Abstract
Interaction with social peers may increase rates of drug self-administration, but a recent study from our laboratory showed that social interaction may serve as a type of alternative reward that competes with drug taking in adolescent male rats. Based on those previous results, the current study examined sex differences in preference for social interaction compared to amphetamine (AMPH) in adolescent rats using the conditioned place preference (CPP) paradigm. Similar to previous results with males, females showed AMPH CPP regardless whether they were individual- or pair-housed. In contrast to males, however, females failed to show social CPP, and they did not prefer a peer-associated compartment over an AMPH-associated compartment in a free-choice test. In separate experiments, dopamine (DA) and serotonin (5-HT) metabolite levels were measured in adolescent males and females that were exposed acutely to peer interaction, no peer interaction, AMPH, or saline. In amygdala, levels of the DA metabolite dihydroxyphenylacetic acid (DOPAC) were altered more in response to peer interaction in males than females; in contrast, there was a greater amygdala DOPAC response to AMPH in females. Furthermore, there were greater changes in the 5-HT metabolite 5-HIAA in females than in males following social interaction. These results indicate that the ability of peer interactions to reduce drug reward is greater in adolescent males than females, perhaps due to a greater ability of social cues to activate limbic reward mechanisms in males or a greater ability of AMPH cues to activate limbic reward mechanisms in females.
Keywords: amphetamine, monoamines, social interaction, adolescents, sex differences
Adolescence is a developmental period characterized by increased novelty-seeking (Spear, 2000); as a consequence, adolescents are most likely to try addictive drugs for the first time during their teenage years. Another defining feature of adolescence is a transition from spending time with parents to spending time with peers, a transition that occurs with many mammalian species (Spear, 2000). The nature of these peer relationships helps determine an individual’s propensity to initiate drug use. In fact, a major predictor of teenage drug use is negative social influence (Bahr, Hoffmann, & Yang, 2005; Branstetter, Low, & Furman, 2011).
Numerous animal studies suggest that interactions with drug-treated age-matched conspecifics increases drug intake and conditioned place preference (CPP) across a wide range of drug classes, including opioids (Cole, Hofford, Evert, Wellman, & Eitan, 2013; Kennedy, Panksepp, Runckel, & Lahvis, 2012), stimulants (Watanabe, 2011), and ethanol (Hunt, Holloway, & Scordalakes, 2001; Logue, Chein, Gould, Holliday, & Steinberg, 2014), with the effect being most robust among adolescent male rodents (Cole et al., 2013; Hunt et al., 2001; Kennedy et al., 2012; Logue et al., 2014). For example, in young adults that are trained individually to self-administer amphetamine (AMPH, 0.1 mg/kg/infusion) to a stable rate, introduction of a social partner in an adjacent chamber produces a temporary increase in self-administration (Gipson et al., 2011). In addition, when housed in adjacent operant conditioning chambers, acquisition of drug self-administration is enhanced when both peers are allowed to self-administer, whereas acquisition is reduced when only one peer is allowed to self-administer (Smith, 2012). These preclinical studies suggest that the drug experience of social peers can moderate drug taking behavior.
Evidence from humans suggests that having healthy non-drug peer relationships is protective during adolescence (Forster, Grigsby, Bunyan, Unger, & Valente, 2015). Similar to humans, rodents engage in more social behaviors during adolescence (Spear, 2000). While social play fighting during this developmental period tends to be greater in males than in females (Olioff & Stewart, 1978), not all studies have observed a sex difference (Veenema, Bredewold, & De Vries, 2013). Regardless of any sex differences, however, adolescent rats find social interaction more rewarding than adults. For example, rats maintained in individual or group housing conditions show a preference for a peer-associated CPP compartment compared to a control compartment (no peer association), and this CPP is stronger for adolescent males compared to either adolescent females or adult males (Douglas, Varlinskaya, & Spear, 2004). The preference for a peer-paired compartment also is able to negate the ability of cocaine to induce CPP in adults (Zernig, Kummer, & Prast, 2013). In a recent study from our laboratory, we showed further that individually-housed adolescent males given a choice between an AMPH-paired and peer-paired compartment preferred the peer-paired chamber, whereas adult males did not (Yates, Beckmann, Meyer, & Bardo, 2013). Thus, adolescent males appear to be most sensitive to social reward, and this reward is able to compete with drug-related cues in a free-choice test.
Most studies comparing adolescent to adult social reward have been conducted in males. The same is true for experiments regarding the neurobiology of social interaction and social reward. Studies using the cellular activity marker c-fos demonstrate that nucleus accumbens (NAcc), dorsal striatum (Str), medial prefrontal cortex (mPFC), and amygdala (Amyg) are likely involved in social reward (Gordon, Kollack-Walker, Akil, & Panksepp, 2002; van Kerkhof et al., 2014). These areas all receive input from both dopamine (DA) and serotonin (5-HT) projections, with 5-HT-signaling in NAcc being especially important in social reward (Dolen, Darvishzadeh, Huang, & Malenka, 2013). However, some of the neural circuitry involved in social activity differs between males and females. For example, hypothalamus (Hypo), bed nucleus of stria terminalis (BNST) and lateral septum (LS) differentially modulate social play in adolescent males and females (Paul et al., 2014; Veenema et al., 2013).
We have shown previously that peer-associated cues are preferred over AMPH-related cues when adolescent males are individually-housed, but not when pair-housed (Yates et al., 2013). The main purpose of this study was to determine if a similar effect is observed in individually- and pair-housed adolescent females. Moreover, given the role of DA and 5-HT systems in drug and social reward, the current study also determined if there is a differential activation of DA and 5-HT systems in male and female adolescents exposed to AMPH or a social peer. Activation of these monoamine systems was estimated by measuring DA and 5-HT metabolite levels in various brain regions relevant to AMPH and/or social reward, including NAcc, Str, mPFC, Amyg, Hypo and midbrain (MB).
Methods
Animals
Sprague-Dawley rats (32 male and 66 female total) were used in three separate experiments. Experiments 1–3 each used 12 females; Experiments 4 and 5 used 32 rats each (16 male and 16 female). Animals arrived from Harlan Industries (Indianapolis, IN) at 21 days of age. Upon arrival, rats were housed in a colony room that was on a 12-hr light-dark cycle, and all experimentation occurred during the light cycle. In Experiments 1–3, one half of the females was individually housed and the other half was pair-housed; in Experiments 4 and 5, males and females were individually-housed. Rats had unlimited access to food and water in their home cage and were cared for in accordance with the National Institutes of Health Guide for the Care and Use of Laboratory Animals (8th edition, 2011). Experimental protocols were approved by the Institutional Animal Care and Use Committee at the University of Kentucky.
Apparatus
A 3-compartment CPP apparatus (68 × 21 × 21cm; ENV-013; Med Associates, St. Albans, VT) located inside a sound-attenuating chamber (ENV-020 M; Med Associates) was used for all experiments. Sliding guillotine doors separated the three compartments. The middle compartment (12 × 21 × 21 cm) had gray walls with a smooth gray PVC floor. The end compartments (28 × 21 × 21 cm) provided distinct contexts. One compartment had black walls with a stainless steel grid floor, and the other compartment had white walls with a stainless steel mesh floor. A computer controlled the experimental trial using Med-IV software. A series of infrared photobeams were used to detect the rats’ presence in a particular compartment and record the amount of time spent in each compartment.
Procedure
Experiment 1 (AMPH CPP)
Adolescent females were tested for AMPH CPP using the same 10-day procedure described previously for individually- and paired-housed adolescent males in our laboratory (Yates et al., 2013). On day 1 (pre-conditioning test), the guillotine doors were opened, and rats were placed in the center gray compartment and were allowed to explore all three compartments for 15 min. The duration spent in each compartment was recorded. Following the pre-conditioning test, rats went through 8 days of conditioning (days 2–9) in which rats were confined by the guillotine door to either the black or white compartment. On every other day, each rat was given an injection of AMPH (1.0 mg/kg, s.c.) and was placed immediately into either the white or black (counterbalanced) compartment. On alternating days, each rat received saline (s.c.) and was placed immediately into the opposite compartment. On the post-conditioning test (day 10), each rat was placed in the center gray compartment with the guillotine doors open and was allowed to explore all three compartments for 15 min. The time spent in each compartment was recorded
Experiment 2 (Social CPP)
The pre- and post-conditioning sessions were similar to that described for Exp 1. However, for conditioning days 2–9, each rat was given an injection of saline (s.c) and was placed immediately into either the white or black compartment (counterbalanced) that contained a weight-, and age-matched female peer. On alternating days, each rat was given an injection of saline and placed immediately into the opposite compartment without any peer. For paired-housed females, the peer was not the cage mate and all rats received the same peer during each conditioning session.
Experiment 3 (AMPH vs. Social CPP)
The pre- and post-conditioning sessions were similar to that described for Exp 1. However, for conditioning days 2–9, each rat was given an injection of saline (s.c.) and was allowed social interaction in the white or black compartment for 30 min every other day. On alternating days, each rat received AMPH (1.0 mg/kg; s.c.) and was placed immediately into the opposite compartment. The compartment in which rats received AMPH or peer interaction was counterbalanced (i.e., unbiased) and the order in which rats received AMPH or social interaction was counterbalanced within each group.
Experiment 4 (Neurochemical effect of AMPH)
At 28 days of age, male and female rats were placed individually in one end compartment (counterbalanced across rats) for 30 min in order to habituate them to the compartment. On the following day, male and female rats were injected with AMPH (1 mg/kg; s.c.) or saline (1 ml/kg) and placed individually in the same compartment as the previous session for 30 min. Within 5 min following the session, rats were killed by rapid decapitation. Brains were extracted and areas of interest (NAcc, Str, mPFC, Amyg, Hypo, MB) were dissected on an ice-cold glass plate. Each tissue sample was stored in 0.1 N HClO4 (at a concentration of 0.1 g/ml) at −80 °C until the assay was performed.
Experiment 5 (Neurochemical effect of peer interaction)
At 28 days of age, male and female rats were placed individually in one end compartment (counterbalanced across rats) for 30 min to habituate them to the compartment. On the following day, rats were placed in the same compartment as the previous session, either individually or with a same-sex peer for 30 min. Within 5 min following the session, rats were killed and brains dissected as described in Experiment 4.
Assay Method
Tissue samples were thawed, sonicated, and then centrifuged for 15 min at 4 °C. Supernatants were placed on ice and samples were analyzed for DA, 3,4-dihydroxyphenylacetic acid (DOPAC), 5-HT, and 5-hydroxyindoleacetic acid (5-HIAA) using high-performance liquid chromatography with electrochemical detection (HPLC-EC) using a 150 × 3.2 mm analytical column (BetaBasic-18 column, Keystone Scientific, Bellefonte, PA, USA). The computer-controlled HPLC-EC system consisted of a solvent delivery pump (ESA model 582), an ESA 542 HPLC autosampler, and a Coulochem III 5200A electrochemical detector equipped with an ESA 5014B analytical cell and a 5020 guard cell. The guard cell was set at 350 mV, electrode 1 at −150 mV, and electrode 2 at 220 mV. The mobile phase consisted of 75 mM NaH2PO4, 1.7 nM 1-octanesulfonic acid, 25 µM EDTA, 100 µL/L triethylamine and 10% acetonitrile (pH 3.0, adjusted with phosphoric acid; flow rate was 0.5 ml/min). Samples (20 µl) were loaded into a 100 µl loop by an autosampler and peaks were compared with external standards using an ESA Chromatography Data System (EZChrom Elite; ESA, Chelmsford, MA, USA).
Drug
d-Amphetamine sulfate (Sigma, St. Louis, MO) was prepared in sterile 0.9% NaCl (saline) and was injected subcutaneously in a volume of 1 ml/kg. The dose was calculated based on the salt weight.
Statistical Analyses
For Experiments 1–3, initial preferences for the white or black compartment were analyzed with t-tests to compare absolute durations spent in each compartment for individually- and pair-housed groups. For the post-conditioning preferences, a preference ratio was calculated by dividing the amount of time spent in the compartment paired with AMPH (Exp 1) or peer (Exps 2 and 3) by the total time spent in both the white and black end compartments. A preference ratio of 0.5 indicated no preference for either compartment. In Experiment 1, a preference ratio above 0.5 designating a preference for AMPH; in Experiment 2, a preference ratio above 0.5 indicated a preference for peer. In Experiment 3, a preference ratio above 0.5 indicated a preference for peer and ratio below 0.5 designated a preference for AMPH. One-sample t tests were performed to determine if each preference ratio was significantly different from 0.5. All tests were considered significant at p < 0.05.
For Experiments 4 and 5, ANOVAs were conducted for DA, DOPAC, 5-HT, and 5-HIAA values (µg/g tissue wet weight) with brain region as a within-subjects factor, and sex and treatment (AMPH vs. saline; peer vs. no peer) as between-subjects factors. This was followed by individual ANOVAs for each region, with sex and treatment as between-subjects factors. Significant interactions were probed with a Student’s t test. All tests were considered significant at p < 0.05.
Results
Experiment 1 (AMPH CPP)
Pre-conditioning preference scores revealed no compartment bias in individually housed adolescent females, but there was a significant compartment bias towards the white chamber in pair housed rats (t(10) = 6.45, p < 0.001; see Figure 1a). After conditioning, preference ratios showed a significant preference for the AMPH-paired compartment over the saline-paired compartment in both individually-housed (t(5) = 16.43, p < 0.0001) and pair-housed (t(5) = 4.94, p < 0.01) adolescent females (see Figure 2a).
Figure 1.
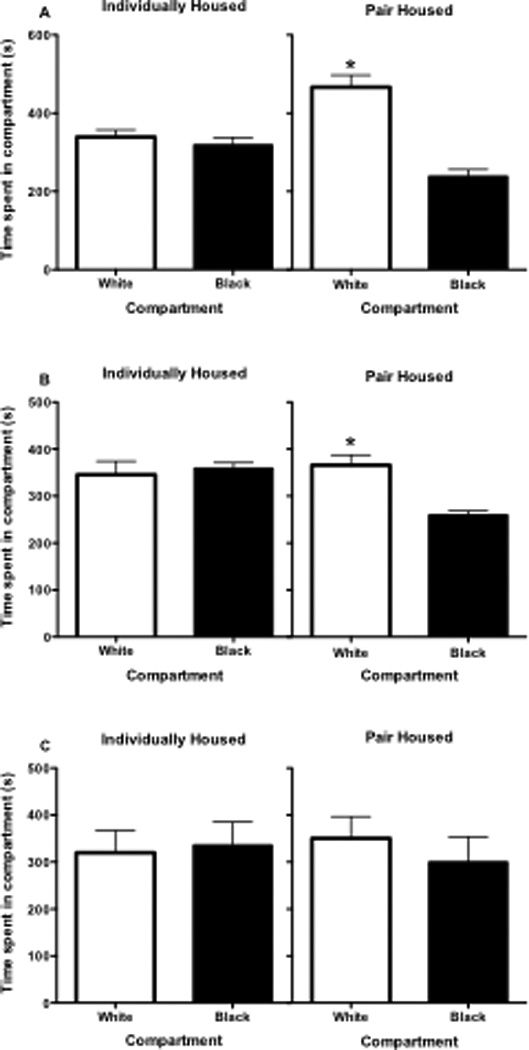
Time spent in white and black compartments of CPP chamber for individuality- and pair-housed females during pre-conditioning test in Experiment 1 (Panel A), Experiment 2 (Panel B) and Experiment 3 (Panel C). In all three panel figures, bar represents mean (±SEM) for time (sec) spent in compartment. Asterisk (*) represents significantly difference compared to time spent in black compartment, p<0.05.
Figure 2.
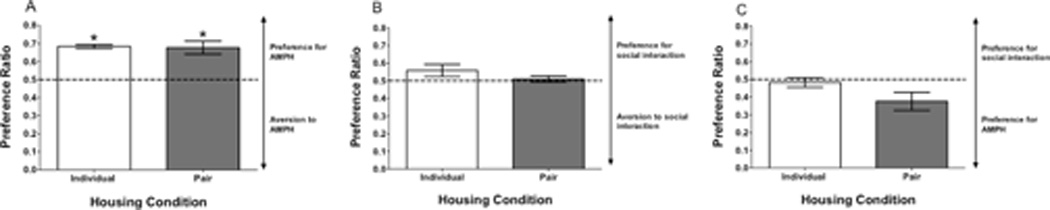
(A) Preference ratio for individually- and pair-housed females following AMPH in Experiment 1. (B) Preference ratio for individually- and pair-housed females following social peer interaction in Experiment 2. (C) Preference ratio for individually- and pair-housed females following AMPH vs. social peer interaction in Experiment 3. In all three figures, bar represents mean (±SEM) preference ratio, with the dashed line indicating equal preference for both compartments. Asterisk (*) represents a within-subject difference relative to a 0.5 preference ratio p < 0.05.
Experiment 2 (Social CPP)
Pre-conditioning preference scores revealed no compartment bias in individually housed adolescent females, but there was a significant compartment bias towards the white chamber in pair housed rats (t(10) = 4.49, p = 0.001; see Figure 1b). After conditioning, preference ratios showed no significant preference for the peer-paired compartment over the empty compartment in either individually- or pair-housed adolescent females (see Figure 2b).
Experiment 3 (Social vs. AMPH CPP)
Pre-conditioning preference scores revealed no compartment bias in either the individually- or pair-housed adolescent females (see Figure 1c). After conditioning, preference ratios showed no significant preference for the compartment paired with AMPH or the social peer for either individually- or pair-housed adolescent females, although there was a near significant preference for AMPH in the pair-housed group (t(5) = 2.42, p = 0.06; see Figure 2c).
Experiment 4 (Neurochemical effects of AMPH)
Table 1 provides all values for DOPAC, 5-HIAA, DA and 5-HT across each brain region in males and females from Experiment 4 (AMPH treatment). The overall ANOVA revealed a significant main effect of region for DOPAC (F(5, 19) = 124.4, p < 0.001) and 5-HIAA (F(5, 20) = 54.8, p < 0.001). For DOPAC, subsequent two-way ANOVAs conducted for each individual region revealed a significant effect of treatment for DOPAC levels in Amyg (F(1, 26) = 16.22, p < 0.001), with AMPH producing an overall decrease in DOPAC; for comparison, DOPAC levels from Str and mPFC are also depicted in Figure 3; these regions were selected for graphic presentation because they each yielded significant effects as depicted in Table 1. To further probe differences between males and females in Amyg, pairwise comparisons revealed a sex difference in the AMPH-induced decrease in DOPAC, with only females showing a statistically significant decrease (t(13) = 4.01, p < 0.01); however the sex difference in Amyg was due to greater control (saline) DOPAC levels in females compared to males. No significant effects were observed in 5-HIAA levels in these same regions (Figure 4).
Table 1.
Mean (±SEM) levels of DOPAC, 5-HIAA, DA and 5-HT from Experiments 4 and 5, expressed as µg/g wet weight tissue.
| DOPAC | ||||||||
| Experiment 4 | Experiment 5 | |||||||
| Male | Female | Male | Female | |||||
| Saline | AMPH | Saline | AMPH | Alone | Social Int. | Alone | Social Int. | |
| Region | Mean (±SEM) | Mean (±SEM) | Mean (±SEM) | Mean (±SEM) | Mean (±SEM) | Mean (±SEM) | Mean (±SEM) | Mean (±SEM) |
| Nacc | 0.96 (±0.07) | 0.91 (±0.10) | 1.13 (±0.12) | 0.85 (±0.09) | 0.91 (±0.07) | 0.82 (±0.09) | 0.54 (±0.04) | 0.65 (±0.07) |
| Str | 1.62 (±0.32) | 1.47 (±0.23) | 1.49 (±0.24) | 1.21 (±0.16) | 0.98 (±0.11) | 0.83 (±0.06) | 0.73 (±0.04) | 1.05 (±0.07)* |
| mPFC | 0.04 (±0.00) | 0.04 (±0.00) | 0.05 (±0.01) | 0.03 (±0.00) | 0.02 (±0.00) | 0.03 (±0.00) | 0.02 (±0.00) | 0.04 (±0.01) |
| Amyg | 0.03 (±0.00) | 0.02 (±0.00) | 0.06 (±0.00) | 0.02 (±0.00)* | 0.03 (±0.00) | 0.07 (±0.01)* | 0.03 (±0.00) | 0.05 (±0.00) |
| Hypo | 0.11 (±0.03) | 0.11 (±0.03) | 0.13 (±0.02) | 0.09 (±0.02) | 0.04 (±0.00) | 0.05 (±0.00) | 0.07 (±0.00) | 0.08 (±0.00) |
| MB | 0.08 (±0.01) | 0.06 (±0.00) | 0.08 (±0.01) | 0.08 (±0.00) | 0.06 (±0.00) | 0.06 (±0.00) | 0.06 (±0.01) | 0.06 (±0.00) |
| 5-HIAA | ||||||||
| Experiment 4 | Experiment 5 | |||||||
| Male | Female | Male | Female | |||||
| Saline | AMPH | Saline | AMPH | Alone | Social Int. | Alone | Social Int. | |
| Region | Mean (±SEM) | Mean (±SEM) | Mean (±SEM) | Mean (±SEM) | Mean (±SEM) | Mean (±SEM) | Mean (±SEM) | Mean (±SEM) |
| Nacc | 0.56 (±0.04) | 0.68 (±0.08) | 0.50 (±0.07) | 0.71 (±0.07) | 0.45 (±0.02) | 0.42 (±0.03) | 0.25 (0.02) | 0.31 (±0.02) |
| Str | 0.53 (±0.04) | 0.62 (±0.07) | 0.63 (±0.50) | 0.63 (±0.04) | 0.33 (±0.01) | 0.40 (±0.02)* | 0.28 (±0.02) | 0.42 (±0.03)* |
| mPFC | 0.31 (±0.04) | 0.26 (±0.05) | 0.39 (±0.05) | 0.35 (±0.04) | 0.17 (±0.01) | 0.19 (±0.02) | 0.18 (±0.01) | 0.24 (±0.02)* |
| Amyg | 0.54 (±0.06) | 0.41 (±0.07) | 0.59 (±0.05) | 0.51 (±0.06) | 0.24 (±0.02) | 0.21 (±0.01) | 0.30 (±0.01) | 0.33 (±0.00)* |
| Hypo | 0.85 (±0.18) | 0.87 (±0.17) | 1.00 (±0.15) | 0.86 (±0.14) | 0.34 (±0.04) | 0.42 (±0.01) | 0.41 (±0.03) | 0.5 (±0.02) |
| MB | 1.13 (±0.17) | 1.25 (±0.21) | 1.17 (±0.16) | 1.33 (±0.15) | 0.70 (±0.02) | 0.70 (±0.04) | 0.72 (±0.03) | 0.79 (±0.01) |
| DA | ||||||||
| Experiment 4 | Experiment 5 | |||||||
| Male | Female | Male | Female | |||||
| Saline | AMPH | Saline | AMPH | Alone | Social Int. | Alone | Social Int. | |
| Region | Mean (±SEM) | Mean (±SEM) | Mean (±SEM) | Mean (±SEM) | Mean (±SEM) | Mean (±SEM) | Mean (±SEM) | Mean (±SEM) |
| Nacc | 3.28 (±0.33) | 3.94 (±0.33) | 3.38 (±0.29) | 4.03 (±0.25) | 4.14 (±0.17) | 3.23 (±0.42) | 3.49 (±0.19) | 3.41 (±0.24) |
| Str | 0.44 (±0.18) | 0.21 (±0.05) | 0.36 (±0.10) | 0.18 (±0.04) | 4.36 (±0.38) | 3.16 (±0.48) | 3.62 (±0.34) | 5.20 (±0.26)* |
| mPFC | 0.04 (±0.00) | 0.06 (±0.01) | 0.04 (±0.00) | 0.06 (±0.01) | 0.04 (±0.00) | 0.03 (±0.00) | 0.05 (±0.01) | 0.08 (±0.03) |
| Amyg | 0.10 (±0.02) | 0.08 (±0.01) | 0.17 (±0.03) | 0.11 (±0.02)* | 0.13 (±0.01) | 0.24 (±0.04) | 0.14 (±0.01) | 0.18 (±0.03) |
| Hypo | 0.20 (±0.01) | 0.25 (±0.01) | 0.23 (±0.02) | 0.19 (±0.02) | 0.25 (±0.03) | 0.24 (±0.02) | 0.31 (±0.03) | 0.33 (±0.02) |
| MB | 0.28 (±0.03) | 0.29 (±0.02) | 0.28 (±0.04) | 0.29 (±0.04) | 0.25 (±0.01) | 0.22 (±0.01) | 0.26 (±0.01) | 0.24 (±0.01) |
| 5-HT | ||||||||
| Experiment 4 | Experiment 5 | |||||||
| Male | Female | Male | Female | |||||
| Saline | AMPH | Saline | AMPH | Alone | Social Int. | Alone | Social Int. | |
| Region | Mean (±SEM) | Mean (±SEM) | Mean (±SEM) | Mean (±SEM) | Mean (±SEM) | Mean (±SEM) | Mean (±SEM) | Mean (±SEM) |
| Nacc | 0.49 (±0.05) | 0.52 (±0.05) | 0.52 (±0.06) | 0.60 (±0.07) | 0.43 (±0.03) | 0.35 (±0.02) | 0.33 (±0.02) | 0.36 (±0.02) |
| Str | 0.24 (±0.01) | 0.31 (±0.01) | 0.31 (±0.02) | 0.36 (±0.02) | 0.18 (±0.01) | 0.40 (±0.02)* | 0.14 (±0.01) | 0.21 (±0.02)* |
| mPFC | 0.15 (±0.02) | 0.16 (±0.02) | 0.15 (±0.02) | 0.23 (±0.03) | 0.15 (±0.01) | 0.15 (±0.01) | 0.21 (±0.01) | 0.19 (±0.01) |
| Amyg | 0.53 (±0.04) | 0.42 (±0.03) | 0.62 (±0.09) | 0.57 (±0.06) | 0.28 (±0.04) | 0.21 (±0.02) | 0.48 (±0.01) | 0.46 (±0.01) |
| Hypo | 0.36 (±0.05) | 0.42 (±0.02) | 0.58 (±0.05) | 0.67 (±0.04) | 0.53 (±0.06) | 0.67 (±0.08) | 0.58 (±0.07) | 0.61 (±0.09) |
| MB | 0.64 (±0.02) | 0.59 (±0.04) | 0.70 (±0.03) | 0.73 (±0.03) | 0.59 (±0.05) | 0.75 (±0.05) | 0.68 (±0.08) | 0.72 (±0.07) |
p<0.05 compared to same-sex control group in that region.
Figure 3.
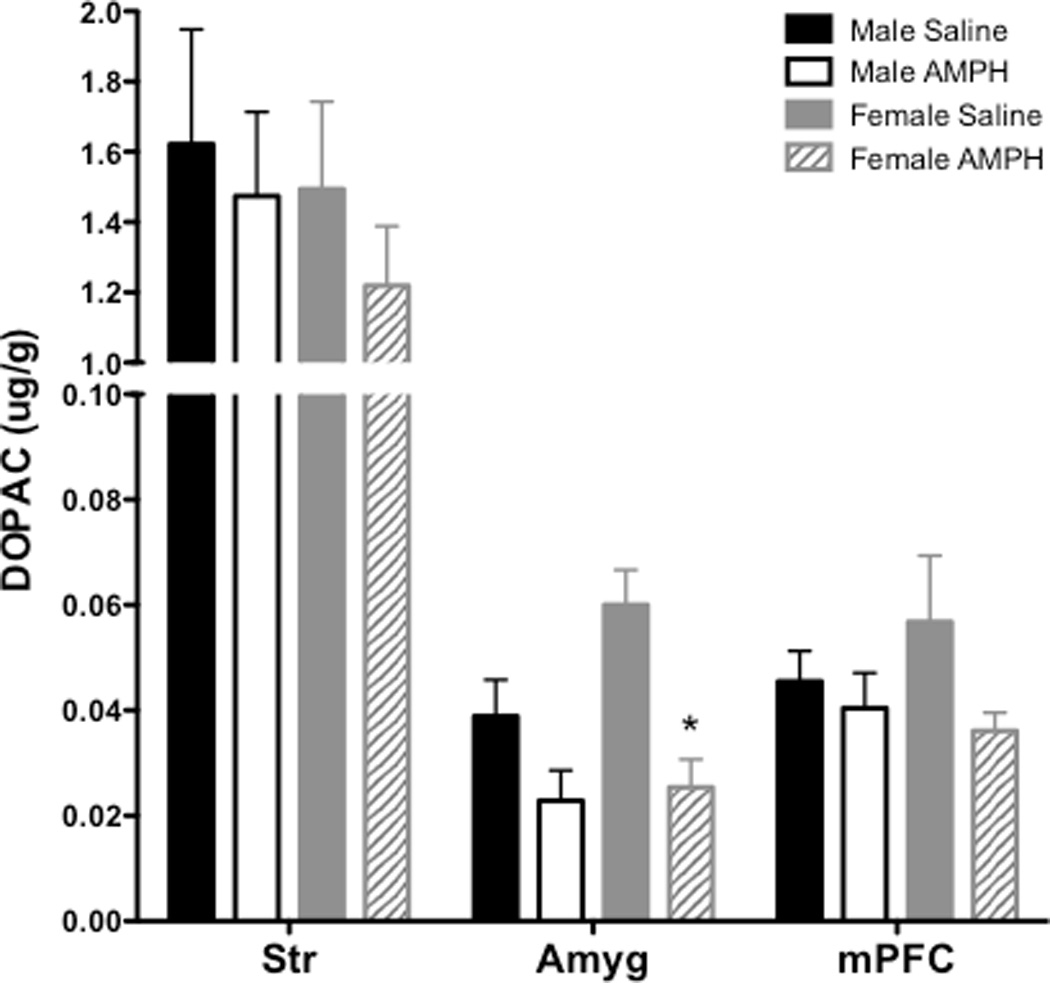
DOPAC levels (±SEM) in Str, Amyg and mPFC 30 min following s.c. injection of saline or AMPH. Asterisk (*) represents a within-subject difference relative to female saline control group, p<0.05.
Figure 4.
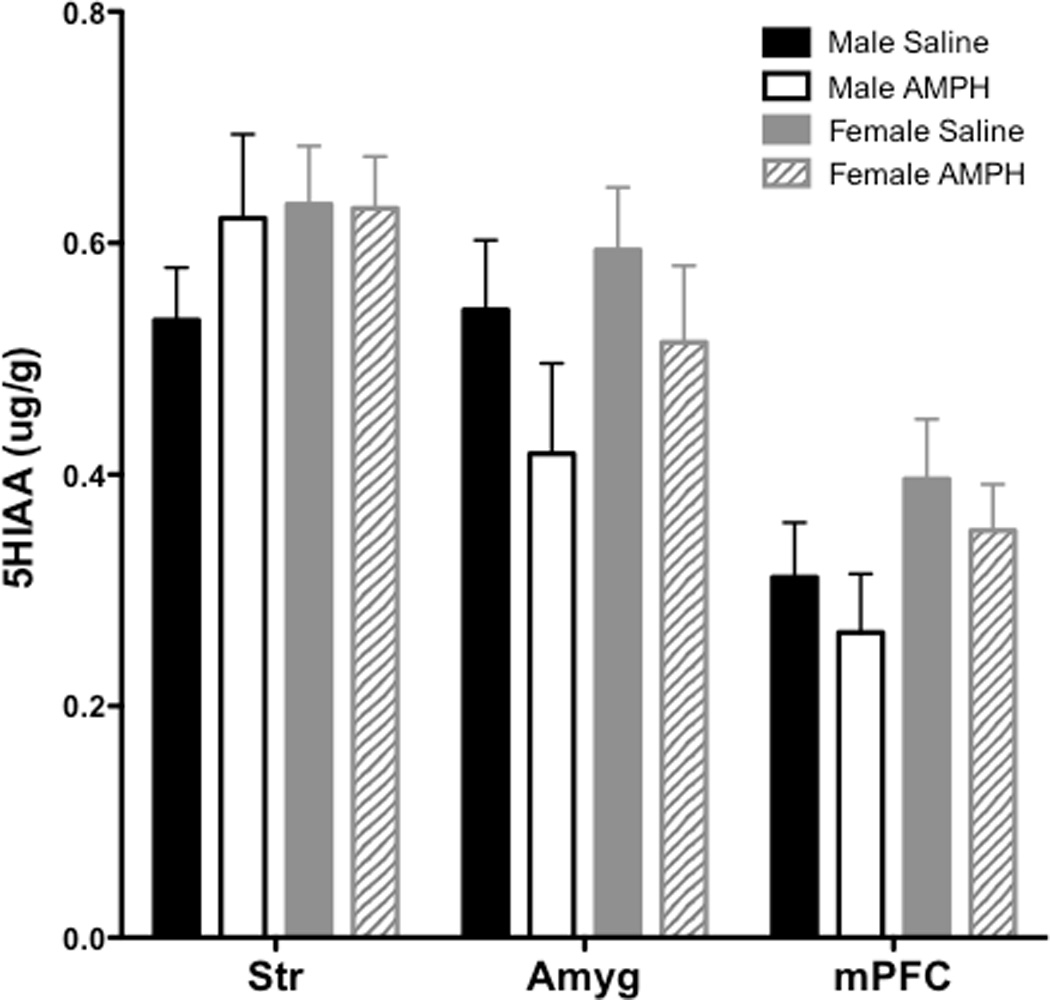
5-HIAA levels (±SEM) in Str, Amyg and mPFC 30 min following s.c. injection of saline or AMPH.
Experiment 5 (Neurochemical effects of peer interaction)
Table 1 provides all values for DOPAC, 5-HIAA, DA and 5-HT across each brain region in males and females from Experiment 5 (peer treatment). The overall ANOVA for DOPAC revealed significant effects of region (F(5, 20) = 341.4 p < 0.001) and a region × sex × treatment interaction (F(5, 20) = 6.448, p < 0.05). The overall ANOVA for 5-HIAA revealed significant main effects of region (F(5, 21) = 159.1, p < 0.001) and sex (F(1, 25) = 17.8, p < 0.001), as well as region × sex (F(5, 20) = 12.85, p < 0.001), region × treatment (F(5, 21) = 2.691, p < 0.05) and sex × treatment interactions (F(1, 25) = 6.721, p < 0.05). For DOPAC, subsequent two-way ANOVAs conducted for each individual region revealed a significant sex × treatment interaction in Str (F(1, 28) = 7.08, p < 0.05), as well as a main effect of treatment in Amyg (F(1, 27) = 11.09, p < 0.01; Figure 5). To further probe differences between males and females in these regions, pairwise comparisons revealed a sex difference in the peer-induced increased in DOPAC in Str and Amyg, with only females showing a peer-induced increase in Str (t(14) = 3.04, p < 0.01 and only males showing a peer-induced increase in Amyg (t(13) = 2.82, p < 0.05. For 5-HIAA, subsequent two-way ANOVAs conducted for each individual region revealed a main effect of treatment in Str (F(1, 28) = 14.66, p < 0.001), a main effect of sex in Amyg (F(1, 27) = 29.93, p < 0.001), and a main effect of treatment in mPFC (F(1,28) = 4.74, p < 0.05; Figure 6). To further probe differences between males and females in these regions, pairwise comparisons revealed a sex difference in Amyg and mPFC, with only females showing an increase in 5-HIAA in Amyg (t(14) = 1.913, p < 0.05) and mPFC (t(14) = 2.15, p < 0.05).
Figure 5.
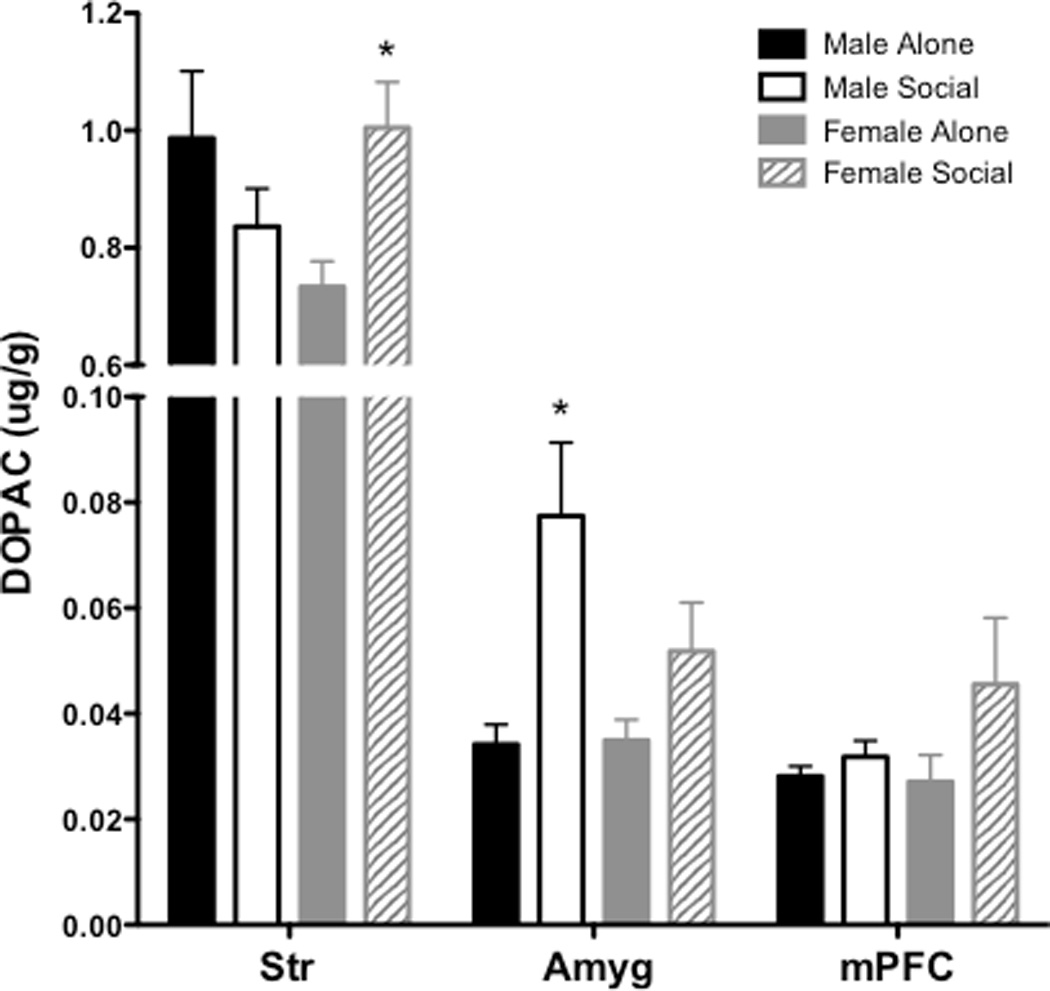
DOPAC levels (±SEM) Str, Amyg and mPFC following 30 min with or without social interaction. Asterisk (*) represents a between-subject difference compared to same-sex control group that received no social interaction, p<0.05.
Figure 6.
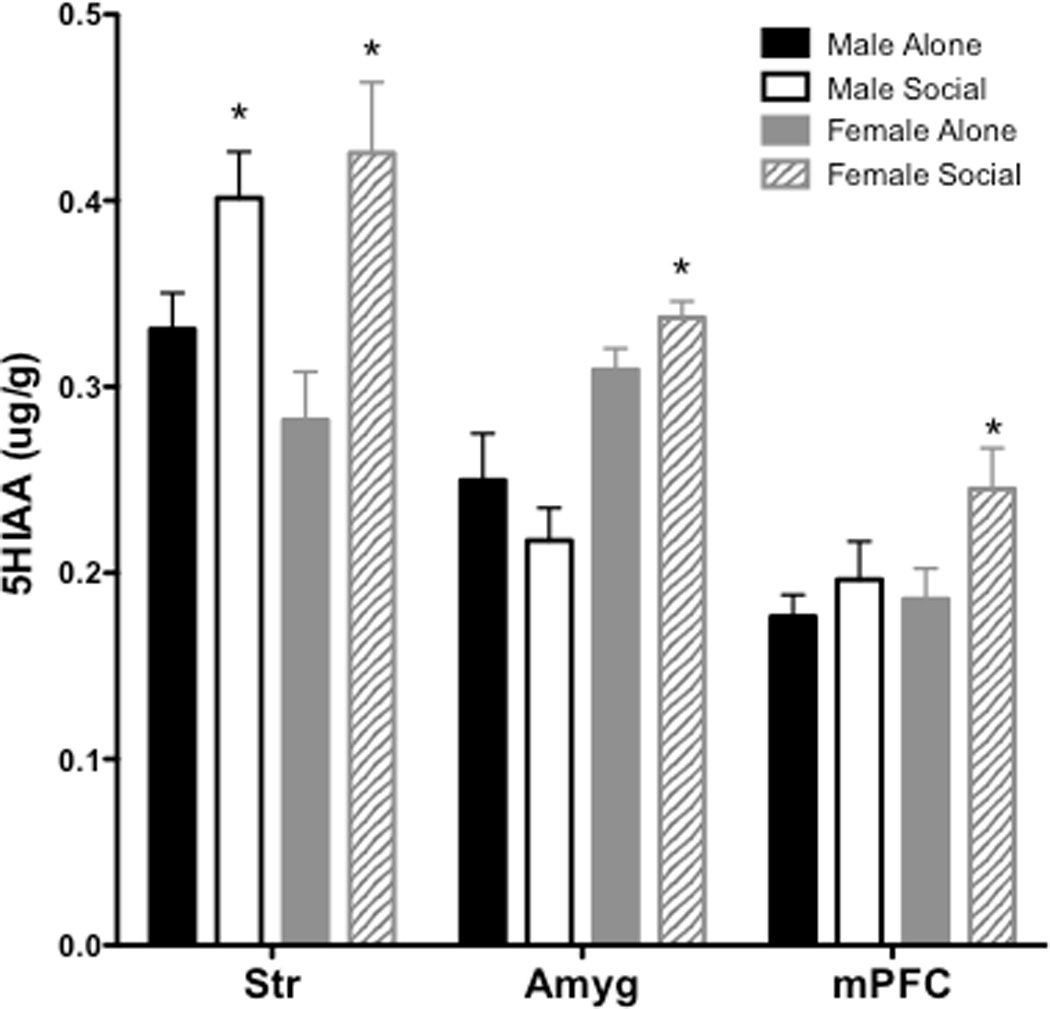
5-HIAA levels (±SEM) in Str, Amyg and mPFC following 30 min with or without social interaction. Asterisk (*) represents a between-subject difference compared to same-sex control group that received no social interaction, p<0.05.
Discussion
The current study was conducted based on a previous report from our laboratory in adolescent males (Yates et al., 2013). In that report, adolescent males showed social CPP when individually-housed, but not when pair-housed. Moreover, individually-housed males preferred a peer-paired compartment over an AMPH-paired compartment when given free-choice access to both compartments simultaneously. In contrast, using procedures similar to that previous report, the current study showed that neither individually- nor pair-housed adolescent females showed social CPP, and they did not prefer the peer-paired context over the AMPH-paired context on a free choice test. In both the previous study (Yates et al., 2013) and the current study, the dose of AMPH was the same (1 mg/kg) and the social peer used was sex- and age-matched. Thus, the sex differences in behavior observed across studies likely reflects inherent neurobiological differences between males and females rather than any procedural differences.
Given the sex differences in choice between AMPH- and peer-paired contexts, the current study examined monoamine function in reward- and social-relevant brain regions, including the NAcc, Str, Amyg, mPFC, Hypo and MB following AMPH or peer interaction in both males and females. While the results regarding sex differences were somewhat complex, at least three general findings were noted. First, while AMPH did not alter either DOPAC or 5-HIAA reliably across all of the various brain regions, females were more sensitive than males to the AMPH-induced decrease in DA metabolism in Amyg. Second, as for the effect of peer interaction, males were more sensitive than females to the peer-induced increase in DOPAC levels in Amyg. Third, in contrast to the results obtained with DOPAC, females were more sensitive than males to peer-induced increase in 5-HIAA levels in both Amyg and mPFC. Taken together, these results suggest that the greater ability of social interaction to protect against AMPH CPP in adolescent males compared to females may relate to greater DA activation in Amyg in males following social interaction and/or to a reduced DA activation in Amyg in males following AMPH.
However, there are some limitations in the current study. In particular, the sex difference observed in DOPAC in Amyg was not apparent in any other brain region examined. In addition, only a single dose of AMPH was examined and, in contrast to the 4-trial CPP behavioral results, the neurochemical effects were examined following either one AMPH trial or one social interaction. An acute procedure was chosen because previous findings indicate that the first trial is most important for obtaining drug (Bardo & Neisewander, 1986; Bardo, Rowlett, & Harris, 1995); in addition, only one social interaction trial was used to avoid any potential habituation to repeated social interactions.
Previous work in adults has shown that females are more sensitive to the rewarding and neurochemical effects of AMPH and other stimulants (Chin et al., 2001; Lynch & Carroll, 1999). However, there is relatively little information available examining the sex differences during the adolescent period. In adults, evidence indicates that females show greater stimulant-induced locomotor activity (Bisagno, Ferguson, & Luine, 2003; Hensleigh, Smedley, & Pritchard, 2011), CPP (Russo et al., 2003), and stimulant self-administration (Roth & Carroll, 2004). Controlled studies using ovariectomized females with or without hormone replacement treatment have demonstrated a significant role for adult circulating estrogen levels (Hu & Becker, 2008; Lynch & Carroll, 1999; Lynch, Roth, Mickelberg, & Carroll, 2001), in addition to progesterone (Feltenstein, Byrd, Henderson, & See, 2009). During the proestrus period, some evidence indicates that adolescent females are also more sensitive to cocaine-induced hyperactivity and CPP (Zakharova, Wade, & Izenwasser, 2009), although not all studies support this conclusion (Caine et al., 2004; Kantak, Goodrich, & Uribe, 2007). Sex differences are thought to be modulated by the number of cage mates provided in the housing environment (Zakharova, Starosciak, Wade, & Izenwasser, 2012).
While there is little known about stimulant-induced DA activity in adolescent males and females, one report showed that methamphetamine induced levels of 5-HT are greater in left mPFC of males compared to females (Staiti et al., 2011). In contrast to that previous microdialysis study, our results did not reveal any metabolite differences in the same brain region. This discrepancy in results may reflect a methodological difference between microdialysis and tissue metabolite levels measured via HPLC-EC analysis. In contrast to measurement of extracellular DA and 5-HT directly, tissue metabolite levels reflect not only release and reuptake, but also MAO activity, which is inhibited by AMPH to reduce DOPAC (Eisenhofer, Kopin, & Goldstein, 2004; Sulzer, Sonders, Poulsen, & Galli, 2005). Although the lack of difference in metabolism following AMPH administration in adolescents was unexpected, this finding may relate to the relative immaturity of monoamine systems in adolescents relative to adults (Benes, Taylor, & Cunningham, 2000).
Although there is little known about sex differences in adolescents following stimulant administration, there is increasing literature on the rewarding properties of social interaction among adolescent rats. An initial study showed that social interaction is more rewarding to adolescent males than adolescent females (Douglas et al., 2004). This likely reflects greater levels of play behavior in males relative to females (Olioff & Stewart, 1978). More recently, social reward in adolescent and adult males has been shown to reduce cocaine reward when tested using CPP (Fritz, El Rawas, Klement, et al., 2011; Fritz, El Rawas, Salti, et al., 2011; Thiel, Okun, & Neisewander, 2008), suggesting that social interaction competes with cocaine reward. The current results extend these latter findings by showing that AMPH CPP is also reduced by competing social cues in adolescent females, although the peer-associated compartment was not preferred over the AMPH-associated compartment as shown previously in adolescent males (Yates et al., 2013).
A number of studies have focused on vasopressin as a mediator of sexually dimorphic social play (Paul et al., 2014; Veenema et al., 2013). Vasopressin and other hormone activity in areas of lateral septum, bed nucleus of the stria terminalis, and Amyg have been implicated in social play (Meaney & Stewart, 1981; Paul et al., 2014; Veenema et al., 2013). Within Amyg, dendritic arborization and glial volume are increased in males relative to females, suggesting a role in the greater play behavior exhibited in males (Cooke, Stokas, & Woolley, 2007). Further, vasopressin microinjection enhances DA utilization and systemic injections of vasopressin increases excitatory postsynaptic currents of 5-HT neurons in Amyg (Rood & Beck, 2014), suggesting a critical interaction between social play-related hormones and monoamine neural systems (van Heuven-Nolsen, de Kloet, De Wied, & Versteeg, 1984).
The neurochemical results from the current study revealed sex-dependent differences in monoamine systems following social interaction. Specifically, males showed an increase in DOPAC levels in Amyg, indicative of greater release and turnover, whereas females did not. Conversely, females showed increased levels of 5-HIAA following social interaction in mPFC and Hypo, whereas males did not. Since increased 5-HT levels are known to decrease social play (Homberg, Schiepers, Schoffelmeer, Cuppen, & Vanderschuren, 2007), these results are consistent with previous research that shows females engage in less social play. Finally, increases in 5-HIAA levels were found in Str following social interaction in both males and females, but only females showed an increase in DOPAC. This result is consistent with previous literature showing increased c-fos activation in the dorsal striatum following social interaction/play (Gordon et al., 2002; van Kerkhof et al., 2014).
In translating this work to potential preventive interventions for stimulant abuse, one can view social interaction as an alternative reward that competes with stimulant reward. Based on this notion, our results suggest the possibility that psychosocial interventions that are designed to enhance social interactions could be more effective in males than in females. In humans, adolescent males have smaller social networks and weaker peer attachments relative to females (Gorrese & Ruggieri, 2012). Males also are more prone to be diagnosed with anti-social personality disorder and autism spectrum disorder (Paris, 2004; Werling & Geschwind, 2013), which reflect deficiencies in social communication and peer bonding. Given the generally lower baseline rate of peer attachment in males compared to females, psychosocial and behavioral strategies designed to appeal to males might produce more robust effects than the same strategies in females.
Contributor Information
Virginia G Weiss, Dept Psychology, BBSRB, University of Kentucky, 741 S. Limestone, Lexington, KY 40536-0509, Phone: 859-257-4641, vwe222@g.uky.edu.
Rebecca S Hofford, Dept Psychology, BBSRB, University of Kentucky, 741 S. Limestone Lexington, KY 40536-0509, Phone: 859-257-4641, rebecca.hofford@uky.edu.
Justin R Yates, Department of Psychological Science, MEP 301, Northern Kentucky University, Nunn Drive, Highland Heights, KY 41099. Phone: 859-572-7821 yatesj1@nku.edu.
Faith C Jennings, College of Pharmacy, Biological Pharmaceutical Complex, University of Kentucky, 789. S. Limestone, Lexington, KY 40536-0509, Phone: 859-806-3493 fcje223@email.uky.edu.
Michael T Bardo, Dept Psychology, BBSRB, University of Kentucky, 741 S. Limestone, Lexington, KY 40536-0509, Phone: 859-257-6456, mbardo@uky.edu.
References
- Bahr S, Hoffmann J, Yang X. Parental and Peer Influences on the Risk of Adolescent Drug Use. Journal of Primary Prevention. 2005;26(6):529–551. doi: 10.1007/s10935-005-0014-8. [DOI] [PubMed] [Google Scholar]
- Bardo MT, Neisewander JL. Single-trial conditioned place preference using intravenous morphine. Pharmacol Biochem Behav. 1986;25(5):1101–1105. doi: 10.1016/0091-3057(86)90092-4. [DOI] [PubMed] [Google Scholar]
- Bardo MT, Rowlett JK, Harris MJ. Conditioned place preference using opiate and stimulant drugs: a meta-analysis. Neurosci Biobehav Rev. 1995;19(1):39–51. doi: 10.1016/0149-7634(94)00021-r. [DOI] [PubMed] [Google Scholar]
- Benes FM, Taylor JB, Cunningham MC. Convergence and plasticity of monoaminergic systems in the medial prefrontal cortex during the postnatal period: implications for the development of psychopathology. Cereb Cortex. 2000;10(10):1014–1027. doi: 10.1093/cercor/10.10.1014. [DOI] [PubMed] [Google Scholar]
- Bisagno V, Ferguson D, Luine VN. Chronic d-amphetamine induces sexually dimorphic effects on locomotion, recognition memory, and brain monoamines. Pharmacology Biochemistry and Behavior. 2003;74(4):859–867. doi: 10.1016/s0091-3057(03)00017-0. doi: http://dx.doi.org/10.1016/S0091-3057(03)00017-0. [DOI] [PubMed] [Google Scholar]
- Branstetter SA, Low S, Furman W. The Influence of Parents and Friends on Adolescent Substance Use: A Multidimensional Approach. Journal of substance use. 2011;16(2):150–160. doi: 10.3109/14659891.2010.519421. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Caine SB, Bowen CA, Yu G, Zuzga D, Negus SS, Mello NK. Effect of gonadectomy and gonadal hormone replacement on cocaine self-administration in female and male rats. Neuropsychopharmacology. 2004;29(5):929–942. doi: 10.1038/sj.npp.1300387. [DOI] [PubMed] [Google Scholar]
- Chin J, Sternin O, Wu HB, Fletcher H, Perrotti LI, Jenab S, Quiñones-Jenab V. Sex differences in cocaine-induced behavioral sensitization. Cell Mol Biol (Noisy-le-grand) 2001;47(6):1089–1095. [PubMed] [Google Scholar]
- Cole SL, Hofford RS, Evert DJ, Wellman PJ, Eitan S. Social influences on morphine conditioned place preference in adolescent mice. Addiction Biology. 2013;18(2):274–285. doi: 10.1111/j.1369-1600.2011.00426.x. [DOI] [PubMed] [Google Scholar]
- Cooke BM, Stokas MR, Woolley CS. Morphological sex differences and laterality in the prepubertal medial amygdala. The Journal of Comparative Neurology. 2007;501(6):904–915. doi: 10.1002/cne.21281. [DOI] [PubMed] [Google Scholar]
- Dolen G, Darvishzadeh A, Huang KW, Malenka RC. Social reward requires coordinated activity of nucleus accumbens oxytocin and serotonin. Nature. 2013;501(7466):179–184. doi: 10.1038/nature12518. [Article]. http://www.nature.com/nature/journal/v501/n7466/abs/nature12518.html#supplementary-information. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Douglas LA, Varlinskaya EI, Spear LP. Rewarding properties of social interactions in adolescent and adult male and female rats: Impact of social versus isolate housing of subjects and partners. Dev Psychobiol. 2004;45(3):153–162. doi: 10.1002/dev.20025. [DOI] [PubMed] [Google Scholar]
- Eisenhofer G, Kopin IJ, Goldstein DS. Catecholamine metabolism: a contemporary view with implications for physiology and medicine. Pharmacological reviews. 2004;56(3):331–349. doi: 10.1124/pr.56.3.1. [DOI] [PubMed] [Google Scholar]
- Feltenstein MW, Byrd EA, Henderson AR, See RE. Attenuation of cocaine-seeking by progesterone treatment in female rats. Psychoneuroendocrinology. 2009;34(3):343–352. doi: 10.1016/j.psyneuen.2008.09.014. http://dx.doi.org/10.1016/j.psyneuen.2008.09.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Forster M, Grigsby TJ, Bunyan A, Unger JB, Valente TW. The Protective Role of School Friendship Ties for Substance Use and Aggressive Behaviors Among Middle School Students. Journal of School Health. 2015;85(2):82–89. doi: 10.1111/josh.12230. [DOI] [PubMed] [Google Scholar]
- Fritz M, El Rawas R, Klement S, Kummer K, Mayr MJ, Eggart V, Zernig G. Differential effects of accumbens core vs. shell lesions in a rat concurrent conditioned place preference paradigm for cocaine vs. social interaction. PLoS One. 2011;6(10):e26761. doi: 10.1371/journal.pone.0026761. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fritz M, El Rawas R, Salti A, Klement S, Bardo MT, Kemmler G, Zernig G. Reversal of cocaine-conditioned place preference and mesocorticolimbic Zif268 expression by social interaction in rats. Addict Biol. 2011;16(2):273–284. doi: 10.1111/j.1369-1600.2010.00285.x. [DOI] [PubMed] [Google Scholar]
- Gipson CD, Yates JR, Beckmann JS, Marusich JA, Zentall TR, Bardo MT. Social facilitation of d-amphetamine self-administration in rats. Exp Clin Psychopharmacol. 2011;19(6):409–419. doi: 10.1037/a0024682. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gordon NS, Kollack-Walker S, Akil H, Panksepp J. Expression of c-fos gene activation during rough and tumble play in juvenile rats. Brain Research Bulletin. 2002;57(5):651–659. doi: 10.1016/s0361-9230(01)00762-6. doi: http://dx.doi.org/10.1016/S0361-9230(01)00762-6. [DOI] [PubMed] [Google Scholar]
- Gorrese A, Ruggieri R. Peer attachment: a meta-analytic review of gender and age differences and associations with parent attachment. J Youth Adolesc. 2012;41(5):650–672. doi: 10.1007/s10964-012-9759-6. [DOI] [PubMed] [Google Scholar]
- Hensleigh E, Smedley L, Pritchard LM. Sex, but not repeated maternal separation during the first postnatal week, influences novel object exploration and amphetamine sensitivity. Developmental Psychobiology. 2011;53(2):132–140. doi: 10.1002/dev.20499. [DOI] [PubMed] [Google Scholar]
- Homberg J, Schiepers OG, Schoffelmeer AM, Cuppen E, Vanderschuren LMJ. Acute and constitutive increases in central serotonin levels reduce social play behaviour in peri-adolescent rats. Psychopharmacology. 2007;195(2):175–182. doi: 10.1007/s00213-007-0895-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hu M, Becker JB. Acquisition of cocaine self-administration in ovariectomized female rats: Effect of estradiol dose or chronic estradiol administration. Drug and Alcohol Dependence. 2008;94(1–3):56–62. doi: 10.1016/j.drugalcdep.2007.10.005. doi: http://dx.doi.org/10.1016/j.drugalcdep.2007.10.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hunt PS, Holloway JL, Scordalakes EM. Social interaction with an intoxicated sibling can result in increased intake of ethanol by periadolescent rats. Dev Psychobiol. 2001;38(2):101–109. doi: 10.1002/1098-2302(200103)38:2<101::aid-dev1002>3.0.co;2-4. [DOI] [PubMed] [Google Scholar]
- Kantak KM, Goodrich CM, Uribe V. Influence of sex, estrous cycle, and drug-onset age on cocaine self-administration in rats (Rattus norvegicus) Exp Clin Psychopharmacol. 2007;15(1):37–47. doi: 10.1037/1064-1297.15.1.37. [DOI] [PubMed] [Google Scholar]
- Kennedy B, Panksepp J, Runckel P, Lahvis G. Social influences on morphine-conditioned place preference in adolescent BALB/cJ and C57BL/6J mice. Psychopharmacology. 2012;219(3):923–932. doi: 10.1007/s00213-011-2421-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Logue S, Chein J, Gould T, Holliday E, Steinberg L. Adolescent mice, unlike adults, consume more alcohol in the presence of peers than alone. Developmental Science. 2014;17(1):79–85. doi: 10.1111/desc.12101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lynch WJ, Carroll ME. Sex differences in the acquisition of intravenously self-administered cocaine and heroin in rats. Psychopharmacology (Berl) 1999;144(1):77–82. doi: 10.1007/s002130050979. [DOI] [PubMed] [Google Scholar]
- Lynch WJ, Roth ME, Mickelberg JL, Carroll ME. Role of estrogen in the acquisition of intravenously self-administered cocaine in female rats. Pharmacology Biochemistry and Behavior. 2001;68(4):641–646. doi: 10.1016/s0091-3057(01)00455-5. doi: http://dx.doi.org/10.1016/S0091-3057(01)00455-5. [DOI] [PubMed] [Google Scholar]
- Meaney MJ, Stewart J. Neonatal androgens influence the social play of prepubescent rats. Horm Behav. 1981;15(2):197–213. doi: 10.1016/0018-506x(81)90028-3. [DOI] [PubMed] [Google Scholar]
- Olioff M, Stewart J. Sex differences in the play behavior of prepubescent rats. Physiol Behav. 1978;20(2):113–115. doi: 10.1016/0031-9384(78)90060-4. [DOI] [PubMed] [Google Scholar]
- Paris J. Gender differences in personality traits and disorders. Curr Psychiatry Rep. 2004;6(1):71–74. doi: 10.1007/s11920-004-0042-8. [DOI] [PubMed] [Google Scholar]
- Paul MJ, Terranova JI, Probst CK, Murray EK, Ismail NI, De Vries GJ. Sexually dimorphic role for vasopressin in the development of social play. [Original Research] Frontiers in Behavioral Neuroscience. 2014;8 doi: 10.3389/fnbeh.2014.00058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rood BD, Beck SG. Vasopressin indirectly excites dorsal raphe serotonin neurons through activation of the vasopressin1A receptor. Neuroscience. 2014;260(0):205–216. doi: 10.1016/j.neuroscience.2013.12.012. doi: http://dx.doi.org/10.1016/j.neuroscience.2013.12.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roth ME, Carroll ME. Sex differences in the escalation of intravenous cocaine intake following long- or short-access to cocaine self-administration. Pharmacology Biochemistry and Behavior. 2004;78(2):199–207. doi: 10.1016/j.pbb.2004.03.018. doi: http://dx.doi.org/10.1016/j.pbb.2004.03.018. [DOI] [PubMed] [Google Scholar]
- Russo SJ, Jenab S, Fabian SJ, Festa ED, Kemen LM, Quinones-Jenab V. Sex differences in the conditioned rewarding effects of cocaine. Brain Research. 2003;970(1–2):214–220. doi: 10.1016/s0006-8993(03)02346-1. doi: http://dx.doi.org/10.1016/S0006-8993(03)02346-1. [DOI] [PubMed] [Google Scholar]
- Smith M. Peer influences on drug self-administration: Social facilitation and social inhibition of cocaine intake in male rats. Psychopharmacology. 2012;224(1):81–90. doi: 10.1007/s00213-012-2737-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spear LP. The adolescent brain and age-related behavioral manifestations. Neurosci Biobehav Rev. 2000;24(4):417–463. doi: 10.1016/s0149-7634(00)00014-2. [DOI] [PubMed] [Google Scholar]
- Staiti AM, Morgane PJ, Galler JR, Grivetti JY, Bass DC, Mokler DJ. A microdialysis study of the medial prefrontal cortex of adolescent and adult rats. Neuropharmacology. 2011;61(3):544–549. doi: 10.1016/j.neuropharm.2011.04.005. doi: http://dx.doi.org/10.1016/j.neuropharm.2011.04.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sulzer D, Sonders MS, Poulsen NW, Galli A. Mechanisms of neurotransmitter release by amphetamines: a review. Prog Neurobiol. 2005;75(6):406–433. doi: 10.1016/j.pneurobio.2005.04.003. [DOI] [PubMed] [Google Scholar]
- Thiel KJ, Okun AC, Neisewander JL. Social reward-conditioned place preference: A model revealing an interaction between cocaine and social context rewards in rats. Drug and Alcohol Dependence. 2008;96(3):202–212. doi: 10.1016/j.drugalcdep.2008.02.013. doi: http://dx.doi.org/10.1016/j.drugalcdep.2008.02.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van Heuven-Nolsen D, de Kloet ER, De Wied D, Versteeg DHG. Microinjection of vasopressin and two related peptides into the amygdala: enhancing effect on local dopamine neurotransmission. Brain Research. 1984;293(1):191–195. doi: 10.1016/0006-8993(84)91470-7. doi: http://dx.doi.org/10.1016/0006-8993(84)91470-7. [DOI] [PubMed] [Google Scholar]
- van Kerkhof LM, Trezza V, Mulder T, Gao P, Voorn P, Vanderschuren LMJ. Cellular activation in limbic brain systems during social play behaviour in rats. Brain Structure and Function. 2014;219(4):1181–1211. doi: 10.1007/s00429-013-0558-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Veenema AH, Bredewold R, De Vries GJ. Sex-specific modulation of juvenile social play by vasopressin. Psychoneuroendocrinology. 2013;38(11):2554–2561. doi: 10.1016/j.psyneuen.2013.06.002. doi: http://dx.doi.org/10.1016/j.psyneuen.2013.06.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Watanabe S. Drug-social interactions in the reinforcing property of methamphetamine in mice. Behav Pharmacol. 2011;22(3):203–206. doi: 10.1097/FBP.0b013e328345c815. [DOI] [PubMed] [Google Scholar]
- Werling DM, Geschwind DH. Sex differences in autism spectrum disorders. Curr Opin Neurol. 2013;26(2):146–153. doi: 10.1097/WCO.0b013e32835ee548. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yates JR, Beckmann JS, Meyer AC, Bardo MT. Concurrent choice for social interaction and amphetamine using conditioned place preference in rats: Effects of age and housing condition. Drug and Alcohol Dependence. 2013;129(3):240–246. doi: 10.1016/j.drugalcdep.2013.02.024. doi: http://dx.doi.org/10.1016/j.drugalcdep.2013.02.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zakharova E, Starosciak A, Wade D, Izenwasser S. Sex differences in the effects of social and physical environment on novelty-induced exploratory behavior and cocaine-stimulated locomotor activity in adolescent rats. Behavioural Brain Research. 2012;230(1):92–99. doi: 10.1016/j.bbr.2012.01.052. doi: http://dx.doi.org/10.1016/j.bbr.2012.01.052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zakharova E, Wade D, Izenwasser S. Sensitivity to cocaine conditioned reward depends on sex and age. Pharmacology Biochemistry and Behavior. 2009;92(1):131–134. doi: 10.1016/j.pbb.2008.11.002. doi: http://dx.doi.org/10.1016/j.pbb.2008.11.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zernig G, Kummer KK, Prast JM. Dyadic social interaction as an alternative reward to cocaine. Front Psychiatry. 2013;4:100. doi: 10.3389/fpsyt.2013.00100. [DOI] [PMC free article] [PubMed] [Google Scholar]


