Abstract
In order to identify antifungal compounds from natural sources to be used as ingredients in the bakery industry, water/salt-soluble extracts (WSE) from different legume flour hydrolysates obtained by the use of a fungal protease were assayed against Penicillium roqueforti DPPMAF1. The agar diffusion assays allowed the selection of the pea (Pisum sativum) hydrolysate as the most active. As shown by the hyphal radial growth rate, the WSE had inhibitory activity towards several fungi isolated from bakeries. The MIC of the WSE was 9.0 mg/ml. Fungal inhibition was slightly affected by heating and variations in pH. The antifungal activity was attributed to three native proteins (pea defensins 1 and 2 and a nonspecific lipid transfer protein [nsLTP]) and a mixture of peptides released during hydrolysis. The three proteins have been reported previously as components of the defense system of the plant. Five peptides were purified from WSE and were identified as sequences encrypted in leginsulin A, vicilin, provicilin, and the nsLTP. To confirm antifungal activity, the peptides were chemically synthesized and tested. Freeze-dried WSE were used as ingredients in leavened baked goods. In particular, breads made by the addition of 1.6% (wt/wt) of the extract and fermented by baker's yeast or sourdough were characterized for their main chemical, structural, and sensory features, packed in polyethylene bags, stored at room temperature, and compared to controls prepared without pea hydrolysate. Artificially inoculated slices of a bread containing the WSE did not show contamination by fungi until at least 21 days of storage and behaved like the bread prepared with calcium propionate (0.3%, wt/wt).
INTRODUCTION
Contamination by fungi is the most common cause of microbial spoilage and a costly problem for bakeries. In many cases, it is the major factor governing shelf life. In addition to the repellent sight of visible growth, fungi may be responsible for off-flavors and may synthesize mycotoxins and allergenic compounds (1, 2).
Chemical preservatives, novel ingredients with fungus-inhibiting properties, and packaging techniques are all technology options that, together with hygiene during processing, may contribute to decreasing the growth of fungi in baked goods (3). Routinely, salts of propionic, sorbic, and benzoic acids, as well as ethanol, are used as chemical preservatives. Nevertheless, consumers require high-quality, preservative-free, safe but mildly processed foods with extended shelf life and preserved sensory properties. These requirements have led to a search for new preservatives derived mainly from natural sources. In nature, antifungal compounds from plants comprise proteins and peptides that are thought to play an important role in plant disease resistance, serving as protective agents against fungal invasion (4). In particular, leguminous plants represent an important reservoir to be investigated owing to their abundance of proteins with important biological activities (5). Overall, leguminous proteins are classified as protease and amylase inhibitors, lectins, and ribosome-inactivating and antifungal agents (5). The latter are divided into many types, comprising thaumatin-like proteins, chitinases, glucanases, embryo-abundant proteins, miraculin-like proteins, cyclophilin-like proteins, allergen-like proteins, thionins, and nonspecific lipid transfer proteins (nsLTPs) (5). Frequently, a combination of different antifungal proteins is found in one leguminous species (4, 5).
The use of antifungal compounds from legumes for extending the shelf life of baked goods has already been proposed (1). Indeed, it has been demonstrated that a water-soluble extract from Phaseolus vulgaris cv. Pinto inhibited a large number of fungal species isolated from bakeries due to the activity of phaseolin alpha-type precursor, phaseolin, and erythroagglutinating phytohemagglutinin (1). The combination of water extracts from pinto beans and sourdough fermentation with Lactobacillus brevis AM7 allowed fungal inhibition on bread slices until at least 14 days of storage at room temperature. This level of protection was comparable to that found with 0.3% (wt/wt) calcium propionate (1). Sourdough is one example of a traditional and natural starter that deserves great interest for its multiple functional features. Sourdough was also considered for extending the shelf life of baked goods. In particular, sourdough lactic acid bacteria synthesize a broad range of antifungal compounds, such as phenyllactic acid, peptides, cyclic dipeptides, and short or medium-chain fatty acids (2, 6, 7, 8, 9, 10, 11).
Several recent studies have addressed the use of metabolites from sourdough lactic acid bacteria and yeasts alone and in combination with natural preservatives, which were extracted from plant materials or were contained in pseudocereals used in the formula for making baked goods (1, 2, 6, 7, 8, 12, 13). Despite promising results, the problem of fungal contamination, of great concern to both consumers and bakeries, still remains unsolved.
This work describes the antifungal activity of a pea (Pisum sativum) flour hydrolysate and its use to extend the shelf life of wheat flour bread. The effects of the pea flour hydrolysate on the rheological and sensory features of the bread were determined. Antifungal proteins and peptides were identified by nano-liquid chromatography–electrospray ionization–tandem mass spectrometry (nano-LC–ESI–MS-MS) analysis, and their inhibitory activities were confirmed over a long-term shelf life under pilot plant conditions.
MATERIALS AND METHODS
Microorganisms, culture media, and growth conditions.
Penicillium roqueforti DPPMAF1 was used as the indicator microorganism for antifungal assays, since it is one of the more resistant fungi to chemical preservatives (1, 13). Penicillium polonicum CBS 112490, Penicillium chrysogenum CBS 111214, Penicillium paneum CBS 101032, Penicillium albocoremium CBS 109582, Penicillium chermesinum CBS 117279, Penicillium carneum CBS 112297, Eurotium herbariorum CBS 117336, Eurotium rubrum CBS 150.92, Aspergillus parasiticus CBS 971.97, Aspergillus versicolor CBS 117286, Penicillium bialowiezense CBS 110102, and Penicillium brevicompactum CBS 28997 were from the Culture Collection of the Centraalbureau voor Schimmelcultures (Utrecht, Netherlands); Penicillium aethiopicum DPPMAF2 and Aspergillus niger DPPMAF3 were from the Culture Collection of the Department of Soil, Plant, and Food Science (University of Bari Aldo Moro, Bari, Italy). All these species correspond to some of the most relevant spoilage fungi in baked goods (7). Fungi were grown in potato dextrose agar (pH 5.6) (PDA; Oxoid Laboratories) at 25°C for 24 to 72 h. Wheat flour hydrolysate (WFH) was used for the determination of conidial germination (14). A suspension of wheat flour (20% [wt/vol] in tap water) was incubated for 18 h at 30°C under stirring conditions (ca. 200 rpm). After incubation, the suspension was filtered onto a Whatman apparatus (Polycarp 75 SPF; Whatman International, Maidstone, England), and yeast extract (0.3%, wt/vol), sucrose, glucose, and maltose (total concentration, 1.5% [wt/vol]) were added. The WFH was sterilized by filtration on 0.22-μm membrane filters (Millipore Corporation, Bedford, MA) and was stored at 4°C before use. WFH was chosen as the substrate because it is representative of the chemical composition of wheat flour.
Lactobacillus plantarum 1A7, which was previously selected for its antifungal activity (2, 6) and belongs to the Culture Collection of the Department of Soil, Plant, and Food Science (University of Bari Aldo Moro, Bari, Italy), was used for making sourdough bread. L. plantarum 1A7 was propagated for 24 h at 30°C in MRS medium (Oxoid Laboratories, Hampshire, United Kingdom), modified by the addition of fresh yeast extract (5%, vol/vol) and 28 mM maltose, at a final pH of 5.6 (mMRS medium). Lactic acid bacteria were enumerated by plating (30°C for 48 h) on mMRS medium.
Enzymes and legume flour hydrolysates.
The commercial proteolytic enzyme preparations (AB Enzymes GmbH, Darmstadt, Germany) used in this study were as follows: Corolase 7089 (177 nkat PRO/ml, where PRO is the endopeptidase activity on azocasein and 1 nkat is defined as the amount of enzyme required to raise the rate of reaction by 1 nmol/s under the assay conditions described by Heiniö et al. [15]); Corolase LAP (63.9 U/ml, where 1 U of protease activity corresponds to the release of 1 μg/min of tyrosine under the conditions described by Dias et al. [16]); Veron HPP (1,116 UHb/g, where 1 UHb/g corresponds to the release of 1 μmol/min of tyrosine from hemoglobin at 37°C and pH 5.0); and Veron PS (227 UHb/g). Fungal proteases from Aspergillus oryzae (500,000 UHb/g) and Aspergillus niger (3,000 spectrophotometric acid protease units/g), routinely used as improvers in the bakery industry and purchased from Bio-Cat Inc. (Troy, VA), were also used.
Soy (Glycine max), lentil (Lens culinaris), pea (Pisum sativum), faba bean (Vicia faba), and chickpea (Cicer arietinum) seed samples were purchased at local organic markets. Flours were produced from pools of seeds (for each species, seeds from three different batches were used) with a laboratory mill (Ika M 20; Ika-Werke GmbH and Co. KG, Staufen, Germany). Protein (total nitrogen × 5.7), ash, and moisture contents were determined according to AACC approved methods 46-11A, 08-01, and 44-15A, respectively (17). The etheric extract was analyzed by the Soxhlet method to determine the amount of lipids. The chemical compositions of legume flours are reported in Table 1.
TABLE 1.
Chemical characteristics of soy, lentil, pea, faba bean, and chickpea floursa
| Flour | Moisture (%) | Content (% of dry matter) |
||||
|---|---|---|---|---|---|---|
| Proteins | Lipids | Carbohydrates | Dietary fiber | Ash | ||
| Soy (Glycine max) | 8.8 ± 0.3 | 36.9 ± 0.9 A | 19.1 ± 1.2 A | 23.2 ± 0.7 C | 11.9 ± 0.4 E | 1.4 ± 0.3 D |
| Lentil (Lens culinaris) | 8.9 ± 0.5 | 23.2 ± 0.9 C | 3.5 ± 0.1 C | 62.3 ± 4.7 B | 17.4 ± 1.1 D | 2.2 ± 0.1 C |
| Pea (Pisum sativum) | 9.1 ± 0.8 | 19.4 ± 1.1 C | 2.1 ± 0.1 D | 64.9 ± 2.3 A | 35.3 ± 2.8 A | 4.6 ± 0.2 A |
| Faba bean (Vicia faba) | 9.3 ± 0.5 | 26.1 ± 0.7 B | 2.5 ± 0.3 D | 60.2 ± 1.5 B | 21.5 ± 1 C | 2.6 ± 0.2 C |
| Chickpea (Cicer arietinum) | 8.7 ± 0.6 | 15.4 ± 1.2 D | 6.3 ± 0.5 B | 66.3 ± 3.9 A | 27.2 ± 1.9 B | 3.4 ± 0.3 B |
Data are means for three independent experiments ± standard deviations (n = 3). Values in the same column followed by different capital letters differ significantly (P < 0.05).
Doughs (600 g) with a dough yield (DY; calculated as dough weight × 100/flour weight) of 160 (corresponding to 62.5% flour and 37.5% water) were mixed at 60 × g for 5 min with an IM 5-8 high-speed mixer (Mecnosud, Flumeri, Italy) and were incubated at 30°C for 24 h. AB enzymes were added to doughs at the dosages suggested by the manufacturer: Corolase 7989 at 10 g/100 kg of protein, Corolase LAP at 20 g/100 kg of protein, Veron HPP at 10 g/100 kg of protein, and Veron PS at 25 g/100 kg of protein. Enzymes from Bio-Cat were used at 200 ppm (18, 19).
Aliquots (50 g) from each legume flour hydrolysate were taken after 0, 3, 6, 9, 12, and 24 h of incubation and were used for obtaining water/salt-soluble extracts (WSE) according to the method originally described by Osborne (20) and modified by Weiss et al. (21). The extracts were treated at 90°C for 5 min for enzyme denaturation and were stored at −20°C before the antifungal assay. The concentration of peptides in the WSE and related fractions (see below) was determined by the o-phthaldialdehyde (OPA) method (22). The concentration of proteins was determined by the Bradford method (23). WSE from legume hydrolysates were freeze-dried and were used as ingredients for bread making.
Assays for antifungal activity.
Three different methods were used to assay antifungal activity. The agar diffusion assay was carried out as described by Coda et al. (1). Petri plates (diameter, 90 mm) containing 20 ml of PDA (Oxoid) were inoculated with the fungus. The plates were incubated at 25°C for 72 h. After the mycelial colony had developed, a sterile blank paper disk (diameter, 0.5 cm) was placed ca. 0.5 cm away from the rim of the mycelial colony. Ten microliters of the sample to be assayed was added to the disk.
The inhibitory spectra of the WSE were assayed on the basis of the hyphal radial growth rate of fungi (24). The WSE were sterilized by filtration on 0.22-μm membrane filters (Millipore Corporation, Bedford, MA) and were added (final concentration, 30% [vol/vol]) to sterilized PDA. After mixing, aliquots of 20 ml were poured onto petri plates (diameter, 90 mm). Control plates contained PDA supplemented with 30% (vol/vol) Tris HCl (50 mM; pH 8.8). The assay was carried out by placing a 3-mm-diameter plug of growing mycelia on the center of a petri dish containing the culture medium. Plates were incubated aerobically at 25°C. Three replicates were run simultaneously. The radial growth of mycelia (expressed as the colony diameter, in millimeters) on all plates was measured 8 days after inoculation. Each datum point is the mean of at least four measurements of a growing colony. The percentage of growth inhibition was calculated from mean radial growth values as follows: percentage of inhibition = [(mycelial growth under control conditions − mycelial growth in the presence of WSE)/(mycelial growth under control conditions)] × 100.
The effect of WSE on the germination of conidia was also determined (25). After growth for 7 days on PDA plates, conidia of P. roqueforti DPPMAF1 were harvested in sterile water containing 0.05% (vol/vol) Tween 80. The conidia in the suspension were counted by using a Petroff-Hausser counting chamber. A fixed number of ca. 106 to 107 conidia/ml was added to 5 ml of the mixture of WFH containing 30% (vol/vol) WSE. The mixtures were incubated in 60-mm petri dishes for 24 h at 25°C under stirring conditions. WFH alone and WFH with 0.3% (wt/vol) calcium propionate added were used as the controls. To determine the percentage of germinated conidia (length/width ratio, ≥2), slides of the suspension were examined under a microscope (magnification, ×40) at 4-h intervals. Three separate replicates of at least 100 conidia were used for each assay. All assays for antifungal activity were carried out at least in triplicate.
To determine the MIC by the effect on conidial germination, WSE and related fractions (see below) were freeze-dried and were redissolved in WFH. Concentrations from 0.1 to 10 mg of proteins or peptides/ml (0.5-mg/ml intervals) were tested as described above. The MIC was defined as the lowest concentration of the WSE that inhibited fungal growth at 25°C for 12 h. All assays for antifungal activity were carried out at least in triplicate.
Proteolysis, heat stability, and pH stability of antifungal compounds.
The WSE from pea flour hydrolysate was treated with trypsin (EC 3.4.21.4; Sigma-Aldrich Co.) as described by Atanassova et al. (26). Heat stability was determined by heating at 100°C for 5 min. After treatments, the residual activity was determined by an agar diffusion assay. The dependence of the antifungal activity on the pH was investigated by measuring the hyphal radial growth rate of the indicator fungus P. roqueforti DPPMAF1 in PDA adjusted to pH 4.0 to 8.0.
Purification of antifungal compounds.
Active WSE were fractionated by ultrafiltration (Ultrafree-MC centrifugal filter units; Millipore) using membranes with cutoffs of 50, 30, 10, and 3 kDa according to the manufacturer's instructions. After ultrafiltration, fractions were used for the agar diffusion assay and were analyzed by electrophoresis. In particular, aliquots containing ca. 15 μg of protein were diluted 1:1 with sample buffer (0.125 M Tris-C1 [pH 6.8], 10% glycerol, 5% β-mercaptoethanol, and 2% SDS), treated at 100°C for 5 min, and analyzed by SDS-polyacrylamide gel electrophoresis (SDS-PAGE) according to the Laemmli procedure (27).
An aliquot of the 3-kDa partially purified fraction was further fractionated (37 fractions) by reversed-phase fast performance liquid chromatography (RP-FPLC), using a Resource RPC column and an ÄKTA FPLC equipment with the UV detector operating at 214 nm (GE Healthcare Bio-Sciences AB, Uppsala, Sweden), as described previously by Rizzello et al. (8). Solvents were removed from collected fractions by freeze-drying. The fractions were redissolved in sterile water and were subjected to an agar diffusion assay. The freeze-dried preparation of the fractions with antifungal activity was used for the identification of peptides.
Identification of antifungal compounds. (i) Proteins.
Protein bands from the active fraction with a molecular mass lower than 10 kDa were cut from the gel, digested in-gel by trypsin (Promega, Mannheim, Germany), and subjected to nano-liquid chromatography–electrospray ionization–tandem mass spectrometry (nano-LC–ESI–MS-MS).
Protein identification using nano-LC–ESI–MS-MS was performed by Proteome Factory AG (Berlin, Germany). The MS system consisted of an Agilent 1100 NanoLC system (Agilent, Waldbronn, Germany), a PicoTip electrospray emitter (New Objective, Woburn, MA) and an Orbitrap XL or LTQ-FT Ultra mass spectrometer (Thermo Fisher, Bremen, Germany). Peptides were trapped and desalted on the enrichment column (Zorbax SB C18; 0.3 by 5 mm; Agilent) for 5 min using 2.5% acetonitrile–0.5% formic acid as the eluent; then peptides were separated on a Zorbax 300 SB C18 column (75 μm by 150 mm; Agilent) using an acetonitrile–0.1% formic acid gradient from 5% to 35% acetonitrile within 40 min. MS-MS spectra were recorded data-dependently by the mass spectrometer according to the manufacturer's recommendations. Proteins were identified using an MS-MS ion search with the Mascot search engine (Matrix Science, London, England) and the nonredundant (nr) protein database (National Center for Biotechnology Information, Bethesda, MD, USA). For database research, the ion charge parameter was set to 1+, 2+, or 3+ according to the common charge state distribution of the instrument.
(ii) Peptides.
Peptides from the FPLC fractions with antifungal activities were identified by nano-LC–ESI–MS-MS by using a Finnigan LCQ Deca XP Max ion trap mass spectrometer (Thermo Electron) through the nano-ESI interface. According to the manufacturer's instrument settings for nano-LC–ESI–MS-MS analyses, MS spectra were automatically taken by Xcalibur software (Thermo Electron) in positive-ion mode. MS-MS spectra were processed using BioWorks software, version 3.2 (Thermo Electron), generating peak lists suitable for database searches. Peptides were identified using an MS-MS ion search with the Mascot search engine (Matrix Science, London, England) and the NCBI nr protein database (National Center for Biotechnology Information, Bethesda, MD, USA). For the identification of peptides, the following parameters were considered: enzyme, “none”; instrument type, “ESI-trap”; peptide mass tolerance, ±0.1%; fragment mass tolerance, ±0.5 Da. Peptide identification results were subjected to manual evaluation, as described by Chen et al. (28), and the validated peptide sequences explained all the major peaks in the MS-MS spectrum.
All peptides identified were chemically synthesized (PolyPeptide Laboratories, Strasbourg, France) and were tested for antifungal activity by the effect on conidial germination (at concentrations of 1 to 10 mg of peptides/ml), singly or in mixtures, as described above. The purity of the synthesized peptides was higher than 95% as determined by high-performance liquid chromatographic analysis by the manufacturer.
Bread making.
To confirm the antifungal activity of pea flour hydrolysate, five wheat flour breads (dough yield, 160) were manufactured at the pilot plant of the Department of Soil, Plant, and Food Science (Bari, Italy): wheat flour bread containing 1.6% (wt/wt) freeze-dried pea flour hydrolysate (PWB), sourdough wheat flour bread containing 1.6% freeze-dried pea flour hydrolysate (PSB), wheat flour bread (WB), sourdough wheat flour bread (SB), and wheat flour bread with 0.3% (wt/wt) calcium propionate added (WBcp). The amount of freeze-dried WSE from pea flour hydrolysate added to doughs (1.6%) was calculated by considering the theoretical addition of 30% WSE to dough (the same percentage that was used for in vitro assays). Fifty-five grams of freeze-dried matter was collected from each liter of WSE. WB and SB were manufactured according to the protocol routinely used for typical Italian bread (2, 29) and were used as the controls. The formulas for bread making are listed in Table 2.
TABLE 2.
Formulas used for bread making
| Breada | Amtb of the following ingredient: |
|||||
|---|---|---|---|---|---|---|
| Wheat flour | Tap water | Pea flour hydrolysate | Sourdough S1A7c | Calcium propionate | Baker's yeast | |
| WB | 250 | 150 | 8 | |||
| WBcp | 250 | 150 | 1.2 | 8 | ||
| SB | 175 | 105 | 120 | 8 | ||
| PWB | 243.6 | 150 | 6.4 | 8 | ||
| PSB | 168.6 | 105 | 6.4 | 120 | 8 | |
WB, bread started with baker's yeast; WBcp, bread started with baker's yeast, containing calcium propionate; SB, bread started with baker's yeast and Lactobacillus plantarum 1A7 sourdough (S1A7); PWB, bread started with baker's yeast, containing pea flour hydrolysate; PSB, bread started with baker's yeast and sourdough S1A7, containing pea flour hydrolysate. The dough yield for all breads was 160, and fermentation with baker's yeast was allowed to proceed for 1.5 h at 30°C.
All ingredients except tap water are measured in grams; tap water is measured in milliliters.
Sourdough containing 62.5% flour and 37.5% water (dough yield, 160), inoculated with Lactobacillus plantarum 1A7 (ca. 7 log CFU/g) and fermented at 30°C for 24 h.
The characteristics of the flour (Triticum aestivum cv. Appulo) used were as follows: moisture, 14.2%; protein (N × 5.70), 11.5% of dry matter (d.m.); fat, 1.6% of d.m.; ash, 0.6% of d.m.; total soluble carbohydrates, 1.5% of d.m.
L. plantarum 1A7 was used for preparing sourdough (S1A7). The starter was cultivated in mMRS broth at 30°C for 24 h. Cells were harvested by centrifugation (10,000 × g, 10 min, 4°C) until the late phase of growth was reached (ca. 10 h). The optical density at 620 nm (OD620) was 2.5. Cells were washed twice in 50 mM sterile potassium phosphate buffer (pH 7.0) and were resuspended in tap water at a cell density of ca. 8.0 log CFU/ml. Wheat flour was mixed with tap water containing the bacterial suspension (initial cell density in sourdoughs, 7.0 log CFU/g). Doughs, with a dough yield of 160 (corresponding to 62.5% flour and 37.5% water), were mixed at 60 × g for 5 min with an IM 58 high-speed mixer (Mecnosud, Flumeri, Italy) and were incubated at 30°C for 24 h. After fermentation, sourdough (30%, wt/wt) was used as an ingredient for making SB and PSB. All the doughs for bread making were mixed at 60 × g for 5 min with an IM 58 high-speed mixer (Mecnosud). Baker's yeast was always added (2% [wt/wt], corresponding to a final cell density of ca. 7 log CFU/g). Fermentation was carried out at 30°C for 1.5 h. Before baking, doughs were characterized for pH and total titratable acidity (TTA; expressed as ml of 0.1 M NaOH needed to reach the value of pH of 8.3). All breads were baked at 220°C for 30 min using a Combo 3 oven (Zucchelli, Verona, Italy). Fermentations were carried out in triplicate, and breads were analyzed twice. For each bread, 3 slices were cut. Each slice was 12 cm high and 1.5 cm wide. Slices were inoculated by nebulization with a suspension of 102 conidia/ml of P. roqueforti DPPMAF1, packed in polyethylene bags to maintain constant moisture, and incubated at room temperature for 21 days. The moisture content was determined according to the standard AACC method (17). Water activity (aw) was determined at 25°C with an AquaLab 4TE Dew Point water activity meter (Decagon Devices Inc., USA).
Texture, color, and image analyses of breads.
Instrumental texture profile analysis (TPA) was carried out with a TVT-300XP texture analyzer (TexVol Instruments, Viken, Sweden) equipped with a P-Cy25S cylinder probe and a BVM test system, using Texture Analyzer TVT-XP software, version 3.8.0.5 (30, 31). The following textural parameters were obtained by the texturometer software: hardness (maximum peak force), fracturability (the first significant peak force during the probe compression of the bread), and resilience (ratio of the first decompression area to the first compression area).
The chromaticity coordinates of the bread crust (obtained by a Minolta CR-10 camera) were also reported in the form of a color difference, dE, calculated as , where dL, da, and db are the differences in the L, a, and b values between the sample and the reference (a white ceramic plate has an L value of 93.4, an a value of −1.8, and a b value of 4.4) (32).
The crumb features of breads were evaluated after 24 h of storage using image analysis technology and the UTHSCSA ImageTool program (version 2.0; University of Texas Health Science Center, San Antonio, TX), as described previously by Rizzello et al. (31).
Sensory analysis.
Sensory analysis of breads was carried out by 10 panelists (5 male and 5 female; mean age, 35 years; range, 18 to 54 years) according to the method described by Haglund et al. (33) and others (34, 35). Elasticity, the color of the crust and crumb, acid taste, acid flavor, sweetness, dryness, and taste were considered as sensory attributes on a scale from 0 to10, with 10 as the highest score. Salty taste, previously described as another wheat sourdough bread attribute, was also included (34, 35).
Statistical analysis.
Data were subjected to one-way analysis of variance (ANOVA); pairwise comparison of treatment means was achieved by Tukey's procedure at a P value of <0.05 by using the statistical software Statistica, version 8.0 (StatSoft Inc., Tulsa, OK, USA).
RESULTS
Antifungal activity of water/salt-soluble extracts from legume flour hydrolysates.
As reported in Table 1, the legume flours used in this study showed different chemical compositions. Chickpea flour had the lowest protein concentration, while the other legume flours showed protein contents higher than ca. 18%. Soy flour had the highest protein concentration. The lipid content was also highest in soy. The lowest concentrations of lipids were found in pea, faba bean, and lentil flours. Except for soy, similar concentrations of total carbohydrates were found for all legume flours. The dietary fiber content was highest for pea flour, which also showed the highest content of ash.
All the flours were mixed with water to produce doughs, and commercial proteolytic enzymes were added. WSE were obtained at different times of incubation and were assayed for antifungal activity. Before enzyme treatment, only WSE from pea hydrolysate had weak antifungal activity against P. roquefortii DPPMAF1. Treatment with Corolase 7089, Corolase LAP, or either of the two Aspergillus sp. proteases did not lead to increases in antifungal activity for any matrix. Treatment with Veron HPP caused the appearance of antifungal activity in the lentil flour hydrolysate after 6 h of incubation and a slight increase in the antifungal activity of the pea flour hydrolysate after 3 and 6 h of incubation (data not shown). After 6 h of incubation with Veron HPP, no activity was found for the WSE from the other legume flours. The use of Veron PS led to the appearance of antifungal activity for lentil and faba bean flours after 3 h of incubation, and in both cases, slight increases were also found after 6 h of incubation (Table 3). Longer incubation did not lead to increases in antifungal activity. When Veron PS was used to hydrolyze pea flour, the weak activity already present at 0 h and after 3 h of incubation increased markedly at 6 h and persisted after 9 and 12 h of incubation. Hydrolysis for more than 12 h caused a loss of antifungal activity.
TABLE 3.
Inhibitory activities of water/salt-soluble extracts from legume flour hydrolysatesa against Penicillium roqueforti DPPMAF1 after 0, 3, 6, 9, 12, and 24 h of incubation at 30°C
| Hydrolysate | Inhibitory activityb after the following incubation time (h): |
|||||
|---|---|---|---|---|---|---|
| 0 | 3 | 6 | 9 | 12 | 24 | |
| Soy flour | − | − | − | − | − | − |
| Lentil flour | − | + | ++ | − | − | − |
| Pea flour | + | + | +++ | ++ | ++ | − |
| Chickpea flour | − | − | − | − | − | − |
| Faba bean flour | − | + | ++ | − | − | − |
Obtained with a neutral protease from Aspergillus oryzae (Veron PS; dosage, 25 g/100 kg of protein).
Determined by an agar diffusion assay and scored as follows: −, no inhibition; +, very weak inhibition; ++, low-level inhibition, with a small clear zone near the rim of the colony; +++, strong inhibition, with a large clear zone near the rim of the colony.
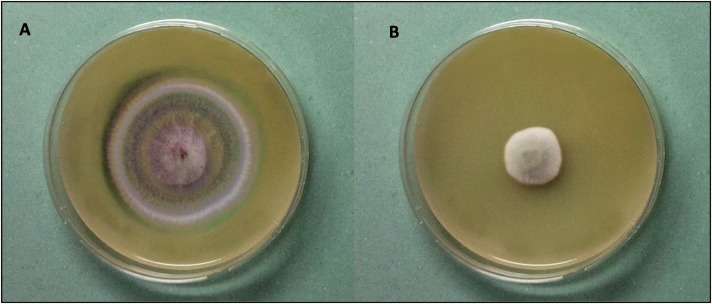
Since the highest levels of inhibition of the indicator fungus were found for WSE from pea, lentil, and faba bean flours after 6 h of incubation, these were further characterized for the spectrum of activity. The inhibitory activities of the hydrolysates were evaluated by monitoring the hyphal radial growth rates of several fungi isolated from bakeries (Table 4 and Fig. 1). Among the 15 species of fungi assayed, P. carneum CBS 112297, P. aethiopicum DPPMAF2, A. niger DPPMAF3, P. chrysogenum CBS 112490, P. bialowiezense CBS110102, P. albocoremium CBS109582, P. brevicompactum CBS 28997, E. herbariorum CBS117336, E. rubrum CBS150.92, A. parasiticus CBS971.97, and A. versicolor CBS 117286 were inhibited only by the WSE from the pea flour hydrolysate. The level of antifungal activity of the pea hydrolysate against P. roqueforti DPPMAF1 was 59%.
TABLE 4.
Inhibitory spectra of water/salt-soluble extracts from lentil, pea, and faba bean flour hydrolysatesa after 6 h of incubation at 30°C
| Fungus | Source | Inhibitory activity of legume flour hydrolysate (%)b |
||
|---|---|---|---|---|
| Lentil | Pea | Faba bean | ||
| Penicillium roqueforti DPPMAF1 | Bread, Italy | 22 | 59 | 73 |
| Penicillium aethiopicum DPPMAF2 | Bakery, Belgium | 5 | ||
| Aspergillus niger DPPMAF3 | Bakery, Belgium | 11 | ||
| Penicillium polonicum CBS 112490 | Bread, Italy | 23 | 76 | 73 |
| Penicillium chrysogenum CBS 111214 | Bread, England | 15 | ||
| Penicillium paneum CBS 101032 | Rye bread, Denmark | 60 | 95 | 67 |
| Penicillium bialowiezense CBS 110102 | Bread, Italy | 15 | ||
| Penicillium brevicompactum CBS 28997 | Cake, Denmark | 23 | ||
| Penicillium albocoremium CBS 109582 | Cake factory, Denmark | 18 | ||
| Penicillium chermesinum CBS 117279 | Bakery plant, Netherlands | 50 | 95 | 62 |
| Penicillium carneum CBS 112297 | Rye bread, Denmark | 5 | ||
| Eurotium herbariorum CBS 117336 | Chocolate cake, Netherlands | 21 | ||
| Eurotium rubrum CBS 150.92 | Cake | – | 53 | |
| Aspergillus parasiticus CBS 971.97 | Indian sweets, India | 11 | ||
| Aspergillus versicolor CBS 117286 | Wall in bakery, Netherlands | 12 | ||
Obtained with a neutral protease from Aspergillus oryzae (Veron PS; dosage, 25 g/100 kg of protein).
Determined by a hyphal radial growth rate assay. The radial growth of mycelia (expressed as the colony diameter, in millimeters) was measured 8 days after inoculation. The percentage of growth inhibition was calculated from mean radial growth values as [(mycelial growth under control conditions − mycelial growth in the presence of the water/salt soluble extract)/(mycelial growth under control conditions)] × 100. Each datum point is the mean from at least four measurements of a growing colony.
FIG 1.
Antifungal activity of the water/salt-soluble extract from pea flour hydrolysate, obtained with a neutral protease from Aspergillus oryzae (Veron PS; dosage, 25 g/100 kg of protein), as determined by hyphal radial growth inhibition after 8 days of incubation at 25°C. Penicillium roqueforti DPPMAF1 was used as the indicator. (A) PDA alone (control); (B) PDA containing 30% (vol/vol) water/salt-soluble extract.
The pH of the WSE from pea flour hydrolysate was 6.13 ± 0.12. The concentrations of proteins and peptides were 4.55 ± 0.21 and 12.53 ± 0.27 mg/ml, respectively.
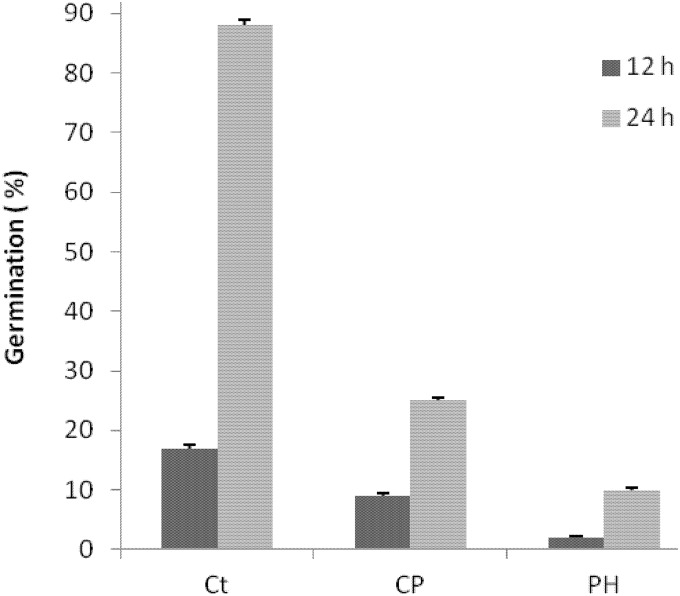
The antifungal activity of the WSE from pea flour hydrolysate was further characterized by conidial germination using WFH medium. The pH was ca. 5.6, comparable to that for the common baker's yeast bread. After incubation at 25°C for 12 h in WFH, the mean percentage of spore germination by P. roqueforti DPPMAF1 was ca. 17.3% ± 0.5%. Incubation in the presence of 0.3% (wt/vol) calcium propionate or WSE (30%, vol/vol) caused a significant (P < 0.05) decrease in conidial germination to 9.3% ± 0.4% or 2.7% ± 0.4%, respectively (Fig. 2). The same trend was found after 24 h of incubation.
FIG 2.
Conidial germination of Penicillium roqueforti DPPMAF1 incubated in WFH for 12 or 24 h at 25°C under different conditions. Treatments were as follows: Ct, control (WFH alone); CP, WFH with 0.3% (wt/vol) calcium propionate added; PH, WFH with 30% water/salt-soluble extract from pea flour hydrolysate added. Data are means for three independent experiments.
As determined by the hyphal growth rate of P. roqueforti, trypsin digestion slightly affected the antifungal activity of the pea flour hydrolysate, which decreased ca. 10% from that for the untreated hydrolysate. The variations in the pH values from 4.5 to 8.0 and the heating at 100°C for 5 min did not decrease the inhibitory effectiveness.
Identification of antifungal compounds.
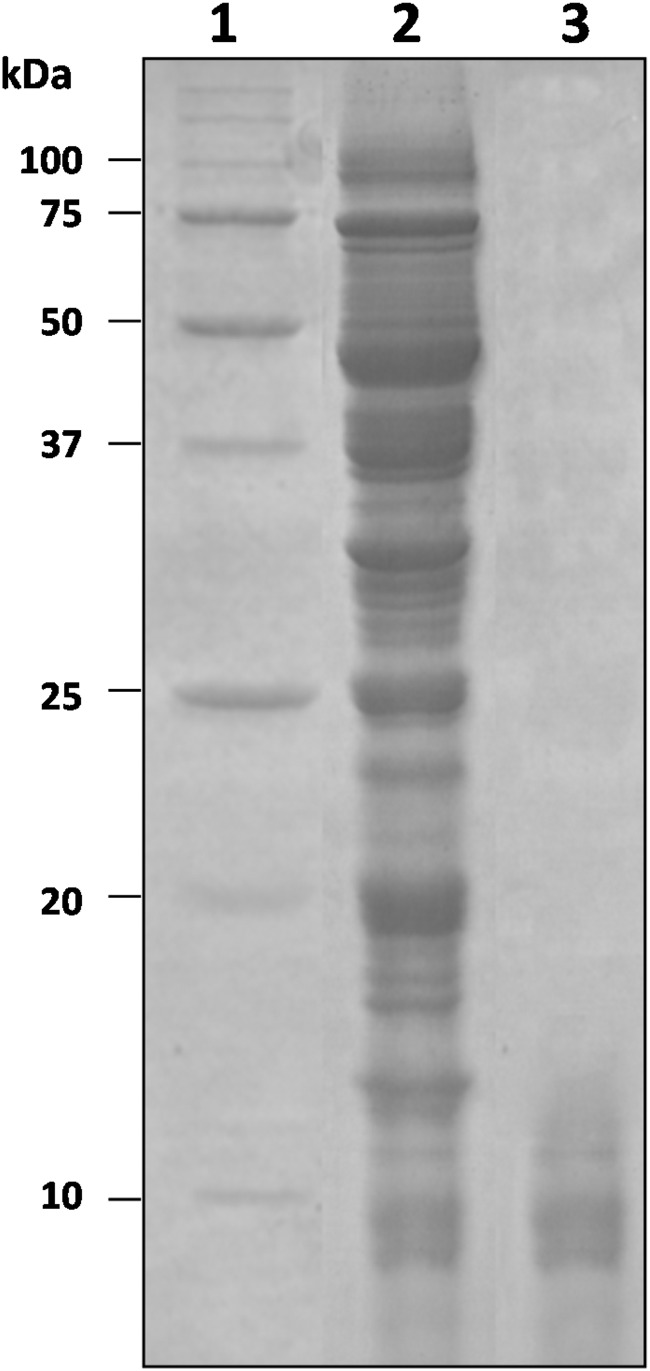
As shown by SDS-PAGE, the WSE from pea flour hydrolysate contained protein bands with a wide range of molecular masses (Fig. 3). The WSE was fractionated by ultrafiltration. Antifungal activity was found in all the fractions. In particular, those with molecular masses of <50, <30, and <10 kDa had 80% antifungal activity. The fraction with a molecular mass of <3 kDa had 50% antifungal activity. On the basis of these results, it can be hypothesized that overall, active compounds were characterized by molecular masses lower than 10 kDa; among these, some had masses lower than 3 kDa.
FIG 3.
SDS-PAGE of the water/salt-soluble extract from the pea flour hydrolysate. Lanes: 1, protein standard (Bio-Rad, Hercules, CA, USA); 2, water/salt-soluble extract; 3, polypeptides after purification by ultrafiltration using a membrane with a 10-kDa cutoff. The polypeptides identified are reported in Table 5.
The protein bands from the fraction with a molecular mass of <10 kDa (Fig. 3) were separated by SDS-PAGE and identified. Identification by nano-LC–ESI–MS-MS (Table 5) showed that defensin-1 (NCBI accession number P81929) and defensin-2 (NCBI accession number P81930), with molecular masses of 5.2 and 5.4 kDa, were the small proteins contained in this fraction. A nonspecific lipid transfer protein (nsLTP) (accession number O24309), with a molecular mass of 10.49 kDa, was also identified.
TABLE 5.
Antifungal polypeptides identified by nano-LC–ESI–MS-MS in the water/salt-soluble extract from the pea flour hydrolysatea after 6 h of incubation at 30°C
| Pisum sativum protein | NCBI accession no. | Sequenceb | Theoretical mass (kDa) | Coverage (%) | Mascot score |
|---|---|---|---|---|---|
| Defensin-1 | P81929 | KTCEHLADTYRGVCFTNASCDDHCKNKAHLISGTCHNWKCFCTQNC | 5.21 | 82 | 55 |
| Defensin-2 | P81930 | KTCENLSGTFKGPCIPDGNCNKHCRNNEHLLSGRCRDDFRCWCTNRC | 5.40 | 75 | 54 |
| Nonspecific lipid transfer protein | O24309 | MVVVSAPMAEAAISCGAVTAAVAPCFAYLKGAPSPSLQCCGGVRRLNGIPDRKGVCNCLKGAAGSVPGLKPGVAALPAN VVLDFLSRLA PLPTVMPSVFKKKCG | 10.49 | 60 | 65 |
Obtained with a neutral protease from Aspergillus oryzae (Veron PS; dosage, 25 g/100 kg of protein).
Single-letter amino acid code is used.
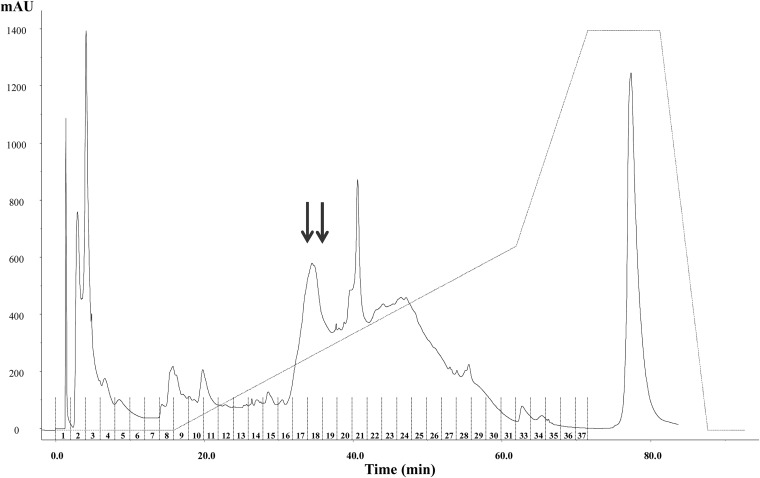
The active fraction with a molecular mass of <3 kDa was further purified by RP-FPLC, separating 37 fractions (Fig. 4). Antifungal activity was found only in fractions 17 and 18. The peptides were identified by nano-LC–ESI–MS-MS, followed by an MS-MS ion search with the Mascot search engine. Five peptides, with 9 to 18 amino acid residues, were identified (Table 6). The LNTNNAAALPGK peptide (1,182.6 Da), with a total net charge of +1, was found in both the active fractions. This peptide is encrypted in the sequences of lipid transfer proteins (LTP2, LTP5, LTP6, LTP4). The SEAEDFFSKITPK peptide (1,497.7 Da; hydrophobic ratio, 30%; total net charge, −1), encrypted in the 13-kDa pea albumin (PA1 A, chain b), was found in fraction 17. The ELAFPGSSHEVDR sequence (1,442.7 Da; total net charge, −2; total hydrophobic ratio, 30%), encrypted in pea provicilin, was also found in fraction 17. LSSGDVFVIPAGHPVAVK, which was identified in fraction 18, was the only neutral peptide, with an experimental molecular mass of 1,791.9 Da and a total hydrophobic charge of 50%. This peptide is part of the vicilin protein. Fraction 18 also contained SEAEDFFSK (1,058.4 Da; hydrophobic ratio, 33%; total net charge, −2), similar to a peptide found in fraction 17 but without the last four amino acid residues at the C terminus.
FIG 4.
RP-FPLC chromatogram of the purified fraction (ultrafiltration through a membrane with a 3-kDa cutoff) from the water/salt-soluble extract from the pea flour hydrolysate. Arrows indicate peptide fractions with the highest antifungal activity.
TABLE 6.
Sequences of peptides contained in partially purified fractions 17 and 18 of the water/salt-soluble extract of the pea flour hydrolysatea after 6 h of incubation at 30°C
| Fraction no.b | Sequencec | Score | Mass (Da) |
NCBI accession no.|source protein | |
|---|---|---|---|---|---|
| Calculated | Expected | ||||
| 17 | SEAEDFFSKITPK | 55 | 1,497.7351 | 1,497.7375 | P62926.1|ALB1A_PEA, albumin-1 A, chain b (leginsulin A) |
| ELAFPGSSHEVDR | 59 | 1,442.6790 | 1,442.6777 | P02854.1|VCLB_PEA, provicilin | |
| LNTNNAAALPGK | 73 | 1,182.6357 | 1,182.6362 | A0AT29.1|NLTP2_LENCU, nonspecific lipid transfer protein 2 | |
| 18 | LSSGDVFVIPAGHPVAVK | 28 | 1,791.9883 | 1,791.9899 | P13918|VCLC_PEA, vicilin |
| SEAEDFFSK | 52 | 1,058.4556 | 1,058.4564 | P62926.1|ALB1A_PEA, albumin-1 A, chain b (leginsulin A) | |
| LNTNNAAALPGK | 65 | 1,182.6357 | 1,182.6351 | A0AT29.1|NLTP2_LENCU, nonspecific lipid-transfer protein 2 | |
Obtained with a neutral protease from Aspergillus oryzae (Veron PS; dosage, 2 5g/100 kg of protein).
Fraction numbers are from Fig. 4.
Single-letter amino acid code is used.
The WSE from the pea flour hydrolysate, the fraction with molecular masses from 3 to 10 kDa, and purified FPLC fractions 17 and 18 were freeze-dried and were redissolved in WFH to determine the MIC. No germination of P. roqueforti DPPMAF1 conidia was found at the following concentrations: 9.0 mg protein/ml for WSE, 2.2 mg protein/ml for the 3- to 10-kDa fraction, and 2.5 mg peptide/ml for pooled fractions 17 and 18.
The peptides identified were chemically synthesized and were assayed at concentrations of 1 to 10 mg of peptides/ml. At the concentrations tested, the inhibitory activities of all peptides except for LSSGDVFVIPAGHPVAVK were confirmed. The MICs ranged from 6.0 (LNTNNAAALPGK) to 9.0 (ELAFPGSSHEVDR) mg/ml. When the peptides were used together, a MIC of 2 mg of peptides/ml was found.
Baking test.
Five different breads were manufactured at the pilot plant according to the formulas in Table 2. At the end of fermentation, the cell density of L. plantarum 1A7 was ca. 9 log CFU/g of sourdough.
The pH values were in the range from 5.10 ± 0.05 to 5.50 ± 0.12 for breads fermented with baker's yeast alone. Breads containing sourdough (SB and PSB) had pH values ca. 1 unit lower (Table 7). Addition of the freeze-dried pea flour hydrolysate did not affect the pH. Slightly but significantly (P < 0.05) higher TTA was found for PWB and PSB than for WB and SB, respectively. No significant differences (P > 0.05) were found for the moisture and aw values (Table 7).
TABLE 7.
Chemical and textural characteristics, and image and color analyses, of breads
| Characteristic | Valuea for the following breadb: |
||||
|---|---|---|---|---|---|
| WB | WBcp | SB | PWB | PSB | |
| Chemical characteristics | |||||
| pH | 5.50 ± 0.12 A | 5.48 ± 0.03 A | 4.23 ± 0.02 B | 5.39 ± 0.05 A | 4.12 ± 0.03 B |
| Total titratable acidity | 3.0 ± 0.1 B | 3.1 ± 0.1 B | 6.1 ± 0.2 A | 3.6 ± 0.1 B | 6.4 ± 0.2 A |
| Moisture (%) | 31.5 ± 0.3 | 31.3 ± 0.3 | 31.4 ± 0.2 | 31.2 ± 0.3 | 31.4 ± 0.2 |
| aw | 0.951 ± 0.02 | 0.950 ± 0.02 | 0.949 ± 0.02 | 0.952 ± 0.02 | 0.951 ± 0.02 |
| Textural characteristics | |||||
| Specific vol (cm3/g) | 2.38 ± 0.02 B | 2.36 ± 0.01 B | 2.47 ± 0.03 A | 2.39 ± 0.03 B | 2.50 ± 0.05 A |
| Hardness (g) | 3,410 ± 12 A | 3,405 ± 16 A | 3,367 ± 11 B | 3,414 ± 13 A | 3,365 ± 9 B |
| Resilience | 0.65 ± 0.03 A | 0.67 ± 0.01 A | 0.55 ± 0.02 B | 0.65 ± 0.01 A | 0.57 ± 0.02 B |
| Fracturability (g) | 3,049 ± 4 A | 3,053 ± 8 A | 2,952 ± 7 B | 3,032 ± 8 A | 2,956 ± 8 B |
| Image analysis, black pixel area (%) | 39.7 ± 0.3 B | 39.4 ± 0.3 B | 42.1 ± 0.1 A | 40.6 ± 0.4 B | 42.3 ± 0.4 A |
| Color analysis (crust) | |||||
| L | 65.2 ± 0.3 A | 65.0 ± 0.2 A | 57.4 ± 0.2 C | 63.0 ± 0.2 B | 56.9 ± 0.2 C |
| a | 7.1 ± 0.1 C | 7.4 ± 0.2 C | 10.8 ± 0.2 A | 9.5 ± 0.1 B | 11.2 ± 0.2 A |
| b | 31.9 ± 0.2 A | 31.6 ± 0.3 A | 32.4 ± 0.3 A | 30.9 ± 0.2 B | 32.1 ± 0.3 A |
| dE | 40.5 ± 0.2 B | 40.3 ± 0.2 B | 47.3 ± 0.1 A | 41.8 ± 0.1 B | 47.6 ± 0.1 A |
pH and total titratable acidity were determined before baking. Data are means for three independent fermentations analyzed twice. Values in the same row followed by different capital letters differ significantly (P < 0.05).
WB, bread started with baker's yeast; WBcp, bread started with baker's yeast, containing calcium propionate; SB, bread started with baker's yeast and Lactobacillus plantarum 1A7 sourdough (S1A7); PWB, bread started with baker's yeast, containing pea flour hydrolysate; PSB, bread started with baker's yeast and sourdough S1A7, containing pea flour hydrolysate. The ingredients used for bread making are listed in Table 2.
After baking, slices of each bread were cut and were inoculated with a suspension (102 conidia/ml) of P. roqueforti DPPMAF1. The slices were packed in polyethylene film and were stored at room temperature. The bread slices were observed for fungal contamination for 21 days (Table 8). During storage, the moisture of all the slices was in the range of 29.5 to 31.0%. After 7 days of storage, ca. 40% of the surface of the WB slice was colonized by P. roqueforti DPPMAF1. At the same time, no growth of P. roqueforti DPPMAF1 was found for WBcp, PWB, or PSB. Weak fungal contamination appeared in the slices of SB. After 14 days of storage, fungal contamination was clearly visible in WBcp and SB also, and a fungal mycelium started to grow in PWB. No fungal contamination was found in PSB before 21 days of storage.
TABLE 8.
Fungal contamination of slices of breads
| Days | Contaminationa of the following breadb: |
||||
|---|---|---|---|---|---|
| WB | WBcp | SB | PWB | PSB | |
| 7 | ++ | − | +/− | − | − |
| 14 | +++ | + | + | +/− | − |
| 21 | ++++ | ++ | +++ | +/− | − |
Scored as follows: −, 0% contamination of the slice surface; +/−, 10% contamination; +, 20% contamination; ++, 40% contamination; +++, 80% contamination; ++++, 100% contamination. Data are means for three independent fermentations analyzed twice.
WB, bread started with baker's yeast; WBcp, bread started with baker's yeast, containing calcium propionate; SB, bread started with baker's yeast and Lactobacillus plantarum 1A7 sourdough (S1A7); PWB, bread started with baker's yeast, containing pea flour hydrolysate; PSB, bread started with baker's yeast and sourdough S1A7, containing pea flour hydrolysate. The ingredients used for bread making are listed in Table 2.
Characterization of breads.
The specific volumes of breads produced without sourdough did not show significant differences (P > 0.05) (Table 7), whereas SB and PSB had higher specific volumes. SB and PSB also showed hardness values, corresponding to the peak force of the first compression of the product, slightly but significantly (P < 0.05) lower than those of the other breads. The same trend was found for resilience and fracturability, which, respectively, indicated how well the bread fights to regain its original position and the first significant peak force during compression of the bread.
Digital images were preprocessed to detect total crumb cell area by binary conversion (black/white pixels). The total cell area of sourdough breads was higher than those of the corresponding breads fermented with baker's yeast alone. However, for this parameter also, no differences were found when pea flour hydrolysate was used as an ingredient.
The crust showed lightness (L) values ranging from 65.2 ± 0.3 (WB) to 56.9 ± 0.2 (PSB) (Table 7). The color difference (dE), calculated based on the chromaticity coordinates (L, a, and b), did not differ significantly (P > 0.05) between WB, WBcp, and PWB. Only breads produced with sourdough (SB and PSB) significantly (P < 0.05) differed from those samples. In any case, no significant differences (P > 0.05) were found when pea flour hydrolysate was added to baker's yeast or sourdough breads (PWB versus WB and PSB versus SB, respectively).
A few hours after baking, sensory analysis was carried out (Table 9). The sourdough breads (SB and PSB) received the lowest scores for elasticity. The scores for color were similar for WB, WBcp, and PWB, and for SB and PSB. Scores for sourdough bread were significantly (P < 0.05) higher than those for baker's yeast breads. As expected, the same was found also for acid taste and flavor. Baker's yeast breads had the highest values for sweetness and dryness. The addition of pea flour hydrolysate in PWB was judged as determining a slight but significant (P < 0.05) decrease in the dryness score from those for WB and WBcp. The overall perception of taste was highest for SB and PSB, followed by PWB. The lowest scores were assigned to WB and WBcp.
TABLE 9.
Sensory analysis of breads
| Breada | Scoreb for the following sensory attribute: |
||||||
|---|---|---|---|---|---|---|---|
| Elasticity | Color | Acid taste | Acid flavor | Sweetness | Dryness | Taste | |
| WB | 7.5 ± 0.2 A | 4.5 ± 0.5 C | 2.3 ± 0.2 B | 2.5 ± 0.5 B | 6.4 ± 0.5 A | 5.8 ± 0.3 A | 3.9 ± 0.6 C |
| WBcp | 7.4 ± 0.3 A | 4.5 ± 0.4 C | 2.5 ± 0.5 B | 2.5 ± 0.3 B | 6.2 ± 0.3 A | 5.6 ± 0.3 A | 3.8 ± 0.4 C |
| SB | 7.0 ± 0.6 B | 7.7 ± 0.5 A | 7.0 ± 0.6 A | 6.4 ± 0.8 A | 5.4 ± 0.3 B | 3.6 ± 0.2 C | 7.8 ± 0.5 A |
| PWB | 7.5 ± 0.6 A | 5.0 ± 0.6 B | 2.8 ± 0.6 B | 2.9 ± 0.8 B | 6.1 ± 0.3 A | 5.2 ± 0.2 B | 4.3 ± 0.5 B |
| PSB | 7.2 ± 0.2 B | 7.8 ± 0.6 A | 7.3 ± 0.4 A | 6.0 ± 0.8 A | 5.2 ± 0.3 B | 3.8 ± 0.2 C | 7.9 ± 0.7 A |
WB, bread started with baker's yeast; WBcp, bread started with baker's yeast, containing calcium propionate; SB, bread started with baker's yeast and Lactobacillus plantarum 1A7 sourdough (S1A7); PWB, bread started with baker's yeast, containing pea flour hydrolysate; PSB, bread started with baker's yeast and sourdough S1A7, containing pea flour hydrolysate. The ingredients used for bread making are listed in Table 2.
Scored on a scale from 0 to 10. Data are means for three independent fermentations analyzed twice. Means in the same column followed by different capital letters are significantly different (P < 0.05).
DISCUSSION
Staling, and especially fungal spoilage, still remains the major factor responsible for huge economic losses in the bakery industries (36). The European directive on preservatives (37) has decreased the allowable concentrations of sorbate (0.2%, wt/wt) and propionate (0.3%, wt/wt) due to potential negative effects on consumer health (38). Moreover, it has been shown that prolonged use of calcium propionate at a high concentration stimulated resistant strains of P. roqueforti (39). Ethanol has inhibitory activity against fungi in baked goods, and it has GRAS (generally recognized as safe) status, but it may have weak antifungal activity, depending on the fungal species, even at the maximum allowable concentration (2%, wt/wt) (40).
With the aim of identifying novel natural preservatives with marked antifungal activity, hydrolysates from legume flours with high protein contents were produced and screened. Overall, proteolysis may enhance food digestibility and decrease the effects of toxic, allergenic and antinutritional compounds (41). Although food proteins are sources of indispensable nutrients (42), it has been well established that they may release encrypted biologically active sequences due to enzyme degradation (43), thus assuming technological and functional significance (42).
Among the samples screened, the hydrolysate made from pea flour with a neutral protease from Aspergillus oryzae (Veron PS) showed the highest antifungal activity against the indicator, P. roqueforti DPPMAF1, while hydrolysates from lentil and faba bean flours showed lower activity. Untreated pea flour showed weak antifungal activity, which increased markedly after moderate hydrolysis. It could be hypothesized that the inhibitory activity was dependent on proteolysis and that extensive hydrolysis, related to prolonged incubation, had a detrimental effect on antifungal sequences already released during the first step of the process. A similar phenomenon was observed previously for bioactive peptides from other food matrices (44).
Fractionation and purification of the water/salt-soluble extract from the pea flour hydrolysate allowed the identification of compounds that were responsible for antifungal activity: defensin-1 and defensin-2, nonspecific lipid transfer proteins (nsLTPs), and a mixture of low-molecular-mass peptides. Defensin-1 and defensin-2 have been identified in pea seeds (45) and were already recognized as markedly inhibitory toward A. niger, A. versicolor, and Fusarium solani (45). Pea defensins are localized predominantly in vascular bundles and epidermal tissues, indicating their contribution to the defense system, because these tissues represent the first barriers to pathogen invasion (epidermal tissues) and dissemination (vascular tissues) (45). According to sequence homologies, pea defensins may be included in a large family of small, basic, Cys-rich proteins that are conserved throughout the plant kingdom (e.g., wheat, barley, spinach, and several members of the Brassicaceae) and have similar defense functions (45). nsLTPs are basic, 9- to 10-kDa proteins that have the capacity to bind to a variety of different lipids and transfer them between membranes (5). Plant nsLTPs are synthesized as precursors, with an N-terminal extension showing the characteristic sequence of a signal peptide (46). Barley nsLTPs are upregulated in response to infection by various fungal pathogens (47). nsLTPs with antifungal activity have also been isolated from radish and onion seeds, sugar beets, barley, and maize (5). Among legumes, an antifungal nsLTP isolated from mung beans (Phaseolus mungo) induced swelling in pathogens (cell wall disruption, release of cell sap, cytoplasmic leakage) (5). The antifungal activity of the WSE from pea flour before treatment with protease is probably due to pea defensins and nsLTP in their native form.
After proteolysis, purification of the active fractions with molecular masses lower than 3 kDa allowed the identification of a mixture of peptides, which markedly improved antifungal activity. Two of the five peptides identified (SEAEDFFSKITPK and SEAEDFFSK) are encrypted in pea albumin-1 A, chain b, a well-characterized 11-kDa protein called leginsulin A. The pea leginsulin is considered the most promising bioinsecticide of plant origin (48). Its insecticidal activity has been shown against cereal weevils (genus Sitophilus), the mosquitoes Culex pipiens and Aedes aegypti, and some aphids (48). This study first reported the hydrolysis products of this protein as antifungal agents. The sequences LSSGDVFVIPAGHPVAVK and ELAFPGSSHEVDR are encrypted in pea vicilin and provicilin, respectively. Vicilin is one of the major pea storage proteins (49), and antifungal peptides with high homology to vicilin have already been isolated from other seeds, such as cowpea (Vigna unguiculata) and melon (Cucumis melo) seeds (50, 51). Also, LNTNNAAALPGK, a sequence encrypted in nsLTPs, was identified in the pea flour hydrolysate. Peptides derived from the hydrolysis of antifungal proteins have often shown higher activity than their precursors (52). The MIC of the mixture of chemically synthesized peptides was similar to that found for the pool of the two purified fractions; nevertheless, when singly tested, all peptides showed higher MIC values, thus indicating a synergistic effect of the compounds.
Antifungal activity was not affected by thermal treatment, which mimicked the temperature (below 100°C) of the bread crumb during baking (53). Although the effectiveness of many antifungal compounds is pH dependent (39), the activity of the pea flour hydrolysate was not affected by a range of pH values that are compatible with those of leavened baked goods (35).
The water/salt-soluble extract from the pea flour hydrolysate had a higher fungistatic effect than calcium propionate (0.3%, wt/vol) on conidial germination by P. roqueforti DPPMAF1, suggesting a different mechanism of inhibition (1). The MICs of the partially purified fractions were comparable to those of similar proteinaceous substances (1, 13, 39). Inhibitory activity was shown toward several fungal species, which are commonly isolated from bakeries and baked goods. The level of inhibition was higher than 10% for 13 of the 15 fungal species that were assayed. The spectrum of activity differed from those found previously for other natural substances, antifungal yeasts (Meyerozyma guilliermondii, Wickerhamomyces anomalus), and sourdough lactic acid bacteria (1, 6).
The effect of the combination of sourdough fermentation and the addition of freeze-dried pea flour hydrolysate was investigated. L. plantarum 1A7, previously selected for antifungal activity and successfully used for prolonging the shelf life of baked goods (6), was used to produce sourdough. L. plantarum 1A7 had the capacity to release antifungal peptides from native proteins during fermentation (6). Bread was made with the freeze-dried pea flour hydrolysate (1.6%, wt/wt) as an ingredient in the formula. From the perspective of industrial scale, the use of the freeze-dried hydrolysate can markedly simplify the technology, as reported already for other plant extracts (1, 8, 13). As shown under pilot plant conditions, the bread made with the combination of sourdough and pea flour hydrolysate had the longest shelf life. The simplest addition of the freeze-dried pea flour hydrolysate also gave promising results. Under conditions of a high artificial conidial inoculum, fungal growth was delayed at least until 21 days of storage at room temperature. It was found that the inhibitory activity was higher than that for calcium propionate (0.3%, wt/wt). The use of pea flour hydrolysate as an ingredient did not affect the textural characteristics of baker's yeast and sourdough breads. Also, sensory analysis demonstrated that pea flour hydrolysate did not cause any detectable effects on either type of bread. However, relative to those for baker's yeast bread, many of the textural and sensory features improved with the use of sourdough.
The process proposed here for the preparation and the use of pea flour hydrolysate, as for the selected sourdough fermentation, can easily be transferred to an industrial scale, since it does not require specific adaptations of classical bakery plants.
Overall, the pea is rich in proteins, carbohydrates, fiber, vitamins, and minerals, even though it contains low levels of antinutritional factors (42); moreover, as one of the most cultivated legumes in the world, it has marked nutritional and economic significance (42, 54).
It has been found previously that enzyme digestion of pea proteins may improve their digestibility, lower allergy symptoms, and improve the physiological activity of human intestinal bacteria (42). In this study, the promising use of a pea flour hydrolysate as a natural biopreservative to considerably extend the shelf life of bread, without affecting its rheological and sensory properties, was demonstrated.
ACKNOWLEDGMENTS
We thank Ferrero S.p.a. (Alba, Cuneo, Italy), Stefano Bordignon (Ferrero S.p.a.), and Davide Mivervini (Molini Tandoi Pellegrino, Corato, Italy) for their kind collaboration.
REFERENCES
- 1.Coda R, Rizzello CG, Nigro F, De Angelis M, Arnault P, Gobbetti M. 2008. Long-term fungal inhibitory activity of water-soluble extracts of Phaseolus vulgaris cv. Pinto and sourdough lactic acid bacteria during bread storage. Appl Environ Microbiol 74:7391–7398. doi: 10.1128/AEM.01420-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Coda R, Rizzello CG, Di Cagno R, Trani A, Cardinali G, Gobbetti M. 2013. Antifungal activity of Meyerozyma guilliermondii: identification of active compounds synthesized during dough fermentation and their effect on long-term storage of wheat bread. Food Microbiol 33:243–251. doi: 10.1016/j.fm.2012.09.023. [DOI] [PubMed] [Google Scholar]
- 3.Dal Bello F, Clarke CI, Ryan LAM, Ulmer H, Schober TJ, Ström K, Sjögren J, van Sinderen D, Schnürer J, Arendt EK. 2007. Improvement of the quality and shelf life of wheat bread by fermentation with the antifungal strain Lactobacillus plantarum FST 1.7. J Cereal Sci 45:309–318. doi: 10.1016/j.jcs.2006.09.004. [DOI] [Google Scholar]
- 4.Ng TB. 2004. Antifungal proteins and peptides of leguminous and non-leguminous origins. Peptides 25:1215–1222. doi: 10.1016/j.peptides.2004.03.012. [DOI] [PubMed] [Google Scholar]
- 5.Wang SY, Wu JH, Ng TB, Ye XY, Rao PF. 2004. A non-specific lipid transfer protein with antifungal and antibacterial activities from the mung bean. Peptides 25:1235–1242. doi: 10.1016/j.peptides.2004.06.004. [DOI] [PubMed] [Google Scholar]
- 6.Coda R, Cassone A, Rizzello CG, Nionelli L, Cardinali G, Gobbetti M. 2011. Antifungal activity of Wickerhamomyces anomalus and Lactobacillus plantarum during sourdough fermentation: identification of novel compounds and long-term effect during storage of wheat bread. Appl Environ Microbiol 77:3484–3492. doi: 10.1128/AEM.02669-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Lavermicocca P, Valerio F, Evidente A, Lazzaroni S, Corsetti A, Gobbetti M. 2000. Purification and characterization of novel antifungal compounds from the sourdough Lactobacillus plantarum strain 21B. Appl Environ Microbiol 66:4084–4090. doi: 10.1128/AEM.66.9.4084-4090.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Rizzello CG, Cassone A, Coda R, Gobbetti M. 2011. Antifungal activity of sourdough fermented wheat germ used as an ingredient for bread making. Food Chem 127:952–959. doi: 10.1016/j.foodchem.2011.01.063. [DOI] [PubMed] [Google Scholar]
- 9.Schnürer J, Magnusson J. 2005. Antifungal lactic acid bacteria as biopreservatives. Trends Food Sci Technol 16:70–78. doi: 10.1016/j.tifs.2004.02.014. [DOI] [Google Scholar]
- 10.Sjögren J, Magnusson J, Broberg A, Schnürer J, Kenne L. 2003. Antifungal 3-hydroxy fatty acids from Lactobacillus plantarum MiLAB 14. Appl Environ Microbiol 69:7554–7557. doi: 10.1128/AEM.69.12.7554-7557.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Ström K, Sjögren J, Broberg A, Schnürer J. 2002. Lactobacillus plantarum MiLAB 393 produces the antifungal cyclic dipeptides cyclo(l-Phe-l-Pro) and cyclo(l-Phe-trans-4-OH-l-Pro) and 3-phenyllactic acid. Appl Environ Microbiol 68:4322–4327. doi: 10.1128/AEM.68.9.4322-4327.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Niku-Paavola ML, Laitila A, Mattila-Sandholm T, Haikara A. 1999. New types of antimicrobial compounds produced by Lactobacillus plantarum. J Appl Microbiol 86:29–35. doi: 10.1046/j.1365-2672.1999.00632.x. [DOI] [PubMed] [Google Scholar]
- 13.Rizzello CG, Coda R, De Angelis M, Di Cagno R, Carnevali P, Gobbetti M. 2009. Long-term fungal inhibitory activity of water-soluble extract from Amaranthus spp. seeds during storage of gluten-free and wheat flour breads. Int J Food Microbiol 131:189–196. doi: 10.1016/j.ijfoodmicro.2009.02.025. [DOI] [PubMed] [Google Scholar]
- 14.Gobbetti M, Corsetti A, Rossi J. 1994. The sourdough microflora. Interactions between lactic acid bacteria and yeasts: metabolism of carbohydrates. Appl Environ Microbiol 41:456–460. [DOI] [PubMed] [Google Scholar]
- 15.Heiniö RL, Lehtinen P, Oksman-Caldentey KM, Poutanen K. 2002. Differences between sensory profiles and development of rancidity during long-term storage of native and processed oat. Cereal Chem 79:367–375. doi: 10.1094/CCHEM.2002.79.3.367. [DOI] [Google Scholar]
- 16.Dias DR, Vilela DM, Silvestre MPC, Schwan RF. 2008. Alkaline protease from Bacillus sp. isolated from coffee bean grown on cheese whey. World J Microbiol Biotechnol 24:2027–2034. doi: 10.1007/s11274-008-9706-6. [DOI] [Google Scholar]
- 17.AACC. 2000. Approved methods of the American Association of Cereal Chemists, 10th ed AACC, St. Paul, MN. [Google Scholar]
- 18.Rizzello CG, De Angelis M, Di Cagno R, Camarca A, Silano M, Losito I, De Vincenzi M, De Bari MD, Palmisano F, Maurano F, Gianfrani C, Gobbetti M. 2007. Highly efficient gluten degradation by lactobacilli and fungal proteases during food processing: new perspectives for celiac disease. Appl Environ Microbiol 73:4499–4507. doi: 10.1128/AEM.00260-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Rizzello CG, Curiel JA, Nionelli L, Vincentini O, Di Cagno R, Silano M, Gobbetti M, Coda R. 2014. Use of fungal proteases and selected sourdough lactic acid bacteria for making wheat bread with an intermediate content of gluten. Food Microbiol 37:59–68. doi: 10.1016/j.fm.2013.06.017. [DOI] [PubMed] [Google Scholar]
- 20.Osborne TB. 1907. The proteins of the wheat kernel. Carnegie Institute of Washington, publication 84. Judd and Detweiler, Washington, DC. [Google Scholar]
- 21.Weiss W, Vogelmeier C, Görg A. 1993. Electrophoretic characterization of wheat grain allergens from different cultivars involved in bakers' asthma. Electrophoresis 14:805–816. doi: 10.1002/elps.11501401126. [DOI] [PubMed] [Google Scholar]
- 22.Church FC, Swaisgood HE, Porter DH, Catignani GL. 1983. Spectrophotometric assay using o-phthaldialdehyde for determination of proteolysis in milk and isolated milk proteins. J Dairy Sci 66:1219–1227. doi: 10.3168/jds.S0022-0302(83)81926-2. [DOI] [Google Scholar]
- 23.Bradford MM. 1976. A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal Biochem 72:248–254. doi: 10.1016/0003-2697(76)90527-3. [DOI] [PubMed] [Google Scholar]
- 24.Quiroga EN, Sampietro AR, Vattuone MA. 2001. Screening antifungal activities of selected medicinal plants. J Ethnopharmacol 74:89–96. doi: 10.1016/S0378-8741(00)00350-0. [DOI] [PubMed] [Google Scholar]
- 25.Magnusson J, Schnürer J. 2001. Lactobacillus coryniformis subsp. coryniformis strain Si3 produces a broad-spectrum proteinaceous antifungal compound. Appl Environ Microbiol 67:1–5. doi: 10.1128/AEM.67.1.1-5.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Atanassova M, Choiset Y, Dalgalarrondo M, Chobert JM, Dousset X, Ivanova I, Haertlé T. 2003. Isolation and partial biochemical characterization of a proteinaceous anti-bacteria and anti-yeast compound produced by Lactobacillus paracasei subsp. paracasei strain M3. Int J Food Microbiol 87:63–73. doi: 10.1016/S0168-1605(03)00054-0. [DOI] [PubMed] [Google Scholar]
- 27.Laemmli UK. 1970. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature 227:680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- 28.Chen Y, Kwon SW, Kim SC, Zhao Y. 2005. Integrated approach for manual evaluation of peptides identified by searching protein sequence databases with tandem mass spectra. J Proteome Res 4:998–1005. doi: 10.1021/pr049754t. [DOI] [PubMed] [Google Scholar]
- 29.Gobbetti M. 1998. The sourdough microflora: interactions of lactic acid bacteria and yeasts. Trends Food Sci Technol 9:267–274. doi: 10.1016/S0924-2244(98)00053-3. [DOI] [Google Scholar]
- 30.Gámbaro A, Fiszman S, Giménez A, Varela P, Salvador A. 2004. Consumer acceptability compared with sensory and instrumental measures of white pan bread: sensory shelf-life estimation by survival analysis. J Food Sci 69:401–405. doi: 10.1111/j.1365-2621.2004.tb09957.x. [DOI] [Google Scholar]
- 31.Rizzello CG, Coda R, Mazzacane F, Minervini D, Gobbetti M. 2012. Micronized by-products from debranned durum wheat and sourdough fermentation enhanced the nutritional, textural and sensory features of bread. Food Res Int 46:304–313. doi: 10.1016/j.foodres.2011.12.024. [DOI] [Google Scholar]
- 32.Ahrné L, Andersson CG, Floberg P, Rosen J, Lingnert H. 2007. Effect of crust temperature and water content on acrylamide formation during baking of white bread: steam and falling temperature baking. LWT 40:1708–1715. doi: 10.1016/j.lwt.2007.01.010. [DOI] [Google Scholar]
- 33.Haglund Å, Johansson L, Dahlstedt L. 1998. Sensory evaluation of wholemeal bread from ecologically and conventionally grown wheat. J Cereal Sci 27:199–207. doi: 10.1006/jcrs.1997.0155. [DOI] [Google Scholar]
- 34.Lotong V, Iv EC, Chambers DH. 2000. Determination of the sensory attributes of wheat sourdough bread. J Sens Stud 15:309–326. doi: 10.1111/j.1745-459X.2000.tb00273.x. [DOI] [Google Scholar]
- 35.Rizzello CG, Nionelli L, Coda R, Di Cagno R, Gobbetti M. 2010. Use of sourdough fermented wheat germ for enhancing the nutritional, texture and sensory characteristics of the white bread. Eur Food Res Technol 230:645–654. doi: 10.1007/s00217-009-1204-z. [DOI] [Google Scholar]
- 36.Gray JA, Bemiller JN. 2003. Bread staling: molecular basis and control. Compr Rev Food Sci Food Saf 2:1–21. doi: 10.1111/j.1541-4337.2003.tb00011.x. [DOI] [PubMed] [Google Scholar]
- 37.European Union. 18 March 1995. European Parliament and Council Directive No 95/2/EC of 20 February 1995 on food additives other than colours and sweeteners. OJ L 61. http://eur-lex.europa.eu/legal-content/EN/TXT/PDF/?uri=OJ:L:1995:061:FULL&from=EN.
- 38.Pattison TL, Lindsay D, von Holy A. 2004. Natural antimicrobials as potential replacements for calcium propionate in bread. S Afr J Sci 100:339–342. [Google Scholar]
- 39.Suhr KI, Nielsen PV. 2004. Effect of weak acid preservatives on growth of bakery product spoilage fungi at different water activities and pH values. Int J Food Microbiol 95:67–78. doi: 10.1016/j.ijfoodmicro.2004.02.004. [DOI] [PubMed] [Google Scholar]
- 40.Dantigny P, Guilmart A, Radoi F, Bensoussan M, Zwietering M. 2005. Modelling the effect of ethanol on growth rate of food spoilage moulds. Int J Food Microbiol 98:261–269. doi: 10.1016/j.ijfoodmicro.2004.07.008. [DOI] [PubMed] [Google Scholar]
- 41.Szymkiewicz A, Jêdrychowski L, Wagner A. 2007. Wpływ obróbki termicznej i hydrolizy enzymatycznej na alergennoœć białek grochu. Żywnoœć Nauka Technologia Jakoœć 3:147–158. [Google Scholar]
- 42.Światecka D, Światecki A, Kostyra H, Marciniak-Darmochwal K, Kostyra E. 2010. The impact of pea protein hydrolysates on bacterial physiological activity—an in vitro study. Int J Food Microbiol 140:263–270. doi: 10.1016/j.ijfoodmicro.2010.03.015. [DOI] [PubMed] [Google Scholar]
- 43.Coda R, Rizzello CG, Pinto D, Gobbetti M. 2012. Selected lactic acid bacteria synthesize antioxidant peptides during sourdough fermentation of cereal flours. Appl Environ Microbiol 78:1087–1096. doi: 10.1128/AEM.06837-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Rizzello CG, Cassone A, Di Cagno R, Gobbetti M. 2008. Synthesis of angiotensin I-converting enzyme (ACE)-inhibitory peptides and γ-aminobutyric acid (GABA) during sourdough fermentation by selected lactic acid bacteria. J Agric Food Chem 56:6936–6943. doi: 10.1021/jf800512u. [DOI] [PubMed] [Google Scholar]
- 45.Almeida MS, Cabral KM, Zingali RB, Kurtenbach E. 2000. Characterization of two novel defense peptides from pea (Pisum sativum) seeds. Arch Biochem Biophys 378:278–286. doi: 10.1006/abbi.2000.1824. [DOI] [PubMed] [Google Scholar]
- 46.Bernhard WR, Thoma S, Botella J, Somerville CR. 1991. Isolation of a cDNA clone for spinach lipid transfer protein and evidence that the protein is synthesized by the secretory pathway. Plant Physiol 95:164–170. doi: 10.1104/pp.95.1.164. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.García-Olmedo F, Molina A, Segura A, Moreno M. 1995. The defensive role of nonspecific lipid-transfer proteins in plants. Trends Microbiol 3:72–74. doi: 10.1016/S0966-842X(00)88879-4. [DOI] [PubMed] [Google Scholar]
- 48.Gressent F, de Silva P, Eyraud V, Karaki L, Royer C. 2011. Pea albumin 1 subunit b (PA1b), a promising bioinsecticide of plant origin. Toxins (Basel) 3:1502–1517. doi: 10.3390/toxins3121502. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Barac M, Cabrilo S, Pesic M, Stanojevic S, Zilic S, Macej O, Ristic N. 2010. Profile and functional properties of seed proteins from six pea (Pisum sativum) genotypes. Int J Mol Sci 11:4973–4990. doi: 10.3390/ijms11124973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Gomes VM, Mosqueda MI, Blanco-Labra A, Sales MP, Fernandes KVS, Cordeiro RA, Xavier-Filho J. 1997. Vicilin storage proteins from Vigna unguiculata (legume) seeds inhibit fungal growth. J Agric Food Chem 45:4110–4115. doi: 10.1021/jf960942g. [DOI] [Google Scholar]
- 51.Ribeiro SFF, Agizzio AP, Machado OLT, Neves-Ferreira AGC, Oliveira MA, Fernandes KVS, Carvalho AO, Perales J, Gomes VM. 2007. A new peptide of melon seeds which shows sequence homology with vicilin: partial characterization and antifungal activity. Sci Hortic 111:399–405. doi: 10.1016/j.scienta.2006.11.004. [DOI] [Google Scholar]
- 52.Rizzello CG, Losito I, Gobbetti M, Carbonara T, De Bari MD, Zambonin PG. 2005. Antibacterial activities of peptides from the water-soluble extracts of Italian cheese varieties. J Dairy Sci 88:2348–2360. doi: 10.3168/jds.S0022-0302(05)72913-1. [DOI] [PubMed] [Google Scholar]
- 53.Zanoni B, Peri C, Pierucci S. 1993. A study of the bread-baking process. I. A phenomenological model. J Food Eng 19:389–398. [Google Scholar]
- 54.Adsule RN, Kadam SS. 1989. Proteins, pp. 75–97. In Salunkhe DK, Kadam SS (ed), Handbook of world food legumes: nutritional chemistry, vol II Processing technology and utilization CRC Press, Boca Raton, FL. [Google Scholar]