ABSTRACT
Polyamines are found in all groups of cyanobacteria, but their role in environmental adaptation has been barely investigated. In Synechocystis sp. strain PCC 6803, inactivation of spermidine synthesis genes significantly reduced the survivability under chill (5°C)-light stress, and the survivability could be restored by addition of spermidine. To analyze the effects of spermidine on gene expression at 5°C, lacZ was expressed from the promoter of carboxy(nor)spermidine decarboxylase gene (CASDC) in Synechocystis. Synechocystis 6803::PCASDC-lacZ pretreated at 15°C showed a high level of LacZ activity for a long period of time at 5°C; without the pretreatment or with protein synthesis inhibited at 5°C, the enzyme activity gradually decreased. In a spermidine-minus mutant harboring PCASDC-lacZ, lacZ showed an expression pattern as if protein synthesis were inhibited at 5°C, even though the stability of its mRNA increased. Four other genes, including rpoA that encodes the α subunit of RNA polymerase, showed similar expression patterns. The chill-light stress led to a rapid increase of protein carbonylation in Synechocystis. The protein carbonylation then quickly returned to the background level in the wild type but continued to slowly increase in the spermidine-minus mutant. Our results indicate that spermidine promotes gene expression and replacement of damaged proteins in cyanobacteria under the chill-light stress in winter.
IMPORTANCE Outbreak of cyanobacterial blooms in freshwater lakes is a worldwide environmental problem. In the annual cycle of bloom-forming cyanobacteria, overwintering is the least understood stage. Survival of Synechocystis sp. strain PCC 6803 under long-term chill (5°C)-light stress has been established as a model for molecular studies on overwintering of cyanobacteria. Here, we show that spermidine, the most common polyamine in cyanobacteria, promotes the survivability of Synechocystis under long-term chill-light stress and that the physiological function is based on its effects on gene expression and recovery from protein damage. This is the first report on the role of polyamines in survival of overwintering cyanobacteria. We also analyzed spermidine synthesis pathways in cyanobacteria on the basis of bioinformatic and experimental data.
INTRODUCTION
Cyanobacteria are a large group of prokaryotes that carry out oxygenic photosynthesis. In freshwater eutrophic lakes, some cyanobacterial species may vigorously propagate and cause the outbreak of water blooms. Bloom-forming cyanobacteria overwinter in the sediment (1–3) and reinitiate growth in shallow areas in spring providing inocula for the pelagic region (4–6). Studies using Synechocystis sp. strain PCC 6803 (Synechocystis 6803) showed that preconditioning at a low temperature, such as 15°C, could greatly enhance its survivability under long-term chill (5°C)-light stress (7, 8). This finding suggests that cyanobacteria acquire the capability to overwinter in late autumn and early winter. Also, it allows the overwintering mechanism to be investigated under simulating conditions in laboratory.
Studies on how cyanobacteria adapt to low temperatures can be roughly divided into two categories: (i) cold acclimation of cells exposed to a suboptimal growth temperature, such as 22 to 24°C (9, 10) or 15°C (11) and (ii) survival under long-term (8 to 10 days) chill (5°C)-light stress as a simulation of overwintering (7, 8, 12). In previous reports, we showed that α-tocopherol, which is membrane localized, is essential for the acquired chill-light tolerance (ACLT) of cyanobacteria (7) and that accumulation of RNA-binding protein 1 (Rbp1) leads to the formation of overwintering capability of cyanobacteria (8, 12). Nonetheless, information about the biology of overwintering cyanobacteria is still very limited. This is mainly due to cessation of growth and lack of photosynthetic oxygen evolution in mesophilic cyanobacteria (such as Synechocystis 6803) under long-term chill-light stress.
Polyamines are polycationic hydrocarbon molecules with multiple amino groups. In prokaryotic cells, most polyamines are bound to phosphate groups of RNA, even though they can also bind to DNA, free nucleotides, and phospholipids (13). In conjunction with Mg2+, polyamines may cause conformational changes of certain mRNAs, leading to enhancement of protein synthesis; as scavengers of free radicals, they may reduce oxidative damage of macromolecules; specific binding of a polyamine molecule may also modulate the activity of a protein (13, 14). For these characteristics, polyamines are involved in variable cellular activities of prokaryotes, such as biofilm formation (15, 16), modification of outer membrane permeability (17, 18), and enhancement of acid resistance (19, 20). In cyanobacteria, there are certain clues that may relate polyamines to osmotic tolerance (21), but little is known about the role of polyamines in adaptation to freshwater environments. Because polyamines in plants are involved in cold responses and can enhance tolerance to cold and freezing temperatures (22), those in cyanobacteria may relate to overwintering.
The most common polyamines in bacteria include putrescine, spermidine, norspermidine, spermine, etc. (14, 23). Putrescine is formed through the decarboxylation of arginine or ornithine (14, 15, 24, 25). Arginine is decarboxylated into agmatine by arginine decarboxylase, and further converted into putrescine by agmatine ureohydrolase (AUH) or agmatine iminohydrolase (AIH) and N-carbamoylputrescine amidohydrolase (NCPAH). The decarboxylation of ornithine directly produces putrescine. There are two known pathways for the synthesis of spermidine from putrescine (Fig. 1). In the classical pathway, spermidine synthase (SPDS) transforms putrescine and decarboxylated S-adenosylmethionine (DC-SAM) into spermidine (14, 15). DC-SAM is generated from SAM by SAM decarboxylase (SAMDC). In the alternative pathway, carboxy(nor)spermidine dehydrogenase (CASDH) transforms putrescine and aspartic β-semialdehyde (ASA) into carboxyspermidine, which is then transformed into spermidine by carboxy(nor)spermidine decarboxylase (CASDC) (15, 26). In microbes with diaminobutyrate aminotransferase/decarboxylase, from ASA and glutamate, diaminopropane (DAP) is produced via the intermediate diaminobutyrate. ASA and DAP are transformed by CASDH into carboxynorspermidine and then by CASDC into norspermidine.
FIG 1.
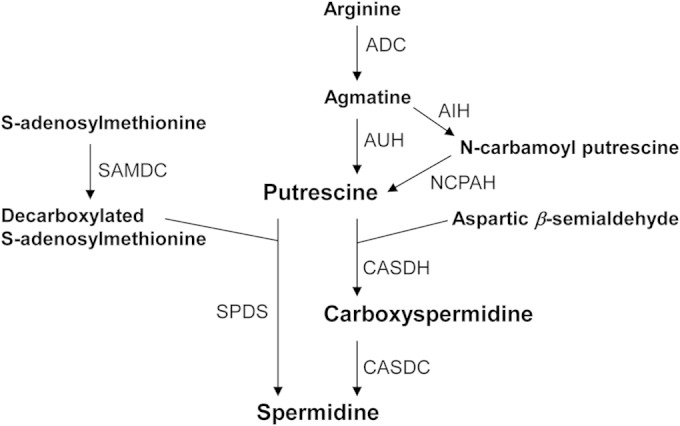
Pathways for synthesis of spermidine in prokaryotes. Abbreviations: ADC, arginine decarboxylase; AIH, agmatine iminohydrolase; AUH, agmatine ureohydrolase; CASDC, carboxyspermidine decarboxylase; CASDH, carboxyspermidine dehydrogenase; NCPAH, N-carbamoylputrescine amidohydrolase; SAMDC, S-adenosylmethionine decarboxylase; SPDS, spermidine synthase.
Spermidine and homospermidine (both triamines) are the two most common polyamines found in cyanobacteria (27). In most unicellular species that belong to Chroococcales, spermidine is the major polyamine; in most heterocyst-forming species that belong to Nostocales, homospermidine is the major polyamine; in nonheterocystous species that belong to Oscillatoriales and heterocyst-forming species that belong to Stigonematales, spermidine and/or homospermidine are the major polyamines. Genome sequence data indicate that both classical and alternative pathways for spermidine synthesis can be found in cyanobacteria. Homospermidine synthase is not found, but deoxyhypusine synthase may transform putrescine into homospermidine, in certain groups of cyanobacteria (28). Like other bacteria, cyanobacteria can take up polyamines from the environment (29–32) and may secrete polyamines (32).
In the study of overwintering mechanism of cyanobacteria, we used microarray analysis to identify genes upregulated in Synechocystis 6803 exposed to chill-light stress. CASDC, a gene involved in synthesis of spermidine, was upregulated in response to the stress. Further analyses revealed roles of spermidine in survivability and transcription/translation activity of mesophilic cyanobacteria at 5°C in the light.
MATERIALS AND METHODS
Strains, culture conditions, and measurements of RACLT.
Synechocystis 6803 was a gift from J. Zhao at the Institute of Hydrobiology. The wild type and its derivative strains were grown in BG11 as previously described (33). Measurements of the relative acquired chill-light tolerance (RACLT) were performed according to the method of Yang et al. (7). Polyamines except homospermidine were purchased from Sigma Chemicals. Homospermidine was synthesized in this lab and confirmed using nuclear magnetic resonance spectroscopy. The data (means ± the standard deviations [SD]) were generated from the results of three replicate experiments.
Construction of plasmids.
Molecular manipulations were performed according to standard methods or according to the manufacturers' instructions. Clones of PCR products were confirmed by sequencing. The details of plasmid construction are described in Table S1 in the supplemental material. Plasmid pHB2193 was used to inactivate sll0873 (CASDC in Synechocystis) with a kanamycin resistance (Kmr) cassette excised from pRL446 (NCBI GenBank accession no. EU346690). Plasmid pHB4239 was used to inactivate slr0049 (CASDH in Synechocystis) with the Kmr cassette; plasmid pHB5450 was used to inactivate Synpcc7942_0628 (SPDS in Synechococcus) with the Kmr cassette. Plasmid pHB2972 was used to insert Ω-PrbcL-sll0873 into an integrative neutral platform of Synechocystis 6803 (34, 35) to complement the sll0873::Kmr mutation. Plasmid pHB4768 was used to insert Ω-Pslr0049-slr0049 into the integrative platform to complement the slr0049::Kmr mutation. Plasmid pHB4324 was used to insert Ω-Psll0873-lacZ into the integrative platform of Synechocystis 6803. Ω is a spectinomycin/streptomycin resistance cassette excised from pRL57 (36).
Generation of cyanobacterial strains.
Transformation of Synechocystis 6803 was performed according to the method of Williams (34). Complete segregation of mutants was confirmed by PCR. To complement a mutant, the wild-type gene with a spectinomycin resistance marker was inserted into the integrative platform in the genome. Transformation of Synechococcus 7942 was performed as described previously by Güler et al. (37) with modifications. Fifty milliliters of Synechococcus cells (4 days old, optical density at 730 nm [OD730] of ∼1.2) were collected by centrifugation (4,000 rpm, 10 min), washed with 50 ml of 10 mM NaCl, and resuspended in 1.5 ml of BG11. Plasmid DNA was added to cell suspension at 2 μg of DNA/ml. After incubation overnight in the dark at 30°C on a rotating shaker, the cells were spread on BG11 plates with 5 μg of kanamycin/ml. Cyanobacterial strains are described in Table 1, and the primers are listed in Table S1 in the supplemental material.
TABLE 1.
Cyanobacterial strains
| Synechocystis strain | Derivation and/or relevant characteristicsa | Source or reference |
|---|---|---|
| PCC 6803 | ||
| Synechocystis 6803 (wild type) | Wild type, a glucose-tolerant strain | J. Zhao, Peking University/Institute of Hydrobiology |
| DRHB2193 | Kmr; sll0873::Kmr (CASDC insertion mutant), Synechocystis 6803 transformed with pHB2193 | This study |
| DRHB2193/DRHB2972 | Kmr Spr, sll0873::Kmr complemented with wild-type sll0873, DRHB2193 transformed with pHB2972 | This study |
| DRHB2193/DRHB4324 | Kmr Spr; Ω-PCASDC-lacZ integrated into the neutral platform in the genome of CASDC mutant, DRHB2193 transformed with pHB4324 | This study |
| DRHB4239 | Kmr; slr0049::Kmr (CASDH insertion mutant), Synechocystis 6803 transformed with pHB4239 | This study |
| DRHB4239/DRHB4768 | Kmr Spr; slr0049::Kmr complemented with wild-type slr0049, DRHB4239 transformed with pHB4768 | This study |
| DRHB4324 | Spr; Ω-PCASDC-lacZ integrated into the neutral platform in the genome of wild type, Synechocystis 6803 transformed, with pHB4324 | This study |
| PCC 7942 | ||
| Synechococcus 7942 (wild type) | Wild type | Freshwater Algae Culture Collection at the Institute of Hydrobiology |
| DRHB5450 | Kmr; Synpcc7942_0628::Kmr (SPDS insertion mutant), Synechococcus 7942 transformed with pHB5450 | This study |
Spr, spectinomycin resistance; Kmr, kanamycin resistance.
Microarray analysis.
Synechocystis 6803 grown at 30°C was preconditioned at 15°C for 6 days, cooled to 4°C in a water bath, and then incubated in a refrigerator at 4°C with illumination of 100 μE m−2 s−1 for 2 h. Total RNA was extracted using TRIzol reagent (Life Technologies), treated with RNase-free DNase I to eliminate contaminating chromosomal DNA, and examined by agarose gel electrophoresis.
CyanoCHIP v.2.0 (TaKaRa Biotechnology) was used for analyses of genome-wide transcriptional activities. Labeling of cDNA, hybridization, rinsing and scanning of microarrays, and data analyses were performed by TaKaRa Biotechnology at Dalian. The data (means ± the SD) were generated from three independent experiments, each with two repeats (“3 × 2”).
Northern blot analysis.
Total RNA was extracted from Synechocystis 6803 preconditioned at 15°C and exposed to chill and light at different time points as indicated, separated by electrophoresis on an agarose-formaldehyde gel, and blotted onto an Immobilon-Ny+ membrane (Millipore) by capillary transfer. Digoxigenin-labeled DNA probes were prepared by PCR using the primer pairs sll0873n-1/sll0873n-2 and rnpBn-1/rnpBn-2. Hybridization and immunological detection were performed with a DIG High Prime DNA labeling and detection starter kit I (Roche) according to the manufacturer's protocol.
RACE, qRT-PCR, and RNA stability.
Rapid amplification of cDNA ends (RACE) was performed according to the method of Zhang et al. (38). The gene-specific primer used for the first PCR was sll0873up-7, and the gene-specific primer used for the second PCR was sll0873up-2. PCR products were cloned into pMD18-T and sequenced. Quantitative reverse transcription-PCR (qRT-PCR) was performed according to the method of Gao and Xu (39). rnpB (RNase P subunit B) (40) was used as the internal control. RNA stability analyses were performed according to the method of Wang and Xu (41) with modifications. Cells were pretreated at 15°C for 2 days and exposed to chill-light stress for 1 days, and then rifampin was added to inhibit mRNA synthesis. Total RNA was extracted at 0, 1, and 2 h. The PCR primers are listed in Table S1 in the supplemental material.
Assays of β-galactosidase.
The β-galactosidase activity of Synechocystis 6803 cells was measured as described by Miller (42) with modifications. Portions (1.5 ml) of cells were spun down by centrifugation (12,000 rpm, 1 min), resuspended in 1 ml of Z buffer, and permeabilized by addition of 50 μl of 0.1% sodium dodecyl sulfate (SDS) and 50 μl of chloroform, and then 200 μl of 13.28 mM ONPG (o-nitrophenyl-β-d-galactopyranoside) was added to initiate the reaction. After incubation at 30°C for 30 min, the reaction was terminated by addition of 500 μl of 1 M Na2CO3. Cells were removed from the reaction mixture by centrifugation (12,000 rpm, 2 min), and the absorbance at 420 nm was measured to calculate the β-galactosidase activity (OD420 min−1 μl−1 OD730−1).
Western blot analysis.
Synechocystis cells grown at 30°C were preconditioned at 15°C and exposed to chill and light for different periods of time. Protein samples were prepared from Synechocystis cells by sonication in the presence of 1 mM phenylmethylsulfonyl fluoride (PMSF). Western blot analyses were performed as previously described (12) using rabbit antisera against Rbp1, EF-Ts, and LacZ. The former two antisera were generated by this laboratory, while the anti-LacZ antiserum was purchased from Creative Diagnostics. Portions (20 μg) of the proteins were loaded into each lane. The protein concentrations were determined by the Bradford method (43).
Measurements of polyamines.
Polyamines were extracted from Synechocystis 6803 with 5% cold HClO4 and derivatized with benzoyl chloride as reported earlier (21). The derivatization of polyamine standards (Sigma Chemicals) was performed similarly. The polyamine contents were analyzed by high-performance liquid chromatography using a C18 reversed-phase column and a UV-Vis detector at 254 nm. The system was run using a 60% methanol over 25 min with a flow rate of 1 ml min−1, and the oven temperature was 35°C.
Measurement of protein carbonylation.
Synechocystis cells were broken by sonication on ice in the presence of 1 mM PMSF. Cell debris was removed by centrifugation (12,000 rpm, 15 min) at 4°C. Protein carbonylation was measured spectrophotometrically by using an OxiSelect protein carbonyl spectrophotometric assay kit (Cell Biolabs).
RESULTS
Upregulation of sll0873 (CASDC) in response to chill-light stress.
Pretreatment at 15°C can greatly enhance the chill-light survivability of Synechocystis 6803 (7). Previously, we had analyzed the response of 30°C-grown Synechocystis 6803 to 15°C using microarrays (12). In the present study, we further performed a transcriptomic analysis of the response of 15°C-treated cells to chill-light stress. Many genes showed significant changes in mRNA level within 2 h of exposure to chill-light, including 44 genes upregulated (4°C/15°C ratio of ≥2.0) and 39 genes downregulated (4°C/15°C ratio of ≤0.5) (see Table S2 in the supplemental material).
By generating mutants of genes that are upregulated upon exposure to chill-light (denoted by a or b in Table S2 in the supplemental material) and testing their chill-light survivability, we identified slr0083 (crhR) and sll0873 (CASDC) as two important genes for the survivability (data not shown). slr0083 that encodes an RNA helicase has been investigated for its role in cold acclimation (temperature downshift from 34 to 24°C) before (9). In our study, we focused on sll0873, a gene predicted to encode the carboxy(nor)spermidine decarboxylase, for its specific role in chill-light survivability.
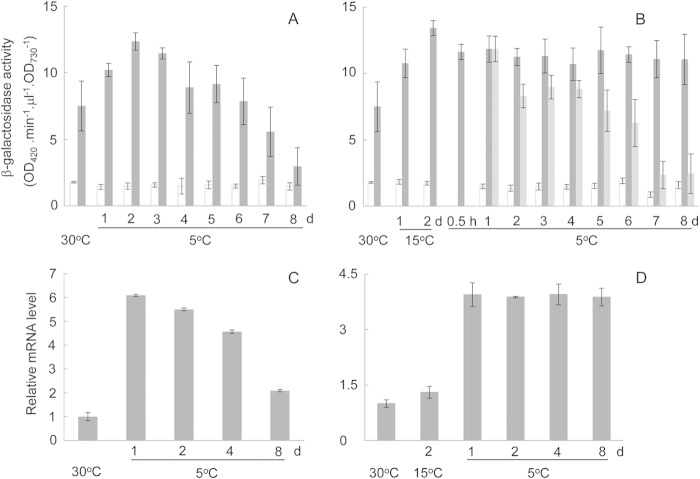
Northern blot analysis showed that sll0873 mRNA was gradually accumulated after transfer from 15°C to 5°C and reached the maximal level at 24 h (Fig. 2A). The mRNA level gradually decreased afterwards. qRT-PCR analysis confirmed this finding (Fig. 2B). The marked increase of mRNA abundance could be due to either activation of the promoter or enhancement of mRNA stability. Here, we analyzed the mRNA stability by measuring the rate of decay in the presence of rifampin. In cells exposed to chill light for 1 day, sll0873 mRNA showed much more enhanced stability than in cells at 15°C (Fig. 2C). The promoter activity was analyzed later using the lacZ reporter.
FIG 2.
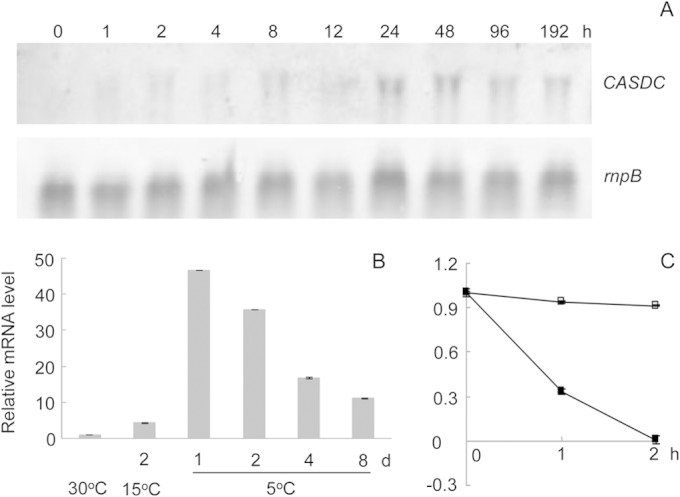
mRNA levels of CASDC in Synechocystis 6803 at different stages of acquisition of chill-light tolerance. (A) Northern blot analysis of expression of CASDC in cells exposed to chill-light stress. (B) qRT-PCR measurement of CASDC mRNA levels in cells grown at 30°C, treated at 15°C, and exposed to chill (5°C)-light stress. (C) qRT-PCR analysis of CASDC mRNA decay at 15°C (■) or under chill-light stress (□).
Requirement of spermidine for survival of Synechocystis 6803 under chill-light stress.
Jantaro et al. (21) reported that Synechocystis 6803 possesses spermidine, spermine, and putrescine, with spermidine as the most abundant polyamine (21). However, spermidine was the only detectable polyamine in Synechocystis 6803 maintained in our laboratory (see Fig. S1 in the supplemental material). Similarly, substrains of Synechocystis 6803 differ in tocopherol contents (7). Unlike the mRNA level of CASDC, the spermidine content decreased after transfer from 30 to 15°C and then remained unchanged or increased slightly after further transfer to 5°C in the light (Fig. 3). In cells directly exposed to chill-light stress for 8 days, spermidine decreased to a much lower level than in cells pretreated at 15°C.
FIG 3.
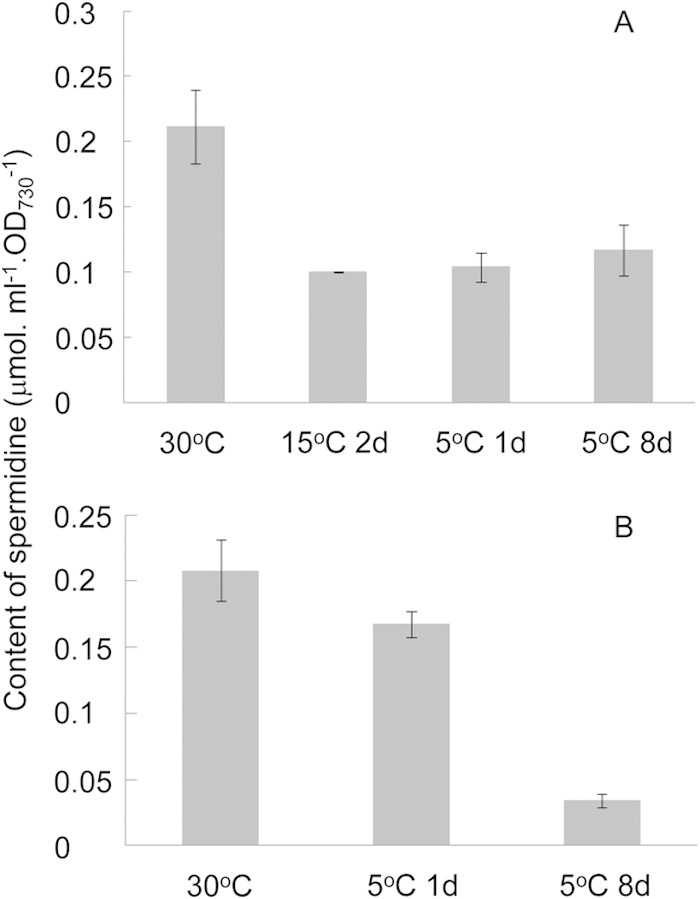
Content of spermidine in Synechocystis 6803 at different temperatures. (A) Cells grown at 30°C were treated at 15°C for 2 days before exposure to chill-light stress. (B) Cells grown at 30°C were directly exposed to chill-light stress. d, day(s).
To investigate the role of spermidine in chill-light survivability, we generated the CASDC-null mutant of Synechocystis 6803, namely, sll0873::Kmr (DRHB2193). CASDC can catalyze the synthesis of spermidine as well as norspermidine. However, no norspermidine was detected in Synechocystis 6803. Inactivation of sll0873 abolished the synthesis of spermidine; after complementation, the synthesis of spermidine was resumed in the mutant (see Fig. S1 in the supplemental material). The spermidine-minus mutant lost over 50% RACLT (relative acquired chill-light tolerance, calculated based on survivability of the mutant under the chill-light stress relative to the wild type treated in parallel [7]), while complementation with wild-type sll0873 almost fully restored the RACLT (Fig. 4A).
FIG 4.
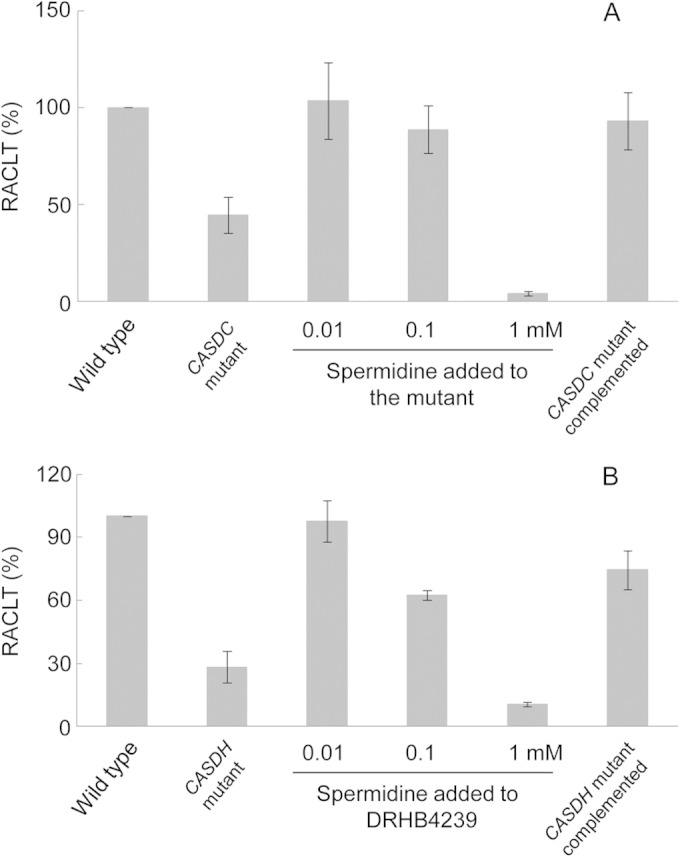
Effect of spermidine on the chill-light survivability of Synechocystis 6803. RACLT, relative acquired chill-light tolerance. (A) Requirement of CASDC for the chill-light survivability. (B) Requirement of CASDH for the chill-light survivability.
It has been reported that Synechocystis 6803 can take up spermidine (31). We tested the effect of exogenous spermidine on the RACLT of sll0873::Kmr. Spermidine was added to the culture medium at 0.01 or 0.1 mM, and the CASDC-null mutant was grown in the medium with spermidine at 15°C for 6 days before transfer to the chill-light stress. The exogenous spermidine fully restored the RACLT of the mutant to the wild-type level (Fig. 4A). However, 1 mM spermidine appeared to be toxic to Synechocystis cells. These results indicated that spermidine is required to promote the chill-light survivability of Synechocystis 6803.
To confirm the role of spermidine in chill-light survivability, we also generated the CASDH-null mutant, slr0049::Kmr (DRHB4239). Inactivation of this gene showed similar effects on synthesis of spermidine (see Fig. S2 in the supplemental material) and chill-light survivability (Fig. 4B), and the RACLT was reduced to ca. 30%. Complementation with wild-type slr0049 or addition of spermidine fully or partially restored the RACLT (Fig. 4B). Even though spermidine is required for the chill-light survivability, inactivation of CASDH or CASDC showed no effect on the growth of Synechocystis 6803 in BG11 at 30 and 15°C.
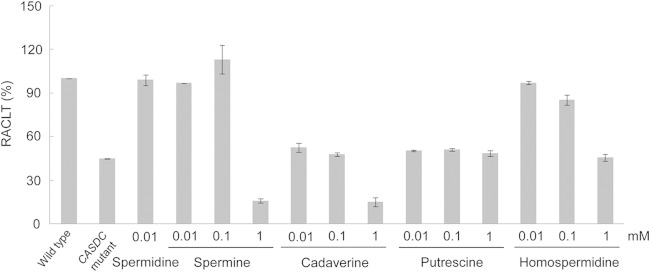
Using the CASDC mutant, we also tested whether spermidine, a triamine, can be replaced with other polyamines. We tested spermine (tetramine), homospermidine (triamine), cadaverine (diamine), and putrescine (diamine). Of these polyamines, at least putrescine has been shown to be taken up by Synechocystis 6803 (30), and the uptake of spermine has been shown in another cyanobacterium (29). The addition of 0.0l or 0.1 mM spermine/homospermidine restored the RACLT of CASDC mutant to the wild-type level, while cadaverine and putrescine only slightly increased the RACLT (Fig. 5). Apparently, diamines cannot serve as substitutes for spermidine in promoting chill-light survivability.
FIG 5.
Effects of spermine, cadaverine, putrescine, and homospermidine on the RACLT of the CASDC mutant of Synechocystis 6803.
Role of spermidine in expression of PCASDC-lacZ under chill-light stress.
Polyamines have been shown to affect gene expression in other bacteria (14). The requirement of spermidine for the chill-light survivability of Synechocystis 6803 may relate to its role in gene expression under the chill-light stress. We attempted to use the in vitro translation system (44) to test this hypothesis but found it technically unfeasible due to the precipitation of constituents at the low temperature. Alternatively, we used lacZ as a reporter gene to test gene expression in vivo. CASDC was shown to be actively expressed at 5°C. RACE analysis indicated that transcription of this gene started 324 bp (“A” at bp 1141984 of the chromosome) upstream of the start codon. On the basis of this information, we constructed the transcriptional fusion PCASDC-lacZ and introduced this construct into the wild type and the sll0873::Kmr mutant of Synechocystis 6803 via double-crossover integration into a neutral platform in the genome.
In the wild-type strain carrying PCASDC-lacZ, the expression of lacZ was consistent with the acquisition of chill-light tolerance. Without preconditioning at 15°C, the β-galactosidase activity increased in first 2 days after exposure to chill and light and then gradually decreased to a low level in next 6 days (Fig. 6A); with preconditioning, the enzyme activity was maintained at the high level within the same range of time under chill-light stress (Fig. 6B). Measurement of the relative mRNA level of lacZ with qRT-PCR showed similar results (Fig. 6C and D). When chloramphenicol was added to preconditioned cells at the beginning of chill-light stress, the β-galactosidase activity gradually decreased to the close-to-background level (Fig. 6B). It indicated that the high and stable β-galactosidase activity in such cells depended upon the activity of protein synthesis under the chill-light stress.
FIG 6.
Expression of lacZ in Synechocystis 6803::PCASDC-lacZ under the chill-light stress with (B and D) or without (A and C) preconditioning. The expression of lacZ was determined as shown by the β-galactosidase activity (A and B) or qRT-PCR (C, D). Bars in panels A and B: dark gray, Synechocystis 6803::PCASDC-lacZ; light gray, Synechocystis 6803::PCASDC-lacZ supplemented with chloramphenicol upon transfer to 5°C; open, Synechocystis 6803 treated in parallel (background activity).
Using the lacZ reporter system, we examined the role of spermidine in gene expression under chill-light stress. After the transfer of cells from 30 to 15°C, Synechocystis 6803 sll0873::Kmr with PCASDC-lacZ, like the wild type with PCASDC-lacZ, showed an increase of β-galactosidase activity (Fig. 7A). After exposure to chill-light stress, the enzyme activity in the spermidine-minus strain gradually decreased as if chloramphenicol were added; exogenous supplementation of spermidine restored the ability to maintain the enzyme activity under the stress (Fig. 7A).
FIG 7.
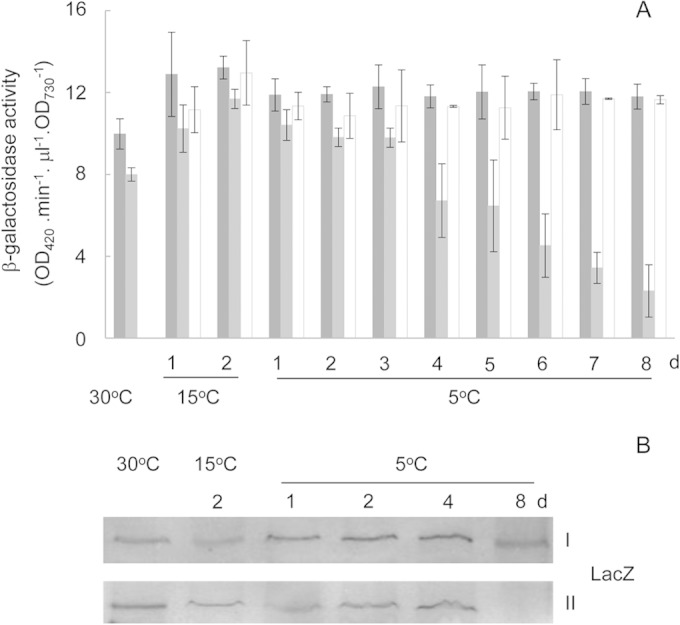
Assays of β-galactosidase activity and LacZ abundance showed the effect of spermidine on expression of PCASDC-lacZ in Synechocystis strains. (A) β-Galactosidase activity in Synechocystis 6803::PCASDC-lacZ (dark gray), Synechocystis 6803 DRHB2193::PCASDC-lacZ without (light gray), or with (open) exogenous spermidine. (B) Western blot detection of LacZ in Synechocystis 6803::PCASDC-lacZ (row I) and Synechocystis 6803 DRHB2193::PCASDC-lacZ (row II). DRHB2193, the CASDC mutant.
Using Western blot analysis, we also detected LacZ at the protein level. Within 4 days under the chill-light stress, the two strains showed no difference in abundance of LacZ; on day 8, LacZ remained almost unchanged in the wild type with PCASDC-lacZ but disappeared in the spermidine-minus strain (Fig. 7B).
The decrease in β-galactosidase activity and LacZ abundance in the mutant may reflect the role of spermidine in translation or transcription or both. Using qRT-PCR, we compared mRNA levels of lacZ in the two strains and found results similar to when we assayed the β-galactosidase activity (Fig. 8A): the lack of spermidine led to a gradual decrease in the mRNA level. Because the decay of lacZ mRNA was slower in the spermidine-minus strain (Fig. 8B), spermidine was required for the transcription of PCASDC-lacZ rather than the stability of lacZ mRNA.
FIG 8.
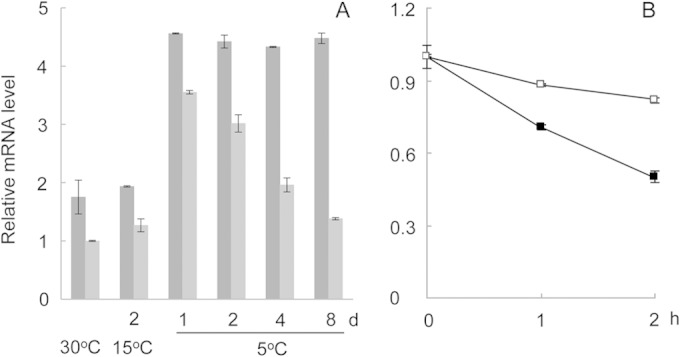
Measurements of lacZ mRNA level showing the effect of spermidine on expression of PCASDC-lacZ in Synechocystis strains. (A) qRT-PCR analysis of lacZ mRNA levels in Synechocystis 6803::PCASDC-lacZ (dark gray) and Synechocystis 6803 DRHB2193::PCASDC-lacZ (light gray). (B) qRT-PCR analysis of lacZ mRNA decay in Synechocystis 6803::PCASDC-lacZ (■) and Synechocystis 6803 DRHB2193::PCASDC-lacZ (□) under chill-light stress. DRHB2193, the CASDC mutant.
Role of spermidine in expression of other genes under chill-light stress.
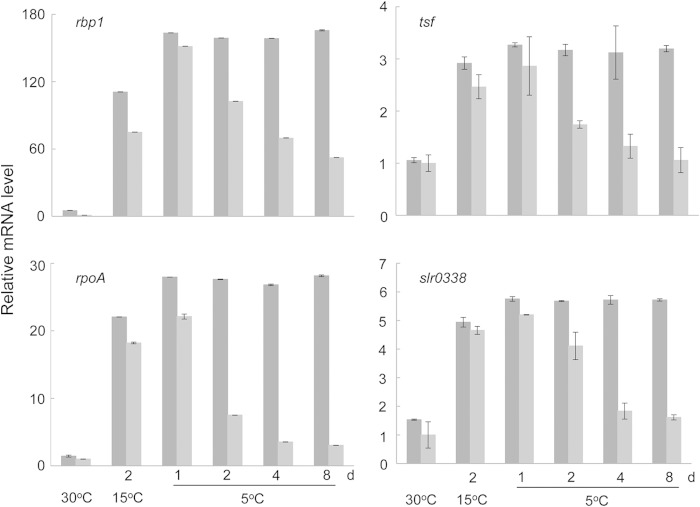
To further investigate the role of spermidine in gene expression under chill-light stress, we compared the relative mRNA levels of four other genes—rbp1, tsf, rpoA, and slr0338—in the wild type and the CASDC mutant of Synechocystis 6803 using qRT-PCR (Fig. 9). These genes encode RNA-binding protein 1 (12), elongation factor Ts (EF-Ts), RNA polymerase α-subunit, and a potential oxidoreductase, respectively. In wild-type cells transferred from 30 to 15°C and then to 5°C, the mRNA levels of these genes showed stepwise increases and remained unchanged within 8 days of chill-light stress. In contrast, these mRNAs in the spermidine-minus mutant gradually decreased after exposure to chill and light.
FIG 9.
qRT-PCR measurement of mRNA levels showing the role of spermidine synthesis in gene expression under chill-light stress. Bars: dark gray, Synechocystis 6803; light gray, the CASDC mutant. rbp1, RNA-binding protein 1; tsf, EF-Ts; rpoA, α subunit of RNA polymerase; slr0338, probable oxidoreductase.
Using rbp1 as an example, we tested the effect of spermidine on the stability of mRNAs and found a slight increase in the spermidine-minus mutant compared to the wild type (see Fig. S3 in the supplemental material). Therefore, in Synechocystis cells exposed to chill-light stress, spermidine enhances transcription activity rather than mRNA stability.
Unlike LacZ, the abundance of Rbp1 and EF-Ts remained unchanged within 8 days of chill-light stress in strains with or without spermidine (see Fig. S4 in the supplemental material). Apparently, in cyanobacterial cells exposed to chill-light stress, proteins differ from each other in stability.
Role of spermidine in recovery from protein oxidative damage.
Synechocystis 6803 shows no growth and no photosynthetic O2 evolution activity after exposure to the chill-light stress for over 24 h (data not shown). Turnover of macromolecules, such as replacement of damaged proteins with newly synthesized ones, is critical for maintaining the viability of cyanobacterial cells under such conditions. In Fig. 7, the β-galactosidase activity decreased before the degradation of LacZ in the CASDC mutant with PCASDC-lacZ. This could be due to protein oxidative damage under the chill-light stress. Spermidine may reduce protein oxidative damage by scavenging free radicals (14) or promote the recovery from oxidative damage by enhancing protein synthesis. We compared protein carbonylation in the wild type and the CASDC mutant of Synechocystis 6803. In the wild type transferred from 30 to 15°C and then to 5°C, protein carbonylation rapidly increased on the first day of exposure to 5°C but dropped back to the background level on the second day (Fig. 10). In the spermidine-minus mutant, protein carbonylation also rapidly increased on the first day at 5°C (to a slightly lower level in comparison to the wild type) but continued to slowly increase in next several days. Spermidine appeared to play an important role in recovery from oxidative damage under the chill-light stress.
FIG 10.
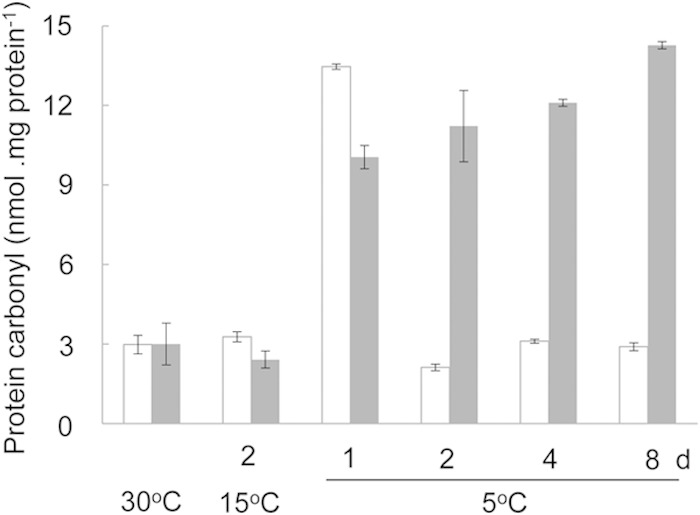
Comparison of protein carbonylation in the wild type and the CASDC mutant of Synechocystis 6803. Bars: open, wild type; gray, CASDC mutant.
Requirement of spermidine for chill-light survivability of Synechococcus 7942.
Many cyanobacteria possess SAMDC and spermine/spermidine synthase (SPDS) rather than CASDH and CASDC (Table 2). In other words, the classical pathway of polyamine synthesis is much more widely distributed in cyanobacteria. To confirm that spermidine is also required for the chill-light survivability of cyanobacteria with the classical pathway, we generated a SPDS-null mutant of Synechococcus sp. PCC 7942 (Synechococcus 7942), namely, DRHB5450 (SPDS::Kmr). In this mutant, spermidine was no longer synthesized, but putrescine was detected at a low level (see Fig. S5 in the supplemental material). Elimination of spermidine from Synechococcus 7942 reduced its RACLT to ca. 40%, and the addition of 0.1 mM spermidine to the medium restored the RACLT to ca. 80% (Fig. 11).
TABLE 2.
Distribution of genes involved in spermidine synthesis in cyanobacteria
| Strain | Presence (+) or absence (–) of gene for: |
|||||||
|---|---|---|---|---|---|---|---|---|
| ADC | AUH | AIH | NCPAH | SAMDC | SPDSa | CASDH | CASDC | |
| Acaryochloris marina MBIC11017 | + | + | + | + | – | + | – | – |
| Anabaena sp. PCC 7120 | + | + | – | – | – | – | – | – |
| Anabaena variabilis ATCC 29413 | + | + | – | – | – | – | – | – |
| Arthrospira platensis | + | + | – | – | + | + | – | – |
| Chamaesiphon minutus | + | + | – | – | – | – | + | + |
| Cyanothece sp. ATCC 51142 | – | – | – | – | – | – | – | – |
| Cyanothece sp. PCC 7424 | + | + | – | – | + | + | – | – |
| Cyanothece sp. PCC 7425 | + | – | + | + | – | – | – | – |
| Cyanothece sp. PCC 8801 | + | – | + | + | + | + | – | – |
| Fischerella sp. PCC 9605 | + | + | – | – | + | + | – | – |
| Gloeobacter violaceus PCC 7421 | + | – | + | + | – | – | – | – |
| Leptolyngbya boryana | + | – | – | – | – | – | + | + |
| Mastigocladopsis repens | + | + | – | – | + | + | – | – |
| Microcystis aeruginosa NIES-843 | + | + | – | – | + | + | – | – |
| Nostoc punctiforme ATCC 29133 | + | + | – | – | – | – | – | – |
| Prochlorococcus marinusb | + | + | – | – | + | + | – | – |
| Synechococcus sp. WH8102 | + | + | – | – | + | + | – | – |
| Synechococcus elongatus PCC 7942 | + | – | + | + | + | + | – | – |
| Synechococcus sp. PCC 9311 | + | + | – | – | + | + | – | – |
| Synechocystis sp. PCC 6803 | + | + | – | – | – | – | + | + |
| Synechocystis sp. PCC 6714 | + | + | – | – | – | – | + | + |
| Scytonema hofmanni | + | + | – | – | + | + | + | + |
| Trichodesmium erythraeum IMS101 | + | + | – | – | + | + | – | – |
Because spermidine rather than spermine is one of the major polyamines in cyanobacteria, the spermine/spermidine synthase gene is tentatively abbreviated as SPDS (spermidine synthase).
Prochlorococcus marinus includes all 12 strains listed in the Cyanobase (http://genome.microbedb.jp/cyanobase/).
FIG 11.
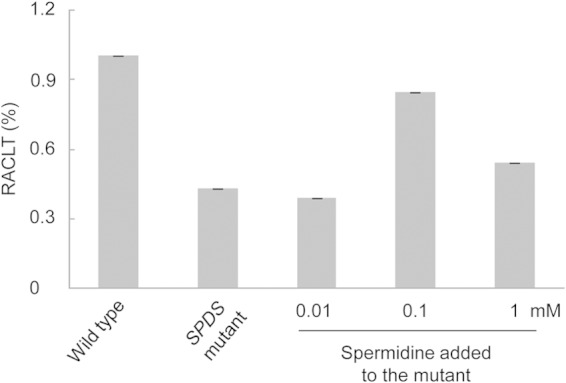
Effect of spermidine on chill-light survivability of Synechococcus 7942.
DISCUSSION
Polyamines are involved in various cellular activities in heterotrophic bacteria, but their role in environmental adaptation of cyanobacteria has been barely investigated. In the present study, we found that spermidine is required for the chill-light survivability of cyanobacteria and that the physiological function is based on its effects on gene expression and recovery from protein damage.
Before the finding of the alternative polyamine synthesis pathway in bacteria (15), spermidine synthesis in Synechocystis 6803 was thought to occur via SAMDC, and spermine was reported to be detected in Synechocystis samples (21, 45). Our study, however, showed that polyamine synthesis in this cyanobacterium should be via the alternative pathway rather than the classical pathway because the inactivation of either CASDH or CASDC abolished the synthesis of spermidine, and no gene has been predicted to encode SAMDC in the genome. Also, no spermine was detectable in Synechocystis 6803 under our conditions. This is supported by the lack of spermine synthase gene in the genome. Furthermore, in an investigation of polyamine distribution profiles, spermine was only found at very low levels in 7 of 63 cyanobacterial strains (Hosoya et al. [27], with no information of axenicity of these strains).
According to the gene information shown in Table 2, many cyanobacteria can be predicted to synthesize spermidine via either classical or alternative pathway or both. Synechocystis 6803, and several cyanobacterial strains that possess the alternative pathway might have acquired CASDH and CASDC genes from heterotrophic bacteria by horizontal gene transfer. Without the two enzymes (diaminobutyrate aminotransferase, diaminobutyrate decarboxylase) for the synthesis of diaminopropane (15), Synechocystis 6803 produces spermidine rather than norspermidine. In some cyanobacteria, such as Anabaena sp. and most other species of Nostocales, the major polyamine is homospermidine (27). Table 2 also lists one strain that lacks both AUH and AIH/NCPAH (Leptolyngbya boryana) and one strain that lacks all of the enzymes involved in spermidine synthesis (Cyanothece sp. strain ATCC 51142). Possibly, such cyanobacterial species can acquire polyamines via import from environments or from coexisting bacteria.
The initial prompt for us to relate spermidine synthesis to chill-light survivability of cyanobacteria was the upregulation of CASDC gene in response to chill and light. Preconditioning at 15°C enabled Synechocystis cells to maintain a much higher level of spermidine after 8 days of chill-light stress. Similar effects of preconditioning had been found on α-tocopherol (7) and Rbp1 (12) in Synechocystis. A downshift of the temperature to 5°C greatly increased the stability of CASDC mRNA, leading to accumulation of the mRNA. Unlike the mRNA abundance, the spermidine content decreased at 15°C to about half of that at 30°C and remained unchanged or slightly increased after transfer to 5°C. The decrease at 15°C could be due to a slowed uptake of nitrate (46) because the synthesis of spermidine is probably responsive to nitrogen status that affects the availability of arginine. At 5°C, spermidine synthesis might have significantly decreased if CASDC were not upregulated.
Our results clearly indicated that spermidine promoted the chill-light survivability. Inactivation of either CASDH or CASDC in Synechocystis 6803 or SPDS in Synechococcus 7942 remarkably reduced the chill-light survivability, and supplementation with spermidine restored the survivability of mutants. The chain length of polyamine appeared to be important for such a physiological function because the chill-light survivability of the Synechocystis CASDC mutant could be restored by spermine (a tetramine) and spermidine and homospermidine (triamines) but not putrescine and cadaverine (both diamines). This result suggests that homospermidine, a major polyamine in some cyanobacterial groups, should also play a role in chill-light survivability.
To analyze the underlying molecular mechanism for the role of spermidine in promoting chill-light survivability, we introduced PCASDC-lacZ into the wild type and the CASDC mutant of Synechocystis 6803. After preconditioning, the wild type with PCASDC-lacZ showed a high level of β-galactosidase activity over 8 days under the chill-light stress. Inhibition of protein synthesis by chloramphenicol and elimination of spermidine synthesis by gene inactivation both led to a decrease in β-galactosidase activity to a very low level. On one hand, this showed the persistence of protein synthesis activity in Synechocystis 6803 at 5°C over a relatively long period of time; on the other hand, this indicated the role of spermidine in gene expression. In parallel to the β-galactosidase activity, the abundance of lacZ mRNA decreased without compromised mRNA stability, and examination of the mRNA abundance for tsf, rbp1, rpoA, and slr0338 showed similar results. The decrease in β-galactosidase activity is seemingly due to the decline of transcription in spermidine-minus cells. Equally possible, the effect of spermidine on transcription is attributed to its role in translation because polyamines may enhance the synthesis of RNA polymerase at 5°C by binding to its mRNA.
Alternatively, polyamines can reduce oxidative stress by scavenging free radicals; therefore, lack of spermidine may intensify oxidative damage of LacZ and RNA polymerase under the chill-light stress. Oxidative modification of amino acid side chains to carbonyl derivatives results in protein carbonylation. Only a portion of protein molecules are carbonylated (0.05 to 0.4 carbonyl per 50-kDa protein) in cells (47), and the carbonylation appears to prefer some proteins over others (48–50). Oxidative damage could inhibit protein functions by modifying critical sites, destabilizing tertiary structure and promoting protein aggregation (51, 52). Due to its irreversible nature, carbonylation is widely used to indicate the degree of protein oxidation. On the first day at 5°C, the CASDC mutant showed a lower protein carbonylation level than the wild type, indicating that spermidine was actually not an important free radical scavenger in this case. After acclimation on the first day, free radicals presumably diminished in Synechocystis 6803. At this stage, protein carbonylation rapidly returned to the background level in the wild type but continued to slowly increase in the CASDC mutant (Fig. 10). This difference can be explained by the lack of protein turnover in the mutant at 5°C; under the same conditions, damaged proteins in the wild type can be actively replaced with newly synthesized ones. The continually increased protein carbonylation would inactivate RNA polymerase and other enzymes in the mutant under chill-light stress. Consistently, in a Synechocystis spermidine-minus background, the transcription activity (as seen with mRNA abundance in Fig. 8 and 9) and β-galactosidase activity (Fig. 7) continued to decrease within 8 days, and LacZ was even completely degraded between days 4 and 8 (Fig. 7).
On the basis of these analyses, we propose that spermidine is required for maintaining gene expression in cyanobacteria under chill-light stress. At 5°C, spermidine may promote translation by binding to mRNA, promote transcription by binding to DNA, or both. In any case, gene expression under the chill-light stress promotes replacement of damaged proteins, so that cyanobacterial cells can maintain viability in the winter. Therefore, spermidine should play an important role in overwintering of cyanobacteria, even though it is not required for their growth at favorable temperatures.
Supplementary Material
ACKNOWLEDGMENTS
This study was supported by the National Natural Science Foundation of China (30825003) and the State Key Laboratory of Freshwater Ecology and Biotechnology at the Institute of Hydrobiology, Chinese Academy of Sciences (2014FBZ).
We thank Anthony J. Michael of University of Texas Southwestern Medical Center for critical reading of the manuscript.
Footnotes
Supplemental material for this article may be found at http://dx.doi.org/10.1128/JB.00153-15.
REFERENCES
- 1.Preston T, Steward WDP, Reynolds CS. 1980. Bloom-forming cyanobacterium Microcystis aeruginosa overwinters on sediment surface. Nature 288:365–367. doi: 10.1038/288365a0. [DOI] [Google Scholar]
- 2.Takamura N, Yasuno M, Sugahara K. 1984. Overwintering of Microcystis aeruginosa Kutz in a shallow lake. J Plankton Res 6:1019–1029. doi: 10.1093/plankt/6.6.1019. [DOI] [Google Scholar]
- 3.Visser PM, Ibelings BW, Mur LR. 1995. Autumnal sedimentation of Microcystis spp. as result of an increase in carbohydrate ballast at reduced temperature. J Plankton Res 17:919–933. doi: 10.1093/plankt/17.5.919. [DOI] [Google Scholar]
- 4.Tsujimura S, Tsukada H, Nakahara H, Nakajima T, Nishino M. 2000. Seasonal variations of Microcystis populations in sediments of Lake Biwa, Japan. Hydrobiology 434:183–192. doi: 10.1023/A:1004077225916. [DOI] [Google Scholar]
- 5.Brunberg AK, Blomqvist P. 2003. Recruitment of Microcystis (Cyanophyceae) from lake sediments: the importance of littoral inocula. J Phycol 39:58–63. doi: 10.1111/j.0022-3646.2003.03906001_169.x. [DOI] [Google Scholar]
- 6.Verspagen JMH, Snelder EOFM, Visser PM, Huisman J, Mur LR, Ibelings BW. 2004. Recruitment of benthic Microcystis (Cyanophyceae) to the water column: internal buoyancy changes or resuspension? J Phycol 40:260–270. doi: 10.1111/j.1529-8817.2004.03174.x. [DOI] [Google Scholar]
- 7.Yang Y, Yin C, Li W, Xu X. 2008. α-Tocopherol is essential for acquired chill-light tolerance in the cyanobacterium Synechocystis sp. strain PCC 6803. J Bacteriol 190:1554–1560. doi: 10.1128/JB.01577-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Zhu T, Xu X. 2013. Efficacy of a dual fluorescence method in detecting the viability of overwintering cyanobacteria. Lett Appl Microbiol 57:174–180. doi: 10.1111/lam.12095. [DOI] [PubMed] [Google Scholar]
- 9.Prakash JS, Krishna PS, Sirisha K, Kanesaki Y, Suzuki I, Shivaji S, Murata N. 2010. An RNA helicase, CrhR, regulates the low-temperature-inducible expression of heat-shock genes groES, groEL1, and groEL2 in Synechocystis sp. PCC 6803. Microbiology 156:442–451. doi: 10.1099/mic.0.031823-0. [DOI] [PubMed] [Google Scholar]
- 10.Los DA, Ray MK, Murata N. 1997. Differences in the control of the temperature-dependent expression of four genes for desaturases in Synechocystis PCC 6803. Mol Microbiol 25:1167–1175. doi: 10.1046/j.1365-2958.1997.5641912.x. [DOI] [PubMed] [Google Scholar]
- 11.Li W, Gao H, Yin C, Xu X. 2012. Identification of a novel thylakoid protein gene Involved in cold acclimation in cyanobacteria. Microbiology 158:2440–2449. doi: 10.1099/mic.0.060038-0. [DOI] [PubMed] [Google Scholar]
- 12.Tan X, Zhu T, Shen S, Yin C, Gao H, Xu X. 2011. The role of Rbp1 in the acquired chill-light tolerance of cyanobacteria. J Bacteriol 193:2675–2683. doi: 10.1128/JB.01454-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Igarashi K, Kashiwagi K. 2000. Polyamines: mysterious modulators of cellular functions. Biochem Biophys Res Commun 271:559–564. doi: 10.1006/bbrc.2000.2601. [DOI] [PubMed] [Google Scholar]
- 14.Shah P, Swiatlo E. 2008. A multifaceted role of polyamines in bacterial pathogens. Mol Microbiol 68:4–16. doi: 10.1111/j.1365-2958.2008.06126.x. [DOI] [PubMed] [Google Scholar]
- 15.Lee J, Sperandio V, Frantz DE, Longgood J, Camilli A, Phillips MA, Michael AJ. 2009. An alternative polyamine biosynthetic pathway is widespread in bacteria and essential for biofilm formation in Vibrio cholerae. J Biol Chem 284:9899–9907. doi: 10.1074/jbc.M900110200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.McGinnis MW, Parker ZM, Walter NE, Rutkovsky AC, Cartaya-Marin C, Karatan E. 2009. Spermidine regulates Vibrio cholerae biofilm formation via transport and signaling pathways. FEMS Microbiol Lett 299:166–174. doi: 10.1111/j.1574-6968.2009.01744.x. [DOI] [PubMed] [Google Scholar]
- 17.Dela-Vega AL, Delcour AH. 1996. Polyamines decrease Escherichia coli outer membrane permeability. Biochem J 178:3715–3721. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Iyer R, Wu Z, Woster PM, Delcour AH. 2000. Molecular basis for the polyamine-OmpF porin interactions: inhibitor and mutant studies. J Mol Biol 297:933–945. doi: 10.1006/jmbi.2000.3599. [DOI] [PubMed] [Google Scholar]
- 19.Jung IL, Kim IG. 2003. Polyamines and glutamate decarboxylase-based acid resistance in Escherichia coli. J Biol Chem 278:22846–22852. doi: 10.1074/jbc.M212055200. [DOI] [PubMed] [Google Scholar]
- 20.Chattopadhyay MK, Tabor H. 2013. Polyamines are critical for the induction of the glutamate decarboxylase-dependent acid resistance system in Escherichia coli. J Biol Chem 288:33559–33570. doi: 10.1074/jbc.M113.510552. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Jantaro S, Mäenpää P, Mulo P, Incharoensakdi A. 2003. Content and biosynthesis of polyamines in salt and osmotically stressed cells of Synechocystis sp. PCC 6803. FEMS Microbiol Lett 228:129–135. doi: 10.1016/S0378-1097(03)00747-X. [DOI] [PubMed] [Google Scholar]
- 22.Alcázar R, Cuevas JC, Planas J, Zarza X, Bortolotti C, Carrasco P, Salinas J, Tiburcio AF, Altabella T. 2011. Integration of polyamines in the cold acclimation response. Plant Sci 180:31–38. doi: 10.1016/j.plantsci.2010.07.022. [DOI] [PubMed] [Google Scholar]
- 23.Tait GH. 1985. Bacterial polyamines, structures, and biosynthesis. Biochem Soc Trans 13:316–318. [DOI] [PubMed] [Google Scholar]
- 24.Nakada Y, Itoh Y. 2003. Identification of the putrescine biosynthetic genes in Pseudomonas aeruginosa and characterization of agmatine deiminase and N-carbamoylputrescine amidohydrolase of the arginine decarboxylase pathway. Microbiology 149:707–714. doi: 10.1099/mic.0.26009-0. [DOI] [PubMed] [Google Scholar]
- 25.Burrell M, Hanfrey CC, Murray EJ, Stanley-Wall NR, Michael AJ. 2010. Evolution and multiplicity of arginine decarboxylases in polyamine biosynthesis and essential role in Bacillus subtilis biofilm formation. J Biol Chem 285:39224–39238. doi: 10.1074/jbc.M110.163154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Hanfrey CC, Pearson BM, Hazeldine S, Lee J, Gaskin DJ, Woster PM, Phillips MA, Michael AJ. 2011. Alternative spermidine biosynthetic route is critical for growth of Campylobacter jejuni and is the dominant polyamine pathway in human gut microbiota. J Biol Chem 286:43301–43312. doi: 10.1074/jbc.M111.307835. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Hosoya R, Hamana K, Isobe M, Yokota A. 2005. Polyamine distribution profiles within cyanobacteria. Microbiol Cult Coll 21:3–8. [Google Scholar]
- 28.Shaw FL, Elliot KA, Kinch LN, Fuell C, Phillips MA, Michael AJ. 2010. Evolution and multifarious horizontal transfer of an alternative biosynthetic pathway for the alternative polyamine sym-homospermidine. J Biol Chem 285:14711–14723. doi: 10.1074/jbc.M110.107219. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Ramakrishna S, Guarino L, Cohen SS. 1978. Polyamines of Anacystis nidulans and metabolism of exogenous spermidine and spermine. J Bacteriol 134:744–750. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Raksajit W, Mäenpää P, Incharoensakdi A. 2006. Putrescine transport in a cyanobacterium Synechocystis sp. PCC 6803. J Biochem Mol Biol 39:394–399. [DOI] [PubMed] [Google Scholar]
- 31.Raksajit W, Yodsang P, Maenpaa P, Incharoensakdi A. 2009. Characterization of spermidine transport system in a cyanobacterium, Synechocystis sp. PCC 6803. J Microbiol Biotechnol 19:447–454. [DOI] [PubMed] [Google Scholar]
- 32.Yodsang P, Pothipongsa A, Mäenpää P, Incharoensakdi A. 2014. Involvement of polyamine binding protein D (PotD) of Synechocystis sp. PCC 6803 in spermidine uptake and excretion. Curr Microbiol 69:417–422. doi: 10.1007/s00284-014-0605-9. [DOI] [PubMed] [Google Scholar]
- 33.Yin C, Li W, Du Y, Kong R, Xu X. 2007. Identification of a gene, ccr-1 (sll1242), required for chill-light tolerance and growth at 15°C in Synechocystis sp. PCC 6803. Microbiology 153:1261–1267. [DOI] [PubMed] [Google Scholar]
- 34.Williams JGK. 1988. Construction of specific mutations in photosystem II photosynthetic reaction center by genetic engineering methods in Synechocystis 6803. Methods Enzymol 167:766–778. doi: 10.1016/0076-6879(88)67088-1. [DOI] [Google Scholar]
- 35.Gao H, Xu X. 2009. Depletion of Vipp1 in Synechocystis sp. PCC 6803 affects photosynthetic activity prior to the loss of thylakoid membranes. FEMS Microbiol Lett 292:63–70. doi: 10.1111/j.1574-6968.2008.01470.x. [DOI] [PubMed] [Google Scholar]
- 36.Elhai J, Wolk CP. 1988. A versatile class of positive-selection vectors basted on the nonviability of palindrome-containing plasmids that allows the cloning into long polylinkers. Gene 68:119–138. doi: 10.1016/0378-1119(88)90605-1. [DOI] [PubMed] [Google Scholar]
- 37.Güler S, Seeliger A, Härtel H, Renger G, Benning C. 1996. A null mutant of Synechococcus sp. PCC 7942 deficient in the sulfolipid sulfoquinovosyl diacylglycerol. J Biol Chem 271:7501–7507. [DOI] [PubMed] [Google Scholar]
- 38.Zhang W, Du Y, Khudyakov I, Fan Q, Gao H, Ning D, Wolk CP, Xu X. 2007. A gene cluster that regulates both heterocyst differentiation and pattern formation in Anabaena sp. strain PCC 7120. Mol Microbiol 66:1429–1443. doi: 10.1111/j.1365-2958.2007.05997.x. [DOI] [PubMed] [Google Scholar]
- 39.Gao H, Xu X. 2012. The cyanobacterial NAD kinase gene sll1415 is required for photoheterotrophic growth and cellular redox homeostasis in Synechocystis sp. strain PCC 6803. J Bacteriol 194:218–224. doi: 10.1128/JB.05873-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Vioque A. 1992. Analysis of the gene encoding the RNA subunit of ribonuclease P from cyanobacteria. Nucleic Acids Res 20:6331–6337. doi: 10.1093/nar/20.23.6331. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Wang Y, Xu X. 2013. Effects of Rbp3 on lipid peroxidation and salt tolerance in Synechocystis sp. PCC 6803. FEBS Lett 587:1446–1451. doi: 10.1016/j.febslet.2013.03.028. [DOI] [PubMed] [Google Scholar]
- 42.Miller JH. 1972. Experiments in molecular genetics. Cold Spring Harbor Laboratory Press, Cold Spring Harbor, NY. [Google Scholar]
- 43.Kruger NJ. 2002. The Bradford method for protein quantitation, p 15–21. Walker JM. (ed), The protein protocols handbook, 2nd ed Humana Press, Totowa, NJ. [Google Scholar]
- 44.Kojima K, Oshita M, Nanjo Y, Kasai K, Tozawa Y, Hayashi H, Nishiyama Y. 2007. Oxidation of elongation factor G inhibits the synthesis of the D1 protein of photosystem II. Mol Microbiol 65:936–947. doi: 10.1111/j.1365-2958.2007.05836.x. [DOI] [PubMed] [Google Scholar]
- 45.Jantaro S, Kidron H, Chesnel D, Incharoensakdi A, Mulo P, Salminen T, Mäenpää P. 2006. Structural modeling and environmental regulation of arginine decarboxylase in Synechocystis sp. PCC 6803. Arch Microbiol 184:397–406. [DOI] [PubMed] [Google Scholar]
- 46.Sakamoto T, Bryant DA. 1999. Nitrate transport and not photoinhibition limits growth of the freshwater cyanobacterium Synechococcus species PCC 6301 at low temperature. Plant Physiol 119:785–794. doi: 10.1104/pp.119.2.785. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Møller IM, Rogowska-Wrzesinska A, Rao RSP. 2011. Protein carbonylation and metal-catalyzed protein oxidation in a cellular perspective. J Proteomics 74:2228–2242. doi: 10.1016/j.jprot.2011.05.004. [DOI] [PubMed] [Google Scholar]
- 48.Das N, Levine RL, Orr WC, Sohal RS. 2001. Selectivity of protein oxidative damage during aging in Drosophila melanogaster. Biochem J 360:209–216. doi: 10.1042/0264-6021:3600209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Jana CK, Das N, Sohal RS. 2002. Specificity of age-related carbonylation of plasma proteins in the mouse and rat. Arch Biochem Biophys 397:433–439. doi: 10.1006/abbi.2001.2690. [DOI] [PubMed] [Google Scholar]
- 50.Choi J, Rees HD, Weintraub ST, Levey AI, Chin LS, Li L. 2005. Oxidative modifications and aggregation of Cu, Zn-superoxide dismutase associated with Alzheimer and Parkinson diseases. J Biol Chem 280:11648–11655. doi: 10.1074/jbc.M414327200. [DOI] [PubMed] [Google Scholar]
- 51.Ding QX, Dimayuga E, Keller JN. 2007. Oxidative damage, protein synthesis, and protein degradation in Alzheimer's disease. Curr Alzheimer Res 4:73–79. doi: 10.2174/156720507779939788. [DOI] [PubMed] [Google Scholar]
- 52.Petrov D, Zagrovic B. 2011. Microscopic analysis of protein oxidative damage: effect of carbonylation on structure, dynamics, and aggregability of villin headpiece. J Am Chem Soc 133:7016–7024. doi: 10.1021/ja110577e. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.