ABSTRACT
Herpes simplex virus 1 (HSV-1) and HSV-2 establish latency in sensory and autonomic neurons after ocular or genital infection, but their recurrence patterns differ. HSV-1 reactivates from latency to cause recurrent orofacial disease, and while HSV-1 also causes genital lesions, HSV-2 recurs more efficiently in the genital region and rarely causes ocular disease. The mechanisms regulating these anatomical preferences are unclear. To determine whether differences in latent infection and reactivation in autonomic ganglia contribute to differences in HSV-1 and HSV-2 anatomical preferences for recurrent disease, we compared HSV-1 and HSV-2 clinical disease, acute and latent viral loads, and viral gene expression in sensory trigeminal and autonomic superior cervical and ciliary ganglia in a guinea pig ocular infection model. HSV-2 produced more severe acute disease, correlating with higher viral DNA loads in sensory and autonomic ganglia, as well as higher levels of thymidine kinase expression, a marker of productive infection, in autonomic ganglia. HSV-1 reactivated in ciliary ganglia, independently from trigeminal ganglia, to cause more frequent recurrent symptoms, while HSV-2 replicated simultaneously in autonomic and sensory ganglia to cause more persistent disease. While both HSV-1 and HSV-2 expressed the latency-associated transcript (LAT) in the trigeminal and superior cervical ganglia, only HSV-1 expressed LAT in ciliary ganglia, suggesting that HSV-2 is not reactivation competent or does not fully establish latency in ciliary ganglia. Thus, differences in replication and viral gene expression in autonomic ganglia may contribute to differences in HSV-1 and HSV-2 acute and recurrent clinical disease.
IMPORTANCE Herpes simplex virus 1 (HSV-1) and HSV-2 establish latent infections, from which the viruses reactivate to cause recurrent disease throughout the life of the host. However, the viruses exhibit different manifestations and frequencies of recurrent disease. HSV-1 and HSV-2 establish latency in both sensory and autonomic ganglia. Autonomic ganglia are more responsive than sensory ganglia to stimuli associated with recurrent disease in humans, such as stress and hormone fluctuations, suggesting that autonomic ganglia may play an important role in recurrent disease. We show that HSV-1 can reactivate from autonomic ganglia, independently from sensory ganglia, to cause recurrent ocular disease. We found no evidence that HSV-2 could reactivate from autonomic ganglia independently from sensory ganglia after ocular infection, but HSV-2 did replicate in both ganglia simultaneously to cause persistent disease. Thus, viral replication and reactivation in autonomic ganglia contribute to different clinical disease manifestations of HSV-1 and HSV-2 after ocular infection.
INTRODUCTION
Herpes simplex virus 1 (HSV-1) and HSV-2 infect and establish latency in peripheral sensory ganglia, including the trigeminal ganglia (TG) after orofacial infection and the dorsal root ganglia (DRG) after genital infection. While both viruses can reactivate from latency to cause recurrent lesions throughout the life of the host, HSV-1 and HSV-2 demonstrate different patterns of recurrent disease. HSV-1 is more likely to produce recurrent lesions in the orofacial region, while HSV-2 rarely, if ever, recurs orofacially, even if primary infection occurs in the mouth, nose, or eyes. HSV-1 is becoming more common as a cause of genital herpes, but HSV-2 recurs much more efficiently after genital infection. Although 60 to 90% of individuals with genital HSV-2 demonstrate symptomatic recurrences, only 25% with genital HSV-1 infections experience symptomatic recurrences (1, 2), demonstrating that HSV-1 and HSV-2 characteristic recurrent disease patterns are not simply a result of differences in the site of initial infection.
Upon reaching the sensory ganglia after peripheral inoculation, the viruses replicate in some neurons, while establishing latent infection in others. The sensory neuronal populations that are permissive for productive infection differ between HSV-1 and HSV-2. Sensory neurons recognized by monoclonal antibody Fe-A5 (A5+) limit productive HSV-1 infection (3, 4). In contrast, sensory neurons bound by the monoclonal antibody KH10 or isolectin IB4 (IB4+) limit productive infection of HSV-2 (3–5). Similar percentages of these nonoverlapping populations of sensory neurons are found in TG (10 to 12%) and DRG (13 to 15%). HSV-1 and HSV-2 demonstrate neuronal specificity for A5+ and IB4+ neurons, respectively, regardless of the route of infection. One-half of the LAT-positive latent HSV-1 reservoir is found in A5+ neurons, and one-half of the LAT-positive latent HSV-2 reservoir is found in IB4+ neurons, in the TG after ocular infection or in the DRG after genital infection (5–9). Thus, different patterns of HSV-1 and HSV-2 recurrence cannot be adequately explained by preferential establishment of latency in different types of sensory neurons.
The orofacial and genital regions innervated by the sensory TG and DRG are also extensively innervated by autonomic nerve endings. Sympathetic neurons of the superior cervical ganglia (SCG) and parasympathetic neurons in the ciliary ganglia (CG) innervate the conjunctival epithelium and stroma (6), and mixed autonomic neurons in the major pelvic ganglia (MPG) innervate the genitourinary tract. In both humans and animal models, HSV-1 and HSV-2 latent viral DNA has been detected in autonomic ganglia, including the SCG, CG, pterygopalatine ganglia (PTG), and MPG (also referred to as the paracervical ganglia) (7–12). The autonomic pathways are intimately involved in physiological activities associated with symptomatic recurrences in humans, including the stress response, febrile response, and hormone regulation. Although sensory ganglia are considered to be the site of reactivating HSV, reactivation of latent virus residing in autonomic ganglia could contribute to recurrent symptoms.
To determine whether differences between HSV-1 and HSV-2 in autonomic ganglia may contribute to the viruses' different patterns of recurrent disease, we used a guinea pig ocular infection model to evaluate clinical signs and to analyze viral DNA load and gene expression in sensory and autonomic ganglia at various time points postinoculation. After ocular infection, reactivation of HSV-1 occurred from autonomic ganglia to cause recurrent disease symptoms, but there was no evidence that HSV-2 could independently reactivate from autonomic ganglia to cause recurrences. We also provide evidence that differences in viral gene expression in sympathetic and parasympathetic ganglia are likely responsible for differences between HSV-1 and HSV-2 virulence and reactivation.
MATERIALS AND METHODS
Virus strains.
HSV-1 strain 17+ was originally transferred from John Hay (SUNY Buffalo, Buffalo, NY) and HSV-2 strain 333 from Gary Hayward (Johns Hopkins, MD) to the Krause lab (FDA, Bethesda, MD). Viruses were propagated in Vero cells (ATCC), and first-passage stocks were transferred to the Margolis lab (UCSF, San Francisco, CA). Viruses were propagated in Vero cells, and first-passage stocks were transferred to the Bertke lab (Virginia Tech, Blacksburg, VA). Viruses were propagated in Vero cells and titrated in duplicate by plaque assay in Vero cells. Stock viruses were diluted in Dulbecco's modified Eagle medium (DMEM) for guinea pig and mouse inoculations.
Guinea pig ocular infection and sample collection.
Female 3-week-old Hartley guinea pigs (HillTop Laboratories) were infected with 5 × 105 PFU of HSV-1 or HSV-2 by corneal inoculation after scarification. Guinea pigs were observed daily for 60 days postinfection (dpi). The severity of acute infection was graphed as the mean lesion score for each group of animals through day 14, based on a scale of 0 to 4 (0, no symptoms; 1, inflammation or redness; 2, one or two lesions; 3, three to five lesions; 4, more than five lesions, coalescence of lesions, or deep stromal involvement). The frequencies of recurrence were graphed as cumulative recurrences per guinea pig from days 15 to 60 postinfection (p.i.). At various time points (days 1, 2, 3, 4, 7, 10, 14, 30, and 60), guinea pigs were sacrificed, and SCG, CG, and TG were collected into RLT buffer (Qiagen), homogenized, and frozen until further processed for quantitative PCR or reverse transcription (RT)-PCR. Experiments were performed twice, and data from the two experiments were combined. Acute severity was statistically analyzed by Mann-Whitney test (SPSS) using the area under the curve per guinea pig. Cumulative recurrences were statistically analyzed by Mann-Whitney test (SPSS) using cumulative recurrences per individual animal. All studies were approved by and conducted in accordance with the Virginia Tech Institutional Care and Use Committee (IACUC# 13-008-CVM).
Quantitation of HSV load and gene expression.
Viral DNA and RNA were extracted from homogenized tissues with the Qiagen AllPrep DNA/RNA minikit (Qiagen) according to the instructions of the manufacturer. After RNA extraction, cDNA was synthesized using the iScript cDNA Synthesis kit (Bio-Rad). Quantitative PCR was performed on a Viia7 real-time PCR machine (Applied Biosystems), using the iTaq universal probe mix (Bio-Rad) and ZEN primer/probe sets (IDT) specific for genes encoding HSV-1 or HSV-2 thymidine kinase (TK) and latency-associated transcript (LAT) and immediate early (IE) genes encoding ICP0 (infected cell protein 0), ICP27, and ICP4, as follows: HSV-1 TK forward, 5′-AAAACCACCACCACGCAACT-3′, reverse, 5′-TCATCGGCTCGGGTACGTA-3′, and probe, 5′-TGGGTTCGCGCGACGATATCG-3′; HSV-1 LAT forward, 5′-ACCCACGTACTCCAAGAAGGC-3′, reverse, 5′-TAAGACCCAAGCATAGAGAGCCA-3′, and probe, 5′-TCCCACCCCGCCTGTGTTTTTGT-3′; HSV-1 ICP0 forward, 5′-GATGCAATTGCGCAACAC-3′, reverse, 5′-GCGTCACGCCCACTATCAG-3′, and probe, 5′-GCTGTGCAACGCCAAGCTGGTGTA-3′; HSV-1 ICP27 forward, 5′-GCGGCTGTGCTGGATAA-3′, reverse, 5′-GCGAACACAGTTCGTCCA-3′, and probe, 5′-TTTCTCCAGTGCTACCTGAAGGCGCGA-3′; HSV-1 ICP4 forward, 5′-CATGGCGTAGCCCAGGT-3′, reverse, 5′-GGCCTGCTTCCGGATCT-3′, and probe, 5′-CCGGTGATGAAGGAGCTGCTGTT-3′; HSV-2 TK forward, 5′-TAATGACCAGCGCCCAGAT-3′, reverse, 5′-CGATATGAGGAGCCAAAACG-3′, and probe, 5′-ACAATGAGCACGCCTTATGCGGC-3′; HSV-2 LAT forward, 5′-GTCAACACGGACACACTCTTTTT-3′, reverse, 5′-CGAGGCCTGTTGGTCTTTATC-3′, and probe, 5′-CACCCACCAAGACAGGGAGCCA-3′; HSV-2 ICP0 forward, 5′-GGTCACGCCCACTATCAGGTA-3′, reverse, 5′-CCTGCACCCCTTCTGCAT-3′, and probe, 5′-CAACGGAATCCAGGTCTTCATGCACG-3′; HSV-2 ICP27 forward, 5′-CTTTCTGCAGTGCTACCTGAA-3′, reverse, 5′-CAGGATGACCAACACAAAGGA-3′, and probe, 5′-CGACGCCTGTCGGACATTAAGGAT-3′; and HSV-2 ICP4 forward, 5′-GTCGTCGTCGTCGTCAG-3′, reverse, 5′-CCGCCTCTGACTCATCAAA-3′, and probe, 5′-ATGCAGACGAGGAGGAGGAG-3′. Viral DNA load was determined by quantifying viral DNA by quantitative PCR (qPCR) using HSV-1 and HSV-2 TK gene-specific primers and probes (13, 14). All assays were normalized to 18s rRNA (Applied Biosystems) and reported as quantity in 200 ng of DNA or RNA.
In vitro infection.
Trigeminal ganglia (TG), superior cervical ganglia (SCG), and ciliary ganglia (CG) were removed from 6-week-old Swiss Webster mice and cultured on Matrigel-coated 8-well Lab-Tek II chamber slides (Thermo Scientific), as previously described (3). Briefly, ganglia were digested in papain, collagenase, and dispase (Worthington), followed by mechanical trituration with a pipette. TG were passed through an OptiPrep (BD Biosciences) gradient to enrich for neurons; SCG and CG were plated without the gradient step, since they contain minimal axonal debris in the cell suspension. Cells were washed and plated in Neurobasal A medium supplemented with 2% B27, 1% penicillin-streptomycin, l-glutamine, neurotrophic factors, and mitotic inhibitors (Life Technologies). Four days after 3,000 neurons per well were plated, medium was removed, neurons were inoculated with HSV-1 (strain 17+) or HSV-2 (strain 333), viruses were allowed to adsorb for 1 h, and complete Neuro medium (Neurobasal A, B27, l-glutamine, and neurotrophic factors, with no mitotic inhibitors) was added. Neurons were fixed with 2% paraformaldehyde and immunostained for HSV antigens with polyclonal antisera (Dako). Neurons were counted to determine the percentage of HSV-positive neurons. Infections were repeated in duplicate: TG (15 cultures), SCG (4 cultures), CG (2 cultures).
Ex vivo reactivation.
Six-week-old Swiss Webster mice (Harlan) were ocularly infected with HSV-1 VP26-GFP or HSV-2 VP26-GFP (where GFP is green fluorescent protein). Mice were euthanized 21 days postinoculation, and TG, SCG, and CG were collected into Neurobasal A medium supplemented with B27 and penicillin-streptomycin. Ganglia were dissociated as described above and plated onto 24-well plates coated with Matrigel (BD Biosciences). Human immunoglobulin (hIgG) was included in the medium to prevent viral spread from infectious virus released into the medium from reactivating neurons. Reactivation was determined by daily observation of GFP expression in neurons for the first 3 days after plating. Locations of reactivating neurons, as detected by GFP expression, were carefully recorded, and superimposed signals were excluded on subsequent days to ensure that reactivating neurons were counted only once. Data represent the mean results from three separate infection experiments, using 10 mice per virus for each experiment. All studies were approved by and conducted in accordance with the Virginia Tech Institutional Care and Use Committee (IACUC# 13-003-CVM).
RESULTS
Acute disease and cumulative recurrences in the ocular guinea pig model.
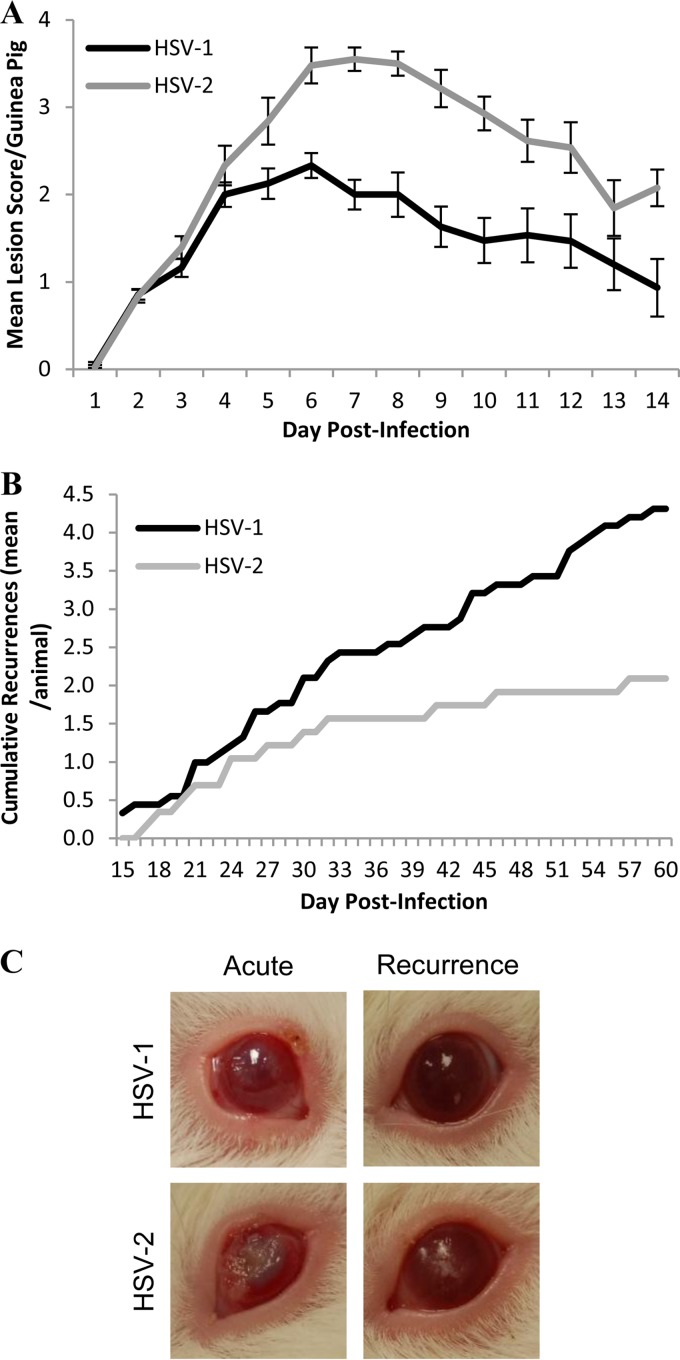
To characterize HSV-1 and HSV-2 ocular disease in a guinea pig model, female guinea pigs were inoculated by topical application of virus (5 × 105 PFU of virus) and observed for 60 days for clinical signs. During the acute phase of infection (1 to 14 dpi), HSV-2 produced significantly more severe ocular disease than did HSV-1 infection (Fig. 1A) (P = 0.002 by Mann-Whitney test). Both viruses produced corneal and periorbital lesions, corneal clouding, conjunctivitis, and blepharitis (Fig. 1C). However, HSV-2 produced deep stromal ulcerations in 18 of 42 guinea pigs (42.9%), of which 12 were bilateral, while similar deep lesions were observed in only 1 of 40 (2.5%) guinea pigs infected with HSV-1. Between days 5 and 9 p.i., head tilting, rotational head movement, and postural instability consistent with vertigo were observed in 13 guinea pigs infected with HSV-2; these symptoms resolved in 2 to 4 days, and similar signs were not observed in HSV-1-infected guinea pigs.
FIG 1.
Acute severity and cumulative recurrences in the guinea pig ocular model. (A) Severity of acute infection from 1 to 14 dpi, graphed as the mean lesion score for each group of guinea pigs on each day of observation, based on a scale of 0 to 4 (0, no symptoms; 1, inflammation or redness; 2, 1 or 2 lesions; 3, 3 to 5 lesions; 4, >5 lesions or coalescence of lesions); HSV-1, n = 40; HSV-2, n = 42; P = 0.002 by Mann-Whitney test. (B) Cumulative recurrences per guinea pig for each group during latent infection from 15 to 60 dpi; HSV-1, n = 11; HSV-2, n = 9; P = 0.020 by Mann-Whitney test. (C) Representative images of HSV-1 and HSV-2 acute (day 7) and recurrent (HSV-1, day 22; HSV-2, day 39) ocular disease.
HSV-1 produced recurrent corneal and periocular lesions at a significantly higher frequency from days 15 to 60 p.i. than did HSV-2 (Fig. 1B) (P = 0.020 by Mann-Whitney test). HSV-1 produced asymptomatic latent infection with defined episodes of symptomatic recurrences, consisting of 1 or 2 corneal and/or periocular lesions that cleared in 2 to 4 days (Fig. 1C). However, HSV-2 produced a more persistent form of symptomatic disease, characterized by continuous eruption of lesions and corneal clouding over a period of 5 to 18 days with minimal clearance between episodes. In addition, all nine of the HSV-2-infected guinea pigs observed throughout the 60-day period developed vesicular lesions on the nose, while none of the animals infected with HSV-1 developed nose lesions, suggesting a more extensive zosteriform spread with HSV-2 than HSV-1.
Viral DNA in sensory and autonomic ganglia.
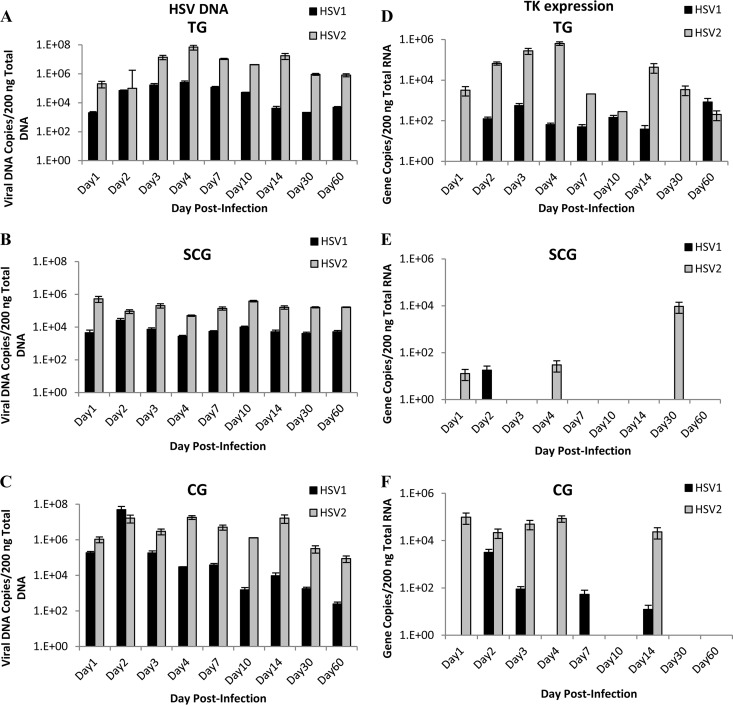
To determine if differences in viral DNA load were responsible for differences in lesion severity and recurrence frequency, viral DNA levels were evaluated at various time points in sensory and autonomic ganglia, including the sensory trigeminal ganglia (TG), sympathetic superior cervical ganglia (SCG), and parasympathetic ciliary ganglia (CG). Both HSV-1 and HSV-2 efficiently infected sensory neurons in the TG after ocular infection, as expected (Fig. 2A). Viral DNA increased during the first 4 days of infection, correlating with increasing clinical severity of the infections. By day 14 p.i., a latent viral DNA reservoir was established, and it was maintained throughout the 60-day experiment (Fig. 2A). Although the quantity of HSV-1 DNA was consistently lower than the quantity of HSV-2 (P = 0.0001), the viruses produced nearly identical patterns within the TG, reaching a peak on day 4 p.i. and decreasing thereafter.
FIG 2.
Viral DNA quantities and thymidine kinase gene expression of HSV-1 and HSV-2 in sensory and autonomic ganglia of guinea pigs. HSV-1 and HSV-2 viral DNA extracted from ganglia was quantified by qPCR in sensory trigeminal ganglia (TG) (A), sympathetic superior cervical ganglia (SCG) (B), and parasympathetic ciliary ganglia (CG) (C). HSV-1 and HSV-2 viral gene thymidine kinase (TK) copy number was quantified by qRT-PCR in sensory trigeminal ganglia (D), sympathetic superior cervical ganglia (E), and parasympathetic ciliary ganglia (F). (n = 2 to 4 samples per group per time point.)
In the sympathetic SCG, HSV-1 viral DNA increased transiently on day 2 postinfection and then maintained a static quantity of viral DNA in the ganglia, suggesting that the virus replicated briefly within the ganglia early after infection and then established a latent reservoir in the SCG, which remained stable throughout the 60-day observation period (Fig. 2B). HSV-2 viral DNA showed minor variability between time points but remained relatively stable throughout the infection period. While there was a significant difference in the quantities of viral DNA detected in the SCG (P = 0.0001), the overall patterns were similar, with both viruses maintaining a similar quantity of viral DNA at all time points.
In the parasympathetic CG, viral DNA was detected 1 day after inoculation and both HSV-1 and HSV-2 viral DNA increased on day 2 p.i. However, HSV-2 DNA remained elevated from day 3 through day 14 p.i., while HSV-1 DNA began decreasing on day 3 p.i. (P = 0.0001) (Fig. 2C).
A significantly greater quantity of HSV-2 viral DNA was detected in all three sensory and autonomic ganglia analyzed, implying that the viral load could have been responsible for the difference in severity of disease but not for the difference in recurrence frequency.
Thymidine kinase expression in sensory and autonomic ganglia.
HSV encodes the enzyme TK, which is important for HSV replication in neurons (15). Expression of TK was analyzed to verify active viral replication in the ganglia, as opposed to just the presence of viral DNA.
During acute infection (1 to 14 dpi), HSV-2 expressed higher levels of TK in TG, SCG, and CG than did HSV-1, correlating with the more severe acute disease symptoms observed in the guinea pigs. In the sensory TG, HSV-1 and HSV-2 expressed relatively similar patterns of TK expression (Fig. 2D), although HSV-2 TK expression was generally higher than HSV-1 expression (P = 0.0001). In the sympathetic SCG, however, TK expression was detected from HSV-2-infected animals only on days 1 and 4 p.i. and from HSV-1-infected animals on day 2 (Fig. 2E). Considering the quantity of viral DNA detected in the SCG, these results indicate that adult sympathetic SCG limit both HSV-1 and HSV-2 replication during acute infection of guinea pigs after ocular infection. In the parasympathetic CG, HSV-2 TK expression was sustained at high levels for the first 4 days after infection (Fig. 2F), which was significantly different from HSV-1 TK expression during the same time period (P = 0.025). HSV-1 TK expression was detected at a high level on day 2 and at decreased levels on days 3 and 7. Combined with the detected DNA quantities, these patterns of TK expression suggest that sensory TG and parasympathetic CG support both HSV-1 and HSV-2 replication, resulting in large quantities of viral DNA in the ganglia at latent time points. However, CG preferentially support HSV-2 replication rather than HSV-1 during the first 4 days of acute infection. Even though large quantities of HSV-1 and HSV-2 viral DNA are present in the SCG, neither virus replicates efficiently in the SCG after ocular infection.
During latent time periods, HSV-2 TK expression was detected in TG, SCG, and CG (Fig. 2D, E, and F), coincident with observed recurrent lesions. On day 14, TK expression was detected in 2 of 4 TG; one of these guinea pigs concurrently expressed TK in the CG. On day 30, TK was detected in a single guinea pig in both TG and SCG. On day 60, TK was detected in a single guinea pig in the TG only. HSV-2-infected guinea pigs that had lesions at the time of tissue analysis expressed TK in just TG or in both TG and autonomic ganglia; thus, the recurrent lesions could have originated from replicating virus in either the TG or the autonomic ganglia. HSV-1 TK was detected in TG and CG, but not SCG during latent time points. On day 14, one guinea pig with no lesions expressed TK in the TG only, demonstrating that HSV-1 can replicate in the TG without producing peripheral lesions. Another guinea pig with lesions on day 14 p.i. expressed TK in CG only, demonstrating that HSV-1 can reactivate from the CG independently of the TG to cause recurrent ocular lesions. HSV-1 TK expression was also detected on day 60 in the TG of a single animal, which had lesions but no detectable TK expression in other ganglia. Thus, HSV-1 can reactivate from either TG or CG to produce recurrent ocular lesions.
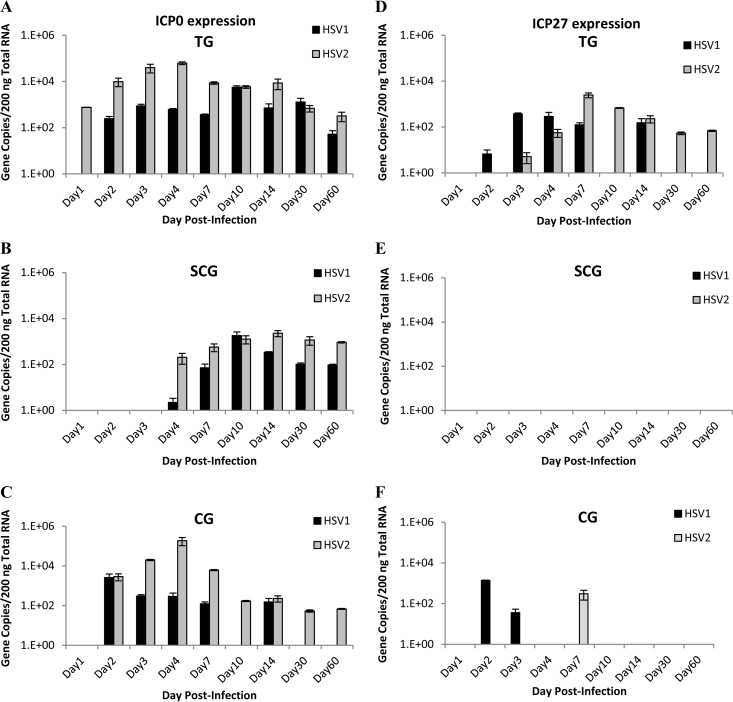
IE gene expression in sensory and autonomic ganglia.
HSV produces immediate early (IE) genes, which manipulate the host cell replication and antiviral mechanisms to promote viral early (E) and late (L) gene expression. Infected cell protein 0 (ICP0) is a ubiquitin ligase involved in both lytic and latent infections and has been implicated in reactivation from latency (16, 17). ICP0 expression was detected in guinea pig TG very early after infection, on day 1 p.i. for HSV-2 and day 2 p.i. for HSV-1, but there was no significant difference in expression profiles (Fig. 3A). In sympathetic SCG, ICP0 expression was delayed compared to expression in TG, but the expression profiles for HSV-1 and HSV-2 were similar (Fig. 3B). Expression profiles differed in CG, however; while both HSV-1 and HSV-2 ICP0 transcripts were detected on day 2 p.i., HSV-2 continued to express ICP0 at all time points but HSV-1 expressed ICP0 at detectable levels during acute infection only, and at lower levels than HSV-2 (Fig. 3C). These results suggest that the functions of ICP0 may differ between HSV-1 and HSV-2 in parasympathetic neurons.
FIG 3.
Immediate early gene expression in sensory and autonomic ganglia of guinea pigs. HSV-1 and HSV-2 viral immediate early (IE) ICP0 gene expression was quantified by qRT-PCR in sensory trigeminal ganglia (A), sympathetic superior cervical ganglia (B), and parasympathetic ciliary ganglia (C). HSV-1 and HSV-2 viral IE ICP27 gene expression was quantified by qRT-PCR in sensory trigeminal ganglia (D), sympathetic superior cervical ganglia (E), and parasympathetic ciliary ganglia (F). (n = 2 to 4 samples per group per time point.)
Another viral IE gene encodes a multifunctional protein, ICP27, that contributes to host cell shutoff, downregulates the interferon response, and promotes viral transcription and translation (18). In guinea pig TG, HSV-1 and HSV-2 both expressed ICP27 during acute infection (days 1 to 14 p.i.), but expression was also detected in HSV-2-infected ganglia analyzed at latent time points, suggestive of an ongoing persistent infection (Fig. 3D). No ICP27 was detected in SCG from either virus (Fig. 3E), and expression was sporadically detected in CG (Fig. 3F), suggesting that ICP27 expression is not required for lytic infection in autonomic neurons.
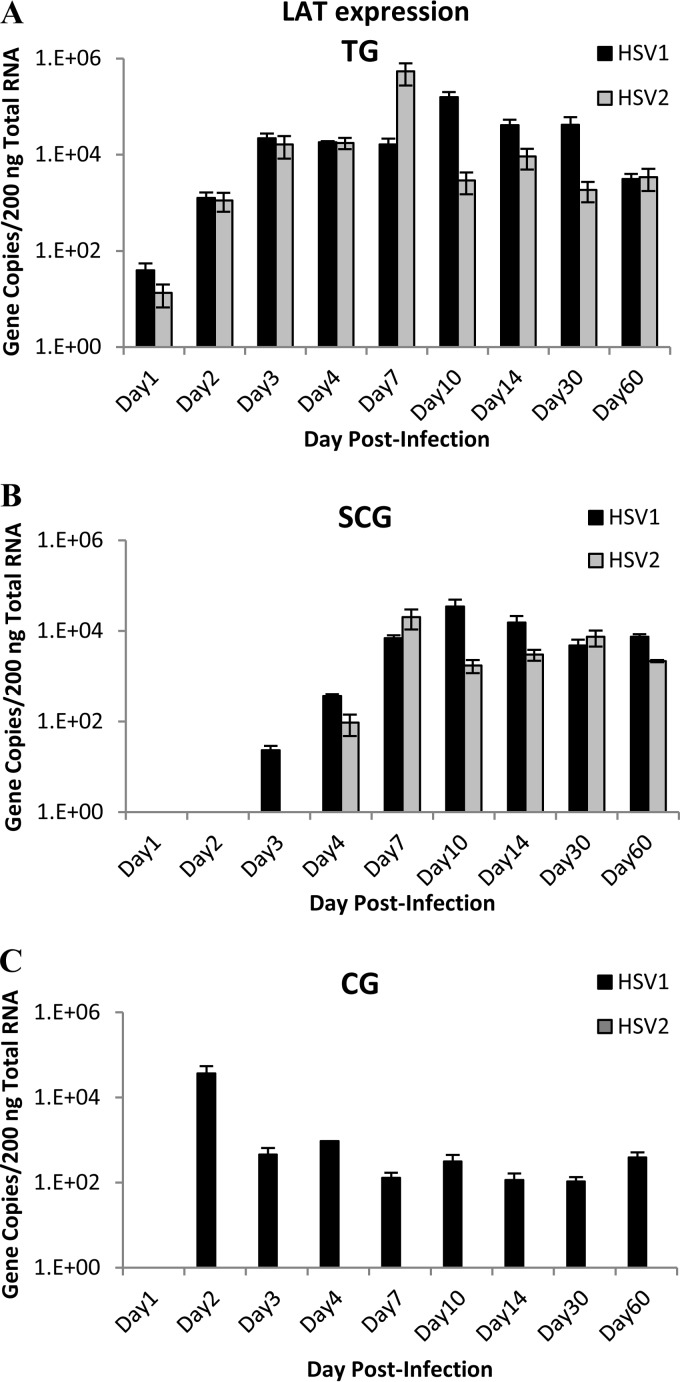
LAT expression in sensory and autonomic ganglia.
The latency-associated transcript (LAT) is the most abundant gene expressed during HSV latency. While not required for establishment of latency, LAT exon 1 is necessary for HSV type-specific neuron specificity and characteristic patterns of reactivation (4, 13, 19). To determine if differences in LAT expression play a role in differences between HSV-1 and HSV-2 recurrence frequency, LAT expression was analyzed in the sensory and autonomic ganglia.
In the TG, LAT expression was detected on day 1 p.i. and increased thereafter, as expected, demonstrating the establishment of latent infection in the TG (Fig. 4A). A similar pattern of LAT expression was detected in the sympathetic SCG, although LAT expression was first detected later after infection on day 3 for HSV-1 and day 4 for HSV-2 (Fig. 4B). The sustained presence of viral DNA and LAT expression in the SCG demonstrates that both HSV-1 and HSV-2 established latency in sympathetic ganglia as effectively as in sensory TG, and there were no significant differences between HSV-1 and HSV-2 LAT expression in the TG or SCG (P = 0.231 and 0.200, respectively).
FIG 4.
LAT gene expression in sensory and autonomic ganglia of guinea pigs. HSV-1 and HSV-2 viral gene LAT copy number was quantified by qRT-PCR in sensory trigeminal ganglia (A), sympathetic superior cervical ganglia (B), and parasympathetic ciliary ganglia (C). (n = 2 or 3 samples per group per time point.)
A different pattern of LAT expression was identified in parasympathetic CG (Fig. 4C). HSV-1 LAT expression was detected at a high level on day 2 p.i. and persisted throughout the 60-day period of analyses. Since both viral DNA and LAT expression persisted, these data show that HSV-1 established a LAT-positive latent infection in the guinea pig CG after ocular infection. However, HSV-2 LAT expression was not detected in the CG at any time point (P = 0.0001), suggesting either that HSV-2 is not capable of reactivation from ciliary ganglion neurons or that LAT is not required for reactivation from ciliary ganglia.
Viral infection and reactivation in primary cultured neurons.
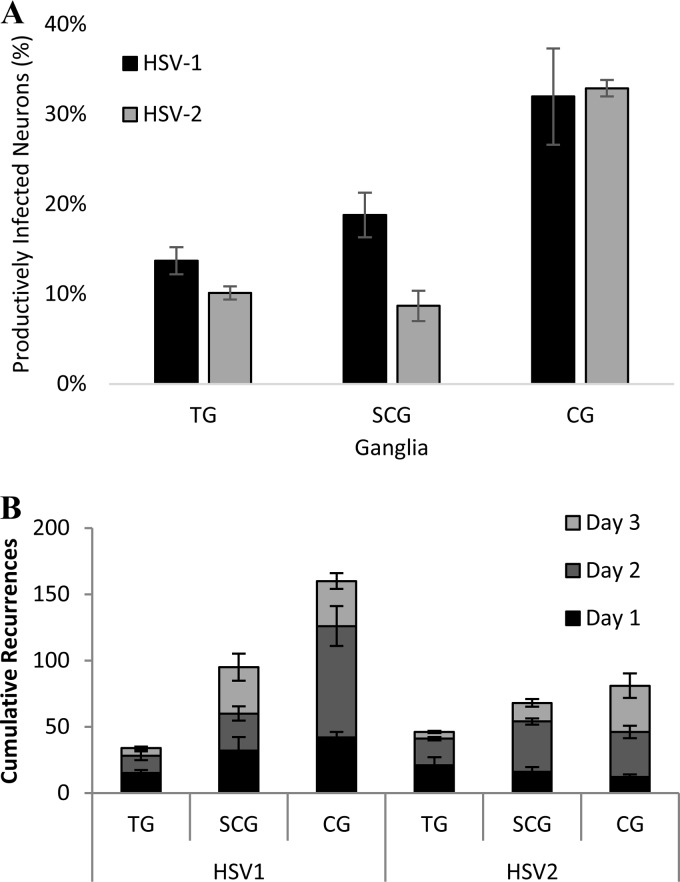
Animal models of HSV infection are necessary to identify differences in severity of disease and recurrence frequencies of HSV-1 and HSV-2. In an animal model, however, hormone levels and the immune response are involved in regulating disease severity and also contribute to viral reactivation to either limit or promote symptomatic recurrences. To identify any differences in the abilities of HSV-1 and HSV-2 to productively infect sensory and autonomic neurons without the influence of the adaptive immune response or exogenous hormone induction, cultured primary adult murine ganglionic neurons from TG, SCG, and CG were infected with HSV-1 or HSV-2 at a multiplicity of infection (MOI) of 10. Nine hours after infection, the percentages of cultured neurons that were productively infected with HSV-1 or HSV-2 were determined, using polyclonal antisera to visualize HSV productive cycle antigens. HSV-1 and HSV-2 were detected in similar percentages of TG and CG cultured neurons (Fig. 5A). In cultured SCG, however, HSV-1 productively infected a significantly greater percentage of cultured neurons (18.8%) than did HSV-2 (8.7%). In the guinea pigs, neither HSV-1 nor HSV-2 productively infected SCG efficiently, suggesting that extracellular factors limit HSV-1 productive infection of SCG neurons in vivo in the guinea pig model. Additional studies are required to determine whether these extracellular factors are immune system- or hormone-related factors or a host-specific restriction mechanism.
FIG 5.
HSV-1 and HSV-2 in cultured murine primary neurons. (A) Percentage of cultured primary adult murine neurons productively infected in vitro with HSV-1 or HSV-2 at an MOI of 10 for 10 h, immunostained for HSV antigens with polyclonal antisera, in trigeminal ganglia (TG, P = 0.045, n = 30 cultures/virus), superior cervical ganglia (SCG, P = 0.015, n = 8 cultures/virus), and ciliary ganglia (CG, P = 0.880, n = 4 cultures/virus). (B) Cumulative number of reactivating neurons over 3 days ex vivo from ganglia harvested and cultured from adult mice latently infected with GFP-expressing HSV-1 or HSV-2. The graph represents three separate experiments, including 10 mice/group for each experiment.
The guinea pig ocular infection studies demonstrated that HSV-1 could reactivate from CG independently from TG to cause symptomatic recurrences. However, the guinea pig studies did not provide evidence that HSV-2 recurrences could be caused by viral reactivation in autonomic ganglia independently from TG. To determine if HSV-1 and HSV-2 could reactivate from individual autonomic neurons, mice were infected with HSV-1 or HSV-2 viruses that express a VP26-GFP fusion protein during replication. VP26, expressed by a late gene, is a small capsid protein that decorates the outer surface of the mature capsids, bound to VP5 (20–22); previous studies have demonstrated that the HSV VP26-GFP reporter viruses effectively represent productive infection (3, 5). Twenty-one days postinfection, when the viruses had established latency, TG, SCG, and CG were removed from infected mice, cultured, and observed for viral reactivation, visualized by expression of GFP in individual neurons. Cumulatively over 3 days after culture, HSV-1 and HSV-2 both reactivated in a greater number of autonomic neurons than of TG neurons (Fig. 5B), demonstrating that autonomic neurons effectively support viral reactivation. HSV-1 also reactivated much more efficiently from CG neurons, with an average of 160 cumulative reactivations, than did HSV-2, with an average of 61 cumulative reactivations in individual neurons, demonstrating the viral type selectivity of HSV reactivation.
DISCUSSION
Although HSV-1 and HSV-2 infect the same tissues and produce lesions with similar characteristics, there are significant differences in recurrent disease patterns between HSV-1 and HSV-2. HSV-1 is most often associated with recurrent orolabial lesions and keratitis, while HSV-2 is more commonly associated with recurrent genital lesions. Ocular infections caused by herpesviruses present a serious clinical problem throughout the world, and an estimated 500,000 people have recurrent ocular HSV infections in the United States alone. While both HSV-1 and HSV-2 can cause ocular disease, HSV-1 is far more likely to spread to the eyes and cause recurrent disease, causing herpes simplex keratitis characterized by dendritic lesions and inflammation of the cornea, eventually leading to irreversible blindness. While HSV-1 orolabial lesions and herpes keratitis are commonly seen in healthy adults, reports of recurrent HSV-2 oral or ocular disease are rare and typically associated with immunocompromised status (23, 24). HSV-2 ocular disease most often manifests as acute retinal necrosis, rather than the recurrent keratitis characteristic of HSV-1 (24, 25). While the incidence of HSV-1 genital disease is increasing due to changes in sexual behavior, HSV-2 is more likely to cause recurrent genital lesions, and the anatomical specificity of recurrent disease is not simply due to the site of infection; 60 to 90% of individuals with genital HSV-2 experience recurrences, while only about 25% of people with genital HSV-1 develop recurrent lesions (1, 2). It is not clear why these similar viruses reactivate preferentially in an anatomical site-specific manner to cause different patterns of recurrent disease.
In our guinea pig model, HSV-1 produced acute disease symptoms, which cleared in most animals by day 14, and then produced periodic recurrences in the form of corneal and/or periocular lesions. The corneas became hazy during these episodic recurrences, suggestive of inflammation. Thus, guinea pig ocular disease caused by HSV-1 was clinically similar to human disease, which is characterized by recurrent corneal and/or periocular lesions along with inflammation and corneal clouding. After HSV-2 ocular infection, the guinea pigs experienced a more persistent form of symptomatic infection with deep stromal involvement, rather than defined episodic recurrences. The rare occurrences of human HSV-2 ocular disease typically take the form of persistent retinal necrosis. Although we did not evaluate the retinas of our guinea pigs, the persistent, necrotic disease characteristics observed in our guinea pigs were consistent with human HSV-2 ocular disease. HSV-1 reactivated in the guinea pigs spontaneously to cause episodic symptomatic recurrences, while HSV-2 rarely reactivated after day 30 p.i., also consistent with recurrent disease frequencies in humans. Therefore, the guinea pig ocular model is a valuable model for investigating differences between HSV-1 and HSV-2 ocular disease.
Previous studies have demonstrated that HSV-1 and HSV-2 viral DNA is regularly found in the autonomic ganglia of humans and also in animal models after ocular or genital infection (7, 10, 12, 26–32). Although many investigators have reported findings related to HSV activity in the sympathetic and parasympathetic autonomic ganglia, it is still not clear whether virus in the autonomic ganglia contributes to the pathogenesis of herpetic disease, either for severity of acute disease symptoms or for recurrent disease episodes. Since autonomic innervation and response patterns differ significantly between the face and the genitalia, it is highly likely that differences in viral behavior within autonomic ganglia account for the anatomical differences in HSV-1 and HSV-2 recurrent disease.
Our results in the guinea pig model demonstrate that HSV-1 and HSV-2 infected and established latency in autonomic and sensory ganglia after ocular infection. HSV-1 and HSV-2 behaved similarly in sensory trigeminal ganglia after ocular infection, with respect to patterns of viral DNA loads and gene expression throughout the time of analyses. However, the viruses behaved very differently in autonomic ganglia, which likely contributed to differences in the pathogenesis of acute disease as well as differences in reactivation. Both viruses established latency in sympathetic SCG, with comparable viral DNA loads and expression of LAT. However, HSV-1 did not express TK in the SCG except in a single animal on day 2 p.i., and HSV-2 TK expression was sporadic in only a few animals. In addition, neither HSV-1 nor HSV-2 expressed ICP27 in the SCG. Thus, neither virus replicated efficiently in adult SCG after ocular inoculation. Although some viral replication of HSV-2 did occur in the sympathetic ganglia of three guinea pigs during symptomatic recurrent disease, there was no evidence that the recurrence originated in the SCG, since virus was simultaneously replicating in the TG. HSV-1 was, however, capable of productively infecting cultured SCG neurons in vitro, suggesting that extracellular factors may act on the SCG neurons to inhibit HSV-1 viral replication in vivo in the guinea pig model. Additional studies are needed to determine whether those factors are immunological or hormonal in nature or whether they represent a species-specific restriction.
In the ciliary ganglia, both HSV-1 and HSV-2 replicated during acute infection, shown by increases in viral DNA and expression of TK and ICP0. In several animals, HSV-1 and HSV-2 TK levels were higher in the CG than in the TG, particularly for HSV-2, suggesting that virus replicating in the CG during acute infection was contributing to acute disease symptoms. However, HSV-2 failed to express LAT in the CG at latent time points, suggesting either that HSV-2 was incapable of reactivating from the CG or that LAT is not necessary for HSV-2 reactivation from the CG. During HSV-2 recurrences, TK expression was detected in TG and CG simultaneously in a single animal, providing no evidence that recurrences originated from the CG since virus was also replicating in the TG. In contrast, HSV-1 expressed LAT in the CG throughout the 60-day period of analyses. At latent time points, guinea pigs displaying recurrent HSV-1 lesions expressed TK in either TG or CG, but never both, demonstrating that HSV-1 symptomatic recurrences could originate from either the TG or the CG. To develop more effective antivirals that can inhibit reactivation and prevent recurrent herpetic disease and viral transmission to new hosts, it is imperative to fully understand the processes involved in viral reactivation from neurons. HSV-1 and HSV-2 demonstrate preferences for productively infecting and establishing latency in specific types of neurons, and not only sensory neurons but autonomic neurons as well. Different populations of neurons are dependent on, and responsive to, a broad range of neurotrophic factors and hormones, depending on the repertoire of receptors and host factors expressed. Physiological stimuli that are known to reactivate HSV-1 and/or HSV-2, in vivo or in vitro, have a greater effect on activation and signaling cascades in autonomic neurons than in sensory neurons, and autonomic neurons harbor latent virus. Our studies demonstrate that HSV-1 is capable of reactivation from autonomic ciliary ganglia, independently from sensory trigeminal ganglia, to cause recurrent lesions after ocular infection, while actively replicating HSV-2 was not detected in CG independent of TG. Although additional studies are necessary, the ability of HSV-1, but not HSV-2, to independently reactivate from ciliary ganglia to cause recurrent disease may explain the greater orofacial recurrence frequency of HSV-1 than of HSV-2.
ACKNOWLEDGMENTS
This work was supported by research grant K22AI097299 from the National Institute of Allergy and Infectious Diseases.
We thank Kathleen Apakupakul for technical assistance and Leita Estes for critical reviews of the manuscript.
We declare that we have no competing financial interest in the research presented.
REFERENCES
- 1.Lafferty WE, Coombs RW, Benedetti J, Critchlow C, Corey L. 1987. Recurrences after oral and genital herpes simplex virus infection. Influence of site of infection and viral type. N Engl J Med 316:1444–1449. [DOI] [PubMed] [Google Scholar]
- 2.Reeves WC, Corey L, Adams HG, Vontver LA, Holmes KK. 1981. Risk of recurrence after first episodes of genital herpes. Relation to HSV type and antibody response. N Engl J Med 305:315–319. [DOI] [PubMed] [Google Scholar]
- 3.Bertke AS, Swanson SM, Chen J, Imai Y, Kinchington PR, Margolis TP. 2011. A5-positive primary sensory neurons are nonpermissive for productive infection with herpes simplex virus 1 in vitro. J Virol 85:6669–6677. doi: 10.1128/JVI.00204-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Bertke AS, Ma A, Margolis MS, Margolis TP. 2013. Different mechanisms regulate productive herpes simplex virus 1 (HSV-1) and HSV-2 infections in adult trigeminal neurons. J Virol 87:6512–6516. doi: 10.1128/JVI.00383-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Bertke AS, Apakupakul K, Ma A, Imai Y, Gussow AM, Wang K, Cohen JI, Bloom DC, Margolis TP. 2012. LAT region factors mediating differential neuronal tropism of HSV-1 and HSV-2 do not act in trans. PLoS One 7:e53281. doi: 10.1371/journal.pone.0053281. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Dartt DA, McCarthy DM, Mercer HJ, Kessler TL, Chung EH, Zieske JD. 1995. Localization of nerves adjacent to goblet cells in rat conjunctiva. Curr Eye Res 14:993–1000. doi: 10.3109/02713689508998520. [DOI] [PubMed] [Google Scholar]
- 7.Richter ER, Dias JK, Gilbert JE II, Atherton SS. 2009. Distribution of herpes simplex virus type 1 and varicella zoster virus in ganglia of the human head and neck. J Infect Dis 200:1901–1906. doi: 10.1086/648474. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Bustos DE, Atherton SS. 2002. Detection of herpes simplex virus type 1 in human ciliary ganglia. Invest Ophthalmol Vis Sci 43:2244–2249. [PubMed] [Google Scholar]
- 9.Parr MB, Parr EL. 2003. Intravaginal administration of herpes simplex virus type 2 to mice leads to infection of several neural and extraneural sites. J Neurovirol 9:594–602. [DOI] [PubMed] [Google Scholar]
- 10.Sanjuan NA, Lascano EF. 1986. Autonomic nervous system involvement in experimental genital infection by herpes simplex virus type 2. Arch Virol 91:329–339. doi: 10.1007/BF01314291. [DOI] [PubMed] [Google Scholar]
- 11.Shimeld C, Tullo AB, Hill TJ, Blyth WA, Easty DL. 1985. Spread of herpes simplex virus and distribution of latent infection after intraocular infection of the mouse. Arch Virol 85:175–187. doi: 10.1007/BF01314229. [DOI] [PubMed] [Google Scholar]
- 12.Martin JR, Jenkins FJ, Henken DB. 1991. Targets of herpes simplex virus type 1 infection in a mouse corneal model. Acta Neuropathol 82:353–363. doi: 10.1007/BF00296546. [DOI] [PubMed] [Google Scholar]
- 13.Bertke AS, Patel A, Imai Y, Apakupakul K, Margolis TP, Krause PR. 2009. Latency-associated transcript (LAT) exon 1 controls herpes simplex virus species-specific phenotypes: reactivation in the guinea pig genital model and neuron subtype-specific latent expression of LAT. J Virol 83:10007–10015. doi: 10.1128/JVI.00559-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Bertke AS, Patel A, Krause PR. 2007. Herpes simplex virus latency-associated transcript sequence downstream of the promoter influences type-specific reactivation and viral neurotropism. J Virol 81:6605–6613. doi: 10.1128/JVI.02701-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Tenser RB, Ressel S, Dunstan ME. 1981. Herpes simplex virus thymidine kinase expression in trigeminal ganglion infection: correlation of enzyme activity with ganglion virus titer and evidence of in vivo complementation. Virology 112:328–341. doi: 10.1016/0042-6822(81)90638-3. [DOI] [PubMed] [Google Scholar]
- 16.Hagglund R, Roizman B. 2004. Role of ICP0 in the strategy of conquest of the host cell by herpes simplex virus 1. J Virol 78:2169–2178. doi: 10.1128/JVI.78.5.2169-2178.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Boutell C, Everett RD. 2013. Regulation of alphaherpesvirus infections by the ICP0 family of proteins. J Gen Virol 94:465–481. doi: 10.1099/vir.0.048900-0. [DOI] [PubMed] [Google Scholar]
- 18.Sandri-Goldin RM. 2011. The many roles of the highly interactive HSV protein ICP27, a key regulator of infection. Future Microbiol 6:1261–1277. doi: 10.2217/fmb.11.119. [DOI] [PubMed] [Google Scholar]
- 19.Bloom DC, Hill JM, Devi-Rao G, Wagner EK, Feldman LT, Stevens JG. 1996. A 348-base-pair region in the latency-associated transcript facilitates herpes simplex virus type 1 reactivation. J Virol 70:2449–2459. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Desai P, Person S. 1998. Incorporation of the green fluorescent protein into the herpes simplex virus type 1 capsid. J Virol 72:7563–7568. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Chi JH, Wilson DW. 2000. ATP-dependent localization of the herpes simplex virus capsid protein VP26 to sites of procapsid maturation. J Virol 74:1468–1476. doi: 10.1128/JVI.74.3.1468-1476.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Desai P, Akpa JC, Person S. 2003. Residues of VP26 of herpes simplex virus type 1 that are required for its interaction with capsids. J Virol 77:391–404. doi: 10.1128/JVI.77.1.391-404.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Sontakke SA, Umarji HR, Karjodkar F. 2011. Comparison of oral manifestations with CD4 count in HIV-infected patients. Indian J Dent Res 22:732. doi: 10.4103/0970-9290.93470. [DOI] [PubMed] [Google Scholar]
- 24.Cottet L, Kaiser L, Hirsch HH, Baglivo E. 2009. HSV2 acute retinal necrosis: diagnosis and monitoring with quantitative polymerase chain reaction. Int Ophthalmol 29:199–201. doi: 10.1007/s10792-008-9198-2. [DOI] [PubMed] [Google Scholar]
- 25.Sugita S, Shimizu N, Watanabe K, Mizukami M, Morio T, Sugamoto Y, Mochizuki M. 2008. Use of multiplex PCR and real-time PCR to detect human herpes virus genome in ocular fluids of patients with uveitis. Br J Ophthalmol 92:928–932. doi: 10.1136/bjo.2007.133967. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Gilden DH, Gesser R, Smith J, Wellish M, Laguardia JJ, Cohrs RJ, Mahalingam R. 2001. Presence of VZV and HSV-1 DNA in human nodose and celiac ganglia. Virus Genes 23:145–147. doi: 10.1023/A:1011883919058. [DOI] [PubMed] [Google Scholar]
- 27.Sanjuan NA, Zimberlin MN. 2001. Pathogenesis of herpes simplex virus type 2 experimental genital infection in pregnant mice. FEMS Immunol Med Microbiol 30:197–202. doi: 10.1111/j.1574-695X.2001.tb01570.x. [DOI] [PubMed] [Google Scholar]
- 28.Labetoulle M, Kucera P, Ugolini G, Lafay F, Frau E, Offret H, Flamand A. 2000. Neuronal propagation of HSV1 from the oral mucosa to the eye. Invest Ophthalmol Vis Sci 41:2600–2606. [PubMed] [Google Scholar]
- 29.Labetoulle M, Kucera P, Ugolini G, Lafay F, Frau E, Offret H, Flamand A. 2000. Neuronal pathways for the propagation of herpes simplex virus type 1 from one retina to the other in a murine model. J Gen Virol 81:1201–1210. [DOI] [PubMed] [Google Scholar]
- 30.Wagner EK, Bloom DC. 1997. Experimental investigation of herpes simplex virus latency. Clin Microbiol Rev 10:419–443. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Rodahl E, Stevens JG. 1992. Differential accumulation of herpes simplex virus type 1 latency-associated transcripts in sensory and autonomic ganglia. Virology 189:385–388. doi: 10.1016/0042-6822(92)90721-Z. [DOI] [PubMed] [Google Scholar]
- 32.Martin JR, Suzuki S. 1989. Targets of infection in a herpes simplex-reactivation model. Acta Neuropathol 77:402–411. doi: 10.1007/BF00687375. [DOI] [PubMed] [Google Scholar]