF-1α) [20]. Thus, hypoxia due to the exercise has been reported to have a positive effect on angiogenesis. Exercise training leads to an increase of capillaries, and this can be confirmed by stimulation of motor neurons after chronic exercise [40]. In fact, difference between the capillaries and fiber rate can be explained as the result of not only differences in muscle fiber, but also the angiogenesis response of tissue type through exercise training [41]. Exercise increases the blood flow, thereby providing supplies of additional oxygen and nutrition to the skeletal muscles [42]. In particular, endurance exercise causes an increase in the vessel diameter in order to adapt the vessel for a long time in moving the skeletal muscle, which affects angiogenesis through increasing blood flow. Thus, increase of muscle capillaries occurs, and VEGF being the potential mitogen of endothelial cells, may be referred to as being the cause of angiogenesis in response to exercise.
In summary, the findings in this study are that high-fat diet negatively effects angiogenesis, as concluded from the observation that both myokine mRNA and angiogenesis mRNA expression ae decreased. However, regular exercise is effective in upregulating the mRNA expression of both myokines and angiogenesis factors in the skeletal muscle. We contemplate that mRNA expression of myokines was increased by muscle contraction, and mRNA expression of angiogenesis was promoted by activation of mTOR mRNA. Thus, in a healthy state, as well as in obesity and in plethora of nutrition, regular exercise is considered to act positively in controlling the glucose and insulin sensitivity, via the angiogenesis signaling pathway.
Acknowledgments
This work was supported by the National Research Foundation of Korea Grant funded by the Korean Government (NRF-2013S1A5B5A07047609)
REFERENCES
- 1.Trujillo ME, Scherer PE. Adipose tissue-derived factors: impact on health and disease. Endocr Rev. 2006;27:762–78. doi: 10.1210/er.2006-0033. [DOI] [PubMed] [Google Scholar]
- 2.Tilg H, Moschen AR. Adipocytokines: mediators linking adipose tissue, inflammation and immunity. Nat Rev Immunol. 2006;6:772–83. doi: 10.1038/nri1937. [DOI] [PubMed] [Google Scholar]
- 3.Gesta S, Tseng YH, Kahn CR. Developmental origin of fat; tracking obesity to its source. Cell. 2007;131:242–56. doi: 10.1016/j.cell.2007.10.004. [DOI] [PubMed] [Google Scholar]
- 4.Petersen AM, Pedersen BK. The anti-inflammatory effect of exercise. J Appl Physiol. 2005;4:1154–62. doi: 10.1152/japplphysiol.00164.2004. [DOI] [PubMed] [Google Scholar]
- 5.Kern PA, Ranganathan S, Li C, Wood L, Ranganathan G. Adipose tissue tumor necrosis factor and interleukin-6 expression in human obesity and insulin resistance. Am J Physiol Endocrinol Metab. 2001;5:745–51. doi: 10.1152/ajpendo.2001.280.5.E745. [DOI] [PubMed] [Google Scholar]
- 6.Steensberg A, van Hall G, Osada T, Sacchetti M, Saltin B, Pedersen BK. Production of interleukin-6 in contracting human skeletal muscles can account for the exercise-induced increase in plasma interleukin-6. J Physiol. 2000;1:237–42. doi: 10.1111/j.1469-7793.2000.00237.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Mohamed-Ali V, Goodrick S, Rawesh A, Katz DR, Miles JM, Yudkin JS, Klein S, Coppack SW. Subcutaneous adipose tissue releases interleukin-6, but not tumor necrosis factor-alpha, in vivo. J Clin Endocrinol Metab. 1997;12:4196–200. doi: 10.1210/jcem.82.12.4450. [DOI] [PubMed] [Google Scholar]
- 8.Keller C, Steensberg A, Pilegaard H, Osada T, Saltin B, Pedersen BK, Neufer PD. Transcriptional activation of th IL-6 gene in human contracting skeletal muscle: influence of muscle glycogen content. FASEB. 2001;14:2748–50. doi: 10.1096/fj.01-0507fje. [DOI] [PubMed] [Google Scholar]
- 9.Pedersen BK, Febbraio M. Muscle as an endocrine organ: focus on muscle-derived interleukin-6. Physiol Rev. 2008;4:1379–406. doi: 10.1152/physrev.90100.2007. [DOI] [PubMed] [Google Scholar]
- 10.Martínez-Hernández PL, Hernanz-Macías Á, Gómez-Candela C, Grande-Aragón C, Feliu-Batlle J, Castro-Carpeño J, Martínez-Muñoz I, Zurita-Rosa L, Villarino-Sanz M, Prados-Sánchez C, Sánchez García-Girón J. Serum interleukin-15 levels in cancer patients with cachexia. Oncol Rep. 2012;28:1443–52. doi: 10.3892/or.2012.1928. [DOI] [PubMed] [Google Scholar]
- 11.Quinn LS, Anderson BG, Drivdahl RH, Alvarez B, Argilés JM. Overexpression of interleukin-15 induces skeletal muscle hypertrophy in vitro: implications for treatment of muscle wasting disorders. Exp Cell R. 2002;280:55–63. doi: 10.1006/excr.2002.5624. [DOI] [PubMed] [Google Scholar]
- 12.Carbó N, López-Soriano J, Costelli P, Alvarez B, Busquets S, Baccino FM, Quinn LS, López-Soriano FJ, Argilés JM. Interleukin-15 mediates reciprocal regulation of adipose and muscle mass: a potential role in body weight control. Biochim Biophys Acta. 2001;1526:17–24. doi: 10.1016/s0304-4165(00)00188-4. [DOI] [PubMed] [Google Scholar]
- 13.Nicholas LM, Rattanatray L, Morrison JL, Kleemann DO, Walker SK, Zhang S, MacLaughlin S, McMillen IC. Maternal obesity or weight loss around conception impacts hepatic fatty acid metabolism in the offspring. Obesity (Silver Spring) 2014;22:1685–93. doi: 10.1002/oby.20752. [DOI] [PubMed] [Google Scholar]
- 14.Handschin C, Kobayashi YM, Chin S, Seale P, Campbell KP, Spiegelman BM. PGC-1α regulates the neuromuscular junction program and ameliorates Duchenne muscular dystrophy. Genes Dev. 2007;21:770–83. doi: 10.1101/gad.1525107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Calvo JA, Daniels TG, Wang X, Paul A, Lin J, Spiegelman BM, Stevenson SC, Rangwala SM. Muscle-specific expression of PPARgamma coactivator-1alpha improves exercise performance and increases peak oxygen uptake. J Appl Physiol (1985) 2008;104:1304–12. doi: 10.1152/japplphysiol.01231.2007. [DOI] [PubMed] [Google Scholar]
- 16.Tremblay F, Marette A. Amino acid and insulin signaling via the mTOR/p70 S6 kinase pathway. A negative feedback mechanism leading to insulin resistance in skeletal muscle cells. J Biol Chem. 2001;276:38052–60. doi: 10.1074/jbc.M106703200. [DOI] [PubMed] [Google Scholar]
- 17.Thoreen CC, Kang S, Chang J, Liu Q, Zhang J, Gao Y, Reichling LJ, Sim T, Sabatini DM, Gray NS. An ATP-competitive mammalian target of rapamycin inhibitor reveals rapamycin-resistant functions of mTORC1. J. Biol. Chem. 2009;284:8023–32. doi: 10.1074/jbc.M900301200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Rivas DA, Yaspelkis BB, III Hawley JA, Lessard SJ. Lipid-induced mTOR activation in rat skeletal muscle reversed by exercise and 5'-aminoimidazole-4-carboxamide-1-{beta}-D- ribofuranoside. J. Endocrinol. 2009;202:441–51. doi: 10.1677/JOE-09-0202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Takahashi A, Kureishi Y, Yang J, Luo Z, Guo K, Mukhopadhyay D, Ivashchenko Y, Branellec D, Walsh K. Myogenic Akt signaling regulates blood vessel recruitment during myofiber growth. Mol Cell Biol. 2002;22:4803–14. doi: 10.1128/MCB.22.13.4803-4814.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Semenza GL. HIF-1 and human disease: one highly involved factor. Genes Dev. 2000;14:1983–91. [PubMed] [Google Scholar]
- 21.Kang S, Kim KB, Shin KO. Exercise training improve leptin sensitivity in peripheral tissue of obese rats. Biochem. Biophys. Res. Commun. 2013;435:454–9. doi: 10.1016/j.bbrc.2013.05.007. [DOI] [PubMed] [Google Scholar]
- 22.Jun JK, Lee WL, Park HG, Lee SK, Jeong SH, Lee YR. Moderate intensity exercise inhibits macrophage infiltration and attenuates adipocyte inflammation in ovariectomized rats. J Exerc Nutrition Biochem. 2014;18(1):119–27. doi: 10.5717/jenb.2014.18.1.119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Friedewald WT, Levy RI, Fredrickson DS. Estimation of the concentration of low-density lipoprotein cholesterol in plasma, without use of the preparative ultracentrifuge. Clin Chem. 1972;18(6):499–502. [PubMed] [Google Scholar]
- 24.Kim DH, Sarbassov DD, Ali SM, King JE, Latek RR, Erdjument-Bromage H, Tempst P, Sabatini DM. mTOR interacts with raptor to form a nutrient-sensitive complex that signals to the cell growth machinery. Cell. 2002;110(2):163–75. doi: 10.1016/s0092-8674(02)00808-5. [DOI] [PubMed] [Google Scholar]
- 25.Coffey VG, Hawley JA. The molecular bases of training adaptation. Spors Med. 2007;37(9):737–63. doi: 10.2165/00007256-200737090-00001. [DOI] [PubMed] [Google Scholar]
- 26.Febbraio MA, Pedersen BK. Muscle-derived interleukin-6: mechanisms for activation and possible biological roles. FASEB. 2002;11:1335–47. doi: 10.1096/fj.01-0876rev. [DOI] [PubMed] [Google Scholar]
- 27.Pedersen BK, Hoffman-Goetz L. Exercise and the immune system: regulation, intergration, and adaptation. Physiol Rev. 2000;3:1055–81. doi: 10.1152/physrev.2000.80.3.1055. [DOI] [PubMed] [Google Scholar]
- 28.Nieman DC, Davis JM, Brown VA, Henson DA, Dumke CL, Utter AC, Vinci DM, Downs MF, Smith JC, Carson J, Brown A, McAnulty SR, McAnulty LS. Influence of carbohydrate ingestion on immune changes after 2h of intensive resistance training. J Appl Physiol. 2004;4:1292–8. doi: 10.1152/japplphysiol.01064.2003. [DOI] [PubMed] [Google Scholar]
- 29.Chan MH, Carey AL, Watt MJ, Febbraio MA. Cytokine gene expression in human skeletal muscle during concentric contraction: evidence that IL-8, like IL-6, is influenced by glycogen availability. Am J Physiol Regul Integr Comp Physiol. 2004;287(2):R322–7. doi: 10.1152/ajpregu.00030.2004. [DOI] [PubMed] [Google Scholar]
- 30.Henson DA, Nieman DC, Nehlsen-Cannarella SL, Fagoaga OR, Shannon M, Bolton MR, Davis JM, Gaffney CT, Kelln WJ, Austin MD, Hjertman JM, Schilling BK. Influence of carbohydrate on cytokine and phagocytic responses to 2 h of rowing. Med Sci Sports Exerc. 2000;32(8):1384–9. doi: 10.1097/00005768-200008000-00005. [DOI] [PubMed] [Google Scholar]
- 31.Nieman DC, Davis JM, Henson DA, Walberg-Rankin J, Shute M, Dumke CL, Utter AC, Vinci DM, Carson JA, Brown A, Lee WJ, McAnulty SR, McAnulty LS. Carbohydrate ingestion influences skeletal muscle cytokine mRNA and plasma cytokine levels after a 3-h run. J Appl Physiol. 2003;94(5):1917–25. doi: 10.1152/japplphysiol.01130.2002. [DOI] [PubMed] [Google Scholar]
- 32.Nielsen AR, Mounier R, Plomgaard P, Mortensen OH, Penkowa M, Speerschneider T, Pilegaard H. Pedersen BK. Expression of interleukin-15 in human skeletal muscle effect of exercise and muscle fibre type composition. J Physiol. 2007;584(Pt 1):305–12. doi: 10.1113/jphysiol.2007.139618. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Kumar A, Harris TE, Keller SR, Choi KM, Magnuson MA, Lawrence JC Jr. Muscle-specific deletion of rictor impairs insulin-stimulated glucose transport and enhances Basal glycogen synthase activity. Mol Cell Biol. 2008;28(1):61–70. doi: 10.1128/MCB.01405-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Liao B, Xu Y. Exercise improves skeletal muscle insulin resistance without reduced basal mTOR/S6K1 signaling in rats fed a high-fat diet. Eur J Appl Physiol. 2011;111(11):2743–52. doi: 10.1007/s00421-011-1892-5. [DOI] [PubMed] [Google Scholar]
- 35.Mascher H, Andersson H, Nilsson PA, Ekblom B, Blomstrand E. Changes in signalling pathways regulating protein systhesis in human muscle in the recovery period after endurance exercise. Acta Physiol(Oxf) 2007;191(1):67–75. doi: 10.1111/j.1748-1716.2007.01712.x. [DOI] [PubMed] [Google Scholar]
- 36.Dreyer HC, Fujita S, Cadenas JG, Chinkes DL, Volpi E, Rasmussen BB. Resistance exercise increases AMPK activity and reduces 4E-BP1 phossphorylation and protein synthesis in human skeletal muscle. J. Phsiol. 2006;576(2):613–24. doi: 10.1113/jphysiol.2006.113175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Kirwan JP, del Aguila LF, Hernandez JM, Williamson DL, O'Gorman DJ, Lewis R, Krishnan RK. Regular exercise enhances insulin activation of IRS-1-associated PI3-kinase in human skeletal muscle. J Appl Physiol. 2000;88(2):797–803. doi: 10.1152/jappl.2000.88.2.797. [DOI] [PubMed] [Google Scholar]
- 38.Datta SR, Brunet A, Greenberg ME. Cellular survival: a play in three Akts. Genes Dev. 1999;13(22):2905–27. doi: 10.1101/gad.13.22.2905. [DOI] [PubMed] [Google Scholar]
- 39.Chen WS, Xu PZ, Gottlob K, Chen ML, Sokol K, Shiyanova T, Roninson I, Weng W, Suzuki R, Tobe K, Kadowaki T, Hay N. Growth retardation and increased apoptosis in mice with homozygous disruption of the Akt1 gene. Genes Dev. 2001;15(17):2203–08. doi: 10.1101/gad.913901. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Reichmann H, Hoppeler H, Mathieu-Costello O, von Bergen F, Pette D. Biochemical and ultrastructural changes of skeletal muscle mitochondria after chronic electrical stimulation in rabbits. Pflugers Arch. 1985;404(1):1–9. doi: 10.1007/BF00581484. [DOI] [PubMed] [Google Scholar]
- 41.Gute D, Fraga C, Laughlin MH, Amann JF. Regional changes in capillary supply in skeletal muscle of high-intensity endurance-trained rats. J Appl Physiol (1985) 1996;81(2):619–26. doi: 10.1152/jappl.1996.81.2.619. [DOI] [PubMed] [Google Scholar]
- 42.Andersen P, Saltin B. Maximal perfusion of skeletal muscle in man. J Physiol. 1985;366:233–49. doi: 10.1113/jphysiol.1985.sp015794. [DOI] [PMC free article] [PubMed] [Google Scholar]