Abstract
Three ZnAl2O4 samples were prepared via a modified polyacrylamide gel method using a citric acid solution with different aluminum salt starting materials, including AlCl3∙6H2O, Al2(SO4)3∙18H2O, and Al(NO3)3∙9H2O under identical conditions. The influence of different aluminum salts on the morphologies, phase purity, and optical and fluorescence properties of the as-prepared ZnAl2O4 nanoparticles were studied. The experimental results demonstrate that the phase purity, particle size, morphology, and optical and fluorescence properties of ZnAl2O4 nanoparticles can be manipulated by the use of different aluminum salts as starting materials. The energy bandgap (Eg) values of ZnAl2O4 nanoparticles increase with a decrease in particle size. The fluorescence spectra show that a major blue emission band around 400 nm and two weaker side bands located at 410 and 445 nm are observed when the excitation wavelength is 325 nm. The ZnAl2O4 nanoparticles prepared from Al(NO3)3∙9H2O exhibit the largest emission intensity among the three ZnAl2O4 samples, followed in turn by the ZnAl2O4 nanoparticles prepared from Al2(SO4)3∙18H2O and AlCl3∙6H2O. These differences are attributed to combinational changes in Eg and the defect types of the ZnAl2O4 nanoparticles.
Spinel ZnAl2O4 is known to have a wide energy bandgap (Eg), high mechanical resistance, high fluorescence efficiency, high chemical and thermal stability, high photocatalytic activity, and low surface acidity, all of which make it a suitable material for a wide range of applications, including use in photoelectronic devices, catalysts, electroluminesence displays, stress imaging devices, optical coatings, and highly efficient phosphors1,2,3. Based on the above mentioned applications, various morphologies of spinel ZnAl2O4 have been prepared, including one-dimensional microfibers, porous structures, nanoparticles, nanorods, nanotubes, and so on4,5,6,7,8. It has been noted that the optical properties of these materials are strongly dependent on their morphologies and preparation methods. In particular, ZnAl2O4 nanostructures are expected to exhibit enhanced optical and fluorescence properties usually absent in bulk ZnAl2O4. Therefore, the preparation and study of the optical and fluorescence properties of ZnAl2O4 nanostructure powder is of great interest.
Spinel ZnAl2O4 semiconductors have been synthesized using a variety of different methods, such as the solid-state reaction method9,10, a self-generated template pathway9, the combustion synthesis route11, the sol–gel method12, a co-precipitation approach6, the polymeric precursor method13, the citrate precursor method4, a hydrothermal process7, a solvothermal approach14, and the microwave-hydrothermal route15. The particle size of ZnAl2O4 has a large effect on its optical and fluorescence properties. Generally, smaller particles have a relatively larger specific surface area, and therefore have a larger amount of dangling and unsaturated bonds on the particle surface. This in turn affects the defect levels and fluorescence properties of the powder16. However, the main disadvantage of preparing spinel ZnAl2O4 by the traditional synthesis routes, such as the co-precipitation approach, the solid-state reaction method, and others, is the large particle size of the product.
The polyacrylamide gel route is a very good sol-gel method for the preparation of superfine nanoparticles17. Appropriate selection of a chelating agent, monomer systems, initiator, pH value, and sintering temperature can significantly improve the quality of the prepared nanoparticles17. In addition, different aluminum salts, i.e. different anionic species in the precursor solutions, can greatly influence the morphology, phase purity, and optical and fluorescence properties of the ZnAl2O4. However, most previously reported studies have only used a single aluminum salt as a starting material and have not investigated the influences of different aluminum salts on the morphology, structure, and optical and fluorescence properties of the obtained ZnAl2O4.
In this study, three different aluminum salts are used as starting materials to synthesize three ZnAl2O4 gels via a polyacrylamide gel route, specifically aqueous solutions of citric acid with Al2(SO4)3∙18H2O, AlCl3∙6H2O, or Al(NO3)3∙9H2O were used under identical conditions. In order to obtain superfine nanoparticles, N,N’-methylene-bisacrylamide was used as a cross-linking agent, and glucose was used to prevent gel collapse. After sintering these xerogels, three ZnAl2O4 nanostructure samples were obtained. Their phase purity, morphologies, and optical and fluorescence properties were then characterized and compared. The objective of the present work is to investigate the influence of different aluminum salt starting materials on the resulting ZnAl2O4 nanostructures and on their optical and fluorescence properties.
Results
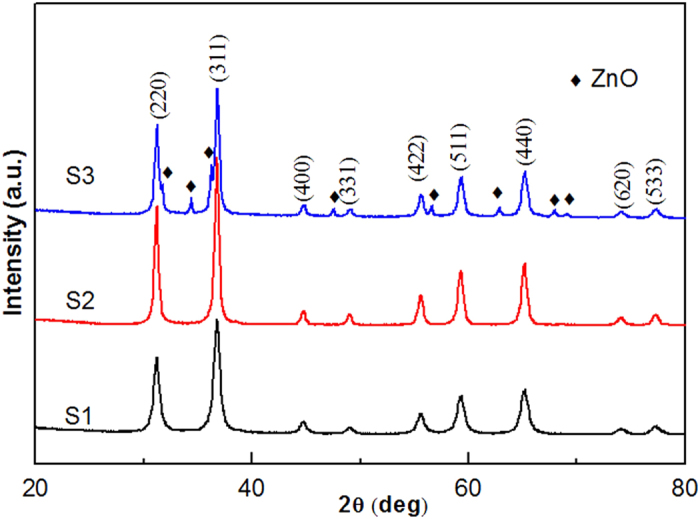
The obtained ZnAl2O4 xerogels decomposed into products after being sintered at 600 °C for 5 h in air. Figure 1 shows the XRD patterns of ZnAl2O4 nanoparticles prepared from (S1) Al2(SO4)3∙18H2O, (S2) AlCl3∙6H2O, and (S3) Al(NO3)3∙9H2O. It can be seen that samples S1 and S2 have crystallized in a single phase with a spinel structure and with space group O7h, but sample S3 contains small amounts of ZnO (JCPDS card No. 36–1451) impurities in addition to the major phase of spinel ZnAl2O4 structure (JCPDS card No. 05–0669). According to the literature2,18, the relevant reactions can be described by the following equations:
Figure 1. XRD patterns of ZnAl2O4 nanoparticles prepared from (S1) Al2(SO4)3∙18H2O, (S2) AlCl3∙6H2O, and (S3) Al(NO3)3∙9H2O and sintered at 600 °C.
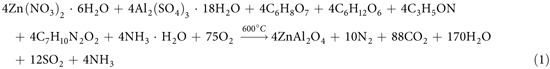 |
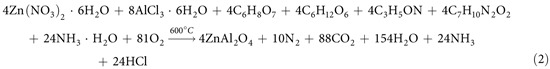 |
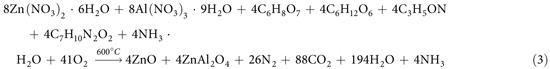 |
For samples S1 and S2, the observed diffraction peaks at 2θ are 31.22, 36.77, 44.69, 48.98, 55.52, 59.27, 65.06, 73.97, and 77.12 and can be ascribed, respectively, to the (220), (311), (400), (331), (422), (511), (440), (620), and (533) planes of ZnAl2O4. The mean grain size of samples S1, S2, and S3 were quantitatively evaluated based on the line broadening of the (220), (311), (511), and (440) peaks using the Scherrer formula, to be 13, 16, and 24 nm, respectively. XRD results indicate that the choice of the aluminum salts also has an influence on the phase purity of the final product. A possible reason for the formation of impurity phases when using citric acid as a chelating agent is that citric acid has a relatively weak coordinating capacity toward the metal ion of Al(NO3)3∙9H2O, and hence the formed metal complexonate is not expected to be highly stable.
Fourier transform infrared (FT-IR) spectra of the ZnAl2O4 nanoparticles prepared from (S1) Al2(SO4)3∙18H2O, (S2) AlCl3∙6H2O, and (S3) Al(NO3)3∙9H2O are shown in Fig. 2. The FT-IR spectra show a series of absorption peaks in the range of 400–2000 cm−1. According to the specific frequencies of the absorption peaks, the functional groups existing in the samples can be deduced. Peaks at 1633, 656, 552, and 493 cm−1 are present in all samples, and are assigned to the H-O-H bending vibration of adsorbed water19, Al-O symmetric stretching vibration (ν1)19,20,21,22, Al-O symmetric bending vibration (ν2)19,20,21,22, and Al-O asymmetric stretching vibration (ν3)21,22, respectively. For sample S2, the peaks located at 1186 and 1117 cm−1 are attributed to the S=O asymmetric stretching vibration23 and the S-O symmetric stretching vibration23,24, respectively.
Figure 2. FT-IR spectrum of ZnAl2O4 nanoparticles prepared from (S1) Al2(SO4)3∙18H2O, (S2) AlCl3∙6H2O, and (S3) Al(NO3)3∙9H2O and sintered at 600 °C.
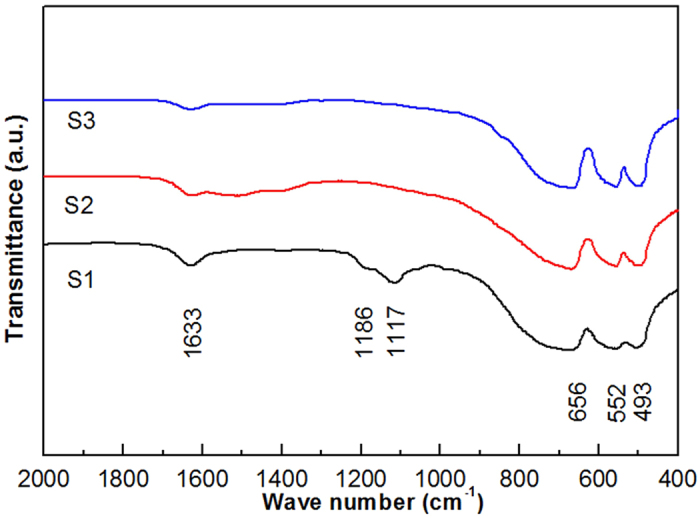
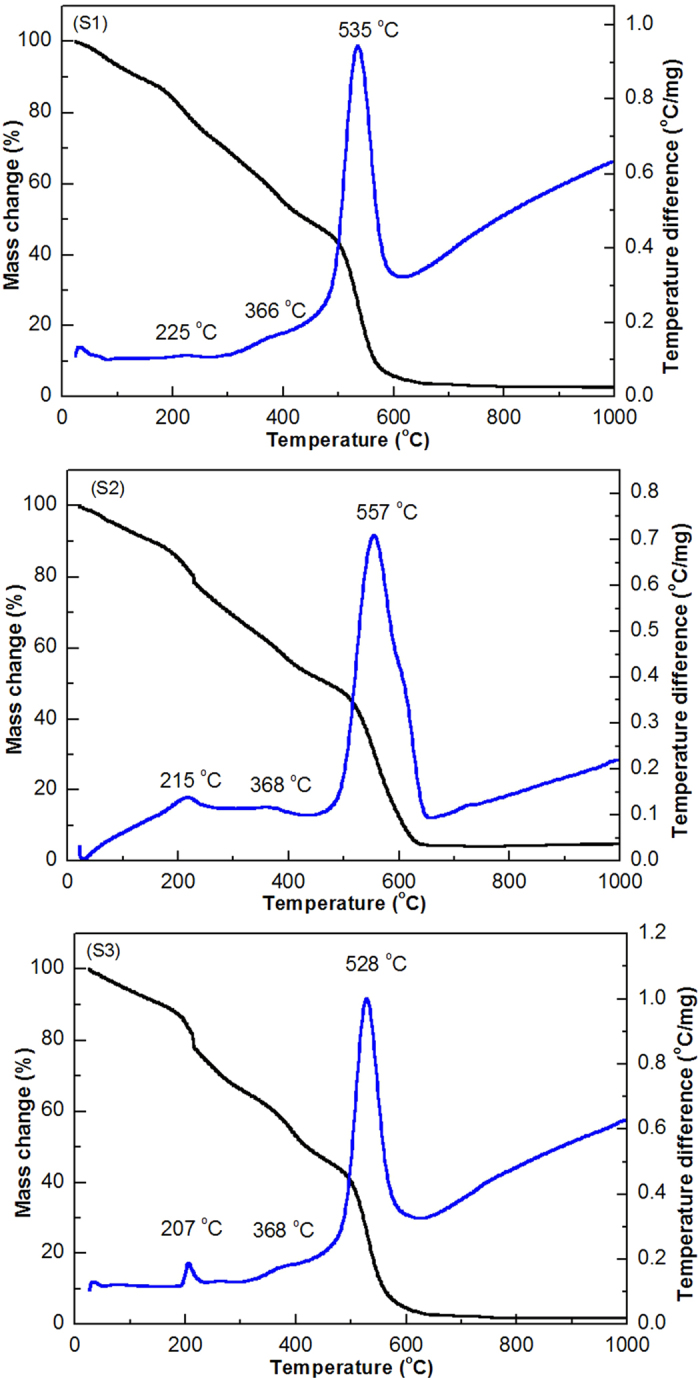
Figure 3 shows the TG/DTA curves of the ZnAl2O4 xerogels obtained from (S1) Al2(SO4)3∙18H2O, (S2) AlCl3∙6H2O, and (S3) Al(NO3)3∙9H2O. There are four weight loss stages observed for each sample. The first weight loss stage is seen at a low temperature range (before 200 °C) and corresponds to the evaporation of surface water in the ZnAl2O4 xerogel precursors25,26. The second weight loss stage (around 200–250 °C) is due to the evaporation of structural water25,26. The third weight loss stage (between 250–400 °C) is due to the decomposition of small molecular organic compounds. The largest and final weight loss stage (around 400–620 °C) is due to decomposition of complexes, glucose, and the polyacrylamide side-chain, as well as combustion of the polyacrylamide backbone and other residues27,28. The total weight loss measured for the ZnAl2O4 xerogel precursors were 97.31% for (S1) Al2(SO4)3∙18H2O, 95.201% for (S2) AlCl3∙6H2O, and 98.234% for (S3) Al(NO3)3∙9H2O. In Fig. 3 (S1), the main endothermic peak appeared at around 535 °C and corresponds to the thermal decomposition of the complexes, polyacrylamide backbone, and other residues originating from Al2(SO4)3 ∙18H2O. Meanwhile, the main endothermic peaks appeared at 557 °C (Fig. 3(S2)) for the ZnAl2O4 xerogel precursor obtained from AlCl3∙6H2O, and at 528 °C (Fig. 3(S3)) for (S3) Al(NO3)3∙9H2O. The slightly different decomposition temperatures of the three ZnAl2O4 xerogels may be related to differences in the microstructures caused by the presence of different anionic species (Cl−, SO42−, and NO3−) in the synthesis process. The chemical reaction is complete at ~ 600/620 °C and results in the formation of ZnAl2O4 nanoparticles. However, for ZnAl2O4 nanoparticles prepared from Al2(SO4)3∙18H2O, a higher heat treatment temperature is usually needed to improve the phase purity.
Figure 3. TG/DTA curves of ZnAl2O4 xerogel prepared from (S1)Al2(SO4)3∙18H2O, (S2) AlCl3∙6H2O, and (S3) Al(NO3)3∙9H2O.
To confirm whether the formation of ZnAl2O4 nanoparticles prepared from Al2(SO4)3∙18H2O needed a higher heat treatment temperature, FT-IR measurements were carried out using a Bruker IFS 66 v/S spectrometer. The FT-IR spectra of the ZnAl2O4 xerogel prepared form Al2(SO4)3∙18H2O and sintered at different temperatures are presented in Fig. 4. Here is can be seen that the S=O asymmetric stretching vibration (1186 cm−1) and S-O symmetric stretching vibration (1117 cm−1) peak intensities decrease with the increase of sintering temperature. This result indicates that the SO42 − anion coordinates to Zn and Al cations and forms a bridged bidentate structure23,29,30.
Figure 4. FT-IR spectrum of ZnAl2O4 xerogel prepared from Al2(SO4)3∙18H2O and sintered at 700, 800, and 900 °C.
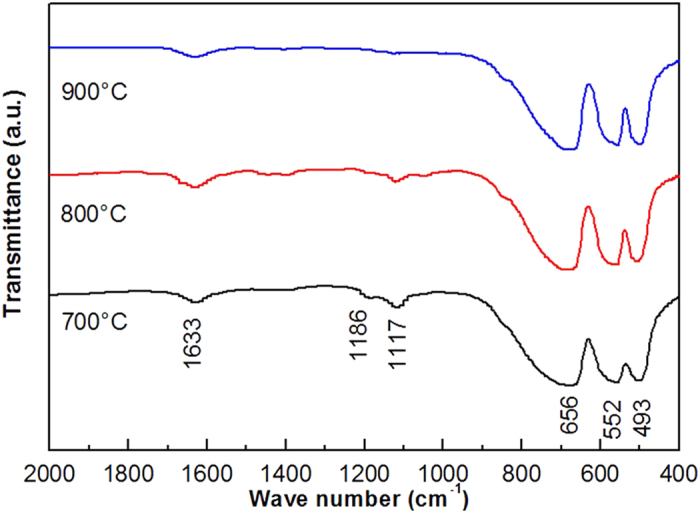
In the case of the sample obtained by sintering the xerogel at 900 °C, all of the organic peaks disappear except for the H−O−H peak (1633 cm −1). These results indicate that the effects of aluminum salts and sintering temperature on the phase purity of ZnAl2O4 cannot be neglected. Based on the subtle information gathered from the XRD and FT-IR results, the relevant reactions can be described as follows:
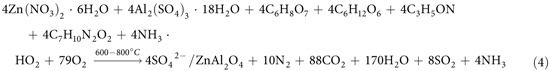 |
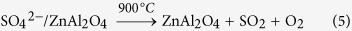 |
The results indicate that the reaction (1) cannot occur at 600 °C, and that a higher sintering temperature is needed for the formation of pure ZnAl2O4 nanoparticles.
In order to investigate the effects of different aluminum salts on the formation of ZnAl2O4, including the particle size and surface morphology, SEM and TEM images were collected of the ZnAl2O4 samples prepared using different aluminum salts and sintered at 700 °C, and are shown in Fig. 5 and Fig. 6. The SEM images of the ZnAl2O4 samples reveal that the particles are almost spherical in shape and have a narrow particle size distribution (Fig. 5(S1–S3)). When AlCl3∙6H2O is added into the ZnAl2O4 precursor, large particles form, however, if Al2(SO4)3∙18H2O is added into the precursor, small particles with little adhesion are observed, as shown in Fig. 5(S1-S2).
Figure 5. SEM images and particle size distribution of ZnAl2O4 xerogel prepared from (S1) Al2(SO4)3∙18H2O, (S2) AlCl3∙6H2O, and (S3) Al(NO3)3∙9H2O and sintered at 700 °C.
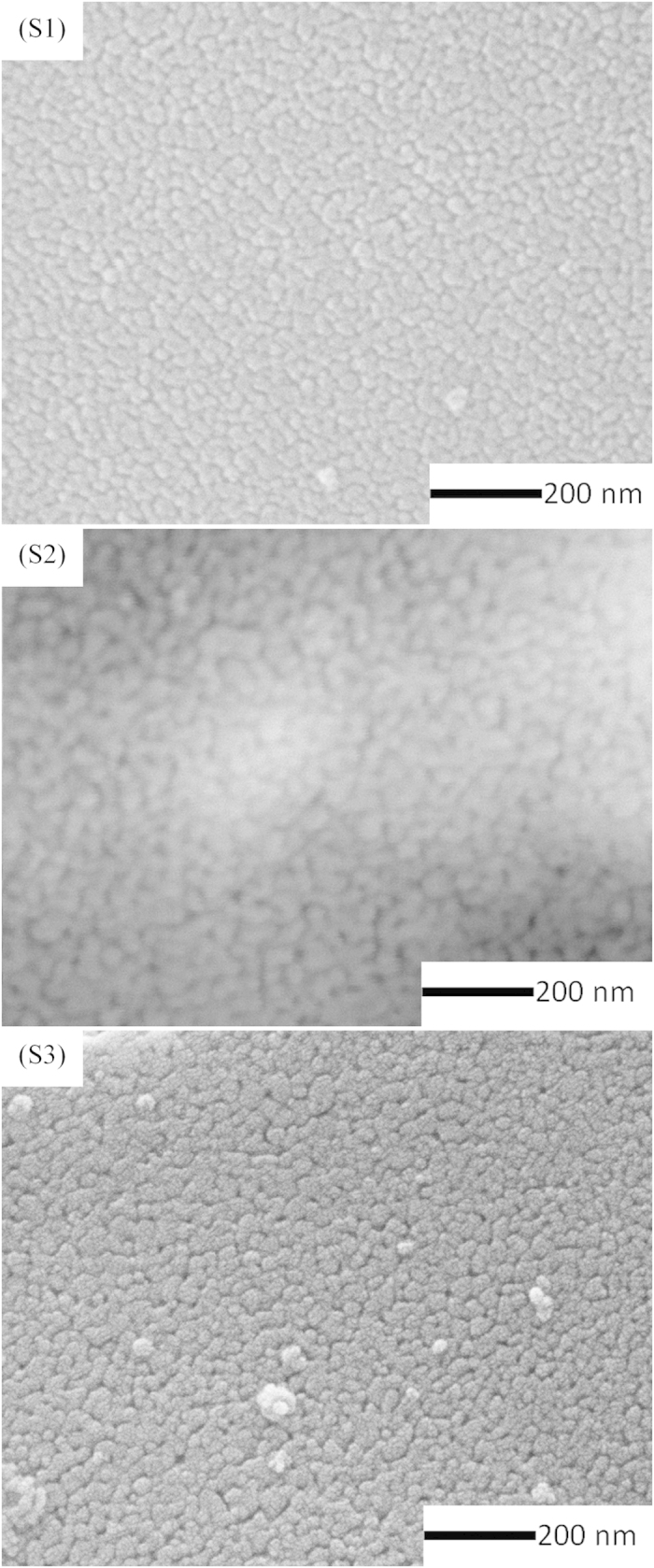
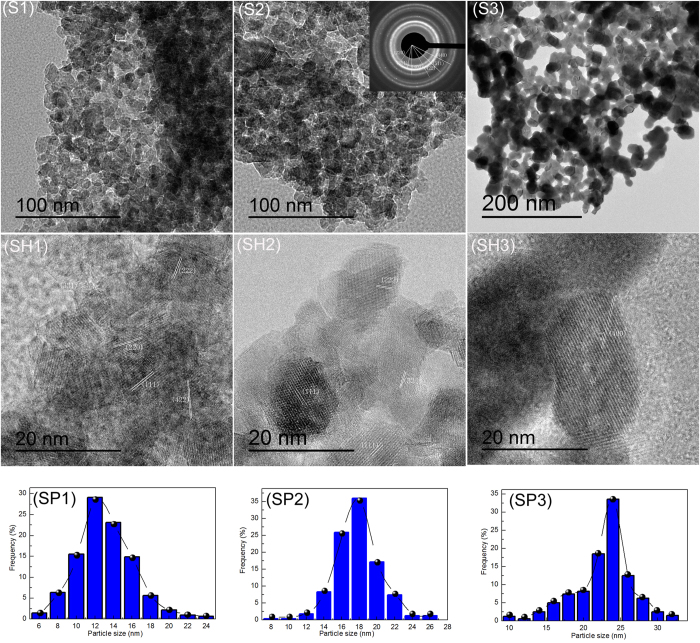
Figure 6. (S1–S3) TEM image, (SH1- SH3) HRTEM image, and (SP1–SP3) Particle size distribution of ZnAl2O4 nanoparticles prepared from (S1) Al2(SO4)3∙18H2O, (S2) AlCl3∙6H2O, and (S3) Al(NO3)3∙9H2O and sintered at 700 °C.
The inset presents the SAED pattern taken from a portion of the ZnAl2O4 nanoparticles shown in Fig. 6(S2).
Figure 6 shows (S1–S3) TEM image, (SH1- SH3) HRTEM image, and (SP1–SP3) the particle size distribution of ZnAl2O4 nanoparticles prepared from (S1) Al2(SO4)3∙18H2O, (S2) AlCl3∙6H2O, and Al(NO3)3∙9H2O. The ZnAl2O4 nanoparticles are spherical in shape with a narrow particle size distribution, as shown in Fig. 6(S1–S3). The corresponding particle size distribution patterns are given in the Fig. 6 (SP1–SP3). The average particle sizes of samples S1, S2, and S3 are around 12, 18, and 24 nm, respectively. TEM results show that the particle size variation tendencies for samples S1, S2, and S3 are consistent with those calculated from XRD patterns (Fig. 1). In addition, the BET surface area of the sample decreases with the increase of particle size as shown in Table 1. Compared with sample S2, the average particle size and BET surface area of sample S1 is significantly reduced, an effect which may be due to the SO42 − anion forming a bridged bidentate structure31. Fig. 6(S2) inset presents the SAED pattern taken from a portion of the ZnAl2O4 nanoparticles shown in Fig. 6(S2). The associated electron diffraction pattern is consistent with that of pure ZnAl2O4 crystals of a spinel structure, indexed as shown in Fig. 1(a). The SAED pattern revealed that the ZnAl2O4 nanoparticles possess interplanar spacings of 2.8235, 2.4156, 2.0205, 1.6328, 1.5531, and 1.4203 Å corresponding to the (220), (311), (400), (422), (511), and (440) planes, respectively.
Table 1. Crystallite size, particle size, Eg and BET specific surface area of samples S1, S2 and S3.
| Sample | Crystallite size (nm) | Particle size (nm) | Eg (eV) | BET specific surface area (m2/g) |
|---|---|---|---|---|
| S1 | 13 | 12 | 3.98 | 40.13 |
| S2 | 16 | 18 | 3.92 | 27.75 |
| S3 | 24 | 24 | 3.22 | 19.38 |
Figure 6(SH1)-(SH3) shows the HRTEM image of ZnAl2O4 nanoparticles prepared from (S1) Al2(SO4)3∙18H2O, (S2) AlCl3∙6H2O, and (S3) Al(NO3)3∙9H2O. For these samples, the lattice planes of ZnAl2O4 nanoparticles were (111) with a lattice space of 4.6687 Å, (220) with a lattice space of 2.8437 Å, (222) with a lattice space of 2.3345 Å, (400) with a lattice space of 2.0210 Å, (331) with a lattice space of 1.8550 Å, and (440) with a lattice space of 1.4356 Å. It has been noted that (111), (220), (222), (400), (331) and (440) planes can be attributed to spinel ZnAl2O4 structure corresponding to JCPDS card No. 05–0669.
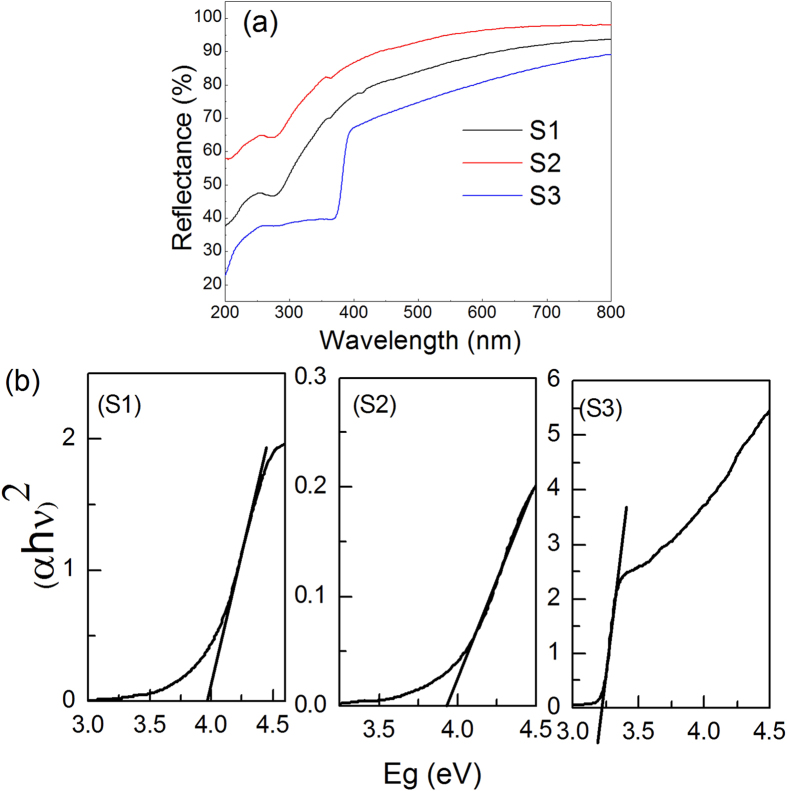
Figure 7(a) shows the UV-Vis diffuse reflectance spectra of ZnAl2O4 samples prepared from (S1) Al2(SO4)3∙18H2O, (S2) AlCl3∙6H2O, and (S3) Al(NO3)3∙9H2O. Considering that ZnAl2O4 is a direct bandgap semiconductor32, the Eg of the ZnAl2O4 samples can be estimated from plots of (αhν)2 versus hν using the Tauc relation33. This is shown in Fig. 7(b), where h is the Planck constant, α is the Kubelka–Munk (K–M) absorption coefficient, and ν is the frequency. The linear portions of the plots are extrapolated to the hν axis to give the values of Eg. The calculated Eg values of samples S1, S2, and S3 are 3.98 ± 0.01, 3.92 ± 0.01 and 3.22 ± 0.01 eV, respectively. Generally, the Eg values of nano-sized semiconductors increase with a decrease in particle size. In this case, the observed phenomenon is consistent with what has been previously reported.
Figure 7.
(a) UV-Vis diffuse reflectance spectra and (b) Eg values of ZnAl2O4 samples prepared from (S1) Al2(SO4)3∙18H2O, (S2) AlCl3∙6H2O, and (S3) Al(NO3)3∙9H2O and sintered at 700 °C.
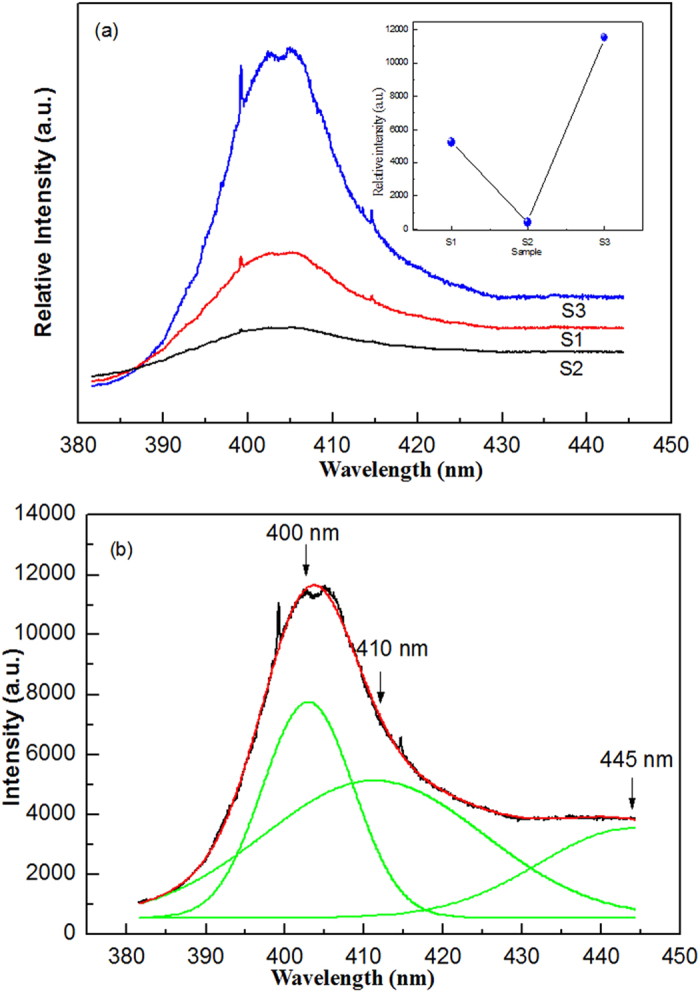
Figure 8(a) show the fluorescence spectra of ZnAl2O4 samples prepared from (S1) Al2(SO4)3∙18H2O, (S2) AlCl3∙6H2O, and (S3) Al(NO3)3∙9H2O and sintered at 700 °C. The fluorescence spectra with a wavelength range of 385 ~ 445 nm for all samples are presented after excitation with light, λexc = 325 nm. The fluorescence spectrum of the ZnAl2O4 sample is wide and can be resolved using three Gaussian peaks at 400, 410, and 445 nm (Fig. 8b). The three emission peaks at 400, 410, and 445 nm can be ascribed to intra band gap defects such as oxygen vacancies34. It has been noted that there is an obvious increase in the fluorescence intensity of the peak located at 400 nm when the Eg value decreases (see Fig. 8(a) inset). Compared with the ZnAl2O4 nanoparticles prepared from Al2(SO4)3∙18H2O, the ZnAl2O4 nanoparticles obtained from AlCl3∙6H2O have a smaller Eg value, however, their fluorescence intensity is also smaller. Therefore, in this case we cannot simply attribute the differences in the fluorescence intensity for the three ZnAl2O4 samples only to the differences in their Eg values. The difference in the fluorescence intensity of the two ZnAl2O4 samples may also be related to the different phase purities of ZnAl2O4 samples prepared from (S1) Al2(SO4)3∙18H2O and (S2) AlCl3∙6H2O. In the case of the ZnAl2O4 sample prepared from Al2(SO4)3∙18H2O, traces of the S=O and S-O are visible in the FT-IR spectrum after sintering at 700 °C.
Figure 8. Fluorescence spectra of ZnAl2O4 samples prepared from (S1)Al2(SO4)3∙18H2O, (S2) AlCl3∙6H2O, and (S3) Al(NO3)3∙9H2O and sintered at 700 °C.
Discussion
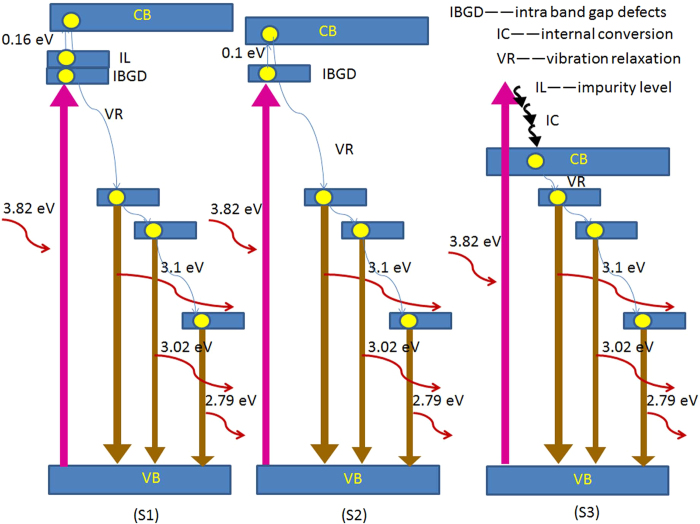
In order to understand the fluorescence mechanism of the prepared ZnAl2O4 samples, it is necessary to propose a schematic band diagram to illustrate the process of excitation and emission for the system. Figure 9 shows a schematic band diagram for the fluorescence mechanism of ZnAl2O4 samples obtained from (S1) Al2(SO4)3∙18H2O, (S2) AlCl3∙6H2O, and (S3) Al(NO3)3∙9H2O. It is known that Al3+ 2p orbitals and s orbitals located at the upper part of the Al3+ 2p orbitals make up the major conduction band (CB) edge of ZnAl2O4, and the hybridization band composed of O2− 2p and Zn2+ 3d orbitals makes up the upper valence band (VB)35. When the Eg value >λexc, (3.82 eV vs. 325 nm) it can be seen that one electron transition occurs from VB onto the intra band gap defects (IBGD) energy level (Fig. 9(S1) and (S2)). After that, the electron will be driven by continued transition from IBGD to CB. Then, the electron on the CB drops down to the low energy level through loss of energy by vibration relaxation (VR). Finally, the electron on the low energy level undergoes a radiative recombination with a hole in the valence band, accompanied by three blue-light emissions. For sample S1, the impurity (SO42−) plays a crucial role to promote the electron transition from IL (impurity level) to CB and improve the fluorescence properties. When Eg < λexc, one electron transition occurs from the VB to the high energy level (Fig. 9(S3)). Then, the electron on the high energy level drops down to the CB by internal conversion. At the same time, the electron on the CB by VR drops down to the low energy level with an accompanying loss of energy. Finally, the electron on the low energy level undergoes a radiative recombination with a hole in the valence band, accompanied by a series of blue-light emissions.
Figure 9. Fluorescence mechanism of ZnAl2O4 samples prepared from (S1) Al2(SO4)3∙18H2O, (S2) AlCl3∙6H2O, and (S3) Al(NO3)3∙9H2O.
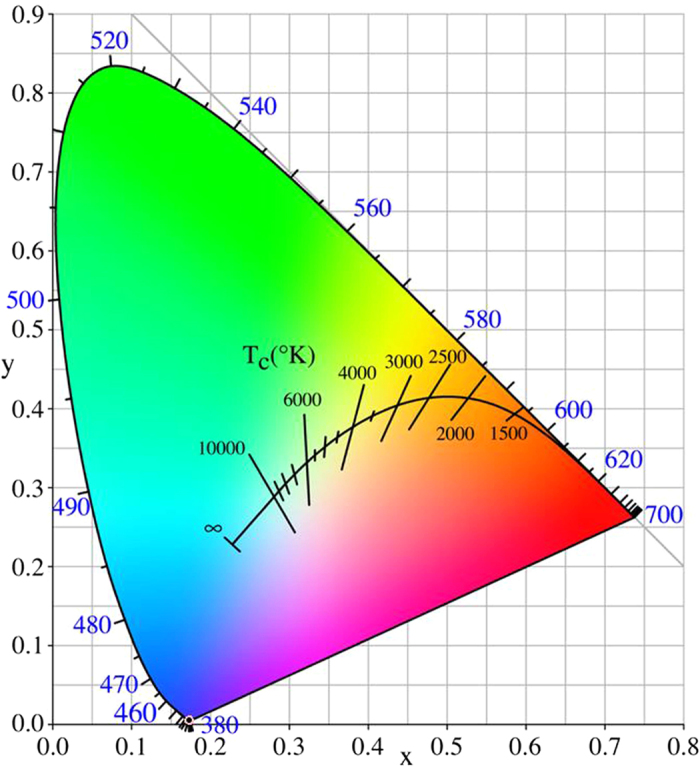
Figure 10 shows the Commission International De I’Eclairage (CIE) diagram of a ZnAl2O4 phosphor under 325 nm laser excitations. The CIE color coordinates (x, y) of the ZnAl2O4 phosphor was calculated using the fluorescence spectra. A typical CIE color coordinate of a ZnAl2O4 phosphor was found to be x, y equals 0.1729, 0.0048 respectively under 325 nm laser excitations.
Figure 10. CIE diagram of ZnAl2O4 phosphor under 325 nm laser excitations.
According to equation (7)36, the fluorescence quantum efficiency of the ZnAl2O4 phosphor can be calculated from the experimental data.
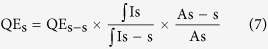 |
Where QEs and QEs-s are the fluorescence quantum efficiencies of the prepared sample and standard sample, respectively, under an excitation wavelength of 325 nm. ∫Is and ∫Is-s are the integrated emission intensities of the prepared sample and standard sample under an excitation wavelength of 325 nm. As and As-s are the absorbance of the prepared sample and standard sample under an excitation wavelength of 325 nm. The standard sample is 5-sulfosalicylic acid, which has a quantum efficiency of about 58% under an excitation wavelength of 325 nm. The fluorescence quantum efficiencies of samples S1, S2, and S3 were calculated to be 6.38%, 4.24%, and 10.11%, respectively. Observations made during these experiments show that the spinel ZnAl2O4 phosphor could be useful for blue light-emitting materials.
In summary, ZnAl2O4 nanoparticles with different particle sizes were prepared using a modified polyacrylamide gel route from different aluminum salt starting materials: Al2(SO4)3∙18H2O, AlCl3∙6H2O, and Al(NO3)3∙9H2O. The type of aluminum salt used can markedly influence the phase purity, particle size, and optical and fluorescence properties of the final product. Under 325 nm excitation, samples of ZnAl2O4 phosphor show blue emissions and the CIE colour coordinate was found to be (0.1729, 0.0048). The fluorescence mechanisms of the ZnAl2O4 phosphor have been discussed based on the experimental results. The fluorescence experiments revealed that the as-prepared ZnAl2O4 phosphor exhibits interesting abilities for application in blue light-emitting materials. Interestingly, similar preparation methods may be employed for the synthesis of other metal oxides nanoparticles, including fluorescence materials, multiferroic materials, oxide thermoelectric materials, photocatalytic materials, solid oxide fuel cell materials, and high-temperature superconducting materials.
Methods
Materials
Zn(NO3)2∙6H2O, AlCl3∙6H2O, Al2(SO4)3∙18H2O, and Al(NO3)3∙9H2O were purchased from DaMao Chem. Ltd., Tianjing. Citric acid (C6H8O7, AR), glucose (C6H12O6.H2O, 99%), acrylamide (C3H5NO, AR), and N, N’-methylene-bisacrylamide (C7H10N2O2, 99%) were purchased from Kemiou Chem. Ltd., Tianjing and used without further purification.
Synthesis
A certain stoichiometric amounts of Zn(NO3)2∙6H2O and different aluminum salts (Al2(SO4)3∙18H2O, AlCl3∙6H2O, and Al(NO3)3∙9H2O are labeled sample S1, S2 and S3, respectively) were dissolved in the deionized water to obtain a final solution of 0.015 mol/L with the total cations. Starting compositions of samples S1, S2 and S3 are given in Table 2. After the solution was transparent, a stoichiometric amount of chelating agent (citric acid) was added into the solution in the mole ratio 1.5:1 with respect to the total cations (Zn2+ and Al3+) to complex the cations. After that, 20 g glucose was dissolved into the solution. Finally, the acrylamide and N, N’-methylene-bisacrylamide monomers were added into the solution. The resultant solution was heated to 90 °C on a hot plate to initiate the polymerization reaction, and a few minutes later a polyacrylamide gel was formed. The gel was dried at 120 °C for 24 h in a thermostat drier. The obtained xerogel precursor was ground into powder and some powder was sintered at 600, 700, 800, and 900 °C for 5 h in air to prepare the objective products.
Table 2. Starting compositions of samples S1, S2 and S3.
| Sample | Al2(SO4)3∙18H2O | AlCl3∙6H2O | Al(NO3)3∙9H2O | Zn(NO3)2∙6H2O | citric acid | glucose | acrylamide | Bis-acrylamide |
|---|---|---|---|---|---|---|---|---|
| S1 | 6.6644g | / | / | 1.4875 g | 4.7282g | 20 g | 9.5958g | 1.9192 g |
| S2 | / | 2.4143g | / | 1.4875 g | 4.7282g | 20 g | 9.5958g | 1.9192 g |
| S3 | / | / | 3.7513g | 1.4875 g | 4.7282g | 20 g | 9.5958g | 1.9192 g |
Sample characterization
The ZnAl2O4 xerogel precursor sintered at 600 and 700 °C were analyzed by X-ray diffractometer (DX-2700) with Cu Kα radiation. Fourier transform infrared (FTIR) spectra in the range 400–2000 cm−1 were recorded using a Bruker IFS 66 v/S spectrometer. Thermogravimetric (TG) and differential thermal analysis (DTA) analyses were performed in a SDT Q600 (TA instruments, Inc. USA) simultaneous thermal analyzer at a heating rate of 10 °C/min. The surface morphology of the synthesized ZnAl2O4 sample was characterized by scanning electron microscopy (SEM) and transmission electron microscopy (TEM). The surface area of the samples were characterized by a 3H-2000BET-M instrument. The absorption spectra of the samples were examined on a Shimadzu UV-2500 UV-Visible spectrophotometer. The fluorescence spectra were collected at room temperature in a confocal Raman system using a He-Cd laser (325 nm, RGB laser system, NovaPro 30 mW, Germany).
Additional Information
How to cite this article: Wang, S.-F. et al. A comparative study of ZnAl2O4 nanoparticles synthesized from different aluminum salts for use as fluorescence materials. Sci. Rep. 5, 12849; doi: 10.1038/srep12849 (2015).
Acknowledgments
This work was supported by the NSAF joint Foundation of China (U1330103) and by Outstanding Doctoral Student Support Plan (A1098524023901001074).
Footnotes
Author Contributions S.W. and G.S. contributed to the preparation and characterization of the nanoparticles. L.L. measured the fluorescence spectra. S.W., G.S., L.F. and X.X. analyzed data from experiments and wrote the main manuscript. X.Z. led the project.
References
- Duan X. L., Yuan D. R. & Yu F. P. Cation distribution in Co-doped ZnAl2O4 nanoparticles studied by X-ray photoelectron spectroscopy and 27Al solid-state NMR spectroscopy. Inorg. Chem. 50, 5460–5467 (2011). [DOI] [PubMed] [Google Scholar]
- Ianos R., Borcǎnescu S. & Lazaǎu R. Large surface area ZnAl2O4 powders prepared by a modified combustion technique. Chem. Eng. J. 240, 260–263 (2014). [Google Scholar]
- Foletto E. L. et al. Synthesis of ZnAl2O4 nanoparticles by different routes and the effect of its pore size on the photocatalytic process. Micropor. Mesopor. Mat. 163, 29–33 (2012). [Google Scholar]
- Li X. Y., Zhu Z. R., Zhao Q. D. & Wang L. Z. Photocatalytic degradation of gaseous toluene over ZnAl2O4 prepared by different methods: A comparative study. J. Hazard. Mater. 186, 208–2096 (2011). [DOI] [PubMed] [Google Scholar]
- Peng C. et al. Fabrication and luminescence properties of one-dimensional ZnAl2O4 and ZnAl2O4: A3+ (A = Cr, Eu, Tb) microfibers by electrospinning method. Mater. Res. Bull. 47, 3592–3599 (2012). [Google Scholar]
- Cheng B. C., Ouyang Z. Y., Tian B. X., Xiao Y. H. & Lei S. J. Porous ZnAl2O4 spinel nanorods: high sensitivity humidity sensors. Ceram. Int. 39, 7379–7386 (2013). [Google Scholar]
- Zhu Z. R. et al. Photocatalytic performances and activities of ZnAl2O4 nanorods loaded with Ag towards toluene. Chem. Eng. J. 203, 43–51 (2012). [Google Scholar]
- Yang Y. et al. Hierarchical three-dimensional ZnO and their shape-preserving transformation into hollow ZnAl2O4 nanostructures. Chem. Mater. 20, 3487–3494 (2008). [Google Scholar]
- Zou L., Li F., Xiang X., Evans D. G. & Duan X. Self-generated template pathway to high-surface-area zinc aluminate spinel with mesopore network from a single-source inorganic precursor. Chem. Mater. 18, 5852–585 (2006). [Google Scholar]
- Van der Laag N.J., Snel M.D., Magusin P. C. M. M. & De With G. Structural, elastic, thermophysical and dielectric properties of zinc aluminate (ZnAl2O4). J. Eur. Ceram. Soc. 24, 2417–2424 (2004). [Google Scholar]
- Ianos R., Lazǎu R., Lazǎu I. & Pǎcurariu C. Chemical oxidation of residual carbon from ZnAl2O4 powders prepared by combustion synthesis. J. Eur. Ceram. Soc. 32, 1605–1611 (2012). [Google Scholar]
- Wei X. & Chen D. Synthesis and characterization of nanosized zinc aluminate spinel by sol–gel technique. Mater. Lett. 60, 823–827 (2006). [Google Scholar]
- Gama L. et al. Synthesis and characterization of the NiAl2O4, CoAl2O4 and ZnAl2O4 spinels by the polymeric precursors method. J. Alloys Comp. 483, 453–455 (2009). [Google Scholar]
- Fan G. L., Wang J. & Li F. Synthesis of high-surface-area micro/mesoporous ZnAl2O4 catalyst support and application in selective hydrogenation of o-chloronitrobenzene. Catal. Commun. 15, 113–117 (2011). [Google Scholar]
- Zawadzki M., Staszak W., López-Suárez F.E., Illán-Gómez M. J. & Bueno-López A. Preparation, characterisation and catalytic performance for soot oxidation of copper-containing ZnAl2O4 spinels. Appl. Catal. A: Gen. 371, 92- 98 (2009). [Google Scholar]
- Yu Z.Q., Li C. & Zhang N. Size dependence of the luminescence spectra of nanocrystal alumina. J. Lumin. 99, 29–34 (2002). [Google Scholar]
- Wang S. F., Lv H. B., Zhou X. S., Fu Y. Q. & Zu X. T. Magnetic nanocomposites through polyacrylamide gel route. Nanosci. Nanotech. Let. 6, 758–771 (2014). [Google Scholar]
- Theophil Anand G., John Kennedy L., Aruldoss U. & Judith Vijaya J. Structural, optical and magnetic properties of Zn1-xMnxAl2O4 (0≤x≤5) spinel nanostructures by one-pot microwave combustion technique. J. Mol. Struct. 1084, 244–253 (2015). [Google Scholar]
- Ge D. L., Fan Y. J., Qi C.L. & Sun Z. X. Facile synthesis of highly thermostable mesoporous ZnAl2O4 with adjustable pore size. J. Mater. Chem. A 1, 1651–1658 (2013). [Google Scholar]
- Silva A. A. D., De Souza Goncalves A. & Davolos M. R. Characterization of nanosized ZnAl2O4 spinel synthesized by the sol–gel method. J. Sol-Gel Sci. Technol. 49, 101–105 (2009). [Google Scholar]
- Huang I. B. et al. Preparation and luminescence of green-emitting ZnAl2O4:Mn2+ phosphor thin films. Thin Solid Films 570, 451–456 (2014). [Google Scholar]
- TheophilAnand G., JohnKennedy L., JudithVijaya J., Kaviyarasan K. & Sukumar M. Structural,optical and magnetic characterization of Zn1-xNixAl2O4 (0≤x≤5) spinel nanostructures synthesized by microwave combustion technique. Ceram. Int. 41, 603–615 (2015). [Google Scholar]
- Yamaguchi T., Jin T. & Tanabe K. Structure of acid sites on sulfur-promoted iron oxide. J. Phys. Chem. 90, 3148–3152 (1986). [Google Scholar]
- Wang J. X. et al. Synthesis and characterization of S2O82 −/ZnFexAl2 − xO4 solid acid catalysts for the esterification of acetic acid with n-butanol. Catal. Commun. 62, 29–33 (2015). [Google Scholar]
- Zhang Y., Zhao C.Y., Liang H. & Liu Y. Macroporous monolithic Pt/α -Al2O3 and K-Pt/α-Al2O3 catalysts used for preferential oxidation of CO. Catal. Lett. 127, 339–347 (2009). [Google Scholar]
- Zhang Y., Liang H., Zhao C. Y. & Liu Y. Macroporous alumina monoliths prepared by filling polymer foams with alumina hydrosols. J. Mater. Sci. 44, 931–938 (2009). [Google Scholar]
- Xian T. et al. Preparation of high-quality BiFeO3 nanopowders via a polyacrylamide gel route. J. Alloy. Comp. 480, 889–892 (2009). [Google Scholar]
- Wu S. Q., Liu Y. Y., He L. N. & Wang F. P. Preparation of β-spodumene-based glass–ceramic powders by polyacrylamide gel process, Mater. Lett. 58, 2772–2775 (2004). [Google Scholar]
- Eskenazi R., Raskovan J. & Levitus R. Sulphato complexes of palladium (II). J. Inorg. Nucl. Chem. 28, 521–526 (1966). [Google Scholar]
- Schoonheydt R. A. & Lunsford J. H. Infrared spectroscopic investigation of the adsorption and reactions of SO2 on MgO. J. Catal. 26, 261–271 (1972). [Google Scholar]
- Coma A. Inorganic solid acids and their use in acid-catalyzed hydrocarbon reactions. Chem. Rev. 95, 559–614 (1995) . [Google Scholar]
- Dixit H. et al. First-principles study of possible shallow donors in ZnAl2O4 spinel, Phys. Rev. B 87, 174101 (2013). [Google Scholar]
- Ragupathi C., Judith Vijaya J., Manikandan A. & John Kennedy L. Phytosynthesis of nanoscale ZnAl2O4 by using sesamum (Sesamum indicum L.) optical and catalytic properties. J. Nanosci. Nanotechno. 13, 8298–8306 (2013). [DOI] [PubMed] [Google Scholar]
- Ragupathi C., Kennedy L. J. & Vijaya J. J. A new approach: Synthesis, characterization and optical studies of nano-zinc aluminate. Adv. Powder Technol. 25, 267–273 (2014). [Google Scholar]
- Khenata R. et al. Full-potential calculations of structural, elastic and electronic properties of MgAl2O4 and ZnAl2O4 compounds. Phys. Lett. A 344, 271–279 (2005). [Google Scholar]
- Demasa J. N. & Crosby G. A. The measurement of photoluminescence quantum yields. A review. J. Phys. Chem. 75, 991–1024 (1971). [Google Scholar]