Abstract
Enterohemorrhagic Escherichia coli (EHEC) O121:H19 belong to a specific clonal type distinct from other classical EHEC and major enteropathogenic E. coli groups and is regarded as one of the major EHEC serogroups involved in severe infections in humans. Sequencing of the fliC genes associated with the flagellar antigen H19 (fliCH19) revealed the genetic diversity of the fliCH19 gene sequences in E. coli. A cluster analysis of 12 fliCH19 sequences, 4 from O121 and 8 from non-O121 E. coli strains, revealed five different genotypes. All O121:H19 strains fell into one cluster, whereas a second cluster was formed by five non-O121:H19 strains. Cluster 1 and cluster 2 strains differ by 27 single nucleotide exchanges in their fliCH19 genes (98.5% homology). Based on allele discrimination of the fliCH19 genes, a real-time PCR test was designed for specific identification of EHEC O121:H19. The O121 fliCH19 PCR tested negative in 73 E. coli H19 strains that belonged to serogroups other than O121, including 28 different O groups, O-nontypeable H19, and O-rough:H19 strains. The O121 fliCH19 PCR reacted with all 16 tested O121:H19 strains and 1 O-rough:H19 strain which was positive for the O121 wzx gene. A cross-reaction was observed only with E. coli H32 strains which share sequence similarities in the target region of the O121 fliCH19 PCR. The combined use of O-antigen genotyping (O121 wzx) and the detection of O121 fliCH19 allele type contributes to improving the identification and molecular serotyping of EHEC O121:H19 motile and nonmotile strains and variants of these strains lacking stx genes.
INTRODUCTION
The contamination of food and the environment with enterohemorrhagic Escherichia coli (EHEC) constitutes a serious public health problem (1, 2). EHEC as a subgroup of Shiga-toxin (stx) producing E. coli (STEC) can be present in the gut flora of domestic animals and wildlife (3). Typical EHEC strains were defined on the basis of the severity of disease they cause, their frequency in outbreaks of disease, and the presence of virulence markers (2, 4–7). EHEC can be spread into the environment and onto food through fecal shedding from animal carriers, with the contamination of food products frequently occurring at primary stages of production such as slaughter and milking (8, 9). In principle, all types of foodstuff may be contaminated with EHEC either by direct transmission from an animal carrying EHEC or by infected humans who are involved in all steps of food processing and distribution (2, 3, 10). Early detection and identification of these pathogens are a crucial step in the control of foodborne outbreaks (11). For this reason, real-time PCR reference methods for detecting EHEC in food have been developed (12, 13). These reference methods operate in a cascade-like process where samples are first tested for the presence of both stx and eae. If samples are positive, the presence of genes encoding the O antigen (lipopolysaccharide) of the suspected EHEC O serogroup is investigated. The set of the virulence markers and O serogroups tested can be adjusted based on the epidemiology and occurrence of new virulent EHEC types, as was the case with the massive outbreak with enteroaggregative E. coli (EAEC)-STEC O104:H4 in 2011 in Europe (14, 15). At present, five EHEC serotypes (O26:H11, O103:H2, O111:H8, O145:H25/H28, and O157:H7) are covered by the ISO/TS 13136 method (16). In addition to these, two more EHEC serotypes, namely O45:H2 and O121:H19, are targeted by the reference method used in the United States (13).
EHEC O121:H19 strains have been found in cattle and reported as severe human pathogens in both North and South America (17–20), in Japan (21, 22), and in European countries (6, 23–26). As EHEC O121:H19 strains are not specifically covered by the existing ISO/TS 13136 method, we became interested in developing suitable real-time PCR assays for the specific detection and identification of O121:H19 EHEC strains, which could improve the existing ISO/TS 13136 reference method. A number of different real-time PCR assays were developed for detecting EHEC O121 strains. These methods have used E. coli O121 O-antigen-specific wzx or wzy sequences as targets for specific detection of these strains (6, 27–29). However, a screening system for EHEC O121:H19 goes beyond the mere detection of the O121 antigen gene. Searching for only the O serogroup makes the screening less specific since a number of non-EHEC E. coli O121 strains containing H antigens other than H19 can be present (9, 30–33). These strains could interfere with a proper detection of EHEC O121:H19 in food and other complex matrices, which can contain heavy mixtures of different bacteria.
The fliC genes of the 53 known flagellar antigens of E. coli have been sequenced, and investigation of flagellar (fliC) genes has been used for the detection and further characterization of motile and nonmotile EHEC strains (23, 34–38). Sequence diversity in the fliC genes of E. coli strains that express different O antigens but share the same flagellar antigenic type, such as H6 and H7 (39, 40), has been used for the specific identification of EHEC O157:H7 (40). More recently, the sequence diversity in the flagellar types H25 and H28 has been used for identification of EHEC O145:H25 and O145:H28 strains (38). In this work, specific features of the O121 fliC gene encoding the flagellar antigen H19 (fliCH19) allowed the development of a real-time PCR test to be used in combination with an O121 wzx PCR assay for the rapid and specific identification of EHEC O121:H19 motile and nonmotile strains.
MATERIALS AND METHODS
Bacteria.
E. coli strains used in this study were derived from the collections of the National Reference Laboratory for E. coli (NRL E. coli) at the Federal Institute for Risk Assessment (BfR) in Berlin, Germany, and from the French Agency for Food, Environmental and Occupational Health and Safety (ANSES) in Maisons-Alfort, France. E. coli strains (n = 285) used for specificity study included the E. coli reference strains belonging to serogroups O1 to O186. All strains have been previously described for their serotypes and virulence genes (6, 32, 41–44). Specificity of the O121 wzx real-time PCR assay was evaluated on the E. coli reference strains belonging to O groups O1 to O186 (32, 45) and on 26 serologically confirmed O121 strains carrying different H antigens. A collection of 73 E. coli non-O121 strains expressing and/or carrying the genes for flagellar antigen H19 were investigated to study the specificity of the O121 fliCH19 real-time PCR assays. These 73 strains included 28 different O groups and O-antigen nontypeable (ONT) or O-rough (Or) strains. E. coli strains were cultivated overnight at 37°C in Luria broth, and DNA was extracted using InstaGene matrix (Bio-Rad Laboratories, Marnes-La-Coquette, France) according to the manufacturers guidelines.
Real-time PCR amplifications were performed in duplicate with an ABI 7500 instrument (Applied Biosystems, Foster City, CA, USA) in 25-μl reaction volumes or with a LightCycler 1536 (Roche Diagnostics, Meylan, France) in 1.5-μl reaction volumes according to the recommendations of the suppliers. Briefly, primers and TaqMan probes were used at 300 nM final concentrations for the PCR. The following thermal profile was used: 95°C for 1 min, followed by 40 cycles of 95°C for 10 s and 60°C for 30s.
PCR detection and mapping of E. coli O-antigen and H-antigen genes.
Mapping of fliC gene variants to their respective H types was performed by PCR, and analysis of restriction fragment length polymorphism (RFLP) of HhaI-digested PCR products (PCR/RFLP) was performed as previously described (23, 46). Nucleotide sequence data obtained from different fliCH19 genes was used for designing TaqMan real-time PCR probes and XS probes (minor groove binder replacement; Biolegio, Nijmegen, The Netherlands) and primers for common detection of all genetic variants of fliCH19 genes (6; this work) and for the specific detection of O121 fliCH19 genes (this work). The real-time PCR assay specific for the wzx (O-antigen flippase) gene of E. coli O121 has been described previously (6). Real-time PCR probes and primers used in this work were designed with the software Primer Express, version 3.0 (Applied Biosystems), and are described in Table 1.
TABLE 1.
Primers and probes for the real-time PCR assays
| Target gene and primer or probe | Sequence (5′–3′)c |
|---|---|
| fliCH19a | |
| Forward primer | AATGACCATCACTTCTGCTGG |
| Reverse primer | TAATTGAGGTCGCATATGCGGC |
| Probe | 6-FAM-AATGCTCAGGTGTTAAAAGACGCGGC-BHQ1 |
| O121 fliCH19b | |
| Forward primer | CAACAACTTACAGCGTATCCGTG |
| Reverse primer | CACCTCTGACCTGGACTCCATT |
| Probe | 6-FAM-TTCAGGCCACTACCGGT-BHQ1 |
| O121 wzxa | |
| Forward primer | TGGTCTCTTAGACTTAGGGC |
| Reverse primer | TTAGCAATTTTCTGTAGTCCAGC |
| Probe | 6-FAM-TCCAACAATTGGTCGTGAAACAGCTCG-BHQ1 |
Nucleotide sequencing.
Relevant characteristics of E. coli strains used for nucleotide sequence analysis of their fliC genes are listed in Table 2. The nucleotide sequences of the fliC genes were determined by Sanger sequencing and analyzed with the Accelrys DS Gene software package (Accelrys, Inc., USA). The nucleotide sequences of the fliC homologs are reported in Table 2.
TABLE 2.
Escherichia coli strains used for nucleotide sequencing of fliC genes
| Strain designation (former no.) | Serotype | fliC gene GenBank accession no.a | STEC virulence gene(s)b | Sample source and country of origin (reference) |
|---|---|---|---|---|
| CB12043 (MB57) | O121:H19 | LN555737 | —c | Human feces, France (42) |
| CB12631 (1850-04) | O121:H19 | LN555735 | stx2a, eae | Human feces, Switzerland (49) |
| CB14655 | O121:H19 | LN555736 | stx2a, eae | Human feces, Germany (this work) |
| CB12008 | O178:H19 | LN810063 | stx1a, stx2a, stx2d | Raw milk, Germany (50) |
| CB12322 | O8:H19 | LN810064 | stx2e | Pork, Germany (9) |
| CB12363 | O174:H19 | LN810065 | Salad, Germany (51)d | |
| CB12368 | O158:H19 | LN810066 | Salad, Germany (51)d | |
| CB12802 (A07) | O111:H19 | LN810067 | eae | Unknown, Italy (52)e |
All genes had coding sequences of 1,833 bp.
Presence of major STEC virulence genes eae and stx.
This strain was negative for stx and eae genes but carried the typical virulence signature of EHEC O121:H19 strains (42).
Isolated in 2009.
Isoalted in 2007.
Nucleotide sequence accession numbers.
Sequences of E. coli flagellin fliC genes were submitted to the European Nucleotide Archive (ENA) and were deposited in GenBank under accession numbers LN555735 to LN555737 and LN810063 to LN810067.
RESULTS
Specificity of the E. coli O121 serogroup wzx gene assay for detection of E. coli O121 strains.
The O121 wzx real-time PCR was tested for its specificity on reference strains belonging to E. coli O groups O1 to O186 and was found to react with only the O121 reference strain (39w, O121:H10). Also, the O121 wzx PCR had positive results with a collection of 26 serologically confirmed O121 strains carrying different H antigens (Table 3). As the O121 wzx PCR proved to be specific and sensitive, it was used to confirm results obtained by O serotyping and for screening for the presence of O121 O-antigen genes in serologically O-rough:H19 strains (see below).
TABLE 3.
Specificity of the O121 wzx (O-antigen flippase) real-time PCR within strains belonging to serogroup O121
| Serotypea | No. of strains | Country of isolation and date (yr) or reference | Sample typeb | STEC virulence gene(s)c |
|---|---|---|---|---|
| O121:H7 | 1 | Germany, 2008 | Wild boar meat | stx1, stx2 |
| O121:H9 | 1 | Germany, 2014 | Raw sausage | stx2e |
| O121:H10 | 2 | Germany, 2011 and 2014 | Raw sausage | stx2e |
| O121:H10 | 4 | Germany, 2007–2013 | Pig feces (n = 2), ground meat (n = 1), pig organ (n = 1) | |
| O121:H11 | 1 | Germany, 2009 | Calf feces | |
| O121:H14 | 1 | Germany, 2008 | Red deer meat | stx1c |
| O121:H19 | 1 | France (6) | ||
| O121:H19 | 2 | Switzerland (25); Germany, 2014 | Sheep raw milk | eae |
| O121:H19 | 13 | Germany (23); Switzerland (25); France (6) | stx2, eae | |
| O rough:H19d | 1 | Switzerland (25) | stx2, eae |
All strains reacted with the O121 wzx real-time PCR. Some of these strains were nonmotile, and their fliC genotype was detected by nucleotide sequencing of fliC PCR products.
n, number of samples.
Presence of stx and eae genes as major markers of EHEC. In brackets: numbers of strains with corresponding genotype.
This O-rough strain gave a positive result in the O121 wzx real-time PCR.
Detection of O-serogroup-specific nucleotide sequences in the fliC genes of EHEC O121:H19 strains.
The nucleotide sequence of fliCH19 (flagellar antigen H19) genes of E. coli O121:H19 and non-O121:H19 strains, isolated from different sources, revealed characteristic features of these genes. The fliCH19 nucleotide sequences of three O121:H19 strains (CB12043, CB12631, and CB14655) and five H19 strains (CB12008, CB12322, CB12363, CB12802, and CB12368) which belonged to five different O groups (O178, O8, O174, O111, and O158) were determined and compared to fliCH19 sequences already deposited in the GenBank. The fliCH19 sequences derived from E. coli O121:H19 strains CB12043 (GenBank accession number LN555737), CB12631 (GenBank accession number LN555735), and CB14655 (GenBank accession number LN555736) were found to be identical to each other, and the length of the fliCH19 coding region of each was 1,833 bp (610 amino acids [aa]). fliCH19 sequences identical to those of CB12043, CB12631, and CB14655 were found in genomes of E. coli O121 strains deposited in the GenBank (accession numbers LM996927.1, LN554924.1, LM996065.1, and LM996022.1). Differences between the fliCH19 sequences of E. coli O121 were found with those from non-O121:H19 strains such as the reference strain for E. coli flagellar type H19 (A18d, O9:H19; GenBank AY250002; length, 1,842 bp, 614 aa) (32), strain IAI1 (E. coli O8:H19; GenBank CU928160; length, 1,833 bp, 611 aa) (47), and strain PC19 (E. coli O157:H19; GenBank AY337479; length, 1,833 bp, 611 aa) (38).
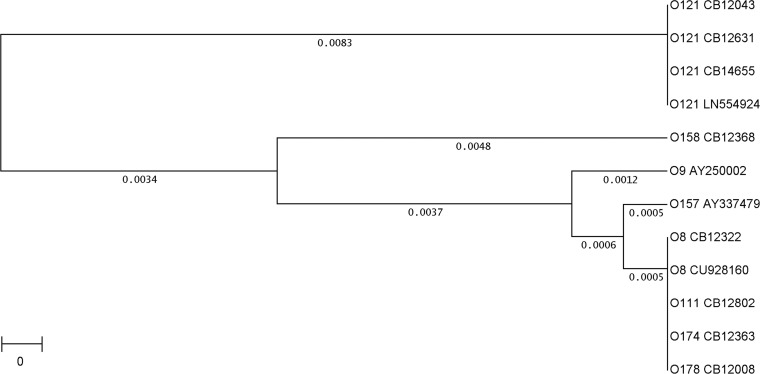
A cluster analysis of 12 fliCH19 sequences, 4 from O121 and 8 from non-O121 strains, is shown in Fig. 1. Five different genotypes were detected among the 12 fliCH19 strains. All O121:H19 strains investigated in this work (CB12043, CB12631, and CB14655) and by other investigators (GenBank accession numbers LM996927.1, LN554924.1, LM996065.1, and LM996022.1) fall into one cluster. A second cluster is formed by the strains CB12008, CB12322, CB12363, and CB12802 (Table 2) and strain IAI (GenBank accession number CU928160) (44). Cluster 1 and cluster 2 strains differ by 27 single nucleotide exchanges in their fliC genes (98.5% homology); however, these have no effect on the amino acid composition of H19 flagellin, which is conserved in these two clusters (see Table S1 in the supplemental material). Three strains (PC19, CB12368, and A18d) present single fliCH19 genotypes. These are characterized by alterations in the amino acid composition: strain PC19 (GenBank accession number AY337479) has T/A at amino acid position 198, strain CB12368 has T/A at amino acid position 476, and strain A18d (AY250002) carries a repeat of three amino acids (GAA) at positions 402 to 404 (see Table S1 in the supplemental material).
FIG 1.
Genetic relationship between fliCH19 genes in different strains and serotypes of E. coli. Cluster analysis was performed using the fliCH19 genes present in the following E. coli strains: serotype O121:H19, CB12043 (GenBank accession number LN555737), CB12631 (LN555735), CB14655 (LN555736), and GenBank accession number LN554924; serotype O158:H19, CB12368 (LN810066); serotype O9:H19, GenBank accession number AY250002; serotype O157:H19, GenBank accession number AY337479; serotype O8:H19, CB12322 (LN810064) and GenBank accession number CU928160; serotype O111:H19, CB12802 (LN810067); serotype O174:H19 (LN810065); serotype O178:H19, CB12008 (LN810063). The unweighted-pair group method using average linkages was used as the tree building mode, and the distances were calculated according to Tajima and Nei (48).
Development of an fliC-specific real-time PCR assay for EHEC O121:H19 strains.
The real-time PCR assay developed from the O121 fliCH19 sequence was tested on 73 E. coli strains identified as H19 positive by serotyping or nucleotide sequence typing of fliC genes and by a generic fliCH19 PCR assay described previously (6). These 73 strains belonged to 28 different O groups, as well as to ONT and O-rough strains. The results are summarized in Table 4. The O121 fliCH19 PCR reacted with all O121:H19 and one O-rough:H19 strain, which was positive for the O121 wzx gene. In contrast, all other flagellar type H19 strains belonging to non-O121 serogroups were clearly negative in the O121 fliCH19 real-time PCR.
TABLE 4.
Reaction of the O121 fliCH19 real-time PCR on different E. coli H19 antigen strains
| Serotypeb | No. of strains |
CT valuea |
|
|---|---|---|---|
| fliCH19 | O121 fliCH19 | ||
| O2:H19 | 1 | 21.6 | 0 |
| O8:H19 | 3 | 18.7–22.9 | 0 |
| O9:H19 | 2 | 19.9–21.6 | 0 |
| O22:H19 | 1 | 20.6 | 0 |
| O32:H19 | 1 | 19.3 | 0 |
| O36:H19 | 1 | 17.9 | 0 |
| O45:H19 | 2 | 20.1–20.2 | 0 |
| O55:H19 | 1 | 18.1 | 0 |
| O61:H19 | 1 | 21.9 | 0 |
| O76:H19 | 6 | 18.4–21.1 | 0 |
| O79:H19 | 1 | 20.6 | 0 |
| O87:H19 | 1 | 19.1 | 0 |
| O93:H19 | 1 | 20.9 | 0 |
| O96:H19 | 1 | 22.9 | 0 |
| O102:H19 | 1 | 20.8 | 0 |
| O111:H19 | 2 | 19.5–21.9 | 0 |
| O112ac:H19 | 1 | 17.2 | 0 |
| O121:H19 | 16 | 17.4–22.8 | 15.1–22.9 |
| O127:H19 | 1 | 19.3 | 0 |
| O138:H19 | 1 | 21.0 | 0 |
| O141:H19 | 1 | 21.5 | 0 |
| O147:H19 | 1 | 22.4 | 0 |
| O157:H19 | 1 | 20.2 | 0 |
| O158:H19 | 1 | 18.4 | 0 |
| O163:H19 | 2 | 17.9–22.9 | 0 |
| O174:H19 | 1 | 21.8 | 0 |
| O176:H19 | 1 | 21.9 | 0 |
| O178:H19 | 6 | 16.4–22.4 | 0 |
| ONT:H19 | 13 | 18.2–22.3 | 0 |
| O rough:H19c | 1 | 19.4 | 20.1 |
Mean of real-time PCR cycle threshold (CT) values calculated from duplicate PCRs. The efficiency of the O121 fliCH19 real-time PCR was determined (slope = −3.504, y inter = 23,223; R2 = 0.999; efficiency, 92,938%), and the PCR was effective for detecting reliably small quantities of 0.026 ng/ml O121:H19 DNA (CT of 28.8).
Some of the strains were nonmotile, and the fliC genotype was detected by nucleotide sequencing of fliC PCR products.
This strain was positive in the real-time PCR for the E. coli O121 wzx gene.
The O121 fliCH19 real-time PCR was further tested for reaction with DNA prepared from E. coli H-serotype reference strains encoding the 53 flagellar antigens of E. coli (H1 to H56) (32, 38). A positive PCR result was obtained only with the E. coli reference strain for flagellar type H32 (strain 26w, O114:H32) (32). In order to confirm the cross-reaction with flagellar type H32-encoding strains, we tested 12 more H32 strains from our collection, which were split into serotypes O2:H32, O26:H32, O62:H32, O85:H32, O167:H32, and ONT:H32. All of these strains reacted positively in the O121 fliCH19 PCR, indicating that the cross-reaction was due to the fliC gene present in these strains (data not shown). A comparison with the fliCH32 sequence deposited in the GenBank (accession number AY250014; 1,713 bp) revealed that the region targeted by the forward primer (positions 258 to 280 in sequence AY250014) and the probe sequence (positions 290 to 306) designed in our study were identical, whereas the backward primer (positions 315 to 336) showed only three nucleotide differences near the 3′ end (positions 316 to 318) and one at the 5′ end (position 336). This makes it likely that the cross-reaction of O121 fliCH19 PCR with E. coli H32 strains is due to similarities in the fliC sequences at the DNA stretch covering the O121 fliCH19 primers and probe.
DISCUSSION
Shiga-toxin producing E. coli O121:H19 carrying the eae (intimin) gene are known as the causative agents of severe illness, such as hemorrhagic colitis (HC) and hemolytic uremic syndrome (HUS), worldwide. These EHEC O121:H19 strains were previously assigned to a clonal type which is distinct from other lineages of EHEC and EPEC strains (20). Serogroup O121 strains were different for virulence markers associated with STEC and EHEC (9, 30–33; this work). Some of these O121 strains produce stx2e and were isolated from pigs or food containing pork (Table 3). Other O121 non-H19 strains produce stx but are negative for eae and were isolated from wild game (Table 3). However, strains fulfilling the criteria for EHEC (presence of stx and eae) were found only in the group of O121:H19 strains.
Many of the so far described methods used for identification of EHEC O121:H19 rely on O121 wzy or O121 wzx real-time PCR assays which are potentially suitable to detect all variants of serogroup O121 strains, including EHEC O121:H19 and non-EHEC E. coli O121 strains containing H antigens other than H19 (27–29). Therefore, these tests lack specificity for EHEC O121:H19. Combined assays including detection of eae-ε (one variant of the intimin genes), wzx O121, and fliCH19 sequences were developed to improve specificity of detection of EHEC O121:H19 strains, but they are applicable only on pure isolates (6). Multidetection of these genetic markers in complex samples may result in false-positive interpretations since these genes can be shared by various E. coli pathogroups. Identification of new genetic markers more specific for EHEC O121:H19 is a desirable goal. Our study is the first using the sequence diversity of fliCH19 genes to develop a more specific diagnostic assay for EHEC O121:H19 strains. The fliCH19 genes are widespread in different E. coli strains, including EHEC O121:H19. By comparing different E. coli strains carrying the fliCH19 gene, we detected an O121:H19 clone-specific signature in the nucleotide sequence of the fliCH19 gene present in E. coli O121:H19 strains of different origins and sources. We used this specific alteration in the fliCH19 gene of O121:H19 strains to develop a real-time PCR suitable for the specific detection of these strains. The O121 fliCH19 PCR can be used in combination with an O121 wzx gene-specific real-time PCR for rapid and clear identification of EHEC O121:H19 and is therefore suitable to be included into the ISO/TS 13136 method (12).
Interestingly, we could identify three O121:H19 strains that were devoid of stx genes (Table 3). It has previously been shown that stx-negative E. coli O121:H19 strains maintain EHEC-associated effector genes located on genomic islands on the chromosome (42, 43). In a clinical study, loss of the stx gene(s) was observed in EHEC strains isolated from about 5% of HUS patients in the course of disease, and it was suggested that markers other than stx have to be employed for detection of these strains from the patients for adequate clinical management (24). Here, we showed that the detection of O121 fliCH19 and O121 wzx is suitable for identification of EHEC O121:H19 and variants of these strains lacking stx genes. We could clearly discern these from other E. coli O121 STEC and non-STEC strains, and we were also able to identify serologically O-rough strains, which cannot be identified by conventional serotyping, as EHEC O121:H19.
The generic fliCH19 PCR assay described previously (6) gave no cross-reaction with the 53 E. coli H types described so far, but a clear cross-reaction between the O121 fliCH19 real-time PCR described here and E. coli fliCH32 sequences was observed. This could lead to the misidentification of an O121:H32 strain as O121:H19. However, to our knowledge, E. coli O121:H32 strains have not yet been described. A positive PCR result for O121 wzx and the O121 fliCH19 genes should therefore most probably indicate the identification of EHEC O121:H19 strains. As far as we know, the sequence diversity of fliCH19 alleles has not been previously studied in E. coli. The fliCH19 alleles described in this work contribute to a better characterization of the flagellar antigens of E. coli. The O121 fliCH19 PCR assay improves the specificity of the diagnostic systems available for detection of EHEC O121:H19 strains.
Supplementary Material
ACKNOWLEDGMENTS
We are grateful to Sarah Ann Ison from Texas Tech University (Lubbock, TX, USA) for proofreading.
The project was partially financed by the French Joint Ministerial Program of R&D against CBRNE Risks (grant number C17609-2).
Footnotes
Supplemental material for this article may be found at http://dx.doi.org/10.1128/AEM.00591-15.
REFERENCES
- 1.Franz E, Delaquis P, Morabito S, Beutin L, Gobius K, Rasko DA, Bono J, French N, Osek J, Lindstedt BA, Muniesa M, Manning S, LeJeune J, Callaway T, Beatson S, Eppinger M, Dallman T, Forbes KJ, Aarts H, Pearl DL, Gannon VP, Laing CR, Strachan NJ. 2014. Exploiting the explosion of information associated with whole genome sequencing to tackle Shiga toxin-producing Escherichia coli (STEC) in global food production systems. Int J Food Microbiol 187:57–72. doi: 10.1016/j.ijfoodmicro.2014.07.002. [DOI] [PubMed] [Google Scholar]
- 2.Karmali MA, Gannon V, Sargeant JM. 2010. Verocytotoxin-producing Escherichia coli (VTEC). Vet Microbiol 140:360–370. doi: 10.1016/j.vetmic.2009.04.011. [DOI] [PubMed] [Google Scholar]
- 3.Beutin L, Fach P. 2014. Detection of Shiga toxin-producing Escherichia coli from nonhuman sources and strain typing. Microbiol Spectr 2(3):EHEC-0001-2013. doi: 10.1128/microbiolspec.EHEC-0001-2013. [DOI] [PubMed] [Google Scholar]
- 4.Kaper JB, Nataro JP, Mobley HL. 2004. Pathogenic Escherichia coli. Nat Rev Microbiol 2:123–140. doi: 10.1038/nrmicro818. [DOI] [PubMed] [Google Scholar]
- 5.Scheutz F. 2014. Taxonomy meets public health: the case of Shiga toxin-producing Escherichia coli. Microbiol Spectr 2(3):EHEC-0019-2013. doi: 10.1128/microbiolspec.EHEC-0019-2013. [DOI] [PubMed] [Google Scholar]
- 6.Bugarel M, Beutin L, Martin A, Gill A, Fach P. 2010. Micro-array for the identification of Shiga toxin-producing Escherichia coli (STEC) seropathotypes associated with hemorrhagic colitis and hemolytic uremic syndrome in humans. Int J Food Microbiol 142:318–329. doi: 10.1016/j.ijfoodmicro.2010.07.010. [DOI] [PubMed] [Google Scholar]
- 7.Beutin L, Martin A. 2012. Outbreak of Shiga toxin-producing Escherichia coli (STEC) O104:H4 infection in Germany causes a paradigm shift with regard to human pathogenicity of STEC strains. J Food Prot 75:408–418. doi: 10.4315/0362-028X.JFP-11-452. [DOI] [PubMed] [Google Scholar]
- 8.Duffy G, Burgess CM, Bolton DJ. 2014. A review of factors that affect transmission and survival of verocytotoxigenic Escherichia coli in the European farm to fork beef chain. Meat Sci 97:375–383. doi: 10.1016/j.meatsci.2014.01.009. [DOI] [PubMed] [Google Scholar]
- 9.Martin A, Beutin L. 2011. Characteristics of Shiga toxin-producing Escherichia coli from meat and milk products of different origins and association with food producing animals as main contamination sources. Int J Food Microbiol 146:99–104. doi: 10.1016/j.ijfoodmicro.2011.01.041. [DOI] [PubMed] [Google Scholar]
- 10.Berger CN, Sodha SV, Shaw RK, Griffin PM, Pink D, Hand P, Frankel G. 2010. Fresh fruit and vegetables as vehicles for the transmission of human pathogens. Environ Microbiol 12:2385–2397. doi: 10.1111/j.1462-2920.2010.02297.x. [DOI] [PubMed] [Google Scholar]
- 11.Werber D, Krause G, Frank C, Fruth A, Flieger A, Mielke M, Schaade L, Stark K. 2012. Outbreaks of virulent diarrheagenic Escherichia coli: are we in control? BMC Medicine 10:11. doi: 10.1186/1741-7015-10-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.International Organization for Standardization. 2012. Microbiology of food and animal feed—real-time polymerase chain reaction (PCR)-based method for the detection of food-borne pathogens—horizontal method for the detection of Shiga toxin-producing Escherichia coli (STEC) and the determination of O157, O111, O26, O103 and O145 serogroups. ISO/TS 13136:2012. International Organization for Standardization, Geneva, Switzerland. [Google Scholar]
- 13.USDA Food Safety and Inspection Service. 2011. Detection and identification of non-O157 Shiga-toxin producing Escherichia coli strains (STEC) from meat products. USDA/FSIS document MLG 5B.01. U.S. Department of Agriculture, Washington, DC. [Google Scholar]
- 14.European Centre for Disease Prevention and Control, European Food Safety Authority. 2011. Shiga toxin/verotoxin-producing Escherichia coli in humans, food and animals in the EU/EEA, with special reference to the German outbreak strain STEC O104. European Centre for Disease Prevention and Control, Stockholm, Sweden. [Google Scholar]
- 15.Scheutz F, Moller NE, Frimodt-Moller J, Boisen N, Morabito S, Tozzoli R, Nataro J, Caprioli A. 2011. Characteristics of the enteroaggregative Shiga toxin/verotoxin-producing Escherichia coli O104:H4 strain causing the outbreak of haemolytic uraemic syndrome in Germany, May to June 2011. Euro Surveill 16(24):pii=19889. http://www.eurosurveillance.org/ViewArticle.aspx?ArticleId=19889. [DOI] [PubMed]
- 16.European Food Safety Authority. 2007. Monitoring of verotoxigenic Escherichia coli (VTEC) and identification of human pathogenic VTEC types. Scientific opinion of the Panel on Biological Hazards. EFSA J 579:1–61. doi: 10.2903/j.efsa.2007.579. [DOI] [Google Scholar]
- 17.Couturier MR, Lee B, Zelyas N, Chui L. 2011. Shiga-toxigenic Escherichia coli detection in stool samples screened for viral gastroenteritis in Alberta, Canada. J Clin Microbiol 49:574–578. doi: 10.1128/JCM.01693-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Meichtri L, Miliwebsky E, Gioffré A, Chinen I, Baschkier A, Chillemi G, Guth BEC, Masana MO, Cataldi A, Rodriguez HR, Rivas M. 2004. Shiga toxin-producing Escherichia coli in healthy young beef steers from Argentina: prevalence and virulence properties. Int J Food Microbiol 96:189–198. doi: 10.1016/j.ijfoodmicro.2004.03.018. [DOI] [PubMed] [Google Scholar]
- 19.Rivero MA, Passucci JA, Rodriguez EM, Parma AE. 2010. Role and clinical course of verotoxigenic Escherichia coli infections in childhood acute diarrhoea in Argentina. J Med Microbiol 59:345–352. doi: 10.1099/jmm.0.015560-0. [DOI] [PubMed] [Google Scholar]
- 20.Tarr CL, Large TM, Moeller CL, Lacher DW, Tarr PI, Acheson DW, Whittam TS. 2002. Molecular characterization of a serotype O121:H19 clone, a distinct Shiga toxin-producing clone of pathogenic Escherichia coli. Infect Immun 70:6853–6859. doi: 10.1128/IAI.70.12.6853-6859.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Fukushima H, Seki R. 2004. High numbers of Shiga toxin-producing Escherichia coli found in bovine faeces collected at slaughter in Japan. FEMS Microbiol Lett 238:189–197. doi: 10.1111/j.1574-6968.2004.tb09755.x. [DOI] [PubMed] [Google Scholar]
- 22.Kikuchi K, Ueno H, Tomari K, Kobori S, Kaetsu A, Miyazaki M. 2014. Identification of resistance and susceptibility to cefotaxime in EHEC O121 strains isolated from an outbreak at two nurseries. Kansenshogaku Zasshi 88:430–437. (In Japanese.) [DOI] [PubMed] [Google Scholar]
- 23.Beutin L, Krause G, Zimmermman S, Kaulfuss S, Gleier K. 2004. Characterization of Shiga toxin-producing Escherichia coli strains isolated from human patients in Germany over a 3-year period. J Clin Microbiol 42:1099–1108. doi: 10.1128/JCM.42.3.1099-1108.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Bielaszewska M, Kock R, Friedrich AW, von Eiff C, Zimmerhackl LB, Karch H, Mellmann A. 2007. Shiga toxin-mediated hemolytic uremic syndrome: time to change the diagnostic paradigm? PLoS One 2:e1024. doi: 10.1371/journal.pone.0001024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Kappeli U, Hachler H, Giezendanner N, Beutin L, Stephan R. 2011. Human infections with non-O157 Shiga toxin-producing Escherichia coli, Switzerland, 2000–2009. Emerg Infect Dis 17:180–185. doi: 10.3201/eid1702.100909. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Mora A, Lopez C, Dhabi G, Lopez-Beceiro AM, Fidalgo LE, Diaz EA, Martinez-Carrasco C, Mamani R, Herrera A, Blanco JE, Blanco M, Blanco J. 2012. Seropathotypes, phylogroups, Stx subtypes, and intimin types of wildlife-carried, Shiga toxin-producing Escherichia coli strains with the same characteristics as human-pathogenic isolates. Appl Environ Microbiol 78:2578–2585. doi: 10.1128/AEM.07520-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Anklam KS, Kanankege KS, Gonzales TK, Kaspar CW, Dopfer D. 2012. Rapid and reliable detection of Shiga toxin-producing Escherichia coli by real-time multiplex PCR. J Food Prot 75:643–650. doi: 10.4315/0362-028X.JFP-11-392. [DOI] [PubMed] [Google Scholar]
- 28.Fratamico PM, Bagi LK, Cray WC, Narang N, Yan X, Medina M, Liu Y. 2011. Detection by multiplex real-time polymerase chain reaction assays and isolation of Shiga toxin-producing Escherichia coli serogroups O26, O45, O103, O111, O121, and O145 in ground beef. Foodborne Pathog Dis 8:601–607. doi: 10.1089/fpd.2010.0773. [DOI] [PubMed] [Google Scholar]
- 29.Lin A, Sultan O, Lau HK, Wong E, Hartman G, Lauzon CR. 2011. O serogroup specific real time PCR assays for the detection and identification of nine clinically relevant non-O157 STECs. Food Microbiol 28:478–483. doi: 10.1016/j.fm.2010.10.007. [DOI] [PubMed] [Google Scholar]
- 30.Bettelheim KA. 1978. The sources of “OH” serotypes of Escherichia coli. J Hyg (Lond) 80:83–113. doi: 10.1017/S0022172400053420. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Fratamico PM, Bagi LK, Bush EJ, Solow BT. 2004. Prevalence and characterization of Shiga toxin-producing Escherichia coli in swine feces recovered in the National Animal Health Monitoring System's Swine 2000 study. Appl Environ Microbiol 70:7173–7178. doi: 10.1128/AEM.70.12.7173-7178.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Orskov F, Orskov I. 1984. Serotyping of Escherichia coli, p 43–112. In Bergan T. (ed), Methods in microbiology, vol 14 Academic Press, London, United Kingdom. [Google Scholar]
- 33.Zweifel C, Blanco JE, Blanco M, Blanco J, Stephan R. 2004. Serotypes and virulence genes of ovine non-O157 Shiga toxin-producing Escherichia coli in Switzerland. Int J Food Microbiol 95:19–27. doi: 10.1016/j.ijfoodmicro.2004.01.015. [DOI] [PubMed] [Google Scholar]
- 34.Beutin L, Strauch E. 2007. Identification of sequence diversity in the Escherichia coli fliC genes encoding flagellar types H8 and H40 and its use in typing of Shiga toxin-producing E. coli O8, O22, O111, O174, and O179 strains. J Clin Microbiol 45:333–339. doi: 10.1128/JCM.01627-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Fields PI, Blom K, Hughes HJ, Helsel LO, Feng P, Swaminathan B. 1997. Molecular characterization of the gene encoding H antigen in Escherichia coli and development of a PCR-restriction fragment length polymorphism test for identification of E. coli O157:H7 and O157:NM. J Clin Microbiol 35:1066–1070. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Iguchi A, Iyoda S, Ohnishi M. 2012. Molecular characterization reveals three distinct clonal groups among clinical Shiga toxin-producing Escherichia coli strains of serogroup O103. J Clin Microbiol 50:2894–2900. doi: 10.1128/JCM.00789-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Sonntag AK, Prager R, Bielaszewska M, Zhang W, Fruth A, Tschape H, Karch H. 2004. Phenotypic and genotypic analyses of enterohemorrhagic Escherichia coli O145 strains from patients in Germany. J Clin Microbiol 42:954–962. doi: 10.1128/JCM.42.3.954-962.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Wang L, Rothemund D, Curd H, Reeves PR. 2003. Species-wide variation in the Escherichia coli flagellin (H-antigen) gene. J Bacteriol 185:2936–2943. doi: 10.1128/JB.185.9.2936-2943.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Reid SD, Selander RK, Whittam TS. 1999. Sequence diversity of flagellin (fliC) alleles in pathogenic Escherichia coli. J Bacteriol 181:153–160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Wang L, Rothemund D, Curd H, Reeves PR. 2000. Sequence diversity of the Escherichia coli H7 fliC genes: implication for a DNA-based typing scheme for E. coli O157:H7. J Clin Microbiol 38:1786–1790. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Beutin L, Jahn S, Fach P. 2009. Evaluation of the “GeneDisc” real-time PCR system for detection of enterohaemorrhagic Escherichia coli (EHEC) O26, O103, O111, O145 and O157 strains according to their virulence markers and their O- and H-antigen-associated genes. J Appl Microbiol 106:1122–1132. doi: 10.1111/j.1365-2672.2008.04076.x. [DOI] [PubMed] [Google Scholar]
- 42.Bugarel M, Beutin L, Fach P. 2010. Low-density macroarray targeting non-locus of enterocyte effacement effectors (nle genes) and major virulence factors of Shiga toxin-producing Escherichia coli (STEC): a new approach for molecular risk assessment of STEC isolates. Appl Environ Microbiol 76:203–211. doi: 10.1128/AEM.01921-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Bugarel M, Martin A, Fach P, Beutin L. 2011. Virulence gene profiling of enterohemorrhagic (EHEC) and enteropathogenic (EPEC) Escherichia coli strains: a basis for molecular risk assessment of typical and atypical EPEC strains. BMC Microbiol 11:142. doi: 10.1186/1471-2180-11-142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Delannoy S, Beutin L, Fach P. 2013. Towards a molecular definition of enterohemorrhagic Escherichia coli (EHEC): detection of genes located on O island 57 as markers to distinguish EHEC from closely related enteropathogenic E. coli strains. J Clin Microbiol 51:1083–1088. doi: 10.1128/JCM.02864-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Scheutz F, Cheasty T, Woodward D, Smith HR. 2004. Designation of O174 and O175 to temporary O groups OX3 and OX7, and six new E. coli O groups that include verocytotoxin-producing E. coli (VTEC): O176, O177, O178, O179, O180 and O181. APMIS 112:569–584. doi: 10.1111/j.1600-0463.2004.apm1120903.x. [DOI] [PubMed] [Google Scholar]
- 46.Machado J, Grimont F, Grimont PA. 2000. Identification of Escherichia coli flagellar types by restriction of the amplified fliC gene. Res Microbiol 151:535–546. doi: 10.1016/S0923-2508(00)00223-0. [DOI] [PubMed] [Google Scholar]
- 47.Touchon M, Hoede C, Tenaillon O, Barbe V, Baeriswyl S, Bidet P, Bingen E, Bonacorsi S, Bouchier C, Bouvet O, Calteau A, Chiapello H, Clermont O, Cruveiller S, Danchin A, Diard M, Dossat C, Karoui ME, Frapy E, Garry L, Ghigo JM, Gilles AM, Johnson J, Le BC, Lescat M, Mangenot S, Martinez-Jehanne V, Matic I, Nassif X, Oztas S, Petit MA, Pichon C, Rouy Z, Ruf CS, Schneider D, Tourret J, Vacherie B, Vallenet D, Medigue C, Rocha EP, Denamur E. 2009. Organised genome dynamics in the Escherichia coli species results in highly diverse adaptive paths. PLoS Genet 5:e1000344. doi: 10.1371/journal.pgen.1000344. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Tajima F, Nei M. 1984. Estimation of evolutionary distance between nucleotide sequences. Mol Biol Evol 1:269–285. [DOI] [PubMed] [Google Scholar]
- 49.Kappeli U, Hachler H, Giezendanner N, Cheasty T, Stephan R. 2011. Shiga toxin-producing Escherichia coli O157 associated with human infections in Switzerland, 2000–2009. Epidemiol Infect 139:1097–1104. doi: 10.1017/S0950268810002190. [DOI] [PubMed] [Google Scholar]
- 50.Miko A, Rivas M, Bentancor A, Delannoy S, Fach P, Beutin L. 2014. Emerging types of Shiga toxin-producing E. coli (STEC) O178 present in cattle, deer, and humans from Argentina and Germany. Front Cell Infect Microbiol 4:78. doi: 10.3389/fcimb.2014.00078. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Tzschoppe M, Martin A, Beutin L. 2012. A rapid procedure for the detection and isolation of enterohaemorrhagic Escherichia coli (EHEC) serogroup O26, O103, O111, O118, O121, O145 and O157 strains and the aggregative EHEC O104:H4 strain from ready-to-eat vegetables. Int J Food Microbiol 152:19–30. doi: 10.1016/j.ijfoodmicro.2011.10.009. [DOI] [PubMed] [Google Scholar]
- 52.Community Reference Laboratory for E. coli. 2007. Report of the 1st inter-laboratory study on verocytotoxin-producing E. coli (VTEC) identification and typing–2007. Istituto Superiore di Sanità, Rome, Italy: http://www.iss.it/binary/vtec/cont/Report_PT_2007.pdf. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.