ABSTRACT
In Salmonella enterica, 1,2-propanediol (1,2-PD) utilization (Pdu) is mediated by a bacterial microcompartment (MCP). The Pdu MCP consists of a multiprotein shell that encapsulates enzymes and cofactors for 1,2-PD catabolism, and its role is to sequester a reactive intermediate (propionaldehyde) to minimize cellular toxicity and DNA damage. For the Pdu MCP to function, the enzymes encapsulated within must be provided with a steady supply of substrates and cofactors. In the present study, Western blotting assays were used to demonstrate that the PduL phosphotransacylase is a component of the Pdu MCP. We also show that the N-terminal 20-residue-long peptide of PduL is necessary and sufficient for targeting PduL and enhanced green fluorescent protein (eGFP) to the lumen of the Pdu MCP. We present the results of genetic tests that indicate that PduL plays a role in the recycling of coenzyme A internally within the Pdu MCP. However, the results indicate that some coenzyme A recycling occurs externally to the Pdu MCP. Hence, our results support a model in which a steady supply of coenzyme A is provided to MCP lumen enzymes by internal recycling by PduL as well as by the movement of coenzyme A across the shell by an unknown mechanism. These studies expand our understanding of the Pdu MCP, which has been linked to enteric pathogenesis and which provides a possible basis for the development of intracellular bioreactors for use in biotechnology.
IMPORTANCE Bacterial MCPs are widespread organelles that play important roles in pathogenesis and global carbon fixation. Here we show that the PduL phosphotransacylase is a component of the Pdu MCP. We also show that PduL plays a key role in cofactor homeostasis by recycling coenzyme A internally within the Pdu MCP. Further, we identify a potential N-terminal targeting sequence using a bioinformatic approach and show that this short sequence extension is necessary and sufficient for directing PduL as well as heterologous proteins to the lumen of the Pdu MCP. These findings expand our general understanding of bacterial MCP assembly and cofactor homeostasis.
INTRODUCTION
Bacterial microcompartments (MCPs) are a diverse family of subcellular proteinaceous organelles used to optimize metabolic pathways that have toxic or volatile intermediates (1, 2). MCPs are polyhedral in shape and about 100 to 150 nm in size and consist of a multiprotein shell that encapsulates sequentially acting enzymes of specific metabolic pathways. Overall, they are composed of several thousand protein subunits of 10 to 20 different types and may exceed a gigadalton in mass. Genomic analyses indicate that MCPs are produced by about 20% of bacteria and that there are seven or more different types (which function in diverse metabolic processes) as well as numerous subtypes (3–5). The first MCP identified was the carboxysome (6). This MCP functions to optimize carbon fixation, and it has been estimated that 25% of CO2 fixation on Earth occurs within carboxysomes (2). Two other MCPs that have been studied are used for metabolizing 1,2-propanediol (1,2-PD) and ethanolamine while sequestering an aldehyde intermediate that is toxic to the cell and/or easily lost to the environment (7, 8). 1,2-PD and ethanolamine are important carbon sources in the intestinal environment, and a number of studies have linked the degradation of these compounds to enteric pathogenesis (9–15).
The MCP involved in 1,2-PD utilization (the Pdu MCP) has been most extensively studied in Salmonella enterica (1). In this system, nine different polypeptides (PduABB′JKMNTU) are proposed to self-assemble to form a polyhedral shell that encapsulates enzymes and cofactors for 1,2-PD metabolism (16–18). In current models, 1,2-PD moves across the shell and into the lumen of the Pdu MCP, where it is converted to propionaldehyde by coenzyme B12-dependent diol dehydratase PduCDE (Fig. 1) (19). Next, propionaldehyde is converted to propionyl coenzyme A (propionyl-CoA) or 1-propanol by the PduP aldehyde dehydrogenase or the PduQ alcohol dehydrogenase, respectively (20, 21). 1-Propanol exits the MCP and is excreted into the medium (22). The PduL phosphotransacylase converts propionyl-CoA to propionyl-PO42−, which is converted to propionate by the PduW propionate kinase with the formation of 1 ATP molecule (23). Some propionyl-CoA also diffuses out of the Pdu MCP and enters the methylcitrate pathway to provide carbon and energy for cell growth. The proposed function of the Pdu MCP is to sequester the propionaldehyde intermediate to prevent cell toxicity and carbon loss (7, 24). If the shell of the Pdu MCP is disrupted by mutation, propionaldehyde is excreted into the growth medium at a high level, resulting in growth arrest and increased DNA damage (7, 22).
FIG 1.
Model for Pdu MCPs and the role of PduL. The Pdu MCP consists of 1,2-PD degradative enzymes encapsulated within a multiprotein shell (dashed line).The shell of the Pdu MCP is proposed to act as a diffusion barrier that helps retain propionaldehyde and channel it to downstream enzymes in order to prevent cellular toxicity and DNA damage. For the lumen enzymes to function, they need a steady supply of substrates and cofactors. Current models propose that this need is met by selective pores through the protein shell and/or by internal cofactor recycling. The results of the studies presented in this report indicate that the PduL enzyme has a role in internal HS-CoA recycling but that some HS-CoA also traverses the shell. The encapsulated enzymes are diol dehydratase (PduCDE), propionaldehyde dehydrogenase (PduP), 1-propanol dehydrogenase (PduQ), and phosphotransacylase (PduL). The shell is thought to be composed of nine polypeptides PduABB′JKMNTU. Propionyl-CoA enters central metabolism via the methylcitrate pathway.
The function of the Pdu MCP requires that the 1,2-PD degradative enzymes be localized to the MCP lumen. Recent studies found that N-terminal targeting sequences are used to traffic enzymes to the lumen of MCPs and that such targeting sequences are widespread. Bioinformatic analyses identified on many MCP-associated enzymes short N-terminal sequence extensions that are absent from homologs lacking an MCP-associated genomic context (25). Further studies showed that the N-terminal 18 amino acids of the PduP enzyme are necessary and sufficient for its encapsulation into the Pdu MCP (25) and that short N-terminal sequences are also used to target the PduD enzyme and the EutC and EutE enzymes to the lumen of the Pdu MCP and the MCP for ethanolamine utilization (Eut MCP), respectively (26–28). Hence, the use of N-terminal targeting sequences is a common method for trafficking proteins to the lumen of bacterial MCPs, but many MCP lumen enzymes lack identifiable targeting sequences, raising the possibility of alternative mechanisms.
Current models for the Pdu MCP propose that its shell acts as a diffusion barrier that helps to channel propionaldehyde to downstream enzymes (1). However, at the same time, the shell must allow the influx of substrates and cofactors required for the activity of the lumen enzymes as well as the egress of products for entry into the central metabolism or excretion from the cell (Fig. 1). Studies to date suggest that the shells of MCPs are selectively permeable and/or that cofactor pools are internally recycled (1). The main building blocks of MCP shells are a family of small proteins that have bacterial microcompartment (BMC) domains (29–31). Many hexameric BMC-domain proteins contain small central pores that are proposed to mediate the movement of substrates and products into and out of MCPs (31–33). In addition, a family of trimeric tandem-BMC-domain shell proteins (EutL, CsoS1D, and CcmP) has been crystallized in pore-open and pore-closed conformations, suggesting a gated pore (34–37). In these proteins, the pores open wide enough (diameter, about 14 Å) to allow the movement of enzymatic cofactors, such as ATP, NAD+, coenzyme A (HS-CoA), and coenzyme B12. On the other hand, Cheng et al. recently showed that NAD+ and NADH are regenerated internally within the Pdu MCP by the PduP and PduQ enzymes, which reduce NAD+ and oxidize NADH, respectively (20). In addition, Huseby and Roth demonstrated that HS-CoA is recycled internally within the Eut MCPs by the EutD phosphotransacetylase during ethanolamine catabolism (28). However, on the basis of the studies described above, two viewpoints have emerged: one is that recycling and transport through specific pores work in conjunction to maintain MCP cofactor homeostasis, and the other is that recycling may occur with or without transport through pores (20, 28).
Prior studies showed that the PduL enzyme is a phosphotransacylase that converts propionyl-CoA to propionyl-PO42− during 1,2-PD degradation (Fig. 1) (38). This enzymatic activity is redundant with a housekeeping phosphotransacetylase (Pta) which uses both acetyl-CoA and propionyl-CoA as the substrates. Furthermore, PduL was not identified to be an MCP component; hence, it was previously proposed that the role of PduL is to increase the total phosphotransacylase activity available for 1,2-PD degradation (17). In this report, we show that PduL is a component of the Pdu MCP and that it is directed to the MCP lumen by an N-terminal targeting sequence. We also present evidence that PduL plays a role in recycling HS-CoA internally within the Pdu MCP, although results suggest that other mechanisms (such as specific pores) may also be used to supply HS-CoA to the lumen enzymes of the Pdu MCP.
MATERIALS AND METHODS
Chemicals and reagents.
Antibiotics, vitamin B12 (cyanocobalamin [CN-Cbl]), and DNase I were from Sigma-Aldrich (St. Louis, MO). Coenzyme A was from MP Biomedicals (Santa Ana, CA). Isopropyl-β-D-1-thiogalactopyranoside (IPTG) was from Diagnostic Chemicals Limited (Charlottetown, PEI, Canada). KOD DNA polymerase was from Novagen (Cambridge, MA). Restriction enzymes and T4 DNA ligase were from New England BioLabs (Beverly, MA). Bacterial protein extraction reagent (B-PER II) was from Pierce (Rockford, IL). The cOmplete protease inhibitor was from Roche Diagnostics Corporation (Indianapolis, IN). Other chemicals were from Fisher Scientific (Pittsburgh, PA).
Bacterial strains and growth conditions.
The bacterial strains used in this study are derivatives of Salmonella enterica serovar Typhimurium LT2 (Table 1). The rich medium used was LB-Lennox medium (Difco, Detroit, MI) (39). The minimal medium used was no-carbon-E (NCE) medium (40).
TABLE 1.
Bacterial strains used in this study
| Straina | Genotype | Source |
|---|---|---|
| BE188 | ΔpduL670 | T. A. Bobik lab collection |
| BE291 | ΔpduL670 pta209::Tn10 | T. A. Bobik lab collection |
| BE527 | pta209::Tn10 | T. A. Bobik lab collection |
| BE647 | ΔpduABB′ | T. A. Bobik lab collection |
| BE717 | pta209::Tn10 ΔpduABB′ | This study |
| BE881 | ΔpduABB′ ΔpduL670 | This study |
| BE790 | ΔpduL670 pta209::Tn10/pLAC22 | T. A. Bobik lab collection |
| BE791 | ΔpduL670 pta209::Tn10/pLAC22-pduL | T. A. Bobik lab collection |
| BE1112 | ΔpduL670 pta209::Tn10/pLAC22-pduLΔ2–5 | This study |
| BE1114 | ΔpduL670 pta209::Tn10/pLAC22-pduLΔ2–10 | This study |
| BE1766 | ΔpduL670 ΔpduABB′ pta209::Tn10 | This study |
| BE2021 | ΔpduL670 pta209::Tn10/pLAC22-eGFP | This study |
| BE2022 | ΔpduL670 pta209::Tn10/pLAC22-pduL1–20-eGFP | This study |
| BE2025 | ΔpduL670 pta209::Tn10/pLAC22-pduL-linker-eGFP | This study |
All strains are derivatives of Salmonella enterica serovar Typhimurium LT2.
MCP purification, SDS-PAGE, Western blotting, and PduL enzymatic assay.
Pdu MCPs were purified by detergent treatment and differential centrifugation as previously described (18). Protein concentrations were determined by use of a protein assay reagent (Bio-Rad, Hercules, CA) with bovine serum albumin (BSA) as a standard. SDS-PAGE was performed using 4 to 20% Mini-Protean TGX gels from Bio-Rad, followed by staining using Bio-Safe Coomassie stain (Bio-Rad). For Western blotting assays, the nitrocellulose membranes were probed using mouse monoclonal anti-green fluorescent protein (anti-GFP; Fisher Scientific) or rabbit polyclonal anti-PduL (GenScript, Piscataway, NJ) at 0.5 μg/ml in TBST buffer (50 mM Tris-HCl, pH 7.4, 150 mM NaCl, 0.05% Tween 20) and goat anti-mouse or anti-rabbit IgG–horseradish peroxidase conjugate at 40 ng/ml in TBST buffer (Santa Cruz Biotechnology, Santa Cruz, CA). Signal development was carried out by using SuperSignal West Pico chemiluminescent substrate (Pierce, Rockford, IL) according to the manufacturer's instructions. The result was analyzed by use of a ChemiDoc XRS+ imager (Bio-Rad). Phosphotransacylase (PduL) assays were performed as described previously (38).
Growth studies.
Growth studies were carried out using a Synergy HT microplate reader (BioTek, Winooski, VT) to shake, incubate, and measure the absorbance of the cultures as previously described (38). Each growth curve was a representative of the curves obtained from three independent experiments performed in triplicate. Doubling times were calculated from semilog plots with the formula doubling time = 0.693/(2.303 × slope of the linear region of the plot).
Bioinformatics.
To identify possible targeting sequences for PduL, homologous sequences were retrieved from the Swiss-Prot database via BLAST analysis (41) and subsequently aligned by use of the ClustalW program (42). Putative N-terminal extensions were identified through sliding of a 10-residue window, starting from the first column in the alignment, until the window contained less than 20% indels and its first column had at least an 80% occupancy in terms of residues (Fig. 2A). If, upon stopping, the scanned regions upstream of the window were longer than 10 residues, those were considered N terminal.
FIG 2.
Sequence analysis and structure prediction of PduL N terminus. (A) Sequence alignment of 100 PduL homologs from different bacterial organisms, here reduced to 8 representative sequences (indicated in black). Terminal sequence extensions, which were identified via a sliding window, were present in some PduL homologs and absent in others. Statistical analyses indicated that PduL enzymes with N-terminal extensions were more likely to be encoded by MCP operons than PduL enzymes lacking identifiable extensions, which is a property consistent with known MCP-targeting sequences. The PduL of S. enterica, highlighted in red in the alignment, belongs to the PduL superfamily (cl05584). The sequence contains two tandem domains. Several members in this superfamily catalyze the interconversion of acetyl-CoA and acetyl phosphate and the interconversion of propionyl-CoA and propionyl phosphate. The KEGG gene identifiers shown in the figure correspond to the following organisms: Yersinia enterocolitica subsp. enterocolitica 8081 (yen), Shigella sonnei 53G (ssj), Klebsiella variicola At-22 (kva), S. enterica subsp. enterica serovar Typhimurium LT2 (stm), Citrobacter rodentium ICC168 (cro), Paenibacillus terrae HPL-003 (pta), Paenibacillus sp. strain Y412MC10 (gym), Thermotoga maritima MSB8 (tmm), and Geobacillus sp. strain JF8 (gjf) are provided. (B) Prediction of tertiary structure of the N-terminal region of PduL by the PEP-FOLD web server.
RESULTS
Sequence analysis.
Prior studies showed that some enzymes are targeted to the MCP lumen by N-terminal extensions that are generally absent from homologs not associated with MCPs (25). Bioinformatic analyses were carried out to determine whether the PduL enzyme had such an extension. We considered two binary variables: (i) the existence of a terminal extension in the enzyme sequence (as described in Materials and Methods) and (ii) whether its corresponding gene occurred in the context of an MCP operon. Using these two variables, we built a contingency table to assess their Pearson's correlation coefficient, here called the phi coefficient. A common interpretation is to consider values above 0.3 to be significantly correlated (a value of 1 reflects a perfect correlation). In the PduL case, we determined a phi coefficient of 0.53 between the presence of a terminal extension and its collocation with MCP genes. Thus, PduL homologs that are associated with an MCP genomic context are significantly more likely to have terminal extensions (as identified by a sliding window) than PduL homologs lacking this association. Hence, these findings suggest that the N-terminal extensions present on certain PduL enzymes are lumen-targeting sequences. As a validation step, a prediction of the tertiary structure of the PduL peptide was obtained using the PEP-FOLD web server (43) (Fig. 2B). Eighteen residues on the N terminus were predicted to have a propensity for α-helix formation, which was consistent with the recent reports that PduP and encapsulated proteins from more than one type of MCP have terminal targeting sequences which are prone to form α helices (44, 45). These analyses suggest that PduL may be a component of the Pdu MCP, even though it was not identified by prior proteomics analyses of purified MCPs (17).
PduL is a component of the Pdu MCP.
To investigate the cellular location of PduL, MCPs purified from wild-type S. enterica as well as a pduL deletion mutant (BE291) were analyzed by SDS-PAGE and Western blotting. By Western blotting with anti-PduL sera, a strong band near 24 kDa was detected in MCPs purified from Salmonella, while a weaker band of the same size was detected in crude extracts (Fig. 3). No bands of this size were detected in either whole-cell extracts or MCPs purified from a pduL deletion mutant, verifying that the band near 24 kDa was PduL. We also attempted to purify PduL (using the MCP purification protocol) using a strain that produces PduL from plasmid pLAC22 but does not produce MCPs. In this case, PduL could not be detected by Western blotting. These results indicate that PduL is a component of the Pdu MCP. A specific band with a molecular mass close to that of PduL was not identified by SDS-PAGE. Most likely, PduL was obscured by major components of Pdu MCPs, PduB′ and PduD, which had molecular masses similar to the molecular mass of PduL (24.1 kDa for PduB′ and 24.2 kDa for PduD versus 23.1 kDa for PduL).
FIG 3.
PduL is a component of the Pdu MCP. Cell extracts and MCPs purified from wild-type Salmonella and a pduL deletion mutant were analyzed for the presence of PduL by Western blotting and SDS-PAGE. (A) Western blotting with anti-PduL sera. Lanes 1 and 2, 10 μg whole-cell extract and 10 μg purified MCPs from the wild type, respectively; lanes 3 and 4, 10 μg whole-cell extract and 10 μg purified MCPs from a pduL deletion mutant (BE188), respectively. (B) A 10 to 20% SDS-polyacrylamide gel stained with Coomassie. Lane M, molecular mass markers; lane 1, 10 μg Pdu MCPs purified from LT2; lane 2, 10 μg Pdu MCPs purified from BE188. PduL (molecular mass = 22,972 Da) was not resolved by SDS-PAGE.
PduL is capable of encapsulating eGFP into the Pdu MCPs.
To further investigate its cellular location, we tested whether PduL could mediate the encapsulation of enhanced GFP (eGFP) into Pdu MCPs. A PduL-eGFP fusion protein was expressed from plasmid pLAC22 in a pduL deletion mutant. PduL fused to eGFP was readily detected in purified Pdu MCPs as a strong band near 50 kDa using an anti-GFP monoclonal antibody (Fig. 4, lane 4). Native eGFP was not detected in MCPs purified from wild-type Salmonella or a ΔpduL mutant expressing native eGFP (Fig. 4, lanes 2 and 3). Western blots also showed that the PduL-eGFP fusion protein and eGFP were present in the whole-cell extract, indicating normal expression. We point out that the amount of the PduL-eGFP fusion protein present in MCPs was larger than the amount present in the whole-cell lysate, indicating that PduL-eGFP copurified with the Pdu MCPs.
FIG 4.
Targeting of a PduL-eGFP fusion protein to the Pdu MCP. eGFP and a PduL-eGFP fusion protein were expressed from pLAC22 in a ΔpduL background under conditions that induce MCP formation, and eGFP was detected by Western blotting. Lane 1, molecular mass standards (M); lanes 2 to 4, 10 μg Pdu MCPs purified from wild-type Salmonella, BE2021 ΔpduL/pLAC22-eGFP, and BE2025 ΔpduL/pLAC22-pduL-eGFP, respectively; lanes 5 to 7, 10 μg whole-cell extracts of the wild type, BE2021, and BE2025, respectively.
The N-terminal region of PduL is required for targeting to the Pdu MCP.
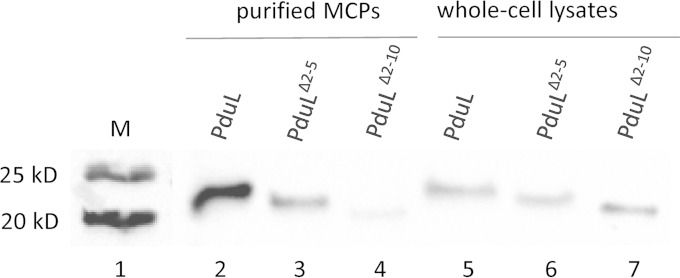
To test for an N-terminal targeting sequence, we expressed truncated PduL lacking N-terminal amino acids 2 to 5 or 2 to 10 (PduLΔ2–5 and PduLΔ2–10) from vector pLAC22 in a pduL deletion background under conditions that induced the Pdu MCP. MCPs were then purified and analyzed by Western blotting for PduL and its truncated derivatives (Fig. 5). Compared to the amount of wild-type PduL detected, significantly smaller amounts of PduLΔ2–5 and PduLΔ2–10 were detected in purified MCPs when the amount of protein loaded on the gel was adjusted so that comparable amounts of PduL, PduLΔ2–5, and PduLΔ2–10 were found in the whole-cell extracts. As a control, we expressed PduL, PduLΔ2–5, and PduLΔ2–10 from pLAC22 and measured their phosphotransacylase activities in crude cell extracts. This was done in strains carrying the ΔpduL and pta::Tn10 mutations to eliminate background phosphotransacylase activity (38). The results showed similar activities for PduL and PduLΔ2–5 (19.24 ± 1.8 and 18.55 ± 1.8 μmol min−1 mg−1, respectively) but a somewhat higher level of activity for PduLΔ2–10 (27.73 ± 3.2 μmol min−1 mg−1). The important finding here was that PduLΔ2–5 and PduLΔ2–10 were expressed and enzymatically active. Thus, the results presented above indicate that the deletion of residues 2 to 5 or 2 to 10 from the N terminus of PduL does not substantially affect its enzymatic activity or folding but does impair targeting to the MCP. We also attempted to examine targeting of PduLΔ2–20; however, none could be detected by Western blotting, suggesting that this mutant protein was unstable.
FIG 5.
Short N-terminal deletions impair encapsulation of PduL into Pdu MCPs. PduL and truncated derivatives were expressed from pLAC22 in a ΔpduL strain background under conditions that induce MCP formation, and PduL was detected by Western blotting with anti-PduL sera. Lane 1, molecular mass markers (M); lanes 2 to 4, 10, 25, and 7.5 μg purified Pdu MCPs from BE791 ΔpduL/pLAC22-pduL, BE1112 ΔpduL/pLAC22-pduLΔ2–5, and BE1114 ΔpduL/pLac22-pduLΔ2–10, respectively; lanes 5 to 7, 10, 25, and 7.5 μg whole-cell lysates from BE791, BE1112, and BE1114, respectively. The amount of protein loaded was varied to obtain similar amounts of PduL in the crude cell extract.
The 20 N-terminal amino acids of PduL can target eGFP to the Pdu MCP.
To determine whether the N-terminal extension of PduL is sufficient for encapsulating proteins into the Pdu MCP, we fused the N-terminal 20 amino acids of PduL (PduL1–20) to eGFP. We then expressed eGFP and PduL1–20-eGFP from pLAC22 in a background carrying a pduL deletion mutation under conditions that induced MCP formation. Western blotting assays indicated that PduL1–20-eGFP (∼28 kDa) copurified with Pdu MCPs, while eGFP did not (Fig. 6, lanes 1 and 2). Control assays detected similar amounts of eGFP and PduL1–20-eGFP in soluble whole-cell lysates, indicating the normal production and folding of both proteins. These results indicate that the N-terminal 20 amino acids of PduL are sufficient for packaging proteins into the Pdu MCP.
FIG 6.

eGFP fused to 20 N-terminal amino acids of PduL is targeted to the Pdu MCP. eGFP and eGFP fused to the 20 N-terminal amino acids of PduL (PduL1–20-eGFP) were expressed from pLAC22 in a ΔpduL background, and eGFP was detected by Western blotting with anti-GFP sera. Lanes 1 and 2, 15 μg MCPs purified from BE2021 ΔpduL670 pta209::Tn10/pLAC22-eGFP and BE2022 ΔpduL670 pta209::Tn10/pLAC22-pduL1–20-eGFP, respectively; lanes 3 and 4, 15 μg whole-cell extracts from BE2021 and BE2022, respectively.
Effects of preventing MCP formation on the growth of a pduL deletion mutant.
The role of PduL in 1,2-PD degradation is to catalyze the conversion of propionyl-CoA to propionyl-PO42− (Fig. 1) (38). Prior studies showed that a pduL deletion mutant had a reduced growth rate on 1,2-PD, even though Salmonella expresses a housekeeping Pta enzyme which can also convert propionyl-CoA to propionyl-PO42− (38). Previously, it was proposed that PduL provides the additional enzyme activity needed to support growth on 1,2-PD (38). However, an alternative explanation is that PduL might have an MCP-specific function, such as internal HS-CoA recycling (Fig. 1). To test for an MCP-specific function, we looked at the effect of breaking the shell of the Pdu MCP on the phenotype of a pduL deletion mutant. To break the shell we used a deletion (ΔpduABB′) that eliminates three major BMC-domain proteins, PduA, PduB, and PduB′. Controls showed that a pduL deletion mutant had a substantially increased doubling time on 1,2-PD minimal medium compared to that of the wild type (7.41 ± 0.12 h compared to 4.8 ± 0.3 h), which was similar to the findings of our previous studies (38). In contrast, the ΔpduABB′ ΔpduL double mutant grew faster than a ΔpduABB′ mutant (doubling times, 3.4 ± 0.1 h versus 4.2 ± 0.1 h, respectively) (Fig. 7). Thus, PduL was required for optimal growth on 1,2-PD when the MCP was intact. However, PduL did not improve growth (it actually slowed growth) when the MCP was broken. This finding argues against the idea that the role of PduL is simply to provide more phosphotransacylase activity and suggests that PduL is essential for optimal MCP function. It was unexpected that a ΔpduABB′ ΔpduL strain would grow faster than the ΔpduABB′ PduL+ strain. One possible tentative interpretation is that the ΔpduABB′ ΔpduL strain accumulated propionyl-CoA to higher levels, thereby increasing flux through the methylcitrate pathway, while the ΔpduABB′ PduL+ strain directed more carbon to propionate, which results in a lower ATP yield (Fig. 1).
FIG 7.
Growth of a pduL deletion mutant on 1,2-PD in backgrounds having intact and broken MCPs. The strains used were wild-type Salmonella, a pduL deletion mutant (BE188 ΔpduL670) that forms intact MCPs (ΔpduL), a strain (BE647 ΔpduABB′) unable to form MCPs due to the deletion of three major shell proteins (ΔpduAB [broken MCPs]), and a pduL deletion mutant (BE881 ΔpduABB′ ΔpduL670) unable to form MCPs (ΔpduAB ΔpduL). Strains were grown on 1,2-PD minimal medium supplemented with 150 nM vitamin B12. This experiment was repeated ≥3 times, and representative data are shown. OD600, optical density at 600 nm.
Growth tests indicate that PduL localizes to the lumen of the Pdu MCP.
The studies described above showed that PduL is an MCP component but did not directly address whether it is located in the lumen or on the MCP surface. To examine this, we looked at the access of PduL to its substrates. Encapsulation of PduL within the Pdu MCP might restrict substrate access, while surface localization would not. Salmonella can grow on acetate by converting it first to acetyl-PO42− and then to acetyl-CoA by the ordered action of acetate kinase (AckA) and Pta. Prior studies showed that a pta mutant grows very poorly on acetate, but this phenotype was corrected by production of PduL from a plasmid (PduL can also convert acetyl-PO42− to acetyl-CoA) (38). Here, we tested whether induction of the Pdu MCP could complement the growth defect of a pta mutant on acetate. In this case, complementation would require the conversion of acetate to acetyl-PO42− by AckA in the cytoplasm. If PduL is located in the lumen of the Pdu MCP, acetyl-PO42− together with HS-CoA would need to enter the Pdu MCP, where they would be converted to acetyl-CoA by PduL. Lastly, acetyl-CoA would exit the MCP and enter central metabolism via the tricarboxylic acid cycle. Thus, if PduL is encapsulated, complementation of a pta mutant by induction of the Pdu MCP would require the diffusion of acetyl-PO42−, HS-CoA, and acetyl-CoA across the MCP shell, whereas the surface localization of PduL would not. Results showed that induction of the pdu operon (which leads to MCP formation) allowed only modest growth of a pta strain on acetate (doubling time, 7.03 ± 0.1 h) (Fig. 8). In contrast, when the pdu operon was induced in a background that could not form the MCP shell (ΔpduABB′), a significantly faster growth of a pta strain on acetate was observed (3.46 ± 0.1 h) (Fig. 8). Although other explanations are possible, these findings support the idea that PduL is encapsulated within the Pdu MCP and that the shell of the Pdu MCP restricts the movement of its substrates to some degree. This is consistent with a need for the internal recycling of HS-CoA, as shown in Fig. 1. For the experiments described above, expression of the pdu operon was induced by supplementation of the growth medium with 1,2-PD. No 1,2-PD metabolism occurred under these conditions because coenzyme B12 (a cofactor required for 1,2-PD degradation) was not present.
FIG 8.
Complementation of a pta mutant for growth on acetate minimal medium by PduL that is encapsulated within the MCP or unencapsulated. A ΔpduABB′ mutant was unable to form intact MCPs. Addition of 1,2-PD was used to induce the genes for 1,2-PD degradation (including PduL) and the formation of the Pdu MCP. This experiment was repeated ≥3 times, and representative data are shown.
DISCUSSION
Prior studies showed that the PduL phosphotransacylase catalyzes the conversion of propionyl-CoA to propionyl-PO42− during 1,2-PD degradation (38). However, prior proteomic studies did not identify PduL as a component of the Pdu MCP, and it was proposed that the main role of PduL is to provide additional phosphotransacylase activity above that supplied by the housekeeping Pta enzyme (17). In this study, we have provided evidence that PduL is a component of the Pdu MCP; Western blotting assays clearly showed the enrichment of PduL and PduL fused to eGFP in purified MCPs. We also conducted studies to investigate whether PduL has an MCP-specific role. The function of the Pdu MCP requires that the enzymes encapsulated within be provided with a steady supply of their required substrates and cofactors. On the basis of crystallographic studies, it was proposed that the central pores seen in BMC-domain shell proteins provide specific conduits for metabolites (31–35, 46), and recent studies showed that the PduA shell protein forms a selective pore tailored to allow the influx of 1,2-PD while restricting the efflux of propionaldehyde (47). Moreover, recent studies showed that enzymatic cofactors can be regenerated internally within MCPs to maintain steady cofactor supplies. In the Pdu system, the PduQ 1-propanol dehydrogenase helps regenerate NAD+ from NADH internally within Pdu MCPs (20). In the ethanolamine utilization system, the EutD phosphotransacetylase recycles HS-CoA within Eut MCPs (28). Results presented here indicate that HS-CoA is also regenerated internally within the Pdu MCP, in this case, by the PduL phosphotransacylase. Genetic tests showed that a pduL deletion mutant was impaired for growth on 1,2-PD when the Pdu MCP was intact. In contrast, a pduL deletion mutant did not impair growth on 1,2-PD when the MCP was broken by a shell protein deletion mutation (growth was actually faster than that of the wild type in this case). This indicates that PduL is required for the optimal function of an intact Pdu MCP and is not simply used to supply extra phosphotransacylase activity. Further investigations suggested that PduL is encapsulated within the Pdu MCP and that encapsulation restricts the access of PduL to its substrates, which would explain the need for internal HS-CoA recycling (Fig. 8). Thus, the results indicate that PduL has a role in the recycling of HS-CoA internally within the Pdu MCP, and internal cofactor recycling may be common in MCPs that function in heterotrophic metabolism.
While there is a consensus that cofactor recycling is important to MCP function, its role relative to the transport of cofactors through pores that span the MCP shell is uncertain. Studies on the ethanolamine utilization system indicated that EutD phosphotransacetylase is essential for HS-CoA recycling and growth on ethanolamine is abolished in a eutD mutant (28). Hence, it was proposed that the Eut MCP has private cofactor pools separate from the cytoplasm and that the transport of cofactors across the shell may or may not be required (28). On the other hand, investigations of the Pdu system suggested that both pores and recycling are needed to maintain cofactor homeostasis (20). A PduQ deletion grew at a rate that was about 54% of the wild-type rate, indicating that a portion of the NADH was recycled back to NAD+ by the electron transport chain, which would require the substantial flux of NAD+ and NADH across the MCP shell (20). Here, we found that a pduL deletion mutant grew at a rate that was about 65% of the wild-type rate, suggesting that substantial amounts of HS-CoA traverse the MCP shell, possibly through pores that span the shell. Thus, studies of PduL support the model that both internal cofactor recycling and transport across the shell are required for the proper function of the Pdu MCP. Furthermore, recent in vitro studies demonstrated that purified Pdu MCPs restrict the influx of 1,2-PD but do not measurably impair the inward movement of HS-CoA and NAD+ (47). This strongly supports the idea of specific routes of cofactor entry into the Pdu MCP. However, the mechanism by which large cofactors traverse the shell of the Pdu MCP remains a major unanswered question of MCP physiology. Multiple cofactors (HS-CoA, propionyl-CoA, NAD+, NADH, coenzyme B12, ATP, and ADP) must cross the shell, which restricts the outward diffusion of propionaldehyde.
It is also known that proper MCP function requires the encapsulation of specific enzymes. Prior studies showed that short N-terminal extensions target diverse enzymes to the lumen of bacterial MCPs (1). Here, our sequence alignments suggested that PduL might have an N-terminal targeting sequence since PduL homologs predicted to be MCP associated on the basis of genomic context featured a short N-terminal extension compared to the sequences of non-MCP-associated enzymes. Western blotting assays showed that PduL lacking N-terminal amino acids 2 to 5 or 2 to 10 was impaired for encapsulation (but normally expressed and active), indicating that its N terminus has a role in targeting. In addition, we were also able to target a PduL1–20-eGFP fusion protein to the Pdu MCP. This indicates that the N-terminal 20 amino acids of PduL are sufficient to mediate the encapsulation of an unrelated protein into the Pdu MCP.
Several groups have proposed that bacterial MCPs might be developed for use as intracellular bioreactors for and the production of biofuels, renewable chemicals, and pharmaceuticals (1, 2, 48, 49). Pathway encapsulation could help with diffusion restrictions, toxic intermediates, metabolite flux, and enzyme stability. However, achieving such applications will require a detailed understanding of the operational principles and mechanisms of MCPs. In the study described here we have added to our understanding of the bacterial MCPs by identifying a new targeting sequence that can be used to encapsulate heterologous proteins in the Pdu MCP and by showing that the PduL enzyme is a component of the Pdu MCP that plays a role in cofactor recycling.
ACKNOWLEDGMENTS
This work was supported by grant MCB0956451 from the National Science Foundation to T.A.B. and grant AI081146 from the National Institutes of Health to T.O.Y. and T.A.B.
We thank the Iowa State University DNA Sequencing and Synthesis Facility for assistance with DNA analyses.
REFERENCES
- 1.Chowdhury C, Sinha S, Chun S, Yeates TO, Bobik TA. 2014. Diverse bacterial microcompartment organelles. Microbiol Mol Biol Rev 78:438–468. doi: 10.1128/MMBR.00009-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Rae BD, Long BM, Badger MR, Price GD. 2013. Functions, compositions, and evolution of the two types of carboxysomes: polyhedral microcompartments that facilitate CO2 fixation in cyanobacteria and some proteobacteria. Microbiol Mol Biol Rev 77:357–379. doi: 10.1128/MMBR.00061-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Abdul-Rahman F, Petit E, Blanchard JL. 2013. The distribution of polyhedral bacterial microcompartments suggests frequent horizontal transfer and operon reassembly. J Phylogen Evolution Biol 1:1000118. doi: 10.4172/2329-9002.1000118. [DOI] [Google Scholar]
- 4.Axen SD, Erbilgin O, Kerfeld CA. 2014. A taxonomy of bacterial microcompartment loci constructed by a novel scoring method. PLoS Comput Biol 10:e1003898. doi: 10.1371/journal.pcbi.1003898. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Jorda J, Lopez D, Wheatley NM, Yeates TO. 2013. Using comparative genomics to uncover new kinds of protein-based metabolic organelles in bacteria. Protein Sci 22:179–195. doi: 10.1002/pro.2196. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Shively JM, Ball F, Brown DH, Saunders RE. 1973. Functional organelles in prokaryotes: polyhedral inclusions (carboxysomes) of Thiobacillus neapolitanus. Science 182:584–586. doi: 10.1126/science.182.4112.584. [DOI] [PubMed] [Google Scholar]
- 7.Havemann GD, Sampson EM, Bobik TA. 2002. PduA is a shell protein of polyhedral organelles involved in coenzyme B12-dependent degradation of 1,2-propanediol in Salmonella enterica serovar Typhimurium LT2. J Bacteriol 184:1253–1261. doi: 10.1128/JB.184.5.1253-1261.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Penrod JT, Roth JR. 2006. Conserving a volatile metabolite: a role for carboxysome-like organelles in Salmonella enterica. J Bacteriol 188:2865–2874. doi: 10.1128/JB.188.8.2865-2874.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Buchrieser C, Rusniok C, Kunst F, Cossart P, Glaser P. 2003. Comparison of the genome sequences of Listeria monocytogenes and Listeria innocua: clues for evolution and pathogenicity. FEMS Immunol Med Microbiol 35:207–213. doi: 10.1016/S0928-8244(02)00448-0. [DOI] [PubMed] [Google Scholar]
- 10.Conner CP, Heithoff DM, Julio SM, Sinsheimer RL, Mahan MJ. 1998. Differential patterns of acquired virulence genes distinguish Salmonella strains. Proc Natl Acad Sci U S A 95:4641–4645. doi: 10.1073/pnas.95.8.4641. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Heithoff DM, Conner CP, Hentschel U, Govantes F, Hanna PC, Mahan MJ. 1999. Coordinate intracellular expression of Salmonella genes induced during infection. J Bacteriol 181:799–807. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Joseph B, Przybilla K, Stuhler C, Schauer K, Slaghuis J, Fuchs TM, Goebel W. 2006. Identification of Listeria monocytogenes genes contributing to intracellular replication by expression profiling and mutant screening. J Bacteriol 188:556–568. doi: 10.1128/JB.188.2.556-568.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Price-Carter M, Tingey J, Bobik TA, Roth JR. 2001. The alternative electron acceptor tetrathionate supports B12-dependent anaerobic growth of Salmonella enterica serovar Typhimurium on ethanolamine or 1,2-propanediol. J Bacteriol 183:2463–2475. doi: 10.1128/JB.183.8.2463-2475.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Thiennimitr P, Winter SE, Winter MG, Xavier MN, Tolstikov V, Huseby DL, Sterzenbach T, Tsolis RM, Roth JR, Baumler AJ. 2011. Intestinal inflammation allows Salmonella to use ethanolamine to compete with the microbiota. Proc Natl Acad Sci U S A 108:17480–17485. doi: 10.1073/pnas.1107857108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Winter SE, Thiennimitr P, Winter MG, Butler BP, Huseby DL, Crawford RW, Russell JM, Bevins CL, Adams LG, Tsolis RM, Roth JR, Baumler AJ. 2010. Gut inflammation provides a respiratory electron acceptor for Salmonella. Nature 467:426–429. doi: 10.1038/nature09415. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Cheng S, Sinha S, Fan C, Liu Y, Bobik TA. 2011. Genetic analysis of the protein shell of the microcompartments involved in coenzyme B12-dependent 1,2-propanediol degradation by Salmonella. J Bacteriol 193:1385–1392. doi: 10.1128/JB.01473-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Havemann GD, Bobik TA. 2003. Protein content of polyhedral organelles involved in coenzyme B12-dependent degradation of 1,2-propanediol in Salmonella enterica serovar Typhimurium LT2. J Bacteriol 185:5086–5095. doi: 10.1128/JB.185.17.5086-5095.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Sinha S, Cheng S, Fan C, Bobik TA. 2012. The PduM protein is a structural component of the microcompartments involved in coenzyme B12-dependent 1,2-propanediol degradation by Salmonella enterica. J Bacteriol 194:1912–1918. doi: 10.1128/JB.06529-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Bobik TA, Xu Y, Jeter RM, Otto KE, Roth JR. 1997. Propanediol utilization genes (pdu) of Salmonella typhimurium: three genes for the propanediol dehydratase. J Bacteriol 179:6633–6639. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Cheng S, Fan C, Sinha S, Bobik TA. 2012. The PduQ enzyme is an alcohol dehydrogenase used to recycle NAD+ internally within the Pdu microcompartment of Salmonella enterica. PLoS One 7:e47144. doi: 10.1371/journal.pone.0047144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Leal NA, Havemann GD, Bobik TA. 2003. PduP is a coenzyme-A-acylating propionaldehyde dehydrogenase associated with the polyhedral bodies involved in B12-dependent 1,2-propanediol degradation by Salmonella enterica serovar Typhimurium LT2. Arch Microbiol 180:353–361. doi: 10.1007/s00203-003-0601-0. [DOI] [PubMed] [Google Scholar]
- 22.Sampson EM, Bobik TA. 2008. Microcompartments for B12-dependent 1,2-propanediol degradation provide protection from DNA and cellular damage by a reactive metabolic intermediate. J Bacteriol 190:2966–2971. doi: 10.1128/JB.01925-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Palacios S, Starai VJ, Escalante-Semerena JC. 2003. Propionyl coenzyme A is a common intermediate in the 1,2-propanediol and propionate catabolic pathways needed for expression of the prpBCDE operon during growth of Salmonella enterica on 1,2-propanediol. J Bacteriol 185:2802–2810. doi: 10.1128/JB.185.9.2802-2810.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Stojiljkovic I, Baeumler AJ, Heffron F. 1995. Ethanolamine utilization in Salmonella typhimurium: nucleotide sequence, protein expression, and mutational analysis of the cchA cchB eutE eutJ eutG eutH gene cluster. J Bacteriol 177:1357–1366. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Fan C, Cheng S, Liu Y, Escobar CM, Crowley CS, Jefferson RE, Yeates TO, Bobik TA. 2010. Short N-terminal sequences package proteins into bacterial microcompartments. Proc Natl Acad Sci U S A 107:7509–7514. doi: 10.1073/pnas.0913199107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Choudhary S, Quin MB, Sanders MA, Johnson ET, Schmidt-Dannert C. 2012. Engineered protein nano-compartments for targeted enzyme localization. PLoS One 7:e33342. doi: 10.1371/journal.pone.0033342. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Fan C, Bobik TA. 2011. The N-terminal region of the medium subunit (PduD) packages adenosylcobalamin-dependent diol dehydratase (PduCDE) into the Pdu microcompartment. J Bacteriol 193:5623–5628. doi: 10.1128/JB.05661-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Huseby DL, Roth JR. 2013. Evidence that a metabolic microcompartment contains and recycles private cofactor pools. J Bacteriol 195:2864–2879. doi: 10.1128/JB.02179-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Kerfeld CA, Sawaya MR, Tanaka S, Nguyen CV, Phillips M, Beeby M, Yeates TO. 2005. Protein structures forming the shell of primitive bacterial organelles. Science 309:936–938. doi: 10.1126/science.1113397. [DOI] [PubMed] [Google Scholar]
- 30.Tanaka S, Kerfeld CA, Sawaya MR, Cai F, Heinhorst S, Cannon GC, Yeates TO. 2008. Atomic-level models of the bacterial carboxysome shell. Science 319:1083–1086. doi: 10.1126/science.1151458. [DOI] [PubMed] [Google Scholar]
- 31.Yeates TO, Jorda J, Bobik TA. 2013. The shells of BMC-type microcompartment organelles in bacteria. J Mol Microbiol Biotechnol 23:290–299. doi: 10.1159/000351347. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Tsai Y, Sawaya MR, Cannon GC, Cai F, Williams EB, Heinhorst S, Kerfeld CA, Yeates TO. 2007. Structural analysis of CsoS1A and the protein shell of the Halothiobacillus neapolitanus carboxysome. PLoS Biol 5:e144. doi: 10.1371/journal.pbio.0050144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Tsai Y, Sawaya MR, Yeates TO. 2009. Analysis of lattice-translocation disorder in the layered hexagonal structure of carboxysome shell protein CsoS1C. Acta Crystallogr D Biol Crystallogr 65(Pt 9):980–988. doi: 10.1107/S0907444909025153. [DOI] [PubMed] [Google Scholar]
- 34.Cai F, Sutter M, Cameron JC, Stanley DN, Kinney JN, Kerfeld CA. 2013. The structure of CcmP, a tandem bacterial microcompartment domain protein from the beta-carboxysome, forms a subcompartment within a microcompartment. J Biol Chem 288:16055–16063. doi: 10.1074/jbc.M113.456897. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Klein MG, Zwart P, Bagby SC, Cai F, Chisholm SW, Heinhorst S, Cannon GC, Kerfeld CA. 2009. Identification and structural analysis of a novel carboxysome shell protein with implications for metabolite transport. J Mol Biol 392:319–333. doi: 10.1016/j.jmb.2009.03.056. [DOI] [PubMed] [Google Scholar]
- 36.Sagermann M, Ohtaki A, Nikolakakis K. 2009. Crystal structure of the EutL shell protein of the ethanolamine ammonia lyase microcompartment. Proc Natl Acad Sci U S A 106:8883–8887. doi: 10.1073/pnas.0902324106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Tanaka S, Sawaya MR, Yeates TO. 2010. Structure and mechanisms of a protein-based organelle in Escherichia coli. Science 327:81–84. doi: 10.1126/science.1179513. [DOI] [PubMed] [Google Scholar]
- 38.Liu Y, Leal NA, Sampson EM, Johnson CL, Havemann GD, Bobik TA. 2007. PduL is an evolutionarily distinct phosphotransacylase involved in B12-dependent 1,2-propanediol degradation by Salmonella enterica serovar Typhimurium LT2. J Bacteriol 189:1589–1596. doi: 10.1128/JB.01151-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Bertani G. 1951. Studies on lysogenesis. I. The mode of phage liberation by lysogenic Escherichia coli. J Bacteriol 62:293–300. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Vogel HJ, Bonner DM. 1956. Acetylornithinase of Escherichia coli: partial purification and some properties. J Biol Chem 218:97–106. [PubMed] [Google Scholar]
- 41.Altschul SF, Gish W, Miller W, Myers EW, Lipman DJ. 1990. Basic local alignment search tool. J Mol Biol 215:403–410. doi: 10.1016/S0022-2836(05)80360-2. [DOI] [PubMed] [Google Scholar]
- 42.Larkin MA, Blackshields G, Brown NP, Chenna R, McGettigan PA, McWilliam H, Valentin F, Wallace IM, Wilm A, Lopez R, Thompson JD, Gibson TJ, Higgins DG. 2007. Clustal W and Clustal X version 2.0. Bioinformatics 23:2947–2948. doi: 10.1093/bioinformatics/btm404. [DOI] [PubMed] [Google Scholar]
- 43.Thevenet P, Shen Y, Maupetit J, Guyon F, Derreumaux P, Tuffery P. 2012. PEP-FOLD: an updated de novo structure prediction server for both linear and disulfide bonded cyclic peptides. Nucleic Acids Res 40:W288–W293. doi: 10.1093/nar/gks419. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Fan C, Cheng S, Sinha S, Bobik TA. 2012. Interactions between the termini of lumen enzymes and shell proteins mediate enzyme encapsulation into bacterial microcompartments. Proc Natl Acad Sci U S A 109:14995–15000. doi: 10.1073/pnas.1207516109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Lawrence AD, Frank S, Newnham S, Lee MJ, Brown IR, Xue W-F, Rowe ML, Mulvihill DP, Prentice MB, Howard MJ, Warren MJ. 2014. Solution structure of a bacterial microcompartment targeting peptide and its application in the construction of an ethanol bioreactor. ACS Synth Biol 3:454–465. doi: 10.1021/sb4001118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Tanaka S, Sawaya MR, Phillips M, Yeates TO. 2009. Insights from multiple structures of the shell proteins from the beta-carboxysome. Protein Sci 18:108–120. doi: 10.1002/pro.14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Chowdhury C, Chun S, Pang A, Sawaya MR, Sinha S, Yeates TO, Bobik TA. 2015. Selective molecular transport through the protein shell of a bacterial microcompartment organelle. Proc Natl Acad Sci U S A 112:2990–2995. doi: 10.1073/pnas.1423672112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Frank S, Lawrence AD, Prentice MB, Warren MJ. 2013. Bacterial microcompartments moving into a synthetic biological world. J Biotechnol 163:273–279. doi: 10.1016/j.jbiotec.2012.09.002. [DOI] [PubMed] [Google Scholar]
- 49.Tsai SJ, Yeates TO. 2011. Bacterial microcompartments insights into the structure, mechanism, and engineering applications. Prog Mol Biol Transl Sci 103:1–20. doi: 10.1016/B978-0-12-415906-8.00008-X. [DOI] [PubMed] [Google Scholar]