ABSTRACT
The ability to persist and grow under alkaline conditions is an important characteristic of many bacteria. In order to survive at alkaline pH, Escherichia coli must maintain a stable cytoplasmic pH of about 7.6. Membrane cation/proton antiporters play a major role in alkaline pH homeostasis by catalyzing active inward proton transport. The DedA/Tvp38 family is a highly conserved membrane protein family of unknown function present in most sequenced genomes. YqjA and YghB are members of the E. coli DedA family with 62% amino acid identity and partially redundant functions. We have shown that E. coli with ΔyqjA and ΔyghB mutations cannot properly maintain the proton motive force (PMF) and is compromised in PMF-dependent drug efflux and other PMF-dependent functions. Furthermore, the functions of YqjA and YghB are dependent upon membrane-embedded acidic amino acids, a hallmark of several families of proton-dependent transporters. Here, we show that the ΔyqjA mutant (but not ΔyghB) cannot grow under alkaline conditions (ranging from pH 8.5 to 9.5), unlike the parent E. coli. Overexpression of yqjA restores growth at alkaline pH, but only when more than ∼100 mM sodium or potassium is present in the growth medium. Increasing the osmotic pressure by the addition of sucrose enhances the ability of YqjA to support growth under alkaline conditions in the presence of low salt concentrations, consistent with YqjA functioning as an osmosensor. We suggest that YqjA possesses proton-dependent transport activity that is stimulated by osmolarity and that it plays a significant role in the survival of E. coli at alkaline pH.
IMPORTANCE The ability to survive under alkaline conditions is important for many species of bacteria. Escherichia coli can grow at pH 5.5 to 9.5 while maintaining a constant cytoplasmic pH of about 7.6. Under alkaline conditions, bacteria rely upon proton-dependent transporters to maintain a constant cytoplasmic pH. The DedA/Tvp38 protein family is a highly conserved but poorly characterized family of membrane proteins. Here, we show that the DedA/Tvp38 protein YqjA is critical for E. coli to survive at pH 8.5 to 9.5. YqjA requires sodium and potassium for this function. At low cation concentrations, osmolytes, including sucrose, can facilitate rescue of E. coli growth by YqjA at high pH. These data are consistent with YqjA functioning as an osmosensing cation-dependent proton transporter.
INTRODUCTION
Bacterial alkaline pH tolerance is a key feature of pathogenic, ecological, and industrially important bacteria. Microbes have many naturally occurring alkaline habitats, including the human body (1). Neutralophilic bacteria, including Escherichia coli and Vibrio cholerae, can stay viable in alkaline marine environments and can cause threats to public health (2). They have the ability to maintain their intracellular pH in a range of 7.5 to 7.7 when grown within a wide range of pH values between 5.5 and 9.0. Cytoplasmic pH maintenance is vital for structural integrity and the functions of proteins essential for growth (1, 3). In order to survive at alkaline pH, bacteria employ various strategies, including increased expression and activity of cation/proton antiporters, which play a crucial role in alkaline pH homeostasis (3). To date, five such antiporters (NhaA, NhaB, ChaA, MdfA, and MdtM) have been reported to function in alkaline pH homeostasis in E. coli (3–7). Inward active transport of protons by these antiporters is coupled with export of cations, such as Na+, K+, and Ca2+.
The DedA/Tvp38 family is a highly conserved membrane protein family, with the corresponding genes present in most sequenced genomes (8). However, the functions of these proteins are not well understood. YqjA and YghB are members of the DedA family sharing 62% amino acid identity and partially redundant functions. We have previously shown that an E. coli strain lacking these two genes (strain BC202) is defective in twin arginine transport (Tat) pathway protein export and cell division (9, 10) and maintenance of the proton motive force (PMF) (11) and is sensitive to a number of antibiotics and biocides normally subject to efflux in a PMF-dependent manner (12). Interestingly, all the BC202 phenotypes appear to be corrected if the strain is grown in slightly acidified growth medium at pH 6.0 (11, 12).
Many DedA proteins contain membrane-embedded acidic amino acids in the first predicted transmembrane-spanning region (Glu39 and Asp51 in both YqjA and YghB, as predicted using SOSUI [13]). Such membrane acidic amino acids are found in many proton-dependent transporters belonging to several different families (14–18). We have recently shown that DedA proteins require these acidic amino acids for the ability to complement the phenotypes of BC202 described above, suggesting they may represent a new family of proton-dependent transporters (12).
Here, we show that the E. coli ΔyqjA mutant (but not the ΔyghB mutant) is unable to grow at an external pH range of 8.5 to 9.5, unlike the parent E. coli strain. Overexpression of yqjA from a plasmid restores growth at alkaline pH, but only if monovalent cation sodium or potassium is supplied in the growth medium. Moreover, acidic amino acids within the first transmembrane domain are functionally important for YqjA to support growth at a pH of >9. We also observed that YqjA could rescue growth of the mutant at elevated pH in the absence of any salt in the presence of higher osmotic pressure provided by sucrose, mannitol, or sorbitol, raising the possibility that YqjA possesses an osmosensing capability. Based on these data, we suggest that YqjA is a newly identified proton-dependent transporter that plays a significant role in alkaline pH homeostasis in E. coli.
MATERIALS AND METHODS
Bacterial growth conditions.
Bacterial cultures were grown in LB medium (1% tryptone, 0.5% yeast extract, and 1% NaCl) with antibiotics (ampicillin [Amp], 100 μg/ml; kanamycin [Kan], 30 μg/ml; tetracycline [Tet], 12.5 μg/ml where specified). In certain experiments, the growth medium was additionally supplemented with 0.002% or 0.02% (wt/vol) arabinose. Media were buffered with 70 mM Bis-Tris propane (BTP), and the pH was adjusted as required with HCl. Cultures were grown at 37°C in a shaking incubator unless otherwise indicated.
Strains and molecular techniques.
All the strains and plasmids are listed in Table 1. Mutations from Keio Collection strains (19) were typically introduced into E. coli W3110 by P1 transduction (20), and the correct configuration was verified through PCR and DNA sequencing with primers flanking the appropriate gene. DNA sequencing was conducted at the Louisiana State University (LSU) College of Science Genomics Facility.
TABLE 1.
Strains and plasmids used in this study
| Strain or plasmid | Description | Source or reference |
|---|---|---|
| Strains | ||
| W3110 | Wild type; F− λ− IN(rrnD-rrnE)1 rph-1 | E. coli genetic stock center, Yale University |
| ΔyqjA mutant | W3110 ΔyqjA::Tetr (previously named BC203) | 10 |
| BC202 | W3110 ΔyqjA::Tetr ΔyghB781::kan | 10 |
| XL1-Blue | recA1 endA1 gyrA96 thi-1 hsdR17 supE44 relA1 lac [F′ proAB lacIqZΔM15] Tn10 (Tetr) | Stratagene |
| BW25113 | F− Δ(araD-araB)567 ΔlacZ4787(::rrnB-3) λ− rph-1 Δ(rhaD-rhaB)568 hsdR514 (Keio parent strain) | 19 |
| JW0826-2 | BW25113 Δcmr742::kan | 19 |
| JW2976-2 | BW25113 ΔyghB781::kan | 19 |
| JW4300-1 | BW25113 ΔmdtM736::kan | 19 |
| JW0018-2 | BW25113 ΔnhaA737::kan | 19 |
| JW1175-1 | BW25113 ΔnhaB775::kan | 19 |
| Plasmids | ||
| pBADHisA | Expression vector; araBAD promoter; Ampr | Invitrogen |
| pBADyqjA | H6-yqjA expression vector; araBAD promoter; Ampr | 11 |
| pBADyqjA-E39A | H6-yqjA E39A expression vector; araBAD promoter; Ampr | 12 |
| pBADyqjA-D51A | H6-yqjA D51A expression vector; araBAD promoter; Ampr | 12 |
| pBADyghB | H6-yghB expression vector; araBAD promoter; Ampr | 11 |
| pBADmdfA | H6-mdfA expression vector; araBAD promoter; Ampr | 11 |
Alkaline pH sensitivity growth assay on solid medium.
To test alkaline pH sensitivity on solid medium, overnight cultures of E. coli were freshly diluted 1:100 in LB medium with appropriate antibiotics and additives and grown to an optical density at 600 nm (OD600) of ∼ 0.6 at 37°C in a shaking incubator. Five microliters of serially log10-diluted cells was spotted on LB agar plates at various pHs with appropriate antibiotics and additives. To test the effects of Na+ and K+ ions at alkaline pH on the growth of bacteria, salt-free medium (1% [wt/vol] tryptone, 0.5% [wt/vol] yeast extract) was supplemented with different concentrations of salts, as indicated. Although we refer to this medium as “salt free,” residual amounts (1 to 10 mM) of both sodium and potassium were likely present (7, 21). The bacterial cells were washed at least two times in salt-free medium before making dilutions and plating. Growth was analyzed after incubation for 20 to 24 h at 37°C. All the experiments were repeated at least three times.
Alkaline pH sensitivity growth assay in liquid medium.
Overnight cultures from a single colony were freshly diluted 1:100 in LB medium with appropriate antibiotics and additives and grown to an OD600 of ∼1.0 at 37°C in a shaking incubator; 1 ml of culture was further inoculated into 20 ml of prewarmed fresh LB medium buffered to the indicated pH and containing appropriate antibiotics and additives. During growth at pH 7, 0.002% arabinose was used when inducing expression of yqjA due to toxicity issues. At higher pH, 0.02% arabinose was used. The cells were further grown aerobically at 37°C in a shaking incubator, and the OD600 was measured each hour for 8 h. In order to see the dependency of sodium and potassium ions at alkaline pH, the cells were first washed in salt-free medium twice before growth was initiated in LB medium containing additives at the indicated concentrations.
RESULTS
E. coli lacking yqjA is unable to grow at alkaline pH.
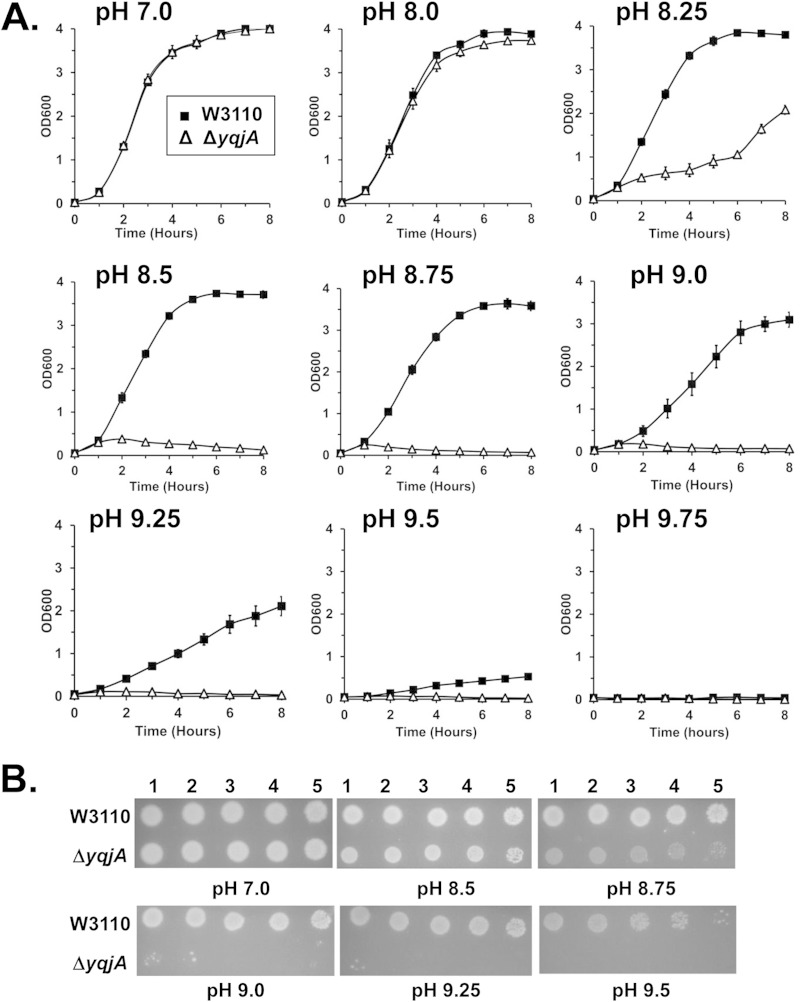
In order to study the physiological roles of YqjA, we tested the growth of wild-type E. coli and a ΔyqjA mutant at different alkaline pHs in liquid medium. At pH 7 and pH 8, both strains grew well at 37°C (Fig. 1A). However, as the pH increased above pH 8.25 to pH 9.5, the growth of the ΔyqjA mutant was diminished compared to the wild-type strain. Similar results were observed when dilutions of these strains were spotted and grown on solid growth medium (Fig. 1B). Above pH 9.5, both the wild type and the ΔyqjA mutant did not show any or showed only marginal growth either on solid or in liquid medium (data not shown). Our results are in agreement with the alkaline sensitivity of a ΔyqjA mutant observed previously (22). These observations show that YqjA plays an important role in providing alkaline tolerance to E. coli. While YghB displays 62% amino acid identity to YqjA and can substitute for YqjA in many processes (8), the ΔyghB mutant did not display sensitivity to growth under alkaline conditions (see Fig. S1 in the supplemental material).
FIG 1.
An E. coli ΔyqjA mutant fails to grow at elevated pH. (A) Parent E. coli W3110 and a ΔyqjA mutant were grown to mid-log phase at pH 7 and inoculated into liquid LB medium at pH 7.0, 8.0, 8.25, 8.5, 8.75, 9.0, 9.25, 9.5, or 9.75. Growth at 37°C was monitored hourly. (B) Parent strain W3110 and a ΔyqjA mutant were grown to mid-log phase at pH 7.0, and then 5 μl of serially diluted cells was spotted onto LB medium plates at pH 7.0, 8.5, 8.75, 9.0, 9.25, and 9.5, and the plates were incubated at 37°C for 20 to 24 h. The error bars indicate standard deviations of three independent measurements.
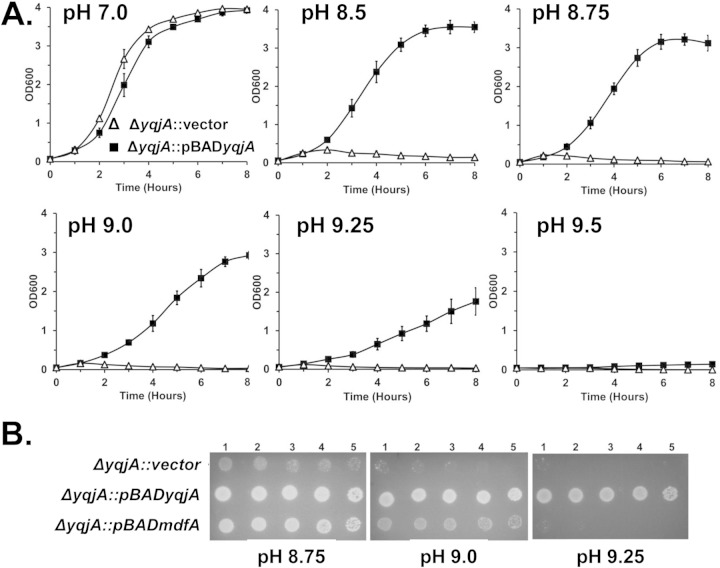
Overexpression of yqjA from a multicopy plasmid corrects the alkaline-sensitive phenotype.
We next examined the ability of overexpression of yqjA from an inducible multicopy plasmid to rescue the alkaline pH sensitivity phenotype. In liquid medium at pH 7.0, the ΔyqjA mutant harboring an empty vector and the ΔyqjA mutant harboring a vector expressing yqjA grew similarly (Fig. 2A). As the pH increased into the range of 8.5 to 9.5, the mutant harboring the control vector grew more and more poorly, while plasmid expression of yqjA was capable of supporting growth of the ΔyqjA mutant to wild-type levels under these conditions. On solid plates, ΔyqjA cells containing vector or expressing wild-type yqjA grew well until about pH 8.5. However, as the pH was increased above 8.5, the cells overexpressing yqjA were able to survive, unlike the cells containing the control vector (Fig. 2B). Taken together, these results suggest that expression of functional YqjA is necessary for a ΔyqjA mutant to survive at alkaline pH. Expression of yghB can restore normal growth and cell division to BC202 (ΔyqjA ΔyghB), suggesting that it possesses functions in common with YqjA (10, 23). We tested the ability of plasmid expression of yghB to facilitate growth of ΔyqjA at elevated pH. At pH 8.75 and 9.0, yghB expression could enhance the growth of the ΔyqjA mutant compared to the vector control, but only yqjA expression could restore growth at the higher pH of 9.25 (see Fig. S2 in the supplemental material). This result suggests a partial but not complete overlap of the functions of these DedA family proteins.
FIG 2.
Expression of yqjA or mdfA displays differing capacities to support growth of the ΔyqjA mutant at elevated pH. (A) ΔyqjA mutants harboring the control vector pBAD or pBADyqjA were grown to mid-log phase at pH 7.0 and inoculated into liquid LB-Amp medium at pH 7.0, 8.5, 8.75, 9.0, 9.25, or 9.5. Growth was monitored hourly by measuring the OD600. (B) ΔyqjA mutants harboring the control vector pBAD, pBADyqjA, or pBADmdfA were grown to mid-log phase at pH 7.0, and 5 μl of serially diluted cells was spotted onto LB-Amp plates at pH 8.75, 9.0, and 9.25. The plates were subsequently incubated at 37°C for 20 to 24 h. In both experiments, growth at pH 7.0 was carried out in the presence of 0.002% arabinose, while growth at higher pH was in the presence of 0.02% arabinose due to toxicity issues with yqjA expression at pH 7.0. The error bars indicate standard deviations of three independent measurements.
Overexpression of MdfA partially complements the alkaline pH sensitivity of the ΔyqjA mutant.
We reported previously that overexpression of mdfA could rescue the growth, cell division, and drug sensitivity of BC202 (ΔyqjA ΔyghB) (11, 12). MdfA is an Na+-K+/H+ antiporter belonging to the major facilitator superfamily and is involved in both alkaline tolerance (5) and drug resistance (24, 25) in E. coli. Therefore, we tested whether mdfA overexpression can correct the alkaline pH sensitivity of the ΔyqjA mutant. We found that MdfA can partially complement the alkaline sensitivity of the ΔyqjA mutant up to about pH 9.0 but not at higher pHs both on solid medium (Fig. 2B) and in liquid medium (see Fig. S3 in the supplemental material), suggesting that YqjA plays a significant role in the survival of E. coli at extremely alkaline pH. We could not perform the reverse experiment, expressing yqjA in a ΔmdfA mutant or, for that matter, a ΔmdtM mutant because both mutants (obtained from the Keio collection [Table 1]) grew well at all pHs in our hands (see Fig. S4 in the supplemental material). These observations are not in agreement with previous reports (4, 5). In contrast, the ΔnhaA mutant did display pH sensitivity in our hands (see Fig. S4 in the supplemental material), consistent with previous reports (26). The reasons for these differences are unclear and may be related to differences in the genetic backgrounds of the strains. We have reported that the ΔmdfA mutant is sensitive to drugs and biocides in our hands (12), consistent with previous reports (27), and the genetic identities of all Keio strains were confirmed by PCR with primers flanking each gene and DNA sequencing. Plasmid overexpression of yqjA did not reverse the pH sensitivity of the ΔnhaA mutant (data not shown).
Transmembrane acidic amino acids are required for YqjA-dependent alkaline tolerance.
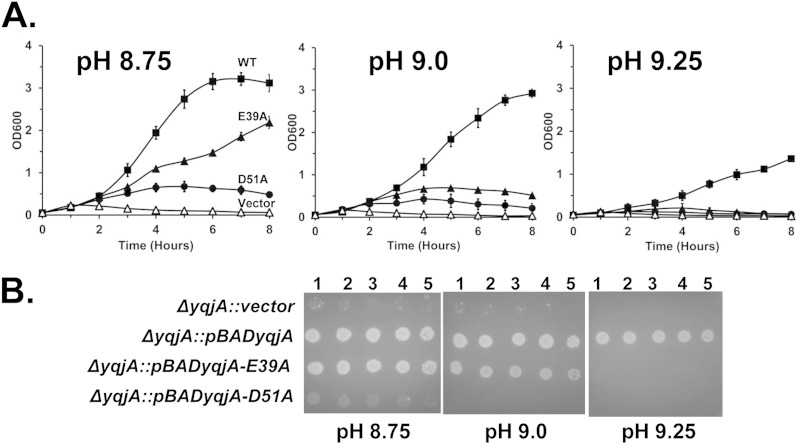
In many proton-dependent transporters, including those involved in drug efflux, it has been demonstrated that membrane-embedded acidic residues play a role in proton transport and recognition of cationic substrates (14–18). Many DedA proteins contain membrane-embedded glutamic acids and/or aspartic acids in the first predicted transmembrane-spanning region (E39 and D51 in both YqjA and YghB). We have shown that these residues are required for YqjA to restore growth, cell division, and drug resistance to BC202 (12). Similar acidic residues (E39 and D40) are found in the Borrelia burgdorferi DedA protein BB0250 that are essential for its ability to complement the growth, cell division, and drug sensitivity phenotypes of BC202 (12). We tested whether YqjA residues E39 and D51 are individually required for YqjA to provide alkaline tolerance to E. coli. Both in liquid and on solid media buffered at pH 9.0 and above, each acidic amino acid is required (Fig. 3). However, at pH 8.75, E39 appears to be dispensable for this activity, as growth was observed for the yqjA mutant expressing YqjA(E39A) at this pH (Fig. 3A and B). This suggests that residue D51 plays a major role in the ability of YqjA to provide alkaline tolerance while E39 is required only at the more extreme alkaline pHs above 9.0.
FIG 3.
YqjA acidic amino acids E39 and D51 are required for YqjA to support growth of E. coli at elevated pH. (A) ΔyqjA mutants harboring either the control vector pBAD (vector), pBADyqjA (WT), pBADyqjA-E39A (E39A), or pBADyqjA-D51A (D51A) were grown to mid-log phase at pH 7.0 and inoculated into liquid LB-Amp medium at pH 8.75, 9.0, and 9.25. (B) ΔyqjA mutants harboring the control vector pBAD, pBADyqjA, pBADyqjA-E39A, or pBADyqjA-D51A were grown to mid-log phase at pH 7.0, and 5 μl of serially diluted cells was spotted onto LB-Amp medium plates at pH 8.75, 9.0, 9.25, and 9.5. The plates were subsequently incubated at 37°C for 20 to 24 h. The error bars indicate standard deviations of three independent measurements.
Sodium or potassium is required for YqjA to support growth under alkaline conditions.
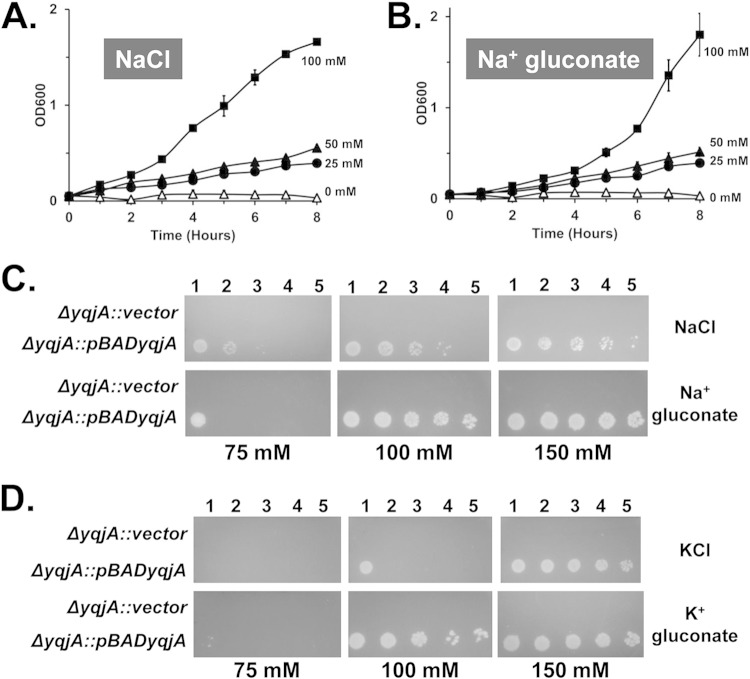
Many of the proteins that have been reported to be necessary for alkaline tolerance (NhaA, NhaB, ChaA, MdtM, and MdfA) in bacteria are antiporters that exchange protons for other cations, such as sodium, potassium, lithium, or calcium (3–7). We tested the cation requirements for YqjA to support alkaline tolerance by growing strains at elevated pH in the presence of various concentrations of extracellular salts. Either sodium or potassium was found to be necessary for this YqjA activity. While no growth was seen in the yqjA-complemented ΔyqjA mutant at pHs above 8.75 in the absence of extracellular salts (Fig. 4A; see Fig. S5 and S6 in the supplemental material), YqjA could support growth of the ΔyqjA mutant at pH 9.25 if 100 mM sodium was included in the growth medium (Fig. 4A to C). Sodium could be provided in the form of either sodium chloride or sodium gluconate, thereby demonstrating that the chloride anion is not necessary. Similarly, potassium, supplied as potassium chloride or potassium gluconate at 100 mM, could support growth at pH 9.25 under these conditions (Fig. 4D; see Fig. S5 in the supplemental material). Choline chloride could not support the ability of YqjA to provide alkaline tolerance at any concentration up to 150 mM (Fig. 5D), further supporting the lack of a role for the chloride anion.
FIG 4.
Sodium or potassium is required for YqjA to support growth at alkaline pH. (A and B) ΔyqjA harboring pBADyqjA was grown to mid-log phase at pH 7.0 and then inoculated into liquid LB-Amp medium at pH 9.25 containing 0 mM, 25 mM, 50 mM, or 100 mM sodium chloride (A) or sodium gluconate (B), and growth was monitored. (C and D) ΔyqjA mutants harboring either the control vector pBAD or pBADyqjA were grown to mid-log phase at pH 7.0, and 5 μl of serially diluted cells was spotted onto LB-Amp medium plates at pH 9.25 containing 75, 100, or 150 mM NaCl or sodium gluconate (C) and KCl or potassium gluconate (D). The plates were incubated at 37°C for 20 to 24 h. For reasons that are not understood, slight differences were observed in the optimal salt concentrations needed for growth when the strains were grown on solid or in liquid media. The error bars indicate standard deviations of three independent measurements.
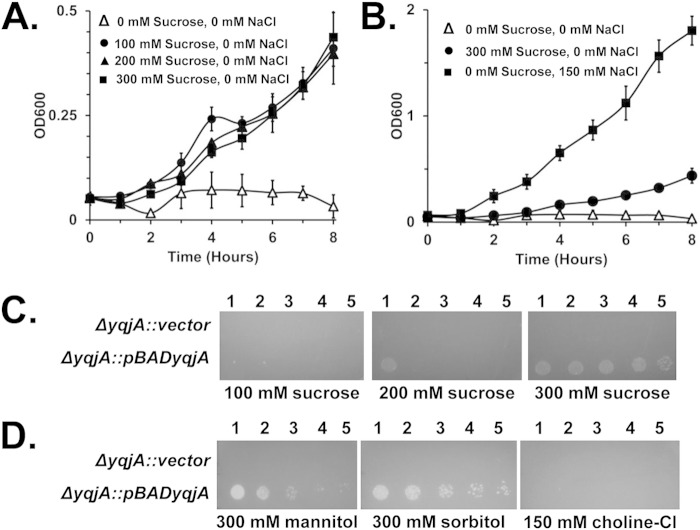
FIG 5.
Increasing the osmotic pressure can partially substitute for cations in supporting the ability of YqjA to permit growth at elevated pH. (A) A ΔyqjA mutant harboring pBADyqjA was grown to mid-log phase at pH 7.0 and then inoculated into salt-free liquid LB-Amp medium at pH 9.25 containing 0 mM, 100 mM, 200 mM, or 300 mM sucrose, and growth was monitored. (B) Comparison of growth rates between ΔyqjA harboring pBADyqjA in salt-free LB-Amp medium, salt-free LB-Amp medium containing 300 mM sucrose, and LB-Amp medium containing 150 mM NaCl. (C and D) A ΔyqjA mutant harboring the control vector pBAD or pBADyqjA was grown to mid-log phase at pH 7.0, and then 5 μl of serially diluted cells was spotted onto LB-Amp medium plates at pH 9.25 containing 100, 200, or 300 mM sucrose (C) or 300 mM mannitol, 300 mM sorbitol, or 150 mM choline chloride (D). The plates were incubated at 37°C for 20 to 24 h. The error bars indicate standard deviations of three independent measurements.
Raising osmotic pressure can enhance YqjA-mediated alkaline tolerance.
While no growth was seen when the ΔyqjA mutant expressing yqjA was grown at pH 9.25 in the absence of extracellular salt (Fig. 4; see Fig. S6 in the supplemental material), we were surprised to find that sucrose, mannitol, or sorbitol could enhance the growth of the strain at this elevated pH (Fig. 5). While the growth rate in the presence of increased osmotic pressure was only ∼25% of that seen in the presence of 150 mM NaCl or KCl (Fig. 5B), it was reproducible and significantly better than the undetectable growth rate seen in the absence of salt (although trace levels of both sodium and potassium are present in the broth [see Materials and Methods]). This growth rate enhancement was seen with 100 to 300 mM sucrose (Fig. 5C), as well as with mannitol or sorbitol (Fig. 5D), but was not seen with a molar equivalent amount of choline chloride (Fig. 5D).
We further tested if increased osmotic pressure could enhance YqjA function by growing the ΔyqjA mutant expressing yqjA at pH 9.25 in the presence of a suboptimal concentration of sodium chloride (50 mM) (Fig. 4) with or without 300 mM sucrose. Both on plates and in liquid medium, sucrose could enhance the growth of the strain in 50 mM NaCl to levels close to what is seen at 100 mM NaCl in the absence of additional osmotic pressure (Fig. 6). No growth was seen under these conditions with the ΔyqjA mutant harboring a control vector. As expected, the wild-type parent strain, W3110, also grows poorly at high pH in the absence of added salt, but growth can be restored by the inclusion of NaCl, KCl, or sucrose in the growth medium (see Fig. S7 in the supplemental material). To our knowledge, this is the first time it has been demonstrated that sucrose can aid the growth of E. coli at elevated pH in the presence of limiting salts, and our data suggest that YqjA alone is responsible for this. These data collectively support the hypothesis that the transport activity of YqjA is required for growth at elevated pH and is stimulated in the presence of increased osmotic pressure.
FIG 6.
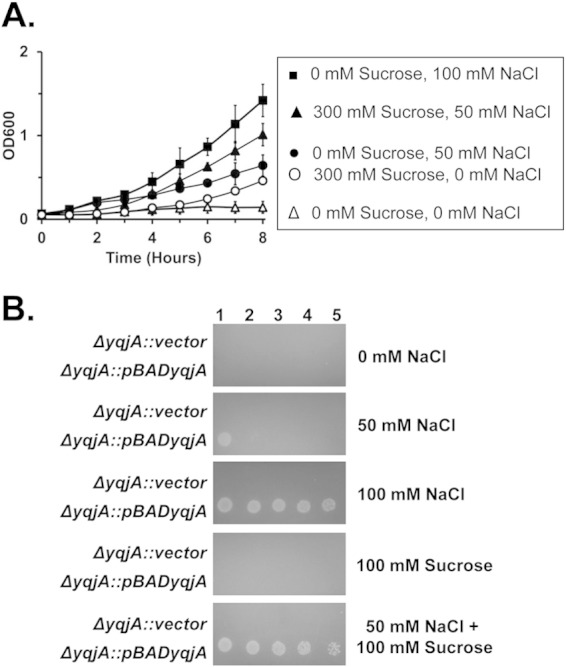
Sucrose enhances the ability of YqjA to provide alkaline tolerance in the presence of a suboptimal concentration of sodium. (A) ΔyqjA harboring pBADyqjA was grown to mid-log phase at pH 7.0 and then inoculated into salt-free liquid LB-Amp medium at pH 9.25 containing 0 mM sucrose, 0 mM NaCl, 300 mM sucrose and 0 mM NaCl, 0 mM sucrose and 50 mM NaCl, 300 mM sucrose and 50 mM NaCl, or 0 mM sucrose and 100 mM NaCl, and growth was monitored. (B) ΔyqjA harboring the control vector pBAD or pBADyqjA was grown to mid-log phase at pH 7.0, and then 5 μl of serially diluted cells was spotted onto LB-Amp medium plates at pH 9.25 containing 0 mM NaCl, 50 mM NaCl, 100 mM sucrose, 50 mM NaCl plus 100 mM sucrose, or 100 mM NaCl. The plates were incubated at 37°C for 20 to 24 h. The error bars indicate standard deviations of three independent measurements.
DISCUSSION
Adaptation to alkaline conditions by neutralophiles remains a poorly understood process. A necessary key process is maintenance of an acidic cytoplasm relative to the pH of the surrounding environment. This can be accomplished in a number of ways, but import of protons from outside the cell is one of the most common. This is usually accomplished by the activity of high-affinity proton-dependent antiporters. To control cytoplasmic pH, the cell by definition must control its internal proton concentration, and it does this by dictating the relative magnitudes of the transmembrane proton gradient (ΔpH) and the membrane electrical potential (ΔΨ), the two components of the PMF. Under conditions of alkaline stress, neutralophiles like E. coli cannot use the ΔpH to drive proton accumulation inside the cell because the cytoplasm is already more acidic than the outside. To overcome this, antiporters use ΔΨ to promote electrogenic Na+/H+ and/or K+/H+ exchange to acidify the cytoplasm (1). In E. coli, there are five known membrane transporters that play a role in adaptation to alkaline conditions: MdfA, NhaA, NhaB, ChaA, and MdtM (3–7). All are proton-dependent antiporters that function under specific environmental conditions. Mutants lacking these genes are often sensitive to alkaline pH (1).
We asked whether E. coli DedA family mutants can grow at elevated pH. Surprisingly, we found that YqjA, but not YghB, is required for growth in the range of pH 8.5 to 9.5, supporting previous observations (22). A ΔyqjA mutant cannot grow above pH 9.0 (Fig. 1), and expression of yqjA from a plasmid can restore growth to the ΔyqjA mutant, but only if sufficient extracellular sodium or potassium is supplied (more than ∼100 mM) (Fig. 2 and 4). Mutation of YqjA acidic amino acid E39 or D51 abolishes this activity (Fig. 3), in spite of the expression and membrane association of the mutant proteins at levels equivalent to the wild-type proteins (12).
We also observed that increasing the osmotic pressure by the addition of sucrose, mannitol, and sorbitol at concentrations of 100 to 300 mM could enhance the ability of YqjA to provide adaptation to alkaline growth conditions (Fig. 5 and 6). Each of these agents is capable of imposing increased osmotic pressure on bacteria (28–30). One interpretation of this surprising result is that YqjA may additionally possess osmosensing capabilities. A number of osmosensing transporters belonging to different membrane transporter families are known. They include ProP of E. coli (major facilitator superfamily), OpuA of Lactococcus lactis (ABC transporter superfamily), and BetP of Corynebacterium glutamicum (betaine-carnitine-choline transporter family) (31). Interestingly, BetP is a homotrimer with 12 transmembrane helix subunits (32) possessing the LeuT fold (33). Members of the DedA family are predicted to be evolutionarily related to LeuT and are predicted to also possess LeuT fold transmembrane helices (34).
The yqjA gene is found in an operon with yqjB, also known as mzrA. MzrA has been reported to modulate the activity of the EnvZ/OmpR two-component system, which responds to osmotic stress (35). MzrA interacts with inner membrane sensor kinases EnvZ and CpxA and promotes enhanced phosphorylation of these proteins, perhaps coordinating the output of the osmolarity-sensing EnvZ and the stress-sensing CpxA. Therefore, it is quite intriguing that we found an osmotic-pressure-stimulated activity of YqjA in promoting adaptation to alkaline growth conditions. EnvZ is not necessary for YqjA to promote adaptation to alkaline growth conditions (S. Kumar and W. T. Doerrler, unpublished observations), consistent with the previous determination that the EnvZ regulator MzrA functions independently of YqjA (35).
YqjA is also a member of the Cpx regulon (22), which responds to periplasmic stress caused by alkaline conditions and overexpression of certain envelope proteins, among other stressors (36). These stresses cause the activation of the sensor kinase CpxA, which in turn results in the activation of the response regulator CpxR. CpxR activation results in the upregulation of a number of periplasmic chaperones and proteases. YqjA is therefore one member of the Cpx regulon required for adaptation to alkaline conditions (22), suggesting a specific environmental niche for YqjA activity.
The DedA/Tvp38 family of membrane proteins are highly conserved and found within all domains of life (8). Nearly all sequenced bacterial genomes possess at least one DedA family member. Our genetic approaches support roles for YqjA and its close homologue YghB in maintenance of the proton motive force, possibly by acting as proton-dependent transporters (11, 12). Our findings reported here support a critical role for YqjA in adaptation to alkaline conditions by the neutralophile E. coli. Furthermore, YqjA appears to possess characteristics similar to those of a number of reported osmosensing transporters (31), although additional work, including in vitro studies, is required to prove this. While other transporters have been reported to counteract alkaline stress (3–7), we believe YqjA, and possibly other DedA family members, is a candidate to play a major role in adaptation to alkaline and possibly other stressful environments.
Supplementary Material
ACKNOWLEDGMENTS
Financial support was provided by the National Science Foundation (MCB-0841853 to W.T.D.).
We thank Rakesh Sikdar, Megan Dugas, and Pradip Panta for valuable discussions and Scott W. Herke of the LSU College of Science Genomics Facility for assistance with DNA sequencing.
Footnotes
Supplemental material for this article may be found at http://dx.doi.org/10.1128/JB.00175-15.
REFERENCES
- 1.Krulwich TA, Sachs G, Padan E. 2011. Molecular aspects of bacterial pH sensing and homeostasis. Nat Rev Microbiol 9:330–343. doi: 10.1038/nrmicro2549. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Herz K, Vimont S, Padan E, Berche P. 2003. Roles of NhaA, NhaB, and NhaD Na+/H+ antiporters in survival of Vibrio cholerae in a saline environment. J Bacteriol 185:1236–1244. doi: 10.1128/JB.185.4.1236-1244.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Padan E, Bibi E, Ito M, Krulwich TA. 2005. Alkaline pH homeostasis in bacteria: new insights. Biochim Biophys Acta 1717:67–88. doi: 10.1016/j.bbamem.2005.09.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Holdsworth SR, Law CJ. 2013. Multidrug resistance protein MdtM adds to the repertoire of antiporters involved in alkaline pH homeostasis in Escherichia coli. BMC Microbiol 13:113. doi: 10.1186/1471-2180-13-113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Lewinson O, Padan E, Bibi E. 2004. Alkalitolerance: a biological function for a multidrug transporter in pH homeostasis. Proc Natl Acad Sci U S A 101:14073–14078. doi: 10.1073/pnas.0405375101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Pinner E, Kotler Y, Padan E, Schuldiner S. 1993. Physiological role of nhaB, a specific Na+/H+ antiporter in Escherichia coli. J Biol Chem 268:1729–1734. [PubMed] [Google Scholar]
- 7.Radchenko MV, Tanaka K, Waditee R, Oshimi S, Matsuzaki Y, Fukuhara M, Kobayashi H, Takabe T, Nakamura T. 2006. Potassium/proton antiport system of Escherichia coli. J Biol Chem 281:19822–19829. doi: 10.1074/jbc.M600333200. [DOI] [PubMed] [Google Scholar]
- 8.Doerrler WT, Sikdar R, Kumar S, Boughner LA. 2013. New functions for the ancient DedA membrane protein family. J Bacteriol 195:3–11. doi: 10.1128/JB.01006-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Sikdar R, Doerrler WT. 2010. Inefficient Tat-dependent export of periplasmic amidases in an Escherichia coli strain with mutations in two DedA family genes. J Bacteriol 192:807–818. doi: 10.1128/JB.00716-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Thompkins K, Chattopadhyay B, Xiao Y, Henk MC, Doerrler WT. 2008. Temperature sensitivity and cell division defects in an Escherichia coli strain with mutations in yghB and yqjA, encoding related and conserved inner membrane proteins. J Bacteriol 190:4489–4500. doi: 10.1128/JB.00414-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Sikdar R, Simmons AR, Doerrler WT. 2013. Multiple envelope stress response pathways are activated in an Escherichia coli strain with mutations in two members of the DedA membrane protein family. J Bacteriol 195:12–24. doi: 10.1128/JB.00762-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Kumar S, Doerrler WT. 2014. Members of the conserved DedA family are likely membrane transporters and are required for drug resistance in Escherichia coli. Antimicrob Agents Chemother 58:923–930. doi: 10.1128/AAC.02238-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Hirokawa T, Boon-Chieng S, Mitaku S. 1998. SOSUI: classification and secondary structure prediction system for membrane proteins. Bioinformatics 14:378–379. doi: 10.1093/bioinformatics/14.4.378. [DOI] [PubMed] [Google Scholar]
- 14.Edgar R, Bibi E. 1999. A single membrane-embedded negative charge is critical for recognizing positively charged drugs by the Escherichia coli multidrug resistance protein MdfA. EMBO J 18:822–832. doi: 10.1093/emboj/18.4.822. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Fluman N, Ryan CM, Whitelegge JP, Bibi E. 2012. Dissection of mechanistic principles of a secondary multidrug efflux protein. Mol Cell 47:777–787. doi: 10.1016/j.molcel.2012.06.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Seeger MA, von Ballmoos C, Verrey F, Pos KM. 2009. Crucial role of Asp408 in the proton translocation pathway of multidrug transporter AcrB: evidence from site-directed mutagenesis and carbodiimide labeling. Biochemistry 48:5801–5812. doi: 10.1021/bi900446j. [DOI] [PubMed] [Google Scholar]
- 17.Sigal N, Fluman N, Siemion S, Bibi E. 2009. The secondary multidrug/proton antiporter MdfA tolerates displacements of an essential negatively charged side chain. J Biol Chem 284:6966–6971. doi: 10.1074/jbc.M808877200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Soskine M, Adam Y, Schuldiner S. 2004. Direct evidence for substrate-induced proton release in detergent-solubilized EmrE, a multidrug transporter. J Biol Chem 279:9951–9955. doi: 10.1074/jbc.M312853200. [DOI] [PubMed] [Google Scholar]
- 19.Baba T, Ara T, Hasegawa M, Takai Y, Okumura Y, Baba M, Datsenko KA, Tomita M, Wanner BL, Mori H. 2006. Construction of Escherichia coli K-12 in-frame, single-gene knockout mutants: the Keio collection. Mol Syst Biol 2:2006.0008. doi: 10.1038/msb4100050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Silhavy TJ, Berman ML, Enquist LW, Cold Spring Harbor Laboratory . 1984. Experiments with gene fusions. Cold Spring Harbor Laboratory, Cold Spring Harbor, NY. [Google Scholar]
- 21.Ohyama T, Igarashi K, Kobayashi H. 1994. Physiological role of the chaA gene in sodium and calcium circulations at a high pH in Escherichia coli. J Bacteriol 176:4311–4315. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Price NL, Raivio TL. 2009. Characterization of the Cpx regulon in Escherichia coli strain MC4100. J Bacteriol 191:1798–1815. doi: 10.1128/JB.00798-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Boughner LA, Doerrler WT. 2012. Multiple deletions reveal the essentiality of the DedA membrane protein family in Escherichia coli. Microbiology 158:1162–1171. doi: 10.1099/mic.0.056325-0. [DOI] [PubMed] [Google Scholar]
- 24.Edgar R, Bibi E. 1997. MdfA, an Escherichia coli multidrug resistance protein with an extraordinarily broad spectrum of drug recognition. J Bacteriol 179:2274–2280. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Lewinson O, Adler J, Poelarends GJ, Mazurkiewicz P, Driessen AJ, Bibi E. 2003. The Escherichia coli multidrug transporter MdfA catalyzes both electrogenic and electroneutral transport reactions. Proc Natl Acad Sci U S A 100:1667–1672. doi: 10.1073/pnas.0435544100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Padan E, Maisler N, Taglicht D, Karpel R, Schuldiner S. 1989. Deletion of ant in Escherichia coli reveals its function in adaptation to high salinity and an alternative Na+/H+ antiporter system(s). J Biol Chem 264:20297–20302. [PubMed] [Google Scholar]
- 27.Tal N, Schuldiner S. 2009. A coordinated network of transporters with overlapping specificities provides a robust survival strategy. Proc Natl Acad Sci U S A 106:9051–9056. doi: 10.1073/pnas.0902400106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Kitko RD, Wilks JC, Garduque GM, Slonczewski JL. 2010. Osmolytes contribute to pH homeostasis of Escherichia coli. PLoS One 5:e10078. doi: 10.1371/journal.pone.0010078. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.van den Bogaart G, Hermans N, Krasnikov V, Poolman B. 2007. Protein mobility and diffusive barriers in Escherichia coli: consequences of osmotic stress. Mol Microbiol 64:858–871. doi: 10.1111/j.1365-2958.2007.05705.x. [DOI] [PubMed] [Google Scholar]
- 30.Verkhovskaya ML, Barquera B, Verkhovsky MI, Wikstrom M. 1998. The Na+ and K+ transport deficiency of an E. coli mutant lacking the NhaA and NhaB proteins is apparent and caused by impaired osmoregulation. FEBS Lett 439:271–274. doi: 10.1016/S0014-5793(98)01380-5. [DOI] [PubMed] [Google Scholar]
- 31.Wood JM. 2011. Bacterial osmoregulation: a paradigm for the study of cellular homeostasis. Annu Rev Microbiol 65:215–238. doi: 10.1146/annurev-micro-090110-102815. [DOI] [PubMed] [Google Scholar]
- 32.Ressl S, Terwisscha van Scheltinga AC, Vonrhein C, Ott V, Ziegler C. 2009. Molecular basis of transport and regulation in the Na(+)/betaine symporter BetP. Nature 458:47–52. doi: 10.1038/nature07819. [DOI] [PubMed] [Google Scholar]
- 33.Forrest LR, Kramer R, Ziegler C. 2011. The structural basis of secondary active transport mechanisms. Biochim Biophys Acta 1807:167–188. doi: 10.1016/j.bbabio.2010.10.014. [DOI] [PubMed] [Google Scholar]
- 34.Khafizov K, Staritzbichler R, Stamm M, Forrest LR. 2010. A study of the evolution of inverted-topology repeats from LeuT-fold transporters using AlignMe. Biochemistry 49:10702–10713. doi: 10.1021/bi101256x. [DOI] [PubMed] [Google Scholar]
- 35.Gerken H, Charlson ES, Cicirelli EM, Kenney LJ, Misra R. 2009. MzrA: a novel modulator of the EnvZ/OmpR two-component regulon. Mol Microbiol 72:1408–1422. doi: 10.1111/j.1365-2958.2009.06728.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Raivio TL, Silhavy TJ. 1999. The sigmaE and Cpx regulatory pathways: overlapping but distinct envelope stress responses. Curr Opin Microbiol 2:159–165. doi: 10.1016/S1369-5274(99)80028-9. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.