Abstract
The importance of neutralizing antibodies (NAbs) in protection against hepatitis C virus (HCV) remains controversial. We infused a chimpanzee with H06 immunoglobulin from a genotype 1a HCV-infected patient and challenged with genotype strains efficiently neutralized by H06 in vitro. Genotype 1a NAbs afforded no protection against genotype 4a or 5a. Protection against homologous 1a lasted 18 weeks, but infection emerged when NAb titers waned. However, 6a infection was prevented. The differential in vivo neutralization patterns have implications for HCV vaccine development.
TEXT
Chimpanzees have been essential for hepatitis C virus (HCV) research (1–3). Understanding the role of neutralizing antibodies (NAbs) in preventing HCV infection is important for vaccine efforts against this important pathogen, which annually infects ∼4 million people and causes chronic liver disease (4–12). We showed that chronic-phase patient serum/plasma prevented HCV infection of chimpanzees if the virus and anti-HCV antibody were incubated prior to inoculation (13, 14). Others showed that immunoglobulin (IgG) purified from anti-HIV antibody-positive blood donors prevented HCV infection when mixed with virus and then administered to a chimpanzee (15). However, the protective effect of preexisting polyclonal HCV NAbs remains to be determined.
Studies with culture systems using pseudotyped particles (HCVpp) or infectious viruses (chimeric cell culture-derived HCV [HCVcc]) confirmed the existence of NAbs in acute- and chronic-phase patients, with high-level cross-neutralization of heterotypic HCV strains (16–20). However, differences exist in neutralization capacities of chronic-phase samples against HCV variants of the same genotype (21), and there is a constant evolution of HCV variants escaping NAbs (14, 22, 23). Nonetheless, these in vitro data suggest that chronic-phase HCV samples might be useful for broad protection against HCV. Effective NAbs could help define critical epitopes for vaccine development (24–27).
We prepared H06 IgG from plasma obtained almost 30 years after disease onset in patient H with persistent HCV infection (28). By infusing H06 IgG with high in vitro neutralizing titers into a chimpanzee (CHA5A009) and then challenging with different HCV strains sensitive to neutralization in vitro (18, 19), our aim was to test the principle of passive immunoprophylaxis against homologous and heterologous strains in vivo.
Animal experimentation and sample collection from chimpanzee CHA5A009 were performed from 2007 to 2008. The housing and care of the chimpanzee met or exceeded all requirements of the National Research Council's 1996 Guide for the Care and Use of Laboratory Animals, 7th ed. (National Academies Press, Washington, DC), which were in effect until 2010. The project license (ASP LID 64) and specific protocol (06-C-482) were separately approved by the Institutional Animal Care and Use Committees of the National Institute of Allergy and Infectious Diseases, NIH, and Bioqual, Inc., the facility housing the chimpanzee. Both facilities were fully accredited by the American Association for Accreditation of Laboratory Animal Care International. Specifically, housing exceeded requirements for square footage and cage height for the chimpanzee's size. Feeding consisted of approved chimpanzee biscuits with supplemental vegetables and fruit and ad libitum water. A comprehensive enrichment program was in place and consisted of foods, puzzles, and environmental enrichment, coupled with visual and auditory contact with other chimpanzees. Sample collection was performed under ketamine hydrochloride anesthesia. Euthanasia was not required.
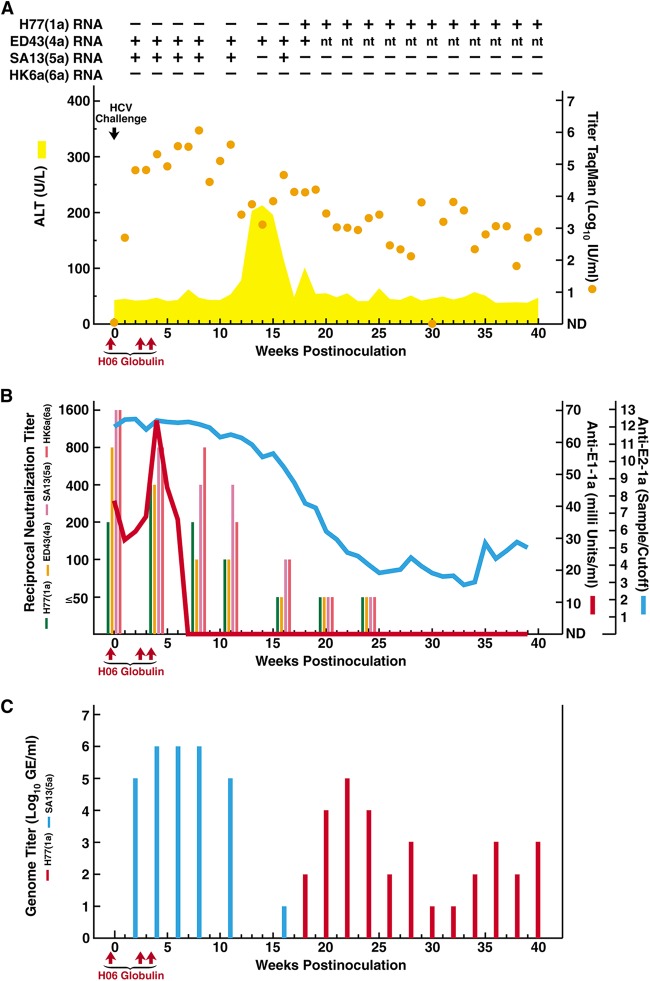
CHA5A009 was infused with H06 IgG intravenously prechallenge (250 mg/kg body weight) and at weeks 2.5 and 3.5 postchallenge (125 mg/kg). Twenty-four hours after the first IgG infusion, CHA5A009 was challenged intravenously with 100 chimpanzee infectious doses of each of the HCV strains (with the genotype in parentheses) H77(1a), ED43(4a), SA13(5a), and HK6a(6a) (29–31), chosen because H77 is the homologous acute-phase patient H strain (31) and chronic-phase patient H sera cross-neutralized all strains in HCVpp and HCVcc assays (18, 19). Based on 5′-untranslated region (UTR)-based TaqMan assay (32), CHA5A009 had serum HCV titers of 2.7 and 4.8 log10 IU/ml at weeks 1 and 2, respectively, and peak titers of 5 to 6 log10 IU/ml at weeks 3 to 11; the animal remained persistently infected (Fig. 1A). 5′-UTR (33) and core-envelope 1 (core-E1) (34) analysis of week 2 viruses, amplified by reverse transcription (RT)-nested PCR detecting the four strains with equal sensitivity (29, 33, 34), detected only SA13(5a). The animal developed acute hepatitis with elevated serum alanine aminotransferase (ALT) levels from weeks 12 to 18 (Fig. 1A).
FIG 1.
Course of hepatitis C infection in a chimpanzee loaded with polyclonal genotype 1a immunoglobulins and challenged with homologous and heterologous HCV strains. Chimpanzee CHA5A009, who was naive for HCV, as well as for other hepatitis viruses, was infused intravenously with immunoglobulins (H06), prepared from plasma obtained from patient H with chronic HCV, at the time points indicated with red arrows; the first infusion was 24 h before HCV challenge. The intravenous challenge (indicated by a black arrow) was 100 chimpanzee infectious doses of each of the HCV strains (with genotype in parentheses) H77(1a), ED43(4a), SA13(5a), and HK6a(6a). (A) Serum samples were tested for HCV RNA by in-house RT-nested PCR with strain-specific primers (Table 1): +, positive; −, negative; nt, not tested. The estimated log10 HCV RNA titers (international units per milliliter), determined in an in-house TaqMan assay (orange dots), were plotted against time; a single sample below the detection limit of ∼10 IU/ml is shown as “not detected” (ND). The area shaded yellow shows serum ALT (units per liter). Results of samples collected after week 40, at weeks 50 and 56, are only referred to in the text. (B) Anti-HCV antibodies against E1 (red line) and E2 (blue line) were detected in serum by 1a-specific ELISAs. The serum neutralizing reciprocal antibody titers, determined in HCVpp assays using H77, ED43, SA13, and HK6a pseudoparticles, are shown in colored bars as indicated. (C) The serum H77(1a) and SA13(5a) genome titers, determined by in-house RT-nested PCR with strain-specific primers (Table 1) on 10-fold serially diluted RNA, are indicated by red and blue bars, respectively.
Serum anti-E2 by enzyme-linked immunosorbent assay (ELISA) (18) was saturated prechallenge through week 16 postchallenge, followed by a steady decrease in titers (Fig. 1B). Anti-E1 (INNO-LIA-HCV) (35) was detected prechallenge through week 6 (Fig. 1B). Serum NAbs were assayed in HCVpp assays using H77, ED43, SA13, and HK6a pseudoparticles, respectively (18). Serum taken prior to IgG infusion had reciprocal neutralization titers of <50 against all HCVpp strains. Following IgG infusion, CHA5A009 had significant NAb titers against all strains, remaining at ≥100 through week 11; at weeks 20 and 24, the titers were ≤50 for all strains (Fig. 1B).
Sera from weeks 2 to 56 were tested for H77, ED43, SA13, and HK6a HCV RNA by RT-nested PCR (Fig. 1A), using strain-specific primers (Table 1) (36). The sensitivities of the H77, SA13, and HK6a assays were equivalent to that using 5′-UTR primers when testing dilutions of H77, SA13, and HK6a pools, respectively (30); the 4a assay was suboptimal. Amplicons were in most cases sequenced, confirming strain authenticity. There was no evidence of H77(1a) infection until week 18, when H77 RNA was detected at 2 log10 genome equivalents (GE)/ml (Fig. 1A and C) (36). The H77(1a) emergence followed the decrease in H77 NAb titers to <50 (Fig. 1B). The week 18 H77(1a) envelope proteins, derived from overlapping amplicons (37), had no changes compared to the polyclonal challenge virus (38, 39), indicating that viral emergence was not due to escape mutations. The animal remained H77 infected, with titers of 4 to 5 log10 GE/ml from weeks 20 to 24; titers fluctuated at low levels thereafter (Fig. 1C).
TABLE 1.
Primers used in the HCV strain-specific RT-nested PCR in this study
| Straina and primer type | Primer | Sequence |
|---|---|---|
| H77(1a) | ||
| RT | 2832R-H77 | AAGCGCCCCTAACTTGATGATG |
| PCR I | 2427S-H77 | ACTGGACACGGAGGTGGCCGCGTC |
| 2832R-H77 | AAGCGCCCCTAACTTGATGATG | |
| PCR II | 2462S-H77 | TTGTTCTTGTCGGGTTAATGGCGC |
| 2645R-H77 | GGGTGTACTACACACATGAGTAAG | |
| ED43(4a) | ||
| RT | HCV4aCoseR3591 | CCGTGGTAGACGGTCCACATCAC |
| PCR I | 2676F-ED43 | AGGGCCGGTTCCCAGCTGCT |
| HCV4aCoseR3591 | CCGTGGTAGACGGTCCACATCAC | |
| PCR II | 2839F-ED43 | GCACTACAAGTTATGGCTGGCTA |
| HCV4aCoseR3318 | CACGCAGCGGTGTCAGCGCCCC | |
| SA13(5a) | ||
| RT | 917R-SA13 | CAGTTGCAGTCCTGCACAACATTA |
| PCR I | 741S-SA13 | AGCCCCGAGCCTCGGAGCGGT |
| 917R-SA13 | CAGTTGCAGTCCTGCACAACATTA | |
| PCR II | 748S-SA13 | AGCCTCGGAGCGGTCACGGCT |
| 898R-SA13 | CATTATGCCGGCGAGGGCTAT | |
| HK6a(6a) | ||
| RT | 1765R-HK6a | TGGGGCACAAAAGCTCATTGG |
| PCR I | 1405S-HK6a | TGGGGCCAAATAACCTACAAA |
| 1765R-HK6a | TGGGGCACAAAAGCTCATTGG | |
| PCR II | 1408S-HK6a | GGCCAAATAACCTACAAAGTC |
| 1762R-HK6a | GGCACAAAAGCTCATTGGCAG |
Genotypes are given in parentheses.
Despite relatively high NAb titers against SA13(5a) (Fig. 1B), there was no apparent in vivo protection against this strain, which appeared at 5 to 6 log10 GE/ml in samples from weeks 2 to 11 (Fig. 1C); infection resolved after week 16 (Fig. 1A and C). The envelope sequence of week 2 SA13(5a), derived from overlapping amplicons (29, 37), had no changes compared to the polyclonal challenge virus (29, 40), indicating failure of neutralization. CHA5A009 became ED43(4a) infected, with sequence-confirmed ED43 RNA positivity from weeks 2 to 18 (Fig. 1A); since we did not develop a 4a-specific assay with optimized sensitivity, titer or outcome analysis was not performed. Finally, the chimpanzee apparently was not infected with HK6a(6a), being HK6a RNA negative from weeks 2 to 56 (Fig. 1A).
This is the first study to address whether polyclonal NAbs, given prechallenge, can prevent HCV infection in chimpanzees. While the homologous virus was suppressed initially, there was failure of neutralization against 2 of 3 heterologous HCV strains. Furthermore, the homologous virus emerged following the disappearance of passively administered NAbs and led to persistent infection. This supports the observation that polyclonal antibodies given postchallenge could control HCV infection only temporarily in the chimpanzee model (41). The infused NAbs might have prevented infection with heterologous 6a virus, and this was the strain previously found to be most efficiently neutralized in HCVcc assays (19, 42).
We previously demonstrated that chronic-phase patient H IgG, infused prechallenge, could control H77 infection in human liver chimeric mice (28, 43). However, the animals could only be followed short term. The reciprocal in vitro neutralization titers detected prechallenge and at weeks 4 and 8 in CHA5A009 (Fig. 1B) were similar to those detected prechallenge in human liver chimeric mice (43). Thus, for the homologous strain, it appeared that polyclonal immunoglobulin with in vitro neutralizing activity can control viremia in vivo. The chronic-phase sera from patient H had high in vitro neutralization titers against the heterologous 4a, 5a, and 6a strains (18, 19), and we previously found partial protection in human liver chimeric mice loaded with H06 IgG against ED43(4a) and HK6a(6a) (28). Here we observed reciprocal neutralization titers of >400 prechallenge and at week 4 postchallenge in the H06-infused chimpanzee using the same HCVpp assays for these 3 strains. Yet, the chimpanzee became infected with the 4a and 5a strains and developed acute hepatitis. For the 5a strain, we showed that this could represent failure of neutralization, since the challenge virus without envelope mutations appeared at high titers at week 2. In evaluating the outcome for the 1a, 4a, 5a, and 6a challenges, it should be recognized that the HCVpp neutralization titers in the chimpanzee were lower than those previously detected for the different genotype stains in chronic-phase sera from patient H (18). Furthermore, it must be recognized that our study in a single chimpanzee does not necessarily permit broad conclusions.
In a recent study, prechallenge infusion with 250 mg/kg of a humanized monoclonal anti-E2 prevented infection in an H77-challenged chimpanzee (44). Using polyclonal immunoglobulin with an HCV-specific antibody concentration of <250 mg/kg, we observed control of H77 viremia but not sterilizing immunity, even though we also infused the animal with IgG postchallenge. In our study, viral interference could have influenced the outcome given the simultaneous infections with 4a and 5a strains.
Recent data indicate that the HCV clearance is associated with NAbs (23, 45, 46). During chronic infection, HCV persists despite the presence of high-titer NAbs, perhaps because it is shielded from neutralization (47–50) or neutralization-resistant mutants are continuously developing (22, 23, 51). It was demonstrated in vitro that chronic-phase patient H sera neutralized only variants from time points early in infection, not later variants (22). Our data confirm in vivo that chronic-phase patient H immunoglobulin can control the acute-phase virus. We previously showed that different recombinant genotype viruses, lacking HVR1, were efficiently neutralized by H06 IgG, indicating that it targets conserved neutralization epitopes within E1 and/or E2 (42).
This study sheds new light on the effectiveness of NAbs in preventing HCV. It is encouraging that the homologous virus apparently could be suppressed long term. However, the lack of protection against heterologous strains highlights significant issues regarding the possibility of developing broadly protective immunoglobulin preparations using patient-derived antibodies alone. More likely, efficient neutralization will require monoclonal antibodies against conserved conformational epitopes and/or combinations of antibodies targeting different HCV epitopes or viral variants (44, 50, 52–54).
ACKNOWLEDGMENTS
We thank Alicia Brockington for technical assistance and Charlene Shaver for animal care.
This study was supported by the Intramural Research Program of the NIAID, NIH, and by NIAID contract NO1-AO-62713, as well as by research grants to J.B. from the Danish Council for Independent Research-Medical Sciences, the Lundbeck Foundation, and the Novo Nordisk Foundation. J.B. is the recipient of an Advanced-Top Researcher grant from the Danish Council for Independent Research (2014) and the 2015 Novo Nordisk Prize.
The authors declare that they have no conflicts of interest to report.
REFERENCES
- 1.Bukh J, Forns X, Emerson SU, Purcell RH. 2001. Studies of hepatitis C virus in chimpanzees and their importance for vaccine development. Intervirology 44:132–142. doi: 10.1159/000050040. [DOI] [PubMed] [Google Scholar]
- 2.Lanford RE, Bigger C, Bassett S, Klimpel G. 2001. The chimpanzee model of hepatitis C virus infections. ILAR J 42:117–126. doi: 10.1093/ilar.42.2.117. [DOI] [PubMed] [Google Scholar]
- 3.Bukh J. 2012. Animal models for the study of hepatitis C virus infection and related liver disease. Gastroenterology 142:1279–1287. doi: 10.1053/j.gastro.2012.02.016. [DOI] [PubMed] [Google Scholar]
- 4.Forns X, Bukh J, Purcell RH. 2002. The challenge of developing a vaccine against hepatitis C virus. J Hepatol 37:684–695. doi: 10.1016/S0168-8278(02)00308-2. [DOI] [PubMed] [Google Scholar]
- 5.Ball JK, Tarr AW, McKeating JA. 2014. The past, present and future of neutralizing antibodies for hepatitis C virus. Antiviral Res 105:100–111. doi: 10.1016/j.antiviral.2014.02.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Houghton M, Abrignani S. 2005. Prospects for a vaccine against the hepatitis C virus. Nature 436:961–966. doi: 10.1038/nature04081. [DOI] [PubMed] [Google Scholar]
- 7.Houghton M. 2011. Prospects for prophylactic and therapeutic vaccines against the hepatitis C viruses. Immunol Rev 239:99–108. doi: 10.1111/j.1600-065X.2010.00977.x. [DOI] [PubMed] [Google Scholar]
- 8.Ray R. 2011. Progress toward development of a hepatitis C vaccine with broad shoulders. Sci Transl Med 3:94ps33. doi: 10.1126/scitranslmed.3002772. [DOI] [PubMed] [Google Scholar]
- 9.Honegger JR, Zhou Y, Walker CM. 2014. Will there be a vaccine to prevent HCV infection? Semin Liver Dis 34:79–88. doi: 10.1055/s-0034-1371081. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Dahari H, Feinstone SM, Major ME. 2010. Meta-analysis of hepatitis C virus vaccine efficacy in chimpanzees indicates an importance for structural proteins. Gastroenterology 139:965–974. doi: 10.1053/j.gastro.2010.05.077. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Alter HJ, Seeff LB. 2000. Recovery, persistence, and sequelae in hepatitis C virus infection: a perspective on long-term outcome. Semin Liver Dis 20:17–35. doi: 10.1055/s-2000-9505. [DOI] [PubMed] [Google Scholar]
- 12.Bukh J, Miller RH, Kew MC, Purcell RH. 1993. Hepatitis C virus RNA in southern African blacks with hepatocellular carcinoma. Proc Natl Acad Sci U S A 90:1848–1851. doi: 10.1073/pnas.90.5.1848. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Farci P, Alter HJ, Wong DC, Miller RH, Govindarajan S, Engle R, Shapiro M, Purcell RH. 1994. Prevention of hepatitis C virus infection in chimpanzees after antibody-mediated in vitro neutralization. Proc Natl Acad Sci U S A 91:7792–7796. doi: 10.1073/pnas.91.16.7792. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Farci P, Bukh J, Purcell RH. 1997. The quasispecies of hepatitis C virus and the host immune response. Springer Semin Immunopathol 19:5–26. doi: 10.1007/BF00945022. [DOI] [PubMed] [Google Scholar]
- 15.Yu MY, Bartosch B, Zhang P, Guo ZP, Renzi PM, Shen LM, Granier C, Feinstone SM, Cosset FL, Purcell RH. 2004. Neutralizing antibodies to hepatitis C virus (HCV) in immune globulins derived from anti-HCV-positive plasma. Proc Natl Acad Sci U S A 101:7705–7710. doi: 10.1073/pnas.0402458101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Bartosch B, Bukh J, Meunier JC, Granier C, Engle RE, Blackwelder WC, Emerson SU, Cosset FL, Purcell RH. 2003. In vitro assay for neutralizing antibody to hepatitis C virus: evidence for broadly conserved neutralization epitopes. Proc Natl Acad Sci U S A 100:14199–14204. doi: 10.1073/pnas.2335981100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Logvinoff C, Major ME, Oldach D, Heyward S, Talal A, Balfe P, Feinstone SM, Alter H, Rice CM, McKeating JA. 2004. Neutralizing antibody response during acute and chronic hepatitis C virus infection. Proc Natl Acad Sci U S A 101:10149–10154. doi: 10.1073/pnas.0403519101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Meunier JC, Engle RE, Faulk K, Zhao M, Bartosch B, Alter H, Emerson SU, Cosset FL, Purcell RH, Bukh J. 2005. Evidence for cross-genotype neutralization of hepatitis C virus pseudo-particles and enhancement of infectivity by apolipoprotein C1. Proc Natl Acad Sci U S A 102:4560–4565. doi: 10.1073/pnas.0501275102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Scheel TK, Gottwein JM, Jensen TB, Prentoe JC, Hoegh AM, Alter HJ, Eugen-Olsen J, Bukh J. 2008. Development of JFH1-based cell culture systems for hepatitis C virus genotype 4a and evidence for cross-genotype neutralization. Proc Natl Acad Sci U S A 105:997–1002. doi: 10.1073/pnas.0711044105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Gottwein JM, Scheel TK, Jensen TB, Lademann JB, Prentoe JC, Knudsen ML, Hoegh AM, Bukh J. 2009. Development and characterization of hepatitis C virus genotype 1-7 cell culture systems: role of CD81 and scavenger receptor class B type I and effect of antiviral drugs. Hepatology 49:364–377. doi: 10.1002/hep.22673. [DOI] [PubMed] [Google Scholar]
- 21.Tarr AW, Urbanowicz RA, Hamed MR, Albecka A, McClure CP, Brown RJ, Irving WL, Dubuisson J, Ball JK. 2011. Hepatitis C patient-derived glycoproteins exhibit marked differences in susceptibility to serum neutralizing antibodies: genetic subtype defines antigenic but not neutralization serotype. J Virol 85:4246–4257. doi: 10.1128/JVI.01332-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.von Hahn T, Yoon JC, Alter H, Rice CM, Rehermann B, Balfe P, McKeating JA. 2007. Hepatitis C virus continuously escapes from neutralizing antibody and T-cell responses during chronic infection in vivo. Gastroenterology 132:667–678. doi: 10.1053/j.gastro.2006.12.008. [DOI] [PubMed] [Google Scholar]
- 23.Dowd KA, Netski DM, Wang XH, Cox AL, Ray SC. 2009. Selection pressure from neutralizing antibodies drives sequence evolution during acute infection with hepatitis C virus. Gastroenterology 136:2377–2386. doi: 10.1053/j.gastro.2009.02.080. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Law JL, Chen C, Wong J, Hockman D, Santer DM, Frey SE, Belshe RB, Wakita T, Bukh J, Jones CT, Rice CM, Abrignani S, Tyrrell DL, Houghton M. 2013. A hepatitis C virus (HCV) vaccine comprising envelope glycoproteins gpE1/gpE2 derived from a single isolate elicits broad cross-genotype neutralizing antibodies in humans. PLoS One 8:e59776. doi: 10.1371/journal.pone.0059776. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Meunier JC, Gottwein JM, Houghton M, Russell RS, Emerson SU, Bukh J, Purcell RH. 2011. Vaccine-induced cross-genotype reactive neutralizing antibodies against hepatitis C virus. J Infect Dis 204:1186–1190. doi: 10.1093/infdis/jir511. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Ray R, Meyer K, Banerjee A, Basu A, Coates S, Abrignani S, Houghton M, Frey SE, Belshe RB. 2010. Characterization of antibodies induced by vaccination with hepatitis C virus envelope glycoproteins. J Infect Dis 202:862–866. doi: 10.1086/655902. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Wong JA, Bhat R, Hockman D, Logan M, Chen C, Levin A, Frey SE, Belshe RB, Tyrrell DL, Law JL, Houghton M. 2014. Recombinant hepatitis C virus envelope glycoprotein vaccine elicits antibodies targeting multiple epitopes on the envelope glycoproteins associated with broad cross-neutralization. J Virol 88:14278–14288. doi: 10.1128/JVI.01911-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Meuleman P, Bukh J, Verhoye L, Farhoudi A, Vanwolleghem T, Wang RY, Desombere I, Alter H, Purcell RH, Leroux-Roels G. 2011. In vivo evaluation of the cross-genotype neutralizing activity of polyclonal antibodies against hepatitis C virus. Hepatology 53:755–762. doi: 10.1002/hep.24171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Bukh J, Apgar CL, Engle R, Govindarajan S, Hegerich PA, Tellier R, Wong DC, Elkins R, Kew MC. 1998. Experimental infection of chimpanzees with hepatitis C virus of genotype 5a: genetic analysis of the virus and generation of a standardized challenge pool. J Infect Dis 178:1193–1197. doi: 10.1086/515683. [DOI] [PubMed] [Google Scholar]
- 30.Bukh J, Meuleman P, Tellier R, Engle RE, Feinstone SM, Eder G, Satterfield WC, Govindarajan S, Krawczynski K, Miller RH, Leroux-Roels G, Purcell RH. 2010. Challenge pools of hepatitis C virus genotypes 1-6 prototype strains: replication fitness and pathogenicity in chimpanzees and human liver-chimeric mouse models. J Infect Dis 201:1381–1389. doi: 10.1086/651579. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Feinstone SM, Alter HJ, Dienes HP, Shimizu Y, Popper H, Blackmore D, Sly D, London WT, Purcell RH. 1981. Non-A, non-B hepatitis in chimpanzees and marmosets. J Infect Dis 144:588–598. doi: 10.1093/infdis/144.6.588. [DOI] [PubMed] [Google Scholar]
- 32.Engle RE, Russell RS, Purcell RH, Bukh J. 2008. Development of a TaqMan assay for the six major genotypes of hepatitis C virus: comparison with commercial assays. J Med Virol 80:72–79. doi: 10.1002/jmv.21043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Yanagi M, Bukh J, Emerson SU, Purcell RH. 1996. Contamination of commercially available fetal bovine sera with bovine viral diarrhea virus genomes: implications for the study of hepatitis C virus in cell cultures. J Infect Dis 174:1324–1327. doi: 10.1093/infdis/174.6.1324. [DOI] [PubMed] [Google Scholar]
- 34.Corbet S, Bukh J, Heinsen A, Fomsgaard A. 2003. Hepatitis C virus subtyping by a core-envelope 1-based reverse transcriptase PCR assay with sequencing and its use in determining subtype distribution among Danish patients. J Clin Microbiol 41:1091–1100. doi: 10.1128/JCM.41.3.1091-1100.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Rollier C, Depla E, Drexhage JA, Verschoor EJ, Verstrepen BE, Fatmi A, Brinster C, Fournillier A, Whelan JA, Whelan M, Jacobs D, Maertens G, Inchauspe G, Heeney JL. 2004. Control of heterologous hepatitis C virus infection in chimpanzees is associated with the quality of vaccine-induced peripheral T-helper immune response. J Virol 78:187–196. doi: 10.1128/JVI.78.1.187-196.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Yanagi M, Purcell RH, Emerson SU, Bukh J. 1999. Hepatitis C virus: an infectious molecular clone of a second major genotype (2a) and lack of viability of intertypic 1a and 2a chimeras. Virology 262:250–263. doi: 10.1006/viro.1999.9889. [DOI] [PubMed] [Google Scholar]
- 37.Sakai A, Takikawa S, Thimme R, Meunier JC, Spangenberg HC, Govindarajan S, Farci P, Emerson SU, Chisari FV, Purcell RH, Bukh J. 2007. In vivo study of the HC-TN strain of hepatitis C virus recovered from a patient with fulminant hepatitis: RNA transcripts of a molecular clone (pHC-TN) are infectious in chimpanzees but not in Huh7.5 cells. J Virol 81:7208–7219. doi: 10.1128/JVI.01774-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Farci P, Shimoda A, Wong D, Cabezon T, De Gioannis D, Strazzera A, Shimizu Y, Shapiro M, Alter HJ, Purcell RH. 1996. Prevention of hepatitis C virus infection in chimpanzees by hyperimmune serum against the hypervariable region 1 of the envelope 2 protein. Proc Natl Acad Sci U S A 93:15394–15399. doi: 10.1073/pnas.93.26.15394. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Yanagi M, Purcell RH, Emerson SU, Bukh J. 1997. Transcripts from a single full-length cDNA clone of hepatitis C virus are infectious when directly transfected into the liver of a chimpanzee. Proc Natl Acad Sci U S A 94:8738–8743. doi: 10.1073/pnas.94.16.8738. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Jensen TB, Gottwein JM, Scheel TK, Hoegh AM, Eugen-Olsen J, Bukh J. 2008. Highly efficient JFH1-based cell-culture system for hepatitis C virus genotype 5a: failure of homologous neutralizing-antibody treatment to control infection. J Infect Dis 198:1756–1765. doi: 10.1086/593021. [DOI] [PubMed] [Google Scholar]
- 41.Krawczynski K, Alter MJ, Tankersley DL, Beach M, Robertson BH, Lambert S, Kuo G, Spelbring JE, Meeks E, Sinha S, Carson DA. 1996. Effect of immune globulin on the prevention of experimental hepatitis C virus infection. J Infect Dis 173:822–828. doi: 10.1093/infdis/173.4.822. [DOI] [PubMed] [Google Scholar]
- 42.Prentoe J, Jensen TB, Meuleman P, Serre SB, Scheel TK, Leroux-Roels G, Gottwein JM, Bukh J. 2011. Hypervariable region 1 differentially impacts viability of hepatitis C virus strains of genotypes 1 to 6 and impairs virus neutralization. J Virol 85:2224–2234. doi: 10.1128/JVI.01594-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Vanwolleghem T, Bukh J, Meuleman P, Desombere I, Meunier JC, Alter H, Purcell RH, Leroux-Roels G. 2008. Polyclonal immunoglobulins from a chronic hepatitis C virus patient protect human liver-chimeric mice from infection with a homologous hepatitis C virus strain. Hepatology 47:1846–1855. doi: 10.1002/hep.22244. [DOI] [PubMed] [Google Scholar]
- 44.Morin TJ, Broering TJ, Leav BA, Blair BM, Rowley KJ, Boucher EN, Wang Y, Cheslock PS, Knauber M, Olsen DB, Ludmerer SW, Szabo G, Finberg RW, Purcell RH, Lanford RE, Ambrosino DM, Molrine DC, Babcock GJ. 2012. Human monoclonal antibody HCV1 effectively prevents and treats HCV infection in chimpanzees. PLoS Pathog 8:e1002895. doi: 10.1371/journal.ppat.1002895. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Pestka JM, Zeisel MB, Blaser E, Schurmann P, Bartosch B, Cosset FL, Patel AH, Meisel H, Baumert J, Viazov S, Rispeter K, Blum HE, Roggendorf M, Baumert TF. 2007. Rapid induction of virus-neutralizing antibodies and viral clearance in a single-source outbreak of hepatitis C. Proc Natl Acad Sci U S A 104:6025–6030. doi: 10.1073/pnas.0607026104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Osburn WO, Snider AE, Wells BL, Latanich R, Bailey JR, Thomas DL, Cox AL, Ray SC. 2014. Clearance of hepatitis C infection is associated with the early appearance of broad neutralizing antibody responses. Hepatology 59:2140–2151. doi: 10.1002/hep.27013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Falkowska E, Kajumo F, Garcia E, Reinus J, Dragic T. 2007. Hepatitis C virus envelope glycoprotein E2 glycans modulate entry, CD81 binding, and neutralization. J Virol 81:8072–8079. doi: 10.1128/JVI.00459-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Helle F, Goffard A, Morel V, Duverlie G, McKeating J, Keck ZY, Foung S, Penin F, Dubuisson J, Voisset C. 2007. The neutralizing activity of anti-hepatitis C virus antibodies is modulated by specific glycans on the E2 envelope protein. J Virol 81:8101–8111. doi: 10.1128/JVI.00127-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Pantua H, Diao J, Ultsch M, Hazen M, Mathieu M, McCutcheon K, Takeda K, Date S, Cheung TK, Phung Q, Hass P, Arnott D, Hongo JA, Matthews DJ, Brown A, Patel AH, Kelley RF, Eigenbrot C, Kapadia SB. 2013. Glycan shifting on hepatitis C virus (HCV) E2 glycoprotein is a mechanism for escape from broadly neutralizing antibodies. J Mol Biol 425:1899–1914. doi: 10.1016/j.jmb.2013.02.025. [DOI] [PubMed] [Google Scholar]
- 50.Pedersen J, Carlsen TH, Prentoe J, Ramirez S, Jensen TB, Forns X, Alter H, Foung SK, Law M, Gottwein J, Weis N, Bukh J. 2013. Neutralization resistance of hepatitis C virus can be overcome by recombinant human monoclonal antibodies. Hepatology 58:1587–1597. doi: 10.1002/hep.26524. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Farci P, Shimoda A, Coiana A, Diaz G, Peddis G, Melpolder JC, Strazzera A, Chien DY, Munoz SJ, Balestrieri A, Purcell RH, Alter HJ. 2000. The outcome of acute hepatitis C predicted by the evolution of the viral quasispecies. Science 288:339–344. doi: 10.1126/science.288.5464.339. [DOI] [PubMed] [Google Scholar]
- 52.Giang E, Dorner M, Prentoe JC, Dreux M, Evans MJ, Bukh J, Rice CM, Ploss A, Burton DR, Law M. 2012. Human broadly neutralizing antibodies to the envelope glycoprotein complex of hepatitis C virus. Proc Natl Acad Sci U S A 109:6205–6210. doi: 10.1073/pnas.1114927109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Keck Z, Wang W, Wang Y, Lau P, Carlsen TH, Prentoe J, Xia J, Patel AH, Bukh J, Foung SK. 2013. Cooperativity in virus neutralization by human monoclonal antibodies to two adjacent regions located at the amino terminus of hepatitis C virus E2 glycoprotein. J Virol 87:37–51. doi: 10.1128/JVI.01941-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Keck ZY, Xia J, Wang Y, Wang W, Krey T, Prentoe J, Carlsen T, Li AY, Patel AH, Lemon SM, Bukh J, Rey FA, Foung SK. 2012. Human monoclonal antibodies to a novel cluster of conformational epitopes on HCV E2 with resistance to neutralization escape in a genotype 2a isolate. PLoS Pathog 8:e1002653. doi: 10.1371/journal.ppat.1002653. [DOI] [PMC free article] [PubMed] [Google Scholar]