Abstract
Accumulating evidence suggests that bacteriocin production represents a probiotic trait for intestinal strains to promote dominance, fight infection, and even signal the immune system. In this respect, in a previous study, we isolated from the porcine intestine a strain of Streptococcus hyointestinalis DPC6484 that displays antimicrobial activity against a wide range of Gram-positive bacteria and produces a bacteriocin with a mass of 3,453 Da. Interestingly, the strain was also found to be immune to a nisin-producing strain. Genome sequencing revealed the genetic determinants responsible for a novel version of nisin, designated nisin H, consisting of the nshABTCPRKGEF genes, with transposases encoded between nshP and nshR and between nshK and nshG. A similar gene cluster is also found in S. hyointestinalis LMG14581. Notably, the cluster lacks an equivalent of the nisin immunity gene, nisI. Nisin H is proposed to have the same structure as the prototypical nisin A but differs at 5 amino acid positions—Ile1Phe (i.e., at position 1, nisin A has Ile while nisin H has Phe), Leu6Met, Gly18Dhb (threonine dehydrated to dehydrobutyrine), Met21Tyr, and His31Lys—-and appears to represent an intermediate between the lactococcal nisin A and the streptococcal nisin U variant of nisin. Purified nisin H inhibits a wide range of Gram-positive bacteria, including staphylococci, streptococci, Listeria spp., bacilli, and enterococci. It represents the first example of a natural nisin variant produced by an intestinal isolate of streptococcal origin.
INTRODUCTION
Bacteriocins of lactic acid bacteria have received extensive attention in recent years given their structural diversity and activity and their potential as biopreservatives and anti-infectives. Indeed, the production of bacteriocins by intestinal bacteria is considered a probiotic trait and has been shown to be associated with strain dominance, infection control, and host cell signaling (1). One of the oldest and undoubtedly the most extensively characterized bacteriocins is nisin A, which was discovered by Rogers in 1928 (2). Nisin A is produced by many strains of Lactococcus lactis, a species widely used for cheese manufacture. It has a broad antimicrobial spectrum against a wide range of Gram-positive genera, including staphylococci, streptococci, Listeria spp., bacilli, and enterococci (3). Nisin A has been used in the food industry as a biopreservative for more than 50 years without inducing widespread microbial resistance (4, 5). The bacteriocin has multiple antimicrobial actions; it binds to the precursor of peptidoglycan, lipid II, to inhibit cell wall biosynthesis and then forms pores in the cell membrane, leading to the release of essential ions and, ultimately, cell death (6–8).
The nisin gene cluster in L. lactis is associated with a conjugative transposon and consists of nisABTCIPRKFEG, where nisA encodes the nisin prepropeptide. Immunity to nisin is provided by a specific immunity protein, NisI, and a specialized ABC transporter, NisFEG (9). The lipoprotein, NisI, most probably orients to the outside of the cytoplasmic membrane and binds nisin, preventing it from binding to lipid II and forming pores in the cell membrane (10, 11). NisFEG are thought to transport nisin from the cytoplasmic membrane to the external environment, thus preventing the accumulation of the high number of nisin molecules necessary for pore formation (12, 13). The extent to which nisin is produced is affected by the level of immunity of the producing microorganism. For maximal nisin immunity, both the lipoprotein and the nisin transporters are required (12, 14–16).
To date, eight natural nisin variants have been discovered (Fig. 1). These include nisins Z, F, and Q, which have been isolated from lactococci, nisins U and U2, from Streptococcus uberis, and nisin P, which is encoded on nisin operons present in both Streptococcus gallolyticus subsp. pasteurianus (17) and Streptococcus suis (18). Nisin Z producers are very common, and the amino acid sequence differs from that of nisin A at a single position (His27Asn) (19, 20), a change that improves the solubility of the peptide at a neutral pH (21). The operon encoding nisin F was found on a plasmid in L. lactis F10, isolated from the intestinal tract of a freshwater catfish in South Africa. Nisin F differs from nisin A at 2 amino acid positions: His27Asn, as seen in nisin Z, and Ile30Val (22). Nisin Q is produced by L. lactis 61-14, isolated from a river in Japan, and differs from nisin A at 4 amino acid positions, i.e., those observed in nisin F as well as Ala15Val and Met21Leu (23). The Streptococcus-associated variants differ more considerably from nisin A. The S. uberis producers of nisin U and U2 were isolated in cases of bovine mastitis in the United States. Nisin U differs from nisin A at 9 positions—Ile4Lys, Ala15Ile, Gly18Thr, Asn20Pro, Met21Leu, His27Gly, Ser29His, Ile30Phe, and His31Gly—and also lacks the three C-terminal amino acids of nisin A. In addition to these changes, nisin U2 contains a further Ile1Val change (24). Finally, and most recently, a phylogenetic study of lanthipeptide synthetases by Zhang et al. (2012) (17) revealed an S. gallolyticus subsp. pasteurianus strain that encodes a structural gene with the potential to produce a new nisin analogue, nisin P. Nisin P is closely related to nisin U2, differing with respect to just 2 amino acids: Phe20 and Leu21 in nisin U2 are changed to Ala20 and Ile21 in nisin P. The more distantly related nisin-like lantibiotic salivaricin D, isolated from Streptococcus salivarius 5M6c, a human isolate, differs from nisin A at 17 positions, with most differences seen at the C-terminal end of the molecule (25).
FIG 1.

Alignment of natural nisin variants, with amino acid changes in boldface. Asterisks mark conserved amino acid residues.
In this study, we have identified a new nisin variant, designated nisin H, produced by a strain of Streptococcus hyointestinalis isolated from the porcine intestine. The name S. hyointestinalis was first employed in 1988 to reassign a number of strains that had previously been classified as Streptococcus salivarius (26) and was derived from the Greek noun hyos, meaning pig, and the Latin adjective intestinalis, which reflects the association of the strains with the porcine intestine. Previously, an S. hyointestinalis isolate producing a broad-spectrum antimicrobial that inhibits bifidobacteria, lactobacilli, Leuconostoc spp., Listeria spp., Staphylococcus aureus, and Streptococcus agalactiae was isolated as part of a mammalian-gut-mining study by O'Shea et al. (2009) (27). Since this represented the first report of an S. hyointestinalis strain that produces an antimicrobial, we sequenced and analyzed the genome of this strain. Ultimately, this led to the isolation, characterization, and identification of a novel nisin variant, which we designate nisin H, produced by a gut-derived strain.
MATERIALS AND METHODS
Bacterial strains and culture conditions.
The bacterial strains used in this study are listed in Table 1. Anaerobic conditions were generated through the use of anaerobic jars containing Anaerocult A gas packs (Merck, Darmstadt, Germany). Agar (Oxoid Ltd., Basingstoke, Hampshire, United Kingdom) was added (1%, wt/vol) to broth media when agar plates were required.
TABLE 1.
Bacterial strains and culture conditions
| Strain | Growth mediuma | Incubation: |
|
|---|---|---|---|
| Temp (°C) | Condition | ||
| Streptococcus hyointestinalis DPC6484 | GM17 or TSB | 37 | Anaerobic |
| Streptococcus hyointestinalis LMG14579 | GM17 or TSB | 37 | Anaerobic |
| Streptococcus hyointestinalis LMG14581 | GM17 or TSB | 37 | Anaerobic |
| Streptococcus hyointestinalis LMG14582 | GM17 or TSB | 37 | Anaerobic |
| Streptococcus hyointestinalis LMG14583 | GM17 or TSB | 37 | Anaerobic |
| Streptococcus hyointestinalis LMG14585 | GM17 or TSB | 37 | Anaerobic |
| Streptococcus hyointestinalis LMG14586 | GM17 or TSB | 37 | Anaerobic |
| Streptococcus hyointestinalis LMG14587 | GM17 or TSB | 37 | Anaerobic |
| Lactococcus lactis NZ9700 | GM17 | 30 | Aerobic |
| Streptococcus uberis strain 42 | GM17 | 37 | Anaerobic |
| Lactococcus lactis DPC3251 | GM17 | 30 | Aerobic |
| Escherichia coli DPC6912 | LB | 37 | Aerobic |
| Bacillus cereus 9139 | BHI | 37 | Aerobic |
| Lactococcus lactis subsp. cremoris HP | GM17 | 30 | Aerobic |
| Enterococcus faecalis 6307 | LB | 37 | Aerobic |
| Streptococcus agalactiae ATCC 13813 | BHI | 37 | Anaerobic |
| Streptococcus agalactiae DPC5338 | BHI | 37 | Anaerobic |
| Streptococcus bovis DPC6491 | BHI | 37 | Anaerobic |
| Streptococcus gallolyticus DPC6501 | BHI | 37 | Anaerobic |
| Listeria innocua DPC3572 | BHI | 37 | Aerobic |
| Listeria monocytogenes 1042 | BHI | 37 | Aerobic |
| Staphylococcus aureus ATCC 25923 | BHI | 37 | Aerobic |
| Staphylococcus aureus DPC5245 | BHI | 37 | Aerobic |
| Lactobacillus delbrueckii subsp. bulgaricus LMG6901 | MRS | 37 | Aerobic |
GM17, M17 medium with 5 g/liter glucose added (Difco Laboratories, Detroit, MI); TSB, tryptic soy broth (Difco Laboratories, Detroit, MI); BHI, brain heart infusion (Merck, Darmstadt, Germany); LB, Luria-Bertani medium or lysogeny broth (Merck, Darmstadt, Germany); MRS, de Man, Rogosa, and Sharpe medium (Difco Laboratories, Detroit, MI).
Isolation of DNA for PCR analysis.
DNA was extracted from culture cell pellets for PCR analysis with a GenElute bacterial genomic DNA kit (Sigma-Aldrich, County Wicklow, Ireland), and molecular manipulation techniques from the work of Sambrook and Russell (2001) (28) were used when required. Oligonucleotide primers were synthesized by Sigma-Genosys (Poole, Dorset, United Kingdom), and purified PCR amplicons were sequenced by Beckman Coulter Genomics (Essex, United Kingdom). DNA was amplified with MyTaq DNA polymerase (Bioline, London, United Kingdom) according to the manufacturer's instructions. PCRs were carried out in a Techne TC-512 thermal cycler (Bibby Scientific, Staffordshire, United Kingdom).
Genome sequencing and analysis of the nisin H gene cluster.
The sequence of the genomic DNA extracted from S. hyointestinalis DPC6484 was determined by 454 pyrosequencing (Beckman Coulter Genomics, USA). The resulting sequence reads were assembled into contigs using the Newbler package. Coding regions in the draft genome assembly were predicted using GLIMMER, version 2.0 (29), and annotation was subsequently determined using the GAMOLA software package (30). Sequence similarity analyses were performed using the gapped BLASTp algorithm and the nonredundant database provided by the NCBI (ftp://ftp.ncbi.nih.gov/blast/db) (31). By using the ARTEMIS genome viewer (32), components of the nisin H gene cluster were identified on two distinct contigs. PCR with the primer pair comprising 5′ GTTGACTTATTGAGCGAGG 3′ and 5′ GCCAACTTATTACGTTCTTCAC 3′, designed to be specific to the sequences flanking the 3′ and 5′ termini of the respective contigs, confirmed the contiguous nature of this gene cluster. The annotation of the gene cluster was then verified manually. The sequence data were aligned and analyzed by using LASERGENE software (DNAStar Inc., Madison, WI).
The genome was searched for the presence of a nisI immunity gene equivalent with primers designed to be specific to the nisI immunity gene of L. lactis NZ9700 and the nsuI immunity gene of S. uberis 42 (Table 2).
TABLE 2.
List of primers used in this study
| Gene | Primer pair sequence | Product size (bp) | Annealing temp (°C) |
|---|---|---|---|
| nshH gap | 5′ GTTGACTTATTGAGCGAGG 3′ | 2,267 | 60 |
| 5′ GCCAACTTATTACGTTCTTCAC 3′ | |||
| nisI | 5′ GGAATAAGTGGCTGTATACTGG 3′ | 903 | 50 |
| 5′ GAGAGTAACTGTTGTGAATTTG 3′ | |||
| nsuI | 5′ TAGTTGCATGTAGATTGGTAG 3′ | 619 | 48 |
| 5′ ATACGTGCTATTCTATATTCA 3′ | |||
| nshT | 5′ CTCTCCTCTTGTTTATTATCCC 3′ | 702 | 53 |
| 5′ GTAGAGAGCCATTAGATTGG 3′ | |||
| nshA | 5′ CTACTATTAGCTAAACAGATTG 3′ | 481 | 50 |
| 5′ GTTGTCCATCTTCATATG 3′ | |||
| nshF | 5′ GCTAGTCAGAATCGCCATAG 3′ | 324 | 60 |
| 5′ GTCTGGCACTGTATGCGG 3′ | |||
| nshR | 5′ TAGAGGAAAGAAGTGTATGTG 3′ | 177 | 53 |
| 5′ TTGGGTTTACTTCATATGTAG 3′ |
Purification of the antimicrobial produced by S. hyointestinalis DPC6484.
The antimicrobial was purified from the cell-free supernatant (CFS) of a 2-liter culture of S. hyointestinalis DPC6484 grown in tryptic soy broth (TSB) at 37°C overnight. The culture supernatant was applied to a 90-ml SP Sepharose column (GE Healthcare, Uppsala, Sweden) preequilibrated with 50 mM sodium acetate buffer, pH 4.4 (buffer A). The column was washed with 300 ml of buffer A, and the antimicrobial activity was eluted in 300 ml of buffer A containing 1 M NaCl. The acetonitrile was removed by rotary evaporation, and the sample was applied to a 5-g, 20-ml Strata C18-E solid-phase extraction (SPE) column (Phenomenex, Cheshire, United Kingdom) preequilibrated with methanol and water. The column was washed with 20 ml of 25% ethanol, and the antimicrobial activity was eluted with 20 ml of 70% 2-propanol–0.1% trifluoroacetic acid (TFA). The antimicrobial activities of cell-free supernatants and eluents from purification protocols were determined via the agar well diffusion assay described by Ryan et al. (1996) (33). Lactobacillus delbrueckii subsp. bulgaricus LMG6901 was used as the indicator strain, and bioactivity was assessed following aerobic incubation of plates overnight at 37°C.
The 2-propanol was removed by rotary evaporation, and the sample was applied to a Phenomenex Jupiter Proteo reversed-phase high-performance liquid chromatography (RP-HPLC) column (length, 10 mm; inside diameter, 250 mm; particle size, 4 μm, pore size, 90 Å) running a 25-to-45% acetonitrile–0.1% TFA gradient over 35 min at 2.5 ml/min. The resultant eluent was monitored at 214 nm, and fractions were collected at 1-min intervals. Fractions were assayed for antimicrobial activity by a well diffusion assay with L. delbrueckii subsp. bulgaricus LMG6901 as the indicator strain, and those containing antimicrobial activity were analyzed via matrix-assisted laser desorption ionization–time of flight mass spectrometry (MALDI-TOF MS) to determine the molecular mass of the antimicrobial peptide and to assess peptide purity. HPLC fractions deemed pure by MALDI-TOF MS were combined and were lyophilized in a Genevac (Suffolk, United Kingdom) lyophilizer. MALDI-TOF mass spectrometry was performed with an Axima TOF2 MALDI-TOF mass spectrometer in positive-ion reflectron mode (Shimadzu Biotech, Manchester, United Kingdom). N-terminal sequencing (Edman degradation) of purified nisin H was performed by Abingdon Health Laboratory Services (Birmingham, United Kingdom).
Pure nisin A peptide was prepared from L. lactis NZ9700 as described for nisin H.
Cross immunity of S. hyointestinalis DPC6484 to other nisin-producing cultures.
The cross immunity of S. hyointestinalis DPC6484 to the bacteriocins produced by L. lactis NZ9700 (nisin A), S. uberis strain 42 (nisin U), and L. lactis DPC3251 (lacticin 3147) and to L. delbrueckii subsp. bulgaricus LMG6901, a non-bacteriocin producer used as a bacteriocin-sensitive strain, was determined by spotting 50-μl aliquots of cell-free culture supernatants onto indicator plates seeded with 1% (vol/vol) of each of these strains. L. delbrueckii subsp. bulgaricus LMG6901, known to be sensitive to each of these nisin variants and to lacticin 3147, was used as an indicator strain to confirm the production of nisins A, H, and U and lacticin 3147 by the respective strains.
Comparison of the inhibitory activities of pure nisin A and nisin H peptides.
Purified nisin A and H peptides were resuspended at 0.22 mg/ml in 35% 2-propanol for optimum solubility. Aliquots (50 μl) of each peptide were tested for antimicrobial activity by well diffusion using the indicator strains listed in Table 4. The culture media and incubation conditions are outlined in Table 1. A 50-μl aliquot of 35% acetonitrile was assayed against each strain to ensure that it did not inhibit any of the test strains.
TABLE 4.
Spectra of inhibition of purified nisin A and nisin H peptides against a range of strains
| Target microorganism | Area of zone of inhibition (cm2)a with purified peptides of: |
|
|---|---|---|
| Nisin A | Nisin H | |
| Escherichia coli DPC6912 | 0.00 ± 0.00 | 0.00 ± 0.00 |
| Bacillus cereus 9139 | 1.42 ± 0.14 | 1.03 ± 0.12 |
| Lactobacillus delbrueckii subsp. bulgaricus LMG6901 | 23.83 ± 0.89 | 16.64 ± 0.38 |
| Lactococcus lactis subsp. cremoris HP | 9.47 ± 0.51 | 6.52 ± 0.25 |
| Enterococcus faecalis 6307 | 6.24 ± 0.00 | 3.94 ± 0.00 |
| Streptococcus agalactiae ATCC 13813 | 6.10 ± 0.25 | 2.66 ± 0.17 |
| Streptococcus agalactiae DPC5338 | 4.06 ± 0.21 | 2.56 ± 0.17 |
| Streptococcus bovis DPC6491 | 3.50 ± 0.38 | 1.75 ± 0.29 |
| Streptococcus gallolyticus DPC6501 | 5.82 ± 0.00 | 3.94 ± 0.00 |
| Listeria innocua DPC3572 | 1.50 ± 0.14 | 1.50 ± 0.14 |
| Listeria monocytogenes 1042 | 2.10 ± 0.00 | 1.58 ± 0.00 |
| Staphylococcus aureus ATCC 25923 | 3.28 ± 0.00 | 2.76 ± 0.17 |
| Staphylococcus aureus DPC5245 | 3.39 ± 0.19 | 4.78 ± 0.21 |
| Lactococcus lactis NZ9700 | 0.32 ± 0.00 | 0.69 ± 0.00 |
| Streptococcus hyointestinalis DPC6484 | 0.69 ± 0.00 | 0.32 ± 0.00 |
| Streptococcus uberis strain 42 | 3.39 ± 0.19 | 2.38 ± 0.28 |
Calculated as (πr12) − (πr22), where r1 is the radius of the zone and r2 is the radius of the well (in centimeters). Peptides were used at a concentration of 0.22 mg/ml.
Genomic profiles of S. hyointestinalis strains.
Molecular fingerprinting of S. hyointestinalis isolates was performed by pulsed-field gel electrophoresis (PFGE) as described by Simpson et al. (2002) (34) using SmaI restriction endonucleases and DNA molecular weight markers (9.42 to 242.50 kb; New England BioLabs, Beverly, MA). DNA fragments were resolved with a CHEF (contour-clamped homogeneous electric field) DRIII pulsed-field system (Bio-Rad Laboratories) at 6 V/cm for 18 h with a 1- to 30-s linear ramp time to resolve bands.
Assessment of the distributions of nshA, nshF, nshR, and nshT in S. hyointestinalis strains.
The presence of the nshA, nshF, nshR, and nshT genes in S. hyointestinalis DPC6484 and S. hyointestinalis strains obtained from the BCCM/LMG culture collection were checked using gene-specific primer pairs. The primer pairs and the sizes of the expected gene products are given in Table 2.
Nucleotide sequence accession number.
The sequence of the nisin H gene cluster of Streptococcus hyointestinalis DPC6484 is available from GenBank/EMBL under accession number KP793707.
RESULTS
Genome sequencing of S. hyointestinalis DPC6484 reveals a nisin-like gene cluster.
In a previous study, which involved the screening of mammalian samples from the gastrointestinal tracts of humans, pigs, and cows, we identified S. hyointestinalis DPC6484, a strain that inhibits bifidobacteria, lactobacilli, Leuconostoc spp., Listeria spp., S. aureus, and S. agalactiae (27). Given that antimicrobial production has not been attributed to an S. hyointestinalis strain previously, the genome of DPC6484 was sequenced with a view to the identification of the gene cluster responsible for this phenotype. Analysis of the draft genome revealed the presence of a nisin homologue and of associated biosynthesis genes on two contiguous sequence regions. The assembled gene cluster, of ∼15.8 kb (Fig. 2), was found to contain a putative nisin variant-encoding structural gene designated nshA (nsh for nisin from S. hyointestinalis, or nisin H) followed by homologues of nisBTCP (designated nshBTCP), a region encoding a streptococcal transposase, the equivalents of nisRK (designated nshRK), another region encoding a streptococcal transposase, and nisFEG-like genes (designated nshFEG). A notable feature was the absence of an equivalent of the nisI immunity gene. Further investigation of the S. hyointestinalis strain via a BLAST analysis on the draft genome sequence and PCR-based approaches suggested the absence of an obvious NisI homologue (data not shown).
FIG 2.
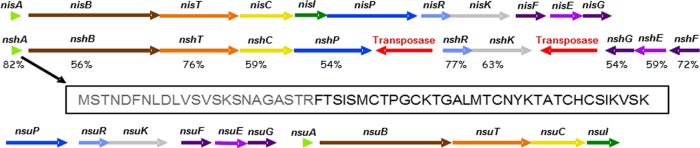
Representation of the bacteriocin-encoding nshA gene cluster as revealed by genome sequencing (center), compared with the nisA (top) and nsuA (bottom) gene clusters. The nshA gene cluster contains the nisin production genes nshABTCP, a gap region encoding a transposase, nshRK, a region encoding a second transposase, and nshFEG. For each gene, the percentage of amino acid identity to the protein encoded by the corresponding nisin A-associated gene is presented. The amino acid sequence of the unmodified NshA peptide is shown below the gene cluster. Residues predicted to be within the leader peptide are shown in gray, and those thought to correspond to the structural peptide are shown in black.
The protein sequences ranged from 54% identity with the lactococcal equivalent for NshG to 82% identity for NshA. The predicted product of nshA is a 57-amino-acid peptide that, on the basis of comparison with other nisin peptides, is likely to consist of a 23-amino-acid leader and a 34-amino-acid propeptide. The putative propeptide differs from the corresponding nisin A peptide at five positions: Ile1Phe, Leu6Met, Gly18Thr, Met21Tyr, and His31Lys (Fig. 1).
Purification and predicted structure of nisin H.
The nisin H peptide was purified using SP Sepharose cation-exchange SPE, C18 SPE, and reversed-phase HPLC. The HPLC chromatogram (Fig. 3) shows a dominant peak corresponding to a fraction that inhibited the indicator strain, L. delbrueckii subsp. bulgaricus LMG6901. This purification strategy typically yielded 0.15 mg/liter, which is lower than the 0.50 mg/liter of nisin A recovered from a corresponding starting volume by using the nisin A producer L. lactis NZ9700.
FIG 3.
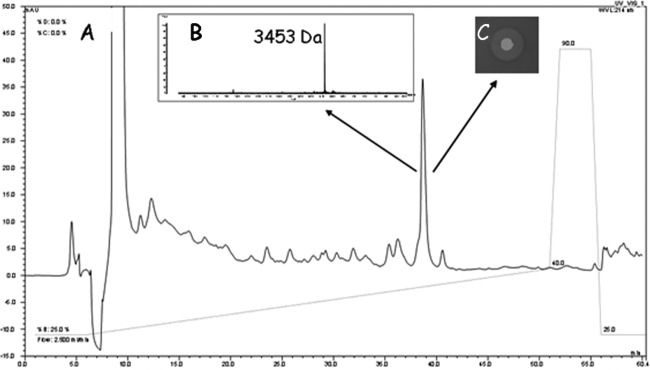
Purification of nisin H from Streptococcus hyointestinalis DPC6484 grown in TSB broth. (A) RP-HPLC chromatogram; (B) MALDI-TOF MS of the active fraction; (C) zone of inhibition of an aliquot of the HPLC fraction on an Lactobacillus delbrueckii subsp. bulgaricus LMG6901 indicator plate.
The first 10 amino acids of the predicted NshA propeptide are FTSISMCTPG (Fig. 2). Lantibiotics can be difficult to sequence using Edman degradation, because the dehydrated amino acids and ring structures are not compatible with this technique. Nonetheless, Edman sequencing of the newly purified antimicrobial revealed a sequence consisting of F-X-X-X-X-M-X-X-P-G. This sequence conforms to the gene predictions of the identifiable residues at positions 1, 6, 9, and 10 and is consistent with the predicted presence of modified residues at positions 2, 3, 5, 7, and 8. MALDI-TOF MS analysis revealed a molecular mass of 3,453 Da, which is consistent with a modified form of the NshA peptide. MALDI-TOF MS also showed a difference of 101 Da between the molecular mass of nisin A (3,352 Da) and that of the antimicrobial produced by DPC6484 (3,453 Da) (data not shown). This difference precisely matches the molecular mass differences expected from the predicted amino acid changes and the likely dehydration of the additional threonine residue. Converting Ile to Phe, Leu to Met, Gly to Thr, and Met to Tyr results in 34-, 18-, 44-, and 32-Da increases, respectively, and results in a total peptide mass of 3,480 Da. In addition, the alteration of His to Lys results in a 9-Da loss, giving a mass of 3,471 Da, 18 Da higher than the 3,453-Da mass for nisin H. However, in nisin molecules, threonine is always dehydrated to dehydrobutyrine (Dhb), thus accounting for this 18 Da. It is thus apparent that the purified antimicrobial, referred to below as nisin H, represents a modified form of the NshA peptide with 5 substitutions. The proposed structure of nisin H, modeled on known structures of nisin variants, is shown in Fig. 4.
FIG 4.
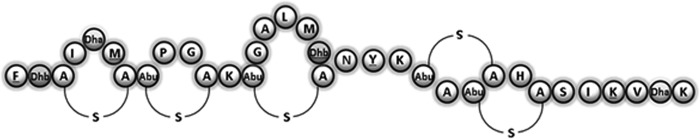
Proposed structure of the new natural nisin variant nisin H.
Nisin A- and nisin H-producing strains are cross immune.
The nisin H producer was tested to assess its cross immunity to CFSs from producers of nisins A and U. CFSs from nisin A-, H-, and U-producing cultures inhibited the growth of the indicator strain L. delbrueckii subsp. bulgaricus LMG6901, as expected (Table 3). Nisin A- and U-containing CFSs did not inhibit S. hyointestinalis DPC6484 or L. lactis NZ9700. However, CFSs from the nisin A and H producers inhibited the nisin U-producing S. uberis strain 42, suggesting that S. uberis is not cross immune to nisin A or nisin H. Further analysis with purified nisins revealed that at the concentrations used (0.22 mg ml−1), the immunity mechanisms are overwhelmed, in that purified nisin A and nisin H generate zones of inhibition with areas of 0.32 cm2 against the strains that produce these peptides and zones that are twice as large (0.69 cm2) against the opposing producer. Notably, however, these peptides produce significantly larger zones, with areas of 3.39 and 2.38 cm2, respectively, against S. uberis strain 42 (Table 4).
TABLE 3.
Cross immunity of Streptococcus hyointestinalis DPC6484 to other nisin-producing strains
| Target organism | Area of zone of inhibition (cm2)a with CFS containing the following bacteriocin: |
|||
|---|---|---|---|---|
| Nisin A | Nisin H | Nisin U | Lacticin 3147 | |
| L. delbrueckii subsp. bulgaricus LMG6901 | 11.41 ± 0.64 | 8.01 ± 0.00 | 8.17 ± 0.28 | 2.66 ± 0.00 |
| L. lactis NZ9700 | 0.00 ± 0.00 | 0.00 ± 0.00 | 0.00 ± 0.00 | 1.66 ± 0.14 |
| S. hyointestinalis DPC6484 | 0.00 ± 0.00 | 0.00 ± 0.00 | 0.00 ± 0.00 | 1.75 ± 0.30 |
| S. uberis strain 42 | 0.32 ± 0.00 | 0.69 ± 0.00 | 0.00 ± 0.00 | 0.97 ± 0.24 |
| L. lactis DPC3251 | 0.32 ± 0.00 | 0.00 ± 0.00 | 0.00 ± 0.00 | 0.00 ± 0.00 |
Calculated as (πr12) − (πr22), where r1 is the radius of the zone and r2 is the radius of the well (in centimeters).
The activities of purified nisin A and nisin H peptides against Escherichia coli DPC6912, Bacillus cereus 9139, L. delbrueckii subsp. bulgaricus LMG6901, Lactococcus lactis subsp. cremoris HP, Enterococcus faecalis 6307, S. agalactiae ATCC 13813, S. agalactiae DPC5338, Streptococcus bovis DPC6491, S. gallolyticus DPC6501, Listeria innocua DPC3572, Listeria monocytogenes 1042, S. aureus ATCC 25923, S. aureus DPC5245, S. hyointestinalis DPC6484, and S. uberis strain 42 were assessed (Table 4). In the majority of instances, nisin A was more inhibitory than nisin H. However, nisin H was more effective than nisin A against one of the S. aureus strains tested, DPC5245, and the two peptides were equally effective against L. innocua DPC3572.
Not all S. hyointestinalis strains produce nisin H.
PFGE of S. hyointestinalis DPC6484 and seven S. hyointestinalis strains from the BCCM/LMG culture collection confirm different banding patterns; thus, these strains are not clonal (Fig. 5A). Efforts to amplify each of the genes nshA, nshF, nshR, and nshT by PCR using specific primers (Table 2) resulted in the generation of amplicons of the appropriate sizes (Fig. 5Bi) for the nisin H producer S. hyointestinalis DPC6484 (positive control) and S. hyointestinalis LMG14581 but not for the other S. hyointestinalis strains. DNA sequencing of the S. hyointestinalis LMG14581 amplicons confirmed the presence of nsh equivalents in this strain. Although S. hyointestinalis LMG14581 contains nsh gene equivalents, this strain did not produce a zone of inhibition against L. delbrueckii subsp. bulgaricus LMG6901 (Fig. 5Bii).
FIG 5.
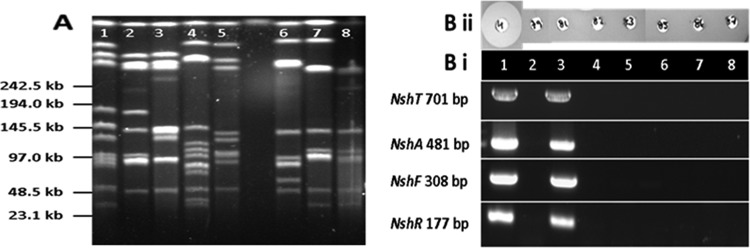
(A) PFGE macrorestriction patterns of Streptococcus hyointestinalis strains restricted with SmaI. Lane 1, S. hyointestinalis DPC6484; lane 2, S. hyointestinalis LMG14579; lane 3, S. hyointestinalis LMG14581; lane 4, S. hyointestinalis LMG14582; lane 5, S. hyointestinalis LMG14583; lane 6, S. hyointestinalis LMG14585; lane 7, S. hyointestinalis LMG14586; lane 8, S. hyointestinalis LMG14587. (B) (i) PCR amplification of strains of S. hyointestinalis template DNA with nshT-, nshH-, nshF-, and nshR-specific primers. Lanes correspond to those in panel A. (ii) Comparison of antimicrobial activities of S. hyointesinalis strains DPC6484 (H), LMG14579, LMG14581, LMG14582, LMG14583, LMG14585, LMG14586, and LMG14587 against the indicator strain, L. delbrueckii subsp. bulgaricus LMG6901. Wells are labeled with H (for nisin H) and with the last two digits of the strain designation for the other strains.
DISCUSSION
Nisin H is of interest for several reasons. First, it is notable by virtue of being a nisin-like bacteriocin that is produced by an intestinal strain. As such, production of the bacteriocin has the potential to give the strain a competitive advantage in the gut environment, either by directly inhibiting competitor bacteria or by facilitating communication with other strains or even the host. In addition, nisin H may also have a role in signaling to the host, since bacteriocin production has been associated with immunomodulatory effects often mediated through cytokine responses (35–37). Second, nisin H seems to represent an evolutionary link between lactococcal and streptococcal nisins in that, while it is quite different from nisin A by virtue of having 5 separate substitutions, it retains key features of the lactococcal peptides, including the three C-terminal amino acids, which are absent from the Streptococcus-associated nisin U. Third, the nisin H gene cluster is the only nisin gene cluster to lack an equivalent of the nisI immunity gene.
The lactococcal nisin A gene cluster (Fig. 2) encodes nisin production genes in the order nisABTCIPRKFEG, and this gene order is conserved in the corresponding nisin Z and nisin Q clusters (38). The gene order is different in streptococcal nisin gene clusters. In the nisin U gene cluster, nsuPRKFEG are at the start of the gene cluster, i.e., before the nsuABTCI genes, suggesting a rearrangement of nsuABTCI and nsuPRKFEG in S. uberis (24). Gene clusters containing structural genes for nisin analogues have also been identified in S. gallolyticus subsp. pasteurianus (17, 39), S. agalactiae (40), and, most recently, S. suis (18). The gene order in these gene clusters is identical to that for nisin U. It would seem most likely that nisin-like clusters have moved between streptococci by horizontal gene transfer, as proposed by Richards et al. (2011) (40). Interestingly, the nisin H gene cluster differs from the Lactococcus and Streptococcus nisin clusters identified previously. Although the order of nshABTCP is the same as that for lactococci, the absence of a nisI gene between nshC and nshP is notable. Attempts to amplify a nisI gene equivalent using primers designed to be specific to the nisI gene of L. lactis and the nsuI gene of S. uberis were unsuccessful. The absence of an obvious nisI gene in the remainder of the draft genome was confirmed by a comprehensive BLAST search; however, the presence of a novel immunity-like gene elsewhere in the genome cannot be ruled out. In addition, the nisin H gene cluster exhibits a number of other significant differences from previously described nisin clusters in gene order and orientation, most likely due to gene rearrangements brought about by the action of transposases.
The lack of an equivalent to the immunity protein, NisI, can have negative implications for bacteriocin production by the producing cell (41, 42). The yield of nisin H from the culture supernatant is low relative to that of nisin A, a finding initially attributed to the lack of a nisI immunity gene. However, further investigations revealed that poor bacteriocin production is most likely due to low cell numbers following 16 h of growth in TSB, typically 6 × 107 CFU/ml for S. hyointestinalis DPC6484 compared to 4 × 108 CFU/ml for L. lactis NZ9700. S. hyointestinalis strain LMG14587, which does not contain the nisin H gene cluster, grew to levels similar to those of DPC6484, suggesting that the absence of nisI was not responsible for a growth defect in DPC6484. In addition, previous nisI knockout studies have shown that a specific immunity gene is not necessary to confer full immunity (10), and Stein et al. (2003) reported that either nisI or nisFEG were able to confer immunity on nisin-sensitive Bacillus subtilis host cells (12). In this study, it would appear that the ABC transporter genes nisFEG are sufficient for self-protection in S. hyointestinalis DPC6484. Of note, other lantibiotic gene clusters, such as those of mersacidin and lacticin 481, also lack a specific immunity protein but possess NisFEG equivalents (13, 43).
The molecular mass difference of 101 Da between nisin A and nisin H can be accounted for by the amino acid differences. Given the highly conserved nature of nisin structures, we propose that nisin H has the same ring structure as nisins A, Z, F, and Q. It is more similar in structure to the lactococcal nisins in that it contains the three terminal amino acids that are missing from the streptococcal nisins U, U2, and P.
Natural lantibiotic variants are likely to arise from point mutations in structural genes; by definition, they should have few amino acid differences and the same ring pattern, and the associated producers should exhibit cross immunity to other variants (44). Nisin H fits this definition in that it differs from nisin A with respect to 5 amino acids, is likely to have an identical ring pattern, and is immune to nisin A.
The lower antimicrobial activity of the CFS of DPC6484 than that of the control nisin A producer used in this study is not related exclusively to poor peptide production levels, since, at equal concentrations and purity, nisin A is more effective than nisin H against many of the target microorganisms investigated. Nisin has been extensively bioengineered in a quest to generate more-active peptides, and this strategy has provided information on the effects of specific amino acid changes (45). It has been stated that an unusual feature of nisin is the absence of aromatic residues and that, to date, any bioengineered nisins containing aromatic residues display reduced activity (46–49). Indeed, Field et al. (2008) (46) have shown that the bioactivity of a derivative of nisin A with Met21Tyr, a change that occurs naturally in nisin H, is reduced to 70% of that of the parental strain against S. aureus strains ST528 and DPC5245, and to 65% against S. agalactiae ATCC 13813. In addition, nisin H also contains a second aromatic residue, Phe1; thus, this is the first report of the presence of such aromatic amino acids in a natural nisin variant. Interestingly, salivaricin D also contains a phenylalanine at position 1. The presence of two aromatic residues may contribute to the potency of nisin H being lower than that of nisin A. It is also notable that the introduction of positively charged amino acids into nisin Z has had a beneficial impact on activity, in that the bioengineered Asn20Lys and Met21Lys variants were more active against the Gram-negative genera Shigella, Pseudomonas, and Salmonella (48). Although nisin H has a histidine-to-lysine change at position 32, this did not confer enhanced antimicrobial activity against Escherichia coli. However, since histidine is positively charged at low pHs, the addition of a lysine at this location does not constitute a significant change from a charge perspective. The Leu6Met amino acid change seen in nisin H is the first amino acid change at this position reported for a natural variant. However, the specific impact of this change on activity will require further investigation.
Notably, nisin H appears as an intermediate between nisins of lactococcal and streptococcal origins, in that it retains the three terminal amino acids found in nisins A, F, Q, and Z while possessing other features, such as a Dhb at position 18, that are associated with nisins U, U2, and P. Alignment of the amino acid sequences of nisins A, Z, F, Q, H, U, U2, and P in Fig. 1 shows an increasing number of amino acid changes from the prototypical nisin A through to nisin P. Overall, nisin H aligns more closely with lactococcal than with streptococcal nisins, a fact that is reflected by amino acid identity.
Very few S. hyointestinalis strains have been deposited in culture collections. Since S. hyointestinalis was designated a new species in 1988, it is possible that isolates were previously catalogued as S. salivarius. PFGE analysis revealed that the seven strains of S. hyointestinalis obtained from the BCCM/LMG culture collection differ in their PFGE patterns (Fig. 5A). S. hyointestinalis LMG14581 conclusively contained the nshA, nshF, nshR, and nshT genes (Fig. 5Bii), but this strain did not display a bacteriocin-producing phenotype (Fig. 5Bi). A thorough analysis of the nisin H gene cluster in this strain would have to be carried out in order to determine the basis for this phenomenon.
In conclusion, we describe nisin H, a novel natural nisin variant produced by an S. hyointestinalis strain of porcine origin. The production of nisin H by a gut strain lends further support to accumulating observations suggesting that bacteriocin production may represent a potential probiotic trait for intestinal strains.
ACKNOWLEDGMENTS
This work was funded by the Alimentary Pharmabiotic Centre, a research center funded by Science Foundation Ireland (SFI), through the Irish Government's National Development Plan. We and our work were supported by SFI (grant 07/CE/B1368).
REFERENCES
- 1.Dobson A, Cotter PD, Ross RP, Hill C. 2012. Bacteriocin production: a probiotic trait? Appl Environ Microbiol 78:1–6. doi: 10.1128/AEM.05576-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Rogers LA. 1928. The inhibiting effect of Streptococcus lactis on Lactobacillus bulgaricus. J Bacteriol 16:321–325. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Delves-Broughton J. 1990. Nisin and its application as a food preservative. Int J Dairy Technol 43:73–76. doi: 10.1111/j.1471-0307.1990.tb02449.x. [DOI] [Google Scholar]
- 4.Cotter PD, Hill C, Ross RP. 2005. Bacteriocins: developing innate immunity for food. Nat Rev Microbiol 3:777–788. doi: 10.1038/nrmicro1273. [DOI] [PubMed] [Google Scholar]
- 5.Lubelski J, Rink R, Khusainov R, Moll GN, Kuipers OP. 2008. Biosynthesis, immunity, regulation, mode of action and engineering of the model lantibiotic nisin. Cell Mol Life Sci 65:455–476. doi: 10.1007/s00018-007-7171-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Hsu ST, Breukink E, Tischenko E, Lutters MA, de Kruijff B, Kaptein R, Bonvin AM, van Nuland NA. 2004. The nisin-lipid II complex reveals a pyrophosphate cage that provides a blueprint for novel antibiotics. Nat Struct Mol Biol 11:963–967. doi: 10.1038/nsmb830. [DOI] [PubMed] [Google Scholar]
- 7.Hasper HE, de Kruijff B, Breukink E. 2004. Assembly and stability of nisin-lipid II pores. Biochemistry 43:11567–11575. doi: 10.1021/bi049476b. [DOI] [PubMed] [Google Scholar]
- 8.Hasper HE, Kramer NE, Smith JL, Hillman JD, Zachariah C, Kuipers OP, de Kruijff B, Breukink E. 2006. An alternative bactericidal mechanism of action for lantibiotic peptides that target lipid II. Science 313:1636–1637. doi: 10.1126/science.1129818. [DOI] [PubMed] [Google Scholar]
- 9.Rodríguez JM, Dodd HM. 1996. Genetic determinants for the biosynthesis of nisin, a bacteriocin produced by Lactococcus lactis. Microbiologia 12:61–74. [PubMed] [Google Scholar]
- 10.Siegers K, Entian KD. 1995. Genes involved in immunity to the lantibiotic nisin produced by Lactococcus lactis 6f3. Appl Environ Microbiol 61:1082–1089. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Takala TM, Koponen O, Qiao M, Saris PEJ. 2004. Lipid-free NisI: interaction with nisin and contribution to nisin immunity via secretion. FEMS Microbiol Lett 237:171–177. doi: 10.1111/j.1574-6968.2004.tb09693.x. [DOI] [Google Scholar]
- 12.Stein T, Heinzmann S, Solovieva I, Entian K-D. 2003. Function of Lactococcus lactis nisin immunity genes nisI and nisFEG after coordinated expression in the surrogate host Bacillus subtilis. J Biol Chem 278:89–94. doi: 10.1074/jbc.M207237200. [DOI] [PubMed] [Google Scholar]
- 13.Draper LA, Ross RP, Hill C, Cotter PD. 2008. Lantibiotic immunity. Curr Protein Pept Sci 9:39–49. doi: 10.2174/138920308783565750. [DOI] [PubMed] [Google Scholar]
- 14.Qiao M, Omaetxebarria MJ, Ra R, Oruetxebarria I, Saris PEJ. 1997. Isolation of a Lactococcus lactis strain with high resistance to nisin and increased nisin production. Biotechnol Lett 19:199–202. doi: 10.1023/A:1018384919362. [DOI] [Google Scholar]
- 15.Ra R, Beerthuyzen MM, de Vos WM, Saris PE, Kuipers OP. 1999. Effects of gene disruptions in the nisin gene cluster of Lactococcus lactis on nisin production and producer immunity. Microbiology 145(Part 5):1227–1233. doi: 10.1099/13500872-145-5-1227. [DOI] [PubMed] [Google Scholar]
- 16.Li H, O'Sullivan DJ. 2006. Identification of a nisI promoter within the nisABCTIP operon that may enable establishment of nisin immunity prior to induction of the operon via signal transduction. J Bacteriol 188:8496–8503. doi: 10.1128/JB.00946-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Zhang Q, Yu Y, Velasquez JE, van der Donk WA. 2012. Evolution of lanthipeptide synthetases. Proc Natl Acad Sci U S A 109:18361–18366. doi: 10.1073/pnas.1210393109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Wu Z, Wang W, Tang M, Shao J, Dai C, Zhang W, Fan H, Yao H, Zong J, Chen D, Wang J, Lu C. 2014. Comparative genomic analysis shows that Streptococcus suis meningitis isolate SC070731 contains a unique 105K genomic island. Gene 535:156–164. doi: 10.1016/j.gene.2013.11.044. [DOI] [PubMed] [Google Scholar]
- 19.Mulders JW, Boerrigter IJ, Rollema HS, Siezen RJ, de Vos WM. 1991. Identification and characterization of the lantibiotic nisin Z, a natural nisin variant. Eur J Biochem 201:581–584. doi: 10.1111/j.1432-1033.1991.tb16317.x. [DOI] [PubMed] [Google Scholar]
- 20.de Vos WM, Mulders JW, Siezen RJ, Hugenholtz J, Kuipers OP. 1993. Properties of nisin Z and distribution of its gene, nisZ, in Lactococcus lactis. Appl Environ Microbiol 59:213–218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Rollema HS, Kuipers OP, Both P, de Vos WM, Siezen RJ. 1995. Improvement of solubility and stability of the antimicrobial peptide nisin by protein engineering. Appl Environ Microbiol 61:2873–2878. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.de Kwaadsteniet M, Ten Doeschate K, Dicks LM. 2008. Characterization of the structural gene encoding nisin F, a new lantibiotic produced by a Lactococcus lactis subsp. lactis isolate from freshwater catfish (Clarias gariepinus). Appl Environ Microbiol 74:547–549. doi: 10.1128/AEM.01862-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Zendo T, Fukao M, Ueda K, Higuchi T, Nakayama J, Sonomoto K. 2003. Identification of the lantibiotic nisin Q, a new natural nisin variant produced by Lactococcus lactis 61-14 isolated from a river in Japan. Biosci Biotechnol Biochem 67:1616–1619. doi: 10.1271/bbb.67.1616. [DOI] [PubMed] [Google Scholar]
- 24.Wirawan RE, Klesse NA, Jack RW, Tagg JR. 2006. Molecular and genetic characterization of a novel nisin variant produced by Streptococcus uberis. Appl Environ Microbiol 72:1148–1156. doi: 10.1128/AEM.72.2.1148-1156.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Birri DJ, Brede DA, Nes IF. 2012. Salivaricin D, a novel intrinsically trypsin-resistant lantibiotic from Streptococcus salivarius 5M6c isolated from a healthy infant. Appl Environ Microbiol 78:402–410. doi: 10.1128/AEM.06588-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Devriese LA, Kilpper-Bälz R, Schleifer KH. 1988. Streptococcus hyointestinalis sp. nov. from the gut of swine. Int J Syst Bacteriol 38:440–441. doi: 10.1099/00207713-38-4-440. [DOI] [Google Scholar]
- 27.O'Shea EF, Gardiner GE, O'Connor PM, Mills S, Ross RP, Hill C. 2009. Characterization of enterocin- and salivaricin-producing lactic acid bacteria from the mammalian gastrointestinal tract. FEMS Microbiol Lett 291:24–34. doi: 10.1111/j.1574-6968.2008.01427.x. [DOI] [PubMed] [Google Scholar]
- 28.Sambrook J, Russell DW. 2001. Molecular cloning: a laboratory manual, 3rd ed Cold Spring Harbor Laboratory Press, Cold Spring Harbor, NY. [Google Scholar]
- 29.Delcher AL, Bratke KA, Powers EC, Salzberg SL. 2007. Identifying bacterial genes and endosymbiont DNA with Glimmer. Bioinformatics 23:673–679. doi: 10.1093/bioinformatics/btm009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Altermann E, Klaenhammer TR. 2003. GAMOLA: a new local solution for sequence annotation and analyzing draft and finished prokaryotic genomes. OMICS 7:161–169. doi: 10.1089/153623103322246557. [DOI] [PubMed] [Google Scholar]
- 31.Altschul SF, Madden TL, Schaffer AA, Zhang J, Zhang Z, Miller W, Lipman DJ. 1997. Gapped BLAST and PSI-BLAST: a new generation of protein database search programs. Nucleic Acids Res 25:3389–3402. doi: 10.1093/nar/25.17.3389. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Carver T, Berriman M, Tivey A, Patel C, Bohme U, Barrell BG, Parkhill J, Rajandream MA. 2008. Artemis and ACT: viewing, annotating and comparing sequences stored in a relational database. Bioinformatics 24:2672–2676. doi: 10.1093/bioinformatics/btn529. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Ryan MP, Rea MC, Hill C, Ross RP. 1996. An application in cheddar cheese manufacture for a strain of Lactococcus lactis producing a novel broad-spectrum bacteriocin, lacticin 3147. Appl Environ Microbiol 62:612–619. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Simpson PJ, Stanton C, Fitzgerald GF, Ross RP. 2002. Genomic diversity within the genus Pediococcus as revealed by randomly amplified polymorphic DNA PCR and pulsed-field gel electrophoresis. Appl Environ Microbiol 68:765–771. doi: 10.1128/AEM.68.2.765-771.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Meijerink M, van Hemert S, Taverne N, Wels M, de Vos P, Bron PA, Savelkoul HF, van Bilsen J, Kleerebezem M, Wells JM. 2010. Identification of genetic loci in Lactobacillus plantarum that modulate the immune response of dendritic cells using comparative genome hybridization. PLoS One 5:e10632. doi: 10.1371/journal.pone.0010632. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.van Hemert S, Meijerink M, Molenaar D, Bron PA, de Vos P, Kleerebezem M, Wells JM, Marco ML. 2010. Identification of Lactobacillus plantarum genes modulating the cytokine response of human peripheral blood mononuclear cells. BMC Microbiol 10:293. doi: 10.1186/1471-2180-10-293. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Kindrachuk J, Jenssen H, Elliott M, Nijnik A, Magrangeas-Janot L, Pasupuleti M, Thorson L, Ma S, Easton DM, Bains M, Finlay B, Breukink EJ, Georg-Sahl H, Hancock RE. 2013. Manipulation of innate immunity by a bacterial secreted peptide: lantibiotic nisin Z is selectively immunomodulatory. Innate Immun 19:315–327. doi: 10.1177/1753425912461456. [DOI] [PubMed] [Google Scholar]
- 38.Piper C, Cotter PD, Ross RP, Hill C. 2009. Discovery of medically significant lantibiotics. Curr Drug Discov Technol 6:1–18. doi: 10.2174/157016309787581075. [DOI] [PubMed] [Google Scholar]
- 39.Lin IH, Liu TT, Teng YT, Wu HL, Liu YM, Wu KM, Chang CH, Hsu MT. 2011. Sequencing and comparative genome analysis of two pathogenic Streptococcus gallolyticus subspecies: genome plasticity, adaptation and virulence. PLoS One 6:e20519. doi: 10.1371/journal.pone.0020519. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Richards VP, Lang P, Bitar PD, Lefébure T, Schukken YH, Zadoks RN, Stanhope MJ. 2011. Comparative genomics and the role of lateral gene transfer in the evolution of bovine adapted Streptococcus agalactiae. Infect Genet Evol 11:1263–1275. doi: 10.1016/j.meegid.2011.04.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Cheigh CI, Park H, Choi HJ, Pyun YR. 2005. Enhanced nisin production by increasing genes involved in nisin Z biosynthesis in Lactococcus lactis subsp. lactis A164. Biotechnol Lett 27:155–160. doi: 10.1007/s10529-004-7661-3. [DOI] [PubMed] [Google Scholar]
- 42.Alkhatib Z, Abts A, Mavaro A, Schmitt L, Smits SH. 2012. Lantibiotics: how do producers become self-protected? J Biotechnol 159:145–154. doi: 10.1016/j.jbiotec.2012.01.032. [DOI] [PubMed] [Google Scholar]
- 43.Dufour A, Hindre T, Haras D, Le Pennec JP. 2007. The biology of lantibiotics from the lacticin 481 group is coming of age. FEMS Microbiol Rev 31:134–167. doi: 10.1111/j.1574-6976.2006.00045.x. [DOI] [PubMed] [Google Scholar]
- 44.Sahl HG, Jack RW, Bierbaum G. 1995. Biosynthesis and biological activities of lantibiotics with unique post-translational modifications. Eur J Biochem 230:827–853. doi: 10.1111/j.1432-1033.1995.tb20627.x. [DOI] [PubMed] [Google Scholar]
- 45.Field D, Hill C, Cotter PD, Ross RP. 2010. The dawning of a ‘Golden era’ in lantibiotic bioengineering. Mol Microbiol 78:1077–1087. doi: 10.1111/j.1365-2958.2010.07406.x. [DOI] [PubMed] [Google Scholar]
- 46.Field D, Connor PM, Cotter PD, Hill C, Ross RP. 2008. The generation of nisin variants with enhanced activity against specific gram-positive pathogens. Mol Microbiol 69:218–230. doi: 10.1111/j.1365-2958.2008.06279.x. [DOI] [PubMed] [Google Scholar]
- 47.Breukink E, van Kraaij C, van Dalen A, Demel RA, Siezen RJ, de Kruijff B, Kuipers OP. 1998. The orientation of nisin in membranes. Biochemistry 37:8153–8162. doi: 10.1021/bi972797l. [DOI] [PubMed] [Google Scholar]
- 48.Yuan J, Zhang ZZ, Chen XZ, Yang W, Huan LD. 2004. Site-directed mutagenesis of the hinge region of nisinZ and properties of nisinZ mutants. Appl Microbiol Biotechnol 64:806–815. doi: 10.1007/s00253-004-1599-1. [DOI] [PubMed] [Google Scholar]
- 49.Martin I, Ruysschaert JM, Sanders D, Giffard CJ. 1996. Interaction of the lantibiotic nisin with membranes revealed by fluorescence quenching of an introduced tryptophan. Eur J Biochem 239:156–164. doi: 10.1111/j.1432-1033.1996.0156u.x. [DOI] [PubMed] [Google Scholar]


