Abstract
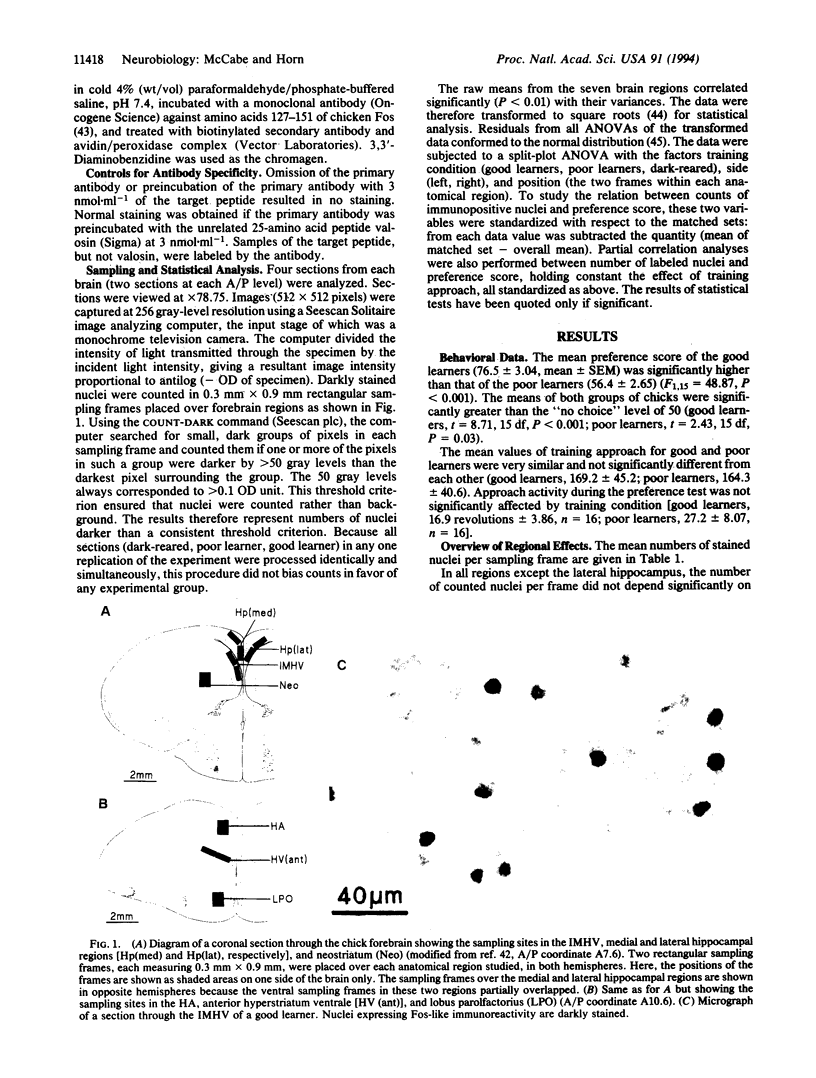
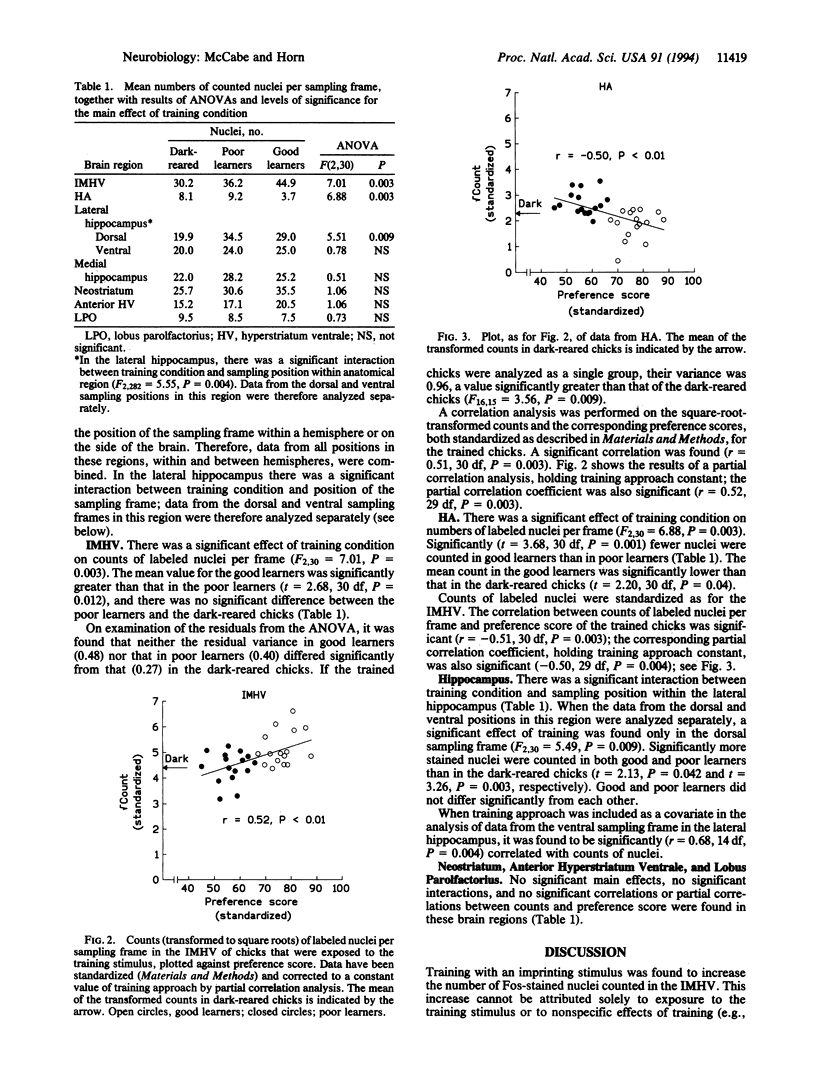
The intermediate and medial part of the hyperstriatum ventrale (IMHV) is a part of the chick forebrain that is critical for the learning process of imprinting and may be a site of information storage. Chicks were either trained on an imprinting stimulus or dark-reared. Trained chicks were classified as good or poor learners by their preference score (a measure of the strength of imprinting). A monoclonal antibody against the immediate early gene product Fos was applied to sections through IMHV and other forebrain regions. In the IMHV, significantly more immunopositive nuclei were counted in good learners than in poor learners or dark-reared chicks. There was a positive correlation between counts of labeled nuclei and preference score that was not attributable to sensory activity per se, locomotor activity during training, or a predisposition to learn well; rather, the results indicated that the change in Fos immunoreactivity in the IMHV was related to learning. In the hyperstriatum accessorium, significantly fewer immunopositive nuclei were counted in good learners than in poor learners or in dark-reared chicks. In the dorsolateral hippocampal region, more immunopositive nuclei were counted in trained than in dark-reared chicks. No significant effects of training were found in the anterior hyperstriatum ventrale, lobus parolfactorius, neostriatum, medial hippocampal region, or ventrolateral hippocampal region, but counts in this last region were positively correlated with training approach. The results for IMHV implicate Fos or Fos-related proteins in memory processes and pave the way for the identification of the cell types that show the learning-related increase in gene expression.
Full text
PDF


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Alkon D. L., Sánchez-Andrés J. V., Ito E., Oka K., Yoshioka T., Collin C. Long-term transformation of an inhibitory into an excitatory GABAergic synaptic response. Proc Natl Acad Sci U S A. 1992 Dec 15;89(24):11862–11866. doi: 10.1073/pnas.89.24.11862. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ambalavanar R., Van der Zee E. A., Bolhuis J. J., McCabe B. J., Horn G. Co-expression of Fos immunoreactivity in protein kinase (PKC gamma)-positive neurones: quantitative analysis of a brain region involved in learning. Brain Res. 1993 Mar 26;606(2):315–318. doi: 10.1016/0006-8993(93)91000-i. [DOI] [PubMed] [Google Scholar]
- Anokhin K. V., Mileusnic R., Shamakina I. Y., Rose S. P. Effects of early experience on c-fos gene expression in the chick forebrain. Brain Res. 1991 Mar 22;544(1):101–107. doi: 10.1016/0006-8993(91)90890-8. [DOI] [PubMed] [Google Scholar]
- Anokhin Konstantin V., Rose Steven P. R. Learning-induced Increase of Immediate Early Gene Messenger RNA in the Chick Forebrain. Eur J Neurosci. 1991;3(2):162–167. doi: 10.1111/j.1460-9568.1991.tb00076.x. [DOI] [PubMed] [Google Scholar]
- Aronin N., Sagar S. M., Sharp F. R., Schwartz W. J. Light regulates expression of a Fos-related protein in rat suprachiasmatic nuclei. Proc Natl Acad Sci U S A. 1990 Aug;87(15):5959–5962. doi: 10.1073/pnas.87.15.5959. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bateson P. P., Horn G., Rose S. P. Effects of early experience on regional incorporation of precursors into RNA and protein in the chick brain. Brain Res. 1972 Apr 28;39(2):449–465. doi: 10.1016/0006-8993(72)90448-9. [DOI] [PubMed] [Google Scholar]
- Bateson P. P., Horn G., Rose S. P. Imprinting: correlations between behaviour and incorporation of (14-C) uracil into chick brain. Brain Res. 1975 Feb 7;84(2):207–220. doi: 10.1016/0006-8993(75)90976-2. [DOI] [PubMed] [Google Scholar]
- Bateson P. P., Rose S. P., Horn G. Imprinting: lasting effects on uracil incorporation into chick brain. Science. 1973 Aug 10;181(4099):576–578. doi: 10.1126/science.181.4099.576. [DOI] [PubMed] [Google Scholar]
- Blough D. S. Reaction time drifts identify objects of attention in pigeon visual search. J Exp Psychol Anim Behav Process. 1993 Apr;19(2):107–120. doi: 10.1037//0097-7403.19.2.107. [DOI] [PubMed] [Google Scholar]
- Blough P. M. Attentional priming and visual search in pigeons. J Exp Psychol Anim Behav Process. 1989 Oct;15(4):358–365. [PubMed] [Google Scholar]
- Bolhuis J. J. Mechanisms of avian imprinting: a review. Biol Rev Camb Philos Soc. 1991 Nov;66(4):303–345. doi: 10.1111/j.1469-185x.1991.tb01145.x. [DOI] [PubMed] [Google Scholar]
- Boxer M. I., Stanford D. Projections to the posterior visual hyperstriatal region in the chick: an HRP study. Exp Brain Res. 1985;57(3):494–498. doi: 10.1007/BF00237836. [DOI] [PubMed] [Google Scholar]
- Bradford C. M., McCabe B. J. Neuronal activity related to memory in the intermediate and medial part of the hyperstriatum ventrale of the chick brain. Brain Res. 1994 Mar 21;640(1-2):11–16. doi: 10.1016/0006-8993(94)91851-1. [DOI] [PubMed] [Google Scholar]
- Bradley P., Davies D. C., Horn G. Connections of the hyperstriatum ventrale of the domestic chick (Gallus domesticus). J Anat. 1985 Jun;140(Pt 4):577–589. [PMC free article] [PubMed] [Google Scholar]
- Bradley P., Horn G., Bateson P. Imprinting. An electron microscopic study of chick hyperstriatum ventrale. Exp Brain Res. 1981;41(2):115–120. doi: 10.1007/BF00236600. [DOI] [PubMed] [Google Scholar]
- Brennan P. A., Hancock D., Keverne E. B. The expression of the immediate-early genes c-fos, egr-1 and c-jun in the accessory olfactory bulb during the formation of an olfactory memory in mice. Neuroscience. 1992 Jul;49(2):277–284. doi: 10.1016/0306-4522(92)90095-j. [DOI] [PubMed] [Google Scholar]
- Brown M. W., Horn G. Are specific proteins implicated in the learning process of imprinting? Brain Res Dev Brain Res. 1990 Mar 1;52(1-2):294–297. doi: 10.1016/0165-3806(90)90248-w. [DOI] [PubMed] [Google Scholar]
- Brown M. W., Horn G. Learning-related alterations in the visual responsiveness of neurons in a memory system of the chick brain. Eur J Neurosci. 1994 Sep 1;6(9):1479–1490. doi: 10.1111/j.1460-9568.1994.tb01009.x. [DOI] [PubMed] [Google Scholar]
- Brown M. W., Horn G. Responsiveness of neurones in the hippocampal region of anaesthetised and unanaesthetised cats to stimulation of sensory pathways. Brain Res. 1977 Mar 11;123(2):241–259. doi: 10.1016/0006-8993(77)90477-2. [DOI] [PubMed] [Google Scholar]
- Cherubini E., Gaiarsa J. L., Ben-Ari Y. GABA: an excitatory transmitter in early postnatal life. Trends Neurosci. 1991 Dec;14(12):515–519. doi: 10.1016/0166-2236(91)90003-d. [DOI] [PubMed] [Google Scholar]
- Cipolla-Neto J., Horn G., McCabe B. J. Hemispheric asymmetry and imprinting: the effect of sequential lesions to the hyperstriatum ventrale. Exp Brain Res. 1982;48(1):22–27. doi: 10.1007/BF00239569. [DOI] [PubMed] [Google Scholar]
- Cole A. J., Saffen D. W., Baraban J. M., Worley P. F. Rapid increase of an immediate early gene messenger RNA in hippocampal neurons by synaptic NMDA receptor activation. Nature. 1989 Aug 10;340(6233):474–476. doi: 10.1038/340474a0. [DOI] [PubMed] [Google Scholar]
- Curran T., Franza B. R., Jr Fos and Jun: the AP-1 connection. Cell. 1988 Nov 4;55(3):395–397. doi: 10.1016/0092-8674(88)90024-4. [DOI] [PubMed] [Google Scholar]
- Davey J. E., Horn G. The development of hemispheric asymmetries in neuronal activity in the domestic chick after visual experience. Behav Brain Res. 1991 Oct 25;45(1):81–86. doi: 10.1016/s0166-4328(05)80183-4. [DOI] [PubMed] [Google Scholar]
- Dragunow M., Faull R. The use of c-fos as a metabolic marker in neuronal pathway tracing. J Neurosci Methods. 1989 Sep;29(3):261–265. doi: 10.1016/0165-0270(89)90150-7. [DOI] [PubMed] [Google Scholar]
- Fujiwara K. T., Ashida K., Nishina H., Iba H., Miyajima N., Nishizawa M., Kawai S. The chicken c-fos gene: cloning and nucleotide sequence analysis. J Virol. 1987 Dec;61(12):4012–4018. doi: 10.1128/jvi.61.12.4012-4018.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gilman M. Z. The c-fos serum response element responds to protein kinase C-dependent and -independent signals but not to cyclic AMP. Genes Dev. 1988 Apr;2(4):394–402. doi: 10.1101/gad.2.4.394. [DOI] [PubMed] [Google Scholar]
- Horn G., Bradley P., McCabe B. J. Changes in the structure of synapses associated with learning. J Neurosci. 1985 Dec;5(12):3161–3168. doi: 10.1523/JNEUROSCI.05-12-03161.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Horn G., Johnson M. H. Memory systems in the chick: dissociations and neuronal analysis. Neuropsychologia. 1989;27(1):1–22. doi: 10.1016/0028-3932(89)90086-9. [DOI] [PubMed] [Google Scholar]
- Horn G., McCabe B. J., Bateson P. P. An autoradiographic study of the chick brain after imprinting. Brain Res. 1979 May 25;168(2):361–373. doi: 10.1016/0006-8993(79)90176-8. [DOI] [PubMed] [Google Scholar]
- Horn G., McCabe B. J., Cipolla-Neto J. Imprinting in the domestic chick: the role of each side of the hyperstriatum ventrale in acquisition and retention. Exp Brain Res. 1983;53(1):91–98. doi: 10.1007/BF00239401. [DOI] [PubMed] [Google Scholar]
- Horn G. Neural bases of recognition memory investigated through an analysis of imprinting. Philos Trans R Soc Lond B Biol Sci. 1990 Aug 29;329(1253):133–142. doi: 10.1098/rstb.1990.0158. [DOI] [PubMed] [Google Scholar]
- Horn G., Rose S. P., Bateson P. P. Monocular imprinting and regional incorporation of tritiated uracil into the brains of intact and "split-brain" chicks. Brain Res. 1973 Jun 29;56:227–237. doi: 10.1016/0006-8993(73)90337-5. [DOI] [PubMed] [Google Scholar]
- Hunt S. P., Pini A., Evan G. Induction of c-fos-like protein in spinal cord neurons following sensory stimulation. Nature. 1987 Aug 13;328(6131):632–634. doi: 10.1038/328632a0. [DOI] [PubMed] [Google Scholar]
- Hunt S. P., Webster K. E. Thalamo-hyperstriate interrelations in the pigeon. Brain Res. 1972 Sep 29;44(2):647–651. doi: 10.1016/0006-8993(72)90328-9. [DOI] [PubMed] [Google Scholar]
- Johnson M. H., Horn G. Dissociation of recognition memory and associative learning by a restricted lesion of the chick forebrain. Neuropsychologia. 1986;24(3):329–340. doi: 10.1016/0028-3932(86)90018-7. [DOI] [PubMed] [Google Scholar]
- Johnson M. H., Horn G. The role of a restricted region of the chick forebrain in the recognition of individual conspecifics. Behav Brain Res. 1987 Mar;23(3):269–275. doi: 10.1016/0166-4328(87)90027-1. [DOI] [PubMed] [Google Scholar]
- Jones S. J., Horn G. Effects of visual experience on photically evoked potentials recorded in the chick forebrain. Brain Res. 1978 Dec 29;159(2):297–306. doi: 10.1016/0006-8993(78)90536-x. [DOI] [PubMed] [Google Scholar]
- Karten H. J., Hodos W., Nauta W. J., Revzin A. M. Neural connections of the "visual wulst" of the avian telencephalon. Experimental studies in the piegon (Columba livia) and owl (Speotyto cunicularia). J Comp Neurol. 1973 Aug;150(3):253–278. doi: 10.1002/cne.901500303. [DOI] [PubMed] [Google Scholar]
- Krebs J. R. Food-storing birds: adaptive specialization in brain and behaviour? Philos Trans R Soc Lond B Biol Sci. 1990 Aug 29;329(1253):153–160. doi: 10.1098/rstb.1990.0160. [DOI] [PubMed] [Google Scholar]
- Maier V., Scheich H. Acoustic imprinting leads to differential 2-deoxy-D-glucose uptake in the chick forebrain. Proc Natl Acad Sci U S A. 1983 Jun;80(12):3860–3864. doi: 10.1073/pnas.80.12.3860. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McCabe B. J., Cipolla-Neto J., Horn G., Bateson P. Amnesic effects of bilateral lesions placed in the hyperstriatum ventrale of the chick after imprinting. Exp Brain Res. 1982;48(1):13–21. doi: 10.1007/BF00239568. [DOI] [PubMed] [Google Scholar]
- McCabe B. J., Davey J. E., Horn G. Impairment of learning by localized injection of an N-methyl-D-aspartate receptor antagonist into the hyperstriatum ventrale of the domestic chick. Behav Neurosci. 1992 Dec;106(6):947–953. doi: 10.1037//0735-7044.106.6.947. [DOI] [PubMed] [Google Scholar]
- McCabe B. J., Horn G., Bateson P. P. Effects of restricted lesions of the chick forebrain on the acquisition of filial preferences during imprinting. Brain Res. 1981 Jan 26;205(1):29–37. doi: 10.1016/0006-8993(81)90717-4. [DOI] [PubMed] [Google Scholar]
- McCabe B. J., Horn G., Bateson P. P. Effects of rhythmic hyperstriatal stimulation on chicks' preferences for visual flicker. Physiol Behav. 1979 Jul;23(1):137–140. doi: 10.1016/0031-9384(79)90133-1. [DOI] [PubMed] [Google Scholar]
- McCabe B. J., Horn G. Learning and memory: regional changes in N-methyl-D-aspartate receptors in the chick brain after imprinting. Proc Natl Acad Sci U S A. 1988 Apr;85(8):2849–2853. doi: 10.1073/pnas.85.8.2849. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McCabe B. J., Horn G. Synaptic transmission and recognition memory: time course of changes in N-methyl-D-aspartate receptors after imprinting. Behav Neurosci. 1991 Apr;105(2):289–294. doi: 10.1037//0735-7044.105.2.289. [DOI] [PubMed] [Google Scholar]
- Miceli D., Peyrichoux J., Repérant J. The retino-thalamo-hyperstriatal pathway in the pigeon (Columba livia). Brain Res. 1975 Dec 12;100(1):125–131. doi: 10.1016/0006-8993(75)90247-4. [DOI] [PubMed] [Google Scholar]
- Morgan J. I., Cohen D. R., Hempstead J. L., Curran T. Mapping patterns of c-fos expression in the central nervous system after seizure. Science. 1987 Jul 10;237(4811):192–197. doi: 10.1126/science.3037702. [DOI] [PubMed] [Google Scholar]
- Morgan J. I., Curran T. Stimulus-transcription coupling in neurons: role of cellular immediate-early genes. Trends Neurosci. 1989 Nov;12(11):459–462. doi: 10.1016/0166-2236(89)90096-9. [DOI] [PubMed] [Google Scholar]
- Morgan J. I., Curran T. Stimulus-transcription coupling in the nervous system: involvement of the inducible proto-oncogenes fos and jun. Annu Rev Neurosci. 1991;14:421–451. doi: 10.1146/annurev.ne.14.030191.002225. [DOI] [PubMed] [Google Scholar]
- Payne J. K., Horn G., Brown M. W. Modifiability of responsiveness in a visual projection area of the chick brain: visual experience is only one of several factors involved. Behav Brain Res. 1984 Aug;13(2):163–172. doi: 10.1016/0166-4328(84)90146-3. [DOI] [PubMed] [Google Scholar]
- Sagar S. M., Sharp F. R., Curran T. Expression of c-fos protein in brain: metabolic mapping at the cellular level. Science. 1988 Jun 3;240(4857):1328–1331. doi: 10.1126/science.3131879. [DOI] [PubMed] [Google Scholar]
- Sheng M., Greenberg M. E. The regulation and function of c-fos and other immediate early genes in the nervous system. Neuron. 1990 Apr;4(4):477–485. doi: 10.1016/0896-6273(90)90106-p. [DOI] [PubMed] [Google Scholar]
- Sheu F. S., McCabe B. J., Horn G., Routtenberg A. Learning selectively increases protein kinase C substrate phosphorylation in specific regions of the chick brain. Proc Natl Acad Sci U S A. 1993 Apr 1;90(7):2705–2709. doi: 10.1073/pnas.90.7.2705. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shimizu T., Karten H. J. Immunohistochemical analysis of the visual wulst of the pigeon (Columba livia). J Comp Neurol. 1990 Oct 15;300(3):346–369. doi: 10.1002/cne.903000307. [DOI] [PubMed] [Google Scholar]




