INTRODUCTION
Anxiety in response to impending surgery is a common emotional phenomenon, but it also leads to perioperative physiological and psychological changes.[1] The major goal of pre-medication is to allay anxiety. An ideal pre-medicant should have a non-invasive route of administration, rapid and reliable onset, rapid elimination, consistent and predictable results and good patient acceptance. At the same time, it should also be free of side effects like haemodynamic instability, respiratory obstruction and delayed recovery.[2]
Oral midazolam fulfills many of these characteristics. It has been extensively evaluated in children and is considered the gold standard pre-medicant for this age group but data regarding its use in adults is scarce.[3] This study was planned to evaluate the role of oral midazolam in adult females undergoing intracavitary cervical implantation for brachytherapy under general anaesthesia. Evaluation of sedation and anxiolysis was our primary objective. Safety profile in terms of haemodynamic stability, respiratory depression and recovery time, and amnestic effects were also assessed.
METHODS
A prospective, double-blind placebo-controlled randomised trial was conducted in a Tertiary Care Centre from August 2010 to July 2012. The study was approved by the Institutional Review Board and Ethics Committee. Written informed consent was taken from each patient. Based on pilot study conducted by us, the sample size was calculated keeping the desired power of study at 80%, α error - 0.05 and β error - 0.02. The study group comprised 70 adult females aged 20–60 years, weighing 40–60 kg, American Society of Anaesthesiologists physical status grade I and II, diagnosed with carcinoma cervix, and undergoing cervical implantation for outpatient intracavitary brachytherapy.
Exclusion criteria included central nervous system abnormality, allergy to benzodiazepines, systemic disorders that might affect drug absorption. Patients were randomised into two groups; Group M (n = 35) received oral midazolam 0.25 mg/kg and Group P (n = 35) received placebo.
Clinical responses (sedation/anxiolysis) were assessed 1-hour after oral pre-medication. Observer's Assessment of Alertness/Sedation scale (OAA/S scale) was used to assess the efficacy of sedation.[4] Similarly, clinical scale based on following four points was used to assess anxiolytic effects: (1) Tearful or combative; (2) anxious but easily reassured; (3) calm; (4) asleep.[5] Two memory tests were administered to assess anterograde amnesia just before induction.[6] Each patient was shown a series of 4 identical posters (pictures of scissors, apple, bottle and pen) and underwent 6 identical events related to procedure; viz shifting of patient from trolley to operation theatre table, intravenous cannulation, protruding out the tongue, squeezing eyes shut and then opening, demonstration of syringe containing milky solution (propofol) and application of mask. On completion of the procedure, recovery from anaesthesia was assessed using modified Aldrete's recovery score. When the patient was fully awake, patients were asked to recall all the posters shown and all the events experienced before induction.
RESULTS
Baseline demographic and clinical characteristics were comparable between the two groups. Overall, 94.3% (33/35) patients achieved clinically detectable sedation (sedation score ≤17) in midazolam group with 62.6% patients being heavily sedated (sedation score 11–14 whereas 2.9% (1/35) patients showed detectable sedation in the control group. With regard to anxiolysis, all patients (35/35) in midazolam group exhibited satisfactory anxiolytic response (anxiolysis score ≥3); 17.1% (6/35) were calm and 82.9% (29/35) reached the maximum level of anxiolysis (asleep). On the other hand, only 65.7% patients achieved anxiolysis score ≥3 in the control group and none of them attained the maximum level of anxiolysis [Table 1].
Table 1.
Sedation and anxiolysis scores

Regarding memory tests, recall rate for posters and events was lower in Group M than in Group P (P < 0.001). Only 28.67% (10/35) patients in midazolam could recall all the four posters in comparison to 85.7% (30/35) patients in placebo group. Similarly, merely 14.3% (5/35) patients could recall all the 6 events after oral midazolam in contrast to 82.9% (29/35) in the control group.
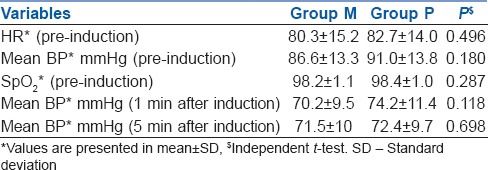
Haemodynamic parameters (prior to induction and after induction) in both the groups were comparable [Table 2].
Table 2.
Haemodynamic stability
Mean recovery time (from the end of procedure to complete recovery that is, an Aldrete score of ≥9) was slightly longer in midazolam group as compared to placebo group (Group M 6.25 ± 4.54 min vs. Group P 3.97 ± 2.51 min, P = 0.011).
DISCUSSION
One hour after pre-medication with oral midazolam, 94.3% patients were sedated as measured by OAA/S scale. Pre-medication with oral midazolam also resulted in significant anxiolysis in all our patients. The dose of 0.25 mg/kg of oral tablet midazolam was an effective sedative and anxiolytic in our patient sample. Brosius and Bannister also observed similar findings.[7] In contrast, in a sample of healthy pre-adolescents and adolescents, clinically detectable sedation (OAA/S ≤17) was observed in merely 40% of patients even at higher doses (20 mg) of oral midazolam.[8]
Tschirch et al. compared oral midazolam (7.5 mg) with nasal midazolam and found that reduction in anxiety was insufficient in 67% in the group that was administered oral midazolam and clinically significant in 97% patients who were administered midazolam nasally. However, they had included only claustrophobic patients and a lower dose of midazolam was used. This could explain inadequate anxiolysis in their patients.[9]
Aldrete's recovery score was used to assess recovery in our study. There was a slight prolongation of recovery time in Group M (6.2 vs. 3.9 min). Similarly in Brosius’ study, with a fixed dose of 20 mg oral midazolam, the median time to discharge readiness was 10 min in placebo versus 11 min in the midazolam group.[8]
Our patients experienced a significant degree of anterograde amnesia after oral midazolam. Similar findings were reported by Bulach et al. in adult patients and by Kain et al. in children using validated series of picture cards at doses of 0.5 mg/kg.[6,10] Haemodynamic parameters (prior to induction and after induction) in both the groups were comparable.
Limitations of our study include; first, the dose of 0.25 mg/kg body weight was fixed based on paediatric data. This could lead to high doses in obese adults, therefore, overweight/obese patients were excluded from the study. Second, street readiness was not compared in both groups. The time for recovery in the Operation Theatre (OT) differed only by 2–3 min (Group M 6.25 ± 4.54 min vs. Group P 3.97 ± 2.51 min, P = 0.011).
There was no difference in the time of discharge readiness in both groups.
Patients receive brachytherapy in the OT and are shifted to the post-anaesthesia care unit and then to the day care ward. As per hospital policy, patients are discharged after oral intake and voiding of urine after 4–6 h.
CONCLUSION
Oral midazolam (at a dose of 0.25 mg/kg) showed sedative, anxiolytic and anterograde amnestic effects with no adverse effects on haemodynamic stability or recovery in adults undergoing outpatient procedures under general anaesthesia.
ACKNOWLEDGEMENTS
The authors acknowledge with gratitude the help extended by Dr. Vishnu Goyal, Dr. Vithal Dhulkhed and Dr. Rajeev Yadav in the execution of this study and the preparation of the manuscript.
REFERENCES
- 1.Hamid M, Khan MA, Khatri A, Akhtar I. Effectiveness of premedication at the time of separation from parent and mask induction in paediatric patients coming for congenital heart disease surgery. J Coll Physicians Surg Pak. 2012;22:280–4. [PubMed] [Google Scholar]
- 2.Parashchanka A, Schelfout S, Coppens M. Role of novel drugs in sedation outside the operating room: Dexmedetomidine, ketamine and remifentanil. Curr Opin Anaesthesiol. 2014;27:442–7. doi: 10.1097/ACO.0000000000000086. [DOI] [PubMed] [Google Scholar]
- 3.Sinha C, Kaur M, Kumar A, Kulkarni A, Ambareesha M, Upadya M. Comparative evaluation of midazolam and butorphanol as oral premedication in pediatric patients. J Anaesthesiol Clin Pharmacol. 2012;28:32–5. doi: 10.4103/0970-9185.92431. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Bauerle K, Greim CA, Schroth M, Geisselbrecht M, Köbler A, Roewer N. Prediction of depth of sedation and anaesthesia by the Narcotrend EEG monitor. Br J Anaesth. 2004;92:841–5. doi: 10.1093/bja/aeh142. [DOI] [PubMed] [Google Scholar]
- 5.Hassani V, Amanni A, Poureslami M, Motilagh SD. Paediatric premedication: A comparison of sublingual buprenorphine and midazolam in children (4–10 years) scheduled for adenotonsillectomy. Iran J Pharmacol Ther. 2002;1:12–6. [Google Scholar]
- 6.Bulach R, Myles PS, Russnak M. Double-blind randomized controlled trial to determine extent of amnesia with midazolam given immediately before general anaesthesia. Br J Anaesth. 2005;94:300–5. doi: 10.1093/bja/aei040. [DOI] [PubMed] [Google Scholar]
- 7.Brosius KK, Bannister CF. Midazolam premedication in children: A comparison of two oral dosage formulations on sedation score and plasma midazolam levels. Anesth Analg. 2003;96:392–5. doi: 10.1097/00000539-200302000-00017. [DOI] [PubMed] [Google Scholar]
- 8.Brosius KK, Bannister CF. Oral midazolam premedication in preadolescents and adolescents. Anesth Analg. 2002;94:31–6. doi: 10.1097/00000539-200201000-00006. [DOI] [PubMed] [Google Scholar]
- 9.Tschirch FT, Göpfert K, Fröhlich JM, Brunner G, Weishaupt D. Low-dose intranasal versus oral midazolam for routine body MRI of claustrophobic patients. Eur Radiol. 2007;17:1403–10. doi: 10.1007/s00330-006-0457-1. [DOI] [PubMed] [Google Scholar]
- 10.Kain ZN, Hofstadter MB, Mayes LC, Krivutza DM, Alexander G, Wang SM, et al. Midazolam: Effects on amnesia and anxiety in children. Anesthesiology. 2000;93:676–84. doi: 10.1097/00000542-200009000-00016. [DOI] [PubMed] [Google Scholar]


