Abstract
Autism spectrum disorder (ASD) is a neurodevelopmental disorder that has a strong genetic basis, and is heterogeneous in its etiopathogenesis and clinical presentation. Neuroimaging studies, in concert with neuropathological and clinical research, have been instrumental in delineating trajectories of development in children with ASD. Structural neuroimaging has revealed ASD to be a disorder with general and regional brain enlargement, especially in the frontotemporal cortices, while functional neuroimaging studies have highlighted diminished connectivity, especially between frontal-posterior regions. The diverse and specific neuroimaging findings may represent potential neuroendophenotypes, and may offer opportunities to further understand the etiopathogenesis of ASD, predict treatment response and lead to the development of new therapies.
Keywords: Autism Spectrum Disorder, DTI, Endophenotypes, Neuroimaging, MRI
Introduction
This paper reviews the application of the endophenotypes concept to neuroimaging in autism spectrum disorder (ASD). To this end, we first review the concept of endophenotypes, the genetic and clinical heterogeneity of ASD and summarize the current understanding of the brain developmental trajectory in ASD. We then present a brief overview of the predominant structural and functional neuroimaging findings in ASD and discuss the potential relevance of the neuroimaging endophenotypes to ASD.
The Endophenotype Concept
Endophenotypes are measurable subclinical biological markers or traits that are internal phenotypic expressions of a genotype. A biomarker that does not represent an expression of a gene is not considered to be an endophenotype1. “An endophenotype may be neurophysiological, biochemical, endocrinological, neuroanatomical, cognitive, or neuropsychological (including configured self-report data) in nature.”2 They are also called intermediate phenotypes as they lie in the pathway between the genome and the external phenotype (clinical or behavioral features). They may be the developmental manifestation of a gene or set of genes; therefore, their study may shed light on the etiopathogenesis of a disorder such as ASD that has strong genetic origins.
Originally proposed in a paper on variability in geographic distribution of grasshoppers3, Gottesman and Shields applied this concept to the genetics of schizophrenia.4, 5 The commonly accepted criteria for a trait or a biomarker to be considered an endophenotype include: a) it is associated with illness in the population; b) it is heritable; c) it is primarily state-independent (manifests in an individual whether or not illness is active); d) within families, endophenotype and illness co-segregate; e) it is found in both the affected and nonaffected family members at a higher rate than in the general population2. There has been great interest in the identification of endophenotypes to study a particular trait in the proband and unaffected relatives in both non-psychiatric and psychiatric conditions. Most psychiatric disorders have a complex genetic architecture, which precludes the identification of single genes that may promote the disorder; identification of endophenotypes may be an alternative approach as an endophenotype may connote a single gene or a set of genes6.
Identifying endophenotypes may, especially, be a more cogent approach to understanding ASD, given its genetic underpinnings and complex nature, than a purely behavioral approach to clinical diagnosis7. It is recognized, however, that many of the putative endophenotypes may not be specific to ASD, but may be generally indicative of either normal variation or atypical development in neural structure or function, and may thus occur in other developmental disorders.8, 9
Autism Spectrum Disorder
Described initially by Leo Kanner at Johns Hopkins in his classic paper in 1943, 10 ASD (or autism) is a neurodevelopmental psychiatric disorder with an estimated prevalence of 1 in 68 children11 that is usually diagnosed in childhood but spans the lifetime.12 It has a male predilection, occurring in 1 in 42 males as compared to 1 in 189 females11. The diagnosis depends upon core diagnostic criteria including delays or deficits in social communication, and restricted areas of interest and repetitive behaviors13. Gold standard measures such as Autism Diagnostic Interview (ADI-R; Lord, Rutter, & Le Couteur, 1994) and Autism Diagnostic Observation Scale (ADOS-G: Lord et al., 2000) are used both clinically and in research to confirm the ASD diagnosis.
Although the concept of an autism spectrum had initially been proposed in 197914, it was not until the publication of DSM-5 (Diagnostic and Statistical Manual: Fifth Edition, APA, 2013), that the term ASD was officially recognized as a diagnosis. Studies comparing the disorders under the DSM-IV (Diagnostic and Statistical Manual: Fourth Edition, Text Revision, APA, 2000) umbrella term pervasive developmental disorders supported incorporating these into a single ASD diagnosis.
ASD is highly heterogeneous both in its etiopathogenesis and in clinical presentation. The term ASD itself recognizes the dimensional differences within the diagnosis. Several factors contribute to the heterogeneity in ASD including high rates of comorbid medical conditions (e.g., sleep disorders, gastrointestinal dysfunction, autoimmune disorders), neurological conditions (such as seizures and sensory and motor system abnormalities), and psychiatric disorders (such as attention deficit hyperactivity disorder and anxiety). Another contributor to the heterogeneity is the intellectual ability of the individual with ASD. Intellectual abilities in ASD may vary from profound intellectual disability to superlative intellect across the spectrum, leading to the concept of “low functioning” and “high functioning” ASD. It is not known whether some of these co-occuring conditions confer their own genetic liability to ASD. It is also not established whether the common co-occurrence of specific disorders in ASD signifies independent phenotypes or if these disorders are truly comorbid disorders in context of ASD. Given the etiopathogenetic, and phenotypic heterogeneity, ASD has been described as “autisms” rather than a unitary “autism”.15
Genetic Underpinnings of ASD
ASD is a highly heritable disorder with estimates as high as 80-90%.16,17 Initial evidence for this came from early twin pair studies.18-20 Subsequent studies found 60% of monozygotic (MZ) twins to be concordant for autism versus none for dizygotic (DZ) pairs; 92% of MZ twins were concordant for a broad spectrum of autism related cognitive and social abnormalities versus 10% for DZ twins.21 Concordance rate for siblings ranges from 3 to 14%.22-25 The frequent finding of subclinical autistic or cognitive traits in close relatives of autistic probands who do not meet the criteria for a diagnosis of ASD (the broader autism phenotype, BAP26), as well as the existence of the such traits in general population27-29 are also cited as evidence for the genetic basis.
Further support for genetic liability is also derived from the fact that 10-20% of individuals with known genetic disorders may have autistic features (“syndromic autism”)30 such as in Fragile X syndrome and Rett syndrome.31,32 Other genetic disorders such as tuberous sclerosis, neurofibromatosis type I, Prader-Willi and Angelman syndromes, Smith-Lemli-Opitz syndrome, Smith-Magenis syndrome, and velocardiofacial syndrome amongst others, may present with autistic features also33 (see Miles 2011 for a thorough review of genetics of ASD).
Newer genetic analytic techniques such as chromosomal microarray (CMA) including single nucleotide polymorphism (SNP) arrays, and whole genome sequencing have highlighted the association of de novo mutations or copy number variants (CNVs) including some at “hot spot” locations on chromosomes, such as 16p11.2, which has underscored their role in ASD.34, 35 Many of these mutations involve genes that are very active in brain developmental processes. These include genes active in synatptogenesis and pruning (such as SHANK, neurexin and neuroligin families), others that regulate growth (such as HOXA1 and PTEN), or are involved in other aspects of brain development such as signaling pathways (e.g. those affecting calcium homeostasis).36 Adding to the complexity, a recent genome wide association study has identified loci of common CNVs across psychiatric disorders such as ASD, attention deficit hyperactivity disorder (ADHD), bipolar disorder, major depressive disorder and schizophrenia37. These genetic factors may play a significant role in influencing the neurobiology of the developing brain in ASD.38
The Developing Brain in ASD
Neuroimaging has ushered in an unprecedented understanding of both typical and atypical neurodevelopment; to understand the neuroimaging findings in context of ASD, it is important to understand the brain development in children with ASD.
It is widely accepted that the brain develops by a dynamic interplay between the genetic factors and experience of the child. Although brain development starts in utero, infancy and toddlerhood are remarkable for peak synaptogenesis and generation of early neural circuitry; in concert with experiential/environmental factors, as well as genetically programmed pruning in childhood and adolescence, the connections and neural circuits are further sculpted.39,40 The brain attains 80% of its adult weight within the first 2 postnatal years41: adult cerebral volume is attained by 5 years of age, with a significant reduction in gray matter (GM) after 12 years of age and a progressive increase in white matter (WM) throughout childhood and adolescence.39 This pattern of decreasing GM and increasing WM is consistent with the sculpting of the neural circuitry as well as laying down of the neural architecture. There is also regional variation in cortical maturation with primary cortices developing earlier than association cortices.42, 43 In general, areas responsible for higher cortical functions mature later, and have a more protracted course of maturation such as in the prefrontal cortex42, 43.
Structural covariation in various cortical/subcortical regions of the maturing brain may also occur at the anatomical (e.g. larger frontal lobe and a smaller cerebellum) 44-46 and the network levels47 and may have a genetic and functional basis48. Abnormal development of a structure may thus influence abnormal development of other structure(s) and consequently, the functional networks49.
Converging evidence over the past three decades has established that the typical trajectory of brain development is altered in children with ASD; the process starts prenatally50,51 and persists into adult life.52,53 Although at birth, the brain size may be normal or even smaller as compared to a typically developing (TD) child's54, there is accelerated growth starting around 6 months of age into toddlerhood55-58; this is followed by slowing in brain growth by school age with plateauing or developmental arrest.52 The early-altered trajectory affects the regional brain growth patterns, neural architecture and connectivity. By late teenage years into adulthood, there may be a normalization of the brain size (referred to by some as “pseudo-normalization”59) or a decline into adulthood.52
Pathology at both the macroscopic and microscopic levels, both in structure and function, that affects the typical development of neural circuitry in the frontal, temporal and cerebellar cortices, contributes to the early developmental and clinical features of ASD.54,60-62 Some have suggested ASD to be a disorder of primarily association cortices and higher order cognitive and developmental functions.63
Given the early accelerated brain growth in infants and toddlers with ASD64, 65, macrocephaly (increased head circumference beyond 97th percentile) is one of the most replicated findings in children with ASD. In infants and toddlers, this correlates with increased total brain volume (TBV); there is a dissociation between macrocephaly and TBV beginning around 4-5 years of age.52,66 However, macrocephaly in ASD is part of a general macrosomia and may not be specific to ASD67-69 as it has been associated with a number of genetic variants, such as those involving the HOX A1 gene70,71, TPH272-75 GLO174,76,77, and PTEN.78-81 Furthermore, family studies have found similar macrocephaly to be present in relatives of ASD probands73,74,82-84.
Neuroimaging in ASD
Neuroimaging has played a crucial role in delineating both the typical and atypical neurodevelopmental trajectories. Given ASD's strong genetic basis with a known developmental trajectory, neuroimaging may further aid identification of endophenotypes as potentially, any consistent structural or functional imaging finding may, putatively, represent an endophenotype.
Over the past 4 decades, there has been a remarkable progress in neuroimaging technology. Several neuroimaging techniques have been developed since the 1980s and most have been applied to ASD research including computerized tomography (CT), structural and functional magnetic resonance imaging (MRI), positron emission tomography (PET), single photon emission computerized tomography (SPECT), magnetic resonance spectroscopy (MRS) and diffusion tensor imaging (DTI). MRI, especially, given its ubiquity in neuroimaging research studies, has been instrumental in proffering new insights and supporting a priori hypotheses about ASD etiopathogenesis, thus aiding the study of structural, neurophysiological/functional and neurocognitive endophenotypes. It has contributed to great advancements in knowledge about typical as well as the atypical brain development, aiding consilience of disparate research findings. The availability of high-resolution scanners and computer based brain atlases for GM and WM (such as the Desikan-Killiany atlas85, JHU-DTI atlas86-88 and others) has also enabled researchers in employing neuroimaging in these studies.
Both functional and structural imaging using magnetic resonance are non-invasive and therefore, preferable for research in children. Neuroimaging combined with network based computational approaches such as the graph theory are shedding light on the network-wide connectivity problems in ASD. Although, any consistent/replicable structural or functional imaging finding may signify a neuroendophenotype, studying neuroimaging endophenotypes, on the other hand has inherent limitations, such as: reliability of findings depending upon the resolution of the image, challenges in training children with developmental and behavioral problems to lie still during imaging, and findings that may not be generalizable to the full spectrum of children with ASD (as most studies have involved higher functioning children and adolescents who may be more easily trained to lie still than those who are lower-functioning). Most ASD neuroimaging studies have used either age matched TD children or those with other developmental delays or learning disabilities as control groups.
The following is a brief overview of some notable and reproducible findings in ASD; more comprehensive reviews are also available by other authors63, 89-96.
A. Structural Neuroimaging
Structural MRI (sMRI) studies, which have focused on the neuroanatomical aspects of brain development, have been instrumental in revealing progression of brain developmental trajectory in ASD. They have been used to examine both cortical and subcortical regions - major findings in each of these areas are described next.
1. Cortex and regional
sMRI has been used to measure total brain volume (TBV), and volume of specific brain structures; it has also enabled study of shapes and other metrics, including growth patterns of specific structures, using regions of interest (ROI) and voxel (“volumetric pixel”) based morphometry (VBM) analyses . Other cortical metrics have included measurement of cortical thickness (CT), surface area (SA) and cortical volumes, (gray matter volume, GMV, and white matter volume, WMV); cortical volume being a product of CT and SA. Notably, CT and SA have been hypothesized to have dissociable developmental trajectories with, putatively, different genetic and neurodevelopmental basis.97, 98
Some initial studies found increases in total brain volume (TBV) in ASD versus TD subjects, with increases noted both in cortical gray matter (GM) and white matter (WM) 64, 99. Other studies have revealed dissociation in the volumes of the cerebrum and the subcortical structures, e.g. a study of 15 year olds with autism compared to TD found increases in volumes of WM, no changes in other structures (caudate, globus pallidus, putamen, diencephalon, cerebellum and brainstem), a reduced volume in others (cerebral cortex, hippocampus-amygdala)66.
The frontal and temporal lobes have been found to show the greatest increases in the mean cortical volumes, both in GM and WM, with limited to no effect on the parietal and occipital lobes100-102. There may also be variations within a specific region of a particular lobe such as enlargement in the dorsolateral prefrontal cortex and medial frontal regions, with limited effect on other areas such as the precentral cortex103. Others have found enlargement in precentral regions104.
2. Subcortical structures
Amygdala
The amygdala has been the focus of several studies in children with ASD, given its important role in attachment security105 socioemotional processing and understanding of social contexts and personal space106-109. The trajectory of growth of amygdala has been correlated to the pattern of early brain overgrowth in ASD110. Overall, the results have been inconsistent, with studies of younger children showing larger volumes111 and those in older children showing no difference compared to TD children112-114.
Cerebellum
The cerebellum (especially, in its posterior regions) is structurally and functionally connected via afferent and efferent pathways to brain regions involved, not only in sensorimotor, but also in language, cognition, executive, and socioemotional functioning115-118. Morphometric studies of ASD subjects have found increased total cerebellar volume66, 114,119-121, reduced mid-sagittal SA for vermal lobules VI-VII122-124 and hypoplasia of the posterior vermis in ASD123, 125-129. Meta-analyses of VBM studies have revealed GM decreases in right Crus I, lobule VIII, and lobule IX of the cerebellum130-132. Others have found decreased GM in left Crus I; or even increased GM in the cerebellum overall130, 131. In one study133 of males, investigators found that cerebellar volume correlated with TBV for ASD and controls.
Basal Ganglia
The basal ganglia (BG) are purported to play an important role in cognition and modulation of motor control via their participation in frontostriatal, thalamocortical and limbic circuits134-137. They have been studied for repetitive behaviors in ASD138, 139. The most consistent finding pertaining to the BG in ASD has been increased caudate volume; this increase may be proportional to the increase in TBV66, 140-143. Such increases have not been consistently reported for other BG structures including globus pallidus and the putamen138. Abnormal shapes of BG structures especially in boys with ASD has been associated with the motor as well as social-communication deficits144.
Corpus callosum
Corpus callosum (CC) contains WM fibers that connect the two hemispheres, and is involved in inter-hemispheric communication145. Decreased CC size in youth with ASD compared to controls has been consistently reported146, 147: reductions have been localized in mid-sagittal area148, anterior corpus callosum149, 150, and body and splenium posteriorly151. Decreased posterior thickness of CC has been reported152. Reduced size of CC has been associated with reduced integration of information153 and slower processing speed154; reduced size of anterior CC may affect connectivity between areas associated with Theory of Mind (ToM) 155. Increased CC volume ASD as compared to non-autistic controls has been reported, especially in those with macrocephaly156; others have not found any difference including a recent study that was adequately powered157, 158.
3. Other areas
Other areas that have been investigated structurally but have shown inconsistencies include, include the hippocampus159-164; the fusiform gyrus involved in face processing165-168; superior temporal gyrus involved in processing of eye movements169-171; left planum temporale involved in auditory processing, which was increased in volume on the left side in ASD in children and adolescents165,172 and decreased in adults172,173; inferior frontal gyrus (Broca's area), with decreased volumes in adults with ASD174; and brainstem175.
B. Diffusion Tensor Imaging
The genetically programmed processes of synaptogenesis and pruning that lead to the development of modular networks, locally as well as regionally, are altered in children with ASD, affecting myelination, thus compromising WM integrity. As a consequence, there may be dysmaturation of the WM characterized by microstructural changes/disorganization176. DTI is a variation of MRI that has been applied to developing brains to study these WM changes – both to study local connectivity as well as long WM tracts and fasciculi that connect regions and lobes177-182. It is based upon the Brownian diffusion of water molecules along the myelinated axons and WM tracts, which may be hampered by crossing fibers. Metrics such as fractional anisotropy (FA), and mean diffusivity (MD) have been used to measure the directionality and the amount of diffusion respectively, in a particular region of interest or at the level of individual voxels. It has been successfully used to confirm anatomical WM findings in ASD, and has yielded rich 3 dimensional maps of the WM circuits and tracts.
DTI has been used to verify the connectivity in ASD based upon the hypothesis that the disconnection in ASD is marked by underconnectivity in distant regions intracortically and corticocortically, with local overconnectivity and predominance of short U (arcuate) fibers within the cortex183, 184. This atypical connectivity should be viewed in context of the structurally and functionally disorganized regional variation in the macrocephalic ASD brain as well as the minicolumnar abnormalities reported in neuropathological studies185-187. A majority of the studies have found reduced FA indicating increased WM microstructural disorganization especially in the frontal and temporal lobe areas188-193 including in young children194; some have found increased MD190, 191. One study found reduced FA in age-matched unaffected siblings as compared to children with ASD, suggesting that it may be a potential marker of genetic risk195. A recent study of school age children196 found widespread increases in MD in many regions of the left hemisphere in children with ASD as compared to TD children, supporting the left hemispheric abnormality/atypical hemispheric dominance that has been hypothesized in ASD for the past three decades197-200.
C. Functional Neuroimaging
Functional MRI (fMRI) has been used to study neural activity and connectivity interregionally, corticocortically and cortico-subcortically. It has been instrumental in establishing ASD as a disorder involving aberrant or diminished functional connectivity and atypical specialization, supporting ASD's characterization as a “disconnection syndrome”15. fMRI has helped correlate neurocognitive abnormalities of ASD with anatomical and functional connections. Both (a) task dependent and (b) resting state fMRI have been used to investigate neurocognitive and behavioral aspects of ASD, yielding information about brain circuitry, which may be dependent upon specific endophenotypes96.
(a) Task dependent fMRI
Task dependent fMRI has been useful in study of deficits that may be region dependent and based upon a specific neurocognitive task such as face processing, emotional processing, attention or executive functions, imitation, sensory perception and processing of auditory or visual information, language functions, motor functions, ToM, and others201. Individuals with ASD show neurocognitive deficits on tasks that may be a reflection of a behavioral dysfunction - hypoactivation or abnormal activation, and involve higher order cognitive functioning processed in association areas rather than the primary cortical areas. fMRI has been used to study aspects of the core features of ASD including social cognition, language deficits and repetitive behaviors (RBs)96.
Possibly, the most well studied of deficits in ASD is the hypoactivation of fusiform gyrus to faces and facial expressions202-207. Findings, though, have been inconsistent, which may be partly due to impaired attention to social cues rather than processing deficits208-210. Reduced interest in interacting with faces may lead to reduced activation of fusiform gyrus; therefore, the lack of social experience may contribute to the hypoactivation.211, 212
Studies of amygdala have also been mixed with wide variability in activation or abnormal/differential activation such as activation to lower faces but not to the whole face203, 211,213-215. The amygdala has been investigated in context of ToM deficits in ASD and it is hypothesized that there may be impaired amygdala modulation than hypoactivation in social contexts106, 107. A recent study used fMRI to study face processing in BAP and found similarities in those with BAP to those in ASD, with hyperactivation in FG and amygdala in individuals with aloof personality vs. hypoactivation in those with non-aloof personality216.
Hypoactivation has been reported in posterior superior temporal sulcus, part of the ventral visuomotor stream, in response to biological motion cues217, 218. Activation of this region may be related to phenotypic expression of social deficits in ASD and may not be a shared genetic liability with unaffected siblings219. Mirror neuron system dysfunction in the inferior frontal gyrus is thought to have a role deficits in mentalizing, empathy and understanding others’ intentions220; it has been found to be hypoactive during imitation, observation of faces221,222 and of emotional expressions223,224.
Communication/language delays and deficits are core features of ASD. As language is a left hemispheric function, delays in language development may be associated with atypical hemispheric lateralization, i.e. decreased left hemispheric dominance225. Others have found reduced synchrony between the language associated areas226, atypical prosody227-229, processing delays230, and recruitment of atypical areas for language processing231, 232.
Although not specific to ASD, RBs in ASD have been associated with dysfunction of the frontostriatal pathways and are thought to reflect atypical cognitive control including response inhibition deficits233, 234 and compensatory/adaptive behaviors in context of sensory deficits235. fMRI studies have used tasks assessing motor control, response inhibition and monitoring, and others. The results have been mixed with both hypoactivation and hyperactivation reported in the frontostriatal pathways, depending upon the task and the analytic methods used236. Notably, genes for RBs are thought to be independent of those influencing social communication and RBs may have a familial inheritance237-239.
Task dependent fMRI has also revealed the presence of functional underconnectivity in frontal-subcortical as well as frontal-posterior networks in ASD240-243. In an fMRI study that used a motor task243, children with ASD, compared to controls, demonstrated diffusely decreased connectivity across the motor execution network including frontal-striatal and frontal-cerebellar. Other studies have revealed “decreased synchronization” or “low bandwidth” between frontal-posterior regions implying decreased connectivity240during a language task242, a task of executive function241, ToM244, working memory245,246, inhibitory control247 and tasks of visuospatial cognition248.
(b) Resting State fMRI
Resting state functional MRI (rs-fMRI) is based upon the finding that even at rest, there is neural activity in disparate brain regions that may be functionally connected249. This activity can be captured by measuring the synchronous fluctuations of blood oxygen level dependent (BOLD) signals in these regions, at rest, without the use of a neurocognitive task250. Rs-fMRI has thereby become an important tool for studying patterns of functional brain connectivity251-253. Various analytic approaches have been applied, leading to new insights, e.g. a recent study found that increased underconnectivity in temporooccipital region was associated with higher symptom severity in adolescents with ASD as compared to underconnectivity in the frontal regions254.
Rs-fMRI has also delineated the Default Mode Network (DMN), a core network which is active when the brain is at rest with nodal regions comprised of ventral medial prefrontal cortex, posterior cingulate cortex, inferior parietal lobule, lateral temporal cortex, dorsomedial prefrontal cortex and hippocampal formation255. Altered functional connectivity has been reported in the DMN in children with ASD256-258 with functional underconnectivity in anterior-posterior connections259. A recent study of a large heterogeneous sample of individuals with ASD, using rs-fMRI, found that the strength of connectivity within and between distinct functional subregions of the precentral gyrus was related to the ASD diagnosis and to the severity of ASD traits260.
Discussion
As described above, there is a wide range of findings in structural and functional neuroimaging studies in ASD, which may indicate potential endophenotypes. Many of these findings, unfortunately, have not been consistent or replicated. Therefore, leveraging these findings from a neuroendophenotype perspective is an endeavor that is still in its conceptual stages. To be clinically meaningful, these findings may require combined efforts of ASD researchers focused on different aspects of basic, genetic, neuroimaging, and clinical research.261 The field is only beginning to scratch the surface, so to speak, of the complex processes that lead to atypical brain development, which may involve nanoscalar aberrations to macroscopic whole brain, regional, lobar and network-wide alterations that occur with the increasing age of the child with ASD. Studies pairing neuroimaging findings with genetics and behavioral findings (imaging genetics), may bridge the genetic complexity of the disorder with the heterogeneity of the phenotypes. Such studies may potentially reveal the endophenotypes in ASD, rooted in the genetic program of the child.
Despite the gains made to date, there are several other areas in ASD research where endophenotype-oriented neuroimaging research can make important contributions. For example, it is imperative that genetic heterogeneity of ASD be taken into account when designing neuroimaging studies. Pairing genetic studies with studies comparing neuroimaging findings in those with syndromic ASD, to those with “idiopathic autism”, common gene variants, or BAP or to TD non-autistic populations may further help correlate the genetic basis to an observed endophenotype.
Elucidating whether the co-occurrence of clinically diagnosable disorders or traits in ASD is true heterotypic comorbidity or if these are distinct endophenotypes that may be indicative of “an autism” is perhaps one of the most pressing areas of investigation. Some disorders, for example, ADHD have a genetic overlap with ASD262-264; occurring in 16% to 78% children with ASD, ADHD is a commonly diagnosed and treated condition in children with ASD265,266, conversely, ASD traits occur in 20% to 50% of children with ADHD267 and their presence may be associated with more impaired functioning268. Yet, in contrast to the trajectory in ASD, children with ADHD have a neurodevelopmental trajectory marked by delayed cortical maturation (and thinner cortices) by several years269. In spite of recent increase in research focused on this issue, it remains to be clarified whether these are co-occurring conditions or if ASD with ADHD is a separate neuroendophenotype of ASD (conversely, whether the presence of ASD traits in ADHD is a separate neuroendophenotype) 270,271. Similarly, anxiety disorders, which co-occur in 40% to 50% of children with ASD, are frequently the target of psychotropic medications272, 273. It remains to be determined whether some anxiety (or forms of anxiety) is (are) a core feature of ASD such as wanting predictability and preference for sameness, and when obsessive-compulsive symptoms should be considered to be beyond merely repetitive and ritualistic behaviors of ASD. One could conjecture that using neuroimaging (for example, studying limbic system connectivity in case of anxiety disorders) to subtype ASD phenotypes with comorbid disorders may help development of more targeted and effective therapies.
This delineation of neuroendophenotypes may also have implications insofar as identifying pretreatment abnormalities, the choice of treatment approaches, monitoring response to psychotropic medications and the outcomes are concerned. Clinical trials tailored to specific subgroups with a particular endophenotype may help with more personalized and effective medication interventions261. Furthermore, subtyping ASD based upon neuroendophenotypes may lead to more informative predictors of psychopathology which could facilitate provision of more appropriate supports for the child with ASD at school, home and community, thus affecting their trajectories of development and consequently, adolescent and adult outcomes.
Despite possible applications, and the explosive increase in the number of studies in ASD over the past two decades, findings from vast majority of the neuroimaging studies have yet to be fully integrated with neuroendophenotype research, which may involve recruiting unaffected relatives of children with ASD, besides the children themselves. Neuroimaging itself is limited by the spatial and temporal limits of the image resolution; although macroscopic level changes have been revealed, by VBM and ROI analyses, visualizing microscopic level changes is still not possible. In ASD, as the neurodevelopmental processes involve cellular level abnormalities, which affect brain development (such as genetically determined distinct pathways leading to increases in surface area and in cortical thickness, and smaller minicolumns), there is a limit to understanding these endophenotypes directly. The challenge to using large-scale neuroimaging in endophenotype studies, is also the prohibitive cost of conducting these studies and recruiting enough non-autistic relatives in addition to the probands, to ensure above adequate statistical power, as most studies so far have had modest-sized cohorts. The cohorts themselves have been high functioning and highly selective and there is a stark lack of population-based studies.
Nevertheless, the current convergent findings lay the groundwork for future research to disentangle the complexities of atypical brain development in children with ASD. The fact that there is increased local, national and global “connectivity” between ASD researchers from disparate fields of research is a positive development that may help further neuroendophenotype research and concomitantly, provide greater insight into the etiopathogenesis of ASD, translating into better therapies.
Conclusions
Endophenotypes are internal biomarkers or traits that represent a gene or a set of genes. Identification of endophenotypes may help further the understanding of the etiology and pathogenesis of a complex, genetically rooted disorder, such as ASD. The wide array of structural and functional neuroimaging findings may represent neural endophenotypes that may be unique to ASD, when paired with research in ASD genetics especially in family based studies and BAP subgroups. Endophenotype oriented neuroimaging research may potentially help with delineating subgroups that may shed light on comorbid disorders in ASD, with monitoring treatment responses or carrying out clinical trials to personalize interventions. Although in its early stages, and despite the technical and practical limitations of neuroimaging, a concerted effort by researchers studying different aspects of ASD may help achieve this goal.
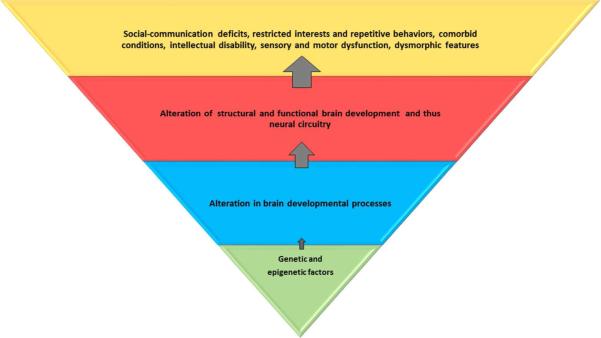
Figure 1. Genetic and epigenetic factors lead to alteration in brain developmental processes at the cellular and microscopic levels.
This affects the global and regional brain structure and function and consequently, the neural circuitry. These alterations result in core and associated behavioral and clinical features of autism spectrum disorder. The blue and red trapezoids represent potential endophenotypes; the red trapezoid can be captured by neuroimaging. Environmental influences may be relevant at all levels.
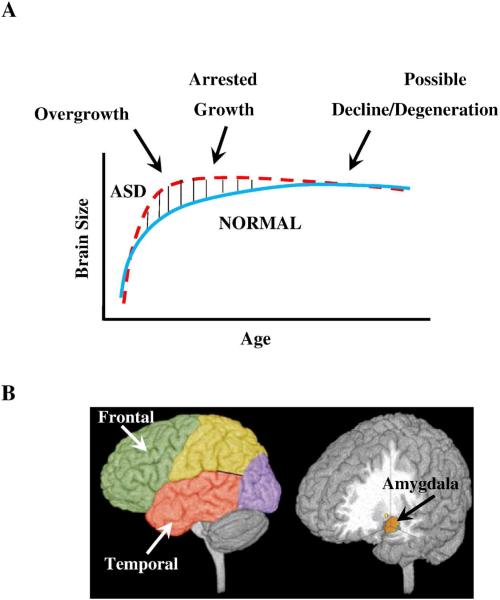
Figure 2. Three phases of growth pathology in autism.
(A) Model of early brain overgrowth in autism that is followed by arrest of growth. Red line represents ASD, while blue line represents age-matched typically developing individuals. In some regions and individuals, the arrest of growth may be followed by degeneration, indicated by the red dashes that slope slightly downward. (B) Sites of regional overgrowth in ASD include frontal and temporal cortices and amygdala (Reproduced from: Brain growth across the life-span in autism: age specific changes in anatomic pathology; Courchesne, Campbell and Solso, Brain Research 1380 (2011)138–145, with permission from Elsevier B.V.)
Table 1.
Summary of notable* neuroimaging findings using magnetic resonance in children and adolescents with autism spectrum disorder delineated by structural MRI (sMRI), diffusion tensor imaging (DTI) and functional MRI (fMRI).
| Structural MRI |
| Cortex and Regional: |
| • Increases in total GM and WM volumes |
| • Dissociation in volumes of cerebral hemispheres and subcortical structures |
| • Frontal-temporal areas GM and WM enlarged; parietal and occipital cortices less often involved |
| • Dorsolateral and medial prefrontal cortex enlargement |
| • Cingulate cortex enlargement |
| • Cortical thickness reflects cortical dysmaturation with atypical regional variation in frontal, temporal and parietal lobes |
| Subcortical Structures: |
| Amygdala |
| • Larger volumes in younger children bilaterally |
| • Trajectory of development of amygdala follows overall trajectory of TBV |
| Cerebellum |
| • Increased total cerebellar volume and GM |
| • Reduced mid-sagittal surface area for vermal lobules VI-VII |
| • Hypoplasia of posterior vermis |
| • Decreased GM in right and left Crus I, lobule VIII and lobule IX |
| Basal Ganglia |
| • Increased volume of caudate |
| • Abnormal shapes of BG structures |
| Corpus callosum |
| • Reduced overall size |
| • Localized reductions in mid-sagittal CC, anterior CC, body and splenium of CC |
| • Increased size of CC (especially in those with macrocephaly) |
| Planum temporale |
| • Increased volume on the left side |
| Diffusion Tensor Imaging |
| Fractional Anisotropy |
| • Reduced FA overall in the whole brain |
| • Reduced FA in frontal lobe regions |
| • Reduced FA in arcuate fasciculus |
| • Reduced FA across entire CC |
| • Reduced FA in anterior thalamic radiation |
| • Increased FA in CC in very young children. |
| • Increased FA and loss of normal lateralization in arcuate fasciculus |
| Mean Diffusivity |
| • Increased MD overall in the whole brain |
| • Increased MD in left hemispheric regions |
| • Increased MD in frontal-temporal regions |
| • Increased MD across entire CC |
| Functional MRI |
| Task dependent fMRI |
| • Hypoactivation of fusiform gyrus to faces and facial expressions |
| • Hypoactivation of posterior superior temporal sulcus in response to biological motion cues. |
| • Hypoactivation of mirror neuron system during imitation, face observation and emotional expression |
| • Atypical language processing on various tasks |
| • Variable activation in frontostriatal pathways for motor control and response inhibition. |
| • Functional underconnectivity in frontal-posterior networks. |
| Resting State fMRI |
| • Altered functional connectivity in the DMN |
| • Functional underconnectivity in the anterior-posterior connections |
GM: gray matter; WM: white matter TBV: total brain volume; BG: basal ganglia; CC: corpus callosum; FA: fractional anisotropy; MD: mean diffusivity; DMN: default mode network.
Please refer to reviews listed in the text for a more exhaustive list.
Acknowledgement
Golda Ginsburg, Ph.D., Professor of Psychiatry and Behavioral Sciences at the University Of Connecticut School Of Medicine, for her comments during the early stages of the manuscript.
• Dr. Mostofsky has received funding from the following grants:
Grant sponsor: NIH/NINDS; Grant number: R01NS048527-08;
Grant sponsor: Autism Speaks Foundation; Grant number: 2506
Biography
Rajneesh Mahajan, M.D. is a faculty pediatric psychiatrist at the Center for Autism and Related Disorders and in the Department of Psychiatry at Kennedy Krieger Institute; he is also an Assistant Professor of Psychiatry in the Department of Psychiatry and Behavioral Sciences at the Johns Hopkins University School of Medicine in Baltimore, Maryland.
Stewart H. Mostofsky, M.D. is a faculty pediatric neurologist, the Batza Family Foundation Research Chair, the Director of the Center for Neurodevelopmental and Imaging Research and the Medical Director of the Center for Autism and Related Disorders at Kennedy Krieger Institute; he is also a Professor in the Department of Neurology, and the Department of Psychiatry and Behavioral Sciences at the Johns Hopkins University School of Medicine, in Baltimore, Maryland.
Footnotes
Disclosure information
• Dr. Mahajan has no biomedical or financial conflicts of interest to declare.
References
- 1.Ruggeri BB, Sarkans UU, Schumann GG, Persico AMA. Biomarkers in autism spectrum disorder: The old and the new. Psychopharmacology (Berl) doi: 10.1007/s00213-013-3290-7. [DOI] [PubMed] [Google Scholar]
- 2.Gottesman, Gould TDT, Gould TDT, Manschreck TCT, Pogue-Geile MFM. The endophenotype concept in psychiatry: Etymology and strategic intentions. Am J Psychiatry. 160(4):636–45. doi: 10.1176/appi.ajp.160.4.636. [DOI] [PubMed] [Google Scholar]
- 3.John BB, Lewis KRK, Caraccio MNM, et al. Chromosome variability and geographic distribution in insects. Science (New York, N.Y.) 152(3723):711–21. doi: 10.1126/science.152.3723.711. [DOI] [PubMed] [Google Scholar]
- 4.Gottesman, Shields JJ. A polygenic theory of schizophrenia. Proc Natl Acad Sci U S A. 58(1):199–205. doi: 10.1073/pnas.58.1.199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Shields JJ, Gottesman Cross-national diagnosis of schizophrenia in twins. The heritability and specificity of schizophrenia. Arch Gen Psychiatry. 27(6):725–30. doi: 10.1001/archpsyc.1972.01750300005001. [DOI] [PubMed] [Google Scholar]
- 6.Cannon TD, Keller MC. Endophenotypes in the genetic analyses of mental disorders. Annual Review of Clinical Psychology. 2:267–290. doi: 10.1146/annurev.clinpsy.2.022305.095232. 267. [DOI] [PubMed] [Google Scholar]
- 7.Viding EE, Blakemore SS. Endophenotype approach to developmental psychopathology: Implications for autism research. Behav Genet. 37(1):51–60. doi: 10.1007/s10519-006-9105-4. [DOI] [PubMed] [Google Scholar]
- 8.Skuse DHD, Skuse DHD. Rethinking the nature of genetic vulnerability to autistic spectrum disorders. Trends in genetics: TIG. 23(8):387–95. doi: 10.1016/j.tig.2007.06.003. [DOI] [PubMed] [Google Scholar]
- 9.DiCicco-Bloom EE, Lord CC, Zwaigenbaum LL, et al. The developmental neurobiology of autism spectrum disorder. The Journal of neuroscience: the official journal of the Society for Neuroscience. 26(26):6897–906. doi: 10.1523/JNEUROSCI.1712-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Kanner L. Autistic disturbances of affective contact. Nervous Child. 1943;2:217–250. [PubMed] [Google Scholar]
- 11.Prevalence of autism spectrum disorder among children aged 8 years - autism and developmental disabilities monitoring network, 11 sites, United States. Morbidity and mortality weekly report. Surveillance summaries (Washington, D.C.: 2002) 2010;63(2):1–21. [PubMed] [Google Scholar]
- 12.Newschaffer CJC, Croen LAL, Daniels JJ, et al. The epidemiology of autism spectrum disorders. Annu Rev Public Health. 28:235–58. doi: 10.1146/annurev.publhealth.28.021406.144007. [DOI] [PubMed] [Google Scholar]
- 13. [1/10/2015];2015 DSM-5 | psychiatry.org. http://www.psychiatry.org/dsm5.
- 14.Wing LL, Gould JJ. Severe impairments of social interaction and associated abnormalities in children: Epidemiology and classification. J Autism Dev Disord. 9(1):11–29. doi: 10.1007/BF01531288. [DOI] [PubMed] [Google Scholar]
- 15.Geschwind DH, Levitt P. Autism spectrum disorders: Developmental disconnection syndromes. Curr Opin Neurobiol. 2007;17(1):103–111. doi: 10.1016/j.conb.2007.01.009. [DOI] [PubMed] [Google Scholar]
- 16.Freitag CM, Staal W, Klauck SM, Duketis E, Waltes R. Genetics of autistic disorders: Review and clinical implications. Eur Child Adolesc Psychiatry. 2010;19(3):169–178. doi: 10.1007/s00787-009-0076-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Lichtenstein Paul, Gillberg Christopher, Carlström Eva, et al. The genetics of autism spectrum disorders and related neuropsychiatric disorders in childhood. American Journal of Psychiatry. 167:1357–1363. doi: 10.1176/appi.ajp.2010.10020223. 1357. [DOI] [PubMed] [Google Scholar]
- 18.Folstein S, Rutter M. Infantile autism: A genetic study of 21 twin pairs. Journal of child psychology and psychiatry, and allied disciplines. 18:297–321. doi: 10.1111/j.1469-7610.1977.tb00443.x. 297. [DOI] [PubMed] [Google Scholar]
- 19.Folstein S, Folstein S, Feuk Lars, et al. Genetic influences and infantile autism. Nature. 265:726–728. doi: 10.1038/265726a0. 726. [DOI] [PubMed] [Google Scholar]
- 20.Folstein SE, Folstein S, Feuk Lars, et al. Autism: Familial aggregation and genetic implications. Journal of Autism and Developmental Disorders. 18:3–30. doi: 10.1007/BF02211815. 3. [DOI] [PubMed] [Google Scholar]
- 21.Bailey AA, Le Couteur AA, Gottesman, et al. Autism as a strongly genetic disorder: Evidence from a British twin study. Psychol Med. 25(1):63–77. doi: 10.1017/s0033291700028099. [DOI] [PubMed] [Google Scholar]
- 22.Bolton PP, Macdonald HH, Pickles AA, et al. A case-control family history study of autism. J Child Psychol Psychiatry. 35(5):877–900. doi: 10.1111/j.1469-7610.1994.tb02300.x. [DOI] [PubMed] [Google Scholar]
- 23.Constantino JNJ, Zhang YY, Frazier TT, et al. Sibling recurrence and the genetic epidemiology of autism. Am J Psychiatry. 167(11):1349–56. doi: 10.1176/appi.ajp.2010.09101470. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Constantino JNJ, Todorov AA, Hilton CC, et al. Autism recurrence in half siblings: Strong support for genetic mechanisms of transmission in ASD. Molecular psychiatry. 18(2):137–8. doi: 10.1038/mp.2012.9. [DOI] [PubMed] [Google Scholar]
- 25.Sumi SS, Taniai HH, Miyachi TT, Tanemura MM, Kishino HH. Sibling risk of pervasive developmental disorder estimated by means of an epidemiologic survey in Nagoya, Japan. J Hum Genet. 51(6):518–22. doi: 10.1007/s10038-006-0392-7. [DOI] [PubMed] [Google Scholar]
- 26.Piven JJ, Palmer PP, Jacobi DD, et al. Broader autism phenotype: Evidence from a family history study of multiple-incidence autism families. Am J Psychiatry. 154(2):185–90. doi: 10.1176/ajp.154.2.185. [DOI] [PubMed] [Google Scholar]
- 27.Constantino JN, Todd RD. Autistic traits in the general population: A twin study. Arch Gen Psychiatry. 60(5):524–530. doi: 10.1001/archpsyc.60.5.524. 524. [DOI] [PubMed] [Google Scholar]
- 28.Constantino JNJ, Todd RDR. Intergenerational transmission of subthreshold autistic traits in the general population. Biol Psychiatry. 57(6):655–60. doi: 10.1016/j.biopsych.2004.12.014. [DOI] [PubMed] [Google Scholar]
- 29.Hoekstra RAR, Bartels MM, Verweij CJHC, Boomsma DID. Heritability of autistic traits in the general population. Arch Pediatr Adolesc Med. 161(4):372–7. doi: 10.1001/archpedi.161.4.372. [DOI] [PubMed] [Google Scholar]
- 30.Geschwind Daniel H., Abrahams Brett S., Herman Edward I., et al. Genetics of autism spectrum disorders. Trends in Cognitive Sciences. 15:409–416. doi: 10.1016/j.tics.2011.07.003. 409. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Schaefer GBG, Mendelsohn NJN. Clinical genetics evaluation in identifying the etiology of autism spectrum disorders: 2013 guideline revisions. Genetics in medicine: official journal of the American College of Medical Genetics. 15(5):399–407. doi: 10.1038/gim.2013.32. [DOI] [PubMed] [Google Scholar]
- 32.Schaefer GBG, Mendelsohn NJN. Clinical genetics evaluation in identifying the etiology of autism spectrum disorders. Genetics in medicine: official journal of the American College of Medical Genetics. 10(4):301–5. doi: 10.1097/GIM.0b013e31816b5cc9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Miles Judith H., George Ian D., Austin Jordan R., et al. Autism spectrum disorders-A genetics review. Genetics in Medicine. 13:278–294. doi: 10.1097/GIM.0b013e3181ff67ba. 278. [DOI] [PubMed] [Google Scholar]
- 34.Sebat J, Lakshmi B, Malhotra D, et al. Strong associations of de novo copy number mutations with autism. Science. 316(5823):445–449. doi: 10.1126/science.1138659. 445. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Michaelson Jacob J., Gujral Madhusudan, Malhotra Dheeraj, et al. Whole-genome sequencing in autism identifies hot spots for de novo germline mutation. Cell. 151:1431–1442. doi: 10.1016/j.cell.2012.11.019. 1431. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Antonio M. Persico, Valerio Napolioni. Autism genetics. Behavioural Brain Research. 251:95–112. doi: 10.1016/j.bbr.2013.06.012. 95. [DOI] [PubMed] [Google Scholar]
- 37.Lee SHS, Ripke SS, Neale BMB, et al. Genetic relationship between five psychiatric disorders estimated from genome-wide SNPs. Nat Genet. 45(9):984–94. doi: 10.1038/ng.2711. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Willsey Jeremy, State Matthew W. Autism spectrum disorders: From genes to neurobiology. Current Opinion in Neurobiology. 30:92–99. doi: 10.1016/j.conb.2014.10.015. 92. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Casey BJ, Casey BJ, Giedd Jay N., et al. Structural and functional brain development and its relation to cognitive development. Biological Psychology. 54:241–257. doi: 10.1016/s0301-0511(00)00058-2. 241. [DOI] [PubMed] [Google Scholar]
- 40.Tau Gregory Z., Peterson Bradley S., Peterson Daniel A., et al. Normal development of brain circuits. Neuropsychopharmacology. 35:147–168. doi: 10.1038/npp.2009.115. 147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Kretschmann HJ, Kammradt G, Krauthausen I, Sauer B, Wingert F. Brain growth in man. Bibliotheca anatomica. :1–26. 1. [PubMed] [Google Scholar]
- 42.Gogtay Nitin, Giedd Jay N., Lusk Leslie, et al. Dynamic mapping of human cortical development during childhood through early adulthood. Proceedings of the National Academy of Sciences of the United States of America. 101:8174–8179. doi: 10.1073/pnas.0402680101. 8174. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Sowell Elizabeth R., Thompson Paul M., Welcome Suzanne E., Kan Eric, Toga Arthur W., Leonard Christiana M. Longitudinal mapping of cortical thickness and brain growth in normal children. Journal of Neuroscience. 24:8223–8231. doi: 10.1523/JNEUROSCI.1798-04.2004. 8223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Mechelli Andrea, Friston Karl J., Frackowiak Richard S., et al. Structural covariance in the human cortex. Journal of Neuroscience. 25:8303–8310. doi: 10.1523/JNEUROSCI.0357-05.2005. 8303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Alexander-Bloch A, Giedd JN, Bullmore E. Imaging structural co-variance between human brain regions. Nature Reviews Neuroscience. 14(5):322. doi: 10.1038/nrn3465. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Li XX, Pu FF, Fan YY, Niu HH, Li SS, Li DD. Age-related changes in brain structural covariance networks. Frontiers in human neuroscience. 7:98. doi: 10.3389/fnhum.2013.00098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Zielinski BA, Gennatas ED, Zhou J, Seeley WW. Network-level structural covariance in the developing brain. PNAS Proceedings of the National Academy of Sciences of the United States of America. 107(42):18191–18196. doi: 10.1073/pnas.1003109107. 18191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Rentería ME, Hansell NK, Strike LT, et al. Genetic architecture of subcortical brain regions: Common and region-specific genetic contributions. Genes, Brain and Behavior. 13:821–830. doi: 10.1111/gbb.12177. 821. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Alexander-Bloch AA, Raznahan AA, Bullmore EE, et al. The convergence of maturational change and structural covariance in human cortical networks. The Journal of neuroscience: the official journal of the Society for Neuroscience. 33(7):2889–99. doi: 10.1523/JNEUROSCI.3554-12.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Stoner RR, Chow MLM, Boyle MPM, et al. Patches of disorganization in the neocortex of children with autism. N Engl J Med. 370(13):1209–19. doi: 10.1056/NEJMoa1307491. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Connors Susan L., Levitt Pat, Matthews Stephen G., et al. Fetal mechanisms in neurodevelopmental disorders. Pediatric Neurology. 38:163–176. doi: 10.1016/j.pediatrneurol.2007.10.009. 163. [DOI] [PubMed] [Google Scholar]
- 52.Courchesne Eric, Redcay Elizabeth, Kennedy Daniel P., et al. The autistic brain: Birth through adulthood. Current Opinion in Neurology. 17:489–496. doi: 10.1097/01.wco.0000137542.14610.b4. 489. [DOI] [PubMed] [Google Scholar]
- 53.Raznahan AA, Toro RR, Daly EE, et al. Cortical anatomy in autism spectrum disorder: An in vivo MRI study on the effect of age. Cerebral cortex (New York, N.Y. 1991;20(6):1332–40. doi: 10.1093/cercor/bhp198. [DOI] [PubMed] [Google Scholar]
- 54.Courchesne Eric, Pierce Karen, Schumann Cynthia M, et al. Brain overgrowth in autism during a critical time in development: Implications for frontal pyramidal neuron and interneuron development and connectivity. International Journal of Developmental Neuroscience. 23:153–170. doi: 10.1016/j.ijdevneu.2005.01.003. 153. [DOI] [PubMed] [Google Scholar]
- 55.Courchesne Eric, Pierce Karen, Courchesne Eric, Hillyard Steven A. Brain overgrowth in autism during a critical time in development: Implications for frontal pyramidal neuron and interneuron development and connectivity. International Journal of Developmental Neuroscience. 23:153–170. doi: 10.1016/j.ijdevneu.2005.01.003. 153. [DOI] [PubMed] [Google Scholar]
- 56.Hazlett Heather Cody, Gu Hongbin, McKinstry Robert C., et al. Brain volume findings in 6-month-old infants at high familial risk for autism. American Journal of Psychiatry. 169:601–608. doi: 10.1176/appi.ajp.2012.11091425. 601. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Hazlett Heather C., Hazlett Heather C., Lightbody Amy A., et al. Early brain development in infants at high risk for autism spectrum disorder. Biological Psychiatry. 73:115S–115S. [Google Scholar]
- 58.Dawson Geraldine, Munson Jeff, Webb Sara Jane, et al. Rate of head growth decelerates and symptoms worsen in the second year of life in autism. Biological Psychiatry. 61:458–464. doi: 10.1016/j.biopsych.2006.07.016. 458. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Zielinski BA, Prigge MBD, Nielsen JA, et al. Longitudinal changes in cortical thickness in autism and typical development. Brain: A Journal of Neurology. 137(6):1799. doi: 10.1093/brain/awu083. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Courchesne EE, Pierce KK. Why the frontal cortex in autism might be talking only to itself: Local over-connectivity but long-distance disconnection. Curr Opin Neurobiol. 15(2):225–30. doi: 10.1016/j.conb.2005.03.001. [DOI] [PubMed] [Google Scholar]
- 61.Casanova Manuel F., Switala E. Andrew, Trippe Juan, et al. The neuropathology of autism. Brain Pathology. 17:422–433. doi: 10.1111/j.1750-3639.2007.00100.x. 422. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Casanova Manuel F., Kooten I. van, Switala AE, et al. Neuropathological and genetic findings in autism: The significance of a putative minicolumnopathy. Neuroscientist. 12:435–441. doi: 10.1177/1073858406290375. 435. [DOI] [PubMed] [Google Scholar]
- 63.Minshew Nancy J., Chakravarti Aravinda, Brune Camille W., et al. The new neurobiology of autism: Cortex, connectivity, and neuronal organization. Archives of Neurology. 64:945–950. doi: 10.1001/archneur.64.7.945. 945. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Courchesne E, Karns CM, Davis HR, et al. Unusual brain growth patterns in early life in patients with autistic disorder: An MRI study. Neurology. 2001;57(2):245–254. doi: 10.1212/wnl.57.2.245. [DOI] [PubMed] [Google Scholar]
- 65.Courchesne E, Carper R, Akshoomoff N. Evidence of brain overgrowth in the first year of life in autism. J Am Med Assoc. 2003;290(3):337–344. doi: 10.1001/jama.290.3.337. [DOI] [PubMed] [Google Scholar]
- 66.Herbert MRM, Ziegler DAD, Deutsch CKC, et al. Dissociations of cerebral cortex, subcortical and cerebral white matter volumes in autistic boys. Brain: a journal of neurology. 126(Pt 5):1182–92. doi: 10.1093/brain/awg110. [DOI] [PubMed] [Google Scholar]
- 67.Williams Charles A., Dagli Aditi, Battaglia Agatino. Genetic disorders associated with macrocephaly. American Journal of Medical Genetics, Part A. 146:2023–2037. doi: 10.1002/ajmg.a.32434. 2023. [DOI] [PubMed] [Google Scholar]
- 68.Chawarska KK, Campbell DD, Chen LL, et al. Early generalized overgrowth in boys with autism. Arch Gen Psychiatry. 68(10):1021–31. doi: 10.1001/archgenpsychiatry.2011.106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Campbell DJ, Chang J, Chawarska K. Early generalized overgrowth in autism spectrum disorder: Prevalence rates, gender effects, and clinical outcomes. Journal of the American Academy of Child & Adolescent Psychiatry. 2014;53(10):1063–1073. e5. doi: 10.1016/j.jaac.2014.07.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Conciatori Monica, Persico Antonio M., Stodgell Christopher J., et al. Association between the HOXA1 A218G polymorphism and increased head circumference in patients with autism. Biological Psychiatry. 55:413–419. doi: 10.1016/j.biopsych.2003.10.005. 413. [DOI] [PubMed] [Google Scholar]
- 71.Muscarella LA, Guarnieri V, Sacco R, et al. HOXA1 gene variants influence head growth rates in humans. American Journal of Medical Genetics Part B: Neuropsychiatric Genetics. 2007;144(3):388–390. doi: 10.1002/ajmg.b.30469. [DOI] [PubMed] [Google Scholar]
- 72.Coon H, Dunn D, Lainhart J, et al. Possible association between autism and variants in the brain-expressed tryptophan hydroxylase gene (TPH2). American Journal of Medical Genetics Part B: Neuropsychiatric Genetics. 2005;135(1):42–46. doi: 10.1002/ajmg.b.30168. [DOI] [PubMed] [Google Scholar]
- 73.Ramoz N, Cai G, Reichert JG, et al. Family-based association study of TPH1 and TPH2 polymorphisms in autism. American Journal of Medical Genetics Part B: Neuropsychiatric Genetics. 2006;141(8):861–867. doi: 10.1002/ajmg.b.30356. [DOI] [PubMed] [Google Scholar]
- 74.Sacco R, Militerni R, Frolli A, et al. Clinical, morphological, and biochemical correlates of head circumference in autism. Biol Psychiatry. 2007;62(9):1038–1047. doi: 10.1016/j.biopsych.2007.04.039. [DOI] [PubMed] [Google Scholar]
- 75.Egawa J, Watanabe Y, Nunokawa A, et al. A detailed association analysis between the tryptophan hydroxylase 2 (TPH2) gene and autism spectrum disorders in a japanese population. Psychiatry Res. 2012;196(2):320–322. doi: 10.1016/j.psychres.2011.09.001. [DOI] [PubMed] [Google Scholar]
- 76.Peculis R, Konrade I, Skapare E, et al. Identification of glyoxalase 1 polymorphisms associated with enzyme activity. Gene. 2013;515(1):140–143. doi: 10.1016/j.gene.2012.11.009. [DOI] [PubMed] [Google Scholar]
- 77.Gabriele S, Lombardi F, Sacco R, et al. The GLO1 C332 (Ala111) allele confers autism vulnerability: Family-based genetic association and functional correlates. J Psychiatr Res. 2014;59:108–116. doi: 10.1016/j.jpsychires.2014.07.021. [DOI] [PubMed] [Google Scholar]
- 78.Varga EA, Pastore M, Prior T, Herman GE, McBride KL. The prevalence of PTEN mutations in a clinical pediatric cohort with autism spectrum disorders, developmental delay, and macrocephaly. Genetics in Medicine. 2009;11(2):111–117. doi: 10.1097/GIM.0b013e31818fd762. [DOI] [PubMed] [Google Scholar]
- 79.Buxbaum JD, Cai G, Chaste P, et al. Mutation screening of the PTEN gene in patients with autism spectrum disorders and macrocephaly. American Journal of Medical Genetics Part B: Neuropsychiatric Genetics. 2007;144(4):484–491. doi: 10.1002/ajmg.b.30493. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.McBride KL, Varga EA, Pastore MT, et al. Confirmation study of PTEN mutations among individuals with autism or developmental delays/mental retardation and macrocephaly. Autism Research. 2010;3(3):137–141. doi: 10.1002/aur.132. [DOI] [PubMed] [Google Scholar]
- 81.Frazier T, Embacher R, Tilot A, Koenig K, Mester J, Eng C. Molecular and phenotypic abnormalities in individuals with germline heterozygous PTEN mutations and autism. Mol Psychiatry. 2014 doi: 10.1038/mp.2014.125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Stevenson RE, Schroer RJ, Skinner C, Fender D, Simensen RJ. Autism and macrocephaly. The Lancet. 1997;349(9067):1744–1745. doi: 10.1016/S0140-6736(05)62956-X. [DOI] [PubMed] [Google Scholar]
- 83.Fidler DJ, Bailey JN, Smalley SL. Macrocephaly in autism and other pervasive developmental disorders. Developmental Medicine & Child Neurology. 2000;42(11):737–740. doi: 10.1017/s0012162200001365. [DOI] [PubMed] [Google Scholar]
- 84.Lainhart JE, Piven J, Wzorek M, et al. Macrocephaly in children and adults with autism. Journal of the American Academy of Child & Adolescent Psychiatry. 1997;36(2):282–290. doi: 10.1097/00004583-199702000-00019. [DOI] [PubMed] [Google Scholar]
- 85.Desikan RS, Ségonne F, Fischl B, et al. An automated labeling system for subdividing the human cerebral cortex on MRI scans into gyral based regions of interest. Neuroimage. 2006;31(3):968–980. doi: 10.1016/j.neuroimage.2006.01.021. [DOI] [PubMed] [Google Scholar]
- 86.Mori S, Oishi K, Jiang H, et al. Stereotaxic white matter atlas based on diffusion tensor imaging in an ICBM template. Neuroimage. 2008;40(2):570–582. doi: 10.1016/j.neuroimage.2007.12.035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Mori S, Oishi K, Faria AV. White matter atlases based on diffusion tensor imaging. Curr Opin Neurol. 2009;22(4):362–369. doi: 10.1097/WCO.0b013e32832d954b. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Wakana S, Jiang H, Nagae-Poetscher LM, Van Zijl PC, Mori S. Fiber tract–based atlas of human white matter anatomy 1. Radiology. 2004;230(1):77–87. doi: 10.1148/radiol.2301021640. [DOI] [PubMed] [Google Scholar]
- 89.Raznahan A, Giedd JN, Bolton PF. The handbook of neuropsychiatric biomarkers, endophenotypes and genes. Springer; 2009. Neurostructural endophenotypes in autism spectrum disorder. pp. 145–169. [Google Scholar]
- 90.Anagnostou E, Taylor MJ. Review of neuroimaging in autism spectrum disorders: What have we learned and where we go from here. Molecular Autism. 2011;2(1):1–9. doi: 10.1186/2040-2392-2-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Jordan I, Murphy D. Update on neuroimaging findings in autism spectrum disorder. Advances in Mental Health and Intellectual Disabilities. 2011;5(6):19–31. [Google Scholar]
- 92.Stigler KA, McDonald BC, Anand A, Saykin AJ, McDougle CJ. Structural and functional magnetic resonance imaging of autism spectrum disorders. Brain Res. 2011;1380:146–161. doi: 10.1016/j.brainres.2010.11.076. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Travers BG, Adluru N, Ennis C, et al. Diffusion tensor imaging in autism spectrum disorder: A review. Autism Research. 2012;5(5):289–313. doi: 10.1002/aur.1243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94. [1/11/2015];Imaging the brain in autism. 2015 http://www.springer.com/biomed/book/978-1-4614-6842-4.
- 95.Barnea-Goraly N, Marzelli MJ. Comprehensive guide to autism. Springer; 2014. Introduction to neuroimaging research in autism spectrum disorders. pp. 893–909. [Google Scholar]
- 96.Dichter GS. Functional magnetic resonance imaging of autism spectrum disorders. Dialogues Clin Neurosci. 2012;14(3):319–351. doi: 10.31887/DCNS.2012.14.3/gdichter. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Goldman-Rakic PS. Development of cortical circuitry and cognitive function. Child Dev. 1987:601–622. [PubMed] [Google Scholar]
- 98.Panizzon MS, Fennema-Notestine C, Eyler LT, et al. Distinct genetic influences on cortical surface area and cortical thickness. Cereb Cortex. 2009;19(11):2728–2735. doi: 10.1093/cercor/bhp026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Hazlett Heather Cody, Gerig Guido, Smith Rachel Gimpel, et al. Magnetic resonance imaging and head circumference study of brain size in autism: Birth through age 2 years. Archives of General Psychiatry. 62:1366–1376. doi: 10.1001/archpsyc.62.12.1366. 1366. [DOI] [PubMed] [Google Scholar]
- 100.Carper RA, Moses P, Tigue ZD, Courchesne E. Cerebral lobes in autism: Early hyperplasia and abnormal age effects. Neuroimage. 2002;16(4):1038–1051. doi: 10.1006/nimg.2002.1099. [DOI] [PubMed] [Google Scholar]
- 101.Schumann CM, Bloss CS, Barnes CC, et al. Longitudinal magnetic resonance imaging study of cortical development through early childhood in autism. J Neurosci. 2010;30(12):4419–4427. doi: 10.1523/JNEUROSCI.5714-09.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Hazlett HC, Poe MD, Gerig G, et al. Early brain overgrowth in autism associated with an increase in cortical surface area before age 2 years. Arch Gen Psychiatry. 2011;68(5):467–476. doi: 10.1001/archgenpsychiatry.2011.39. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Carper RA, Courchesne E. Localized enlargement of the frontal cortex in early autism. Biol Psychiatry. 2005;57(2):126–133. doi: 10.1016/j.biopsych.2004.11.005. [DOI] [PubMed] [Google Scholar]
- 104.Mostofsky SH, Burgess MP, Larson JCG. Increased motor cortex white matter volume predicts motor impairment in autism. Brain: A Journal of Neurology. 2007;130(8):2117–2122. doi: 10.1093/brain/awm129. [DOI] [PubMed] [Google Scholar]
- 105.Lemche E, Giampietro VP, Surguladze SA, et al. Human attachment security is mediated by the amygdala: Evidence from combined fMRI and psychophysiological measures. Hum Brain Mapp. 2006;27(8):623–635. doi: 10.1002/hbm.20206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Baron-Cohen S, Ring HA, Bullmore ET, Wheelwright S, Ashwin C, Williams S. The amygdala theory of autism. Neuroscience & Biobehavioral Reviews. 2000;24(3):355–364. doi: 10.1016/s0149-7634(00)00011-7. [DOI] [PubMed] [Google Scholar]
- 107.Howard MA, Cowell PE, Boucher J, et al. Convergent neuroanatomical and behavioural evidence of an amygdala hypothesis of autism. Neuroreport. 2000;11(13):2931–2935. doi: 10.1097/00001756-200009110-00020. [DOI] [PubMed] [Google Scholar]
- 108.Sweeten TL, Posey DJ, Shekhar A, McDougle CJ. The amygdala and related structures in the pathophysiology of autism. Pharmacology Biochemistry and Behavior. 2002;71(3):449–455. doi: 10.1016/s0091-3057(01)00697-9. [DOI] [PubMed] [Google Scholar]
- 109.Schultz RT. Developmental deficits in social perception in autism: The role of the amygdala and fusiform face area. International Journal of Developmental Neuroscience. 2005;23(2):125–141. doi: 10.1016/j.ijdevneu.2004.12.012. [DOI] [PubMed] [Google Scholar]
- 110.Courchesne E, Pierce K, Schumann CM, et al. Mapping early brain development in autism. Neuron. 2007;56(2):399–413. doi: 10.1016/j.neuron.2007.10.016. [DOI] [PubMed] [Google Scholar]
- 111.Schumann CM, Barnes CC, Lord C, Courchesne E. Amygdala enlargement in toddlers with autism related to severity of social and communication impairments. Biol Psychiatry. 2009;66(10):942–949. doi: 10.1016/j.biopsych.2009.07.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Aylward EH, Minshew NJ, Goldstein G, et al. MRI volumes of amygdala and hippocampus in non-mentally retarded autistic adolescents and adults. Neurology. 1999;53(9):2145–2150. doi: 10.1212/wnl.53.9.2145. [DOI] [PubMed] [Google Scholar]
- 113.Schumann CM, Hamstra J, Goodlin-Jones BL, et al. The amygdala is enlarged in children but not adolescents with autism; the hippocampus is enlarged at all ages. Journal of Neuroscience. 2004;24(28):6392–6401. doi: 10.1523/JNEUROSCI.1297-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Stanfield AC, McIntosh AM, Spencer MD, Philip R, Gaur S, Lawrie SM. Towards a neuroanatomy of autism: A systematic review and meta-analysis of structural magnetic resonance imaging studies. European Psychiatry. 2008;23(4):289–299. doi: 10.1016/j.eurpsy.2007.05.006. [DOI] [PubMed] [Google Scholar]
- 115.Ito M. Control of mental activities by internal models in the cerebellum. Nature Reviews Neuroscience. 2008;9(4):304–313. doi: 10.1038/nrn2332. [DOI] [PubMed] [Google Scholar]
- 116.Strick PL, Dum RP, Fiez JA. Cerebellum and nonmotor function. Annu Rev Neurosci. 2009;32:413–434. doi: 10.1146/annurev.neuro.31.060407.125606. [DOI] [PubMed] [Google Scholar]
- 117.Hodge SM, Makris N, Kennedy DN, et al. Cerebellum, language, and cognition in autism and specific language impairment. J Autism Dev Disord. 2010;40(3):300–316. doi: 10.1007/s10803-009-0872-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Stoodley CJ, Schmahmann JD. Evidence for topographic organization in the cerebellum of motor control versus cognitive and affective processing. Cortex. 2010;46(7):831–844. doi: 10.1016/j.cortex.2009.11.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Piven J, Saliba K, Bailey J, Arndt S. An MRI study of autism: The cerebellum revisited. Neurology. 1997;49(2):546–551. doi: 10.1212/wnl.49.2.546. [DOI] [PubMed] [Google Scholar]
- 120.Hardan AY, Minshew NJ, Harenski K, Keshavan MS. Posterior fossa magnetic resonance imaging in autism. Journal of the American Academy of Child & Adolescent Psychiatry. 2001;40(6):666–672. doi: 10.1097/00004583-200106000-00011. [DOI] [PubMed] [Google Scholar]
- 121.Sparks BF, Friedman SD, Shaw DW, et al. Brain structural abnormalities in young children with autism spectrum disorder. Neurology. 2002;59(2):184–192. doi: 10.1212/wnl.59.2.184. [DOI] [PubMed] [Google Scholar]
- 122.Courchesne E, Yeung-Courchesne R, Hesselink JR, Jernigan TL. Hypoplasia of cerebellar vermal lobules VI and VII in autism. N Engl J Med. 1988;318(21):1349–1354. doi: 10.1056/NEJM198805263182102. [DOI] [PubMed] [Google Scholar]
- 123.Murakami JW, Courchesne E, Press GA, Yeung-Courchesne R, Hesselink JR. Reduced cerebellar hemisphere size and its relationship to vermal hypoplasia in autism. Arch Neurol. 1989;46(6):689–694. doi: 10.1001/archneur.1989.00520420111032. [DOI] [PubMed] [Google Scholar]
- 124.Saitoh O, Courchesne E. Magnetic resonance imaging study of the brain in autism. Psychiatry Clin Neurosci. 1998;52(SUPPL.):S219–S222. doi: 10.1111/j.1440-1819.1998.tb03226.x. [DOI] [PubMed] [Google Scholar]
- 125.Courchesne E, Yeung-Courchesne R, Hesselink J, Jernigan T. Hypoplasia of cerebellar vermal lobules VI and VII in autism. N Engl J Med. 1988;318(21):1349–1354. doi: 10.1056/NEJM198805263182102. [DOI] [PubMed] [Google Scholar]
- 126.Courchesne E, Townsend J, Akshoomoff NA, et al. Impairment in shifting attention in autistic and cerebellar patients. Behav Neurosci. 1994;108(5):848. doi: 10.1037//0735-7044.108.5.848. [DOI] [PubMed] [Google Scholar]
- 127.Carper RA, Courchesne E. Inverse correlation between frontal lobe and cerebellum sizes in children with autism. Brain. 2000;123(Pt 4):836–844. doi: 10.1093/brain/123.4.836. [DOI] [PubMed] [Google Scholar]
- 128.Allen G. The cerebellum in autism. Clinical Neuropsychiatry. 2005;2(6):321–337. [Google Scholar]
- 129.Courchesne E, Campbell K, Solso S. Brain growth across the life span in autism: Age-specific changes in anatomical pathology. Brain Res. 2011;1380:138–145. doi: 10.1016/j.brainres.2010.09.101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.Duerden EG, Mak-Fan KM, Taylor MJ, Roberts SW. Regional differences in grey and white matter in children and adults with autism spectrum disorders: An activation likelihood estimate (ALE) meta-analysis. Autism Research. 2012;5(1):49–66. doi: 10.1002/aur.235. [DOI] [PubMed] [Google Scholar]
- 131.Yu KK, Cheung C, Chua SE, McAlonan GM. Can Asperger syndrome be distinguished from autism? An anatomic likelihood meta-analysis of MRI studies. J Psychiatry Neurosci. 2011;36(6):412–421. doi: 10.1503/jpn.100138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132.Stoodley CJ. Distinct regions of the cerebellum show gray matter decreases in autism, ADHD, and developmental dyslexia. Frontiers in Systems Neuroscience. 2014:8. doi: 10.3389/fnsys.2014.00092. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133.Cleavinger HB, Bigler ED, Johnson JL, Lu J, McMAHON W, Lainhart JE. Quantitative magnetic resonance image analysis of the cerebellum in macrocephalic and normocephalic children and adults with autism. Journal of the International Neuropsychological Society. 2008;14(03):401–413. doi: 10.1017/S1355617708080594. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134.Alexander GE, Crutcher MD. Functional architecture of basal ganglia circuits: Neural substrates of parallel processing. Trends Neurosci. 1990;13(7):266–271. doi: 10.1016/0166-2236(90)90107-l. [DOI] [PubMed] [Google Scholar]
- 135.Middleton FA, Strick PL. Basal ganglia output and cognition: Evidence from anatomical, behavioral, and clinical studies. Brain Cogn. 2000;42(2):183–200. doi: 10.1006/brcg.1999.1099. [DOI] [PubMed] [Google Scholar]
- 136.Bostan AC, Dum RP, Strick PL. Cerebellar networks with the cerebral cortex and basal ganglia. Trends Cogn Sci (Regul Ed) 2013;17(5):241–254. doi: 10.1016/j.tics.2013.03.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137.Utter AA, Basso MA. The basal ganglia: An overview of circuits and function. Neuroscience & Biobehavioral Reviews. 2008;32(3):333–342. doi: 10.1016/j.neubiorev.2006.11.003. [DOI] [PubMed] [Google Scholar]
- 138.Hollander E, Anagnostou E, Chaplin W, et al. Striatal volume on magnetic resonance imaging and repetitive behaviors in autism. Biol Psychiatry. 2005;58(3):226–232. doi: 10.1016/j.biopsych.2005.03.040. [DOI] [PubMed] [Google Scholar]
- 139.Estes A, Shaw DWW, Sparks BF, et al. Basal ganglia morphometry and repetitive behavior in young children with autism spectrum disorder. Autism Research. 2011;4(3):212–220. doi: 10.1002/aur.193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 140.Sears LL, Vest C, Mohamed S, Bailey J, Ranson BJ, Piven J. An MRI study of the basal ganglia in autism. Prog Neuro-Psychopharmacol Biol Psychiatry. 1999;23(4):613–624. doi: 10.1016/s0278-5846(99)00020-2. [DOI] [PubMed] [Google Scholar]
- 141.Voelbel GT, Bates ME, Buckman JF, Pandina G, Hendren RL. Caudate nucleus volume and cognitive performance: Are they related in childhood psychopathology? Biol Psychiatry. 2006;60(9):942–950. doi: 10.1016/j.biopsych.2006.03.071. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 142.Langen M, Durston S, Staal WG, Palmen SJ, van Engeland H. Caudate nucleus is enlarged in high-functioning medication-naive subjects with autism. Biol Psychiatry. 2007;62(3):262–266. doi: 10.1016/j.biopsych.2006.09.040. [DOI] [PubMed] [Google Scholar]
- 143.Hardan AY, Kilpatrick M, Keshavan MS, Minshew NJ. Motor performance and anatomic magnetic resonance imaging (MRI) of the basal ganglia in autism. J Child Neurol. 2003;18(5):317–324. doi: 10.1177/08830738030180050801. [DOI] [PubMed] [Google Scholar]
- 144.Qiu AA, Adler MM, Crocetti DD, Miller MIM, Mostofsky SHS. Basal ganglia shapes predict social, communication, and motor dysfunctions in boys with autism spectrum disorder. J Am Acad Child Adolesc Psychiatry. 49(6):539–51. 551.e1–4. doi: 10.1016/j.jaac.2010.02.012. [DOI] [PubMed] [Google Scholar]
- 145.Gazzaniga MS. Cerebral specialization and interhemispheric communication: Does the corpus callosum enable the human condition? Brain. 2000;123(Pt 7):1293–1326. doi: 10.1093/brain/123.7.1293. [DOI] [PubMed] [Google Scholar]
- 146.Anderson JS, Druzgal TJ, Froehlich A, et al. Decreased interhemispheric functional connectivity in autism. Cereb Cortex. 2011;21(5):1134–1146. doi: 10.1093/cercor/bhq190. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 147.Frazier TW, Hardan AY. A meta-analysis of the corpus callosum in autism. Biol Psychiatry. 2009;66(10):935–941. doi: 10.1016/j.biopsych.2009.07.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 148.Egaas B, Courchesne E, Saitoh O. Reduced size of corpus callosum in autism. Arch Neurol. 1995;52(8):794–801. doi: 10.1001/archneur.1995.00540320070014. [DOI] [PubMed] [Google Scholar]
- 149.Hardan AY, Minshew NJ, Keshavan MS. Corpus callosum size in autism. Neurology. 2000;55(7):1033–1036. doi: 10.1212/wnl.55.7.1033. [DOI] [PubMed] [Google Scholar]
- 150.Waiter GD, Williams JH, Murray AD, Gilchrist A, Perrett DI, Whiten A. Structural white matter deficits in high-functioning individuals with autistic spectrum disorder: A voxel-based investigation. Neuroimage. 2005;24(2):455–461. doi: 10.1016/j.neuroimage.2004.08.049. [DOI] [PubMed] [Google Scholar]
- 151.Piven J, Bailey J, Ranson BJ, Arndt S. An MRI study of the corpus callosum in autism. Am J Psychiatry. 1997;154(8):1051–1056. doi: 10.1176/ajp.154.8.1051. [DOI] [PubMed] [Google Scholar]
- 152.Freitag CM, Luders E, Hulst HE, et al. Total brain volume and corpus callosum size in medication-naive adolescents and young adults with autism spectrum disorder. Biol Psychiatry. 2009;66(4):316–319. doi: 10.1016/j.biopsych.2009.03.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 153.Just MA, Cherkassky VL, Keller TA, Kana RK, Minshew NJ. Functional and anatomical cortical underconnectivity in autism: Evidence from an FMRI study of an executive function task and corpus callosum morphometry. Cereb Cortex. 2007;17(4):951–961. doi: 10.1093/cercor/bhl006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 154.Alexander AL, Lee JE, Lazar M, et al. Diffusion tensor imaging of the corpus callosum in autism. Neuroimage. 2007;34(1):61–73. doi: 10.1016/j.neuroimage.2006.08.032. [DOI] [PubMed] [Google Scholar]
- 155.Mason RA, Williams DL, Kana RK, Minshew N, Just MA. Theory of Mind disruption and recruitment of the right hemisphere during narrative comprehension in autism. Neuropsychologia. 2008;46(1):269–280. doi: 10.1016/j.neuropsychologia.2007.07.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 156.Kilian S, Brown WS, Hallam BJ, et al. Regional callosal morphology in autism and macrocephaly. Dev Neuropsychol. 2007;33(1):74–99. doi: 10.1080/87565640701729821. [DOI] [PubMed] [Google Scholar]
- 157.Rice SA, Bigler ED, Cleavinger HB, et al. Macrocephaly, corpus callosum morphology, and autism. J Child Neurol. 2005;20(1):34–41. doi: 10.1177/08830738050200010601. [DOI] [PubMed] [Google Scholar]
- 158.Lefebvre A, Beggiato A, Bourgeron T, Toro R. Neuroanatomical diversity of corpus callosum and brain volume in the autism brain imaging data exchange (abide) project. bioRxiv. 2014 doi: 10.1016/j.biopsych.2015.02.010. [DOI] [PubMed] [Google Scholar]
- 159.Aylward EH, Minshew NJ, Goldstein G, et al. MRI volumes of amygdala and hippocampus in non-mentally retarded autistic adolescents and adults. Neurology. 1999;53(9):2145–2150. doi: 10.1212/wnl.53.9.2145. [DOI] [PubMed] [Google Scholar]
- 160.Haznedar MM, Buchsbaum MS, Wei T, et al. Limbic circuitry in patients with autism spectrum disorders studied with positron emission tomography and magnetic resonance imaging. Am J Psychiatry. 2000;157(12):1994–2001. doi: 10.1176/appi.ajp.157.12.1994. [DOI] [PubMed] [Google Scholar]
- 161.Bigler ED, Tate DF, Neeley ES, et al. Temporal lobe, autism, and macrocephaly. AJNR Am J Neuroradiol. 2003;24(10):2066–2076. [PMC free article] [PubMed] [Google Scholar]
- 162.Nicolson R, DeVito TJ, Vidal CN, et al. Detection and mapping of hippocampal abnormalities in autism. Psychiatry Research: Neuroimaging. 2006;148(1):11–21. doi: 10.1016/j.pscychresns.2006.02.005. [DOI] [PubMed] [Google Scholar]
- 163.Groen W, Teluij M, Buitelaar J, Tendolkar I. Amygdala and hippocampus enlargement during adolescence in autism. Journal of the American Academy of Child & Adolescent Psychiatry. 2010;49(6):552–560. doi: 10.1016/j.jaac.2009.12.023. [DOI] [PubMed] [Google Scholar]
- 164.Barnea-Goraly N, Frazier TW, Piacenza L, et al. A preliminary longitudinal volumetric MRI study of amygdala and hippocampal volumes in autism. Prog Neuro-Psychopharmacol Biol Psychiatry. 2014;48:124–128. doi: 10.1016/j.pnpbp.2013.09.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 165.Herbert MR, Harris GJ, Adrien KT, et al. Abnormal asymmetry in language association cortex in autism. Ann Neurol. 2002;52(5):588–596. doi: 10.1002/ana.10349. [DOI] [PubMed] [Google Scholar]
- 166.Pierce K, Muller RA, Ambrose J, Allen G, Courchesne E. Face processing occurs outside the fusiform ‘face area’ in autism: Evidence from functional MRI. Brain. 2001;124(Pt 10):2059–2073. doi: 10.1093/brain/124.10.2059. [DOI] [PubMed] [Google Scholar]
- 167.Toal F, Daly E, Page L, et al. Clinical and anatomical heterogeneity in autistic spectrum disorder: A structural MRI study. Psychol Med. 2010;40(07):1171–1181. doi: 10.1017/S0033291709991541. [DOI] [PubMed] [Google Scholar]
- 168.Waiter GD, Williams JH, Murray AD, Gilchrist A, Perrett DI, Whiten A. A voxel-based investigation of brain structure in male adolescents with autistic spectrum disorder. Neuroimage. 2004;22(2):619–625. doi: 10.1016/j.neuroimage.2004.02.029. [DOI] [PubMed] [Google Scholar]
- 169.Levitt JG, Blanton RE, Smalley S, et al. Cortical sulcal maps in autism. Cereb Cortex. 2003;13(7):728–735. doi: 10.1093/cercor/13.7.728. [DOI] [PubMed] [Google Scholar]
- 170.Boddaert N, Chabane N, Gervais H, et al. Superior temporal sulcus anatomical abnormalities in childhood autism: A voxel-based morphometry MRI study. Neuroimage. 2004;23(1):364–369. doi: 10.1016/j.neuroimage.2004.06.016. [DOI] [PubMed] [Google Scholar]
- 171.Zilbovicius M, Meresse I, Chabane N, Brunelle F, Samson Y, Boddaert N. Autism, the superior temporal sulcus and social perception. Trends Neurosci. 2006;29(7):359–366. doi: 10.1016/j.tins.2006.06.004. [DOI] [PubMed] [Google Scholar]
- 172.Rojas DC, Bawn SD, Benkers TL, Reite ML, Rogers SJ. Smaller left hemisphere planum temporale in adults with autistic disorder. Neurosci Lett. 2002;328(3):237–240. doi: 10.1016/s0304-3940(02)00521-9. [DOI] [PubMed] [Google Scholar]
- 173.Rojas DC, Camou SL, Reite ML, Rogers SJ. Planum temporale volume in children and adolescents with autism. J Autism Dev Disord. 2005;35(4):479–486. doi: 10.1007/s10803-005-5038-7. [DOI] [PubMed] [Google Scholar]
- 174.Abell F, Krams M, Ashburner J, et al. The neuroanatomy of autism: A voxel-based whole brain analysis of structural scans. Neuroreport. 1999;10(8):1647–1651. doi: 10.1097/00001756-199906030-00005. [DOI] [PubMed] [Google Scholar]
- 175.Jou RJ, Minshew NJ, Melhem NM, Keshavan MS, Hardan AY. Brainstem volumetric alterations in children with autism. Psychol Med. 2009;39(08):1347–1354. doi: 10.1017/S0033291708004376. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 176.Shukla DK, Keehn B, Müller R. Tract-specific analyses of diffusion tensor imaging show widespread white matter compromise in autism spectrum disorder. Journal of Child Psychology and Psychiatry. 2011;52(3):286–295. doi: 10.1111/j.1469-7610.2010.02342.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 177.Catani M. Diffusion tensor magnetic resonance imaging tractography in cognitive disorders. Curr Opin Neurol. 2006;19(6):599–606. doi: 10.1097/01.wco.0000247610.44106.3f. [DOI] [PubMed] [Google Scholar]
- 178.Alexander AL, Lee JE, Lazar M, Field AS. Diffusion tensor imaging of the brain. Neurotherapeutics. 2007;4(3):316–329. doi: 10.1016/j.nurt.2007.05.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 179.Chanraud S, Zahr N, Sullivan EV, Pfefferbaum A. MR diffusion tensor imaging: A window into white matter integrity of the working brain. Neuropsychol Rev. 2010;20(2):209–225. doi: 10.1007/s11065-010-9129-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 180.Mukherjee P, McKinstry RC. Diffusion tensor imaging and tractography of human brain development. Neuroimaging Clin N Am. 2006;16(1):19–43. doi: 10.1016/j.nic.2005.11.004. [DOI] [PubMed] [Google Scholar]
- 181.White T, Nelson M, Lim KO. Diffusion tensor imaging in psychiatric disorders. Top Magn Reson Imaging. 2008;19(2):97–109. doi: 10.1097/RMR.0b013e3181809f1e. [DOI] [PubMed] [Google Scholar]
- 182.Jou RJ, Jackowski AP, Papademetris X, Rajeevan N, Staib LH, Volkmar FR. Diffusion tensor imaging in autism spectrum disorders: Preliminary evidence of abnormal neural connectivity. Aust N Z J Psychiatry. 2011;45(2):153–162. doi: 10.3109/00048674.2010.534069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 183.Herbert MR, Ziegler DA, Makris N, et al. Localization of white matter volume increase in autism and developmental language disorder. Ann Neurol. 2004;55(4):530–540. doi: 10.1002/ana.20032. [DOI] [PubMed] [Google Scholar]
- 184.Shukla DK, Keehn B, Smylie DM, Müller R- Microstructural abnormalities of short-distance white matter tracts in autism spectrum disorder. Neuropsychologia. 2011;49(5):1378–1382. doi: 10.1016/j.neuropsychologia.2011.02.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 185.Casanova Manuel F., van Kooten Imke A.J., Switala Andrew E., et al. Minicolumnar abnormalities in autism. Acta Neuropathologica. 112:287–303. doi: 10.1007/s00401-006-0085-5. 287. [DOI] [PubMed] [Google Scholar]
- 186.Casanova MF. Intracortical circuitry: One of psychiatry's missing assumptions. European Archives of Psychiatry & Clinical Neuroscience. 254(3):148. doi: 10.1007/s00406-004-0457-6. [DOI] [PubMed] [Google Scholar]
- 187.Buxhoeveden D, Semendeferi K, Buckwalter J, Schenker N, Switzer R, Courchesne E. Reduced minicolumns in the frontal cortex of patients with autism. Neuropathol Appl Neurobiol. 2006;32(5):483–491. doi: 10.1111/j.1365-2990.2006.00745.x. [DOI] [PubMed] [Google Scholar]
- 188.Barnea-Goraly N, Kwon H, Menon V, Eliez S, Lotspeich L, Reiss AL. White matter structure in autism: Preliminary evidence from diffusion tensor imaging. Biol Psychiatry. 2004;55(3):323–326. doi: 10.1016/j.biopsych.2003.10.022. [DOI] [PubMed] [Google Scholar]
- 189.Courchesne E, Pierce K. Why the frontal cortex in autism might be talking only to itself: Local over-connectivity but long-distance disconnection. Curr Opin Neurobiol. 2005;15(2):225–230. doi: 10.1016/j.conb.2005.03.001. [DOI] [PubMed] [Google Scholar]
- 190.Lee JE, Bigler ED, Alexander AL, et al. Diffusion tensor imaging of white matter in the superior temporal gyrus and temporal stem in autism. Neurosci Lett. 2007;424(2):127–132. doi: 10.1016/j.neulet.2007.07.042. [DOI] [PubMed] [Google Scholar]
- 191.Sundaram SK, Kumar A, Makki MI, Behen ME, Chugani HT, Chugani DC. Diffusion tensor imaging of frontal lobe in autism spectrum disorder. Cereb Cortex. 2008;18(11):2659–2665. doi: 10.1093/cercor/bhn031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 192.Kumar A, Sundaram SK, Sivaswamy L, et al. Alterations in frontal lobe tracts and corpus callosum in young children with autism spectrum disorder. Cereb Cortex. 2010;20(9):2103–2113. doi: 10.1093/cercor/bhp278. [DOI] [PubMed] [Google Scholar]
- 193.Shukla DK, Keehn B, Smylie DM, Müller R. Microstructural abnormalities of short-distance white matter tracts in autism spectrum disorder. Neuropsychologia. 2011;49(5):1378–1382. doi: 10.1016/j.neuropsychologia.2011.02.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 194.Ben Bashat D, Kronfeld-Duenias V, Zachor DA, et al. Accelerated maturation of white matter in young children with autism: A high b value DWI study. Neuroimage. 2007;37(1):40–47. doi: 10.1016/j.neuroimage.2007.04.060. [DOI] [PubMed] [Google Scholar]
- 195.Barnea-Goraly N, Lotspeich LJ, Reiss AL. Similar white matter aberrations in children with autism and their unaffected siblings: A diffusion tensor imaging study using tract-based spatial statistics. Arch Gen Psychiatry. 2010;67(10):1052–1060. doi: 10.1001/archgenpsychiatry.2010.123. [DOI] [PubMed] [Google Scholar]
- 196.Peterson D, Mahajan R, Crocetti D, Mejia A, Mostofsky S. Left-Hemispheric microstructural abnormalities in children with High-Functioning autism spectrum disorder. Autism Research. 2014 doi: 10.1002/aur.1413. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 197.Dawson G, Warrenburg S, Fuller P. Cerebral lateralization in individuals diagnosed as autistic in early childhood. Brain Lang. 1982;15(2):353–368. doi: 10.1016/0093-934x(82)90065-7. [DOI] [PubMed] [Google Scholar]
- 198.Dawson G. Lateralized brain dysfunction in autism: Evidence from the halstead-reitan neuropsychological battery. J Autism Dev Disord. 1983;13(3):269–286. doi: 10.1007/BF01531566. [DOI] [PubMed] [Google Scholar]
- 199.Escalante-Mead PR, Minshew NJ, Sweeney JA. Abnormal brain lateralization in high-functioning autism. J Autism Dev Disord. 2003;33(5):539–543. doi: 10.1023/a:1025887713788. [DOI] [PubMed] [Google Scholar]
- 200.Kleinhans NM, Müller R, Cohen DN, Courchesne E. Atypical functional lateralization of language in autism spectrum disorders. Brain Res. 2008;1221:115–125. doi: 10.1016/j.brainres.2008.04.080. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 201. [1/13/2015];Imaging the brain in autism. 2015 http://www.springer.com/biomed/book/978-1-4614-6842-4.
- 202.Hubl D, Bolte S, Feineis-Matthews S, et al. Functional imbalance of visual pathways indicates alternative face processing strategies in autism. Neurology. 2003;61(9):1232–1237. doi: 10.1212/01.wnl.0000091862.22033.1a. [DOI] [PubMed] [Google Scholar]
- 203.Dalton KM, Nacewicz BM, Johnstone T, et al. Gaze fixation and the neural circuitry of face processing in autism. Nat Neurosci. 2005;8(4):519–526. doi: 10.1038/nn1421. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 204.Pelphrey KA, Morris JP, McCarthy G, Labar KS. Perception of dynamic changes in facial affect and identity in autism. Soc Cogn Affect Neurosci. 2007;2(2):140–149. doi: 10.1093/scan/nsm010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 205.Humphreys K, Hasson U, Avidan G, Minshew N, Behrmann M. Cortical patterns of category-selective activation for faces, places and objects in adults with autism. Autism Research. 2008;1(1):52–63. doi: 10.1002/aur.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 206.Klin A. Three things to remember if you are a functional magnetic resonance imaging researcher of face processing in autism spectrum disorders. Biol Psychiatry. 2008;64(7):549–551. doi: 10.1016/j.biopsych.2008.07.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 207.Corbett BA, Carmean V, Ravizza S, et al. A functional and structural study of emotion and face processing in children with autism. Psychiatry Research: Neuroimaging. 2009;173(3):196–205. doi: 10.1016/j.pscychresns.2008.08.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 208.Pierce K, Glad KS, Schreibman L. Social perception in children with autism: An attentional deficit? J Autism Dev Disord. 1997;27(3):265–282. doi: 10.1023/a:1025898314332. [DOI] [PubMed] [Google Scholar]
- 209.Pierce K, Muller RA, Ambrose J, Allen G, Courchesne E. Face processing occurs outside the fusiform ‘face area’ in autism: Evidence from functional MRI. Brain. 2001;124(Pt 10):2059–2073. doi: 10.1093/brain/124.10.2059. [DOI] [PubMed] [Google Scholar]
- 210.Dawson G, Meltzoff AN, Osterling J, Rinaldi J, Brown E. Children with autism fail to orient to naturally occurring social stimuli. J Autism Dev Disord. 1998;28(6):479–485. doi: 10.1023/a:1026043926488. [DOI] [PubMed] [Google Scholar]
- 211.Schultz RT, Grelotti DJ, Klin A, et al. The role of the fusiform face area in social cognition: Implications for the pathobiology of autism. Philos Trans R Soc Lond B Biol Sci. 2003;358(1430):415–427. doi: 10.1098/rstb.2002.1208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 212.Jones W, Carr K, Klin A. Absence of preferential looking to the eyes of approaching adults predicts level of social disability in 2-year-old toddlers with autism spectrum disorder. Arch Gen Psychiatry. 2008;65(8):946–954. doi: 10.1001/archpsyc.65.8.946. [DOI] [PubMed] [Google Scholar]
- 213.Ishitobi M, Kosaka H, Omori M, et al. Differential amygdala response to lower face in patients with autistic spectrum disorders: An fMRI study. Research in Autism Spectrum Disorders. 2011;5(2):910–919. [Google Scholar]
- 214.Paul LK, Corsello C, Tranel D, Adolphs R. Does bilateral damage to the human amygdala produce autistic symptoms? Journal of Neurodevelopmental Disorders. 2010;2(3):165–173. doi: 10.1007/s11689-010-9056-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 215.Aoki Y, Cortese S, Tansella M. Neural bases of atypical emotional face processing in autism: A meta-analysis of fMRI studies. The World Journal of Biological Psychiatry. 2014;(0):1–10. doi: 10.3109/15622975.2014.957719. [DOI] [PubMed] [Google Scholar]
- 216.Yucel Gunes, Parlier Morgan, Adolphs Ralph, et al. Face processing in the broad autism phenotype: An fMRI study. Biological Psychiatry. 67:43S–43S. [Google Scholar]
- 217.Ahmed AA, Vander Wyk BC. Neural processing of intentional biological motion in unaffected siblings of children with autism spectrum disorder: An fMRI study. Brain Cogn. 2013;83(3):297–306. doi: 10.1016/j.bandc.2013.09.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 218.Ciaramidaro A, Bolte S, Schlitt S, et al. Schizophrenia and autism as contrasting minds: Neural evidence for the hypo-hyper-intentionality hypothesis. Schizophr Bull. 2015;41(1):171–179. doi: 10.1093/schbul/sbu124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 219.Kaiser MD, Hudac CM, Shultz S, et al. Neural signatures of autism. Proc Natl Acad Sci U S A. 2010;107(49):21223–21228. doi: 10.1073/pnas.1010412107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 220.Iacoboni M. Imitation, empathy, and mirror neurons. Annu Rev Psychol. 2009;60:653–670. doi: 10.1146/annurev.psych.60.110707.163604. [DOI] [PubMed] [Google Scholar]
- 221.Leslie KR, Johnson-Frey SH, Grafton ST. Functional imaging of face and hand imitation: Towards a motor theory of empathy. Neuroimage. 2004;21(2):601–607. doi: 10.1016/j.neuroimage.2003.09.038. [DOI] [PubMed] [Google Scholar]
- 222.Dapretto M, Davies MS, Pfeifer JH, et al. Understanding emotions in others: Mirror neuron dysfunction in children with autism spectrum disorders. Nat Neurosci. 2005;9(1):28–30. doi: 10.1038/nn1611. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 223.Iacoboni M, Dapretto M. The mirror neuron system and the consequences of its dysfunction. Nature Reviews Neuroscience. 2006;7(12):942–951. doi: 10.1038/nrn2024. [DOI] [PubMed] [Google Scholar]
- 224.Southgate V, Hamilton de C, Antonia F. Unbroken mirrors: Challenging a theory of autism. Trends Cogn Sci (Regul Ed) 2008;12(6):225–229. doi: 10.1016/j.tics.2008.03.005. [DOI] [PubMed] [Google Scholar]
- 225.Kleinhans NM, Müller R, Cohen DN, Courchesne E. Atypical functional lateralization of language in autism spectrum disorders. Brain Res. 2008;1221:115–125. doi: 10.1016/j.brainres.2008.04.080. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 226.Kana RK, Keller TA, Cherkassky VL, Minshew NJ, Just MA. Sentence comprehension in autism: Thinking in pictures with decreased functional connectivity. Brain. 2006;129(Pt 9):2484–2493. doi: 10.1093/brain/awl164. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 227.Wang AT, Lee SS, Sigman M, Dapretto M. Neural basis of irony comprehension in children with autism: The role of prosody and context. Brain. 2006;129(Pt 4):932–943. doi: 10.1093/brain/awl032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 228.Stauder H. Recognition of emotional prosody in autistic children.
- 229.Eigsti I, Schuh J, Mencl E, Schultz RT, Paul R. The neural underpinnings of prosody in autism. Child Neuropsychology. 2012;18(6):600–617. doi: 10.1080/09297049.2011.639757. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 230.Knaus TA, Silver AM, Lindgren KA, Hadjikhani N, Tager-Flusberg H. fMRI activation during a language task in adolescents with ASD. Journal of the International Neuropsychological Society. 2008;14(06):967–979. doi: 10.1017/S1355617708081216. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 231.Eyler LT, Pierce K, Courchesne E. A failure of left temporal cortex to specialize for language is an early emerging and fundamental property of autism. Brain. 2012;135(Pt 3):949–960. doi: 10.1093/brain/awr364. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 232.Mody M, Manoach DS, Guenther FH, et al. Speech and language in autism spectrum disorder: A view through the lens of behavior and brain imaging. Neuropsychiatry. 2013;3(2):223–232. [Google Scholar]
- 233.Mosconi M, Kay M, D'Cruz A, et al. Impaired inhibitory control is associated with higher-order repetitive behaviors in autism spectrum disorders. Psychol Med. 2009;39(09):1559–1566. doi: 10.1017/S0033291708004984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 234.Solomon M, Ozonoff SJ, Ursu S, et al. The neural substrates of cognitive control deficits in autism spectrum disorders. Neuropsychologia. 2009;47(12):2515–2526. doi: 10.1016/j.neuropsychologia.2009.04.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 235.Lewis M, Kim S. The pathophysiology of restricted repetitive behavior. Journal of neurodevelopmental disorders. 2009;1(2):114–132. doi: 10.1007/s11689-009-9019-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 236.Delmonte S, Gallagher L, O'Hanlon E, McGrath J, Balsters JH. Functional and structural connectivity of frontostriatal circuitry in autism spectrum disorder. Frontiers in human neuroscience. 2013;7 doi: 10.3389/fnhum.2013.00430. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 237.Mandy WP, Skuse DH. Research review: What is the association between the social-communication element of autism and repetitive interests, behaviours and activities? Journal of Child Psychology and Psychiatry. 2008;49(8):795–808. doi: 10.1111/j.1469-7610.2008.01911.x. [DOI] [PubMed] [Google Scholar]
- 238.Ronald A, Happé F, Bolton P, et al. Genetic heterogeneity between the three components of the autism spectrum: A twin study. Journal of the American Academy of Child & Adolescent Psychiatry. 2006;45(6):691–699. doi: 10.1097/01.chi.0000215325.13058.9d. [DOI] [PubMed] [Google Scholar]
- 239.Silverman JM, Smith CJ, Schmeidler J, et al. Symptom domains in autism and related conditions: Evidence for familiality. Am J Med Genet. 2002;114(1):64–73. doi: 10.1002/ajmg.10048. [DOI] [PubMed] [Google Scholar]
- 240.Just MA, Keller TA, Malave VL, Kana RK, Varma S. Autism as a neural systems disorder: A theory of frontal-posterior underconnectivity. Neuroscience & Biobehavioral Reviews. 2012;36(4):1292–1313. doi: 10.1016/j.neubiorev.2012.02.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 241.Just MA, Cherkassky VL, Keller TA, Kana RK, Minshew NJ. Functional and anatomical cortical underconnectivity in autism: Evidence from an FMRI study of an executive function task and corpus callosum morphometry. Cereb Cortex. 2007;17(4):951–961. doi: 10.1093/cercor/bhl006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 242.Just MA, Cherkassky VL, Keller TA, Minshew NJ. Cortical activation and synchronization during sentence comprehension in high-functioning autism: Evidence of underconnectivity. Brain. 2004;127(Pt 8):1811–1821. doi: 10.1093/brain/awh199. [DOI] [PubMed] [Google Scholar]
- 243.Mostofsky SH, Powell SK, Simmonds DJ, Goldberg MC, Caffo B, Pekar JJ. Decreased connectivity and cerebellar activity in autism during motor task performance. Brain. 2009;132(Pt 9):2413–2425. doi: 10.1093/brain/awp088. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 244.Kana RK, Keller TA, Cherkassky VL, Minshew NJ, Just MA. Atypical frontal-posterior synchronization of theory of mind regions in autism during mental state attribution. Social Neuroscience. 2009;4(2):135–152. doi: 10.1080/17470910802198510. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 245.Koshino H, Carpenter PA, Minshew NJ, Cherkassky VL, Keller TA, Just MA. Functional connectivity in an fMRI working memory task in high-functioning autism. Neuroimage. 2005;24(3):810–821. doi: 10.1016/j.neuroimage.2004.09.028. [DOI] [PubMed] [Google Scholar]
- 246.Koshino H, Kana RK, Keller TA, Cherkassky VL, Minshew NJ, Just MA. fMRI investigation of working memory for faces in autism: Visual coding and underconnectivity with frontal areas. Cereb Cortex. 2008;18(2):289–300. doi: 10.1093/cercor/bhm054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 247.Kana RK, Keller TA, Minshew NJ, Just MA. Inhibitory control in high-functioning autism: Decreased activation and underconnectivity in inhibition networks. Biol Psychiatry. 2007;62(3):198–206. doi: 10.1016/j.biopsych.2006.08.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 248.Damarla SR, Keller TA, Kana RK, et al. Cortical underconnectivity coupled with preserved visuospatial cognition in autism: Evidence from an fMRI study of an embedded figures task. Autism Research. 2010;3(5):273–279. doi: 10.1002/aur.153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 249.Biswal B, Zerrin Yetkin F, Haughton VM, Hyde JS. Functional connectivity in the motor cortex of resting human brain using echo-planar mri. Magnetic resonance in medicine. 1995;34(4):537–541. doi: 10.1002/mrm.1910340409. [DOI] [PubMed] [Google Scholar]
- 250.Huettel SA, Song AW, McCarthy G. Functional magnetic resonance imaging. Vol. 1. Sinauer Associates; Sunderland, MA: 2004. [Google Scholar]
- 251.Van Den Heuvel, Martijn P, Hulshoff Pol HE. Exploring the brain network: A review on resting-state fMRI functional connectivity. European Neuropsychopharmacology. 2010;20(8):519–534. doi: 10.1016/j.euroneuro.2010.03.008. [DOI] [PubMed] [Google Scholar]
- 252.Van Dijk KR, Hedden T, Venkataraman A, Evans KC, Lazar SW, Buckner RL. Intrinsic functional connectivity as a tool for human connectomics: Theory, properties, and optimization. J Neurophysiol. 2010;103(1):297–321. doi: 10.1152/jn.00783.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 253.Uddin LQ. The self in autism: An emerging view from neuroimaging. Neurocase (Psychology Press) 17(3):201. doi: 10.1080/13554794.2010.509320. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 254.Keown CL, Shih P, Nair A, Peterson N, Mulvey ME, Müller R. Local functional overconnectivity in posterior brain regions is associated with symptom severity in autism spectrum disorders. Cell reports. 2013;5(3):567–572. doi: 10.1016/j.celrep.2013.10.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 255.Buckner RL, Andrews-Hanna JR, Schacter DL. The brain's default network. Ann N Y Acad Sci. 2008;1124(1):1–38. doi: 10.1196/annals.1440.011. [DOI] [PubMed] [Google Scholar]
- 256.Assaf M, Jagannathan K, Calhoun VD, et al. Abnormal functional connectivity of default mode sub-networks in autism spectrum disorder patients. Neuroimage. 2010;53(1):247–256. doi: 10.1016/j.neuroimage.2010.05.067. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 257.Uddin LQ, Supekar K, Menon V. Typical and atypical development of functional human brain networks: Insights from resting-state FMRI. Frontiers in systems neuroscience. 2010;4 doi: 10.3389/fnsys.2010.00021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 258.Supekar K, Uddin LQ, Prater K, Amin H, Greicius MD, Menon V. Development of functional and structural connectivity within the default mode network in young children. Neuroimage. 2010;52(1):290–301. doi: 10.1016/j.neuroimage.2010.04.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 259.Cherkassky VL, Kana RK, Keller TA, Just MA. Functional connectivity in a baseline resting-state network in autism. Neuroreport. 2006;17(16):1687–1690. doi: 10.1097/01.wnr.0000239956.45448.4c. [DOI] [PubMed] [Google Scholar]
- 260.Nebel MB, Joel SE, Muschelli J, et al. Disruption of functional organization within the primary motor cortex in children with autism. Hum Brain Mapp. 2014;35(2):567–580. doi: 10.1002/hbm.22188. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 261.Ecker CC, Murphy DD. Neuroimaging in autism-from basic science to translational research. Nature reviews.Neurology. 10(2):82–91. doi: 10.1038/nrneurol.2013.276. [DOI] [PubMed] [Google Scholar]
- 262.Reiersen AM, Todorov AA. Association between DRD4 genotype and autistic symptoms in DSM-IV ADHD. Journal of the Canadian Academy of Child & Adolescent Psychiatry. 20(1):15. [PMC free article] [PubMed] [Google Scholar]
- 263.Ronald A, Simonoff E, Kuntsi J, Asherson P, Plomin R. Evidence for overlapping genetic influences on autistic and ADHD behaviours in a community twin sample. Journal of Child Psychology and Psychiatry. 2008;49(5):535–542. doi: 10.1111/j.1469-7610.2007.01857.x. [DOI] [PubMed] [Google Scholar]
- 264.Reiersen AM, Constantino JN, Volk HE, Todd RD. Autistic traits in a population-based ADHD twin sample. Journal of Child Psychology & Psychiatry. 48(5):464. doi: 10.1111/j.1469-7610.2006.01720.x. [DOI] [PubMed] [Google Scholar]
- 265.Murray MJ. Attention-deficit/hyperactivity disorder in the context of autism spectrum disorders. Curr Psychiatry Rep. 2010;12(5):382–388. doi: 10.1007/s11920-010-0145-3. [DOI] [PubMed] [Google Scholar]
- 266.Hanson E, Cerban B, Slater C, Caccamo L, Bacic J, Chan E. Brief report: Prevalence of attention deficit/hyperactivity disorder among individuals with an autism spectrum disorder. Journal of Autism & Developmental Disorders. 2013;43(6):1459–1464. doi: 10.1007/s10803-012-1677-7. [DOI] [PubMed] [Google Scholar]
- 267.Rommelse NN, Franke B, Geurts HM, Hartman CA, Buitelaar JK. Shared heritability of attention-deficit/hyperactivity disorder and autism spectrum disorder. Eur Child Adolesc Psychiatry. 2010;19(3):281–295. doi: 10.1007/s00787-010-0092-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 268.Kotte A, Joshi G, Fried R, et al. Autistic traits in children with and without ADHD. Pediatrics. 2013;132(3):e612–22. doi: 10.1542/peds.2012-3947. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 269.Shaw P, Eckstrand K, Sharp W, et al. Attention-deficit/hyperactivity disorder is characterized by a delay in cortical maturation. Proc Natl Acad Sci U S A. 2007;104(49):19649–19654. doi: 10.1073/pnas.0707741104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 270.Rommelse NNJ, Geurts HM, Franke B, Buitelaar JK, Hartman CA. A review on cognitive and brain endophenotypes that may be common in autism spectrum disorder and attention-deficit/hyperactivity disorder and facilitate the search for pleiotropic genes. Neuroscience & Biobehavioral Reviews. 2011;35(6):1363–1396. doi: 10.1016/j.neubiorev.2011.02.015. [DOI] [PubMed] [Google Scholar]
- 271.Gargaro BA, Rinehart NJ, Bradshaw JL, Tonge BJ, Sheppard DM. Autism and ADHD: How far have we come in the comorbidity debate? Neuroscience & Biobehavioral Reviews. 2011;35(5):1081–1088. doi: 10.1016/j.neubiorev.2010.11.002. [DOI] [PubMed] [Google Scholar]
- 272.White SW, Schry AR, Maddox BB. Brief report: The assessment of anxiety in high-functioning adolescents with autism spectrum disorder. Journal of Autism and Developmental Disorders. 2012;42(6):1138–1145. doi: 10.1007/s10803-011-1353-3. [DOI] [PubMed] [Google Scholar]
- 273.Vasa RA, Carroll LM, Nozzolillo AA, et al. A systematic review of treatments for anxiety in youth with autism spectrum disorders. J Autism Dev Disord. 2014;44(12):3215–3229. doi: 10.1007/s10803-014-2184-9. [DOI] [PubMed] [Google Scholar]