Abstract
Background:
Chronic subdural hematoma (CSDH) is one of the most common clinical entities in daily neurosurgical practice which carries a most favorable prognosis. However, because of the advanced age and medical problems of patients, surgical therapy is frequently associated with various complications. This study evaluated the clinical features, radiological findings, and neurological outcome in a large series of patients with CSDH.
Methods:
A classification and regression tree (CART) technique was employed in the analysis of data from 986 patients who were operated at Asclepeion General Hospital of Athens from January 1986 to December 2011. Burr holes evacuation with closed system drainage has been the operative technique of first choice at our institution for 29 consecutive years. A total of 27 prognostic factors were examined to predict the outcome at 3-month postoperatively.
Results:
Our results indicated that neurological status on admission was the best predictor of outcome. With regard to the other data, age, brain atrophy, thickness and density of hematoma, subdural accumulation of air, and antiplatelet and anticoagulant therapy were found to correlate significantly with prognosis. The overall cross-validated predictive accuracy of CART model was 85.34%, with a cross-validated relative error of 0.326.
Conclusions:
Methodologically, CART technique is quite different from the more commonly used methods, with the primary benefit of illustrating the important prognostic variables as related to outcome. Since, the ideal therapy for the treatment of CSDH is still under debate, this technique may prove useful in developing new therapeutic strategies and approaches for patients with CSDH.
Keywords: Chronic subdural hematoma, neurological outcome, prediction tree
INTRODUCTION
Chronic subdural hematoma (CSDH) represents one of the most frequent types of intracranial disorder which carries a most favorable prognosis when diagnosed accurately and treated adequately. A steady increase in the incidence of CSDH has been observed in developing countries due to the rise in life expectancy.[20,35,52,67]
The standard treatment for CSDH is a surgical evacuation, which usually results in improvement of the neurological picture. This condition has been treated by various surgical procedures such as burr holes evacuation, the most popular technique worldwide, twist – drill craniostomy, craniotomy, endoscopic removal, and subdural – peritoneal shunt.[3,19,32,33,34,42,44,46] However, all these procedures are associated with various complications.[6,27,32,33,45] Numerous factors potentially associated with the outcome of patients with CSDH have been reported, although the postoperative neurological outcome of CSDH has not improved substantially over the past 30 years.[1,7,8,9,14,17,20,24,25,35,36,37,38,60,61,62,64,66]
Classification and regression tree (CART) is an alternative statistical method of making predictions from data based on repeated partitioning of the dataset into more homogeneous subgroups.[5,54,55] CART searches for combinations of values of independent variables that best predict the value of the dependent variable and results are presented as “decision trees.”
In our department, burr holes evacuation with closed system drainage has been the operative technique of first choice for 28 consecutive years. The aim of this study is to present our experience of the surgical management of a 986 patients with CSDH and to emphasize the importance of the factors that contribute to neurological outcome, by developing a simple CART model involving a wide set of several variables and parameters possibly related to prognosis. The constructed tree shows that the sequence of important prognostic factors varies among a different group of patients.
MATERIALS AND METHODS
Patient characteristics
This clinical study includes 986 adult patients with CSDH, who were treated surgically at the Neurosurgical Department of Asclepeion General Hospital of Athens, from January 1986 to December 2011. This series represents the experience of our neurosurgical unit, a reference center covering a wide area, in managing 986 CSDH cases with burr holes and a closed drainage system of a total of 1039 CDSH patients. Patients were included in this study if the clinical presentation was because of CSDH alone. We excluded all patients who had been previously operated elsewhere or with incomplete medical records. Subdural hygromas, calcified or ossified CSDHs (the so-called “armored brain”), asymptomatic CSDHs, and patients who were inadequately followed up were also excluded from this study. Information was obtained retrospectively by reviewing the clinical histories.
The sample population was composed of 650 males and 336 females (ratio 1.9:1), with a mean age of 69 years (range 29–96 years). Five-hundred and three patients (51%) had a history of head trauma, most of which were minor or moderate.
The neurological status on admission was classified according to the most common neurological grading scheme for CSDH, as proposed by Markwalder et al.[32,33]: 751 patients were in Grades 0–2 and 235 patients were in Grades 3 and 4. The leading symptom for the over 70s was behavioral disturbance (37.4%), whereas the most frequent symptom in the under 70s was a headache (28.7%).
Predisposing factors included the administration of anticoagulant (AC) or antiaggregant therapy (237 patients), alcohol abuse (132 patients), coagulopathy (89 patients), and ventriculo – peritoneal shunt (6 patients). Arterial hypertension and diabetes mellitus presented in 19% and 14% of patients, respectively. Other concomitant pathological conditions are summarized in Table 1.
Table 1.
Prognostic factors in 986 patients with CSDH

In all cases, diagnosis was based on computed tomography (CT), and CSDHs were classified into four groups according to the density on CT scan: Hypodense (144 cases), isodense (426 cases), hyperdense (61 cases), or mixed (355 cases), on the basis of the density of hematoma relative to brain tissue.[9,26,35,49,66] Radiological measures of the CSDH, including the width of hematoma and midline shift were determined based on CT scans obtained before the operational procedure. The CSDH was right sided in 434 patients (44%), left sided in 473 patients (48%), and bilateral in the remaining 79 (8%). Moreover, evidence of brain atrophy received particular attention; brain atrophy was classified into two grades: No or minor atrophy and medium or severe atrophy.[2,9,30,39,40]
Routine laboratory studies before surgery included a complete blood count, platelet count, international normalized ratio, prothrombin time and activated partial thromboplastin time, and biochemical investigations. Any antiaggregant and AC therapy was temporarily discontinued upon admission and re-established no earlier than 4 weeks after the operation. Coagulopathy, if present, was corrected preoperatively by intravenous (IV) infusion of fresh frozen plasma, Vitamin K, or platelets.
Surgical management
Surgery was performed with mild neuroleptanalgesia and local anesthesia, and perioperative antibiotic prophylaxis was given in all cases. Moreover, all patients received postadmissionally anticonvulsants in the usual way for at least 3-month.
The operative technique of first choice in our clinic for all these years was to deal with the hematoma through burr holes and slow evacuation and irrigation with continuous closed system drainage. Thus, the uniformity of the surgical procedure was maintained. We never tried to remove the subdural hematoma vigorously. A flat Jackson-Pratt drain was introduced under direct vision into the subdural space, and particular precaution was taken not to injure the inner membrane of the hematoma or the brain. A mild vacuum generated suction applied, and the drainage was maintained for a period ranging from two to a maximum of 6 days postoperatively depending on the amount of draining liquid obtained. All bilateral CSDHs were operated upon at the same session. No attempt was made to reinflate the brain by intrathecal injection of the isotonic solution in any patient.
Postoperatively, the patients were kept supine for 48 h to enhance gravitational drainage of residual subdural fluid, were adequately hydrated given 2000cc of IV fluids a day for 3–4 days to promote expansion of the brain, and were mobilized as soon as possible. If early postoperative anticoagulation was required, low molecular weight heparin was administrated.
According to our protocol, a control CT scan was done before taking out the drainage, or earlier as judged clinically appropriate. Postoperative CT scan of all patients were evaluated, and we focused on the maximum residual hematoma thickness, the midline displacement, and the amount of residual air into the subdural cavity.
The patients were assessed periodically, and CT scans were normally repeated at 6 and 12 weeks after discharge if there was any residual collection shown in the first postoperative scan, or earlier if the symptoms reoccurred.
CSDH was considered to have recurred when neurological signs and/or symptoms increased, reappeared, or did not improve within 3-month of the original procedure and the hematoma cavity volume increased. Only patients who fulfilled both criteria underwent repeated operation. Residual hematoma into subdural cavity following the first operational procedure without accompanying signs and symptoms, and with no high-grade mass effect was not recognized as recurrence or as an indication for repeated surgery in this study.
All patients included in this series were followed up for at least 3-month postoperatively. The outcome was assessed at 3-month according to the Glasgow Outcome Scale.[21] Good recovery and moderate disability were considered to be favorable outcomes; severe disability, persistent vegetative state, and dead to be unfavorable.
Statistical analysis
It is well-known that a certain combination of factors yields a more effective prediction of outcome than when factors are used singly. We studied a total of 27 parameters that may be related to outcome [Table 1].
The CART approach is an alternative to the traditional methods for prediction.[5,54,55] It relies on statistically optimum recursive splitting of the patients into smaller and smaller subgroups, based on the critical levels of the prognostic variables. The dataset is split into the two subgroups that are the most different with respect to the outcome. This procedure is continued on each subgroup until some minimum subgroup size is reached. Other desirable properties of CART include incorporation of nonlinear relationships and interactions between predictors, and the ability to predict the outcome of cases despite some missing data, which may be difficult or impossible using traditional multivariate techniques.
For this analysis, the selected method for growing the classification tree was Gini splitting rule, with the extra condition that, whenever possible, at least 10 patients were available at each of the final subgroups. To assess the performance of the prediction tree and the independent predictive accuracy of the model, CART uses cross-validation. The tree presented is the one that minimizes the overall cross-validated relative error estimate that which most accurately predicts data excluded from forming the tree.
RESULTS
Postoperative complications occurred in 224 patients (22.7%). The most common complication was a recurrence of CSDH. In a total of 117 patients (11.8%) further surgery was required to remove a symptomatic recurrence of CSDH, and 9 cases underwent a third operation. The first recurrence was again treated by reopening the burr holes, but in patients with a second symptomatic recurrence a craniotomy was performed. The interval from the primary procedure to the re-operation ranged from 4 days to 9 weeks.
Early major postoperative air accumulation into the subdural cavity was detected on 39 patients. None of these suffered tension pneumocephalus. In 5 patients, surgery was complicated by the development of small intraparenchymatous bleeds in the cerebral hemispheres. All resolved gradually with no need for additional surgery. Two patients developed an acute subdural hematoma due to preexisting severe coagulation disorder and underwent a craniotomy.
Four patients developed a superficial wound infection and underwent local debridement in addition to IV antibiotic therapy. Two patients developed subdural empyema which required a craniotomy.
Although in our series all patients were treated prophylactically with anticonvulsants, 17 (1.7%) developed early postoperative seizures.
Two more patients had a cerebral infarction, in the territory of posterior cerebral and internal carotid artery respectively.
Serious postoperative complicating diseases were recognized in 48 patients (4.8%) and were treated by methods standard at the time. Atelectasis and bronchopneumonia were the most common medical complications in 25 cases. Other postoperative pathological conditions were cardiologic problems (11 cases), thromboembolic (7 cases), renal (4 cases), and septic complications (1 case).
A prediction tree for 986 patients with CSDH based on their known 3-month Glasgow Outcome Scale is presented in Figure 1. The ovals in this diagram denote intermediate subgroups subject to further splitting. The name of the corresponding split variable is recorded within each oval, and the actual split values are indicated in the branches of the tree. The terminal prognostic subgroups are represented by squares; within each square, the predicted rate of unfavorable outcomes and the total number of patients having that pattern in the tree are given. The subgroups, ranked according to the proportion of bad outcomes, are numbered 1–8 as denoted below the squares. Thus, for example, subgroup 1 is the group with the worst prognostic pattern (unfavorable outcome 90.6%) while subgroup 8 is the group with the best prognostic pattern (favorable outcome 97.5%).
Figure 1.
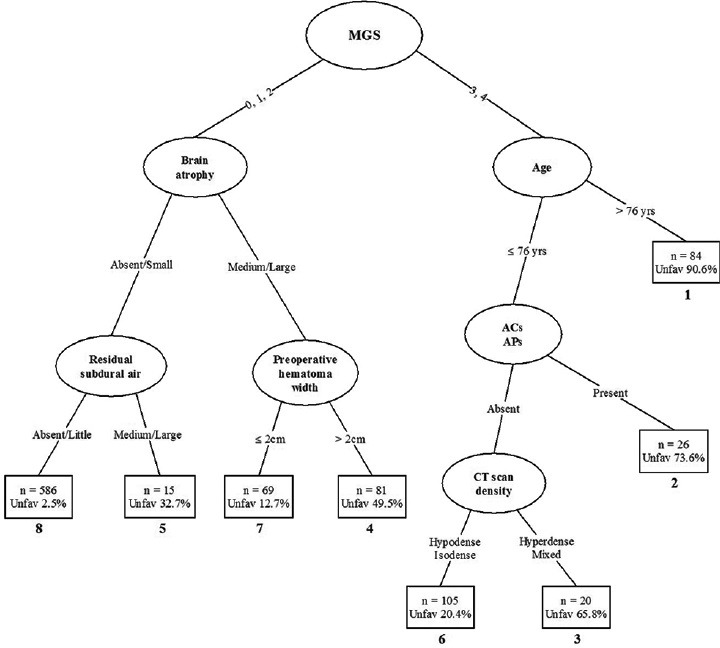
Prediction tree based on 986 patients with chronic subdural hematoma. Ovals denote intermediate subgroups subject to further splitting; squares denote terminal prognostic subgroups. The numbers below the squares represent the prognostic rank of each subgroup based on the proportion of unfavorable outcomes. ACs: Anticoagulants, Aps: Antiplatelet agents, CT: Computed tomography, MGS: Markwalder's grading score
The patients are first split on the basis of their Markwalder's grading score (MGS) on admission, with the cut-off point at 2. Patients with a good neurological status on admission (Grades 0–2) are separated from those having poor neurological grades of 3 and 4.
Subsequent splits in these two major branches of the tree show different patterns. Patients who were very drowsy or worse (Grades 3 and 4) tend to have reasonably an unfavorable outcome unless they have both an absence of AC taking and are not very aged. For those no very elderly patients without ACs and antiplatelets, CT density of CSDH, the next split of the tree, also plays an important role in determining the outcome in certain patients. Thus, two of the earliest splits for the MGS ≥3 are based on age and usage of AC – antiaggregant agents, suggesting that these variables have a greater effect on outcome in these patients.
Patients with a MGS of two or less tend to have a favorable neurological outcome, with pre- and post-operative CT scan findings appearing in subsequent splits. Considerable brain atrophy, high preoperative hematoma thickness, and a large amount of postoperative subdural air predispose an unfavorable outcome.
Prediction of outcome for a patient is accomplished by simply running that patient down the CART tree, according to the values of the prognostic variables. For example, a very elderly patient with a poor MGS of three or four would be placed in subgroup 1. The predicted outcome for such a patient is severely disabled, vegetative, or dead. In contrast, a patient with a better clinical score with neither brain atrophy nor postoperative subdural air would fall into subgroup 8, and the expected outcome is good recovery or moderately disabled.
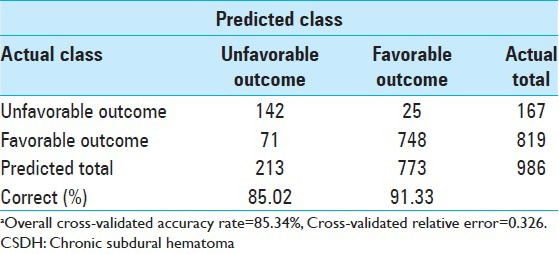
Because the conventional methods of assessing tree accuracy can be wildly optimistic, cross-validation is the method CART normally uses to obtain objective measures for smaller data sets. This CART model, with eight terminal nodes, had a grossly accuracy rate of 89.56%, a cross-validated predictive accuracy of 85.34%, and a cross-validated relative error of 0.326. The proportion of cases correctly classified in each outcome category and for the entire dataset is summarized in Table 2. Moreover, the application of bootstrap aggregation and adaptive resampling and combining methods did not change the predictions from the single CART tree (model stability).
Table 2.
Prediction success table for cross-validated sample based on in 986 patients with CSDHa
DISCUSSION
Treatment of CSDH has improved dramatically in recent years because of advances in diagnostic tools and surgical techniques. However, there is still some debate regarding the best strategy for treatment. The burr holes evacuation, irrigation, and subsequent closed drainage technique is a simple treatment which is able to achieve good results with minimal complications and is at present favored.[2,35,38,40,43,48] Although this method may lead to recurrences, it is preferable to postpone craniotomy until the subdural collections repeatedly re-accumulate, or there is a solid hematoma or marked cerebral swelling underneath the CSDH site.[13,19,33,34,57] Some authors suggest that the applied operative technique is not always of major importance, as long as it is able to suck out the subdural fluid slowly, is performed properly without injuring again the subdural space, and is followed by precise and competent nursing.[29,46,64,65]
Early identification of reliable prognostic factors for patients with CSDH is of great importance. Many of the previous clinical studies have begun with several variables from which the best candidates determined on statistical criteria were selected. However, this approach to the problem ignores the potential differences between the candidate predictors among various patient subgroups. Another common problem is that only a few of the previous clinical studies provide information about the critical point thresholds of each indicator beyond which the risk of a good outcome is substantially increased or decreased.
The results of this study reinforce many previous findings on prediction of outcome for patients with CSDH. Previous studies have clearly reported the bad outcome in patients with lower neurological status on admission.[2,11,12,32,33,45,62] As always in neurosurgery, poor neurological condition on admission has a negative effect on the outcome. In the past, comparisons between the reported series of CSDH were frequently difficult to make as outcome was influenced by the proportion of patients who were drowsy or comatose, with or no neurological symptoms and focal defects, and MGS of clinical state was a valuable contribution to this problem, in the same way that grading has been for head injury and spontaneous subarachnoid hemorrhage.[32,33] This well-recognized scale has been adopted by many authors because of its simplicity and becomes more useful when combined with other variables. Our results suggest that a higher MGS of three and four was closed related to an unfavorable neurological outcome in agreement with earlier reports.
As medical science and public health measures brought the majority of diseases of elderly patients under control, the number of elderly people increased and will continue to do so. Many studies support the belonging of CSDH to brain aging pathology and with the advent of CT scan, an increasing number of aged patients affected by CSDH are diagnosed at an earlier stage of the disease. Age is also generally thought to be a strong predictor of prognosis, and most studies have shown worse prognosis in patients with increasing age.[11,28,35,43] In our series, an unfavorable outcome was associated with increasing age beyond the CART threshold of 76 years. Although elderly did tend to do less well, advancing age was not always bar to success. No elderly patient should be denied low-invasive surgery, as the chances of recovery are not zero.[18,41,67]
After the introduction of CT scanning, diagnosis and outcome of CSDH became much improved. Several authors have proposed various radiological classifications for CSDH, concentrating on the density of hematoma relative to brain tissue,[9,26,35,37,39,49,66] internal architecture which corresponds to possible stages in the natural history of CSDH,[37] and location of hematoma according to the intracranial extension.[37] Our CT variable was designed to be a simple easily identifiable density feature, and CT results were simplified to four diagnostic categories. Evidence of brain atrophy received particular attention since several studies have shown that the presence of brain atrophy in CSDH is associated with a worse outcome.[2,30,39,40]
The density of hematoma on CT scan represents the proportion of fresh blood clots in hematoma cavity. The imaging appearance of the CSDH on CT scans may help identify fresh blood from re-bleeding, suggest the age of the hematoma, and reflect upon the protein concentration from plasma exudation.[16,37,59] Thus, hyperdense and mixed appearances are considered to have a greater tendency to re-bleeding and higher protein exudation rates.[20,39,59] Recent experimental studies have revealed that blood in the subdural space evokes an inflammatory reaction and because of this, the CSDH is more active.[9,15] Our data indicated that high density and mixed hematomas were related to an unfavorable postoperative outcome. In contrast, patients with isodense and hypodense CSDH had a better neurological prognosis. These findings correspond well to some previous results reported in the literature.[2,11,23,24,25,37,39] In our opinion, for the initial treatment of CSDH, complete withdrawal of the subdural fluid, which contains the fibrinolytic agents, by adequate rinsing of the hematoma cavity, is more important per se than the selected operative procedure or membranectomy.
The size of a CSDH at the time of diagnosis can be impressive, and its volume may be a predictor of the postoperative course.[20,40,56] Increased size of hematoma is often attributed to brain atrophy associated with aging, which may provide the CSDH with a potential space in which to grow.[52] The intracranial pressure – volume function reflects the elastic properties of the brain parenchyma, the cerebrovascular bed, and the supporting dural structures within the rigid cranium. Intracranial volume changes are superimposed on the intracranial pressure – volume function of brain elasticity. The elasticity function may be altered by advanced age, brain atrophy, a large amount of CSDH, and prolonged compressed parenchyma. Brains with high elastance tend to re-expand poorly since postoperative subdural space is larger, and this may lead to persistence of postoperative midline shifting and residual air.[17,39,45,50,51,60]
In previous studies, the recurrence rate and the bad outcome of CSDH were higher in patients with greater width of hematoma, cerebral atrophy, and significant subdural air accumulation, and this is in agreement with our series.[2,22,23,35,37,39,40,45,58,60,62,66] In the present study, wider hematomas with a preoperative maximum thickness superior to 20 mm, considerable brain atrophy, and larger amounts of residual air into hematoma cavity were related to the worst prognosis. In these patients, caution not to tear the inner membrane of the CSDH or arachnoid membrane, complete replacement of CSDH fluid by normal saline to prevent intraoperative influx of air into the subdural cavity, postoperative bed rest with adequate IV fluid administration, and maintenance of the drain for a longer period with continuum of antibiotics as long as the catheter remains inserted might prevent a worse outcome by facilitation of brain re-expansion and elimination of the possibility of systemic and intracranial hypotension.[1,34,35,39]
Recently, with increasing numbers of elderly people in the general population, the number of patients who are treated with ACs and antiplatelets is also increasing. These agents are commonly used as prophylactics against cerebral ischemic stroke, myocardial infarction, valvular heart disease or deep venous thrombosis. Bleeding diatheses is well-known risk of these drugs and both have historically been considered as risk factors for CSDH. Anticoagulation and antiplatelet agents are certainly dangerous, because are the causes of the pathology in some cases without evidence of trauma, may add to the risk of CSDH by as much as 42.5 times, have a positive influence on the recurrence of CSDH, and are at least partly responsible for the unfavorable outcome of several patients.[7,8,10,11,14,31,47,62,65] Considering the increasing number of aged patients who use antiplatelet and AC medications, attention should be focused on the possible risks of these treatments. Since the exposure to antiplatelets and ACs increases the risk of developing a CSDH, as well as the risk of reoperation and of a worse prognosis after surgery, the indication to these therapies should strictly follow the current evidence in order to avoid a dangerous undue risk-benefit imbalance.[68] Furthermore, it's of outmost importance the preoperative discontinuation of any therapy with these agents or the correction of any preexisting coagulopathy in patients with CSDH.
It is a basic assumption that CSDH should be removed by simple means. Convincing evidence has accumulated that burr holes technique is a safe, time-saving, and rational treatment which can be performed for elderly patients or those with multiple medical problems using local anesthesia and is usually able to achieve favorable results with minimal complications.[8,12,25,38,43] Postoperative recurrence of CSDH is not rare and has always been a source of frustration for neurosurgeons. Thus, it comes as no surprise that the vast majority of the published articles focus on this matter.[11,26,28,38,40,45,61,62,64,68] Asymptomatic persistent subdural fluid may take up to several weeks for a complete resolution, and can be followed with serial studies until its resolution without surgical intervention. Thus, to evaluate this outcome, longer follow-up periods are necessary.[4,53] Because of these, we were mainly interested in the overall outcome of patients with CSDH based principally on the evolution of the clinical course and all our patients had a postoperative follow-up during an interval up to 3-month. In this period, many cases slowly improved, but some deteriorated or died of related or unrelated causes; multiple internal diseases might affect prognosis more than hematoma's management. A zero mortality rate in any large series is impossible as there will always be late cases on poor MGS or those patients with serious complicating illnesses, particularly in the elderly.
In this analysis, CART was used as an alternative method to predict neurological outcome in 986 patients with CSDH, who were treated surgically with specific inclusion and exclusion criteria. Our results indicate that neurological status on admission (MGS) was the best predictor of outcome. With regard to the other data, the most already widely examined variables such as age, brain atrophy, CT scan findings, or usage of antiplatelet and AC agents proved again to be strong predictors and were found to correlate significantly with prognosis.
The sequence of “splits” points out possible interactions between predictors. For example, effects of aging and AC – antiplatelet therapy appear sooner among patients with a high Markwalder functional score, whereas CT scan findings tend to exert an early influence when the neurological status is less severe. However, these different splits in different branches may just represent variations in small samples with uncertain interaction or clinical importance and need probably to be investigated in a larger prospective clinical study.
Moreover, although the neurological grade on admission emerges as the most powerful early predictor of outcome, radiological variables, such as thickness and density of subdural collection and postoperative accumulation of air into the subdural cavity, might help identify different groups of patients even within the same levels of neurological severity.
Although 27 potential prognostic factors were considered in this study, only seven were actually used in the construction of the CART tree. Several of these have been shown to be effective predictors of outcome in previous studies. The fact that one predictor does not appear in the tree does not necessarily reflect a lack of relationship: This relationship may be subsumed by another variable.[5,54,55] We found that for each split, a short list of “competitor” splits describes the best alternative splits at that point. For example, severe associated diseases, midline displacement, and postoperative pathological complications often appear as competitors in CART analyses and possess a significant predictive power, even though they do not appear in Figure 1.
In our knowledge, this is the first study using a CART model to predict the neurological outcome of patients with CSDH. The overall cross-validated predictive accuracy of our CART tree was 85.34%, with a cross-validated relative error of 0.326. We compared these rates with those obtained with the most traditional method of the logistic regression analysis performed with the same prognostic factors. There was only a slight difference which did not seem to be impressive.
Methodologically, however, CART is quite different from the more commonly used statistical methods. Predictions are read directly from the tree diagram with no requiring specific measurements to derive the patients’ outcome. Based on subgroups, the CART system may indicate important relationships between study variables more clearly than corresponding regression analyses when the relationships are not linear or additive. In addition, one can use CART if some predictors are missing. One disadvantage of the model is the need to split each prognostic factor into two possible groups at each stage of a tree's construction. Although this is fine for variables that could take only two values, it might produce slightly arbitrary categorizations of continuous variables. Thus, care should be taken with patients near the cut-off point, with perhaps the more optimistic route being explored first.[5,54,55]
A large number of papers have been published on the subject of CSDH and many methods of treatment have been proposed, although the ideal standard therapy has not been definitely established and is still under debate. The mechanism by which the subdural collection increases in size and induces anatomical and biological changes is still a fascinating problem. A further research on the complex pathophysiology, methodology of management, and extent of surgical treatment of CSDH might give explanations, but the standard treatment will probably not change much in the near future. Thus, there is a need for properly conducted prospective trials on therapeutic approaches for CSDH. It might also be worthwhile seeking alternative strategies in the treatment of this frequent condition.[63]
Although the present study was retrospective, and, therefore, imposes certain limitations, or may have sources of bias and variations, this CART technique is a visually useful simple way to look at the late prognosis of patients with CSDH. A tree diagram, illustrating the prognostic pattern, provides some threshold values that split the patients into subgroups with varying degrees of risk.
It is of outmost importance, however, that this technique is meant to supplement, not to replace or cloud the neurosurgeon's clinical judgment and other predictive factors. Moreover, course and outcome of patients with a CSDH can be influenced, and often irrevocably altered, by several factors and unsuspected serious medical complications.
Footnotes
Contributor Information
Aristedis Rovlias, Email: arovlias@yahoo.com.
Spyridon Theodoropoulos, Email: spyridon.theodoropoulos@yahoo.gr.
Dimitrios Papoutsakis, Email: dim.tsakis@yahoo.gr.
REFERENCES
- 1.Abouzari M, Rashidi A, Rezaii J, Esfandiari K, Asadollahi M, Aleali H, et al. The role of postoperative patient posture in the recurrence of traumatic chronic subdural hematoma after burr-hole surgery. Neurosurgery. 2007;61:794–7. doi: 10.1227/01.NEU.0000298908.94129.67. [DOI] [PubMed] [Google Scholar]
- 2.Amirjamshidi A, Abouzari M, Eftekhar B, Rashidi A, Rezaii J, Esfandiari K, et al. Outcomes and recurrence rates in chronic subdural haematoma. Br J Neurosurg. 2007;21:272–5. doi: 10.1080/02688690701272232. [DOI] [PubMed] [Google Scholar]
- 3.Aoki N. Chronic subdural hematoma in infancy. Clinical analysis of 30 cases in the CT era. J Neurosurg. 1990;73:201–5. doi: 10.3171/jns.1990.73.2.0201. [DOI] [PubMed] [Google Scholar]
- 4.Asano Y, Hasuo M, Takahashi I, Shimosawa S. Recurrent cases of chronic subdural hematoma – Its clinical review and serial CT findings. No To Shinkei. 1992;44:827–31. [PubMed] [Google Scholar]
- 5.Breiman L, Friedman JH, Olshen RA, Stone CJ. Belmont, CA: Wadsworth International; 1984. Classification and Regression Trees. [Google Scholar]
- 6.Cameron MM. Chronic subdural haematoma: A review of 114 cases. J Neurol Neurosurg Psychiatry. 1978;41:834–9. doi: 10.1136/jnnp.41.9.834. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Chen JC, Levy ML. Causes, epidemiology, and risk factors of chronic subdural hematoma. Neurosurg Clin N Am. 2000;11:399–406. [PubMed] [Google Scholar]
- 8.Choi WW, Kim KH. Prognostic factors of chronic subdural hematoma. J Korean Neurosurg Soc. 2004;35:192–8. [Google Scholar]
- 9.Chon KH, Lee JM, Koh EJ, Choi HY. Independent predictors for recurrence of chronic subdural hematoma. Acta Neurochir (Wien) 2012;154:1541–8. doi: 10.1007/s00701-012-1399-9. [DOI] [PubMed] [Google Scholar]
- 10.De Bonis P, Trevisi G, de Waure C, Sferrazza A, Volpe M, Pompucci A, et al. Antiplatelet/anticoagulant agents and chronic subdural hematoma in the elderly. PLoS One. 2013;8:e68732. doi: 10.1371/journal.pone.0068732. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Delgado PD, Cogolludo FJ, Mateo O, Cancela P, García R, Carrillo R. Early prognosis in chronic subdural hematomas. Multivariate analysis of 137 cases. Rev Neurol. 2000;30:811–7. [PubMed] [Google Scholar]
- 12.El-Kadi H, Miele VJ, Kaufman HH. Prognosis of chronic subdural hematomas. Neurosurg Clin N Am. 2000;11:553–67. [PubMed] [Google Scholar]
- 13.Ernestus RI, Beldzinski P, Lanfermann H, Klug N. Chronic subdural hematoma: Surgical treatment and outcome in 104 patients. Surg Neurol. 1997;48:220–5. doi: 10.1016/s0090-3019(97)80031-6. [DOI] [PubMed] [Google Scholar]
- 14.Forster MT, Mathé AK, Senft C, Scharrer I, Seifert V, Gerlach R. The influence of preoperative anticoagulation on outcome and quality of life after surgical treatment of chronic subdural hematoma. J Clin Neurosci. 2010;17:975–9. doi: 10.1016/j.jocn.2009.11.023. [DOI] [PubMed] [Google Scholar]
- 15.Frati A, Salvati M, Mainiero F, Ippoliti F, Rocchi G, Raco A, et al. Inflammation markers and risk factors for recurrence in 35 patients with a posttraumatic chronic subdural hematoma: A prospective study. J Neurosurg. 2004;100:24–32. doi: 10.3171/jns.2004.100.1.0024. [DOI] [PubMed] [Google Scholar]
- 16.Fujisawa H, Nomura S, Tsuchida E, Ito H. Serum protein exudation in chronic subdural haematomas: A mechanism for haematoma enlargement? Acta Neurochir (Wien) 1998;140:161–5. doi: 10.1007/s007010050077. [DOI] [PubMed] [Google Scholar]
- 17.Fukuhara T, Gotoh M, Asari S, Ohmoto T, Akioka T. The relationship between brain surface elastance and brain reexpansion after evacuation of chronic subdural hematoma. Surg Neurol. 1996;45:570–4. doi: 10.1016/0090-3019(95)00471-8. [DOI] [PubMed] [Google Scholar]
- 18.Fukui S. Evaluation of surgical treatment for chronic subdural hematoma in extremely aged (over 80 years old) patients. No To Shinkei. 1993;45:449–53. [PubMed] [Google Scholar]
- 19.Gonugunta V, Buxton N. Warfarin and chronic subdural haematomas. Br J Neurosurg. 2001;15:514–7. doi: 10.1080/02688690120097822. [DOI] [PubMed] [Google Scholar]
- 20.Gorelick PB, Weisman SM. Risk of hemorrhagic stroke with aspirin use: An update. Stroke. 2005;36:1801–7. doi: 10.1161/01.STR.0000174189.81153.85. [DOI] [PubMed] [Google Scholar]
- 21.Jennett B, Bond M. Assessment of outcome after severe brain damage. A practical scale. Lancet. 1975;1:480–4. doi: 10.1016/s0140-6736(75)92830-5. [DOI] [PubMed] [Google Scholar]
- 22.Kaczmarczyk R, Osuchowski J, Trojanowski T, Turowski K, Rakowski P. Early results of surgical treatment of chronic subdural hematoma in CT images. Neurol Neurochir Pol. 1994;2 8:693–701. [PubMed] [Google Scholar]
- 23.Kim HY, Kwon SC, Kim TH, Shin HS, Hwang YS, Park SK. Analysis of management according to CT findings in chronic subdural hematoma. J Korean Neurosurg Soc. 2005;37:96–100. [Google Scholar]
- 24.Ko BS, Lee JK, Seo BR, Moon SJ, Kim JH, Kim SH. Clinical analysis of risk factors related to recurrent chronic subdural hematoma. J Korean Neurosurg Soc. 2008;43:11–5. doi: 10.3340/jkns.2008.43.1.11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Kong WK, Kim BC, Cho KT, Hong SK. Factors affecting postoperative recurrence of chronic subdural hematoma. Korean J Neurotrauma. 2012;8:122–7. [Google Scholar]
- 26.Kostanian V, Choi JC, Liker MA, Go JL, Zee CS. Computed tomographic characteristics of chronic subdural hematomas. Neurosurg Clin N Am. 2000;11:479–89. [PubMed] [Google Scholar]
- 27.Kotwica Z, Brzezinski J. Chronic subdural haematoma treated by burr holes and closed system drainage: Personal experience in 131 patients. Br J Neurosurg. 1991;5:461–5. doi: 10.3109/02688699108998474. [DOI] [PubMed] [Google Scholar]
- 28.Krupp WF, Jans PJ. Treatment of chronic subdural haematoma with burr-hole craniostomy and closed drainage. Br J Neurosurg. 1991;5:459–60. doi: 10.1080/02688699550040909. [DOI] [PubMed] [Google Scholar]
- 29.Lee GY, Oh CH, Shim YS, Yoon SH, Park HC, Park CO, et al. Comparison of drainage volume of chronic subdural hematoma according to drainage catheter type. Yonsei Med J. 2013;54:1091–7. doi: 10.3349/ymj.2013.54.5.1091. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Lee KS, Bae WK, Doh JW, Bae HG, Yun IG. Origin of chronic subdural haematoma and relation to traumatic subdural lesions. Brain Inj. 1998;12:901–10. doi: 10.1080/026990598121972. [DOI] [PubMed] [Google Scholar]
- 31.Lindvall P, Koskinen LO. Anticoagulants and antiplatelet agents and the risk of development and recurrence of chronic subdural haematomas. J Clin Neurosci. 2009;16:1287–90. doi: 10.1016/j.jocn.2009.01.001. [DOI] [PubMed] [Google Scholar]
- 32.Markwalder TM, Seiler RW. Chronic subdural hematomas: To drain or not to drain? Neurosurgery. 1985;16:185–8. doi: 10.1227/00006123-198502000-00010. [DOI] [PubMed] [Google Scholar]
- 33.Markwalder TM, Steinsiepe KF, Rohner M, Reichenbach W, Markwalder H. The course of chronic subdural hematomas after burr-hole craniostomy and closed-system drainage. J Neurosurg. 1981;55:390–6. doi: 10.3171/jns.1981.55.3.0390. [DOI] [PubMed] [Google Scholar]
- 34.Markwalder TM. Chronic subdural hematomas: A review. J Neurosurg. 1981;54:637–45. doi: 10.3171/jns.1981.54.5.0637. [DOI] [PubMed] [Google Scholar]
- 35.Mori K, Maeda M. Surgical treatment of chronic subdural hematoma in 500 consecutive cases: Clinical characteristics, surgical outcome, complications, and recurrence rate. Neurol Med Chir (Tokyo) 2001;41:371–81. doi: 10.2176/nmc.41.371. [DOI] [PubMed] [Google Scholar]
- 36.Nagatani K, Takeuchi S, Sakakibara F, Otani N, Nawashiro H. Radiological factors related to recurrence of chronic subdural hematoma. Acta Neurochir (Wien) 2011;153:1713. doi: 10.1007/s00701-011-0971-z. [DOI] [PubMed] [Google Scholar]
- 37.Nakaguchi H, Tanishima T, Yoshimasu N. Factors in the natural history of chronic subdural hematomas that influence their postoperative recurrence. J Neurosurg. 2001;95:256–62. doi: 10.3171/jns.2001.95.2.0256. [DOI] [PubMed] [Google Scholar]
- 38.Nakaguchi H, Tanishima T, Yoshimasu N. Relationship between drainage catheter location and postoperative recurrence of chronic subdural hematoma after burr-hole irrigation and closed-system drainage. J Neurosurg. 2000;93:791–5. doi: 10.3171/jns.2000.93.5.0791. [DOI] [PubMed] [Google Scholar]
- 39.Oishi M, Toyama M, Tamatani S, Kitazawa T, Saito M. Clinical factors of recurrent chronic subdural hematoma. Neurol Med Chir (Tokyo) 2001;41:382–6. doi: 10.2176/nmc.41.382. [DOI] [PubMed] [Google Scholar]
- 40.Okada Y, Akai T, Okamoto K, Iida T, Takata H, Iizuka H. A comparative study of the treatment of chronic subdural hematoma – Burr hole drainage versus burr hole irrigation. Surg Neurol. 2002;57:405–9. doi: 10.1016/s0090-3019(02)00720-6. [DOI] [PubMed] [Google Scholar]
- 41.Ooba S, Shiomi N, Shigemori M. Clinical features and surgical results of chronic subdural hematoma in the extremely aged patients. No Shinkei Geka. 2006;34:273–8. [PubMed] [Google Scholar]
- 42.Probst C. Peritoneal drainage of chronic subdural hematomas in older patients. J Neurosurg. 1988;68:908–11. doi: 10.3171/jns.1988.68.6.0908. [DOI] [PubMed] [Google Scholar]
- 43.Ramachandran R, Hegde T. Chronic subdural hematomas – Causes of morbidity and mortality. Surg Neurol. 2007;67:367–72. doi: 10.1016/j.surneu.2006.07.022. [DOI] [PubMed] [Google Scholar]
- 44.Reinges MH, Hasselberg I, Rohde V, Küker W, Gilsbach JM. Prospective analysis of bedside percutaneous subdural tapping for the treatment of chronic subdural haematoma in adults. J Neurol Neurosurg Psychiatry. 2000;69:40–7. doi: 10.1136/jnnp.69.1.40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Robinson RG. Chronic subdural hematoma: Surgical management in 133 patients. J Neurosurg. 1984;61:263–8. doi: 10.3171/jns.1984.61.2.0263. [DOI] [PubMed] [Google Scholar]
- 46.Rodziewicz GS, Chuang WC. Endoscopic removal of organized chronic subdural hematoma. Surg Neurol. 1995;43:569–72. doi: 10.1016/0090-3019(95)00005-4. [DOI] [PubMed] [Google Scholar]
- 47.Rust T, Kiemer N, Erasmus A. Chronic subdural haematomas and anticoagulation or anti-thrombotic therapy. J Clin Neurosci. 2006;13:823–7. doi: 10.1016/j.jocn.2004.12.013. [DOI] [PubMed] [Google Scholar]
- 48.Santarius T, Lawton R, Kirkpatrick PJ, Hutchinson PJ. The management of primary chronic subdural haematoma: A questionnaire survey of practice in the United Kingdom and the Republic of Ireland. Br J Neurosurg. 2008;22:529–34. doi: 10.1080/02688690802195381. [DOI] [PubMed] [Google Scholar]
- 49.Scotti G, Terbrugge K, Melançon D, Bélanger G. Evaluation of the age of subdural hematomas by computerized tomography. J Neurosurg. 1977;47:311–5. doi: 10.3171/jns.1977.47.3.0311. [DOI] [PubMed] [Google Scholar]
- 50.Sklar FH, Beyer CW Jr, Clark WK. Physiological features of the pressure-volume function of brain elasticity in man. J Neurosurg. 1980;53:166–72. doi: 10.3171/jns.1980.53.2.0166. [DOI] [PubMed] [Google Scholar]
- 51.Sklar FH, Diehl JT, Beyer CW Jr, Clark WK. Brain elasticity changes with ventriculomegaly. J Neurosurg. 1980;53:173–9. doi: 10.3171/jns.1980.53.2.0173. [DOI] [PubMed] [Google Scholar]
- 52.Spallone A, Giuffrè R, Gagliardi FM, Vagnozzi R. Chronic subdural hematoma in extremely aged patients. Eur Neurol. 1989;29:18–22. doi: 10.1159/000116370. [DOI] [PubMed] [Google Scholar]
- 53.Steimlé R, Jacquet G, Godard J, Fahrat O, Katranji H. Chronic subdural hematoma in the elderly and computerized tomography. Study of 80 cases. Chirurgie. 1990;116:160–7. [PubMed] [Google Scholar]
- 54.Steinberg D, Colla PL. San Diego, CA: Salford Systems; 1997. CART: CART® – Classification and Regression Trees. [Google Scholar]
- 55.Steinberg D, Colla PL. San Diego, CA: Salford Systems; 1995. CART: Tree – Structured nonparametric data analysis. [Google Scholar]
- 56.Sundstrom T, Helland CA, Aarhus M, Wester K. What is the pressure in chronic subdural hematomas? A prospective, population-based study. J Neurotrauma. 2011;28:1–6. doi: 10.1089/neu.2011.1776. [DOI] [PubMed] [Google Scholar]
- 57.Tabaddor K, Shulmon K. Definitive treatment of chronic subdural hematoma by twist-drill craniostomy and closed-system drainage. J Neurosurg. 1977;46:220–6. doi: 10.3171/jns.1977.46.2.0220. [DOI] [PubMed] [Google Scholar]
- 58.Takayama M, Terui K, Oiwa Y. Retrospective statistical analysis of clinical factors of recurrence in chronic subdural hematoma: Correlation between univariate and multivariate analysis. No Shinkei Geka. 2012;40:871–6. [PubMed] [Google Scholar]
- 59.Tokmak M, Iplikcioglu AC, Bek S, Gökduman CA, Erdal M. The role of exudation in chronic subdural hematomas. J Neurosurg. 2007;107:290–5. doi: 10.3171/JNS-07/08/0290. [DOI] [PubMed] [Google Scholar]
- 60.Torihashi K, Sadamasa N, Yoshida K, Narumi O, Chin M, Yamagata S. Independent predictors for recurrence of chronic subdural hematoma: A review of 343 consecutive surgical cases. Neurosurgery. 2008;63:1125–9. doi: 10.1227/01.NEU.0000335782.60059.17. [DOI] [PubMed] [Google Scholar]
- 61.van Havenbergh T, van Calenbergh F, Goffin J, Plets C. Outcome of chronic subdural haematoma: Analysis of prognostic factors. Br J Neurosurg. 1996;10:35–9. doi: 10.1080/02688699650040502. [DOI] [PubMed] [Google Scholar]
- 62.Villagrasa J, Prat R, Díaz JF, Comuñas F. Analysis of prognostic factors in adults with chronic subdural hematoma. Neurologia. 1998;13:120–4. [PubMed] [Google Scholar]
- 63.Wang D, Li T, Tian Y, Wang S, Jin C, Wei H, et al. Effects of atorvastatin on chronic subdural hematoma: A preliminary report from three medical centers. J Neurol Sci. 2014;336:237–42. doi: 10.1016/j.jns.2013.11.005. [DOI] [PubMed] [Google Scholar]
- 64.Weigel R, Schmiedek P, Krauss JK. Outcome of contemporary surgery for chronic subdural haematoma: Evidence based review. J Neurol Neurosurg Psychiatry. 2003;74:937–43. doi: 10.1136/jnnp.74.7.937. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Weir B, Gordon P. Factors affecting coagulation: Fibrinolysis in chronic subdural fluid collections. J Neurosurg. 1983;58:242–5. doi: 10.3171/jns.1983.58.2.0242. [DOI] [PubMed] [Google Scholar]
- 66.Yamamoto H, Hirashima Y, Hamada H, Hayashi N, Origasa H, Endo S. Independent predictors of recurrence of chronic subdural hematoma: Results of multivariate analysis performed using a logistic regression model. J Neurosurg. 2003;98:1217–21. doi: 10.3171/jns.2003.98.6.1217. [DOI] [PubMed] [Google Scholar]
- 67.Zingale A, Albanese V, Romano A, Distefano G, Chiaramonte J. Traumatic chronic subdural hematoma over 80 years. A preliminary prospective study. J Neurosurg Sci. 1997;41:169–73. [PubMed] [Google Scholar]
- 68.Zingale A, Chibbaro S, Florio A, Distefano G, Porcaro S. Management of chronic subdural hematoma in patients treated with anticoagulation. J Neurosurg Sci. 1999;43:277–84. [PubMed] [Google Scholar]


