Abstract
The Rax homeobox gene plays essential roles in multiple processes of vertebrate retina development. Many vertebrate species possess Rax and Rax2 genes, and different functions have been suggested. In contrast, mice contain a single Rax gene, and its functional roles in late retinal development are still unclear. To clarify mouse Rax function in postnatal photoreceptor development and maintenance, we generated conditional knockout mice in which Rax in maturing or mature photoreceptor cells was inactivated by tamoxifen treatment (Rax iCKO mice). When Rax was inactivated in postnatal Rax iCKO mice, developing photoreceptor cells showed a significant decrease in the level of the expression of rod and cone photoreceptor genes and mature adult photoreceptors exhibited a specific decrease in cone cell numbers. In luciferase assays, we found that Rax and Crx cooperatively transactivate Rhodopsin and cone opsin promoters and that an optimum Rax expression level to transactivate photoreceptor gene expression exists. Furthermore, Rax and Crx colocalized in maturing photoreceptor cells, and their coimmunoprecipitation was observed in cultured cells. Taken together, these results suggest that Rax plays essential roles in the maturation of both cones and rods and in the survival of cones by regulating photoreceptor gene expression with Crx in the postnatal mouse retina.
INTRODUCTION
A number of homeodomain transcription factors, which play significant roles in retinal development, have been identified in vertebrates (1–4). Rax is a homeodomain transcription factor that is essential for various processes in vertebrate retinal development (5). The Rax gene was first identified as a paired-type homeobox gene expressed in the optic vesicle and the presumptive diencephalon area in the early mouse embryo (6, 7). Rax is evolutionarily well conserved from Drosophila melanogaster to humans. Rax is highly expressed in retinal progenitor cells (RPCs), and its expression in the retina gradually decreases as RPCs become postmitotic and begin to differentiate. Rax-null mutant mice exhibit a reduction of brain size and an absence of the optic vesicle (5, 7). Mutations in human RAX are associated with anophthalmia and microphthalmia (8, 9). Rax overexpression promotes the proliferation of RPCs in frogs and zebra fish (7, 10–13). In addition to the function in RPCs, Rax plays significant roles in the development of photoreceptor cells and Müller glial cells (14–19).
Rax paralog genes have been identified in various vertebrate species (20–22). In Xenopus laevis, two Rax genes (xRx and xRx-L/xRx2) have been identified (7, 21), and in zebra fish, three Rax genes (zRx1 to zRx3) have been isolated (7). Interestingly, the expression pattern of zebra fish Rx3 showed more similarity to that of frog and mouse Rax genes than to that of the zebra fish Rx1 and Rx2 genes (23). In chicks, two Rax genes (cRax and cRaxL/cRax2) have been identified (20). The chick Rax2 gene is expressed in both retinal progenitor cells and early-developing photoreceptors, while chick Rax is predominantly expressed in retinal progenitor cells. It was also reported that chick Rax2 is implicated in cone photoreceptor differentiation and that the expression of a putative dominant negative allele of a chick Rax2 gene caused a significant reduction in the level of expression of cone photoreceptor genes (20). Human RAX2/QRX, which is expressed in the outer nuclear layer (ONL) and inner nuclear layer (INL) of the adult human retina, was identified to be a PCE-1-binding protein by acting synergistically with CRX and NRL to modulate the expression of photoreceptor genes. Monkey, cow, and dog genomes also contain two Rax genes. On the other hand, the Rax2 gene is absent from mouse and rat genomes (22). This raises the question of whether mouse Rax plays an essential role in photoreceptor development during postnatal stages like human Rax2 does.
In the current study, we investigated a functional role for Rax in postnatal mouse retinas, which contain a single Rax gene. We report that mouse Rax modulates the expression of photoreceptor genes in the postnatal retina by interacting with Crx. Conditional ablation of Rax in postnatal photoreceptors led to a significant decrease in the level of expression of rod and cone genes and to cone photoreceptor cell death, suggesting that Rax is essential for the maturation of rods and cones as well as for the survival of cones.
MATERIALS AND METHODS
Animal care.
All procedures conformed to the ARVO statement for the use of animals in ophthalmic and vision research, and these procedures were approved by the Institutional Safety Committee on Recombinant DNA Experiments (approval 3380-3) and the Animal Experimental Committees of the Institute for Protein Research (approval 24-05-1), Osaka University, and were performed in compliance with institutional guidelines. Mice were housed in a temperature-controlled room at 22°C with a 12-h light/12-h dark cycle. Fresh water and rodent diet were available at all times.
Raxflox/flox; Crx-CreERT2 mice and tamoxifen.
The Raxflox/flox; Crx-CreERT2 mice (129Sv/Ev background) were generated as described in our previous study (16). Tamoxifen (Sigma, St. Louis, MO) was dissolved in a sunflower oil (Sigma) at 4 mg/ml, and 0.2 mg or 1 mg of tamoxifen was injected intraperitoneally into Raxflox/flox; Crx-CreERT2 mice at postnatal day 4 (P4) or 1 month of age, respectively.
Plasmid constructs.
We subcloned a 3.8-kb upstream genomic fragment of the human S-opsin gene (bp −3769 to +1) into the pGL3-Basic vector (Promega), generating the pS-opsin-Luc reporter plasmid. Mutations of PCE-1 sites in the human rhodopsin, S-opsin, and M-opsin promoters, previously described (15), were introduced by PCR with mutated PCR primers. The resulting constructs were named hRhodopsin-PCE-1-mut1, hS-opsin-PCE-1-mut6, and hM-opsin-PCE-1-mut1. We constructed the expression vectors by subcloning a full-length mouse Rax cDNA into the pME18S (pMIK) expression vector (a gift from K. Maruyama, Tokyo Medical and Dental University, School of Medicine, Tokyo, Japan). An open reading frame fragment of mouse Crx cDNA was amplified by PCR and cloned into the pBSKS-3×FLAG vector containing a 3× FLAG tag. The mouse Crx-3×FLAG fragment obtained was cloned into the modified pCAGGS expression vector, generating the pCAGGS-3×FLAG-Crx expression plasmid. To obtain the pCAGGS-2×HA-Rax expression plasmid containing a 2× hemagglutinin (HA) tag, an open reading frame fragment of the mouse Rax cDNA was amplified by PCR and cloned into the pCAGGS-2×HA vector.
Immunohistochemistry.
For immunohistochemistry, 20-μm retinal tissue sections were washed twice in phosphate-buffered saline (PBS) and boiled in 10 mM sodium citrate buffer (pH 6.0) for 10 min for antigen retrieval. Sections were rinsed twice using PBS and incubated with blocking solution (4% normal donkey serum and 0.1% Triton X-100 in PBS) for 1 h at room temperature. The samples were incubated with a primary antibody at 4°C overnight. After washing with PBS, these samples were incubated with secondary antibodies for 1 h at room temperature. Rhodamine-labeled peanut agglutinin (PNA; Vector Laboratories) was used for staining cone pedicles. DAPI (4′,6-diamidino-2-phenylindole) and Hoechst 33342 were used for staining nuclear DNA.
For whole-mount immunostaining of the retina, each retina was gently peeled off from the sclera, rinsed in PBS, and fixed with 4% (wt/vol) paraformaldehyde in PBS for 2 h. After washing in PBS, samples were blocked with 4% normal donkey serum and 0.05% (wt/vol) Triton X-100 in PBS for 2 h. The retinas were then immunostained with primary antibodies to S-opsin, M-opsin, and rhodopsin at 4°C overnight. Reactions with secondary antibodies were performed for 2 h at room temperature. The specimens were observed under a laser confocal microscope (LSM700; Carl Zeiss).
Antibody production.
By PCR, a cDNA fragment encoding a center portion of mouse Nrl (residues 62 to 130; Nrl-center) was amplified and subcloned into pGEX4T-2 (Amersham Biosciences). The fusion protein was expressed in Escherichia coli strain BL21 and purified with glutathione-Sepharose 4B (Amersham Biosciences) according to the manufacturer's instructions. An antibody against Nrl was obtained by immunizing guinea pigs with purified glutathione S-transferase (GST)-tagged Nrl (GST-Nrl). The guinea pig antiserum against Nrl-center was preabsorbed with GST-Sepharose and affinity purified with an immunizing fusion protein-bound Sepharose column.
Antibodies.
For immunostaining, anti-Rax antibody (guinea pig, 1:1,000) (16), anti-S-opsin antibody (goat, 1:500; Santa Cruz), anti-M-opsin antibody (rabbit, 1:300; Millipore), antirhodopsin antibody (mouse, 1:5,000; Sigma), anti-TRβ2 antibody (rabbit, 1:3,000; a gift from Douglas Forrest, NIDDK), anti-TRβ2 antibody (guinea pig, 1:50) (24), anti-Lhx2 antibody (goat, 1:1,000; Santa Cruz), and antipikachurin antibody (rabbit, 1:500) (25) were used as primary antibodies. We used Cy3-conjugated secondary antibodies (1:500; Jackson), Alexa Fluor 488-conjugated secondary antibodies (1:1,000; Sigma), and Dylight 649-conjugated secondary antibodies (1:500; Jackson). For Western blot analysis, anti-Rax antibody (guinea pig, 1:1,000) (16), anti-Crx antibody (rabbit, 1:3,000) (24), antirhodopsin antibody (mouse, 1:5,000; Sigma), anti-β-actin antibody (mouse, 1:5,000; Sigma), anti-Nrl antibody (guinea pig, 1:500), anti-FLAG antibody (mouse, 1:15,000; catalog number F1804; Sigma), and anti-HA antibody (rat, 1:5,000; Roche) were used as primary antibodies. We used a horseradish peroxidase-conjugated goat antibody against mouse IgG (1:20,000; Zymed), a horseradish peroxidase-conjugated goat antibody against rabbit IgG (1:20,000; Molecular Probes), a horseradish peroxidase-conjugated goat antibody against rat IgG (1:20,000; Jackson), and a horseradish peroxidase-conjugated donkey antibody against guinea pig IgG (1:20,000; Jackson) as secondary antibodies.
Western blot analysis.
Mouse retinas were dissected at P8 and lysed in an SDS-sample buffer. HEK293T cells were washed with PBS twice and lysed in an SDS-sample buffer. Samples were resolved by SDS-PAGE and transferred to polyvinylidene difluoride membranes. Membranes were blocked for 1 h in 5% milk containing Tris-buffered saline with 0.1% Tween 20 and incubated overnight with a primary antibody. The membrane was then incubated for 1 h with a secondary antibody. The signals were measured using ImageJ software (U.S. National Institutes of Health).
TUNEL assay.
Fresh frozen retinas were sectioned to a thickness of 20 μm and fixed with 4% paraformaldehyde in PBS for 1 min. Terminal deoxynucleotidyltransferase-mediated dUTP-biotin nick end labeling (TUNEL) assays were performed using a Click-iT TUNEL Alexa Fluor 488 imaging assay kit (Invitrogen) according to the manufacturer's protocols.
ERG recordings.
Electroretinograms (ERGs) were recorded with a white light-emitting diode luminescent electrode placed on the cornea (PuREC; Mayo, Japan). Two-month-old mice were anesthetized with an intraperitoneal injection of 100 mg ketamine per kg of body weight and 10 mg/kg xylazine. The pupils were dilated with topical 0.5% tropicamide and 0.5% phenylephrine HCl. The mice were stimulated with four levels of stroboscopic stimuli ranging from −4.0 to 1.0 log cd-s/m2 to elicit scotopic ERGs and four levels of stimuli ranging from −0.5 to 1.0 log cd-s/m2 for the photopic ERGs. The photopic ERGs were recorded on a rod-suppressing white background of 1.3 log cd/m2.
In situ hybridization.
Digoxigenin (DIG)-labeled riboprobes were synthesized by T7 and T3 RNA polymerase using Rax cDNA or Cre recombinase cDNA as a template in the presence of 11-digoxigenin UTPs (Roche Molecular Diagnostics, Mannheim, Germany). Postnatal eyeballs from 129Sv/Ev background Raxflox/flox mice and/or Raxflox/flox; Crx-CreERT2 mice were fixed with 4% paraformaldehyde in PBS overnight at 4°C. The samples were cryoprotected with 30% sucrose in PBS and embedded in OCT compound (Sakura Finetechnical, Tokyo, Japan). These tissues were sliced into 20-μm sections with a Microm HM 560 cryostat microtome (Microm Laborgeräte GmbH, Walldorf, Germany). The sliced sections were fixed with 4% paraformaldehyde in PBS containing 0.1% Tween 20 (PBST), treated with 6% H2O2, 5 μg/ml proteinase K, and subsequently treated with 2 mg/ml glycine in PBST. The samples were then postfixed with 4% paraformaldehyde in PBST. Hybridization was carried out overnight at 70°C. After posthybridization washing, they were blocked with 5% sheep serum in 2.5 mM Tris-HCl (pH 7.5), 0.8% NaCl, 0.02% KCl, and 0.1% Tween 20 (TBST) and incubated with an alkaline phosphatase-conjugated anti-DIG antibody (1:2,000; Roche) in TBST with 1% sheep serum. The samples were visualized with nitroblue tetrazolium chloride (Sigma, St. Louis, MO) and 5-bromo-4-chloro-3-indolylphosphate, toluidine salt (Sigma).
Luciferase assay.
We transfected 0.4 μg of the luciferase reporter plasmid DNAs (pGL3b-human rhodopsin promoter-luc, pGL3b-human S-opsin promoter-luc, pGL3b-human M-opsin promoter-luc, pGL3b-human rhodopsin-PCE-1-mut1, pGL3b-human S-opsin-PCE-1-mut6, or pGL3b-human M-opsin-PCE-1-mut1) (26) and 0.25 to 4 μg of mouse Rax or 0.25 to 0.5 μg of mouse Crx or Nrl expression vector DNAs (pME18S, pME18S-Rax, pME18S-Crx, and pME18S-Nrl) per well into NIH 3T3 cells in a 6-well plate using the FuGene reagent (Promega). A β-galactosidase expression vector (β-SV; Promega) was cotransfected for normalization of transfection efficiency. After transfection, the cells were incubated for 48 h and lysed with reporter lysis buffer (Promega). Luciferase activity was measured with a firefly luciferase assay system (Promega) according to the manufacturer's protocol. The luminescence signal was detected using a GloMax Multi+ detection system (Promega).
qPCR.
The mouse retinas were harvested and dissected at P20. Total RNA (1 μg) was isolated from the retina using the TRIzol reagent (Invitrogen) and converted to cDNA using SuperScript II reverse transcriptase (Invitrogen). Real-time PCR was performed using SYBR GreenER qPCR SuperMix (Invitrogen) and thermal cycler Dice real-time system single MRQ TP870 (TaKaRa) according to the manufacturer's instructions. Quantification was performed by thermal cycler Dice real-time system software (version 2.0; TaKaRa). The primer sequences used for quantitative PCR (qPCR) are listed in Table 1.
TABLE 1.
Primer sequences used for qPCR analysis
| Gene | Orientation | Primer sequence (5′-3′) |
|---|---|---|
| M-opsin | Sense | TTGCTTTGCCACTGCTCG |
| Antisense | CTGTGGCCCAGACGTGTTC | |
| S-opsin | Sense | GCTGGACTTACGGCTTGTCACC |
| Antisense | TGTGGCGTTGTGTTTGCTGC | |
| Gnat2 | Sense | ATGCTGACAAGGAAGCCAAGACTG |
| Antisense | GACAGACTTGAACTCTAGGCACTC | |
| Pde6c | Sense | GATCAACGTGATTCCGTCACCTCC |
| Antisense | AGTCTCATCTACCGGTCCTTTCTG | |
| Crx | Sense | CACCAGGCTGTCCCATACTC |
| Antisense | TCGCCCTACGATTCTTGAAC | |
| Rcvrn | Sense | GAGTACGTGATTGCTCTGCACATG |
| Antisense | AGGAGTTTCACATCCTCAGGCTTG | |
| Sag | Sense | AGCCTGCTCAAGAAACTGGGAGACA |
| Antisense | AGCTGGCTGCAACATCACTGAACA | |
| Nrl | Sense | GCTGTGCCTTTCTGGTTCTGA |
| Antisense | GCTCCCGCTTTATTTCGAACT | |
| Nr2e3 | Sense | TTGATGTCACCAGCAATGACC |
| Antisense | TTTTGGCCCATTTGACAGC | |
| Rhodopsin | Sense | GCTTCCCTACGCCAGTGTG |
| Antisense | CAGTGGATTCTTGCCGCAG | |
| Gnat1 | Sense | CGCTCAACATTCAGTATGGAGA |
| Antisense | CGAAGTAGCGATGATTGCAGATG | |
| Pde6g | Sense | CAAACAAGGCAGTTCAAGAGCAAG |
| Antisense | CAGGGATCTGACTAAATGATGCCA | |
| Gapdha | Sense | ACTGGCATGGCCTTCCGTGTTCCTA |
| Antisense | TCAGTGTAGCCCAAGATGCCCTTC |
Gapdh, glyceraldehyde-3-phosphate dehydrogenase gene.
Immunoprecipitation.
We transfected pCAGGS-3×FLAG-Crx and pCAGGS-2×HA-Rax expression plasmids into HEK293T cells in a 35-mm dish using the calcium phosphate method. HEK293T cells were cotransfected with the pCAGGS-3×FLAG-Crx or pCAGGS-3×FLAG expression plasmid together with the pCAGGS-2×HA-Rax or pCAGGS-2×HA expression plasmid. Two days after transfection, the cells were lysed with lysis buffer (50 mM Tris [pH 8.0], 150 mM NaCl, 1% [wt/vol] NP-40, 0.1% [wt/vol] Triton X-100, cOmplete protease inhibitor cocktail [Roche]), and centrifuged for 10 min at 15,100 × g. The supernatants were incubated with an anti-FLAG M2 affinity gel (Sigma) or monoclonal anti-HA-agarose antibody (Sigma) and then eluted with 0.1 M glycine buffer (pH 1.5). After neutralization with 1 M Tris-HCl (pH 9.0), immunoprecipitated samples were analyzed by Western blotting.
Statistical analysis.
Data are presented as means ± standard deviations (SDs). Statistical comparisons of data sets were performed with Student's t test. For multiple comparisons, we performed one-way analysis of variance by the Tukey-Kramer test, and a P value of <0.05 was taken to be statistically significant.
RESULTS
Mouse Rax is expressed in postnatal photoreceptors and Müller glial cells.
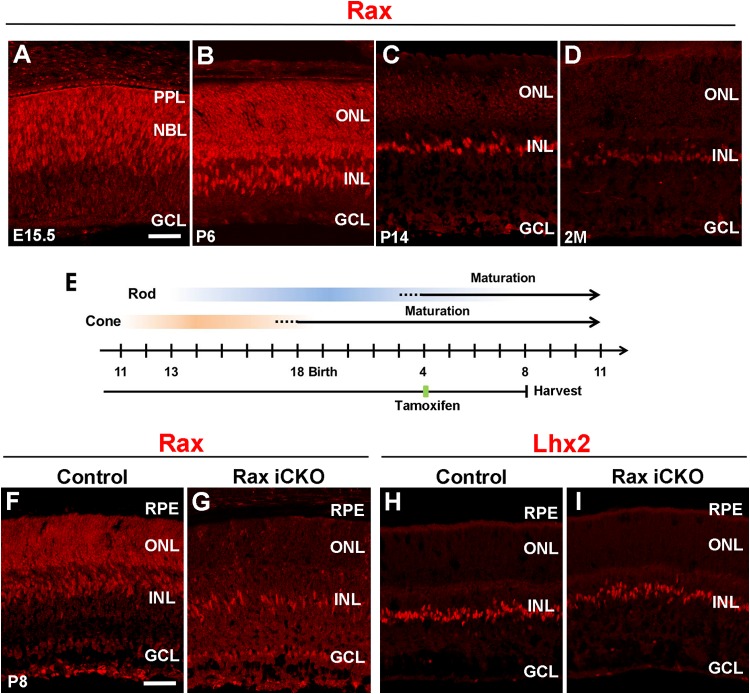
In order to examine Rax protein expression in the mouse retina during development, we immunostained developing and adult mouse retinas with an anti-Rax antibody that we previously generated (16) (Fig. 1A to D). Strong Rax signals were observed in the neuroblastic layer (NBL) and the presumptive photoreceptor layer (PPL) at embryonic day 15.5 (E15.5) (Fig. 1A). At postnatal day 6 (P6), Rax protein expression was detected in the ONL, which corresponds to photoreceptor precursors, and the INL, which corresponds to Müller glial cells (14) (Fig. 1B). Rax protein expression in the ONL decreased at P14 and was weakly detected at age 2 months (Fig. 1C and D). Although the expression level of Rax in the INL was unaltered at P14 compared with that at P6, Rax signals in the INL became substantially fainter at age 2 months (Fig. 1D). These results show that the Rax protein is expressed at high levels in both maturing photoreceptors and Müller glial cells and significantly decreases by maturity.
FIG 1.
Expression and conditional inactivation of Rax in postnatal mouse retinas. (A to D) Mouse retinal sections were immunostained with an anti-Rax antibody (red) at E15.5 (A), P6 (B), P14 (C), and age 2 months (2M) (D). (E) Schematic diagram of schedule for tamoxifen administration and harvest of retinas. To conditionally inactivate Rax in postnatal photoreceptors, we treated Raxflox/flox; Crx-CreERT2 mice with tamoxifen at P4 and harvested the retinas at P8. (F to I) Retinal sections from Rax iCKO (P4 → P8) and control mice were immunostained with antibodies against Rax (red) (F and G) and Lhx2 (a Müller glia marker, red) (H and I). GCL, ganglion cell layer; INL, inner nuclear layer; ONL, outer nuclear layer; NBL, neuroblastic layer; PPL, presumptive photoreceptor layer; RPE, retinal pigment epithelium. Bars, 50 μm.
Generation of maturing photoreceptor-specific Rax iCKO mice.
To examine the possible role of Rax in maturing postnatal photoreceptor cells, we ablated Rax in postnatal photoreceptor cells by using Raxflox/flox; Crx-CreERT2 mice, which we previously reported (16). We inactivated Rax in postmitotic maturing photoreceptor cells by treating these mice with tamoxifen at P4, when the number of retinal progenitors markedly decreases and many photoreceptor precursors begin their maturation (27–30) (Fig. 1E). We also injected tamoxifen into Raxflox/flox littermate mice of the Raxflox/flox; Crx-CreERT2 mice for use as controls in the current study. To assess whether induced Rax inactivation occurs, we analyzed the retinas of P8 control mice and conditional knockout mice in which Rax in maturing or mature photoreceptor cells was inactivated by tamoxifen treatment (Rax iCKO) by immunostaining using the anti-Rax antibody. We refer to the Rax iCKO mice that were treated with tamoxifen at P4 and whose retinas were harvested at P8 as Rax iCKO (P4 → P8) mice. The immunoreactivity to Rax in the ONL, which was strongly detected in the control retina, was undetectable in the Rax iCKO (P4 → P8) mouse retina, while Rax expression in the INL was almost unaffected (Fig. 1F and G). Furthermore, no substantial reduction of the Müller glial cell marker Lhx2 was observed in the Rax iCKO (P4 → P8) mouse retina compared with that in the control mouse retina (Fig. 1H and I). These results show that Rax is specifically deleted in postnatal maturing photoreceptor cells in the Rax iCKO mouse retina.
Rax is required for the expression of opsin proteins in the maturing retina.
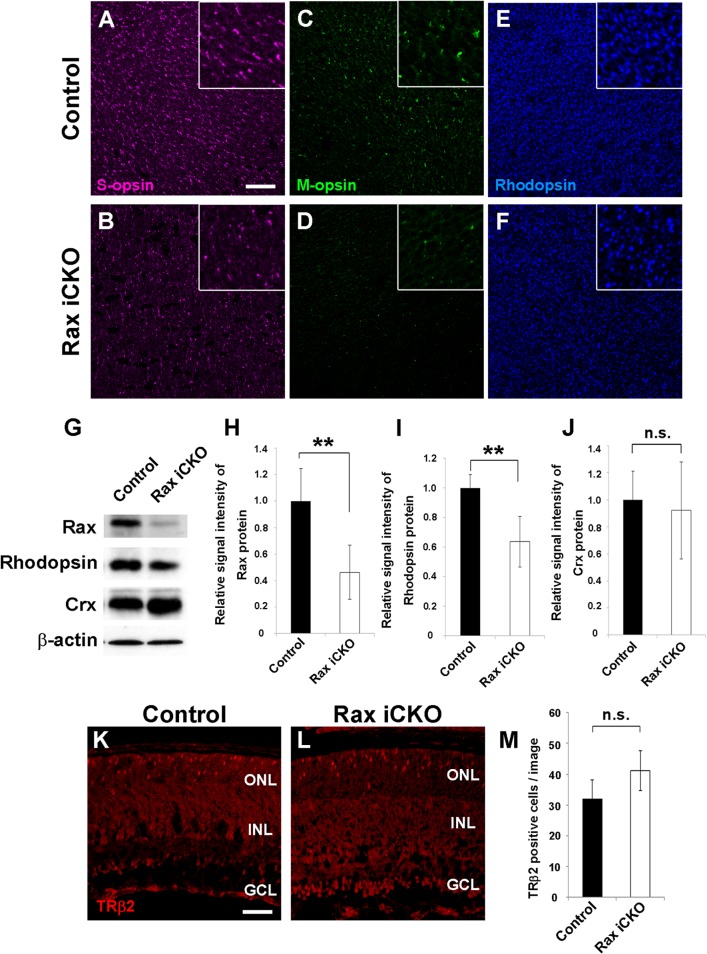
To investigate the retinal phenotypes caused by Rax deficiency in postnatal maturing photoreceptors, we performed flat-mount immunostaining of Rax iCKO (P4 → P8) mouse retinas. We observed that the expression of cone- and rod-specific proteins (S-opsin, M-opsin, and rhodopsin) was slightly downregulated (Fig. 2A to F). Next, we quantified the expression levels of the rhodopsin and Crx proteins in control and Rax iCKO (P4 → P8) mouse retinas by Western blotting. In the Rax iCKO (P4 → P8) mouse retina, the Rax protein level decreased to ∼40% of that in the control retina (Fig. 2G and H). The rhodopsin protein level in the Rax iCKO (P4 → P8) mouse retina was also reduced by ∼40% compared to that in the control retina (Fig. 2G and I). In contrast, Crx protein expression was unaffected in the Rax iCKO (P4 → P8) mouse retina (Fig. 2G and J). To examine whether Rax inactivation affects cone photoreceptors, we performed immunostaining of control and Rax iCKO (P4 → P8) mouse retinas with an anti-TRβ2 antibody that we generated and counted the TRβ2-positive cells on retinal sections (Fig. 2K to M). The numbers of TRβ2-positive cells was unaltered between control and Rax iCKO (P4 → P8) mouse retinas (number of TRβ2-positive cells per image, 32.0 ± 6.0 for control mice and 41.2 ± 6.4 for Rax iCKO mice; P > 0.05) (Fig. 2M). These results suggest that Rax is not required for Crx expression but is essential for the normal expression of opsins in postnatal maturing photoreceptors.
FIG 2.
Expression of opsin genes decreases in the Rax iCKO mouse retina. (A to F) Whole-mount retinas from control and Rax iCKO (P4 → P8) mice were immunostained with antibodies against S-opsin (magenta) (A and B), M-opsin (green) (C and D), and rhodopsin (blue) (E and F). (Insets) S-opsin-, M-opsin-, and rhodopsin-positive cells at high magnification. Bar, 50 μm. (G to J) Comparison of photoreceptor protein levels in control and Rax iCKO (P4 → P8) mouse retinas. (G) Western blots of Rax, rhodopsin, and Crx in the retina are shown. β-Actin was used as a loading control. (H to J) The signal intensities of the Rax, rhodopsin, and Crx proteins, respectively, are shown. Data are means ± SDs (n = 3). **, P < 0.01 by Student's t test; n.s., not significant by Student's t test. (K to M) The number of cone photoreceptor cells was unaltered in the Rax iCKO (P4 → P8) mouse retina. Retinal sections from control (K) and Rax iCKO (P4 → P8) (L) mice were immunostained with an anti-TRβ2 antibody (rabbit; red), a marker for developing cone photoreceptor cells. Bar, 50 μm. (M) The number of TRβ2-positive cells is shown. Data are means ± SDs (n = 4). n.s., not significant by Student's t test. GCL, ganglion cell layer; INL, inner nuclear layer; ONL, outer nuclear layer;.
A defect of Rax in photoreceptor cells affects the expression of photoreceptor genes.
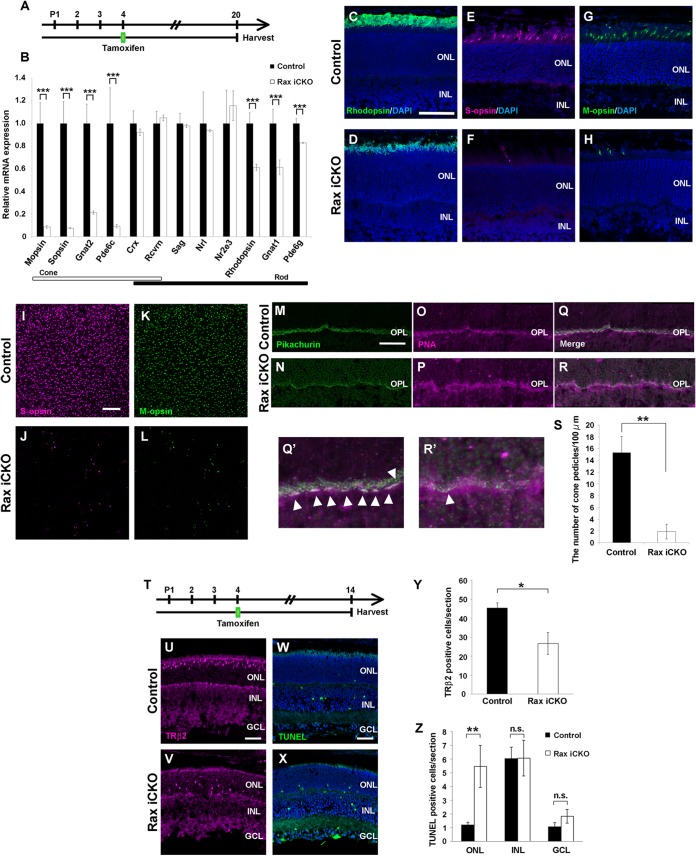
In order to examine whether Rax regulates photoreceptor-specific genes in postnatal maturing photoreceptors, we selected 12 genes involved in photoreceptor development and function and measured the expression levels of these genes in control and Rax iCKO mouse retinas by quantitative PCR (qPCR) analysis. For qPCR, we used Rax iCKO (P4 → P20) mouse retinas, in which retinal neural circuit formation is almost completed (Fig. 3A) (30). We observed that CreERT2 mRNA is predominantly expressed in the ONL of the Rax iCKO (P4 → P20) mouse retina (see Fig. S1 in the supplemental material). We found that the expression levels of cone-specific genes (M-opsin, S-opsin, Gnat2, and Pde6c) decreased by 80 to 90% in Rax iCKO retinas compared with those in control retinas (Fig. 3B). Furthermore, the expression levels of the genes essential for light sensing in rod photoreceptors (Rhodopsin, Gnat1, and Pde6g) also decreased by 20 to 40% from those in control retinas, whereas transcription factors (Crx, Nrl and Nr2e3), which are essential for photoreceptor development in vivo (31, 32), were unaffected (Fig. 3B). Consistent with the qPCR results, we observed a reduced rhodopsin immunostaining signal in the Rax iCKO (P4 → P20) mouse retina (Fig. 3C and D). We also found that the expression of cone photoreceptor proteins (S-opsin and M-opsin) considerably decreased in Rax iCKO (P4 → P20) mouse retinas (Fig. 3E to H). We carried out flat-mount immunostaining of control and Rax iCKO (P4 → P20) mouse retinas using antibodies against S-opsin and M-opsin and observed a significant reduction of the cone cell numbers in the Rax iCKO (P4 → P20) mouse retinas (Fig. 3I to L). To confirm these results, we further immunostained control and Rax iCKO (P4 → P20) mouse retinas with photoreceptor synaptic markers (antipikachurin antibody and rhodamine-labeled peanut agglutinin [PNA]). Immunostaining with pikachurin and PNA revealed that the number of cone pedicles dramatically decreased in the Rax iCKO (P4 → P20) mouse retinas (number of cone pedicles per 100 μm, 15.2 ± 2.7 in control mice and 1.8 ± 1.5 in Rax iCKO mice; P < 0.01) (Fig. 3M to S).
FIG 3.
Rax is required for cone photoreceptor cell survival during maturation. (A) Schematic diagram of schedule for tamoxifen administration and harvest of retinas. We treated Raxflox/flox; Crx-CreERT2 mice with tamoxifen at P4 and harvested the retinas at P20. (B) The expression levels of photoreceptor genes in Rax iCKO (P4 → P20) mouse retinas were analyzed by qPCR. The expression level of each was normalized to the expression level of a housekeeping gene, Gapdh (glyceraldehyde-3-phosphate dehydrogenase). The mean value for each control was set equal to 1.0. Error bars show ±SDs (n = 3). ***, P < 0.001. (C to H) Rax iCKO (P4 → P20) and control retinal sections were immunostained with the antibody against rhodopsin (green) (C and D), S-opsin (magenta) (E and F), or M-opsin (green) (G and H). The nuclei were stained with DAPI (blue). Bar, 50 μm. (I to S) Reduction of cone photoreceptor cells in the Rax iCKO (P4 → P20) mouse retina. (I to L) Whole-mount retinas from control and Rax iCKO (P4 → P20) mice were immunostained with the antibody against S-opsin (magenta) (I and J) or M-opsin (green) (K and L). (M and N) Retinal sections were immunostained with an antipikachurin antibody (a synaptic marker, green). (O and P) Cone pedicles were stained with PNA (magenta). (Q) Merge of panels M and O. (R) Merge of panels N and P. (Q′) and R′) Higher-magnification views of panels Q and R, respectively. Arrowheads, cone pedicles. Bars, 50 μm. (S) The number of cone pedicles decreased in the Rax iCKO (P4 → P20) mouse retina. Data are means ± SDs (n = 3). **, P < 0.01 by Student's t test. (T) Schematic diagram of schedule for tamoxifen administration and harvest of retinas. We treated Raxflox/flox; Crx-CreERT2 mice with tamoxifen at P4 and harvested their retinas at P14. (U to Z) Retinal sections from control (U) and Rax iCKO (P4 → P14) (V) mice were immunostained with an anti-TRβ2 antibody (guinea pig; magenta), a marker for developing cone photoreceptor cells. (W and X) TUNEL staining of retinas from control (W) and Rax iCKO (P4 → P14) (X) mice. Bars, 50 μm. (Y) The number of TRβ2-positive cells is shown. Data are means ± SDs (n = 3). *, P < 0.05. (Z) TUNEL-positive cells in control and Rax iCKO (P4 → P14) mouse retinas were counted. Data are means ± SDs (n = 3). **, P < 0.01 by Student's t test; n.s., not significant by Student's t test. INL, inner nuclear layer; ONL, outer nuclear layer; OPL, outer plexiform layer; GCL, ganglion cell layer.
To examine whether Rax ablation affects the survival of maturing cone photoreceptors, we immunostained cone photoreceptor cells in control and Rax iCKO (P4 → P14) mouse retinas using the anti-TRβ2 antibody (Fig. 3T to V). In addition, we detected apoptotic cell death in Rax iCKO (P4 → P14) and control mouse retinas by the TUNEL assay (Fig. 3W and X). We found that the number of TRβ2-positive cells significantly decreased in the Rax iCKO (P4 → P14) mouse retinas compared to that in the control retinas (number of TRβ2-positive cells per section, 45.4 ± 3.3 in control mice and 26.7 ± 7.0 in Rax iCKO mice; P < 0.01) (Fig. 3Y). We counted the apoptotic cell numbers in the ONL, INL, and ganglion cell layer (GCL) separately and found that apoptotic cell numbers significantly increased in the ONL (Fig. 3Z). Mislocalization of cone photoreceptor cells in the retina has been shown to be associated with a disturbance of cone photoreceptor maturation and consequent degeneration (33, 34). Furthermore, we observed that CreERT2 mRNA was predominantly expressed in the ONL of the Rax iCKO (P4 → P14) mouse retinas (see Fig. S1 in the supplemental material). These results suggest that Rax is essential for cone photoreceptor survival in the postnatal maturing mouse retina.
Rax iCKO mice exhibit abnormal ERGs.
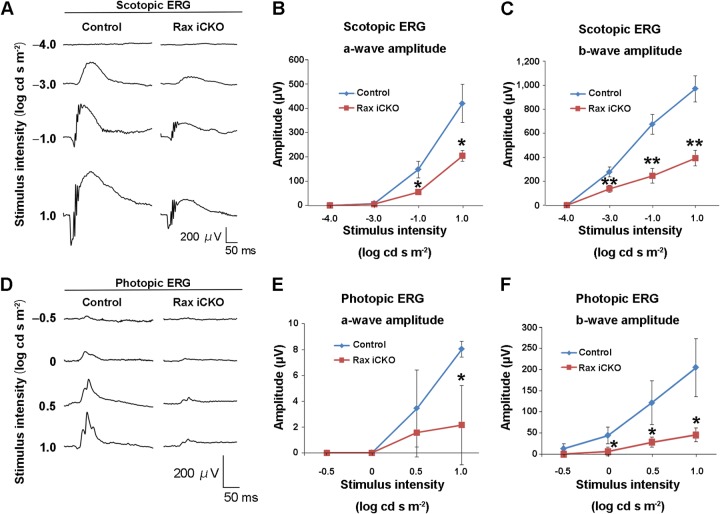
To test visual function in Rax iCKO mice, we performed electroretinogram (ERG) recordings under scotopic and photopic conditions with several light intensities in control mice and Rax iCKO mice that were treated with tamoxifen at P4 and whose retinas were harvested at age 2 months [Rax iCKO (P4 → 2 months) mice] (Fig. 4). In control mice, the negative a-wave, which originates mainly from the activity of rod photoreceptors, appeared at higher stimulus intensities of −1.0 to 1.0 log cd-s/m2 under the scotopic condition (Fig. 4A). A positive b-wave, which originates from rod bipolar cells (35), was seen at an even lower stimulus intensity of −3.0 log cd-s/m2. In contrast, the amplitude of the dark-adapted ERG a-wave in Rax iCKO (P4 → 2 months) mice was reduced at higher stimulus intensities of −1.0 to 1.0 log cd-s/m2 compared with those in control mice (Fig. 4A and B). The amplitude of the scotopic b-wave in Rax iCKO (P4 → 2 months) mice was significantly less than that in control mice at lower and higher stimulus intensities of −3.0 to 1.0 log cd-s/m2 (Fig. 4A and C). These results suggest an essential function of Rax in rod phototransduction in the postnatal mouse retina. Under photopic conditions, Rax iCKO (P4 → 2 months) mice showed markedly decreased amplitudes in a-waves, which originate from cone photoreceptors, at a high stimulus intensity of 1.0 log cd-s/m2 compared with those in control mice (Fig. 4D and E). The amplitude of the photopic ERG b-wave in Rax iCKO (P4 → 2 months) mice, which originates from cone ON-bipolar cells, was significantly smaller than those of control mice at high stimulus intensities of 0 to 1.0 log cd-s/m2 (Fig. 4D and F), suggesting that Rax is also required for cone phototransduction. These results showed that phototransduction in both rod and cone photoreceptor cells is impaired in Rax iCKO (P4 → 2 months) mice.
FIG 4.
ERG analysis of Rax iCKO and control mice. (A to F) ERGs from Rax iCKO (P4 → 2 months) mice were recorded. (A) Scotopic ERGs elicited by four different stimulus intensities are shown. (B and C) The amplitudes of the scotopic ERG a-wave (B) and the b-wave (C) are shown as a function of the stimulus intensity. (D) Photopic ERGs elicited by four different stimulus intensities are shown. (E and F) The amplitudes of the photopic ERG a-wave (E) and b-wave (F) are shown as a function of the stimulus intensity. Data are means ± SDs (n = 3). **, P < 0.01 by Student's t test; *, P < 0.05 by Student's t test.
Rax regulates the maintenance of mature cone photoreceptors.
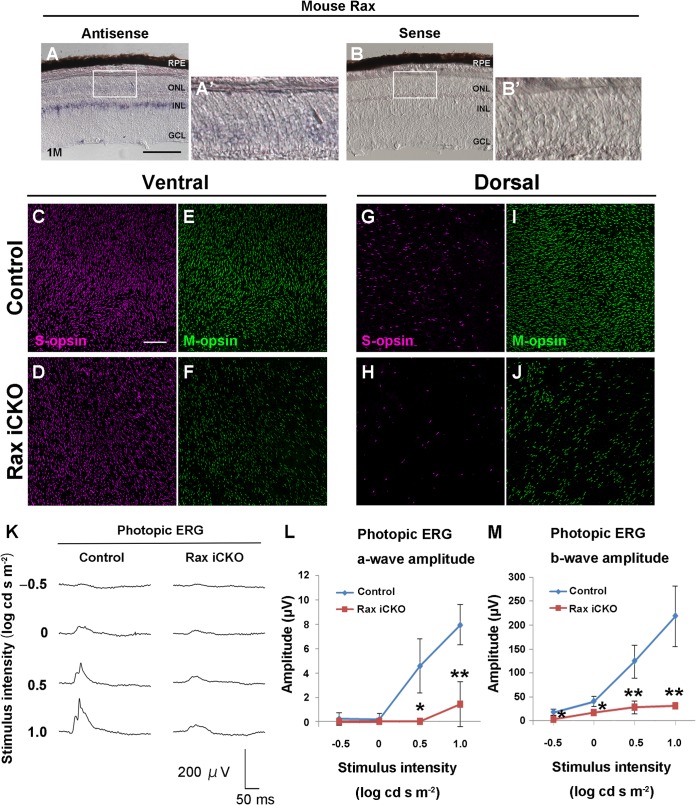
To examine Rax expression in the adult mouse retina, we carried out in situ hybridization of the retina harvested from mice at age 1 month using Rax antisense and sense probes. With the antisense probe, Rax expression was weakly detected in the ONL of the retina, while the Rax sense probe did not give any significant signal (Fig. 5A to B′). To investigate the in vivo function of Rax in mature photoreceptors, we treated Raxflox/flox; Crx-CreERT2 mice with tamoxifen at 1 month to inactivate Rax in mature photoreceptors. We observed that CreERT2 mRNA is predominantly expressed in the ONL of the retina of Rax iCKO mice that were treated with tamoxifen at age 1 month and whose retinas were harvested at age 2 months [Rax iCKO (1 month → 2 months) mice] (see Fig. S1 in the supplemental material). We examined the expressions of S-opsin and M-opsin by whole-mount coimmunostaining of control and Rax iCKO (1 month → 2 months) mouse retinas using anti-S-opsin and anti-M-opsin antibodies (Fig. 5C to J). In mice, S-opsin is expressed predominantly in cones in the ventral retina, and M-opsin is predominantly expressed in cones in the dorsal retina (36, 37). The number of S-opsin-positive cells slightly decreased in both the ventral and dorsal retinas of Rax iCKO (1 month → 2 months) mice (Fig. 5C, D, G, and H), while the number of M-opsin-positive cells notably decreased in both the dorsal and ventral retinas of Rax iCKO (1 month → 2 months) mice (Fig. 5E, F, I, and J). On the other hand, we analyzed rhodopsin expression using retinal sections by immunostaining and observed that the rhodopsin expression level was unaltered between control and Rax iCKO (1 month → 2 months) mouse retinas (data not shown). To evaluate the physiological function of the Rax iCKO mouse retina, we recorded scotopic and photopic ERGs in control and Rax iCKO (1 month → 2 months) mice. Both the waveforms and amplitudes for a- and b-waves in scotopic ERGs were very similar between control and Rax iCKO (1 month → 2 months) mice (data not shown), indicating that rod function in Rax iCKO (1 month → 2 months) mice is unaffected. In contrast, the amplitudes of the photopic ERGs in Rax iCKO (1 month → 2 months) mice were significantly smaller than those in control mice (Fig. 5K to M). These results show that Rax is necessary for the normal expression of cone opsins but not for that of rhodopsin in mature photoreceptors.
FIG 5.
Rax is required for maintenance of cone photoreceptors. (A to B′) Expression of Rax in the Raxflox/flox mouse retina at 1 month was detected by in situ hybridization using an antisense probe (A) or a sense probe (B) for Rax. (A′ and B′, magnifications of boxes in panels A and B, respectively. GCL, ganglion cell layer; INL, inner nuclear layer; ONL, outer nuclear layer; RPE, retinal pigment epithelium; 1M, harvest of retinas at age 1 month. Bar, 100 μm. (C to J) Whole-mount retinas from control and Rax iCKO (1 month → 2 months) mice were immunostained with antibody against S-opsin (magenta) (C, D, G, and H) or M-opsin (green) (E, F, I, and J). Ventral (C to F) and dorsal regions (G to J) are shown. Bar, 100 μm. (K to M) ERGs were recorded from Rax iCKO (1 month → 2 months) mice. (K) Photopic ERGs elicited by four different stimulus intensities are shown. (L and M) The amplitudes of the photopic ERG a-wave (L) and b-wave (M) are shown as a function of the stimulus intensity. For control mice, n = 5; for Rax iCKO mice, n = 3. Data are means ± SDs. **, P < 0.01 by Student's t test; *, P < 0.05 by Student's t test.
Rax promotes Crx transactivation on Rhodopsin, S-opsin, and M-opsin promoters.
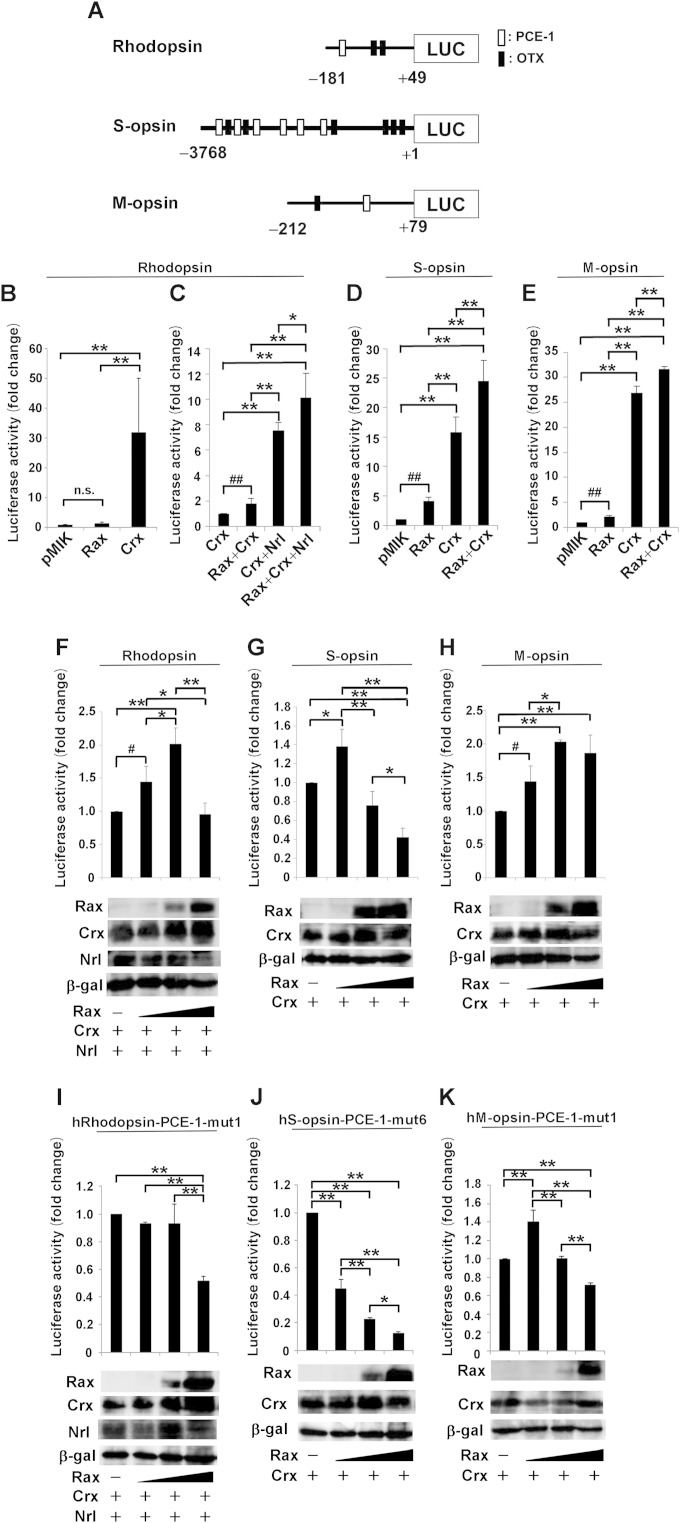
It was reported that Crx, Nrl, and Nr2e3 synergistically regulate the expression of rod-specific genes (31, 38–42). In the current study, we found that although the expression levels of Crx, Nrl, and Nr2e3 were similar between control and Rax iCKO (P4 → P20) mouse retinas, the levels of transcripts of rod-specific genes significantly decreased in the Rax iCKO (P4 → P20) mouse retina (Fig. 3B). Nr2e3 functions downstream of Nrl. Crx and Nrl are major transcription factors regulating Rhodopsin expression, and Crx is the major factor regulating cone opsin expression. Therefore, in the current study we investigated the physiological relevance of Rax with Crx and/or Nrl for photoreceptor gene transactivation. To test whether or not Rax can transactivate opsin genes, we carried out luciferase assays using human Rhodopsin, S-opsin, and M-opsin promoters, which have been better characterized than the mouse Rhodopsin, S-opsin, and M-opsin promoters. These promoters contain both PCE-1 (Ret1) and OTX elements, which are the binding sites for Rax and Crx, respectively. These sites are known to be necessary for photoreceptor-specific gene expression (15). We tested the effect of Rax expression on the promoter activity in transient-transfection experiments using NIH 3T3 cells. The human Rhodopsin promoter region (positions −181 to + 49) contains a single PCE-1 site and two OTX sites. The human S-opsin promoter region (positions −3768 to + 1) possesses six PCE-1 sites and six OTX sites, and the M-opsin promoter region (positions −212 to + 79) possesses a single PCE-1 site and a single OTX site (Fig. 6A). Although Rax alone showed no significant effect on the activity of the human Rhodopsin promoter, Crx alone strongly transactivated the human Rhodopsin promoter (Fig. 6B). Cotransfection of the Rax plasmid together with the Crx plasmid exhibited a slight synergic effect on transactivation activity compared the effect of the Crx plasmid alone (Fig. 6C). In contrast, we observed a prominent effect on the activation of the human Rhodopsin promoter when the Nrl plasmid was cotransfected with the Crx plasmid. Addition of the Rax plasmid to the Crx and Nrl plasmids significantly increased the transactivation activity compared with that induced by the Crx plus Nrl plasmids alone (Fig. 6C).
FIG 6.
Transactivation of Rhodopsin, S-opsin, and M-opsin promoters by Rax, Crx, and/or Nrl. (A to H) Luciferase (LUC) reporter assay using human Rhodopsin, S-opsin, and M-opsin promoter-luciferase constructs. (A) Schematics of luciferase assay promoter construct. (B and C) NIH 3T3 cells were cotransfected with 0.4 μg of the human Rhodopsin promoter (positions −181 to + 49)-luciferase construct together with 0.5 μg Rax, Crx, and Nrl expression plasmids. Luciferase activity was corrected for transfection efficiency using a β-galactosidase internal control (0.3 μg) and is shown as the fold change, which was calculated as the ratio of the value for each combination to the value for the reporter plasmid with an empty vector. Data are means ± SDs (n = 4). (D) As for panels B and C, except that the reporter plasmid was the human S-opsin promoter (positions −3768 to + 1)-luciferase construct with 0.5 μg Rax expression plasmid. Data are means ± SDs (n = 3). (E) As for panel D, except that the reporter plasmid was the human M-opsin promoter (positions −212 to + 79)-luciferase construct. Data are means ± SDs (n = 3). (F to H) NIH 3T3 cells were cotransfected with the human Rhodopsin promoter-luciferase reporter construct plus the Crx and Nrl expression plasmids (0.25 μg) and the Rax expression plasmid (0, 0.25, 1, and 4 μg) (F), the human S-opsin promoter-luciferase reporter construct plus the Crx expression plasmid (0.25 μg) and the Rax expression plasmid (0, 0.25, 1, and 4 μg) (G), or the human M-opsin promoter-luciferase reporter construct plus the Crx expression plasmid (0.25 μg) and the Rax expression plasmid (0, 0.25, 1, and 4 μg) (H). (I to K) NIH 3T3 cells were cotransfected with the human Rhodopsin-PCE-1-mut1 promoter-luciferase reporter construct plus the Crx and Nrl expression plasmids (0.25 μg) and the Rax expression plasmid (0, 0.25, 1, and 4 μg) (I), the human S-opsin-PCE-1-mut6 promoter-luciferase reporter construct plus the Crx expression plasmid (0.25 μg) and the Rax expression plasmid (0, 0.25, 1, and 4 μg) (J), or the human M-opsin-PCE-1-mut1 promoter-luciferase reporter construct plus the Crx expression plasmid (0.25 μg) and the Rax expression plasmid (0, 0.25, 1, and 4 μg) (K). Rax, Crx, and Nrl expression levels were determined by Western blotting after normalization using a β-galactosidase (β-gal) internal control. Data are means ± SDs (n = 3). **, P < 0.01 by the Tukey-Kramer multiple-comparison test; *, P < 0.05 by the Tukey-Kramer multiple-comparison test; n.s., not significant by the Tukey-Kramer multiple-comparison test; ##, P < 0.01 by Student's t test; #, P < 0.05 by Student's t test.
We next tested whether the human Cone opsin promoters (S-opsin and M-opsin) are transactivated by Rax alone or only in the combination with Crx (Fig. 6D and E). While Rax alone weakly transactivated the S-opsin or M-opsin promoters, Crx alone exhibited a strong transactivation effect on both the S-opsin and M-opsin promoters (Fig. 6D and E). The addition of Rax significantly augmented the transactivation activities induced by Crx alone for both the S-opsin and M-opsin promoters (Fig. 6D and E).
We revealed that the Rax protein expression level decreases during mouse photoreceptor maturation (Fig. 1A to D and F). To examine whether the decrease of the Rax expression level during photoreceptor maturation is biologically meaningful, we examined the dose dependency of Rax transactivation activity on opsin promoters. NIH 3T3 cells were cotransfected with fixed amounts of the Crx and Nrl expression plasmids (0.25 μg each) and four different amounts of the Rax expression plasmid (0, 0.25, 1, and 4 μg), together with the human Rhodopsin promoter-luciferase reporter construct. Compared with the luciferase activity obtained by coexpression of Crx and Nrl, about 1.5-fold or 2-fold activation was observed when 0.25 μg or 1 μg of Rax plasmid was additionally transfected, respectively (Fig. 6F). Interestingly, a larger amount of the Rax plasmid (4 μg) markedly suppressed Rhodopsin transactivation by Crx and Nrl (Fig. 6F). Next, we performed a similar assay on the human S-opsin and M-opsin promoters, which are mainly regulated by Crx. Compared with the level of activation achieved by Crx alone, an almost 1.4-fold activation of the human S-opsin promoter was observed when the Rax plasmid (0.25 μg) was cotransfected (Fig. 6G). However, this activation was dose-dependently suppressed when larger amounts of Rax plasmids (1 and 4 μg) were cotransfected (Fig. 6G). Similarly, compared with the level of activation achieved with Crx alone, the human M-opsin promoter showed an approximately 1.5- or 2-fold activation when Rax (0.25 or 1 μg, respectively) was coexpressed, while transfection of a larger amount of the Rax plasmid (4 μg) did not promote the Crx transactivation activity (Fig. 6H). Next, to test whether transactivation of the Rhodopsin, S-opsin, and M-opsin promoters by Rax depends on the PCE-1 sites, we altered the human PCE-1 core from 5′-AATTA-3′ to 5′-AACCA-3′ (in hRhodopsin-PCE-1-mut1, hS-opsin-PCE-1-mut6, and hM-opsin-PCE-1-mut1) as previously described (15). In this study, neither Rax nor Crx bound detectably to this mutant oligonucleotide. When we used hRhodopsin-PCE-1-mut1 as a promoter, cotransfection with Rax and Crx together did not further activate the promoter (Fig. 6I). Intriguingly, a larger amount of Rax plasmid (4 μg) markedly suppressed Rhodopsin transactivation by Crx and Nrl (Fig. 6I). Next, we performed a similar assay on the hS-opsin-PCE-1-mut6 and hM-opsin-PCE-1-mut1 promoters. When we used the hS-opsin-PCE-1-mut6 promoter, the activation was suppressed in a dose-dependent manner (Fig. 6J). Interestingly, compared with the level of activation by the hRhodopsin-PCE-1-mut1 and hS-opsin-PCE-1-mut6 promoters, the hM-opsin-PCE-1-mut1 promoter showed about a 1.4-fold activation when Rax (0.25 μg) was coexpressed, while transfection of a larger amount of the Rax plasmid (4 μg) suppressed M-opsin transactivation by Crx (Fig. 6K). Furthermore, we analyzed the levels of the Rax, Crx, and Nrl proteins in the reporter assays to examine whether a higher level of expression of Rax affects the protein levels of the other factors, Nrl and Crx, which could lead to the activation or suppression of transactivation. We observed that suppression was not due to changes in the Crx and Nrl expression levels (Fig. 6F to K). These results suggest and demonstrate that proper levels of Rax protein and Rax binding to the PCE-1 elements are important for photoreceptor gene transcription.
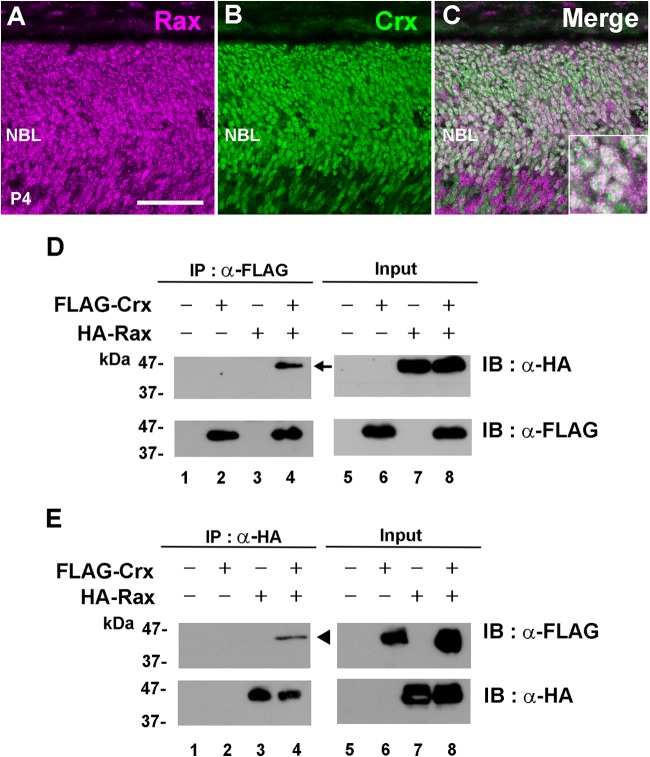
Rax protein interacts with Crx protein.
Since the luciferase assay suggested that Rax is functionally associated with Crx, we then tested whether the Rax protein colocalizes with the Crx protein in the maturing postnatal mouse retina. At P4, we immunostained wild-type mouse retinas using anti-Rax and anti-Crx antibodies. We observed the colocalization of these two proteins in almost all nuclei of photoreceptor cells (Fig. 7A to C). In order to assess whether the Rax protein interacts with the Crx protein, we carried out a coimmunoprecipitation assay. We expressed FLAG-tagged full-length Crx and/or HA-tagged full-length Rax in HEK293T cells and performed an immunoprecipitation assay using anti-FLAG and anti-HA antibodies (Fig. 7D and E). The HEK293T cell lysates were immunoprecipitated using the anti-FLAG antibody, and the HA-tagged Rax was detected in the immunoprecipitates by Western blotting (Fig. 7D, lane 4). To further confirm the interaction between the Rax and Crx proteins, a reciprocal experiment was performed. The HEK293T cell lysates were immunoprecipitated using the anti-HA antibody, and the FLAG-tagged Crx protein was detected in the immunoprecipitates by Western blotting (Fig. 7E, lane 4). These results suggest that the Rax protein interacts with the Crx protein.
FIG 7.
The Rax protein physically associates with the Crx protein. (A to C) Rax colocalizes with Crx in the postnatal retina. (A and B) At P4, retinal sections were immunostained with anti-Rax (magenta) (A) and anti-Crx (green) (B) antibodies. (C) Merge of panels A and B. (Inset) Rax- and Crx-double-positive cells at high magnification. Bar, 50 μm. (D and E) The Rax protein interacts with the Crx protein. The HA-tagged Rax expression plasmid was transfected with the FLAG-tagged Crx expression plasmid into HEK293T cells. Cell lysates were incubated with either an anti-FLAG antibody (D) or an anti-HA antibody (E). Immunoprecipitated proteins were analyzed by Western blotting. Lanes 5 to 8, input proteins without immunoprecipitation (IP). Arrow, protein immunoprecipitated by the anti-FLAG antibody; arrowhead, protein immunoprecipitated by the anti-HA antibody. IB, immunoblot; NBL, neuroblastic layer.
DISCUSSION
Functional roles of Rax in immature and mature photoreceptor cells.
In the current study, we investigated the expression patterns and functional roles of Rax in immature and mature postnatal photoreceptors in mice, which possess a single Rax gene in the genome. In order to investigate the in vivo function of Rax in the postnatal maturing retina, we treated Raxflox/flox; Crx-CreERT2 mice with tamoxifen at P4 and harvested the retinas at P8 or P20 (P4 → P8 or P20) (Fig. 2 and 3). In the Rax iCKO (P4 → P8) mouse retina, the levels of expression of the rhodopsin, S-opsin, and M-opsin proteins were slightly decreased, while the expression of Crx was unaffected (Fig. 2A to J). We observed that TRβ2-positive cells survived in the Rax iCKO (P4 → P8) mouse retina (Fig. 2K to M). In the Rax iCKO (P4 → P20) mouse retina, the levels of expression of cone photoreceptor-specific genes (M-opsin, S-opsin, Gnat2, and Pde6c) markedly decreased compared to those in the control retina, and the levels of expression of rod photoreceptor-specific genes (Rhodopsin, Gnat1, and Pde6g), which are involved in rod phototransduction, moderately decreased (Fig. 3B). By in situ hybridization of Rax, we observed that Rax mRNA expression continues in the ONL until the adult stage (1 month) (Fig. 5A and A′). We therefore investigated the in vivo function of Rax in mature photoreceptors by inactivating Rax after 1 month. We found that the levels of S-opsin and M-opsin expression were remarkably reduced (Fig. 5C to J) but that the level of rhodopsin expression was unaffected in the Rax iCKO (1 month → 2 months) mouse retina (data not shown). These histological observations match well with the results obtained by the use of ERG recordings (Fig. 5K to M). Taken together, these in vivo analyses show that Rax has an essential role in cone photoreceptor gene expression and survival and is also partially required for rod photoreceptor gene expression but is not essential for rod survival in the postnatal mouse retina.
Functional difference between Rax and Rax2.
It is known that various vertebrates contain at least two Rax genes, Rax and Rax2. On the other hand, the genomes of mice and rats encode only a single Rax gene (22). Several studies reported on the in vivo functions of Rax and Rax2 in photoreceptor development at maturing stages. In chicks, it was reported that RaxL/Rax2 is not sufficient to promote the photoreceptor cell fate choice but is required for photoreceptor cell development (20). In this study, the engrailed repressor domain-fused dominant negative Rax and Rax2 expression vectors were electroporated into the optic vesicles of chick embryos to knock down the function of Rax and Rax2, respectively, and the results suggested that chick Rax2, but not Rax, is required for photoreceptor cell development. It was also reported that rhodopsin expression and PNA staining were decreased in Rax short hairpin RNA-expressing transgenic tadpoles in which both Rax and Rax2 were downregulated (18). Interestingly, the expression of mouse Rax can restore reduced rhodopsin expression and PNA staining. In humans, three heterozygous QRX/RAX2 mutant alleles were identified in the genomes of degenerative retinal disease patients (22). R87Q was found in a patient with age-related macular degeneration (AMD), and G137R and 140P_141Gdup (140PGIns) were identified in cone-rod dystrophy (CRD) patients. This study also reported that these mutant RAX2 proteins exhibited alterations in their transactivation activities and the strength of their interaction with CRX; however, it seems that these mutants were heterozygous and had missense mutations which did not result in drastic biochemical abnormalities, so there might not be sufficient evidence to conclude that these mutations are responsible for AMD or CRD. Moreover, a recent study reported that interspecific variation in Rax expression controls opsin expression and causes visual system diversity in African cichlid fishes (43).
Taken together, although previous studies showed that Rax and/or Rax2 is involved in the maturation of photoreceptors in vivo, it is still unclear whether one Rax gene or both of them play essential roles in photoreceptor maturation. In addition, to our best knowledge, there has been no study in which Rax and/or Rax2 function is inactivated or knocked down specifically at the adult stage.
Rax modulates photoreceptor gene expression in cooperation with Crx.
In the present study, we examined the transactivation activity of mouse Rax by luciferase assays using human photoreceptor gene promoters. In the luciferase assays, we observed that the addition of Rax significantly increases the transactivation activities of Crx plus Nrl on the human Rhodopsin promoter or Crx alone on the human S-opsin and M-opsin promoters (Fig. 6A to E). This result matches well with the significant reductions in the level of photoreceptor protein and gene expression in Rax iCKO (P4 → P8 and P20) mouse retinas (Fig. 2A to I and 3B to L). We observed that a specific amount of Rax shows the strongest transactivation effect on the photoreceptor gene promoters in luciferase assays (Fig. 6F to H). Furthermore, our studies also showed that PCE-1 elements are necessary for high levels of photoreceptor-specific gene expression, as described previously (15), while PCE-1 elements are not necessary for the transactivation of Rax on the human M-opsin promoter at low levels of Rax (Fig. 6I to K). The human QRX/RAX2 study also showed that the transactivation effects of RAX2 peak depending on the amount of RAX2 plasmids used in the assays. In addition, it was also reported that human RAX binds to PCE-1 sites in the mouse rod arrestin promoter and activates it with an appropriate proportion of RAX and CRX expression plasmids in the chloramphenicol acetyltransferase assay (15). These results suggest that there exists an appropriate level of Rax protein for proper Rax function in photoreceptor gene transactivation. In addition, our observation that Rax ablation at the adult stage resulted in cone photoreceptor loss suggests that even a relatively small amount of Rax has an important in vivo function (Fig. 5).
We found that the Rax and Crx proteins colocalized in the photoreceptor nuclei at P4 and were coimmunoprecipitated using cultured cells (Fig. 7A to C). Similar results were observed in the study of human RAX2 (22). In coimmunoprecipitation analyses, the G137R and 140PGIns RAX2 proteins showed a reduced interaction with CRX compared to wild-type RAX2. However, this study did not describe any interaction between RAX and CRX. Since both the Rax and Crx proteins have been highly conserved through evolution, our result that the mouse Rax protein interacts with the mouse Crx protein suggests a possible interaction between Rax and Crx in other species as well as in mice. Clarification of this hypothesis awaits future studies.
Rax has a more critical role in cone maturation than in rod maturation.
We detected a significant increase in the level of apoptosis in the Rax iCKO (P4 → P14) mouse retina (Fig. 3T to Z). Various studies reported that a deficiency of a rod photoreceptor gene, including Rhodopsin, Gnat1, and Pde6g, causes rod photoreceptor degeneration (44–46). The cone photoreceptor function loss 1 (cpfl1) mutation in Pde6c causes a lack of cone function and cone photoreceptor degeneration, which are associated with achromatopsia (47). Although we observed a significant reduction in the number of cone photoreceptors, the number of rod photoreceptor cells was unchanged or only very slightly decreased. This cone-specific cell death in the Rax iCKO (P4 → P14) mouse retina may be due to a drastic reduction of cone photoreceptor proteins and a moderate reduction of rod photoreceptor genes. Our results indicate that Rax has a modulatory function in rod gene expression but a crucial function in cone gene expression in postnatal photoreceptors. While Nrl together with other transcription factors, including Crx, transactivates rod genes, Nrl does not participate in cone gene transactivation. The absence of Nrl in cone gene transactivation might explain why Rax has a more essential role in cone maturation than in rod maturation.
Prospective studies on Rax and Rax2 in photoreceptor maturation.
Our findings in the current study demonstrate that mouse Rax plays an essential role not only in the proliferation of retinal progenitor cells, cone cell fate determination, and Müller glia cell development but also in the maturation and survival of photoreceptor cells (12–14, 16). Previous studies seemed to propose that Rax mainly regulates progenitor proliferation and Rax2 mainly functions in photoreceptor maturation. This idea may be supported partly because the human QRX/RAX2 protein (184 amino acid residues) is poorly conserved with the human RAX protein (346 amino acid residues) except in the homeodomain. However, it should be noted that Xenopus Rax1 and zebra fish Rax (Rx1) continue to be expressed in mature photoreceptors during development (11, 17, 18). Thus, together with the current results on Rax function in mice, which contain a single Rax gene, the possibility that Rax and Rax2 have a redundant function in photoreceptor maturation rather than a separate function can be proposed. Further studies in various species are needed to clarify and conclude whether Rax and Rax2 are functionally redundant or are used separately for photoreceptor maturation. Identification of many more mutations in human QRX/RAX2 and RAX will be especially useful. For a future study, it is also important to elucidate how expression level balance among multiple transcription factors is controlled in photoreceptor development. We previously showed that Rax promotes the differentiation of Müller glial cells (5, 14). In the present study, we observed that Rax is expressed in Müller glial cells until age 2 months. What the role of Rax is in maturing Müller glial cells is another interesting question to be addressed in the future.
Supplementary Material
ACKNOWLEDGMENTS
We thank Douglas Forrest for sharing an antibody, Y. Omori and S. Watanabe for helpful comments, and M. Kadowaki, A. Tani, A. Ishimaru, Y. Saioka, Y. Tojima, K. Hasegawa T. Tsujii, H. Abe, and S. Kennedy for technical assistance.
This work was supported by the Japan Science and Technology Agency (JST), Core Research for Evolutional Science and Technology (CREST), Grants-in-Aid for Scientific Research on Priority Areas, a Grant-in-Aid for Scientific Research (B), a Grant in Aid for Young Scientists (B), Specially Designated Research Promotion and Scientific Research on Innovative Areas from the Ministry of Education, Culture, Sports, Science and Technology of Japan, the Novartis Research Foundation, the Takeda Science Foundation, the Kato Memorial Foundation, the Uehara Memorial Foundation, and the Inamori Foundation.
S.I., R.S., and T.F. designed the project. T.F. and Y.M. generated the Raxflox/flox and Crx-CreERT2 mice, and S.I. and Y.M. produced the Rax iCKO mice. S.I., K.K., R.S., and T.C. carried out the molecular and biochemical experiments. S.I., Y.M., and K.K. performed histological and cell biological analysis. S.I. carried out the ERG experiments. S.I., R.S., and T.F. wrote the manuscript. T.F. supervised the project.
Footnotes
Supplemental material for this article may be found at http://dx.doi.org/10.1128/MCB.00048-15.
REFERENCES
- 1.Cayouette M, Poggi L, Harris WA. 2006. Lineage in the vertebrate retina. Trends Neurosci 29:563–570. doi: 10.1016/j.tins.2006.08.003. [DOI] [PubMed] [Google Scholar]
- 2.Livesey FJ, Cepko CL. 2001. Vertebrate neural cell-fate determination: lessons from the retina. Nat Rev Neurosci 2:109–118. doi: 10.1038/35053522. [DOI] [PubMed] [Google Scholar]
- 3.Marquardt T, Gruss P. 2002. Generating neuronal diversity in the retina: one for nearly all. Trends Neurosci 25:32–38. doi: 10.1016/S0166-2236(00)02028-2. [DOI] [PubMed] [Google Scholar]
- 4.Morrow EM, Furukawa T, Cepko CL. 1998. Vertebrate photoreceptor cell development and disease. Trends Cell Biol 8:353–358. [DOI] [PubMed] [Google Scholar]
- 5.Muranishi Y, Terada K, Furukawa T. 2012. An essential role for Rax in retina and neuroendocrine system development. Dev Growth Differ 54:341–348. doi: 10.1111/j.1440-169X.2012.01337.x. [DOI] [PubMed] [Google Scholar]
- 6.Furukawa T, Kozak CA, Cepko CL. 1997. rax, a novel paired-type homeobox gene, shows expression in the anterior neural fold and developing retina. Proc Natl Acad Sci U S A 94:3088–3093. doi: 10.1073/pnas.94.7.3088. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Mathers PH, Grinberg A, Mahon KA, Jamrich M. 1997. The Rx homeobox gene is essential for vertebrate eye development. Nature 387:603–607. doi: 10.1038/42475. [DOI] [PubMed] [Google Scholar]
- 8.Bailey TJ, El-Hodiri H, Zhang L, Shah R, Mathers PH, Jamrich M. 2004. Regulation of vertebrate eye development by Rx genes. Int J Dev Biol 48:761–770. doi: 10.1387/ijdb.041878tb. [DOI] [PubMed] [Google Scholar]
- 9.Voronina VA, Kozhemyakina EA, O'Kernick CM, Kahn ND, Wenger SL, Linberg JV, Schneider AS, Mathers PH. 2004. Mutations in the human RAX homeobox gene in a patient with anophthalmia and sclerocornea. Hum Mol Genet 13:315–322. doi: 10.1093/hmg/ddh025. [DOI] [PubMed] [Google Scholar]
- 10.Andreazzoli M, Gestri G, Angeloni D, Menna E, Barsacchi G. 1999. Role of Xrx1 in Xenopus eye and anterior brain development. Development 126:2451–2460. [DOI] [PubMed] [Google Scholar]
- 11.Chuang JC, Raymond PA. 2001. Zebrafish genes rx1 and rx2 help define the region of forebrain that gives rise to retina. Dev Biol 231:13–30. doi: 10.1006/dbio.2000.0125. [DOI] [PubMed] [Google Scholar]
- 12.Terada K, Furukawa T. 2010. Sumoylation controls retinal progenitor proliferation by repressing cell cycle exit in Xenopus laevis. Dev Biol 347:180–194. doi: 10.1016/j.ydbio.2010.08.023. [DOI] [PubMed] [Google Scholar]
- 13.Terada K, Kitayama A, Kanamoto T, Ueno N, Furukawa T. 2006. Nucleosome regulator Xhmgb3 is required for cell proliferation of the eye and brain as a downstream target of Xenopus rax/Rx1. Dev Biol 291:398–412. doi: 10.1016/j.ydbio.2005.12.029. [DOI] [PubMed] [Google Scholar]
- 14.Furukawa T, Mukherjee S, Bao ZZ, Morrow EM, Cepko CL. 2000. rax, Hes1, and notch1 promote the formation of Muller glia by postnatal retinal progenitor cells. Neuron 26:383–394. doi: 10.1016/S0896-6273(00)81171-X. [DOI] [PubMed] [Google Scholar]
- 15.Kimura A, Singh D, Wawrousek EF, Kikuchi M, Nakamura M, Shinohara T. 2000. Both PCE-1/RX and OTX/CRX interactions are necessary for photoreceptor-specific gene expression. J Biol Chem 275:1152–1160. doi: 10.1074/jbc.275.2.1152. [DOI] [PubMed] [Google Scholar]
- 16.Muranishi Y, Terada K, Inoue T, Katoh K, Tsujii T, Sanuki R, Kurokawa D, Aizawa S, Tamaki Y, Furukawa T. 2011. An essential role for RAX homeoprotein and NOTCH-HES signaling in Otx2 expression in embryonic retinal photoreceptor cell fate determination. J Neurosci 31:16792–16807. doi: 10.1523/JNEUROSCI.3109-11.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Nelson SM, Park L, Stenkamp DL. 2009. Retinal homeobox 1 is required for retinal neurogenesis and photoreceptor differentiation in embryonic zebrafish. Dev Biol 328:24–39. doi: 10.1016/j.ydbio.2008.12.040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Pan Y, Martinez-De Luna RI, Lou CH, Nekkalapudi S, Kelly LE, Sater AK, El-Hodiri HM. 2010. Regulation of photoreceptor gene expression by the retinal homeobox (Rx) gene product. Dev Biol 339:494–506. doi: 10.1016/j.ydbio.2009.12.032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Reks SE, McIlvain V, Zhuo X, Knox BE. 2014. Cooperative activation of Xenopus rhodopsin transcription by paired-like transcription factors. BMC Mol Biol 15:4. doi: 10.1186/1471-2199-15-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Chen CM, Cepko CL. 2002. The chicken RaxL gene plays a role in the initiation of photoreceptor differentiation. Development 129:5363–5375. doi: 10.1242/dev.00114. [DOI] [PubMed] [Google Scholar]
- 21.Pan Y, Nekkalapudi S, Kelly LE, El-Hodiri HM. 2006. The Rx-like homeobox gene (Rx-L) is necessary for normal photoreceptor development. Invest Ophthalmol Vis Sci 47:4245–4253. doi: 10.1167/iovs.06-0167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Wang QL, Chen S, Esumi N, Swain PK, Haines HS, Peng G, Melia BM, McIntosh I, Heckenlively JR, Jacobson SG, Stone EM, Swaroop A, Zack DJ.. 2004. QRX, a novel homeobox gene, modulates photoreceptor gene expression. Hum Mol Genet 13:1025–1040. doi: 10.1093/hmg/ddh117. [DOI] [PubMed] [Google Scholar]
- 23.Rojas-Munoz A, Dahm R, Nusslein-Volhard C. 2005. chokh/rx3 specifies the retinal pigment epithelium fate independently of eye morphogenesis. Dev Biol 288:348–362. doi: 10.1016/j.ydbio.2005.08.046. [DOI] [PubMed] [Google Scholar]
- 24.Katoh K, Omori Y, Onishi A, Sato S, Kondo M, Furukawa T. 2010. Blimp1 suppresses Chx10 expression in differentiating retinal photoreceptor precursors to ensure proper photoreceptor development. J Neurosci 30:6515–6526. doi: 10.1523/JNEUROSCI.0771-10.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Sato S, Omori Y, Katoh K, Kondo M, Kanagawa M, Miyata K, Funabiki K, Koyasu T, Kajimura N, Miyoshi T, Sawai H, Kobayashi K, Tani A, Toda T, Usukura J, Tano Y, Fujikado T, Furukawa T. 2008. Pikachurin, a dystroglycan ligand, is essential for photoreceptor ribbon synapse formation. Nat Neurosci 11:923–931. doi: 10.1038/nn.2160. [DOI] [PubMed] [Google Scholar]
- 26.Sanuki R, Omori Y, Koike C, Sato S, Furukawa T. 2010. Panky, a novel photoreceptor-specific ankyrin repeat protein, is a transcriptional cofactor that suppresses CRX-regulated photoreceptor genes. FEBS Lett 584:753–758. doi: 10.1016/j.febslet.2009.12.030. [DOI] [PubMed] [Google Scholar]
- 27.Carter-Dawson LD, LaVail MM. 1979. Rods and cones in the mouse retina. I. Structural analysis using light and electron microscopy. J Comp Neurol 188:245–262. [DOI] [PubMed] [Google Scholar]
- 28.Cepko CL, Austin CP, Yang X, Alexiades M, Ezzeddine D. 1996. Cell fate determination in the vertebrate retina. Proc Natl Acad Sci U S A 93:589–595. doi: 10.1073/pnas.93.2.589. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Rapaport DH, Wong LL, Wood ED, Yasumura D, LaVail MM. 2004. Timing and topography of cell genesis in the rat retina. J Comp Neurol 474:304–324. doi: 10.1002/cne.20134. [DOI] [PubMed] [Google Scholar]
- 30.Sherry DM, Wang MM, Bates J, Frishman LJ. 2003. Expression of vesicular glutamate transporter 1 in the mouse retina reveals temporal ordering in development of rod vs. cone and ON vs. OFF circuits. J Comp Neurol 465:480–498. doi: 10.1002/cne.10838. [DOI] [PubMed] [Google Scholar]
- 31.Furukawa T, Morrow EM, Li T, Davis FC, Cepko CL. 1999. Retinopathy and attenuated circadian entrainment in Crx-deficient mice. Nat Genet 23:466–470. doi: 10.1038/70591. [DOI] [PubMed] [Google Scholar]
- 32.Hsiau TH, Diaconu C, Myers CA, Lee J, Cepko CL, Corbo JC. 2007. The cis-regulatory logic of the mammalian photoreceptor transcriptional network. PLoS One 2:e643. doi: 10.1371/journal.pone.0000643. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Sanuki R, Onishi A, Koike C, Muramatsu R, Watanabe S, Muranishi Y, Irie S, Uneo S, Koyasu T, Matsui R, Chérasse Y, Urade Y, Watanabe D, Kondo M, Yamashita T, Furukawa T. 2011. miR-124a is required for hippocampal axogenesis and retinal cone survival through Lhx2 suppression. Nat Neurosci 14:1125–1134. doi: 10.1038/nn.2897. [DOI] [PubMed] [Google Scholar]
- 34.Trifunovic D, Dengler K, Michalakis S, Zrenner E, Wissinger B, Paquet-Durand F. 2010. cGMP-dependent cone photoreceptor degeneration in the cpfl1 mouse retina. J Comp Neurol 518:3604–3617. doi: 10.1002/cne.22416. [DOI] [PubMed] [Google Scholar]
- 35.Robson JG, Frishman LJ. 1995. Response linearity and kinetics of the cat retina: the bipolar cell component of the dark-adapted electroretinogram. Vis Neurosci 12:837–850. doi: 10.1017/S0952523800009408. [DOI] [PubMed] [Google Scholar]
- 36.Applebury ML, Antoch MP, Baxter LC, Chun LL, Falk JD, Farhangfar F, Kage K, Krzystolik MG, Lyass LA, Robbins JT. 2000. The murine cone photoreceptor: a single cone type expresses both S and M opsins with retinal spatial patterning. Neuron 27:513–523. doi: 10.1016/S0896-6273(00)00062-3. [DOI] [PubMed] [Google Scholar]
- 37.Szel A, Rohlich P, Caffe AR, van Veen T. 1996. Distribution of cone photoreceptors in the mammalian retina. Microsc Res Tech 35:445–462. doi:. [DOI] [PubMed] [Google Scholar]
- 38.Chen S, Wang QL, Nie Z, Sun H, Lennon G, Copeland NG, Gilbert DJ, Jenkins NA, Zack DJ. 1997. Crx, a novel Otx-like paired-homeodomain protein, binds to and transactivates photoreceptor cell-specific genes. Neuron 19:1017–1030. doi: 10.1016/S0896-6273(00)80394-3. [DOI] [PubMed] [Google Scholar]
- 39.Cheng H, Khanna H, Oh EC, Hicks D, Mitton KP, Swaroop A. 2004. Photoreceptor-specific nuclear receptor NR2E3 functions as a transcriptional activator in rod photoreceptors. Hum Mol Genet 13:1563–1575. doi: 10.1093/hmg/ddh173. [DOI] [PubMed] [Google Scholar]
- 40.Lerner LE, Gribanova YE, Ji M, Knox BE, Farber DB. 2001. Nrl and Sp nuclear proteins mediate transcription of rod-specific cGMP-phosphodiesterase beta-subunit gene: involvement of multiple response elements. J Biol Chem 276:34999–35007. doi: 10.1074/jbc.M103301200. [DOI] [PubMed] [Google Scholar]
- 41.Pittler SJ, Zhang Y, Chen S, Mears AJ, Zack DJ, Ren Z, Swain PK, Yao S, Swaroop A, White JB. 2004. Functional analysis of the rod photoreceptor cGMP phosphodiesterase alpha-subunit gene promoter: Nrl and Crx are required for full transcriptional activity. J Biol Chem 279:19800–19807. doi: 10.1074/jbc.M401864200. [DOI] [PubMed] [Google Scholar]
- 42.Rehemtulla A, Warwar R, Kumar R, Ji X, Zack DJ, Swaroop A. 1996. The basic motif-leucine zipper transcription factor Nrl can positively regulate rhodopsin gene expression. Proc Natl Acad Sci U S A 93:191–195. doi: 10.1073/pnas.93.1.191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Schulte JE, O'Brien CS, Conte MA, O'Quin KE, Carleton KL. 2014. Interspecific variation in Rx1 expression controls opsin expression and causes visual system diversity in African cichlid fishes. Mol Biol Evol 31:2297–2308. doi: 10.1093/molbev/msu172. [DOI] [PubMed] [Google Scholar]
- 44.Calvert PD, Krasnoperova NV, Lyubarsky AL, Isayama T, Nicolo M, Kosaras B, Wong G, Gannon KS, Margolskee RF, Sidman RL, Pugh EN Jr, Makino CL, Lem J. 2000. Phototransduction in transgenic mice after targeted deletion of the rod transducin alpha-subunit. Proc Natl Acad Sci U S A 97:13913–13918. doi: 10.1073/pnas.250478897. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Lem J, Krasnoperova NV, Calvert PD, Kosaras B, Cameron DA, Nicolo M, Makino CL, Sidman RL. 1999. Morphological, physiological, and biochemical changes in rhodopsin knockout mice. Proc Natl Acad Sci U S A 96:736–741. doi: 10.1073/pnas.96.2.736. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Tsang SH, Gouras P, Yamashita CK, Kjeldbye H, Fisher J, Farber DB, Goff SP. 1996. Retinal degeneration in mice lacking the gamma subunit of the rod cGMP phosphodiesterase. Science 272:1026–1029. doi: 10.1126/science.272.5264.1026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Chang B, Grau T, Dangel S, Hurd R, Jurklies B, Sener EC, Andreasson S, Dollfus H, Baumann B, Bolz S, Artemyev N, Kohl S, Heckenlively J, Wissinger B. 2009. A homologous genetic basis of the murine cpfl1 mutant and human achromatopsia linked to mutations in the PDE6C gene. Proc Natl Acad Sci U S A 106:19581–19586. doi: 10.1073/pnas.0907720106. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.