Abstract
Human noroviruses (HuNoVs) are the most common cause of food-borne disease outbreaks, as well as virus-related waterborne disease outbreaks in the United States. Here, we hypothesize that common free-living amoebae (FLA)—ubiquitous in the environment, known to interact with pathogens, and frequently isolated from water and fresh produce—could potentially act as reservoirs of HuNoV and facilitate the environmental transmission of HuNoVs. To investigate FLA as reservoirs for HuNoV, the interactions between two Acanthamoeba species, A. castellanii and A. polyphaga, as well as two HuNoV surrogates, murine norovirus type 1 (MNV-1) and feline calicivirus (FCV), were evaluated. The results showed that after 1 h of amoeba-virus incubation at 25°C, 490 and 337 PFU of MNV-1/ml were recovered from A. castellanii and A. polyphaga, respectively, while only few or no FCVs were detected. In addition, prolonged interaction of MNV-1 with amoebae was investigated for a period of 8 days, and MNV-1 was demonstrated to remain stable at around 200 PFU/ml from day 2 to day 8 after virus inoculation in A. castellanii. Moreover, after a complete amoeba life cycle (i.e., encystment and excystment), infectious viruses could still be detected. To determine the location of virus associated with amoebae, immunofluorescence experiments were performed and showed MNV-1 transitioning from the amoeba surface to inside the amoeba over a 24-h period. These results are significant to the understanding of how HuNoVs may interact with other microorganisms in the environment in order to aid in its persistence and survival, as well as potential transmission in water and to vulnerable food products such as fresh produce.
INTRODUCTION
Noroviruses belong to a highly genetically and antigenically diverse genus Norovirus of the Caliciviridae family. Noroviruses are divided into five genogroups (GI, GII, GIII, GIV, and GV) according to the genetic similarity of highly conserved areas of their genomes, such as the RNA-dependent RNA polymerase and the VP1 protein (1), with GI, GII, and GIV associated with human diseases. They are important enteric pathogens responsible for at least 50% of all gastroenteritis outbreaks worldwide (2) and, partially due to their high infectivity and low infectious dose (18 to 1,000 virus particles) (3), human noroviruses (HuNoVs) are the most common cause of food-borne disease outbreaks in the United States (4).
Transmission of HuNoV occurs primarily through exposure to contaminated food or water, person-to-person contact, aerosolized particles from vomitus, and contact with contaminated fomites (e.g., door handles, toilets, tables, and elevator buttons) (5). Numerous waterborne disease outbreaks have been linked to HuNoV (6–9), and HuNoVs are often detected in surface water (10), wastewater (11), and even finished drinking water (12). With respect to food-borne disease outbreaks, the most common food commodity implicated in HuNoV outbreaks is fresh produce, specifically leafy greens (13); however, the route of transmission and point of contamination are often unknown. For example, national and international outbreaks involving fresh and frozen raspberries or raspberry products (14, 15) and bagged Lollo Bionda lettuce (16) indicated the likelihood of contamination during harvest, transport, or processing of the products. In these instances the source of contamination could be environmental. Another more recent outbreak from 2012 related to frozen strawberries imported by Germany from China caused more than 11,000 cases of acute gastroenteritis in German school children (17). Based on various aspects of fresh produce production—exposure to environmental factors such as soil, compost, and water combined with minimal processing—HuNoVs could be introduced into the supply chain through a variety of routes. In addition, HuNoVs may potentially interact with other microorganisms such as free-living amoebae (FLA) that have been isolated from washed and ready-to-eat fresh produce purchased from retail settings (18, 19). Moreover, our research group has also isolated acanthamoebae from fresh produce available at retail (unpublished data).
Acanthamoeba is a genus of common FLA (20). These amoebae are ubiquitous throughout natural environments (soil, water, and sediments), as well as built environments, including chlorinated swimming pools, drinking water distribution systems, cooling towers, and hospital water networks. There are two stages of the Acanthamoeba life cycle: the active trophozoite stage and the dormant cyst stage. In the trophozoite stage, Acanthamoeba actively feeds on bacteria, yeast, algae, and small organic particles, replicates by mitosis, and has the ability to transform into a cyst when the living environment becomes unfavorable or adverse to survival such as desiccation, nutrient deprivation, or significant temperature change (21). Acanthamoeba cysts are capable of withstanding hostile conditions and a variety of physical (dryness, heat, freezing, and UV radiation) and chemical (chloride and antimicrobials) agents that normally inactivate trophozoites (21–23). Acanthamoeba can also act as an opportunistic pathogen to humans and is responsible for two well-recognized diseases, including (i) Acanthamoeba keratitis, which can lead to blindness, and (ii) fatal granulomatous amoebic encephalitis in immunocompromised populations (20). It is important to note that the diseases caused by Acanthamoeba are primarily associated with contact lens wearers and exposure to amoebae in recreational waters—the latter being quite rare—and are not associated with ingestion of contaminated food products (20).
In addition to its direct infection in humans, more evidence has been reported that Acanthamoeba interacts with other human pathogens, including bacteria and viruses. Even though FLA primarily feed on bacteria, many are known to contain microbial endosymbionts, including human pathogens. This relationship between human pathogens and FLA has been associated with enhanced environmental survival, increased virulence, and increased resistance to biocides and antibiotics in pathogens that undergo intraprotozoal growth (24–26). Bacteria implicated in food-borne disease outbreaks that have been reported to interact with Acanthamoeba include enterohemorrhagic Escherichia coli (27), Salmonella enterica subsp. enterica, Listeria monocytogenes, Clostridium botulinum, and Campylobacter jejuni (26, 28, 29). Studies involving Acanthamoeba interactions with viruses are much more limited, including poliovirus, echoviruses 4 and 30 (30), adenoviruses 11 and 41 (31), and coxsackievirus B3 (25). These amoeba-virus interactions include (i) extended survival (i.e., 21 to at least 75 days depending on the virus type) of viruses by binding to amoeba surface (enteroviruses), (ii) internalization of viruses in amoeba cytoplasm (adenoviruses), and (iii) internalization and survival of viruses through complete life cycle, including trophozoite and cyst forms, of amoebae (coxsackievirus B3). However, to the best of our knowledge, no studies exist on the interaction of FLA with HuNoVs or HuNoV surrogates such as murine norovirus type 1 (MNV-1) and feline calicivirus (FCV).
To investigate whether Acanthamoeba could also serve as a reservoir for the survival and transmission of HuNoV in the environment, we evaluated the interactions between two species of Acanthamoeba (A. castellanii and A. polyphaga) and two HuNoV surrogates (MNV-1 and FCV) under reproducible culture conditions. First, amoeba trophozoites were inoculated with virus, followed by the analysis of virus titers associated with amoebae to evaluate the interactions between amoebae and viruses. Second, the virus-inoculated trophozoites were induced to encyst and then excyst to evaluate the virus titer associated with different stages of the amoeba life cycle. Last, indirect immunofluorescence stain was performed to investigate and determine virus localization in trophozoites.
MATERIALS AND METHODS
Amoeba cultivation.
Two species of Acanthamoeba available at American Type Culture Collection (ATCC; Manassas, VA) were used here: A. castellanii (ATCC 50374) and A. polyphaga (ATCC 30871). The axenic cultivation of the two Acanthamoeba species was in accordance with ATCC protocols. Trophozoites were cultured with 5 ml of peptone-yeast extract-glucose (PYG; ATCC medium 712) medium (pH 6.5) with additives [0.4 mM CaCl2, 4 mM MgSO4·7H2O, 2.5 mM Na2HPO4·7H2O, 2.5 mM KH2PO4, 0.05 mM Fe(NH4)2(SO4)2·6H2O, and 1 g/liter sodium citrate·2H2O] in T-25 flasks at 25°C. When the amoebae formed a nearly 100% confluent sheet of trophozoites on the bottom surface of the flask (i.e., near peak density), the flask was vigorously agitated, and ∼250 μl of the suspended amoebae were transferred to a new tube or flask containing 5 ml of fresh PYG medium.
Virus stock preparation.
Murine norovirus (type 1) and FCV (F9 strain) were prepared as described previously with modifications (32). MNV-1 and FCV stocks, as well as CRFK (Crandell Rees feline kidney) cells, were all kindly provided by Kellogg Schwab at Johns Hopkins School of Public Health, Baltimore, MD. MNV-1 and FCV were propagated in monolayers of RAW 267.4 (mouse leukemic monocyte macrophage [ATCC TIB-71]) and CRFK cells, respectively. RAW 264.7 cells were cultured in Dulbecco modified Eagle medium (DMEM; MediaTech, Inc., Manassas, VA) containing 10% low-endotoxin fetal bovine serum (FBS; Biosera North America, Kansas City, MO), 1% 100× penicillin-streptomycin (pen-strep) solution, and 1% HEPES (Sigma-Aldrich, St. Louis, MO) at 37°C, 5% CO2. After reaching 80 to 90% confluence, the cells were infected with MNV-1 at a multiplicity of infection (MOI) of 0.05. Feline calicivirus was propagated using CRFK cells cultured in minimum essential medium (MediaTech, Inc.) with 10% low-endotoxin FBS (Biosera), 1% 100× pen-strep solution, and 1% nonessential amino acid (Gibco, Grand Land, NY) at an MOI of 0.01. Both viruses were extracted from cell lysate after a complete cytopathic effect was observed as described previously by Gibson and Schwab (33) with modifications. Briefly, the culture medium with cells and viruses were subjected to three freeze-thaw cycles at −80 and 37°C, respectively, followed by centrifugation at 5,000 × g for 20 min, filtration with a 0.01-μm-pore-size filter, and storage at −80°C. To determine the infectious titers of MNV-1 and FCV, a plaque assay reported previously (33) was applied with modifications. Six-well plates were seeded with 2 × 106 or 7 × 105 RAW and CRFK cells, respectively, per well and grown to 80 to 90% (for RAW cells) and 100% (for CRFK cells) confluence in 2 ml of complete growth medium. Cell monolayers were inoculated with virus stocks for 1 h at 37°C, followed by removal of the inocula. Cells were covered with 2 ml of prepared overlay medium containing 1.5% low-melting-point agarose (Invitrogen, Carlsbad, CA) and then incubated for 48 h. After 48 h, 2 ml of 0.1% neutral red (Sigma-Aldrich) in 1× phosphate-buffered saline (PBS) was added to each well to visualize the formed plaques. Plaques were counted 1 to 3 h after the addition of neutral red solution. Plates with 15 to 50 PFU were used to determine the virus titer as the PFU/ml.
Amoeba inoculation with virus.
For amoeba virus inoculation with MNV-1 and FCV, the protocol described by Mattana et al. (25) was used with modifications. Experiments were performed in suspension in 50-ml conical polypropylene tubes inoculated each with 3 × 106 amoebae in 1 ml of DMEM and 1 ml of virus stock suspension to give a virus/amoeba ratio of 0.1 (1:10), 1 (1:1), or 10 (10:1). Amoeba controls without virus received the same volume of DMEM. The amoeba-virus suspensions were then incubated for 1 h at 25°C, followed by centrifugation (100 × g, 5 min) to pellet the amoebic cells. The pellet was subsequently washed with 1× PBS in order to remove unassociated viruses, suspended in 1 ml of DMEM containing 5% FBS and 1% 100× pen-strep solution, and maintained at 25°C. To monitor pH levels (i.e., to ensure a neutral pH range from 7 to 8), DMEM with a phenol red indicator was used for all experiments.
Determination of the virus titer associated with amoebae after virus inoculation.
To determine the number of infectious viruses associated with amoebae after virus inoculation, the method described by Mattana et al. (25) was used with modifications. The virus-amoebic trophozoite suspensions described above were used for these experiments. After 1 h of incubation, followed by washing and resuspension, these amoebic suspensions were centrifuged (400 × g, 10 min) in order to separately recover both cell-free supernatants and pellets. All samples were stored at −80°C until virus titrations were performed. Prior to titration, pellets were suspended in 1 ml of DMEM, the trophozoites were disrupted by six freeze-thaw cycles, and cell debris were removed by centrifugation. Virus titration was performed as described above for virus stock preparation with a cell control (DMEM only), virus positive control (virus only), and amoeba control (amoebae without virus inoculation). All experiments were performed in duplicate and were repeated three times.
Evaluation of amoeba growth after virus inoculation.
Trophozoites inoculated with virus as describe above for amoeba inoculation with virus were washed with sterile 1× PBS, suspended in 2 ml of PYG medium at a concentration of 105 cells/ml, and incubated in six-well plates at 25°C. Amoebae were counted with a hemocytometer at day 2, 4, 6, 8, 10, and 12 after incubation. Amoeba viability was determined by using the trypan blue exclusion method. Amoeba morphology was observed using a Nikon microscope (Nikon Instrument, Inc., Melville, NY) equipped with a ×40 objective lens.
Determining virus titer associated with amoebae over time.
To determine the number of infectious virus associated with amoebae at different time points postinoculation, the method of Mattana et al. (25) was used with modifications. The virus-inoculated amoebic trophozoites described earlier were also used for these experiments. Briefly, immediately (time zero) and at days 1, 2, 4, 6, and 8 after virus incubation, amoebic suspensions were centrifuged (400 × g,10 min) in order to collect amoeba pellets. All samples were stored at −80°C until analyzed to determine the virus concentrations. Before titration, pellets were suspended in 1 ml of DMEM, the trophozoites were disrupted by six freeze-thaw cycles, and the cell debris was removed by centrifugation. The virus titration was performed as described previously with a cell control (DMEM only), virus positive control (virus only), and amoeba control (amoebae without virus inoculation). All tests were performed in duplicate and were repeated three times.
Evaluation of virus associated with amoebae during encystment and excystment.
In these experiments, trophozoites at 2 days after virus inoculation were washed, suspended in 3 ml of sterile PBS, and incubated at 25°C in order to induce their encystment (20). After 2 weeks in cyst form, the trophozoites were recovered. In brief, the cysts were washed twice in PBS and cultured in PYG medium at 25°C for 2 to 4 days to induce their differentiation into the trophozoitic form. Both cysts and recovered trophozoites were then lysed and used for virus titration as described above.
Confirmation of virus localization in amoebae by confocal immunofluorescence.
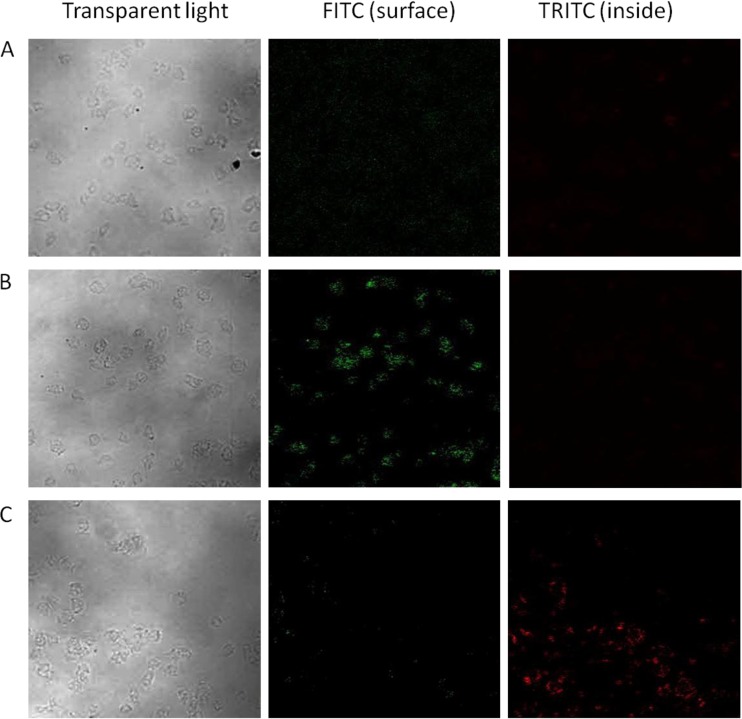
A double indirect immunofluorescence assay was used to determine the location of virus (on the amoeba surface or inside amoebae) as described by Mattana et al. (25) with modifications. At 0 and 24 h postinoculation, suspensions of virus inoculated trophozoites (10:1 virus/amoeba ratio) were centrifuged and then resuspended in 1× PBS, seeded into chamber slides, and incubated for 1 h at 25°C to allow the amoebae to attach to the surface of the slide. Amoebae were then treated with a 1:1,000 dilution of rabbit polyclonal anti-MNV-1 capsid protein 1 antiserum (Alpha Diagnostic International, Inc., San Antonio, TX) for 1 h to detect the virus localization on the surface of trophozoites. After 1 h of incubation at 25°C, the samples were washed with 1× PBS to remove excess antibody, fixed with freezing methanol for 45 min, and incubated with a 1:100 dilution of goat anti-rabbit IgG fluorescein isothiocyanate (FITC)-conjugated antibody (KPL, Gaithersburg, MD). Next, the samples were washed and again exposed to the rabbit polyclonal anti-MNV-1 capsid protein 1 antiserum to detect the virus inside trophozoites followed by incubation with a 1:100 dilution of goat anti-rabbit IgG TRITC (tetramethyl rhodamine isothiocyanate)-conjugated antibody (KPL). After a wash with 1× PBS, the samples were mounted and examined with a Nikon 90i upright scanning laser confocal microscope.
Statistical analyses.
All experiments were performed in either triplicate or duplicate and repeated at least twice. The data were analyzed by using a Student t test using JMP Pro 11 (SAS Institute, Inc., Cary, NC), and the difference between groups was considered significant when P was <0.05.
RESULTS
Qualitative evaluation of virus-amoeba association.
The initial association of HuNoV surrogates with A. castellanii and A. polyphaga was determined based on 1 h of incubation at 25°C. The results indicated that MNV-1 had some interaction with both amoeba species at all three virus/amoeba cell ratios, whereas FCV showed no association with amoebae (data not shown). In order to verify that FCV and amoebae truly had no association as opposed to the possibility of inactivation of FCV, additional control experiments were performed. These experiments included FCV inoculated with Acanthamoeba at a virus/amoeba cell ratio of 1 (3 × 106 of each), and a control with 3 × 106 PFU of FCVs in DMEM—the same medium used for the virus-amoeba interaction studies. After 1 h of incubation at 25°C, followed by centrifugation to pellet amoebic cells, there were no differences in FCVs with 6.48, 6.46, and 6.44 log10 PFU in the initial virus input, DMEM only, and virus-amoeba samples, respectively. These data indicate that FCVs do not associate or interact with A. castellanii and A. polyphaga and remain viable during coincubation. Therefore, based on these results, all remaining experiments were performed with only MNV-1 at a virus/cell ratio of 1 with the exception of the double immunofluorescence assay, which was performed at a virus/cell ratio of 10.
Amoeba growth evaluation.
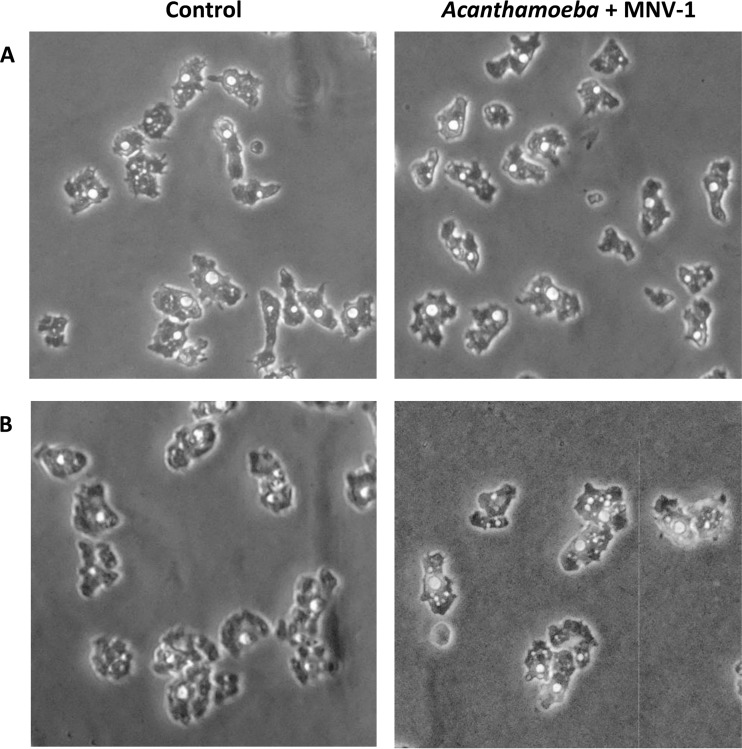
To study the possible effect of MNV-1 inoculation on the growth of A. castellanii and A. polyphaga, the growth rate of noninoculated and virus-inoculated trophozoites, as well as the morphology of trophozoites, was evaluated. After 12 days of incubation at 25°C, control cultures of A. castellanii and A. polyphaga produced about 7.1 × 106 and 4.9 × 106 amoebae/ml, respectively. Under the same conditions, cultures of virus-inoculated trophozoites in both Acanthamoeba species did not show any significant differences (P > 0.05) in growth rate (data not shown). In addition, trophozoites of both amoeba species showed no difference in morphological features between control and virus-inoculated groups under phase-contrast microscopy examination (Fig. 1). Finally, cell viability evaluated by trypan blue was >95% for both amoeba species.
FIG 1.
Light micrographs of Acanthamoeba trophozoites with or without virus inoculation in PYG medium. A. castellanii (A) and A. polyphaga (B) were inoculated with virus (MNV-1) or DMEM only (Control) and then cultured in PYG medium for 8 days.
Quantitative evaluation of virus titer associated with amoebae.
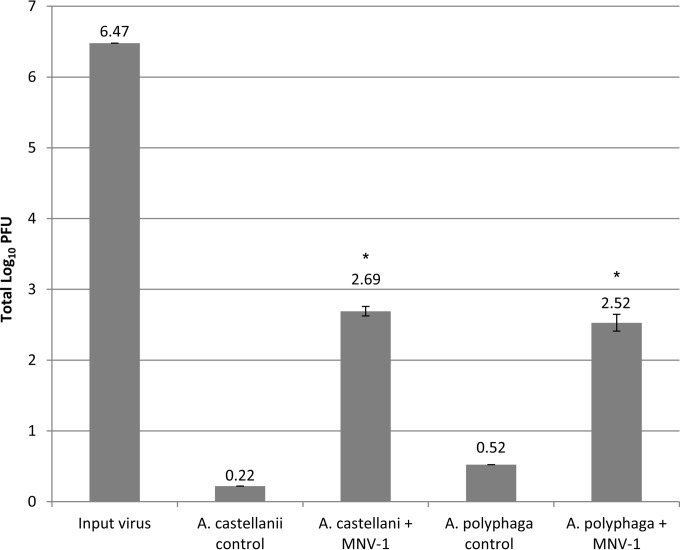
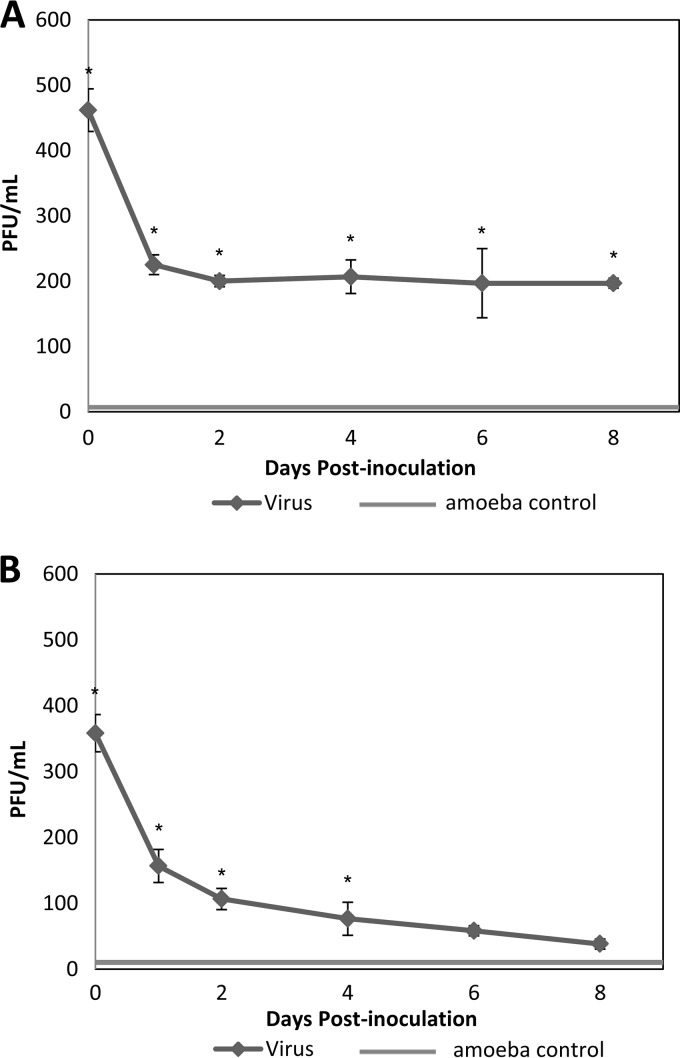
The number of infectious MNV-1 associated with amoebae over time was determined for both amoeba species. The virus titration showed that after 1 h of incubation, 490 and 337 PFU were recovered from A. castellanii and A. polyphaga, respectively (Fig. 2). The input virus value was 6.47 log10, and the total numbers of recovered virus from A. castellanii and A. polyphaga were 2.69 and 2.52 log10, respectively. The kinetic study of infectious MNV-1 associated with amoebae was performed over 8 days. For A. castellanii, MNV-1 decreased during the first 48 h from 2.65 to 2.26 log10; however, from day 4 to day 8 after inoculation, the virus number remained stable at ∼2.27 log10 (Fig. 3A). Conversely, for A. polyphaga, MNV-1 steadily decreased after inoculation. After day 4, there was no significant difference (day 6, P = 0.064; day 8, P = 0.078) between the amoeba control and virus-inoculated groups (Fig. 3B), while the cell negative control showed no plaques. With respect to the amoeba control group samples, amoeba lysates were inoculated onto RAW 264.7 cell monolayers to serve as a negative experimental control. However, occasionally what appeared to be PFU were observed in the amoeba lysate controls. The number of PFU ranged from 1 to 5 per well, although normally no PFU or 1 PFU was observed. Because of the high concentration of MNV-1 used in the experiments, we are confident that these observed PFU levels are not due to contamination with MNV-1, and we consider the reasons for amoeba-only plaques in the Discussion.
FIG 2.
MNV-1 titer associated with A. castellanii and A. polyphaga 1 h after virus-amoeba inoculation at virus/amoeba ratio 1:1. The input virus value was 6.47 log10 (3 × 106 PFU), and the recovered virus value means from A. castellanii and A. polyphaga were 2.69 log10 (490 PFU) and 2.52 log10 (337 PFU), respectively. Values are means ± the standard errors from at least three experiments.*, P < 0.01 versus the amoeba control.
FIG 3.
MNV-1 associated with A. castellanii (A) and A. polyphaga (B) over a period of 8 days after inoculation. Values are means ± the standard errors from at least three experiments. Amoeba control group showed the results of amoeba lysate without virus inoculation. *, P < 0.01 versus the amoeba control.
Quantitative evaluation of virus titer in different stages of amoeba life cycle.
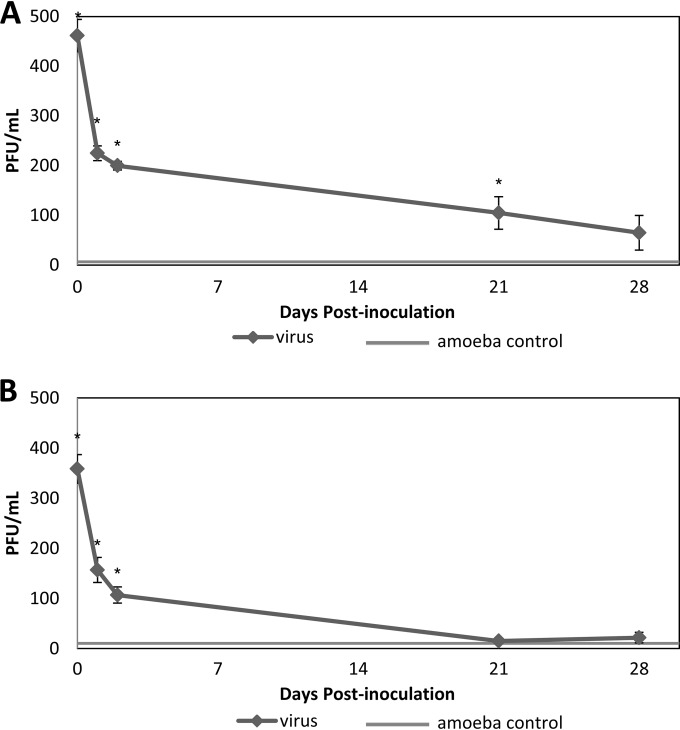
The number of infectious MNV-1 associated with amoebae in different stages of the life cycle was determined for both groups of amoeba (Fig. 4). In A. castellanii, the total number of infectious viruses associated with cysts was 105 PFU, an 80% decrease compared to the virus concentration immediately after virus inoculation. The virus number associated with recovered trophozoites was 65 PFU total and significantly different (P < 0.05) from the amoeba-only group. In A. polyphaga, the virus number in both cysts and recovered trophozoites showed no significant difference (P > 0.05) compared to the amoeba-only group.
FIG 4.
MNV-1 associated with A. castellanii (A) and A. polyphaga (B) through a complete life cycle of the amoeba. Two days after virus inoculation, trophozoites were transferred from PYG medium to PBS to induce encystment. After 2 weeks in cyst form, the cysts were either collected for virus analysis (day 21) or recovered for trophozoites for another week (day 28) before virus analysis. Values are means ± the standard errors from at least three experiments. The amoeba control group showed the results of amoeba lysate without virus inoculation. *, P < 0.05 versus the amoeba control.
Confocal immunofluorescence studies.
To further investigate the location of MNV-1 in A. castellanii trophozoites, a double indirect immunofluorescence stain was performed using rabbit IgG anti-MNV-1 capsid protein 1 as the primary antibody and goat anti-rabbit IgG antibodies conjugated with TRITC or FITC as secondary antibodies. Because of the design of the assay and likely saturation of epitope binding sites during the first application of primary antibody, these two different fluorescence-labeled secondary antibodies are capable of distinguishing viruses adsorbed onto the cell surface (green) and inside the cell (red) (Fig. 5). However, there is a finite possibility that some of the virus epitopes were not saturated during the first application of the primary antibody; therefore, some of the red fluorescence could be due to surface-associated viruses, although this is unlikely based on the controls. Compared to the control group (amoeba incubated with DMEM only) (Fig. 5A), trophozoites incubated with MNV-1 showed FITC signals (green) at the corresponding amoeba position under transparent light observation immediately after 1 h of incubation (Fig. 5B). This result reflects the location of MNV-1 on the surface of trophozoites. However, the trophozoites examined 24 h after virus inoculation showed positive TRITC signals (red) inside the trophozoites, along with a corresponding weaker FITC signal (green) on the cell surface (Fig. 5C).
FIG 5.
Immunofluorescence pictures of uninoculated A. castellanii trophozoites in DMEM only (A) and of MNV-1-inoculated A. castellanii trophozoites after 1 h (B) and 24 h (C) of incubation at room temperature. Magnification, ×200.
DISCUSSION
Acanthamoebae are one of the most ubiquitous protozoa in the environment and are particularly widespread in soil and water systems (33–35). In cyst form, these protozoa are reported to be highly stable after treatment with sanitizing and disinfecting agents such as chlorine and are highly resistant to extremes of pH and temperature (36, 37). Human contact with acanthamoebae is quite frequent since antibodies to Acanthamoeba could be detected in >80% of the human population (38). Human noroviruses are highly contagious and responsible for an estimated 21 million gastrointestinal illnesses annually in the United States (39). A large proportion of HuNoV outbreaks are caused by transmission through water and food, especially leafy greens, possibly contaminated with viruses via polluted water sources or during preparation (40, 41); however, the route of HuNoV transmission within the environment is still not clear. Since both HuNoV and Acanthamoeba spp. can be found in the same environment (i.e., water), as well as on fresh produce (18, 19), and acanthamoebae have been observed to have interactions with human pathogens (bacteria and viruses), the interactions between acanthamoebae and HuNoV surrogates were investigated to determine whether amoebae could play a role in the environmental transmission of HuNoV.
To verify whether FLA can play a role in transmission of HuNoV by serving as either short-term (i.e., hours to several days) or long-term (i.e., through a complete amoeba life cycle) reservoirs, the interactions between acanthamoebae and HuNoV surrogates in vitro were investigated in order to determine (i) the extent of virus adhesion and (ii) the possible absorption and internalization of the virus in protozoan cells. Based on this investigation, we may better understand the role of virus-amoeba contact in transmission of the virus in the environment. Two Acanthamoeba species were chosen based on previous studies. A. castellanii is reported to have interactions with nonenveloped viruses (human enteroviruses and adenoviruses) of significance to public health (25, 30, 31), while both A. castellanii and A. polyphaga play a role in many interactions between acanthamoebae and food-borne bacteria (26–28).
Our results on virus-amoeba associations demonstrated that by 1 h after virus inoculation, MNV-1, but not FCV, has the ability to adhere to both Acanthamoeba species. To our knowledge, this is the first study to show this association of amoeba with MNV-1—the first culturable NoV. This is also of interest since MNV-1 is more similar to HuNoV than other norovirus surrogates to date (42) based on phylogenic analysis of the viral protein and viral genome. The biological and molecular properties MNV-1 shares with other noroviruses are important characteristics since this virus is one of the preferred in vitro surrogates for the analysis of the biology and pathogenesis of HuNoVs (43–47). Based on this, the association of MNV-1 observed with Acanthamoeba spp. may reflect more accurately the interaction between HuNoV and Acanthamoeba than FCV's association with amoebae. However, it is important to note that additional HuNoV surrogates have been discovered more recently compared to MNV-1, including Tulane virus (TuV). Even though MNV-1 is the only culturable norovirus, research has also suggested that TuV would be a suitable surrogate. TuV is also a member of the family Caliciviridae but belongs to the Recovirus genus, as opposed to Norovirus. An important aspect of TuV with respect to similarities to HuNoV is the recognition of histo-blood group antigen receptors—the presumed binding receptor for HuNoV cell entry (65). Cromeans et al. (48) reported that TuV is the more resistant of the HuNoV surrogates—including FCV and MNV-1—across inactivation procedures and aligns closely with results from studies with HuNoV that use molecular assays to suggest inactivation (48). Conversely, Hirneisen and Kniel (49) reported that MNV-1 was likely a better surrogate than TuV. Therefore, there is really no consensus on appropriate HuNoV surrogate selection for studies evaluating any given parameter (50, 51), including our present study on virus interaction with FLA.
To understand how MNV-1 may interact with and be internalized by Acanthamoeba, the relationship amoebae are reported to have with so-called giant viruses, including mimivirus and pandoravirus, was considered, although with the obvious difference of these giant viruses are able to replicate within the cytoplasm of the amoeba (52). Ghigo et al. (52) reported on the interaction between Acanthamoeba polyphaga mimivirus (APMV) and professional macrophages, including RAW 264.7 murine macrophages. These authors observed that APMV entered macrophages through phagocytosis initiated by direct binding at the cell body—a mechanism normally used by bacteria and parasites for cell entry. However, phagocytosis is a more likely scenario for APMV than a typical enteric virus since APMV is an enveloped virus around 750 nm in diameter—i.e., closer to the size of a bacterial cell—whereas enteric viruses are nonenveloped and typically less than 100 nm in size. Moreover, it is known that MNV-1 entry into RAW 264.7 macrophages occurs independently of phagocytosis (53).
Based on the unlikely scenario of A. castellanii phagocytosis of MNV-1, the components of amoeba plasma membrane were investigated through review of published literature. Korn and Wright (54) originally reported on the relatively simple macromolecular composition of A. castellanii plasma membrane with ca. 30% composed of two separate inositol-containing glycosphingolipids (i.e., glycoinositolphosphospingolipids, [GIPSLs]) termed lipophosphonoglycans (54, 55). Briefly, GIPSLs are distinguishable from traditional glycosphingolipids (GSLs) by the relation of the carbohydrate to the ceramide moiety; specifically, the carbohydrate is coupled to the lipophilic portion of the molecule via an intermittent inositol phosphate (56). While GSLs present on vertebrate cells can function as receptors for bacteria and viruses via their carbohydrate structure (57), the role of the closely related GIPSLs present on amoebic cells as functional receptors, or minimal binding sites, for nonenveloped viruses, specifically caliciviruses, is unclear. However, Bowers and Korn (58) indicated the presence of acidic groups on GIPSLs found in A. castellanii plasma membrane, an important consideration given that both MNV-1 and select strains of genogroup II.4 HuNoV have been shown to bind negatively charged sialylated structures that are also present on acidic GSLs (58–61). Overall, further studies are necessary for the identification of possible amoebic surface molecules that may serve as attachment factors involved in MNV-1 binding or adsorption, as well as for characterization of the mechanism(s) involved in virus internalization.
Kinetic findings of virus titers in amoeba culture for a period of 8 consecutive days indicate that MNV-1 maintains its association with amoeba trophozoites with 2.69 and 2.52 log10 viruses recovered from an initial input 6.47 log10 virus in A. castellanii and A. polyphaga, respectively. Compared to the virus recovery from amoeba trophozoites in previous studies, coxsackievirus B3 (25) and polioviruses (31) were recovered at 50 and 30% of the original virus input, respectively, whereas the value of recovered MNV-1 was much less (i.e., <1%). These differences in recovery efficiency may be attributed to a few factors. First, coxsackievirus B3 and poliovirus may simply have a better association with Acanthamoeba than MNV-1, and the recovery is a reflection of the level of association. Second, the strain of A. castellanii may also be an important consideration in the level of enteric virus association. Danes and Cerva (31) used A. castellanii Neff strain, whereas Mattana et al. (25) used an unspecified strain isolated from the corneal ulcer of a contact lens wearer. In the present study, A. castellanii strain C30, a clinical isolate, was selected for virus interaction studies. Although these are all clinical isolates, there could be some minor differences in cell wall structure that may impact the way viruses associate; however, as discussed in more detail below, differences at the species level are difficult to discern. Thus, strain level differences are likely even more elusive. Finally, the present study used a plaque assay to quantify MNV-1 association with amoebae, while previous studies used 50% tissue culture infective dose assays (25, 31). However, these should not impact the relative quantification of virus recovery unless amoeba lysate caused a cytopathic effect in the cell lines used for the detection of coxsackievirus B3 (25) and polioviruses (31). Regardless, the constant virus titer detected in A. castellanii from day 4 to day 8 implies that MNV-1 can still remain infectious and stable at significant concentrations.
To verify the difference in recovered virus titer from amoeba lysate and virus number observed in an immunofluorescence stain, we also analyzed the virus titer in A. castellanii amoeba sediment (i.e., cell debris remaining after lysing the pellet) separated from an amoeba pellet to determine whether there were any unreleased viruses after the freeze-thaw step. The virus titer associated with amoeba sediment was 20 to 60% less than that of pellet lysate but had a similar trend over the period of 6 days (data not shown). Therefore, the association of virus with cellular debris may account for some of the differences in recovery of virus from amoebae. Danes and Cerva (31) previously reported on the relatively “tight” adherence of enteric viruses (i.e., poliovirus and echovirus) to the amoeba surface, possibly accounting for the MNV-1 presence in amoebic cell debris even after six freeze-thaw cycles. In addition, the primary antibody used to detect MNV-1 in the immunofluorescence stain specifically recognizes the VP1 protein of the virus capsid. This means that the antibody detects all virus particles (or capsid protein) inside or on the surface of the amoeba, while a plaque assay only reflects the infectious virus number. Further investigation and analysis of the immunofluorescence stain and MNV-1 inactivation will be needed to clarify this difference.
Moreover, the differences in virus association between the two amoeba species—the MNV-1 titer in A. polyphaga kept decreasing over time, whereas the virus titer remains stable in A. castellanii—may be due to intrinsic differences in the amoeba species. However, these species belong to the same Acanthamoeba group and genotype (i.e., group 2, genotype 4), making them more similar than different (62). Unfortunately, we were not able to determine specific differences within the scope of the present study, nor have we been able to identify potential reasons based on review of the published literature. Moreover, identification of Acanthamoeba spp. at the species level is based on subtle size and morphological differences in the cyst stage as opposed to the trophozoite stage, where the initial virus-amoeba interactions are occurring (62, 63). Finally, as indicated in the results, we also observed a few PFU (1 to 5 PFU) in the RAW cell monolayers that were inoculated with amoeba cell lysate control used in the virus titration experiments. Based on the significantly higher number of PFU in plates with amoeba lysate plus MNV-1, we are confident that these low numbers of plaques are not due to experimental contamination with MNV-1 since plaque numbers would be expected to be much higher if this were the case. However, it is possible that the plaques observed in the RAW cell monolayer inoculated with the amoeba control group were formed by amoeba-associated viruses such as mimiviruses (52), which have been reported to infect the RAW 267.4 cells. Further analysis of these plaques using molecular methods such as reverse transcription-PCR (RT-PCR) for detecting MNV-1, mimivirus, and other possible microorganisms may be needed to investigate the actual cause of the plaques. However, inclusion of these additional analyses would not impact the results of the present study.
To further investigate virus-amoeba interactions in two different amoeba species, a double indirect immunofluorescence technology was applied to study virus adsorption to and absorption into amoebae, as well as the location of the virus in amoebae. The initial immunofluorescence stain showed that 1 h after virus inoculation, MNV-1 was located on the surface of A. castellanii trophozoites. However, after 24 h of amoeba-virus interaction, the immunofluorescence stain revealed less MNV-1 virus on the amoeba surface and the majority of the MNV-1 virus inside the amoeba. It is important to note again the slight possibility that some of the TRITC (red) stain could be due to MNV-1 associated with the amoeba surface, although this is considered highly unlikely based on the assay design and staining patterns observed. Moreover, compared to FITC signals, TRITC signals indicating internal location of MNV-1 appeared more condensed and aggregated to one spot within the trophozoites. We hypothesize that the MNV-1 entering the amoeba may localize to a vacuole or other intracellular region; however, further confocal microscopy analyses with higher magnification are needed to confirm this hypothesis. Last, our study indicates that MNV-1 can still be detected from virus-inoculated trophozoites of A. castellanii after a 2-week period in the cystic form. Previously, it has been shown that encysted amoebae are resistant to various physical and chemical agents, and cysts have been known to survive for several years (64). Therefore, we suggest that encystment of A. castellanii may provide MNV-1—or even human enteric viruses found to be associated with FLA—with shelter if the virus has been internalized. Overall, the data reported here clearly highlight the need for more extensive research investigating human enteric virus interactions with microorganisms (e.g., bacteria, amoeba, ciliates, etc.) present in the same environments.
In conclusion, we have demonstrated that A. castellanii is able to interact with MNV-1; after adhesion to the surface of amoeba, viruses can be internalized by trophozoites in which they survive and maintain infectivity regardless of amoeba replication or encystment. Further experiments are necessary in order to characterize the molecules on the surface of A. castellanii involved with MNV-1 adhesion and the mechanisms of MNV-1 internalization in amoeba trophozoites. Regardless, based on the data reported here, A. castellanii appears to have the potential to carry MNV-1 and play a role as a reservoir for closely related human enteric viruses (i.e., HuNoV) in aquatic environments, as well as a vector in their transmission in the environment.
ACKNOWLEDGMENTS
We especially thank Jeffrey Silberman for his guidance and assistance in Acanthamoeba culture and experimental parameters.
This study was partially supported by a USDA NIFA grant (2013-68003-21288) awarded to K.E.G.
REFERENCES
- 1.Green KY. 2007. Caliciviridae: the norovirus, p 949–979. In Knipe DM, Howley PM (ed), Fields virology, 4th ed Lippincott/The Williams & Wilkins Co, Philadelphia, PA. [Google Scholar]
- 2.Karst SM. 2010. Pathogenesis of noroviruses, emerging RNA viruses. Viruses 2:748–781. doi: 10.3390/v2030748. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Teunis PF, Moe CL, Liu P, Miller SE, Lindesmith L, Baric RS, Le Pendu J, Calderon RL. 2008. Norwalk virus: how infectious is it? J Med Virol 80:1468–1476. doi: 10.1002/jmv.21237. [DOI] [PubMed] [Google Scholar]
- 4.Scallan E, Hoekstra RM, Angulo FJ, Tauxe RV, Widdowson MA, Roy SL, Jones JL, Griffin PM. 2011. Foodborne illness acquired in the United States—major pathogens. Emerg Infect Dis 17:7–15. doi: 10.3201/eid1701.P11101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Lopman B, Gastañaduy P, Park GW, Hall AJ, Parashar UD, Vinjé J. 2012. Environmental transmission of norovirus gastroenteritis. Curr Opin Virol 2:96–102. doi: 10.1016/j.coviro.2011.11.005. [DOI] [PubMed] [Google Scholar]
- 6.Hewitt J, Bell D, Simmons GC, Rivera-Aban M, Wolf S, Greening GE. 2007. Gastroenteritis outbreak caused by waterborne norovirus at a New Zealand ski resort. Appl Environ Microbiol 73:7853–7857. doi: 10.1128/AEM.00718-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Lysén M, Thorhagen M, Brytting M, Hjertqvist M, Andersson Y, Hedlund KO. 2009. Genetic diversity among food-borne and waterborne norovirus strains causing outbreaks in Sweden. J Clin Microbiol 47:2411–2418. doi: 10.1128/JCM.02168-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Kvitsand HM, Fiksdal L. 2010. Waterborne disease in Norway: emphasizing outbreaks in groundwater systems. Water Sci Technol 61:563–571. doi: 10.2166/wst.2010.863. [DOI] [PubMed] [Google Scholar]
- 9.Gibson KE. 2014. Viral pathogens in water: occurrence, public health impact, and available control strategies. Curr Opin Virol 4:50–57. doi: 10.1016/j.coviro.2013.12.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Hernandez-Morga J, Leon-Felix J, Peraza-Garay F, Gil-Salas BG, Chaidez C. 2009. Detection and characterization of hepatitis A virus and norovirus in estuarine water samples using ultrafiltration–RT-PCR integrated methods. J Appl Microbiol 106:1579–1590. doi: 10.1111/j.1365-2672.2008.04125.x. [DOI] [PubMed] [Google Scholar]
- 11.Aw TG, Gin KY. 2010. Environmental surveillance and molecular characterization of human enteric viruses in tropical urban wastewaters. J Appl Microbiol 109:716–730. doi: 10.1111/j.1365-2672.2010.04701.x. [DOI] [PubMed] [Google Scholar]
- 12.Victoria M, Rigotto C, Moresco V, de Abreu Corrêa A, Kolesnikovas C, Leite JP, Miagostovich MP, Barardi CRM. 2010. Assessment of norovirus contamination in environmental samples from Florianópolis City, Southern Brazil. J Appl Microbiol 109:231–238. doi: 10.1111/j.1365-2672.2009.04646.x. [DOI] [PubMed] [Google Scholar]
- 13.Hall AJ, Eisenbart VG, Etingüe AL, Gould LH, Lopman BA, Parashar UD. 2012. Epidemiology of foodborne norovirus outbreaks, United States, 2001–2008. Emerg Infect Dis 18:1566–1573. doi: 10.3201/eid1810.120833. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Pönkä A, Maunula L, von Bonsdorff CH, Lyytikäinen O. 1999. An outbreak of calicivirus associated with consumption of frozen raspberries. Epidemiol Infect 123:469–474. doi: 10.1017/S0950268899003064. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Falkenhorst G, Krusell L, Lisby M, Madsen SB, Böttiger B, Mølbak K. 2005. Imported frozen raspberries cause a series of norovirus outbreaks in Denmark, 2005. Euro Surveill 10:E050922.2. [DOI] [PubMed] [Google Scholar]
- 16.Ethelberg S, Lisby M, Böttiger B, Schultz AC, Villif A, Jensen T, Olsen KE, Scheutz F, Kjelsø C, Müller L. 2010. Outbreaks of gastroenteritis linked to lettuce, Denmark, January 2010. Euro Surveill 15:1–3. [PubMed] [Google Scholar]
- 17.Bernard H, Faber M, Wilking H, Haller S, Höhle M, Schielke A, Ducomble T, Siffczyk C, Merbecks SS, Fricke G, Hamouda O, Stark K, Werber D, Outbreak Investigation Team. 2014. Large multistate outbreak of norovirus gastroenteritis associated with frozen strawberries, Germany, 2012. Euro Surveill 19:20719. [DOI] [PubMed] [Google Scholar]
- 18.Rude RA, Jackson GJ, Bier JW, Sawyer TK, Risty NG. 1984. Survey of fresh vegetables for nematodes, amoebae, and Salmonella. J Assoc Off Anal Chem 67:613–615. [PubMed] [Google Scholar]
- 19.Gourabathini P, Brandl MT, Redding KS, Gunderson JH, Berk SG. 2008. Interactions between food-borne pathogens and protozoa isolated from lettuce and spinach. Appl Environ Microbiol 74:2518–2525. doi: 10.1128/AEM.02709-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Khan NA. 2006. Acanthamoeba: biology and increasing importance in human health. FEMS Microbiol Rev 30:564–595. doi: 10.1111/j.1574-6976.2006.00023.x. [DOI] [PubMed] [Google Scholar]
- 21.Weisman RA. 1976. Differentiation in Acanthamoeba castellanii. Annu Rev Microbiol 30:189–219. doi: 10.1146/annurev.mi.30.100176.001201. [DOI] [PubMed] [Google Scholar]
- 22.Cordingley JS, Wills RA, Villemez CL. 1996. Osmolarity is an independent trigger of Acanthamoeba castellanii differentiation. J Cell Biochem 61:167–171. doi:. [DOI] [PubMed] [Google Scholar]
- 23.Turner NA, Russell AD, Furr JR, Lloyd D. 2000. Emergence of resistance to biocides during differentiation of Acanthamoeba castellanii. J Antimicrob Chemother 46:27–34. doi: 10.1093/jac/46.1.27. [DOI] [PubMed] [Google Scholar]
- 24.Cirillo JD, Falkow S, Tompkins LS. 1994. Growth of Legionella pneumophila in Acanthamoeba castellanii enhances invasion. Infect Immun 62:3254–3261. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Mattana A, Serra C, Mariotti E, Delogu G, Fiori PL, Cappuccinelli P. 2006. Acanthamoeba castellanii promotion of in vitro survival and transmission of coxsackie B3 viruses. Eukaryot Cell 5:665–671. doi: 10.1128/EC.5.4.665-671.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Douesnard-Malo F, Daigle F. 2011. Increased persistence of Salmonella enterica serovar Typhi in the presence of Acanthamoeba castellanii. Appl Environ Microbiol 77:7640–7646. doi: 10.1128/AEM.00699-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Chekabab SM, Daigle F, Charette SJ, Dozois CM, Harel J. 2012. Survival of enterohemorrhagic Escherichia coli in the presence of Acanthamoeba castellanii and its dependence on Pho regulon. Microbiologyopen 1:427–437. doi: 10.1002/mbo3.40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Anacarso I, de Niederhäusern S, Messi P, Guerrieri E, Iseppi R, Sabia C, Bondi M. 2012. Acanthamoeba polyphaga, a potential environmental vector for the transmission of food-borne and opportunistic pathogens. J Basic Microbiol 52:261–268. doi: 10.1002/jobm.201100097. [DOI] [PubMed] [Google Scholar]
- 29.Karst SM, Wobus CE, Lay M, Davidson J, Virgin HW IV. 2003. STAT1-dependent innate immunity to a Norwalk-like virus. Science 299:1575–1578. doi: 10.1126/science.1077905. [DOI] [PubMed] [Google Scholar]
- 30.Huws SA, Morley RJ, Jones MV, Brown MR, Smith AW. 2008. Interactions of some common pathogenic bacteria with Acanthamoeba polyphaga. FEMS Microbiol Lett 282:258–265. doi: 10.1111/j.1574-6968.2008.01123.x. [DOI] [PubMed] [Google Scholar]
- 31.Danes L, Cerva L. 1981. Survival of polioviruses and echoviruses in Acanthamoeba castellanii cultivated in vitro. J Hyg Epidemiol Microbiol Immunol 25:169–174. [PubMed] [Google Scholar]
- 32.Scheid P, Schwarzenberger R. 2012. Acanthamoeba spp. as vehicle and reservoir of adenoviruses. Parasitol Res 111:479–485. doi: 10.1007/s00436-012-2828-7. [DOI] [PubMed] [Google Scholar]
- 33.Gibson KE, Schwab KJ. 2011. Tangential-flow ultrafiltration with integrated inhibition detection for recovery of surrogates and human pathogens from large-volume source water and finished drinking water. Appl Environ Microbiol 77:385–391. doi: 10.1128/AEM.01164-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Khan NA. 2003. Pathogenesis of Acanthamoeba infections. Microb Pathog 34:277–285. doi: 10.1016/S0882-4010(03)00061-5. [DOI] [PubMed] [Google Scholar]
- 35.Marciano-Cabral F, Cabral G. 2003. Acanthamoeba spp. as agents of disease in humans. Clin Microbiol Rev 16:273–307. doi: 10.1128/CMR.16.2.273-307.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Schuster FL, Visvesvara GS. 2004. Free-living amoebae as opportunistic and non-opportunistic pathogens of humans and animals. Int J Parasitol 34:1001–1027. doi: 10.1016/j.ijpara.2004.06.004. [DOI] [PubMed] [Google Scholar]
- 37.Seal DV, Kirkness CM, Bennett HG, Peterson M, Group KS. 1999. Acanthamoeba keratitis in Scotland: risk factors for contact lens wearers. Cont Lens Anterior Eye 22:58–68. doi: 10.1016/S1367-0484(99)80004-6. [DOI] [PubMed] [Google Scholar]
- 38.Abrahão JS, Dornas FP, Silva LCF, Almeida GM, Boratto PVM, Colson P, La Scola B, Kroon EG. 2014. Acnathamoeba polyphaga mimivirus and other giant viruses: an open field to outstanding discoveries. Virol J 11:120. doi: 10.1186/1743-422X-11-120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Chappell CL, Wright JA, Coletta M, Newsome AL. 2001. Standardized method of measuring acanthamoeba antibodies in sera from healthy human subjects. Clin Diagn Lab Immunol 8:724–730. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Centers for Disease Control and Prevention. 2014. U.S. trends and outbreaks. Centers for Disease Control and Prevention, Atlanta, GA: http://www.cdc.gov/norovirus/trends-outbreaks.html. [Google Scholar]
- 41.Berger CN, Sodha SV, Shaw RK, Griffin PM, Pink D, Hand P, Frankel G. 2010. Fresh fruit and vegetables as vehicles for the transmission of human pathogens. Environ Microbiol 12:2385–2397. doi: 10.1111/j.1462-2920.2010.02297.x. [DOI] [PubMed] [Google Scholar]
- 42.Tuan Zainazor C, Hidayah MS, Chai LC, Tunung R, Ghazali FM, Son R. 2010. The scenario of norovirus contamination in food and food handlers. J Microbiol Biotechnol 20:229–237. [PubMed] [Google Scholar]
- 43.Wobus CE, Karst SM, Thackray LB, Chang KO, Sosnovtsev SV, Belliot G, Krug A, Mackenzie JM, Green KY, Virgin HW IV. 2004. Replication of Norovirus in cell culture reveals a tropism for dendritic cells and macrophages. PLoS Biol 2:e432. doi: 10.1371/journal.pbio.0020432. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Ward VK, McCormick CJ, Clarke IN, Salim O, Wobus CE, Thackray LB, Virgin HW IV, Lambden PR. 2007. Recovery of infectious murine norovirus using Pol II-driven expression of full-length cDNA. Proc Natl Acad Sci U S A 104:11050–11055. doi: 10.1073/pnas.0700336104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Yunus MA, Chung LM, Chaudhry Y, Bailey D, Goodfellow I. 2010. Development of an optimized RNA-based murine norovirus reverse genetics system. J Virol Methods 169:112–118. doi: 10.1016/j.jviromet.2010.07.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Subba-Reddy CV, Goodfellow I, Kao CC. 2011. VPg-primed RNA synthesis of norovirus RNA-dependent RNA polymerases by using a novel cell-based assay. J Virol 85:13027–13037. doi: 10.1128/JVI.06191-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Thorne LG, Goodfellow IG. 2014. Norovirus gene expression and replication. J Gen Virol 95:278–291. doi: 10.1099/vir.0.059634-0. [DOI] [PubMed] [Google Scholar]
- 48.Cromeans T, Park GW, Costantini V, Lee D, Wang Q, Farkas T, Lee A, Vinjé J. 2014. Comprehensive comparison of cultivable norovirus surrogates in response to different inactivation and disinfection treatments. Appl Environ Microbiol 80:5743–5751. doi: 10.1128/AEM.01532-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Hirneisen KA, Kniel KE. 2013. Comparing human norovirus surrogates: murine norovirus and Tulane virus. J Food Prot 76:139–143. doi: 10.4315/0362-028X.JFP-12-216. [DOI] [PubMed] [Google Scholar]
- 50.Richards GP. 2011. Critical review of norovirus surrogates in food safety research: rationale for considering volunteer studies. Food Environ Virol 4:6–13. doi: 10.1007/s12560-011-9072-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Arthur SE, Gibson KE. 2015. Comparison of methods for evaluating the thermal stability of human enteric viruses. Food Environ Virol 7:14–26. doi: 10.1007/s12560-014-9178-9. [DOI] [PubMed] [Google Scholar]
- 52.Ghigo E, Kartenbeck J, Lien P, Pelkmans L, Capo C, Mege JL, Raoult D. 2008. Ameobal pathogen mimivirus infects macrophages through phagocytosis. PLoS Pathog 4:e1000087. doi: 10.1371/journal.ppat.1000087. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Aderem A, Underhill DM. 1999. Mechanisms of phagocytosis in macrophages. Annu Rev Immunol 17:593–623. doi: 10.1146/annurev.immunol.17.1.593. [DOI] [PubMed] [Google Scholar]
- 54.Korn ED, Wright PL. 1973. Macromolecular composition of an amoeba plasma membrane. J Biol Chem 248:439–447. [PubMed] [Google Scholar]
- 55.Dearborn DG, Smith S, Korn ED. 1976. Lipophosphonoglycan of the plasma membrane of Acanthamoeba castellanii. J Biol Chem 251:2976–2982. [PubMed] [Google Scholar]
- 56.Karás MA, Russa R. 2013. New long chain bases in lipophosphonoglycan of Acanthamoeba castellanii. Lipids 48:639–650. doi: 10.1007/s11745-013-3794-2. [DOI] [PubMed] [Google Scholar]
- 57.Taube S, Jiang M, Wobus CE. 2010. Glycosphingolipids as receptors for non-enveloped viruses. Viruses 2:1011–1049. doi: 10.3390/v2041011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Bowers B, Korn ED. 1974. Localization of lipophosphonoglycan on both sides of Acanthamoeba plasma membrane. J Cell Biol 62:533–540. doi: 10.1083/jcb.62.2.533. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Rydell GE, Nilsson J, Rodriguez-Diaz J, Ruvoën-Clouet N, Svensson L, Le Pendu J, Larson G. 2009. Human noroviruses recognize sialyl Lewis x neoglycoprotein. Glycobiology 19:309–320. doi: 10.1093/glycob/cwn139. [DOI] [PubMed] [Google Scholar]
- 60.Taube S, Perry JW, Yetming K, Patel SP, Auble H, She L, Nawar HF, Lee CH, Connell TD, Shayman JA, Wobus CE. 2009. Ganglioside-linked terminal sialic acid moieties on murine macrophages function as attachment receptors for murine noroviruses. J Virol 83:4092–4101. doi: 10.1128/JVI.02245-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Han L, Tan M, Xia M, Kitova EN, Jiang X, Klassen JS. 2014. Gangliosides are ligands for human noroviruses. J Am Chem Soc 136:12631–12637. doi: 10.1021/ja505272n. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Visvesvara GS. 1991. Classification of Acanthamoeba. Rev Infect Dis 13:S369–S372. doi: 10.1093/clind/13.Supplement_5.S369. [DOI] [PubMed] [Google Scholar]
- 63.Fuerst PA. 2014. Insights from the DNA databases: approaches to the phylogenetic structure of Acanthamoeba. Exp Parasitol 145:S39–S45. doi: 10.1016/j.exppara.2014.06.020. [DOI] [PubMed] [Google Scholar]
- 64.Mazur T, Hadaś E, Iwanicka I. 1995. The duration of the cyst stage and the viability and virulence of Acanthamoeba isolates. Trop Med Parasitol 46:106–108. [PubMed] [Google Scholar]
- 65.Farkas T, Cross RW, Hargitt E, Lerche NW, Morrow AL, Sestak K. 2010. Genetic diversity and histo-blood group antigen interactions of rhesus enteric caliciviruses. J Virol 84:8617–8625. doi: 10.1128/JVI.00630-10. [DOI] [PMC free article] [PubMed] [Google Scholar]