ABSTRACT
The V3 region of HIV-1 gp120 is important for virus-coreceptor interaction and highly immunogenic. Although most anti-V3 antibodies neutralize only the sensitive tier 1 viruses, anti-V3 antibodies effective against the more resistant viruses exist, and a better understanding of these antibodies and their epitopes would be beneficial for the development of novel vaccine immunogens against HIV. The HIV-1 isolate JRFL with its cryptic V3 is resistant to most V3-specific monoclonal antibodies (MAbs). However, the V3 MAb 2424 achieves 100% neutralization against JRFL. 2424 is encoded by IGHV3-53 and IGLV2-28 genes, a pairing rarely used by the other V3 MAbs. 2424 also has distinct binding and neutralization profiles. Studies of 2424-mediated neutralization of JRFL produced with a mannosidase inhibitor further revealed that its neutralizing activity is unaffected by the glycan composition of the virus envelope. To understand the distinct activity of 2424, we determined the crystal structure of 2424 Fab in complex with a JRFL V3 peptide and showed that the 2424 epitope is located at the tip of the V3 crown (307IHIGPGRAFYT319), dominated by interactions with HisP308, ProP313, and ArgP315. The binding mode of 2424 is similar to that of the well-characterized MAb 447-52D, although 2424 is more side chain dependent. The 2424 epitope is focused on the very apex of V3, away from nearby glycans, facilitating antibody access. This feature distinguishes the 2424 epitope from the other V3 crown epitopes and indicates that the tip of V3 is a potential site to target and incorporate into HIV vaccine immunogens.
IMPORTANCE HIV/AIDS vaccines are crucial for controlling the HIV epidemics that continue to afflict millions of people worldwide. However, HIV vaccine development has been hampered by significant scientific challenges, one of which is the inability of HIV vaccine candidates evaluated thus far to elicit production of potent and broadly neutralizing antibodies. The V3 loop is one of the few immunogenic targets on the virus envelope glycoprotein that can induce neutralizing antibodies, but in many viruses, parts of V3 are inaccessible for antibody recognition. This study examined a V3-specific monoclonal antibody that can completely neutralize HIV-1 JRFL, a virus isolate resistant to most V3 antibodies. Our data reveal that this antibody recognizes the most distal tip of V3, which is not as occluded as other parts of V3. Hence, the epitope of 2424 is in one of the vulnerable sites on the virus that may be exploited in designing HIV vaccine immunogens.
INTRODUCTION
The HIV-1 envelope glycoprotein (Env) is the only virus-encoded protein expressed on the surface of the virus and is the sole target for virus-neutralizing antibodies (Abs). On the virion surface, the HIV Env spike is a compact heterodimeric trimer made up of gp120 and gp41 subunits (1–3). The surface gp120 subunit is responsible for interacting with the host cell through binding to CD4 and the coreceptor, the chemokine receptor CCR5 or CXCR4 (4–7). On the basis of primary amino acid sequences, gp120 is divided into five conserved regions (C1 to C5), which are interspersed with five variable regions (V1 to V5) (8). The CD4-binding site and the chemokine receptor-binding site are both highly conformational and discontinuous. The chemokine receptor binding site in particular is composed of the invariant β2 and β3 strands of the V1V2 stem region, β20 and β21 strands in the conserved C4 region, and the third variable (V3) region of gp120 (3, 9). Vulnerable sites on the HIV Env have been identified based on their recognition by broadly neutralizing human monoclonal antibodies (MAbs). On gp120, these epitope sites include the CD4-binding site (10, 11), a cluster of glycans recognized by MAb 2G12 (12–14), and the glycan-bearing regions in V1V2 and V3 (15–17). These Abs recognize structurally complex epitopes and display an unusual VH domain exchange or an extreme level of somatic hypermutations and/or CDR3 lengths; thus, inducing such Abs by vaccination is not a simple feat.
The crown of the V3 loop, on the other hand, is highly immunogenic; antibodies to the V3 crown are induced in the vast majority of human subjects following HIV infection or after vaccination with HIV gp120 vaccines (18–23). The importance of V3 as a vaccine immunogen is further established by the fact that V3 is essential for HIV-1 infectivity (24, 25) and that antibodies binding to V3 can block the virus infection (26–31) Most V3-specific MAbs isolated from HIV-1-infected individuals are also highly cross-reactive, recognizing gp120 proteins from viruses of different HIV-1 subtypes. However, these V3-specific MAbs neutralize mainly the relatively sensitive tier 1 viruses and are ineffective against tier 2 and tier 3 isolates (2, 26, 32). The failure of anti-V3 MAbs to neutralize tier 2 and tier 3 viruses in the face of recognition of their corresponding soluble gp120 proteins indicates that V3 epitopes are present but inaccessible on the functional Env spikes on the virions (33, 34). Nonetheless, there are distinct epitopes on the V3 loop (17, 35). Many are occluded, but some may be more exposed. Immunogenic V3 epitopes that are accessible on the virus Env would be valuable new targets for HIV vaccine development.
In this study, we present the crystal structure of a V3 epitope recognized by human MAb 2424, which is distinct from the other V3 MAbs in its capacity to neutralize JRFL, a relatively resistant HIV-1 isolate. MAb 2424 was isolated from a chronically HIV-infected subject living in New York City by a cellular method in which peripheral blood mononuclear cells (PBMCs) were transformed by Epstein-Barr virus, fused with heteromyeloma cells (36, 37), and selected based on enzyme-linked immunosorbent assay (ELISA) reactivity with the V3 (consensus B)-MLV gp70 fusion protein. Unlike the other V3 MAbs, 2424 preferentially binds to V3 of subtype B viruses. Moreover, the 2424 gene usage is unusual among human anti-V3 MAbs; 2424 is IgG1 with a kappa light chain and is encoded by IGHV3-53 and IGLV2-28 genes (38). In agreement with functional studies, the crystallographic structure reveals a distinct epitope at the tip of the V3 crown that is not as influenced by glycans as the other V3 epitopes. Although it is unknown if potent 2424-like Abs can be generated by vaccination, this V3 epitope represents another important site that may be incorporated into the immunogens for vaccines designed to elicit HIV-1-neutralizing antibodies.
MATERIALS AND METHODS
Human monoclonal antibodies (MAbs) and HIV-1 Env proteins.
Anti-V3 human MAbs 2424, 447-52D, 2219, 2557, 3074, and 3869 were obtained from an existing panel of HIV-specific MAbs. MAbs 2424, 447-52D, and 2219 were derived from the cells of HIV-1-infected subjects from the United States, while MAbs 2257, 3074, and 3869 were produced from Cameroonian individuals infected with CRF02_AG or other non-B subtypes (22, 38–41). All of these MAbs were generated by the cellular method as described previously (36, 37). The irrelevant human anti-parvovirus B19 MAb 1418 was used as a negative control (42). MAb b12 (provided by Dennis Burton and Carlos Barbas) and MAb 2G12 (provided by Hermann Katinger) were obtained through the NIH AIDS Reagent Program, Division of AIDS, NIAID, NIH, while MAb PG9 was provided by Wayne Koff (International AIDS Vaccine Initiative [IAVI]'s Neutralizing Antibody Consortium) or purchased from Polymun Scientific.
Recombinant gp120 proteins of various subtypes (produced in 293T cells) were purchased from Immune Technology Corp., except for gp120JRFL (produced in CHO cells by Progenics, Inc.) which was obtained from Vaccine Research and Development Branch of Division of AIDS, NIAID, NIH. Stabilized trimeric BG505-SOSIP.664 gp140 was a generous gift from John P. Moore (Weill Cornell Medical College).
Antibody binding assay.
The binding of human V3 MAbs to recombinant gp120s was determined by ELISA. Briefly, 96-well ELISA plates were coated with gp120 (1.0 μg/ml in phosphate-buffered saline [PBS]) at 4°C overnight, blocked with 3% bovine serum albumin (BSA) in PBS, and reacted for 2 h at 37°C with MAbs serially diluted in PBS with 1% BSA. Bound MAbs were detected with alkaline phosphatase-conjugated goat anti-human IgG and p-nitrophenyl phosphate substrate.
Cell lines, plasmids, and viruses.
Cells of the TZM.bl line were obtained through the NIH AIDS Research and Reference Reagent Program (contributed by J. Kappes and X. Wu). 293T/17 cell line was purchased from the American Type Culture Collection (ATCC).
HIV-1 Env-expressing plasmids for generating pseudoviruses were obtained from the NIH AIDS Research and Reference Reagent Program, Division of AIDS, NIAID, NIH. Pseudoviruses were produced by cotransfecting 293T cells with env, rev, and pNL4-3.Luc.R-E- or pSG3 using a ProFection kit (Promega, Madison, WI) or polyethylenimine (PEI) MAX40,000 (Polysciences, Warrington, PA). Glycan-modified HIV-1JRFL was generated as described above in the presence of 25 μM kifunensine or 20 μM swainsonine (Sigma, St. Louis, MO). Supernatants were harvested after 48 h and clarified by centrifugation and 0.22-μm filtration. Single-use aliquots were stored at −80°C until use.
Point mutations in JRFL Env were introduced in pCAGGS JRFL.JB gp160 using the QuikChange II XL site-directed mutagenesis kit (Stratagene, La Jolla, CA, USA), according to the manufacturer's instructions. All mutant constructs were sequenced to confirm the correct amino acid changes. Plasmid pCAGGS JRFL.JB gp160 was kindly supplied by the NIH Vaccine Research Center.
Neutralization assay.
Virus neutralization was measured as described previously (43). Briefly, serially diluted MAbs were incubated with the virus for 1 h at 37°C. TZM.bl cells were then added to virus-MAb mixtures in Dulbecco's modified Eagle medium (DMEM) containing 10% fetal bovine serum and DEAE-dextran (Sigma, St. Louis, MO). After 48 h, virus infection was determined using the Bright-Glo luciferase assay system (Promega, Madison, WI). For neutralization assays with soluble CD4 (sCD4), the virus was preincubated with recombinant human sCD4 (Progenics Pharmaceuticals, Tarrytown, NY) for 30 min at 37°C, before addition of serially diluted MAbs. Virus infection was measured in TZM.bl cells using a β-galactosidase-based assay (Promega, Madison, WI).
Fab production and purification.
The Fab fragment of MAb 2424 was prepared by papain digestion as described previously (35, 44). Briefly, the IgG molecule was mixed with papain (Worthington, Lakewood, NJ) at a 20:1 molar ratio in 100 mM Tris (pH 6.8) with 1 mM cysteine hydrochloride and 4 mM EDTA. The mixture was incubated for 1 h at 37°C, and the reaction was stopped with 10 mM iodoacetamide. The Fab fragment was separated from the Fc fragment and the undigested IgG by a protein A column and further purified by size exclusion chromatography. The Fab fragment was then concentrated to about 10 mg/ml for crystallization.
Crystallization, data collection, structure determination, and refinement.
The V3JRFL peptide was synthesized by Biomatik (Wilmington, DE), dissolved in water, and mixed with Fab 2424 at a 10:1 molar ratio. Crystallization conditions were screened and optimized using the vapor diffusion hanging-drop method. Well-diffracted crystals of Fab alone were obtained with a well solution of 20% polyethylene glycol 8000, 0.1 M Tris (pH 8.5), 36% glycol, whereas those of the Fab/epitope complex were obtained in a well solution of 28% polyethylene glycol 4000, 0.17 M Li2SO4, 0.085 M Tris (pH 8.5), 15% glycerol. X-ray diffraction data sets were collected at the synchrotron beamline GM/CA-CAT of the Advanced Photon Source (APS), Argonne National Laboratory. All data sets were processed using the HKL 2000 package (45) and XDS (46), and structures were determined by molecular replacement using a homologous Fab structure (PDB ID 3KDM) as the initial model. Cycles of refinement for each model were carried out in COOT and Phenix (47, 48). The NCS constraints were imposed for the three complexes in the refinement for the 3.18-Å structure of the Fab 2424/V3 complex. Final structural analyses were carried out using ICM, and figures were generated using PyMOL (Schrödinger, LLC) and ICM (49).
Protein structure accession numbers.
Coordinates and structure factors of Fab 2424/V3 complex and Fab 2424 have been deposited in the Protein Data Bank under accession numbers 4XMK and 4XML, respectively.
Nucleotide sequence accession numbers.
The IGHV and IGKV sequences of 2424 have been deposited in GenBank with accession numbers EU794426 and KP050787, respectively.
RESULTS
V3-specific MAb 2424 potently neutralizes HIV-1 JRFL isolate.
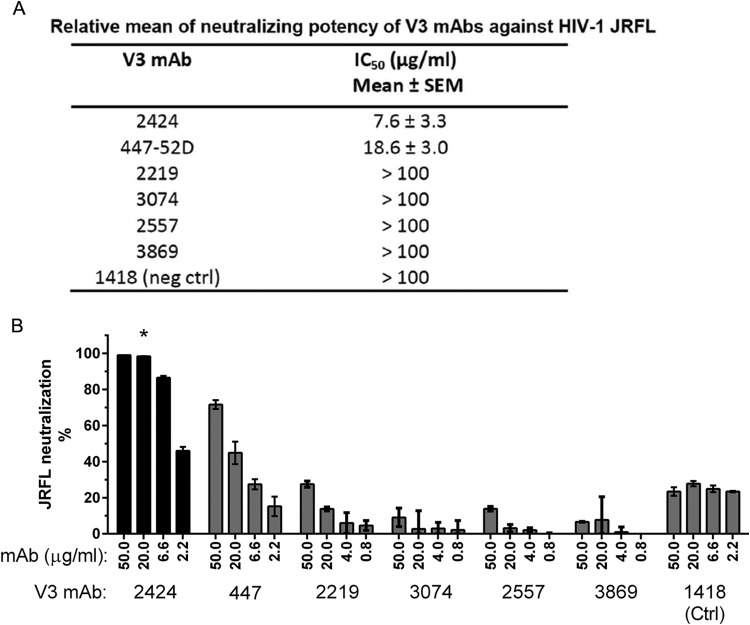
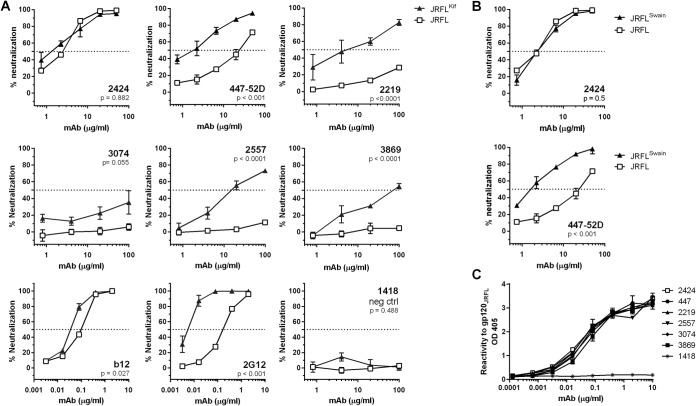
Most V3-specific MAbs isolated from HIV-infected subjects or vaccine recipients are capable of neutralizing tier 1 HIV-1 isolates but not the more resistant tier 2 or tier 3 viruses, due to the cryptic nature of many V3 epitopes on the Env spikes of tier 2 and tier 3 viruses. However, one V3-specific MAb, 2424, shows a potent neutralizing activity against a relatively resistant HIV-1 isolate, JRFL, indicating the presence of an Ab-accessible neutralizing V3 epitope on this virus. As shown in Fig. 1A, among six V3 MAbs tested, 2424 was the most effective, with a 50% inhibitory concentration (IC50) of 7.6 μg/ml, whereas the remaining five had IC50s of 18.7 μg/ml or >100 μg/ml. The IC50s of other V3 MAbs against JRFL were also shown previously to range from 11 to >50 μg/ml (26). More recently, when a panel of 48 V3 MAbs was tested, 2424 was also found to be the most potent against JRFL (50). The greater potency of 2424 was further shown by its ability to achieve a neutralization plateau of 100%, a level unattainable by 447-52D and the other V3 MAbs tested (Fig. 1B; also, see Fig. 3 to 5).
FIG 1.
Neutralization potency of 2424 versus the other V3-specific MAbs against JRFL. (A) Relative neutralizing potency of 2424 and five other V3 MAbs against JRFL. These V3 MAbs were isolated from HIV-infected individuals (22, 38–40). Means and standard errors of IC50s from four independent experiments are shown. (B) Titration curves of the six V3 MAbs against JRFL. Neutralization assay was performed with JRFL pseudovirus and TZM.bl target cells as previously reported (43). MAbs were titrated and incubated with virus at 37°C for 1 h. TZM.bl cells were then added to the virus-MAb mixtures in DMEM containing 10% fetal bovine serum and DEAE-dextran. Neutralization activity was assessed 48 h later using the luciferase detection system. The parvovirus-specific MAb 1418 was included as a negative control. Means and standard deviations were calculated from duplicate wells. Representative data from multiple repeat experiments are shown. Statistical analysis was performed by two-way analysis of variance (ANOVA). *, P < 0.001 compared to MAbs 447-52D, 2219, 3074, 2557, 3869, and 1418.
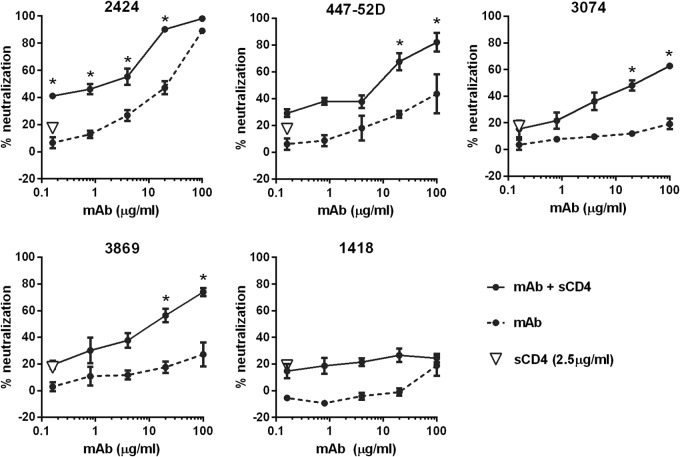
FIG 3.
Effects of CD4 engagement on virus neutralization by 2424 versus the other V3 MAbs. Neutralization of JRFL pseudovirus by V3 MAbs 2424, 447-52D, 3074, and 3869 was evaluated after treatment with or without sCD4. Virus was pretreated with 2.5 μg/ml of sCD4 for 30 min at 37°C or left untreated and then incubated with serially diluted MAb for 1 h at 37°C. TZM.bl cells were added to virus-MAb mixtures in DMEM containing 10% fetal bovine serum and DEAE-dextran. After 48 h, virus infection was measured based on β-galactosidase activity. Means and standard errors from two independent experiments are shown. *, P < 0.01 based on the two-way ANOVA to show a synergistic difference above the calculated sum of percent neutralization attained by MAb and sCD4 on their own.
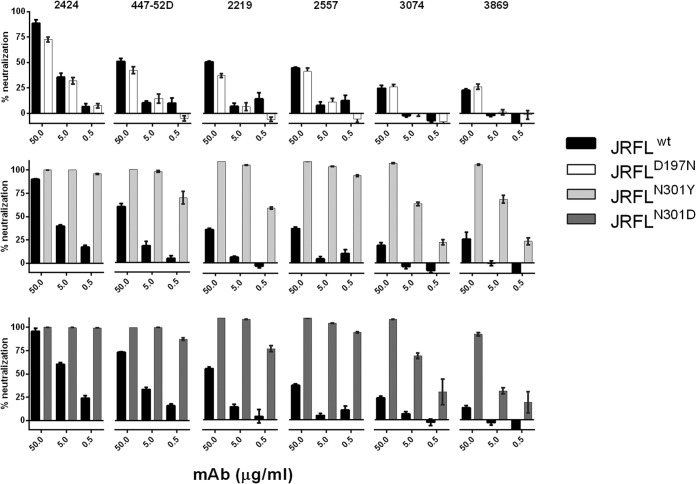
FIG 5.
Neutralization activities of 2424 versus the other V3 MAbs against HIV-1 pseudoviruses expressing wild-type or mutant JRFL Envs. HIV-1 JRFL pseudoviruses bearing wild-type or mutated Envs were produced in transfected 293T cells and incubated by titrated V3 MAbs (2424, 447-52D, 2219, 2557, 3074, and 3869) for 1 h at 37°C. TZM.bl cells were then added to the virus-MAb mixtures in complete DMEM containing DEAE-dextran. Neutralization activity was assessed after 48 h. Results are means and standard errors from two experiments.
2424 is also unlike the other V3 MAbs in terms of its Ig gene usage. This MAb has VH and VL gene pairing not used by the other V3 MAbs (50). The 2424 VH and VL genes are somatically mutated 14.4% and 5.1% from the germ line IGHV3-53 and IGLV2-28 genes, respectively. Heavy-chain complementarity-determining region 3 (CDR H3) is composed of 17 amino acids, similar in length to normal human Igs (51). Moreover, while the majority of V3 MAbs use λ light chains, 2424 uses a κ light chain. Only 17 out of 70 human V3 MAbs with known VH and VL genes use κ light chains (23, 38, 50, 52). Together, these data suggest that 2424 is a unique MAb targeting an epitope in the V3 loop that may be more accessible or occluded differently compared to the other V3 epitopes.
2424 displays subtype B-specific binding and neutralizing activities.
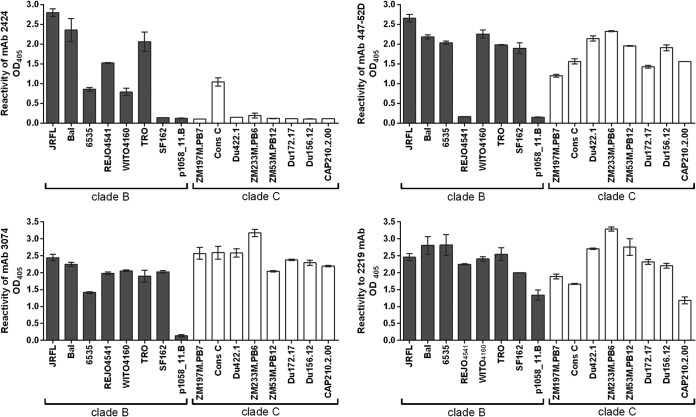
To better characterize MAb 2424 and its epitope, we assessed the cross-reactivity of 2424 in ELISA using recombinant gp120 proteins from HIV-1 subtype B and C (Fig. 2). Three V3 MAbs with weaker JRFL neutralization activities, 447-52D, 3074, and 2219, were also tested for comparison. 2424 displayed a gp120-binding profile distinct from those of the other three MAbs. 2424 was reactive mainly with subtype B gp120s, whereas 447-52D, 3074, and 2219 recognized most of the subtype B and subtype C gp120s tested. 2424 also poorly reacted with recombinant gp140 proteins of subtype C (CN54), subtype A (UG37), and subtype A (BG505-SOSIP.664) (optical density at 405 nm [OD405] = 0.28, 0.15, and 0.313, respectively; OD405 of irrelevant MAb 1418 = 0.15), although it was reactive with few V3 peptides of subtype A viruses, such as KE-Ken29 (OD405 = 1.3) and D687 (OD405 = 0.5).
FIG 2.
Binding of MAb 2424 versus the other V3 MAbs to gp120 proteins of clade B and clade C HIV-1 isolates. The binding of MAbs (1 μg/ml) to gp120 used at 1 μg/ml to coat the well surface was detected by ELISA. An irrelevant control MAb, MAb 1418, was also tested in parallel and displayed no binding to any gp120 in the panel (OD405 ≤ 0.2; data not shown). Data from one representative experiment are shown; the experiments were repeated twice.
The neutralizing breadth and potency of MAb 2424 were evaluated using TZM.bl target cells and pseudoviruses expressing 15 HIV-1 Envs from tier 1 to 3 subtype B and non-B viruses (2, 32) (Table 1). 2424 neutralized six viruses (BX08.16, BaL26, SS1196.1, REJO4541, JRCSF, and JRFL.JB), and three of these viruses (JRFL, JRCSF, and REJO4541) are relatively resistant to other V3 MAbs (26, 50). This neutralization pattern paralleled the 2424 binding to V3 peptides of the corresponding viruses. All six viruses belong to subtype B, confirming the subtype B specificity of this MAb. In contrast, the other V3 MAbs studied here (447-52D, 2219, 2557, 3074, and 3869) neutralized viruses from both B and non-B subtypes (26, 50). These data indicate that MAb 2424 is relatively potent against JRFL, JRCSF, and REJO4541, but its binding and neutralizing breadth are more restricted than those of the other V3 MAbs.
TABLE 1.
Neutralization activity of MAb 2424 against HIV-1 pseudoviruses
| Virus | Tiera | Clade | V3 sequenceb | MAb 2424 |
||
|---|---|---|---|---|---|---|
| IC50 (μg/ml)c | Peptide bindingd | Signature motife | ||||
| Bx08.16 | 1B | B | CTRPNNNTRKSIHIGPGRAFYTTGDIIGDIRQAHC | <0.4 | +++ | + |
| Bal.26 | 1B | B | CTRPNNNTRKSIHIGPGRAFYTTGEIIGDIRQAHC | <0.4 | +++ | + |
| SS1196.1 | 1B | B | CTRPNNNTRKSIHIGPGRAFYATGGVIGDIRQAHC | <0.4 | +++ | + |
| JRFL.JB | 2/Chr | B | CTRPNQNTRKSIHIGPGRAFYTTGEIIGDIRQAHC | 5.4 | +++ | + |
| JRCSF | 2/Chr | B | CTRPSNNTRKSIHIGPGRAFYTTGEIIGDIRQAHC | 6.6 | +++ | + |
| REJO4541 | 2 | B | CTRPNNNTRKSIHIAPGRAFYATGEIIGDIRKAYC | 11 | +++ | + |
| Bal.01 | 1B | B | CTRPNNNTRKSINIGPGRAFYTTGEIIGDIRQAHC | >50 | NT | − |
| SF162.LS | 1A | B | CTRPNNNTRKSITIGPGRAFYATGDIIGDIRQAHC | >50 | ± | − |
| 6535.3 | 1B | B | CTRPNNNTRKSINLGPGRAFYATGDIIGDIRQAHC | >50 | + | − |
| YU2 | 2 | B | CTRPNNNTRKSINIGPGRALYTTGEIIGDIRQAHC | >50 | − | − |
| MW965.26 | 1A | C | CTRPNNNTRKSVRIGPGQTFYATGAIIGDIRQAHC | >50 | − | − |
| HO31.7 | 2 | B | CTRPSNNTRKSITIGPGRAFYTTGDIIGDIRRAHC | >50 | NT | − |
| DJ263.8 | 1B | A | CTRPNNNTRRSVRIGPGQTFYATGDIIGDIRQAHC | >50 | − | − |
| HO61.14 | 3 | B | CTRPNNNTRKSIPIGPGRAFYTTGDIIGDIRQAHC | >50 | NT | − |
| ZM109F | 1B | C | CIRPGNNTRKSIRLGPGQTFYATGDVIGDIRKAYC | >50 | − | − |
Chr, chronic.
Boldface indicates the 2424 epitope signature motif.
MAb concentration needed to reach 50% inhibition in the TZM.bl neutralization assay. IC50s of <50 μg/ml are in bold.
ELISA reactivity against biotinylated V3 peptides. Reactivity with scrambled peptide was an OD405 of ≤0.5. Average OD405s are classified as follows: −, 0.2 to 0.5; ±, 0.5 to 0.75; +, 0.75 to 1.5; ++, 1.5 to 2.0; +++, 2.0 to 3.5. NT, not tested.
His308, Pro313, and Arg315 constitute a signature motif for the 2424 epitope.
We next determined how the addition of 2424 affected virus neutralization of the other V3 MAbs. Neutralizing activities of 2424 and 447-52D were tested individually or in a 1:1 combination against eight HIV-1 pseudoviruses (Table 2). The combination of 2424 and 447-52D was able to neutralize seven of the viruses, while on its own, 447-52D neutralized six viruses. However, the IC50s of the combined MAbs were generally higher than those of the individual MAbs, indicative of potential competition and steric hindrance between the two MAbs.
TABLE 2.
Virus neutralization by 2424 and 447-52D individually or in combination
| Virus | Tiera | Clade | IC50 (μg/ml)b |
||
|---|---|---|---|---|---|
| 2424 | 447-52D | 2424 + 447-52D | |||
| SF162 | 1A | B | >25 | <1 | <1 |
| Bal.01 | 1B | B | >25 | <1 | <1 |
| DJ263.8 | 1B | A | >50 | 8.5 | 25.0 |
| SS1196.1 | 1B | B | <1 | <1 | <1 |
| 6535 | 1B | B | >50 | 2.5 | 4.5 |
| JRFL.JB | 2/Chr | B | 4 | 15 | 10.5 |
| REJO | 2 | B | 18 | >25 | 20 |
| PVO.4 | 3 | B | >50 | >50 | >50 |
| No. positive/total (%) | 3/8 (37.5) | 6/8 (75) | 7/8 (87.5) | ||
Chr, chronic.
Total MAb concentration needed to reach 50% neutralization in the TZM.bl neutralization assay. IC50s of ≤25 μg/ml are in bold.
Neutralizing activity of 2424 is affected by soluble CD4 and removal of the N-glycan at position 301 but not by changes in N-glycan composition of the virus Env.
To further characterize the 2424 epitope, we tested whether 2424 neutralizing activity was affected by virus pretreatment with soluble CD4. CD4 binding to the virus Env causes conformational changes that induce exposure of masked epitopes on V3 (53). A fixed concentration of soluble CD4, which on its own yielded ∼20% neutralization, was incubated with JRFL, prior to addition of titrated amounts of 2424. Three other V3 MAbs (447-52D, 3074, and 3869) and an irrelevant parvovirus-specific MAb (1418) were tested for comparison. CD4 treatment augmented virus neutralization by 2424, similar to that observed with 447-52D, 3074, and 3869 (Fig. 3). The neutralization levels attained were significantly higher than the calculated sums of percent neutralization attained by each MAb alone and CD4. The other V3 MAbs, 2219 and 2557, were similarly affected by CD4 (54). These data indicate that CD4-induced conformational changes modulate MAb accessibility of the 2424 epitope, similar to the other neutralizing V3 epitopes.
Next we examined the contribution of N-glycan composition in influencing the accessibility of 2424 epitope vis-a-vis the other V3 epitopes. Our previous studies demonstrated that V3 epitopes on JRFL were better exposed when the virus was produced in the presence of a mannosidase inhibitor, kifunensine or swainsonine, which enriches the viral Env glycoproteins with high mannose-type N-linked glycans (27). Hence, we evaluated neutralization of JRFL produced with or without kifunensine by 2424 and five other V3 MAbs (Fig. 4A). JRFL neutralization by 2424 was unaltered whether the virus was produced with or without kifunensine. Kifunensine treatment also had minimal effect on 3074, but this MAb did not neutralize JRFL. In contrast, 447-52D, 2219, 2557, and 3869 were more effective against JRFL produced in the presence of kifunensine than untreated JRFL. Swainsonine treatment also had no effect on JRFL neutralization by 2424, although it enhanced virus sensitivity to 447-52D (Fig. 4B). MAb 2G12, which recognizes a cluster of N-glycans bearing the high-mannose type (55), and the CD4-binding site-specific MAb b12 were tested for comparison (Fig. 4A). 2G12 displayed higher neutralization against JRFLKif than untreated JRFL, whereas neutralization by b12 was changed slightly, consistent with previously published data (56). The irrelevant control MAb 1418 had no neutralizing activity against treated or untreated virus.
FIG 4.
Neutralizing activities of MAb 2424 versus the other V3 MAbs against JRFL produced in the presence or absence of a mannosidase inhibitor. (A) HIV-1 JRFL pseudoviruses were produced in transfected 293T cells in the absence or presence of 25 μM kifunensine (JRFLKif) and tested for neutralization by V3 MAbs (2424, 447-52D, 2219, 2557, 3869, and 3074), MAb 2G12, CD4bs MAb b12, or an irrelevant MAb, MAb 1418. (B) HIV-1 JRFL pseudoviruses were produced in 293T cells with or without 20 μM swainsonine (JRFLSwain) and tested for neutralization by V3 MAbs 2424 and 447-52D. Serially diluted MAbs were incubated with virus at 37°C for 1 h. TZM.bl cells were then added to the virus-MAb mixtures in complete DMEM containing DEAE-dextran. Neutralization activity was assessed after 48 h. Results with 2424 and 447-52D were averages from duplicate wells, and data from one representative experiment are shown. Results with other MAbs are shown as averages from two to three experiments. P values were determined by the two-way ANOVA. (C) V3 MAb reactivity with gp120 JRFL was assessed in ELISA. Recombinant gp120 protein (1 μg/ml) was coated on 96-well plates and reacted with serially diluted V3 MAbs. Data from a representative experiment with averages and standard deviations from duplicate wells are shown.
Although various levels of neutralization were observed with the different V3 MAbs against untreated JRFL, the six V3 MAbs had similar activity of binding to the JRFL gp120 protein (Fig. 4C). These data demonstrated that the epitopes recognized by the six V3 MAbs are present on the monomeric JRFL Env but are masked to different degrees when expressed on the virus. Neutralization data of JRFL produced with or without a mannosidase inhibitor further indicate that, while the exposure of many V3 epitopes was modulated by the N-glycan composition of the virus Env, the 2424 epitope was not affected. Two N-glycans previously implicated as controlling V3 exposure include those at position 197 at the C-terminal base of V1V2 and position 301 at the N-terminal base of V3 (57–59), and both glycans were assigned to be the complex type (60). However, for JRFL, there is a glycosylation site at position 301 but not at position 197. To evaluate the importance of N-glycan at these positions, mutations were introduced into JRFL Env at position 301 (N to D or N to Y) to remove the specific glycan or at position 197 (D to N) to add a glycosylation site. The mutations at position 301 rendered the virus sensitive to all V3 MAbs tested, including 2424 (Fig. 5). Nonetheless, mutations that remove the glycan at residue 301 also enhanced sensitivity of different HIV-1 isolates to antibodies against the CD4-binding site and the CD4-induced epitopes, although the MPER epitopes in the gp41 subunit were unaffected (57, 59, 61, 62), indicating that mutations at this position induce global structural alterations which affect not only V3 but also other distant sites. In contrast, the D197N mutation did not affect virus neutralization by any of the six V3 MAbs tested. The contribution of residue 197 in shielding V3 epitopes is not associated with N-glycan; rather, this residue is involved in stabilizing the interprotomer interactions between V1V2 and V3 that allow V1V2 to shield V3 in the Env trimeric spike. Only specific mutations that disrupt these interactions (e.g., D197H and D197Q) are able to release V3 from the V1V2 masking (S. Zolla-Pazner, unpublished data).
The crystal structure of MAb 2424 and its epitope reveals that 2424 targets a vulnerable region at the apex of the V3 crown.
To define the epitope of MAb 2424, we determined the structure of the 2424 antigen-binding fragment (Fab) cocrystallized with a 23-mer V3JRFL peptide, NNTRKSIHIGPGRAFYTTGEIIG (residues 301 to 325 in the HxB2 numbering system [63]). The structure of the 2424 Fab-epitope complex was solved by molecular replacement and refined to 3.18-Å resolution with an Rwork/Rfree value of 22.5%/28.3% (Fig. 6A and Table 3). The crystals grew in an orthorhombic space group with three Fab-epitope complexes in the asymmetric unit. Since the noncrystallographic symmetry constraints (NCS) were imposed in the refinement, only one complex is described in detail here. The residues of the light and heavy chains are numbered following the convention of Kabat and Wu (64), preceded by “L” and “H,” respectively, and the residues of the V3 peptide are preceded by a “P.” Although a 23-mer peptide was used in the crystallization, only 11 residues, with the sequence IHIGPGRAFYT (residues 307 to 319), were observed in the electron densities and thus built into the final model. We also determined the structure of Fab 2424 alone and refined it to a 2.68-Å resolution (Rwork/Rfree = 20.0%/28.5%) (Table 3). The Fab structure is very similar to that in the Fab-epitope complex, with only one residue, PheH58, in the antigen-binding site of the Fab alone having a different side chain orientation.
FIG 6.
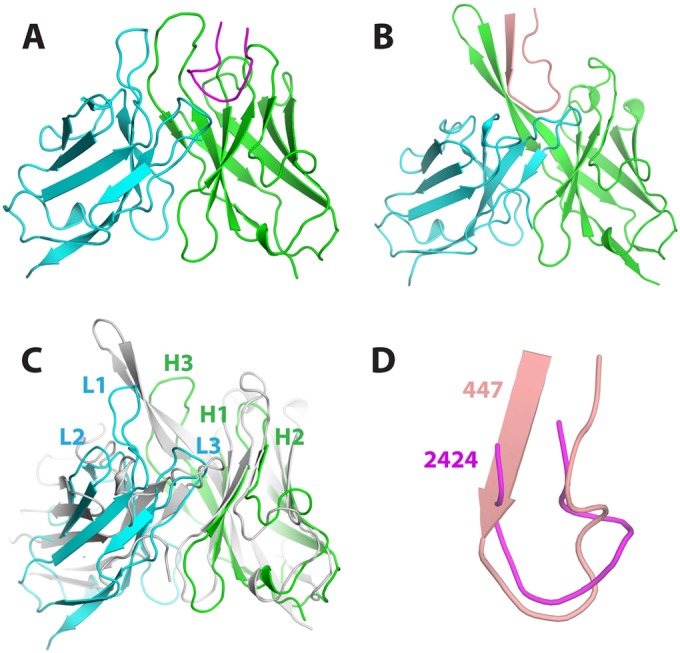
Structural comparison of MAbs 2424 and 447-52D. (A) Ribbon representation of the Fab 2424/V3JRFL complex. The epitope and the light and heavy chains are magenta, cyan, and green, respectively. For simplicity, only the Fv region is shown. (B) Fab 447-52D/V3MN complex. The epitope is shown in salmon. (C) Superimposition of the two Fabs. Both light and heavy chains of 447-52D are in gray. (D) Superimposition of the V3 peptides bound to 2424 and 447-52D. The N terminus of 2424 epitope is shorter than that of 447-52D.
TABLE 3.
Crystallographic data collection and refinement statisticsa
| Parameter | Fab 2424/V3JRFL | Fab 2424 |
|---|---|---|
| Data collection | ||
| Space group | P22121 | P21 |
| Cell dimensions | ||
| a, b, c (Å) | 95.71, 121.62, 139.84 | 69.59, 45.04, 74.78 |
| α, β, γ | 90, 90, 90 | 90, 96.75, 90 |
| Resolution (Å) | 3.18 (3.37–3.18) | 2.68 (2.84–2.68) |
| CC (1/2) | 99.2 (76.5) | 99.3 (70.4) |
| Ι/σΙ | 11.6 (2.0) | 11.5 (2.0) |
| Completeness (%) | 99.9 (99.9) | 99.1 (98.6) |
| Refinement | ||
| Resolution (Å) | 48.28–3.18 | 47.86–2.68 |
| Unique reflections | 53,260 | 13,118 |
| Rwork/Rfree | 22.5/28.3 | 20.0/28.5 |
| No. of atoms | ||
| Protein | 10,085 | 3,287 |
| Solvent | 0 | 33 |
| B factors (Å2) | ||
| Protein | 69.7 | 59.7 |
| Solvent | 47.3 | |
| RMS deviations | ||
| Bond length (Å) | 0.009 | 0.009 |
| Bond angle (°) | 1.183 | 1.225 |
Numbers in parentheses refer to the outer resolution shell. RMS, root mean square.
The Fab/V3 epitope complex revealed that 2424 binds the very apex of the V3 crown (Fig. 6 and 7), forming direct contacts with 9 residues, P308HIGPGRAFYP318. However, IleP309 and PheP317 have only very little backbone contact (∼6.5 Å2 and 4.3 Å2, respectively) with the antibody, and their side chains point away from the antigen-binding surface of 2424. The antibody binds the epitope using the ladle mode, one of the two binding modes typically used by anti-V3 crown MAbs (65). In this binding mode, the arch of the V3 crown (four residues at the apex, typically GPGR for subtype B or GPGQ for subtype C) points directly toward the antigen-binding site (Fig. 6A). This is different from the other binding mode, called the cradle mode, in which the V3 crown lies sideways in the long antigen-binding groove of the antibody (35, 66). Like other anti-V3 crown MAbs, the antigen-binding site is negatively charged, so that it can accommodate the positively charged V3 crown. Interestingly, residue P315-P317 forms a small 310 helical turn, which is rarely observed in V3 crown structures despite the early prediction from a sequence analysis that the C-terminal part of V3 has a propensity to be helical (67). All CDR loops participate in the antigen-antibody interactions, and they form a relatively small but deep binding site basin. There are three specific binding pockets that interact with the side chains of HisP308, ProP313, and ArgP315, respectively (Fig. 7A). The contact areas of these three residues (240 Å2) account for over 60% of the contact areas of all the epitope residues with 2424 (383 Å2). In agreement with these structural data, the presence or absence of these three specific residues dictates whether 2424 binds strongly or poorly to V3 from diverse HIV-1 isolates (Table 1). Thus, the side chains of these three residues dominate the antigen-antibody interaction for 2424, forming the signature motif for 2424 recognition. This is consistent with the 2424 specificity for subtype B viruses, which often have HisP308 and ArgP315.
FIG 7.
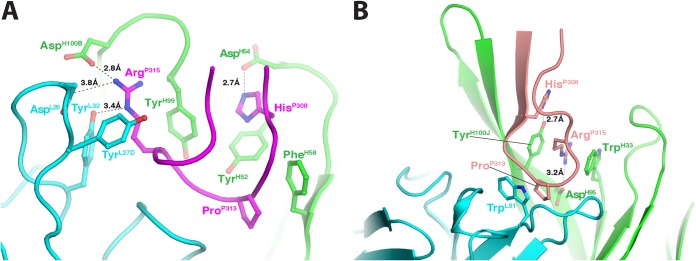
Details of the antigen-antibody interactions in the 2424 and 447-52D Fab/V3 peptide complexes. The color scheme is the same as for Figs. 6A and B. The key residues involved in the antigen-antibody interactions are shown as sticks. (A) Fab 2424/epitope complex. Note that three residues, HisP308, ProP313, and ArgP315, of V3 play key roles in the antigen-antibody interaction: (i) the side chain HisP308 is stacked with that of TyrH52 and forms a potential hydrogen bond with AspH54, (ii) the side chain of ProP313 is stacked with that of PheH58, and (iii) the side chain of ArgP315 is surrounded by three Tyrs and forms a salt bridge with AspH100B. (B) 447-52D Fab/epitope complex. Note again that three residues, HisP308, ProP313, and ArgP315, of V3 play key roles in the antigen-antibody interaction.
Comparison of the antigen-antibody interactions of 2424 with 447-52D.
We compared structurally the antigen-antibody interaction of 2424 with that of 447-52D, a well-characterized anti-V3 crown MAb. The most obvious similarity between these two MAbs is their mode of antigen binding: both use the ladle mode, but 447-52D has a 22-amino-acid CDR H3 that serves as the handle of the ladle (Fig. 6B). They also have some similarities in the details of their antigen binding. For example, the side chain ArgP315 of the epitope in 2424 forms a salt bridge with a heavy-chain aspartic acid, and it is also surrounded by several Tyr residues from both heavy and light chains (Fig. 7A). Similarly, the side chain ArgP315 in 447-52D also forms a salt bridge with a heavy-chain aspartic acid, and it is also sandwiched between the side chains of two aromatic amino acids of the antibody (Fig. 7B). In addition, ProP313 of V3 in both MAbs is placed in a hydrophobic environment with its side chain stacked against the side chain of either a Phe (2424) or a Trp (447-52D). However, the two MAbs also have several differences in their antigen-antibody interactions. First, the epitope of 2424 is two residues shorter in the N terminus than that of 447-52D; this focuses 2424 binding to the very apex of the V3 crown. Second, 2424 is highly side chain specific, burying the side chains of HisP308 and ArgP315 in deep pockets in the antigen-binding site, while 447-52D utilizes a beta-sheet main-chain interaction with the N terminus of the V3 crown, rendering 447-52D more broadly reactive. The limited contacts between 2424 and the V3 crown and the 2424 epitope being farther away from nearby glycans may explain how its binding is less affected by the glycan composition of gp120 (Fig. 4). However, its side chain specificity is likely to limit the neutralization breadth of 2424.
DISCUSSION
This study presents the crystallographic structure of MAb 2424 in complex with its V3 epitope, elucidating the distinctive capacity of this V3 MAb to neutralize HIV-1 JRFL, a virus isolate resistant to many other V3 MAbs (26, 52). The presence of such a MAb indicates that, although epitopes on the V3 crown region of tier 2 or tier 3 viruses are often occluded, some are accessible to Abs. The broadly neutralizing MAb PGT 128 that is highly potent against tier 2 and tier 3 viruses also binds to a complex epitope consisting in part of a short beta-strand segment of the V3 loop and N-linked glycans at the V3 base (17). 2424 uses a different approach to access its epitope in that it targets the very tip of the V3 crown, away from the many N-glycans shrouding the HIV Env surface. In support of this idea, we demonstrate that treating the virus with a mannosidase inhibitor, which changes the sugar composition of N-glycans on the virus Env, did not affect virus neutralization by 2424. On the other hand, most other V3 MAbs were more potent against viruses bearing Env with homogenously high mannose-type glycans, suggesting the important contribution of complex-type N-glycans in masking the epitopes of these V3 MAbs, either by direct steric hindrance or by facilitating formation of more compact Env trimers. All together, the data presented herein indicate that, unlike other V3 epitopes, the 2424 epitope is devoid of and is minimally masked by N-glycans; thus, it constitutes a distinct target which would complement the other neutralizing epitopes on the virus Env.
Another distinctive feature of MAb 2424 is its ability to mediate 100% neutralization against JRFL. Although the mechanistic explanations for this activity remain unknown, the result suggests the possibility that MAb 2424 can attain binding saturation of the virus Env due to the relative distance of its epitope from the surrounding glycans. Nonetheless, the neutralizing activity of MAb 2424 was further enhanced after CD4 binding and also affected by the removal of N-glycan from residue 301 at the base of V3, indicating that the 2424 epitope is not completely exposed on the prefusion Env spikes. The binding affinity of MAb 2424 is not noticeably different from that of the other V3 MAbs when tested with soluble gp120 monomer. On the Env trimers, the V3 distal tip may be constrained to preferentially adopt the conformation recognized by 2424 and/or may be better oriented for binding by 2424 compared to the other V3 crown MAbs.
Together with the current 2424-V3 structure, many crystal structures of human V3 MAbs in complex with their cognate V3 epitopes have been resolved (35, 68–70). They reveal three major modes of Ab-epitope interactions, designated ladle, cradle, and Janus. The ladle mode is exemplified by 447-52D, which grasps V3 by the long CDR H3 handle of the ladle. 2424 belongs to this same category but is distinct from 447-52D in that it interacts only with the tip of the V3 crown with the bowl portion of the ladle. The potency of 2424 to neutralize relatively resistant viruses such as JRFL and REJO and its indifference to mannosidase inhibitors imply that the very tip of V3 targeted by 2424 is minimally shielded by glycans in these viruses. In contrast, the cradle mode is represented by MAbs 2219 and 2557, whose antigen-binding sites are shaped like a cradle, and the V3 crown lies in it. MAbs in this category are effective only when the virus Env is enriched with the high-mannose-type N-glycans, indicating the critical contribution of N-glycans in shielding these MAbs' epitopes. In addition, there is a third binding mode, the Janus mode, which is characterized by MAbs 3074 and 268; they approach V3 from two opposing sides to contact, respectively, the conserved hydrophobic core and the strain-specific hydrophilic face, in the middle segment of the V3 crown (35). MAb 3074 neutralizes JRFL poorly whether the virus is produced with or without a mannosidase inhibitor, indicating that this MAb cannot reach into the hydrophobic core of V3 when presented on the JRFL Env spike and that this conserved site is concealed by factors other than N-glycans.
The distinct epitope and neutralization profile of MAb 2424 correspond to its Ig constant and variable gene usage. MAb 2424 has a kappa light chain and is encoded by IGHV3-53 and IGLV2-28 genes. Among 51 human anti-V3 MAbs evaluated by Gorny et al. (38), the majority (76.5%) use lambda light chains. Only 12 (23.5%) utilize the IGHV3 gene family, and these IGHV3-encoded V3 MAbs are paired preferentially with kappa light chains. However, although V3 Abs are skewed away from using IGHV3, this gene family is common among Abs from healthy uninfected individuals. As shown by Tiller et al. (51), 54.8% of 183 recombinant Abs isolated from uninfected individuals belong to the IGHV3 gene family. It remains to be determined if such V3 Abs can be generated in these individuals by vaccination. 2424-like Abs were not the dominant response elicited in mice upon immunization with gp120 JRFL alone or in complex with MAb, as indicated by the inability of serum Ab pools from the immunized animals to neutralize 2424-sensitive viruses such as JRFL or REJO (27), although the composition of dominant and nondominant Ab responses were not yet delineated and the gp120/MAb complex vaccines tested were not selected and optimized for presenting the 2424 epitope. Nonetheless, many epitopes in the V3 crown has been shown to be highly immunogenic in humans and in animal models. MAb 2424 also is not as highly mutated, with somatic hypermutation rates of about 14% and 5% in its VH and VL genes and a CDR H3 length of 17 amino acids. This level of affinity maturation is lower than those required to generate the extremely potent and broadly neutralizing MAbs targeting other Env sites, such as PG6 and PG19 (15), PGT 141-145, PGT121, PGT127, PGT128, and PGT135 (16), VRC01 (66), NIH45-46 (71), 10E8 (72), and the most recently reported 35O22 (73). Rather, it is in the range observed with influenza virus-specific Abs after repeated administration of influenza vaccines (74), although it remains above the mutation rates achieved after immunization with gp120 protein alone or with prime-boost vaccines of ALVAC and gp120 protein (58).
The crystallographic structures of 2424 and its V3 epitope reveal the important contribution of HisP308 and ArgP315, both of which are characteristic of subtype B V3, and provide an explanation for the 2424 specificity for subtype B Env. In contrast, the consensus Env sequences of subtype A and subtype C viruses, which encompass the vast majority of HIV-1 isolates circulating in the areas of epidemicity in Africa and Asia, commonly contain an Arg at position 308 and a Gln at position 315. Induction of Abs directed to the 2424 epitope site at the apex of V3 with the subtype B and non-B sequences would be desirable. No MAb has been identified thus far that targets the non-B counterpart of the 2424 epitope to determine whether this site is also accessible on non-B HIV-1 isolates. Nonetheless, targeting this site would constitute an additional approach to the other V3-targeting vaccine strategies that focus on the complex PGT128-like peptidoglycan epitopes or the V3 epitopes recognized via the cradle or Janus mode. Abs against disparate targets on V3 and other sites on the HIV Env are needed to prevent the transmission of a diverse array of HIV-1 isolates and block escape variants that would readily emerge under pressure from only one Ab class.
ACKNOWLEDGMENTS
We thank Constance Williams, Vincenza Itri, and Timothy M. O'Neal for preparing the monoclonal antibodies used in the study, Barbara Volsky for sharing technical expertise in pseudovirus generation and neutralization assays, and Michael Tuen for general lab support.
This study was supported in part by NIH grants AI102740, AI100151, and AI082274 and by research funds from the Henry M. Jackson Foundation and the Department of Veterans Affairs (VA), Veterans Health Administration, Office of Research and Development. C.E.H. is the recipient of the VA Research Career Scientist and VA Merit Review Awards. GM/CA-CAT at APS, Argonne National Laboratory, was funded in whole or in part with federal funds from the National Cancer Institute (Y1-CO-1020) and the National Institute of General Medical Sciences (Y1-GM-1104). Use of the Advanced Photon Source was supported by the U.S. Department of Energy, Basic Energy Sciences, Office of Science, under contract no. DE-AC02-06CH11357.
The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health or the Department of Veterans Affairs.
REFERENCES
- 1.Gaschen B, Taylor J, Yusim K, Foley B, Gao F, Lang D, Novitsky V, Haynes B, Hahn BH, Bhattacharya T, Korber B. 2002. Diversity considerations in HIV-1 vaccine selection. Science 296:2354–2360. doi: 10.1126/science.1070441. [DOI] [PubMed] [Google Scholar]
- 2.Seaman MS, Janes H, Hawkins N, Grandpre LE, Devoy C, Giri A, Coffey RT, Harris L, Wood B, Daniels MG, Bhattacharya T, Lapedes A, Polonis VR, McCutchan FE, Gilbert PB, Self SG, Korber BT, Montefiori DC, Mascola JR. 2010. Tiered categorization of a diverse panel of HIV-1 Env pseudoviruses for assessment of neutralizing antibodies. J Virol 84:1439–1452. doi: 10.1128/JVI.02108-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Kwong PD, Wyatt R, Robinson J, Sweet RW, Sodroski J, Hendrickson WA. 1998. Structure of an HIV gp120 envelope glycoprotein in complex with the CD4 receptor and a neutralizing human antibody. Nature 393:648–659. doi: 10.1038/31405. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Lifson JD, Feinberg MB, Reyes GR, Rabin L, Banapour B, Chakrabarti S, Moss B, Wong-Staal F, Steimer KS, Engleman EG. 1986. Induction of CD4-dependent cell fusion by the HTLV-III/LAV envelope glycoprotein. Nature 323:725–728. doi: 10.1038/323725a0. [DOI] [PubMed] [Google Scholar]
- 5.Feng Y, Broder CC, Kennedy PE, Berger EA. 1996. HIV-1 entry cofactor: functional cDNA cloning of a seven-transmembrane, G protein-coupled receptor. Science 272:872–877. doi: 10.1126/science.272.5263.872. [DOI] [PubMed] [Google Scholar]
- 6.Deng H, Liu R, Ellmeier W, Choe S, Unutmaz D, Burkhart M, Di Marzio P, Marmon S, Sutton RE, Hill CM, Davis CB, Peiper SC, Schall TJ, Littman DR, Landau NR. 1996. Identification of a major co-receptor for primary isolates of HIV-1. Nature 381:661–666. doi: 10.1038/381661a0. [DOI] [PubMed] [Google Scholar]
- 7.Dragic T, Litwin V, Allaway GP, Martin SR, Huang Y, Nagashima KA, Cayanan C, Maddon PJ, Koup RA, Moore JP, Paxton WA. 1996. HIV-1 entry into CD4+ cells is mediated by the chemokine receptor CC-CKR-5. Nature 381:667–673. doi: 10.1038/381667a0. [DOI] [PubMed] [Google Scholar]
- 8.Modrow S, Hahn BH, Shaw GM, Gallo RC, Wong-Staal F, Wolf H. 1987. Computer-assisted analysis of envelope protein sequences of seven human immunodeficiency virus isolates: prediction of antigenic epitopes in conserved and variable regions. J Virol 61:570–578. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Rizzuto CD, Wyatt R, Hernandez-Ramos N, Sun Y, Kwong PD, Hendrickson WA, Sodroski J. 1998. A conserved HIV gp120 glycoprotein structure involved in chemokine receptor binding. Science 280:1949–1953. doi: 10.1126/science.280.5371.1949. [DOI] [PubMed] [Google Scholar]
- 10.Wu X, Yang ZY, Li Y, Hogerkorp CM, Schief WR, Seaman MS, Zhou T, Schmidt SD, Wu L, Xu L, Longo NS, McKee K, O'Dell S, Louder MK, Wycuff DL, Feng Y, Nason M, Doria-Rose N, Connors M, Kwong PD, Roederer M, Wyatt RT, Nabel GJ, Mascola JR. 2010. Rational design of envelope identifies broadly neutralizing human monoclonal antibodies to HIV-1. Science 329:856–861. doi: 10.1126/science.1187659. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Klein F, Gaebler C, Mouquet H, Sather DN, Lehmann C, Scheid JF, Kraft Z, Liu Y, Pietzsch J, Hurley A, Poignard P, Feizi T, Morris L, Walker BD, Fatkenheuer G, Seaman MS, Stamatatos L, Nussenzweig MC. 2012. Broad neutralization by a combination of antibodies recognizing the CD4 binding site and a new conformational epitope on the HIV-1 envelope protein. J Exp Med 209:1469–1479. doi: 10.1084/jem.20120423. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Trkola A, Purtscher M, Muster T, Ballaun C, Buchacher A, Sullivan N, Srinivasan K, Sodroski J, Moore JP, Katinger H. 1996. Human monoclonal antibody 2G12 defines a distinctive neutralization epitope on the gp120 glycoprotein of human immunodeficiency virus type 1. J Virol 70:1100–1108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Scanlan CN, Pantophlet R, Wormald MR, Ollmann Saphire E, Stanfield R, Wilson IA, Katinger H, Dwek RA, Rudd PM, Burton DR. 2002. The broadly neutralizing anti-human immunodeficiency virus type 1 antibody 2G12 recognizes a cluster of alpha1→2 mannose residues on the outer face of gp120. J Virol 76:7306–7321. doi: 10.1128/JVI.76.14.7306-7321.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Murin CD, Julien JP, Sok D, Stanfield RL, Khayat R, Cupo A, Moore JP, Burton DR, Wilson IA, Ward AB. 2014. Structure of 2G12 Fab2 in complex with soluble and fully glycosylated HIV-1 Env by negative-stain single-particle electron microscopy. J Virol 88:10177–10188. doi: 10.1128/JVI.01229-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Walker LM, Phogat SK, Chan-Hui PY, Wagner D, Phung P, Goss JL, Wrin T, Simek MD, Fling S, Mitcham JL, Lehrman JK, Priddy FH, Olsen OA, Frey SM, Hammond PW, Kaminsky S, Zamb T, Moyle M, Koff WC, Poignard P, Burton DR. 2009. Broad and potent neutralizing antibodies from an African donor reveal a new HIV-1 vaccine target. Science 326:285–289. doi: 10.1126/science.1178746. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Walker LM, Huber M, Doores KJ, Falkowska E, Pejchal R, Julien JP, Wang SK, Ramos A, Chan-Hui PY, Moyle M, Mitcham JL, Hammond PW, Olsen OA, Phung P, Fling S, Wong CH, Phogat S, Wrin T, Simek MD, Koff WC, Wilson IA, Burton DR, Poignard P. 2011. Broad neutralization coverage of HIV by multiple highly potent antibodies. Nature 477:466–470. doi: 10.1038/nature10373. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Pejchal R, Doores KJ, Walker LM, Khayat R, Huang PS, Wang SK, Stanfield RL, Julien JP, Ramos A, Crispin M, Depetris R, Katpally U, Marozsan A, Cupo A, Maloveste S, Liu Y, McBride R, Ito Y, Sanders RW, Ogohara C, Paulson JC, Feizi T, Scanlan CN, Wong CH, Moore JP, Olson WC, Ward AB, Poignard P, Schief WR, Burton DR, Wilson IA. 2011. A potent and broad neutralizing antibody recognizes and penetrates the HIV glycan shield. Science 334:1097–1103. doi: 10.1126/science.1213256. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Carrow EW, Vujcic LK, Glass WL, Seamon KB, Rastogi SC, Hendry RM, Boulos R, Nzila N, Quinnan GV Jr. 1991. High prevalence of antibodies to the gp120 V3 region principal neutralizing determinant of HIV-1MN in sera from Africa and the Americas. AIDS Res Hum Retroviruses 7:831–838. doi: 10.1089/aid.1991.7.831. [DOI] [PubMed] [Google Scholar]
- 19.Vaine M, Wang S, Liu Q, Arthos J, Montefiori D, Goepfert P, McElrath MJ, Lu S. 2010. Profiles of human serum antibody responses elicited by three leading HIV vaccines focusing on the induction of Env-specific antibodies. PLoS One 5:e13916. doi: 10.1371/journal.pone.0013916. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Gottardo R, Bailer RT, Korber BT, Gnanakaran S, Phillips J, Shen X, Tomaras GD, Turk E, Imholte G, Eckler L, Wenschuh H, Zerweck J, Greene K, Gao H, Berman PW, Francis D, Sinangil F, Lee C, Nitayaphan S, Rerks-Ngarm S, Kaewkungwal J, Pitisuttithum P, Tartaglia J, Robb ML, Michael NL, Kim JH, Zolla-Pazner S, Haynes BF, Mascola JR, Self S, Gilbert P, Montefiori DC. 2013. Plasma IgG to linear epitopes in the V2 and V3 regions of HIV-1 gp120 correlate with a reduced risk of infection in the RV144 vaccine efficacy trial. PLoS One 8:e75665. doi: 10.1371/journal.pone.0075665. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Zolla-Pazner S, Edlefsen PT, Rolland M, Kong XP, deCamp A, Gottardo R, Williams C, Tovanabutra S, Sharpe-Cohen S, Mullins JI, deSouza MS, Karasavvas N, Nitayaphan S, Rerks-Ngarm S, Pitisuttihum P, Kaewkungwal J, O'Connell RJ, Robb ML, Michael NL, Kim JH, Gilbert P. 2014. Vaccine-induced human antibodies specific for the third variable region of HIV-1 gp120 impose immune pressure on infecting viruses. EBioMedicine 1:37–45. doi: 10.1016/j.ebiom.2014.10.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Gorny MK, Williams C, Volsky B, Revesz K, Wang XH, Burda S, Kimura T, Konings FA, Nadas A, Anyangwe CA, Nyambi P, Krachmarov C, Pinter A, Zolla-Pazner S. 2006. Cross-clade neutralizing activity of human anti-V3 monoclonal antibodies derived from the cells of individuals infected with non-B clades of human immunodeficiency virus type 1. J Virol 80:6865–6872. doi: 10.1128/JVI.02202-05. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Scheid JF, Mouquet H, Feldhahn N, Seaman MS, Velinzon K, Pietzsch J, Ott RG, Anthony RM, Zebroski H, Hurley A, Phogat A, Chakrabarti B, Li Y, Connors M, Pereyra F, Walker BD, Wardemann H, Ho D, Wyatt RT, Mascola JR, Ravetch JV, Nussenzweig MC. 2009. Broad diversity of neutralizing antibodies isolated from memory B cells in HIV-infected individuals. Nature 458:636–640. doi: 10.1038/nature07930. [DOI] [PubMed] [Google Scholar]
- 24.Cao J, Sullivan N, Desjardin E, Parolin C, Robinson J, Wyatt R, Sodroski J. 1997. Replication and neutralization of human immunodeficiency virus type 1 lacking the V1 and V2 variable loops of the gp120 envelope glycoprotein. J Virol 71:9808–9812. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Saunders CJ, McCaffrey RA, Zharkikh I, Kraft Z, Malenbaum SE, Burke B, Cheng-Mayer C, Stamatatos L. 2005. The V1, V2, and V3 regions of the human immunodeficiency virus type 1 envelope differentially affect the viral phenotype in an isolate-dependent manner. J Virol 79:9069–9080. doi: 10.1128/JVI.79.14.9069-9080.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Hioe CE, Wrin T, Seaman MS, Yu X, Wood B, Self S, Williams C, Gorny MK, Zolla-Pazner S. 2010. Anti-V3 monoclonal antibodies display broad neutralizing activities against multiple HIV-1 subtypes. PLoS One 5:e10254. doi: 10.1371/journal.pone.0010254. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Kumar R, Tuen M, Liu J, Nadas A, Pan R, Kong X, Hioe CE. 2013. Elicitation of broadly reactive antibodies against glycan-modulated neutralizing V3 epitopes of HIV-1 by immune complex vaccines. Vaccine 31:5413–5421. doi: 10.1016/j.vaccine.2013.09.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Zolla-Pazner S, Cohen S, Pinter A, Krachmarov C, Wrin T, Wang S, Lu S. 2009. Cross-clade neutralizing antibodies against HIV-1 induced in rabbits by focusing the immune response on a neutralizing epitope. Virology 392:82–93. doi: 10.1016/j.virol.2009.05.039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Montefiori DC, Graham BS, Zhou J, Zhou J, Bucco RA, Schwartz DH, Cavacini LA, Posner MR. 1993. V3-specific neutralizing antibodies in sera from HIV-1 gp160-immunized volunteers block virus fusion and act synergistically with human monoclonal antibody to the conformation-dependent CD4 binding site of gp120. NIH-NIAID AIDS Vaccine Clinical Trials Network. J Clin Invest 92:840–847. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Spear GT, Takefman DM, Sharpe S, Ghassemi M, Zolla-Pazner S. 1994. Antibodies to the HIV-1 V3 loop in serum from infected persons contribute a major proportion of immune effector functions including complement activation, antibody binding, and neutralization. Virology 204:609–615. doi: 10.1006/viro.1994.1575. [DOI] [PubMed] [Google Scholar]
- 31.Emini EA, Schleif WA, Nunberg JH, Conley AJ, Eda Y, Tokiyoshi S, Putney SD, Matsushita S, Cobb KE, Jett CM, et al. 1992. Prevention of HIV-1 infection in chimpanzees by gp120 V3 domain-specific monoclonal antibody. Nature 355:728–730. doi: 10.1038/355728a0. [DOI] [PubMed] [Google Scholar]
- 32.Li M, Gao F, Mascola JR, Stamatatos L, Polonis VR, Koutsoukos M, Voss G, Goepfert P, Gilbert P, Greene KM, Bilska M, Kothe DL, Salazar-Gonzalez JF, Wei X, Decker JM, Hahn BH, Montefiori DC. 2005. Human immunodeficiency virus type 1 env clones from acute and early subtype B infections for standardized assessments of vaccine-elicited neutralizing antibodies. J Virol 79:10108–10125. doi: 10.1128/JVI.79.16.10108-10125.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Agarwal A, Hioe CE, Swetnam J, Zolla-Pazner S, Cardozo T. 2011. Quantitative assessment of masking of neutralization epitopes in HIV-1. Vaccine 29:6736–6741. doi: 10.1016/j.vaccine.2010.12.052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Krachmarov CP, Honnen WJ, Kayman SC, Gorny MK, Zolla-Pazner S, Pinter A. 2006. Factors determining the breadth and potency of neutralization by V3-specific human monoclonal antibodies derived from subjects infected with clade A or clade B strains of human immunodeficiency virus type 1. J Virol 80:7127–7135. doi: 10.1128/JVI.02619-05. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Jiang X, Burke V, Totrov M, Williams C, Cardozo T, Gorny MK, Zolla-Pazner S, Kong XP. 2010. Conserved structural elements in the V3 crown of HIV-1 gp120. Nat Struct Mol Biol 17:955–961. doi: 10.1038/nsmb.1861. [DOI] [PubMed] [Google Scholar]
- 36.Gorny MK, Xu J-Y, Gianakakos V, Karwowska S, Williams C, Sheppard HW, Hanson CV, Zolla-Pazner S. 1991. Production of site-selected neutralizing human monoclonal antibodies against the third variable domain of the HIV-1 envelope glycoprotein. Proc Natl Acad Sci U S A 88:3238–3242. doi: 10.1073/pnas.88.8.3238. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Gorny MK. 1994. Production of human monoclonal antibodies via fusion of Epstein-Barr virus-transformed lymphocytes with heteromyeloma, p 276–281. In Celis JE. (ed), Cell biology: a laboratory handbook, vol 2 Academic Press, New York, NY. [Google Scholar]
- 38.Gorny MK, Wang XH, Williams C, Volsky B, Revesz K, Witover B, Burda S, Urbanski M, Nyambi P, Krachmarov C, Pinter A, Zolla-Pazner S, Nadas A. 2009. Preferential use of the VH5-51 gene segment by the human immune response to code for antibodies against the V3 domain of HIV-1. Mol Immunol 46:917–926. doi: 10.1016/j.molimm.2008.09.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Gorny MK, Revesz K, Williams C, Volsky B, Louder MK, Anyangwe CA, Krachmarov C, Kayman SC, Pinter A, Nadas A, Nyambi PN, Mascola JR, Zolla-Pazner S. 2004. The v3 loop is accessible on the surface of most human immunodeficiency virus type 1 primary isolates and serves as a neutralization epitope. J Virol 78:2394–2404. doi: 10.1128/JVI.78.5.2394-2404.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Gorny MK, Williams C, Volsky B, Revesz K, Cohen S, Polonis VR, Honnen WJ, Kayman SC, Krachmarov C, Pinter A, Zolla-Pazner S. 2002. Human monoclonal antibodies specific for conformation-sensitive epitopes of V3 neutralize human immunodeficiency virus type 1 primary isolates from various clades. J Virol 76:9035–9045. doi: 10.1128/JVI.76.18.9035-9045.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Gorny MK, Xu JY, Karwowska S, Buchbinder A, Zolla-Pazner S. 1993. Repertoire of neutralizing human monoclonal antibodies specific for the V3 domain of HIV-1 gp120. J Immunol 150:635–643. [PubMed] [Google Scholar]
- 42.Gigler A, Dorsch S, Hemauer A, Williams C, Kim S, Young NS, Zolla-Pazner S, Wolf H, Gorny MK, Modrow S. 1999. Generation of neutralizing human monoclonal antibodies against parvovirus B19 proteins. J Virol 73:1974–1979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Montefiori DC. 2005. Evaluating neutralizing antibodies against HIV, SIV, and SHIV in luciferase reporter gene assays. Curr Protoc Immunol Chapter 12:Unit 12.11. doi: 10.1002/0471142735.im1211s64. [DOI] [PubMed] [Google Scholar]
- 44.Burke B, Gomez-Roman VR, Lian Y, Sun Y, Kan E, Ulmer J, Srivastava IK, Barnett SW. 2009. Neutralizing antibody responses to subtype B and C adjuvanted HIV envelope protein vaccination in rabbits. Virology 387:147–156. doi: 10.1016/j.virol.2009.02.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Otwinowski Z, Minor W. 1997. Processing of X-ray diffraction data collected in oscillation mode, p 307–326. In Carter CW, Sweet R (ed), Macromolecular crystallography, part A, vol 276 Academic Press, New York, NY. [DOI] [PubMed] [Google Scholar]
- 46.Kabsch WX. 2010. XDS. Acta Crystallogr D Biol Crystallogr 66:125–132. doi: 10.1107/S0907444909047337. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Emsley P, Cowtan K. 2004. Coot: model-building tools for molecular graphics. Acta Crystallogr D Biol Crystallogr 60:2126–2132. doi: 10.1107/S0907444904019158. [DOI] [PubMed] [Google Scholar]
- 48.Adams PD, Afonine PV, Bunkoczi G, Chen VB, Davis IW, Echols N, Headd JJ, Hung LW, Kapral GJ, Grosse-Kunstleve RW, McCoy AJ, Moriarty NW, Oeffner R, Read RJ, Richardson DC, Richardson JS, Terwilliger TC, Zwart PH. 2010. PHENIX: a comprehensive Python-based system for macromolecular structure solution. Acta Crystallogr D Biol Crystallogr 66:213–221. doi: 10.1107/S0907444909052925. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Abagyan RA, Totrov M, Kuznetsov D. 1994. ICM—a new method for protein modeling and design: applications to docking and structure prediction from the distorted native conformation. J Comput Chem 15:488–506. doi: 10.1002/jcc.540150503. [DOI] [Google Scholar]
- 50.Li L, Wang XH, Williams C, Volsky B, Steczko O, Seaman MS, Luthra K, Nyambi P, Nadas A, Giudicelli V, Lefranc MP, Zolla-Pazner S, Gorny MK. 2015. A broad range of mutations in HIV-1 neutralizing human monoclonal antibodies specific for V2, V3, and the CD4 binding site. Mol Immunol 66:364–374. doi: 10.1016/j.molimm.2015.04.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Tiller T, Tsuiji M, Yurasov S, Velinzon K, Nussenzweig MC, Wardemann H. 2007. Autoreactivity in human IgG+ memory B cells. Immunity 26:205–213. doi: 10.1016/j.immuni.2007.01.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Li L, Wang XH, Banerjee S, Volsky B, Williams C, Moody MA, Zolla-Pazner S, Gorny MK. 2012. Clonal analysis of human anti-V3 monoclonal antibodies selected by a V3 tetramer. Hum Antibodies 21:65–73. doi: 10.3233/HAB-130264. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Sattentau QJ, Moore JP. 1991. Conformational changes induced in the human immunodeficiency virus envelope glycoprotein by soluble CD4 binding. J Exp Med 174:407–415. doi: 10.1084/jem.174.2.407. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Upadhyay C, Mayr LM, Zhang J, Kumar R, Gorny MK, Nadas A, Zolla-Pazner S, Hioe CE. 2014. Distinct mechanisms regulate exposure of neutralizing epitopes in the V2 and V3 loops of HIV-1 envelope. J Virol 88:12853–12865. doi: 10.1128/JVI.02125-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Doores KJ, Fulton Z, Huber M, Wilson IA, Burton DR. 2010. Antibody 2G12 recognizes di-mannose equivalently in domain- and nondomain-exchanged forms but only binds the HIV-1 glycan shield if domain exchanged. J Virol 84:10690–10699. doi: 10.1128/JVI.01110-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Doores KJ, Burton DR. 2010. Variable loop glycan dependency of the broad and potent HIV-1-neutralizing antibodies PG9 and PG16. J Virol 84:10510–10521. doi: 10.1128/JVI.00552-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Li Y, Cleveland B, Klots I, Travis B, Richardson BA, Anderson D, Montefiori D, Polacino P, Hu SL. 2008. Removal of a single N-linked glycan in human immunodeficiency virus type 1 gp120 results in an enhanced ability to induce neutralizing antibody responses. J Virol 82:638–651. doi: 10.1128/JVI.01691-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Haynes BF, Moody MA, Alam M, Bonsignori M, Verkoczy L, Ferrari G, Gao F, Tomaras GD, Liao HX, Kelsoe G. 2014. Progress in HIV-1 vaccine development. J Allergy Clin Immunol 134:3–10; quiz 11. doi: 10.1016/j.jaci.2014.04.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Binley JM, Ban YE, Crooks ET, Eggink D, Osawa K, Schief WR, Sanders RW. 2010. Role of complex carbohydrates in human immunodeficiency virus type 1 infection and resistance to antibody neutralization. J Virol 84:5637–5655. doi: 10.1128/JVI.00105-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Leonard CK, Spellman MW, Riddle L, Harris RJ, Thomas JN, Gregory TJ. 1990. Assignment of intrachain disulfide bonds and characterization of potential glycosylation sites of the type 1 recombinant human immunodeficiency virus envelope glycoprotein (gp120) expressed in Chinese hamster ovary cells. J Biol Chem 265:10373–10382. [PubMed] [Google Scholar]
- 61.Wang W, Nie J, Prochnow C, Truong C, Jia Z, Wang S, Chen XS, Wang Y. 2013. A systematic study of the N-glycosylation sites of HIV-1 envelope protein on infectivity and antibody-mediated neutralization. Retrovirology 10:14. doi: 10.1186/1742-4690-10-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Gach JS, Quendler H, Tong T, Narayan KM, Du SX, Whalen RG, Binley JM, Forthal DN, Poignard P, Zwick MB. 2013. A human antibody to the CD4 binding site of gp120 capable of highly potent but sporadic cross clade neutralization of primary HIV-1. PLoS One 8:e72054. doi: 10.1371/journal.pone.0072054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Ratner L, Fisher A, Jagodzinski LL, Mitsuya H, Liou RS, Gallo RC, Wong-Staal F. 1987. Complete nucleotide sequences of functional clones of the AIDS virus. AIDS Res Hum Retroviruses 3:57–69. doi: 10.1089/aid.1987.3.57. [DOI] [PubMed] [Google Scholar]
- 64.Kabat EA, Wu TT. 1991. Identical V region amino acid sequences and segments of sequences in antibodies of different specificities. Relative contributions of VH and VL genes, minigenes, and complementarity-determining regions to binding of antibody-combining sites. J Immunol 147:1709–1719. [PubMed] [Google Scholar]
- 65.Burke V, Williams C, Sukumaran M, Kim S-S, Li H, Wang X-H, Gorny MK, Zolla-Pazner S, Kong X-P. 2009. Structural basis of the cross-reactivity of genetically related human anti-HIV-1 monoclonal antibodies: implications for design of V3-based immunogens. Structure 17:1538–1546. doi: 10.1016/j.str.2009.09.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Sok D, Laserson U, Laserson J, Liu Y, Vigneault F, Julien JP, Briney B, Ramos A, Saye KF, Le K, Mahan A, Wang S, Kardar M, Yaari G, Walker LM, Simen BB, St John EP, Chan-Hui PY, Swiderek K, Kleinstein SH, Alter G, Seaman MS, Chakraborty AK, Koller D, Wilson IA, Church GM, Burton DR, Poignard P. 2013. The effects of somatic hypermutation on neutralization and binding in the PGT121 family of broadly neutralizing HIV antibodies. PLoS Pathog 9:e1003754. doi: 10.1371/journal.ppat.1003754. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.LaRosa GJ, Davide JP, Weinhold K, Waterbury JA, Profy AT, Lewis JA, Langlois AJ, Dreesman GR, Boswell RN, Shadduck P. 1990. Conserved sequence and structural elements in the HIV-1 principal neutralizing determinant. Science 249:932–935. doi: 10.1126/science.2392685. [DOI] [PubMed] [Google Scholar]
- 68.Stanfield RL, Gorny MK, Williams C, Zolla-Pazner S, Wilson IA. 2004. Structural rationale for the broad neutralization of HIV-1 by human monoclonal antibody 447-52D. Structure 12:193–204. [DOI] [PubMed] [Google Scholar]
- 69.Gorny MK, Sampson J, Li H, Jiang X, Totrov M, Wang XH, Williams C, O'Neal T, Volsky B, Li L, Cardozo T, Nyambi P, Zolla-Pazner S, Kong XP. 2011. Human anti-V3 HIV-1 monoclonal antibodies encoded by the VH5-51/VL lambda genes define a conserved antigenic structure. PLoS One 6:e27780. doi: 10.1371/journal.pone.0027780. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Bell CH, Pantophlet R, Schiefner A, Cavacini LA, Stanfield RL, Burton DR, Wilson IA. 2008. Structure of antibody F425-B4e8 in complex with a V3 peptide reveals a new binding mode for HIV-1 neutralization. J Mol Biol 375:969–978. doi: 10.1016/j.jmb.2007.11.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Diskin R, Scheid JF, Marcovecchio PM, West AP Jr, Klein F, Gao H, Gnanapragasam PN, Abadir A, Seaman MS, Nussenzweig MC, Bjorkman PJ. 2011. Increasing the potency and breadth of an HIV antibody by using structure-based rational design. Science 334:1289–1293. doi: 10.1126/science.1213782. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Huang J, Ofek G, Laub L, Louder MK, Doria-Rose NA, Longo NS, Imamichi H, Bailer RT, Chakrabarti B, Sharma SK, Alam SM, Wang T, Yang Y, Zhang B, Migueles SA, Wyatt R, Haynes BF, Kwong PD, Mascola JR, Connors M. 2012. Broad and potent neutralization of HIV-1 by a gp41-specific human antibody. Nature 491:406–412. doi: 10.1038/nature11544. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Huang J, Kang BH, Pancera M, Lee JH, Tong T, Feng Y, Georgiev IS, Chuang GY, Druz A, Doria-Rose NA, Laub L, Sliepen K, van Gils MJ, de la Pena AT, Derking R, Klasse PJ, Migueles SA, Bailer RT, Alam M, Pugach P, Haynes BF, Wyatt RT, Sanders RW, Binley JM, Ward AB, Mascola JR, Kwong PD, Connors M. 2014. Broad and potent HIV-1 neutralization by a human antibody that binds the gp41-gp120 interface. Nature doi: 10.1038/nature13601. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Moody MA, Yates NL, Amos JD, Drinker MS, Eudailey JA, Gurley TC, Marshall DJ, Whitesides JF, Chen X, Foulger A, Yu JS, Zhang R, Meyerhoff RR, Parks R, Scull JC, Wang L, Vandergrift NA, Pickeral J, Pollara J, Kelsoe G, Alam SM, Ferrari G, Montefiori DC, Voss G, Liao HX, Tomaras GD, Haynes BF. 2012. HIV-1 gp120 vaccine induces affinity maturation in both new and persistent antibody clonal lineages. J Virol 86:7496–7507. doi: 10.1128/JVI.00426-12. [DOI] [PMC free article] [PubMed] [Google Scholar]