Abstract
Lactococcus lactis is predominantly associated with dairy fermentations, but evidence suggests that the domesticated organism originated from a plant niche. L. lactis possesses an unusual taxonomic structure whereby strain phenotypes and genotypes often do not correlate, which in turn has led to confusion in L. lactis classification. A bank of L. lactis strains was isolated from various nondairy niches (grass, vegetables, and bovine rumen) and was further characterized on the basis of key technological traits, including growth in milk and key enzyme activities. Phenotypic analysis revealed all strains from nondairy sources to possess an L. lactis subsp. lactis phenotype (lactis phenotype); however, seven of these strains possessed an L. lactis subsp. cremoris genotype (cremoris genotype), determined by two separate PCR assays. Multilocus sequence typing (MLST) showed that strains with lactis and cremoris genotypes clustered together regardless of habitat, but it highlighted the increased diversity that exists among “wild” strains. Calculation of average nucleotide identity (ANI) and tetranucleotide frequency correlation coefficients (TETRA), using the JSpecies software tool, revealed that L. lactis subsp. cremoris and L. lactis subsp. lactis differ in ANI values by ∼14%, below the threshold set for species circumscription. Further analysis of strain TIFN3 and strains from nonindustrial backgrounds revealed TETRA values of <0.99 in addition to ANI values of <95%, implicating that these two groups are separate species. These findings suggest the requirement for a revision of L. lactis taxonomy.
INTRODUCTION
Lactococcus lactis is a member of the lactic acid bacteria (LAB), a group of organisms used worldwide in the production of fermented dairy products. Three Lactococcus lactis subspecies exist: Lactococcus lactis subsp. cremoris, L. lactis subsp. lactis, and L. lactis subsp. hordniae. Many strains of L. lactis subsp. lactis and L. lactis subsp. cremoris are typically associated with dairy fermentations, but evidence suggests that these organisms originated from a plant niche, and they are now considered to be “domesticated” compared to their so-called “wild” counterparts. Conversely, L. lactis subsp. hordniae is unable to utilize lactose and has not been isolated previously from the dairy environment (1). A citrate-metabolizing biovariety also exists, L. lactis biovar diacetylactis, which is capable of imparting buttery aromas in dairy fermentations (2). Information regarding subspecies classification is important for the application of cultures in dairy fermentations, particularly cheese production, as L. lactis subsp. cremoris strains are often considered more suitable due to their association with cheeses free from off-flavors (3).
Before the advent of molecular methods, subspecies classification of L. lactis was based on the possession of a number of phenotypic traits. The ability to grow in the presence of 4% NaCl, at 40°C, and at pH 9.2 and the ability to degrade arginine were assigned as traits for L. lactis subspecies (4), while L. lactis subsp. cremoris does not exhibit these characteristics. The ability to ferment maltose is also considered a phenotypic trait of L. lactis subsp. lactis (5). In more recent years, genotypic characterization has largely replaced phenotypic characterization in subspecies designation of new isolates. Genotypic characterization based on 16S rRNA is commonly used; however, the use of 16S rRNA sequencing alone can lead to discrepancies in subspecies identification (6). This may be due to L. lactis subsp. lactis and L. lactis subsp. cremoris exhibiting differences in the 16S rRNA gene of as little as 0.7% despite differing up to 15% at the whole-genome level (7). In some instances, the phenotype and genotype do not correlate: a strain with an L. lactis subsp. lactis genotype (lactis genotype) possesses L. lactis subsp. cremoris phenotype (cremoris phenotype) (8) and vice versa (9, 10). To attempt to accurately identify subspecies of L. lactis, various assays have been developed (11–13). These assays employ different molecular techniques such as PCR-restriction fragment length polymorphism (PCR-RFLP) and Southern blot hybridization, targeting genes encoding branched-chain amino acid biosynthesis and glutamate decarboxylase, among others (12, 14).
In particular, two such methods have been developed for rapid and simple species genotype identification by PCR, based on the 16S rRNA gene and the histidine biosynthesis operon (11, 13). The method designed by Pu et al. (13) targets a 19-bp region which differs by 5 bp between subspecies. Beimfohr et al. (11) exploited a 200-bp insertion in the hisZ gene to differentiate subspecies, with cremoris and lactis genotype strains forming amplicons of different sizes. With advances in sequencing technology and the development of genome analytical software tools, faster, more straightforward approaches for accurate species classification are emerging. One such software tool, JSpecies (15), can be used in defining species based on average nucleotide identity (ANI) and tetranucleotide frequency correlation coefficients (TETRA). What has become obvious upon analysis of the increasing array of L. lactis genomes from diverse sources is that L. lactis possesses an unusual taxonomy with different genotypes and phenotypes thereof. Indeed, in order to correct for the observed incongruence between phenotype and genotype, Kelly and Ward (16) proposed that genotype and phenotype should be stated when referring to the subspecies of a strain.
In this report, we describe the phenotypic and genotypic characterization of a bank of novel L. lactis isolates from nondairy environments and highlight the extensive phenotype-genotype disparity, or mismatching, at the subspecies level. Previous analysis of these strains has demonstrated their diverse metabolic capacity, forming volatile profiles in milk different from those of L. lactis strains from dairy sources, and their capacity to alter flavor in a Gouda-type cheese model (17).
Our analysis shows that genome-level comparison of these wild strains with long-domesticated dairy strains further complicates traditional classification, and we suggest that a revision of the current species classification of the L. lactis species is warranted. In addition to underlining the shortcomings in subspecies definition of L. lactis, this work also demonstrates the phenotypic and genotypic diversity that exists between L. lactis strains from different environmental niches and the potential of lactococci from nondairy sources to be used in dairy fermentations.
MATERIALS AND METHODS
Strains and growth conditions.
Dairy L. lactis strains were provided by the DPC culture collection (Teagasc Food Research Centre, Moorepark, Cork, Ireland). Lactococcus lactis strains were propagated in M17 medium (Difco) containing 0.5% (wt/vol) lactose monohydrate (VWR, Belgium) (LM17) at 30°C. L. lactis subsp. lactis IL1403 and L. lactis subsp. cremoris MG1363 and HP were propagated in M17 medium (Difco) containing 0.5% glucose (Sigma-Aldrich) (GM17).
Lactococcus isolation from nondairy sources.
Environmental samples used in this study for the isolation of novel lactococci were sourced at the Teagasc Animal and Grassland Research and Innovation Centre (Moorepark, Fermoy, Cork, Ireland). Grass samples were gathered from allotments assigned to animal grazing. Bovine rumen samples were collected from a cannulated cow fed on a grass-based diet, while vegetable samples (baby corn and fresh green peas) were collected from a local supermarket. Fresh sample material (10 g) was added to 100 ml of maximum recovery diluent (MRD) (Oxoid Ltd., England) and homogenized using a stomacher (Seward, United Kingdom). The resulting suspensions were diluted in MRD, plated on LM17 medium, and incubated for 48 h at 30°C. Individual colonies were tested for catalase activity (degradation of 3% hydrogen peroxide) and esculin hydrolysis (formation of black colonies) on kanamycin esculin azide agar (KAA) (Oxoid Ltd.). Individual isolates were subsequently tested for the ability to grow in milk. This was achieved by twice washing and resuspending an overnight culture in sterile water and adding a 1.5% inoculum to 10% reconstituted semiskimmed milk (RSM) (Kerry Foods, Ireland). Each sample was incubated at 30°C, and the pH was measured at 5 and 24 h.
Subspecies phenotype identification.
Lactococcus cultures were tested for the ability to grow at 40°C and in 4% (wt/vol) NaCl for 24 h in LM17 broth. Likewise, growth at 8 to 10°C, 45°C, and 6.5% (wt/vol) NaCl (Sigma) was examined over 2 days. Arginine utilization was assessed using the medium described by Beimfohr et al. (11), with the addition of bromocresol purple (0.001% [wt/vol]) in place of phenol red. Acetoin (acetylmethylcarbinol) production from glucose was determined using the Voges-Proskauer test (Dalynn Biologicals) over a period of 72 h. Citrate fermentation was assessed using Kempler and McKay agar (2) under aerobic conditions for up to 5 days at 30°C. L. lactis subsp. lactis IL1403, L. lactis subsp. lactis biovar diacetylactis DRC3, and L. lactis subsp. cremoris SK11 and MG1363 were used as reference strains for the particular subspecies for all phenotypic assays. Each phenotypic assay was performed in duplicate.
Identification of Lactococcus species and subspecies genotypes.
Genomic DNA was extracted from overnight cultures using the GenElute bacterial genomic DNA kit (Sigma). Using primers employed by Alander et al. (18), 16S rRNA analysis was initially performed to identify putative lactococci (Table 1). Genotypic subspecies designation of Lactococcus strains was determined using two separate PCRs targeting the 16S rRNA gene and the histidine biosynthesis operon (Table 1) (11, 13). PCRs were carried out in 50-μl reaction mixtures using the Platinum hi-fidelity PCR Supermix (Invitrogen) containing 50 ng of DNA per reaction mixture. PCR amplicons for 16S rRNA analysis were purified using the High Pure PCR clean-up microkit (Roche Diagnostics, Germany), with sequence analysis performed by GATC Biotech (Cologne, Germany). Sequences were aligned and trimmed using the MegAlign software program (DNAstar Lasergene, Madison, WI), and a consensus sequence was compared to those available in the NCBI BLASTn database.
TABLE 1.
Oligonucleotide primer sequences used in PCRs for species and subspecies identification and MLST
| Primer purpose and name | Sequence | Target region | Reference |
|---|---|---|---|
| Species identification | |||
| 16S_Fa | 5′-AGAGTTTGATCCTGGCTCAGG-3′ | 16S rRNA | 18 |
| 16S_R | 5′-ACGGCAACCTTGTTACGAGTT-3′ | 16S rRNA | |
| Subspecies identification | |||
| LacF | 5′-GTACTTGTACCGACTGGAT-3′ | 16S rRNA | 13 |
| CreF | 5′-GTGCTTGCACCGATTTGAA-3′ | 16S rRNA | |
| LacreR | 5′-GGGATCATCTTTGAGTGAT-3′ | 16S rRNA | |
| Lhis5F | 5′-CTTCGTTATGATTTTACA-3′ | Histidine operon | 11 |
| Lhis6R | 5′-AATATCAACAATTCCATG-3′ | Histidine operon | |
| MLSTb | |||
| rpoA_F | 5′-ATGATYGARTTTGAAAAACC-3′ | RNA polymerase | 5 |
| rpoA_R | 5′-ACHGTRTTRATDCCDGCRCG-3′ | RNA polymerase | |
| atpA_F | 5′-TAYRTYGGKGAYGGDATYGC-3′ | ATP synthase | |
| atpA_R | 5′- CCRCGRTTHARYTTHGCYTG-3′ | ATP synthase | |
| bcaT_F | 5′-TTTKSHRTGCCDGTWGG-3′ | BCAAc aminotransferase | |
| bcaT_R | 5′-GGWCCHACTTCYGTYTC-3′ | BCAA aminotransferase | |
| pepN_F | 5′-ATKTCTTAYGCWGAYRTYGT-3′ | Aminopeptidase N | |
| pepN_R | 5′-TTKCTTCAAGSMAWGSCC-3′ | Aminopeptidase N | |
| pepX_F | 5′-TTTGGGTTGAAAGTCCAGT-3′ | X-prolyl peptidase | |
| pepX_R | 5′-CCAAGAAGAAATTCCAGC-3′ | X-prolyl peptidase | |
| pheS_F | 5′-CAYCCNGCHCGYGAYATGC-3′ | Phe tRNA synthetase | |
| pheS_R | 5′-CCWARVCCRAARGCAAARCC-3′ | Phe tRNA synthetase | |
| SSU rRNA_F | 5′-GCGGCGTGCCTAATACATGC-3′ | 16S rRNA | |
| SSU rRNA_R | 5′-ATCTACGCATTTCACCGCTAC-3′ | 16S rRNA | |
F, forward; R, reverse.
R represents A or G, Y represents C or T, M represents A or C, K represents G or T, S represents C or G, W represents A or T, H represents A, C, or T, B represents C, G, or T, V represents A, C, or G, D represents A, G, or T, and N represents A, C, G, or T.
BCAA, branched-chain amino acid.
MLST.
PCR primers were used to amplify partial sequences of seven genes of L. lactis for the purpose of multilocus sequence typing (MLST) (Table 1). The genes selected include three housekeeping genes—those for the ATP synthase alpha subunit (atpA), the phenylalanine tRNA synthase alpha subunit (pheS), and the RNA polymerase alpha subunit (rpoA)—and three genes involved in flavor formation during fermentation— those for the lysl aminopeptidase N (pepN), the post-proline dipeptidyl aminopeptidase (pepX), and the branched-chain amino acid transferase (bcaT). A small subunit (SSU) of the 16S rRNA region was also included in the analysis. DNA was extracted using the GenElute bacterial genomic DNA kit (Sigma-Aldrich) according to the manufacturer's instructions for Gram-positive bacteria. PCR amplicons were generated using Platinum hi-fidelity PCR Supermix (Invitrogen) and sequenced in forward and reverse by GATC Biotech (Constance, Germany). Sequences were assembled and a consensus sequence was generated using the Lasergene software package and the Seqman assembly program (DNAstar Inc., Madison, WI). Sequences were aligned and trimmed using the BioEdit biological sequence alignment editor (available at http://www.mbio.ncsu.edu/bioedit/bioedit.html) and entered into the PubMLST nonredundant database (NRDB) in order to identify similar genotypes over a batch of sequences (19). Subsequently, a data set table was created in which the sequence type (ST) was identified and uploaded to the sequence type analysis and recombinational tests program (START2) (20). Sequence analysis was performed with MEGA version 4 using the neighbor-joining method with 1,000 bootstrap replications (21).
Genome sequencing, assembly, and annotation.
Genomic libraries were prepared using the rapid library preparation method recommended by Roche Diagnostics Ltd. (West Sussex, United Kingdom). Emulsion PCR and 454 sequencing were performed in the Teagasc Sequencing Centre (Moorepark, Fermoy, Cork, Ireland) on a 454 FLX sequencer using standard protocols from the manufacturer (Roche Diagnostics Ltd.). The single-end reads were assembled using the SeqMan NGen application of the DNAStar Lasergene Genomics Suite (DNASTAR Inc.), with the software specified to trim any poor-quality read sequences which fall below an average quality score of 20 over a window of 5 bp. Contigs of less than 1,000 nucleotides were removed from analysis. Draft assemblies were annotated using an updated version of the GAMOLA software package (22). Briefly, individual contigs were concatenated with a nonbleeding spacer sequence and open reading frames (ORFs) were predicted using Prodigal (23) and RAST (24). Each ORF was then subjected to BLASTP (standalone BLAST [25]), nonredundant NCBI database), COG (updated 2008 COG database release), Pfam (HMMER3 [26], database release 27), and TIGRfam (database release 14.0) analyses to predict biological function. Sequencing data are shown in Table S1 in the supplemental material.
ANI and TETRA analysis.
Average nucleotide identity (ANI) analysis was performed with the BLAST algorithm (ANIb) using the JSpecies software program (15). In tandem with ANI, this program also allowed for the calculation of tetranucleotide frequency correlation coefficients (TETRA).
Assessment of lactose utilization.
Milk acidification kinetics were assessed over a 17-h period for individual cultures in real time using the iCinac pH monitoring system (Alliance Instruments, France), with readings taken every minute. Lactococcus cultures were washed twice in sterile MRD, added to 10% (wt/vol) RSM (Kerry Foods) to generate a 1.5% inoculum, and incubated at 30°C. Pearce test analysis was performed as described previously (27).
Amino acid transferase activity (methionine and phenylalanine).
The amino acid transferase activities of dairy and nondairy strains were determined for the sulfur-containing amino acid methionine and the aromatic amino acid phenylalanine. Both reaction mixtures contained 50 μM pyridoxal phosphate, 5 mM α-ketoglutaric acid, 0.5 mM sodium arsenate, and 50 mM sodium tetraborate buffer (pH 8.5) with either 5 mM l-phenylalanine or methionine. Standards were prepared at concentrations of 100, 200, 300, 400, 500, and 1,000 mM for phenylpyruvate and α-ketomethylthiobutyrate. For the blank preparation, 1 ml of reaction mixture was mixed with 100 μl of distilled water and incubated at 30°C for 30 min. One milliliter of trichloroacetic acid (TCA) was added and the solution centrifuged at 12,000 × g for 2 min. Analysis was performed using a Cary 100 Bio UV-visible (UV-Vis) spectrophotometer (Varian, Netherlands) with the wavelength set to 300 nm. Test mixtures were analyzed as per the blank with the addition of 100 μl of bacterial culture grown to pH 5.7 and read against the standard curve. The instrument was zeroed prior to each measurement, and each sample was assayed in triplicate. Amino acid transferase activity was expressed as micromoles per minute per milligram of protein.
Antibiotic resistance and biogenic amine formation.
MICs for antibiotics were evaluated using the VetMIC system (National Veterinary Institute of Sweden, Uppsala, Sweden) for LAB. Each microtiter plate contained 2-fold serial dilutions of 16 antibiotics. Following growth in LAB susceptibility test broth, each culture was resuspended in MRD as per the manufacturer's instructions to create the desired cell density (∼3 × 105 CFU/ml). One hundred microliters of cell suspension was added to each well on the microtiter plate and incubated for 48 h at 28°C. The lowest antibiotic concentration at which no growth occurred was defined as the MIC for each antibiotic. Antibiotic resistance was examined in duplicate.
The presence of histidine and tyrosine decarboxylase genes was determined by PCR using the primer sets outlined by Coton and Coton (28) and Coton et al. (29). Lactobacillus brevis DPC6660 and Lactobacillus buchneri DPC6666 were used as tyrosine and histamine decarboxylase positive controls, respectively, with Lactococcus lactis subsp. lactis DRC3 used as a negative control.
BioSample record numbers.
The complete nucleotide sequences of L. lactis genomes have been submitted to the GenBank database and can be found under BioSample record numbers SAMN03396773, SAMN03396924, and SAMN03396976 for strains DPC6853, DPC6856, and DPC6860, respectively.
Accession numbers.
The whole-genome shotgun projects have been deposited at DDBJ/EMBL/GenBank under the accession numbers LAVD00000000 (DPC6853), LAVW00000000 (DPC6856), and LAVX00000000 (DPC6860). The versions described in this paper are versions LAVD01000000 (DPC6853), LAVW01000000 (DPC6856), and LAVX01000000 (DPC6860).
RESULTS
Species confirmation of nondairy isolates.
A bank of novel nondairy strains isolated in the course of this study from grass, vegetables, and the bovine rumen was further characterized. Individual colonies were first examined for catalase activity (to eliminate catalase-positive organisms) and esculin hydrolysis on KAA (to eliminate Enterococcus species). Isolates which were negative for both traits were examined by Gram reaction and for growth in milk. Gram-positive isolates capable of growth in milk were subsequently identified by 16S rRNA analysis. Using the primer set described by Alander et al. (18), a 1.5-kb amplicon was generated for all nondairy strains targeting the 16S rRNA gene. Subsequent analysis of the sequences confirmed a number of isolates as Lactococcus lactis. All novel L. lactis strains were deposited in the DPC culture collection, Teagasc Food Research Centre Moorepark, County Cork, Ireland.
Subspecies phenotype identification.
Subspecies lactis phenotypes are identified by the ability to grow in 4% NaCl and at 40°C and are capable of degrading arginine. In addition, the biovariant diacetylactis has the ability to ferment citrate to produce diacetyl. Subspecies cremoris phenotypes are negative for each of these attributes. To establish subspecies phenotypes of the isolated nondairy lactococci, each isolate was examined for the aforementioned characteristics (Table 2) and compared to a bank of well-characterized dairy lactococci which are commonly used in the dairy industry. All of the nondairy lactococci isolated in this study were able to grow at 40°C and in medium containing 4% NaCl. Seven of the eight nondairy lactococcal strains were able to hydrolyze arginine. Strain DPC6855 was unable to hydrolyze arginine but could grow in 4% NaCl and at 40°C, and it was therefore assigned as subspecies lactis. Only the dairy strain DRC3 was capable of fermenting citrate, which is the hallmark of biovariant diacetylactis strains. Based on these results, all nondairy lactococci were assigned lactis phenotypes.
TABLE 2.
Phenotypic characterization of dairy and nondairy lactococcal strains
| Source | Genotype | Phenotype | Strain | Growth at: |
Arginine hydrolysis | Fermentation of: |
|||
|---|---|---|---|---|---|---|---|---|---|
| 40°Ca | 4% NaCla | Maltose | Lactose | Citrate | |||||
| Nondairy | |||||||||
| Corn | lactis | lactis | DPC6853 | + | + | + | + | + | − |
| Grass | cremoris | lactis | DPC6857 | + | + | + | + | + | − |
| Grass | cremoris | lactis | DPC6858 | + | + | + | + | + | − |
| Grass | cremoris | lactis | DPC6855 | + | + | − | + | + | − |
| Grass | cremoris | lactis | DPC6859 | + | + | + | + | + | − |
| Grass | cremoris | lactis | DPC6860 | + | + | + | + | + | − |
| Bovine rumen | cremoris | lactis | DPC6856 | + | + | + | + | + | − |
| Grass | cremoris | lactis | DPC6854 | + | + | + | + | + | − |
| Dairy | |||||||||
| Cheese | lactis | lactis | DRC3 | + | + | + | + | + | + |
| Starter culture | lactis | lactis | 303 | + | + | + | + | + | − |
| Cheese | lactis | lactis | ML8 | + | + | + | − | + | − |
| Cheese | lactis | lactis | 229 | + | + | + | + | + | − |
| Raw milk | cremoris | cremoris | H88M1 | + | − | − | + | + | − |
| Cheese | cremoris | cremoris | AM1 | − | − | − | − | + | − |
| Cheese | cremoris | cremoris | 310 | − | − | − | − | + | − |
| Cheese | cremoris | cremoris | HP | − | − | − | − | + | − |
| Cheese | cremoris | lactis | SK1G | + | + | − | + | + | − |
| Comparative strains | |||||||||
| Cheese | cremoris | cremoris | SK11 | − | − | − | − | + | − |
| Cheese | lactis | lactis | IL1403 | + | + | + | + | − | − |
Growth at 40°C and in 4% NaCl was monitored for 48 h to determine subspecies phenotype.
Subspecies genotype identification.
The nondairy L. lactis isolates were analyzed at the genotypic level to accurately identify subspecies and were again compared to the bank of dairy lactococci. Two separate PCR-based assays were performed in order to identify the subspecies genotype of each strain. In L. lactis the 16S rRNA gene exhibits a 0.07% variance between subspecies depending on the strain (30). Primers designed by Pu et al. (13) were employed to discriminate between lactis and cremoris genotypes on the basis of this difference. The primers sets comprise a common reverse primer, LacreR, and a subspecies-specific forward primer, LacF, for L. lactis subsp. lactis and CreF for L. lactis subsp. cremoris to generate a 163-bp amplicon. In all cases, amplicons were generated with one or other of the subspecies-specific forward primers but not both (Table 3). To further distinguish between L. lactis subsp. lactis and L. lactis subsp. cremoris, Beimfohr et al. (11) designed a single primer set to amplify specific regions of the histidine biosynthesis operon. Between lactis and cremoris genotypes, the average sequence divergence from positions 480 to 700 within the histidine biosynthesis operon equates to 45%. In addition, L. lactis subsp. cremoris possesses a 200-bp insertion at the end of hisZ gene, within this operon, which encodes a phosphoribosyltransferase regulatory subunit (11). Using the primer set designed by Beimfohr et al. (11), amplification of L. lactis subsp. cremoris results in a 1,149-bp product and that of L. lactis subsp. lactis results in a 934-bp product (Table 3). Subspecies genotypes determined using both assays correlated with one another, verifying the genotype of the strains. In all, seven cremoris genotype, lactis phenotype strains were isolated from grass and rumen samples and one lactis genotype, lactis phenotype strain was isolated from corn (Table 2).
TABLE 3.
Differentiation of L. lactis subspecies by PCRa
| Target region | 16S rRNA geneb |
Histidine operon amplicon sizec (bp) | |
|---|---|---|---|
| LacF | CreF | ||
| L. lactis DPC6853 | + | − | 934 |
| L. lactis DPC6855* | − | + | 1,149 |
| L. lactis DPC6860* | − | + | 1,149 |
| L. lactis DPC6859* | − | + | 1,149 |
| L. lactis DPC6854* | − | + | 1,149 |
| L. lactis DPC6856* | − | + | 1,149 |
| L. lactis DPC6858* | − | + | 1,149 |
| L. lactis DPC6857* | − | + | 1,149 |
| L. lactis ML8 | + | − | 934 |
| L. lactis DRC3 | + | − | 934 |
| L. lactis 303 | + | − | 934 |
| L. lactis 229 | + | − | 934 |
| L. lactis AM1* | − | + | 1,149 |
| L. lactis H88M1* | − | + | 1,149 |
| L. lactis 310* | − | + | 1,149 |
| L. lactis SK1G* | − | + | 1,149 |
PCR was performed using the primers designed by Pu et al. (13) and Beimfohr et al. (11) targeting the 16S rRNA gene and the histidine biosynthesis operon respectively. Asterisks indicate cremoris genotype strains.
A plus sign indicates the formation of an amplicon; a minus sign indicates the absence of formation of an amplicon.
L. lactis subsp. cremoris generates a 1,149-bp product; L. lactis subsp. lactis generates a 934-bp product.
Genetic diversity of L. lactis strains from different environments.
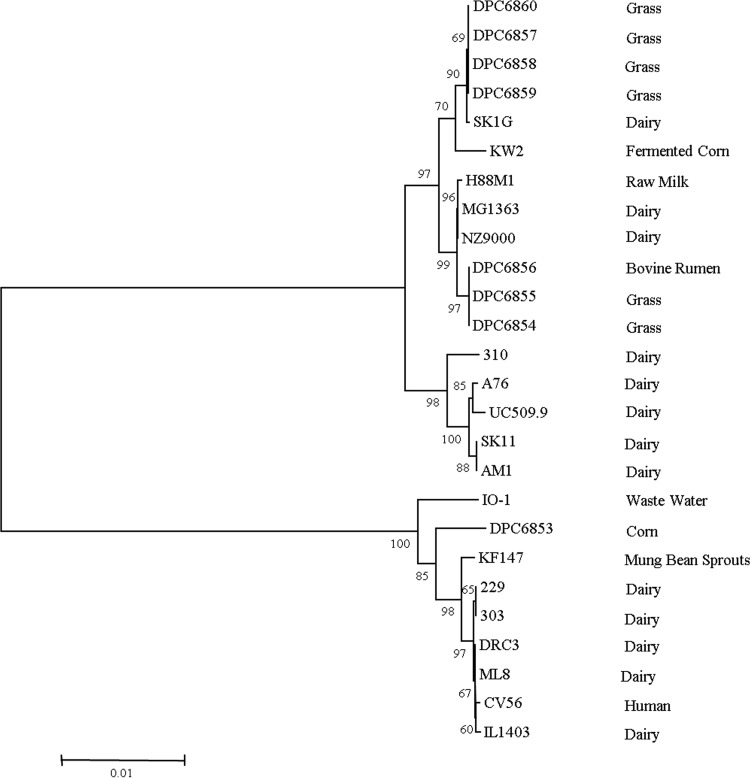
MLST is a comprehensive technique compared to other typing methods, as it provides information on aspects of the population organization and evolution of the species in question (31, 32). Again, dairy (eight) and nondairy (eight) strains were examined for their genetic diversity using comparative sequence analysis of seven genes, including the 16S rRNA gene. The primer sets used in MLST analysis (Table 1) do not generate products for Lactococcus lactis subsp. hordniae or other Lactococcus species (5). Amplicons were generated for segments of each of these genes for all 16 Lactococcus strains tested and sequenced in forward and reverse orientation, and a consensus sequence was generated. Sequence data for each of the seven housekeeping genes were also extracted from the complete genome sequences of L. lactis strains from different habitats available on the NCBI database. Sequence types (STs) were created for all strains using the nonredundant database program (19). For the nondairy lactococci isolated in this study, strains grouped into four STs as follows: (i) DPC6853, (ii) DPC6854 and DPC6855, (iii) DPC6856, and (iv) DPC6857, DPC6858, DPC6859, and DPC6860. MLST analysis, including our nondairy bank and sequenced L. lactis genomes from the NCBI database, identified 20 different STs (8 lactis genotype and 12 cremoris genotype). The frequency of polymorphic sites ranged from 2 in the 16S rRNA gene to 85 in the pepX gene (Table 4). The sequence divergences at these sites were identified as not resulting in amino acid changes, as determined by the low ratio of nonsynonymous to synonymous evolutionary changes (dN/dS ratio) identified for all alleles. Collective analysis of all seven loci (Fig. 1), and each of the genes individually (see Fig. S1 in the supplemental material), showed the presence of two separate clusters corresponding to cremoris and lactis genotype strains, which corresponds to the findings of other studies using the same scheme (Fig. 1) (5, 33, 34). L. lactis NZ9000 is a derivative of L. lactis MG1363, and the two strains possessed the same ST and consistently grouped with each other throughout the analysis, thereby confirming the comparative analysis of the gene sequences (35). Overall, results of our MLST analysis clearly show strains of L. lactis subsp. lactis and L. lactis subsp. cremoris grouping separately, irrespective of the environment from which they were isolated.
TABLE 4.
Genetic diversity of 26 L. lactis strains at seven loci
| Locus | Length (bp) |
% GC content | No. of polymorphic sites | dN/dS ratio | No. of unique alleles | ||
|---|---|---|---|---|---|---|---|
| Gene | Amplicon | Sequence analyzed | |||||
| atpA | 1,503 | 1,141 | 393 | 41.52 | 32 | 0.008 | 10 |
| bcaT | 1,047 | 493 | 320 | 39.47 | 45 | 0.029 | 8 |
| pepN | 1,023 | 482 | 491 | 33.53 | 52 | 0.255 | 8 |
| pepX | 2,269 | 602 | 401 | 39.35 | 85 | 0.076 | 9 |
| rpoA | 939 | 721 | 531 | 40.19 | 13 | 0.092 | 12 |
| pheS | 2,533 | 618 | 361 | 41.51 | 36 | 0.010 | 9 |
| 16S rRNA | 1,548 | 1,465 | 531 | 49.30 | 2 | –a | 5 |
–, not determined.
FIG 1.
Neighbor-joining cluster analysis of a composite data set for seven loci of dairy and nondairy Lactococcus strains. Phylogenetic analysis was carried out with 1,000 bootstrap replications; bootstrap percentages of ≥50 shown. The origin of each strain is indicated on the right side.
Genome overview of nondairy strains.
Draft genome sequences were generated for three nondairy L. lactis strains isolated from grass (DPC6860), corn (DPC6853), and the bovine rumen (DPC6856) with >32× coverage. Following quality control and assembly, multiple contigs were generated for all DPC strains (see the supplemental material). The estimated G+C contents of strains DPC6853, DPC6856, and DPC6860 were approximately 35%, 35.3%, and 35.6%, respectively, comparable to those of previously sequenced L. lactis strains (34.8% to 36.7%). Of the three DPC strains sequenced, the rumen isolate appeared to possess the largest estimated genome size (DPC6853, 2.5 Mb; DPC6856, 2.9 Mb; and DPC6860, 2.62 Mb) in addition to the largest plasmid complement (data not shown). It is unlikely that the plasmid DNA contributes to the entire 400-kb size difference between DPC6860 and DPC6853 but may play an important role in niche adaptation of this organism.
ANI: revision of Lactococcus subspecies as separate species.
To further examine the genetic diversity of the nondairy lactococcal bank, ANI was calculated for L. lactis strains DPC6853 (corn), DPC6856 (bovine rumen), and DPC6860 (grass) using draft genome sequences and compared to ANI for L. lactis genomes available on the NCBI database. ANI is an alternative to DNA-DNA hybridization, using genome sequences as a means of defining a species (15). Table 5 shows ANIb (ANI calculated using the BLAST algorithm) values for L. lactis strains available on the NCBI database plus strains DPC6856, DPC6853, and DPC6860 isolated in this study, calculated using the JSpecies software tool (15). First, as expected, derivatives of the same strain share a 100% ANIb, as in the case of L. lactis subsp. cremoris NZ9000 and L. lactis subsp. cremoris MG1363 (35). Second, all strains belonging to the same subspecies possessed an ANIb value between 96.53 and 99.96%. In contrast, strains reported as different subspecies shared ANIb values of 85.54 to 87.45%, below the cutoff for species circumscription (<95%). Based on ANIb values alone, we propose that strains with the lactis and cremoris genotypes be reclassified as different species.
TABLE 5.
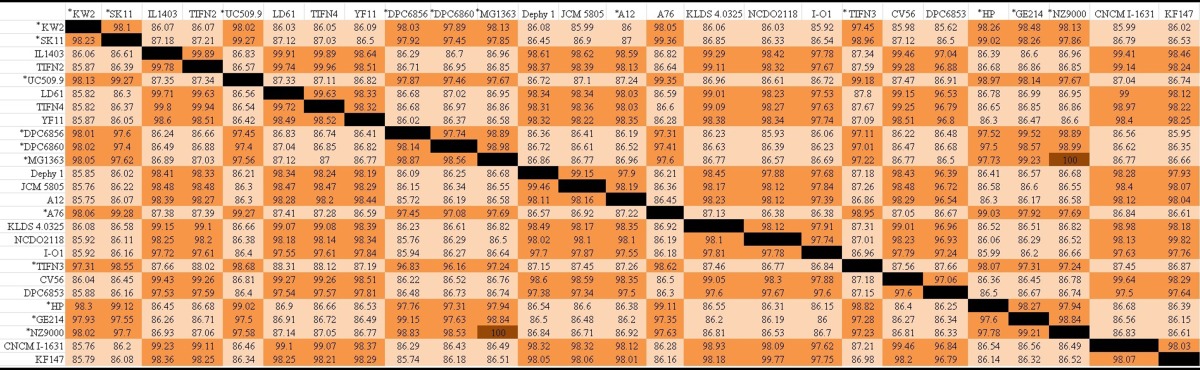
L. lactis subsp. cremoris strains are identified by asterisks. Values are percentages. Colored boxes, from brown to beige, highlight the following values or ranges: 100%, 95 to 99%, 90 to 94%, and 85 to 89%.
In conjunction with an ANI of <95%, a tetranucleotide frequency correlation coefficient (TETRA) of <0.99 is also used in tandem with ANI for species circumscription (15). Compared to L. lactis subsp. cremoris strain TIFN3, used in cheese production, the nondairy strains DPC6860 and DPC6856 (genotype cremoris, phenotype lactis) possessed lower ANIb values, 96.16% and 96.83%, respectively, than other strains designated L. lactis subsp. cremoris (∼97%). Similarly, strain DPC6853 possessed a lower ANIb value, 96.39 to 97.24%, in comparison to other L. lactis subsp. lactis strains. This suggests an increased genetic diversity among strains isolated in this study in comparison to that of previously sequenced strains of the same genotype. In addition, these data also highlight a reduced genetic diversity among dairy starter strains as identified previously (36).
For the vast majority of comparisons between subspecies, a below-threshold ANIb value was coupled with a TETRA value of >0.99, which classifies them as the same species (Table 6). The dairy strain L. lactis subsp. cremoris TIFN3 possessed ANIb and TETRA values below the threshold for species circumscription compared to strain DPC6853. Furthermore, compared to those of wild L. lactis subsp. lactis strains and strain IL1403, similar ANIb and TETRA values were also observed, which were not found between strain TIFN3 and dairy L. lactis subsp. lactis strains. The phenotypes of some of these wild strains are not defined in reports; however, it is tempting to speculate that all of these strains possess lactis phenotypes owing to the environment from which they were isolated. These findings suggest that L. lactis TIFN3 is a species separate from wild L. lactis subsp. lactis strains and IL1403, which may possess lactis phenotypes. The genome sequence status of TIFN3 is insufficient to support this reclassification; however; future sequence analysis of the TIFN3 genome may provide evidence to support these findings.
TABLE 6.
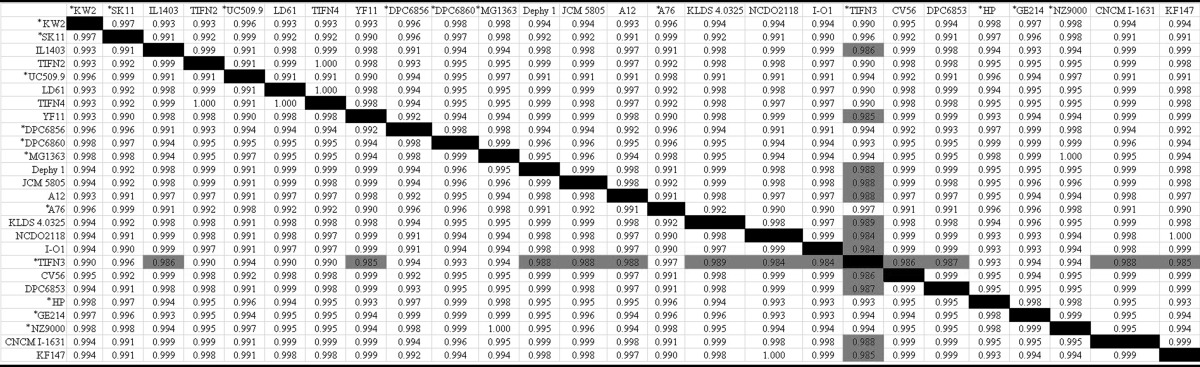
L. lactis subsp. cremoris strains are identified by asterisks.
Values below the threshold TETRA value (<0.99) are highlighted in gray.
Starter activity: milk acidification.
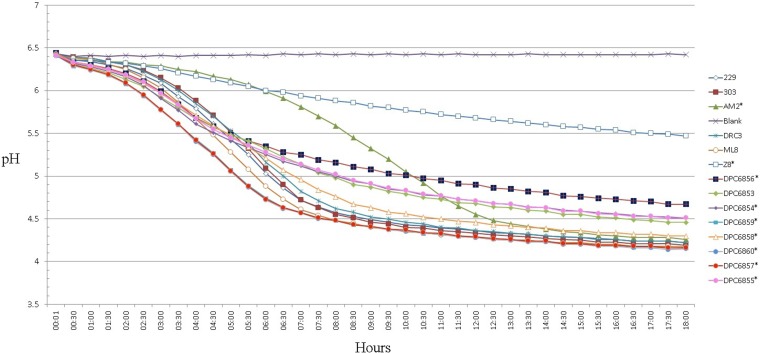
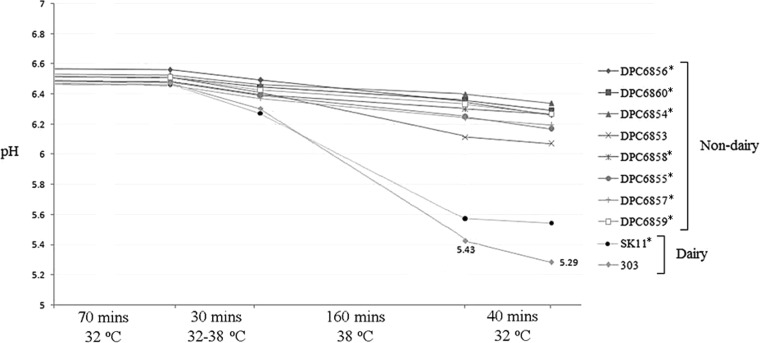
The fast acidification of milk is a crucial trait of dairy cultures for use in the dairy industry (37). All strains isolated in this study possessed good acidification activity when grown in RSM at 30°C (Fig. 2). Strain DPC6856 (isolated from the bovine rumen) showed the slowest production of acid, reaching pH 4.67 after 18 h, compared to pH <4.5 for other strains. This is in comparison to the dairy strains, the fastest of which, strain ML8, reached a final pH of 4.17 in 18 h. The Pearce test simulates conditions that starter cultures are exposed to in cheddar cheese making, which, in turn, allows for the evaluation of strains for use as starter cultures (27). Pearce test analysis (Fig. 3) showed that nondairy strains would be unsuitable for use as starters, as they are unable to reach the desired pH under processing conditions (as mimicked by the Pearce test). Although these strains are unsuitable as starters, they are capable of growth in milk without the use of supplementation, and on this basis, they were used as adjuncts.
FIG 2.
Milk acidification profile of dairy and nondairy strains in 10% reconstituted semiskimmed milk (RSM). Subspecies cremoris genotype strains are indicated by an asterisk.
FIG 3.
Pearce test analysis of nondairy strains. L. lactis 303 and SK11 were used as representative industrial dairy cultures. Results are averages from duplicate experiments. Asterisks indicate Cremoris genotype strains.
All three DPC strains possessed the lacABCDFEGX gene cluster necessary for lactose metabolism, as found in dairy-associated lactococci. The lacABCDFEGX operon encodes a phosphoenolpyruvate phosphotransferase system (PEP-PTS) (lacEF), tagatose-6-phosphate enzymes (lacABCD), and a phospho-β-galactosidase (lacG). The identification of this gene cluster corresponds with the observations that isolates were capable of growth in milk, albeit at various efficiencies. In the case of the grass isolate DPC6860, the lactose operon was located upstream from the genes prtP and prtM. Previously, two L. lactis strains from nondairy environments have been reported to possess lacE, forming part of the lactose PEP-PTS, which was absent in 34.78% of the dairy-associated strains analyzed (36). In the nondairy DPC strains examined in this study, lacE was present in all genomes and an operon containing all other components of the lacABCDFEGX operon was identified.
Key enzyme activity analysis.
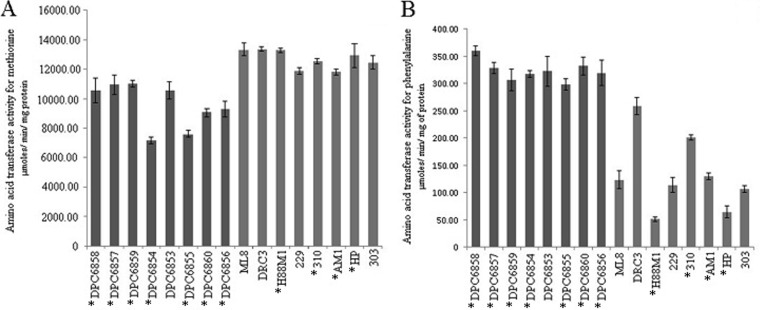
Aminotransferases function in converting amino acids to their respective α-keto acids, which are further converted to flavor compounds during cheese ripening (38–40). Dairy and nondairy cultures were examined for their amino acid transferase activities for the aromatic amino acid phenylalanine and the sulfur amino acid methionine in duplicate experiments (Fig. 4). To enable the comparative assessment of amino acid transferase activity of strains, laboratory medium was used for culturing cells prior to performing enzyme assays. Nondairy isolates displayed increased activity for phenylalanine compared to decreased activity for methionine relative to dairy strains. L. lactis H88M1 showed the lowest transferase activity for phenylalanine, and L. lactis DRC3 and ML8 showed the highest activity for methionine. Conversely, strains DPC6854 and DPC6855 had the lowest activity for methionine, while DPC6858 had the highest activity for phenylalanine.
FIG 4.
Amino acid transferase activities for methionine (A) and phenylalanine (B), with nondairy strains in dark grey and dairy strains in light grey. Results are averages from triplicate experiments. Asterisks indicate cremoris genotype strains.
Genome sequence analysis revealed that in addition to a putative aromatic aminotransferase, AraT, five putative aspartate aminotransferases were identified in DPC6856 and DPC6853 and four in DPC6860. In strains MG1363 and IL1403, three and two aspartate aminotransferases have been identified, respectively. Previously, the aspartate aminotransferase of Brevibacterium linens was shown to function in the transamination of aspartate but was also active on aromatic amino acids (40). Further analysis revealed that in strains DPC6853 and DPC6856, two of the putative aspartate aminotransferases possess PRK07309 domains found in aromatic aminotransferases, while only one ORF, in strain DPC6860, harbored a similar domain. Therefore, the enhanced transferase activity of nondairy isolates could be in part due to an increased number of aminotransferases which are active on phenylalanine.
Antibiotic resistance.
The antibiotic resistance of bacteria destined for use in food needs to be carefully assessed to prevent dissemination of these genes to other bacteria along the food chain. Therefore, the antibiotic resistances of all nondairy strains and three representative dairy strains were determined using VetMIC Lact-1 and Lact-2 plates analyzing a total of 16 antibiotics. Few differences were observed in the MIC profiles between dairy and nondairy strains (Table 7). Nondairy isolates showed lower MICs for ciprofloxacin (DPC6854 and DPC6855), vancomycin (DPC6855, DPC6857, DPC6858, DPC6859, and DPC6860), ampicillin (DPC6855), and clindamycin (DPC6854). All strains tested were highly resistant to trimethoprim (Tm), which corresponds to previous reports on Lactococcus strains isolated from raw milk cheeses. Strain DPC6853 possessed a much higher MIC for tetracycline than any of the other strains tested, above the cutoff values for antimicrobials set by the European Food Safety Authority (EFSA) (41). A tetracycline resistance protein identical to that of L. monocytogenes LM78, Enterococcus faecalis EnGen0311, and Lactococcus garvieae BCC43578 was found on the genome of DPC6853. This protein is commonly associated with plasmids or transposable elements and serves in ribosomal protection. Sequence analysis of the contig also identified a hypothetical protein with 100% amino acid identity to the analogous protein in L. garvieae and a mobile element protein identical to that in Enterococcus faecalis. The association of this protein with a transposable element suggests that this gene may be transferred to other bacteria if used in food production and contribute to the horizontal spread of antibiotic resistance. With the exception of DPC6853, all nondairy strains isolated in this study were below the microbiological cutoff values for antimicrobial resistance set by the EFSA (41).
TABLE 7.
MICs for 16 antibiotics of nondairy strains and three dairy lactococcal strains examined using VetMIC platesa
| Origin and strain | MIC (μg/ml) |
|||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Gm | Km | Sm | Nm | Tc | Em | Cl | Cm | Am | PG | Va | Vi | Lz | Tm | Ci | Ri | |
| Nondairy | ||||||||||||||||
| DPC6857* | 2 | 16 | 32 | 8 | 0.5 | 0.06 | 0.25 | 4 | 0.25 | 0.25 | <0.25 | 2 | 2 | >64 | 4 | 8 |
| DPC6858* | 2 | 8 | 16 | 8 | 0.25 | 0.06 | 0.25 | 4 | 0.25 | 0.25 | <0.25 | 2 | 1 | >64 | 2 | 8 |
| DPC6855* | 1 | 8 | 16 | 4 | 0.25 | 0.06 | 0.06 | 2 | <0.03 | 0.06 | <0.25 | 0.5 | 1 | >64 | <0.25 | 8 |
| DPC6859* | 2 | 16 | 32 | 8 | 0.5 | 0.06 | 0.12 | 4 | 0.25 | 025 | <0.25 | 2 | 2 | >64 | 4 | 16 |
| DPC6860* | 1 | 8 | 16 | 32 | 0.25 | 0.06 | 0.25 | 2 | 0.12 | 0.25 | <0.25 | 2 | 1 | >64 | 8 | 8 |
| DPC6856* | 2 | 16 | 32 | 16 | 1 | 0.06 | 0.06 | 2 | 0.12 | 0.12 | 0.5 | 1 | 2 | >64 | 1 | 2 |
| DPC6854* | 1 | 8 | 16 | 4 | 0.25 | 0.06 | <0.03 | 2 | 0.06 | 0.06 | 0.5 | 1 | 2 | >64 | <0.25 | 2 |
| DPC6853 | 4 | 16 | 32 | 32 | 64 | 0.06 | 0.25 | 4 | 0.5 | 0.25 | 0.5 | 2 | 1 | >64 | 8 | 8 |
| Dairy | ||||||||||||||||
| 303 | 2 | 16 | 16 | 16 | 0.25 | 0.06 | 0.12 | 2 | 0.25 | 0.25 | 0.5 | 1 | 1 | >64 | 8 | 8 |
| SK1G* | 2 | 8 | 16 | 4 | 1 | 0.25 | 0.25 | 8 | 0.5 | 0.5 | 0.5 | 2 | 2 | >64 | 8 | 64 |
| DRC3 | 2 | 16 | 16 | 16 | 0.25 | 0.06 | 0.06 | 2 | 0.25 | 0.25 | 0.5 | 1 | 1 | >64 | 2 | 4 |
Asterisks indicate cremoris genotype strains. MIC assays were carried out in duplicate. Antibiotic abbreviations: Gm, gentamicin; Km, kanamycin; Sm, streptomycin; Nm, neomycin; Tc, tetracycline; Em, erythromycin; Cl, clindamycin; Cm, chloramphenicol; Am, ampicillin; PG, penicillin G; Va, vancomycin; Vi, virginiamycin; Lz, linezolid; Tm, trimethoprim; Ci, ciprofloxacin; Ri, rifampin.
DISCUSSION
With the advancement of molecular tools and high-throughput screening methods, the classification of L. lactis subspecies has come under much scrutiny and has sparked debate as to how the subspecies should be identified. Phenotypic tests have been used in the past to distinguish subspecies; however, in some cases these tests may not account for related organisms that show unusual phenotypic traits (42–44). Numerous reports have identified subspecies genotypes with mismatching phenotypes (10, 34, 45). Salama et al. (45) examined L. lactis strains, isolated from natural environments, which were identified by colony hybridization using species and subspecies-specific probes. Fernández et al. (34) examined the phenotypic and genetic diversity of L. lactis strains from raw milk cheeses, while Parapouli et al. (10) characterized a novel L. lactis strain isolated from raw milk, which produced the bacteriocin nisin A. In this study, seven of the eight strains isolated from various nondairy niches were found to possess cremoris genotypes, with one lactis genotype isolated from corn. All of these strains possessed a lactis phenotype, since seven strains were capable of hydrolyzing arginine and growing in 4% NaCl and at 40°C, with one strain unable to hydrolyze arginine. Rademaker et al. (5) showed that L. lactis strains from alfalfa, radish sprouts, and mung bean sprouts are incapable of hydrolyzing arginine but overall possessed a lactis phenotype. As a result of adaptation to milk, L. lactis is held to have undergone a process of reductive evolution, forfeiting processes no longer required in the milk environment (46). The rarity of cremoris phenotypes outside milk suggests that growth at 40°C and in 4% NaCl and the capacity to utilize arginine are not required in this environment, and it has been hypothesized that the lactis phenotype is required for growth in diverse environments (16, 47). Our findings support this hypothesis: seven L. lactis subsp. cremoris strains from nondairy niches were isolated, all of which possessed lactis phenotypes.
It has been estimated that according to divergence in the 16S rRNA gene, lactis and cremoris genotypes separated approximately 17 million years ago (48). Diversity analysis using the composite data set of the seven loci employed in this study clearly highlighted this separation, with L. lactis subsp. cremoris and L. lactis subsp. lactis grouping separately irrespective of the environments from which they were isolated. Previously, phylogenetic analysis of dairy and nondairy lactococci suggested the relatively recent emergence of dairy lactococcal strains domesticated to milk (5, 36). Therefore, although these lactis and cremoris genotype strains may be phylogenetically close, they are following different directions with respect to their evolution. This may account for the separate grouping of environmental lactis genotype strains with respect to dairy lactis genotype strains during neighbor-joining cluster analysis of individual genes (with the exception of the 16S rRNA gene and rpoA [see Fig. S1 in the supplemental material]) and the seven-locus data set. L. lactis CV56, isolated from human vaginal samples, clustered with strains of dairy origin, hinting that this organism may have originated from a dairy environment (50). Similarly, DPC6856, isolated from the bovine rumen, clustered with grass strains, suggesting that it is derived from a grass niche.
ANI is an alternative to DNA-DNA hybridization (DDH) as a means of species circumscription, using full or partial genome sequences (15). An ANI value of 95% is considered the threshold for species definition, corresponding to a DDH similarity value of 70% (15). It is important to note that software tools such as JSpecies allow for pairwise comparisons only using ANI. With regard to future development of such software tools, there is a call to create a feature where ANI for a cluster of strains (i.e., L. lactis subsp. cremoris strains) can be compared to another cluster (i.e., L. lactis subsp. lactis strains), similar to what is described by Altermann (51) (in this analysis, ORFs are compared rather than average nucleotide sequence). Comparison of ANI values showed that L. lactis subsp. cremoris and L. lactis subsp. lactis possessed approximately 86% DNA similarity, in agreement with the figure established by Wegmann et al. (7) for strains IL1403 and MG1363. Based on our analysis and under the guideline set out by Richter and Rosselló-Móra (15), L. lactis subsp. lactis strains are a different species from L. lactis subsp. cremoris strains, possessing an ANI value of below 95%. Contrastingly, Fernández et al. (34) proposed that cremoris and lactis genotype strains are members of the same species, based on the species concept for prokaryotes (52). In addition, Fernández et al. (34) also put forward that both cremoris and lactis genotype strains with lactis phenotypes represent true subspecies. In comparison to species definition, subspecies definition is quite vague: no established molecular cutoff values exist, and subspecies classification is often at the judgment of the taxonomist (53); i.e., no threshold values for subspecies classification using ANIb or DDH are established. Rosselló-Mora and Amann (52) stated that a species can consist of different genomic groups, and with in-depth phenotypic analysis, better circumscription can be obtained. Therefore, in conjunction with ANI and TETRA, further analyses of both the phenotype and chemotaxonomic markers of analyzed strains are required to support the proposed revision of Lactococcus subspecies as separate species.
Recently, the isolation of novel lactic acid bacterial strains from diverse ecological niches has gained renewed interest, as it has been shown that such strains possess more diverse metabolic traits than established dairy cultures (54). In the present study, we examined technological traits important in the production of fermented dairy products. All nondairy strains grew well in milk and were capable of acidifying milk at a rate similar to that of previously isolated lactococci from dairy and nondairy origins (55). As the strains analyzed in this study were isolated from a nondairy environment, it is unexpected that they possess genetic traits that may enable them to ferment lactose, however; previous reports have identified lacE in L. lactis strains isolated from nondairy sources such as cattle skin (36). Amino acid transferase activity clearly showed that nondairy isolates possess an increased capacity to transanimate phenylalanine compared to the sulfur-derived amino acid methionine. Sulfur compounds derived from methionine impart onion, garlic, and cabbage flavor notes to cheeses such as cheddar and camembert (56, 57). In contrast, aroma compounds originating from aromatic amino acids can contribute to off-flavors in cheeses, such as flowery, bitter almond and rosy aromas (58, 59). The conversion products of phenylalanine have been found in hard- and soft-type cheeses (60), and in small amounts, the production of these compounds may be beneficial in diversifying the flavor profiles of semihard cheeses.
Antibiotics can persist for an extended period in soil- and water-based environments; thus, the widespread use of agricultural antibiotics may select for resistant strains of bacteria in these habitats (61–63). Therefore, if nondairy or wild isolates such as those identified in this study are to be used as cultures in food processing, their antibiotic resistance must be carefully assessed and fall within the guidelines set out by the EFSA (37). These guidelines state the cutoff values for L. lactis for ampicillin (2 mg/liter), vancomycin (4 mg/liter), gentamicin (32 mg/liter), kanamycin (64 mg/liter), streptomycin (32 mg/liter), erythromycin (1 mg/liter), clindamycin (1 mg/liter), tetracycline (4 mg/liter), and chloramphenicol (8 mg/liter). L. lactis DPC6853 possessed a much higher MIC for tetracycline, an antibiotic used commonly in the treatment of human and animal diseases (63). Increased resistance to this compound has previously been identified in L. lactis strains from raw milk cheeses and was found to be plasmid encoded (64). This may raise some doubt about the suitability of DPC6853 for use in food production; however, if this resistance is plasmid encoded, sensitivity of this strain to this antimicrobial could be restored (64).
In conclusion, all nondairy L. lactis strains isolated in this study demonstrated some key technological traits for application in dairy fermentations and were found to possess lactis phenotypes, with seven out of eight isolates possessing cremoris genotypes. MLST clearly separated strains with lactis and cremoris genotypes but highlighted the diversity which exists between wild isolates and their dairy counterparts. Further examination of a number of these strains by ANI and TETRA suggested that the classification of L. lactis requires revision in light of the many nondairy lactococci being isolated and sequenced. With the increased mining of diverse environments for novel L. lactis strains, a standard approach for species and subspecies classification using analysis such as ANI is required. Similarly, it is also crucial to accurately identify the subspecies phenotype and other chemotaxonomic markers. Our analysis suggests the feasibility of reclassifying the L. lactis subsp. lactis and L. lactis subsp. cremoris into two separate species, L. lactis and L. cremoris.
Supplementary Material
ACKNOWLEDGMENTS
This work was supported by Dairy Research Ireland. Daniel Cavanagh was supported by the Teagasc Walsh Fellowship Programme.
Footnotes
Supplemental material for this article may be found at http://dx.doi.org/10.1128/AEM.04092-14.
REFERENCES
- 1.Schleifer K, Kraus J, Dvorak C, Kilpper-Bälz R, Collins M, Fischer W. 1985. Transfer of Streptococcus lactis and related streptococci to the genus Lactococcus gen. nov. Syst Appl Microbiol 6:183–195. doi: 10.1016/S0723-2020(85)80052-7. [DOI] [Google Scholar]
- 2.Kempler G, McKay L. 1981. Biochemistry and genetics of citrate utilization in Streptococcus lactis ssp. diacetylactis. J Dairy Sci 64:1527–1539. doi: 10.3168/jds.S0022-0302(81)82721-X. [DOI] [Google Scholar]
- 3.Taïbi A, Dabour N, Lamoureux M, Roy D, LaPointe G. 2011. Comparative transcriptome analysis of Lactococcus lactis subsp. cremoris strains under conditions simulating Cheddar cheese manufacture. Int J Food Microbiol 146:263–275. doi: 10.1016/j.ijfoodmicro.2011.02.034. [DOI] [PubMed] [Google Scholar]
- 4.Orla-Jensen S. 1919. The lactic acid bacteria. A. F. Høst and Son, Copenhagen, Denmark . [Google Scholar]
- 5.Rademaker JLW, Herbet H, Starrenburg MJC, Naser SM, Gevers D, Kelly WJ, Hugenholtz J, Swings J, van Hylckama Vlieg JET. 2007. Diversity analysis of dairy and nondairy Lactococcus lactis isolates, using a novel multilocus sequence analysis scheme and (GTG)5-PCR fingerprinting. Appl Environ Microbiol 73:7128–7137. doi: 10.1128/AEM.01017-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Xu H, Sun Z, Liu W, Yu J, Song Y, Lv Q, Zhang J, Shao Y, Menghe B, Zhang H. 2014. Multilocus sequence typing of Lactococcus lactis from naturally fermented milk foods in ethnic minority areas of China. J Dairy Sci 97:2633–2645. doi: 10.3168/jds.2013-7738. [DOI] [PubMed] [Google Scholar]
- 7.Wegmann U, O'Connell-Motherway M, Zomer A, Buist G, Shearman C, Canchaya C, Ventura M, Goesmann A, Gasson MJ, Kuipers OP. 2007. Complete genome sequence of the prototype lactic acid bacterium Lactococcus lactis subsp. cremoris MG1363. J Bacteriol 189:3256–3270. doi: 10.1128/JB.01768-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Wouters J, Ayad EH, Hugenholtz J, Smit G. 2002. Microbes from raw milk for fermented dairy products. Int Dairy J 12:91–109. doi: 10.1016/S0958-6946(01)00151-0. [DOI] [Google Scholar]
- 9.Jarvis AW, Jarvis BD. 1981. Deoxyribonucleic acid homology among lactic streptococci. Appl Environ Microbiol 41:77–83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Parapouli M, Delbès-Paus C, Kakouri A, Koukkou A-I, Montel M-C, Samelis J. 2013. Characterization of a wild, novel nisin A-producing Lactococcus strain with an L. lactis subsp. cremoris genotype and an L. lactis subsp. lactis phenotype, isolated from Greek raw milk. Appl Environ Microbiol 79:3476–3484. doi: 10.1128/AEM.00436-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Beimfohr C, Ludwig W, Schleifer K-H. 1997. Rapid genotypic differentiation of Lactococcus lactis subspecies and biovar. Syst Appl Microbiol 20:216–221. doi: 10.1016/S0723-2020(97)80068-9. [DOI] [Google Scholar]
- 12.Godon J-J, Delorme C, Ehrlich SD, Renault P. 1992. Divergence of genomic sequences between Lactococcus lactis subsp. lactis and Lactococcus lactis subsp. cremoris. Appl Environ Microbiol 58:4045–4047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Pu Z, Dobos M, Limsowtin G, Powell I. 2002. Integrated polymerase chain reaction based procedures for the detection and identification of species and subspecies of the Gram positive bacterial genus Lactococcus. J Appl Microbiol 93:353–361. doi: 10.1046/j.1365-2672.2002.01688.x. [DOI] [PubMed] [Google Scholar]
- 14.Nomura M, Kobayashi M, Okamoto T. 2002. Rapid PCR-based method which can determine both phenotype and genotype of Lactococcus lactis subspecies. Appl Environ Microbiol 68:2209–2213. doi: 10.1128/AEM.68.5.2209-2213.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Richter M, Rosselló-Móra R. 2009. Shifting the genomic gold standard for the prokaryotic species definition. Proc Natl Acad Sci U S A 106:19126–19131. doi: 10.1073/pnas.0906412106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Kelly W, Ward L. 2002. Genotypic vs. phenotypic biodiversity in Lactococcus lactis. Microbiology 148:3332–3333. [DOI] [PubMed] [Google Scholar]
- 17.Cavanagh D, Kilcawley KN, O'Sullivan MG, Fitzgerald GF, McAuliffe O. 2014. Assessment of wild non-dairy lactococcal strains for flavour diversification in a mini-Gouda type cheese model. Food Res Int 62:432–440. doi: 10.1016/j.foodres.2014.03.043. [DOI] [Google Scholar]
- 18.Alander M, Satokari R, Korpela R, Saxelin M, Vilpponen-Salmela T, Mattila-Sandholm T, Von Wright A. 1999. Persistence of colonization of human colonic mucosa by a probiotic strain, Lactobacillus rhamnosus GG, after oral consumption. Appl Environ Microbiol 65:351–354. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Gish W, States DJ. 1993. Identification of protein coding regions by database similarity search. Nat Genet 3:266–272. doi: 10.1038/ng0393-266. [DOI] [PubMed] [Google Scholar]
- 20.Jolley KA, Feil E, Chan M-S, Maiden MCJ. 2001. Sequence type analysis and recombinational tests (START). Bioinformatics 17:1230–1231. doi: 10.1093/bioinformatics/17.12.1230. [DOI] [PubMed] [Google Scholar]
- 21.Tamura K, Dudley J, Nei M, Kumar S. 2007. MEGA4: Molecular Evolutionary Genetics Analysis (MEGA) software version 4.0. Mol Biol Evol 24:1596–1599. doi: 10.1093/molbev/msm092. [DOI] [PubMed] [Google Scholar]
- 22.Altermann E, Klaenhammer TR. 2003. GAMOLA: a new local solution for sequence annotation and analyzing draft and finished prokaryotic genomes. OMICS 7:161–169. doi: 10.1089/153623103322246557. [DOI] [PubMed] [Google Scholar]
- 23.Hyatt D, Chen GL, Locascio PF, Land ML, Larimer FW, Hauser LJ. 2010. Prodigal: prokaryotic gene recognition and translation initiation site identification. BMC Bioinformatics 11:119. doi: 10.1186/1471-2105-11-119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Aziz RK, Bartels D, Best AA, Dejongh M, Disz T, Edwards RA, Formsma K, Gerdes S, Glass EM, Kubal M. 2008. The RAST server: rapid annotations using subsystems technology. BMC Genomics 9:75. doi: 10.1186/1471-2164-9-75. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Altschul SF, Gish W, Miller W, Myers EW, Lipman DJ. 1990. Basic local alignment search tool. J Mol Biol 215:403–410. doi: 10.1016/S0022-2836(05)80360-2. [DOI] [PubMed] [Google Scholar]
- 26.Eddy SR. 2009. A new generation of homology search tools based on probabilistic inference. Genome Inform 23:205–211. [PubMed] [Google Scholar]
- 27.Pearce LE. 1969. Activity tests for cheese starter cultures. N Z J Dairy Technol 4:246–247. [Google Scholar]
- 28.Coton E, Coton M. 2005. Multiplex PCR for colony direct detection of Gram-positive histamine- and tyramine-producing bacteria. J Microbiol Methods 63:296–304. doi: 10.1016/j.mimet.2005.04.001. [DOI] [PubMed] [Google Scholar]
- 29.Coton M, Coton E, Lucas P, Lonvaud A. 2004. Identification of the gene encoding a putative tyrosine decarboxylase of Carnobacterium divergens 508. Development of molecular tools for the detection of tyramine-producing bacteria. Food Microbiol 21:125–130. doi: 10.1016/j.fm.2003.10.004. [DOI] [Google Scholar]
- 30.Salama M, Sandine W, Giovannoni S. 1991. Development and application of oligonucleotide probes for identification of Lactococcus lactis subsp. cremoris. Appl Environ Microbiol 57:1313–1318. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Maiden MC. 2006. Multilocus sequence typing of bacteria. Annu Rev Microbiol 60:561–588. doi: 10.1146/annurev.micro.59.030804.121325. [DOI] [PubMed] [Google Scholar]
- 32.Turner KM, Feil EJ. 2007. The secret life of the multilocus sequence type. Int J Antimicrob Agents 29:129–135. doi: 10.1016/j.ijantimicag.2006.11.002. [DOI] [PubMed] [Google Scholar]
- 33.Kuipers OP, de Ruyter PG, Kleerebezem M, de Vos WM. 1998. Quorum sensing-controlled gene expression in lactic acid bacteria. J Biotechnol 64:15–21. doi: 10.1016/S0168-1656(98)00100-X. [DOI] [Google Scholar]
- 34.Fernández E, Alegría Á, Delgado S, Martín MC, Mayo B. 2011. Comparative phenotypic and molecular genetic profiling of wild Lactococcus lactis subsp. lactis strains of the L. lactis subsp. lactis and L. lactis subsp. cremoris genotypes, isolated from starter-free cheeses made of raw milk. Appl Environ Microbiol 77:5324–5335. doi: 10.1128/AEM.02991-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Linares DM, Kok J, Poolman B. 2010. Genome sequences of Lactococcus lactis MG1363 (revised) and NZ9000 and comparative physiological studies. J Bacteriol 192:5806–5812. doi: 10.1128/JB.00533-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Passerini D, Beltramo C, Coddeville M, Quentin Y, Ritzenthaler P, Daveran-Mingot M-L, Le Bourgeois P. 2010. Genes but not genomes reveal bacterial domestication of Lactococcus lactis. PLoS One 5:e15306. doi: 10.1371/journal.pone.0015306. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Marshall VM. 1991. Inoculated ecosystem in a milk environment. J Appl Bacteriol 73:127S–135S. [Google Scholar]
- 38.Yvon M, Thirouin S, Rijnen L, Fromentier D, Gripon JC. 1997. An aminotransferase from Lactococcus lactis initiates conversion of amino acids to cheese flavor compounds. Appl Environ Microbiol 63:414–419. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Smit B, Engels W, Wouters J, Smit G. 2004. Diversity of l-leucine catabolism in various microorganisms involved in dairy fermentations, and identification of the rate-controlling step in the formation of the potent flavour component 3-methylbutanal. Appl Microbiol Biotechnol 64:396–402. doi: 10.1007/s00253-003-1447-8. [DOI] [PubMed] [Google Scholar]
- 40.Rijnen L, Yvon M, van Kranenburg R, Courtin P, Verheul A, Chambellon E, Smit G. 2003. Lactococcal aminotransferases AraT and BcaT are key enzymes for the formation of aroma compounds from amino acids in cheese. Int Dairy J 13:805–812. doi: 10.1016/S0958-6946(03)00102-X. [DOI] [Google Scholar]
- 41.EFSA Panel on Additives and Products or Substances used in Animal Feed (FEEDAP). 2012. Guidance on the assessment of bacterial susceptibility to antimicrobials of human and veterinary importance. EFSA J 10(6):2740. doi: 10.2903/j.efsa.2012.2740. [DOI] [Google Scholar]
- 42.Centeno J, Menéndez S, Rodriguez-Otero J. 1996. Main microbial flora present as natural starters in Cebreiro raw cow's-milk cheese (northwest Spain). Int J Food Microbiol 33:307–313. doi: 10.1016/0168-1605(96)01165-8. [DOI] [PubMed] [Google Scholar]
- 43.Delgado S, Mayo B. 2004. Phenotypic and genetic diversity of Lactococcus lactis and Enterococcus spp. strains isolated from northern Spain starter-free farmhouse cheeses. Int J Food Microbiol 90:309–319. doi: 10.1016/S0168-1605(03)00323-4. [DOI] [PubMed] [Google Scholar]
- 44.Teixeira LM, Merquior VLC, Vianni MDCE, Carvalho MDGS, Fracalanzza SEL, Steigerwalt AG, Brenner DJ, Facklam RR. 1996. Phenotypic and genotypic characterization of atypical Lactococcus garvieae strains isolated from water buffalos with subclinical mastitis and confirmation of L. garvieae as a senior subjective synonym of Enterococcus seriolicida. Int J Syst Bacteriol 46:664–668. doi: 10.1099/00207713-46-3-664. [DOI] [PubMed] [Google Scholar]
- 45.Salama MS, Sandine WE, Giovannoni SJ. 1993. Isolation of Lactococcus lactis subsp. cremoris from nature by colony hybridization with rRNA probes. Appl Environ Microbiol 59:3941–3945. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Kelly WJ, Ward LJH, Leahy SC. 2010. Chromosomal diversity in Lactococcus lactis and the origin of dairy starter cultures. Genome Biol Evol 2:729–744. http://dx.doi.org/10.1093/gbe/evq056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Klijn N, Weerkamp AH, de Vos WM. 1995. Detection and characterization of lactose-utilizing Lactococcus spp. in natural ecosystems. Appl Environ Microbiol 61:788–792. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Bolotin A, Quinquis B, Sorokin A, Ehrlich DS. 2004. Recent genetic transfer between Lactococcus lactis and enterobacteria. J Bacteriol 186:6671–6677. doi: 10.1128/JB.186.19.6671-6677.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Reference deleted.
- 50.Gao Y, Lu Y, Teng K-L, Chen M-L, Zheng H-J, Zhu Y-Q, Zhong J. 2011. Complete genome sequence of Lactococcus lactis subsp. lactis CV56, a probiotic strain isolated from the vaginas of healthy women. J Bacteriol 193:2886–2887. doi: 10.1128/JB.00358-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Altermann E. 2012. Tracing lifestyle adaptation in prokaryotic genomes. Front Microbiol 3:1–17. doi: 10.3389/fmicb.2012.00048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Rosselló-Mora R, Amann R. 2001. The species concept for prokaryotes. FEMS Microbiol Rev 25:39–67. doi: 10.1111/j.1574-6976.2001.tb00571.x. [DOI] [PubMed] [Google Scholar]
- 53.Konstantinidis KT, Stackebrandt E. 2013. Defining taxonomic ranks, p 229–254. In Rosenberg E, DeLong EF, Lory S, Stackebrandt E, Thompson F (ed), The prokaryotes, 4th ed Springer, Germany. [Google Scholar]
- 54.Alemayehu D, Hannon JA, McAuliffe O, Ross RP. 2014. Characterization of plant-derived lactococci on the basis of their volatile compounds profile when grown in milk. Int J Food Microbiol 172:57–61. doi: 10.1016/j.ijfoodmicro.2013.11.024. [DOI] [PubMed] [Google Scholar]
- 55.Nomura M, Kobayashi M, Narita T, Kimoto-Nira H, Okamoto T. 2006. Phenotypic and molecular characterization of Lactococcus lactis from milk and plants. J Appl Microbiol 101:396–405. doi: 10.1111/j.1365-2672.2006.02949.x. [DOI] [PubMed] [Google Scholar]
- 56.McSweeney PLH, Sousa MJ. 2000. Biochemical pathways for the production of flavour compounds in cheeses during ripening: a review. Lait 80:293–324. doi: 10.1051/lait:2000127. [DOI] [Google Scholar]
- 57.Singh T, Drake M, Cadwallader K. 2003. Flavor of Cheddar cheese: a chemical and sensory perspective. Compr Rev Food Sci F 2:166–189. doi: 10.1111/j.1541-4337.2003.tb00021.x. [DOI] [PubMed] [Google Scholar]
- 58.Marilley L, Casey M. 2004. Flavours of cheese products: metabolic pathways, analytical tools and identification of producing strains. Int J Food Microbiol 90:139–159. doi: 10.1016/S0168-1605(03)00304-0. [DOI] [PubMed] [Google Scholar]
- 59.van Kranenburg R, Kleerebezem M, van Hylckama Vlieg J, Ursing BM, Boekhorst J, Smit BA, Ayad EHE, Smit G, Siezen RJ. 2002. Flavour formation from amino acids by lactic acid bacteria: predictions from genome sequence analysis. Int Dairy J 12:111–121. doi: 10.1016/S0958-6946(01)00132-7. [DOI] [Google Scholar]
- 60.Allen HK, Donato J, Wang HH, Cloud-Hansen KA, Davies J, Handelsman J. 2010. Call of the wild: antibiotic resistance genes in natural environments. Nat Rev Microbiol 8:251–259. doi: 10.1038/nrmicro2312. [DOI] [PubMed] [Google Scholar]
- 61.Thiele-Bruhn S. 2003. Pharmaceutical antibiotic compounds in soils—a review. J Plant Nutr Soil Sci 166:145–167. doi: 10.1002/jpln.200390023. [DOI] [Google Scholar]
- 62.Segura PA, François M, Gagnon C, Sauvé S. 2009. Review of the occurrence of anti-infectives in contaminated wastewaters and natural and drinking waters. Environ Health Perspect 117:675. doi: 10.1289/ehp.11776. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Chopra I, Roberts M. 2001. Tetracycline antibiotics: mode of action, applications, molecular biology, and epidemiology of bacterial resistance. Microbiol Mol Biol Rev 65:232–260. doi: 10.1128/MMBR.65.2.232-260.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Fallico V, McAuliffe O, Fitzgerald GF, Ross RP. 2011. Plasmids of raw milk cheese isolate Lactococcus lactis subsp. lactis biovar diacetylactis DPC3901 suggest a plant-based origin for the strain. Appl Environ Microbiol 77:6451–6462. doi: 10.1128/AEM.00661-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.