ABSTRACT
A small number of African green monkeys (AGMs) were introduced into the Caribbean from West Africa in the 1600s. To determine the impact of this population bottleneck on the AGM virome, we used metagenomics to compare the viral nucleic acids in the plasma of 43 wild AGMs from West Africa (Gambia) to those in 44 AGMs from the Caribbean (St. Kitts and Nevis). Three viruses were detected in the blood of Gambian primates: simian immunodeficiency virus (SIVagm; in 42% of animals), a novel simian pegivirus (SPgVagm; in 7% of animals), and numerous novel simian anelloviruses (in 100% of animals). Only anelloviruses were detected in the Caribbean AGMs with a prevalence and levels of viral genetic diversity similar to those in the Gambian animals. A host population bottleneck therefore resulted in the exclusion of adult-acquired SIV and pegivirus from the Caribbean AGMs. The successful importation of AGM anelloviruses into the Caribbean may be the result of their early transmission to infants, very high prevalence in African AGMs, and frequent coinfections with as many as 11 distinct variants.
IMPORTANCE The extent to which viruses can persist in small isolated populations depends on multiple host, viral, and environmental factors. The absence of prior infections may put an immunologically naive population at risk for disease outbreaks. Isolated populations originating from a small number of founder individuals are therefore considered at increased risk following contact with populations with a greater variety of viruses. Here, we compared the plasma virome of West African green monkeys to that in their descendants after importation of a small number of animals to the Caribbean. A lentivirus and a pegivirus were found in the West African population but not in the Caribbean population. Highly diverse anelloviruses were found in both populations. A small founder population, limited to infants and young juvenile monkeys, may have eliminated the sexually transmitted viruses from the Caribbean AGMs, while anelloviruses, acquired at an earlier age, persisted through the host population bottleneck.
INTRODUCTION
Large host populations are required for ongoing transmission of viruses with short generation times, while persistent or chronic infections may persist in smaller populations (1, 2), as observed for human T-cell lymphotropic viruses (HTLVs) in long-isolated populations (3–5). Populations derived from a small group (which have undergone a genetic bottleneck) may experience virus elimination if none of the founders carried that virus. Long-isolated, small groups of human or animals or those founded by a small number of animals may therefore be highly susceptible to viral infections upon contacts with larger populations carrying a greater viral diversity. Reduced HLA diversity in small isolated populations may also increase the risk of pathogenic outbreaks after contact with larger, frequently virus-exposed, more HLA-diverse populations.
Members of the Chlorocebus genus of Old World monkeys are the most common and widely dispersed monkeys in Africa. One of four closely related Chlorocebus species is the African green monkey (AGM) (Chlorocebus sabaeus), which is largely restricted to West Africa (6). In the mid- to late 17th century, a small number of AGMs were imported from West Africa to the Caribbean, alongside the slaves used for the production of sugarcane (7–9). By 1682, the introduced AGMs had become feral and were declared to be vermin in Barbados (10). As a result of this population bottleneck, the Caribbean AGM population has been shown to be genetically highly homogenous (11, 12). The African origin of the Caribbean AGMs has been mapped to West Africa by mitochondrial DNA (9) and specifically to Senegal, Gambia, or Sierra Leone from historical records (10).
In Africa, these highly social, semiterrestrial primates show a high rate of simian immunodeficiency virus (SIV in AGM [SIVagm]) infection but without progression to simian AIDS (SAIDS), despite high levels of viremia (13–15). Infection in the wild is thought to result largely from sexual activity rather than vertical transmission from infected mothers, since adults show a much higher rate of SIV infection (67%) than infants (7%) (16). SIVagm strains in West African AGMs are also highly diverse genetically (17, 18). Related but distinct SIVs are found in the four Chlorocebus species (vervet, grivet, tantalus, and sabaeus) (19). Evidence for ancient recombination between SIVagm and the SIV in sooty mangabey (SIVsm)/human immunodeficiency virus type 2 (HIV-2) lineage has also been reported (20). The high prevalence and genetic diversity of SIVagm, the benign course of infection in its natural host, and the presence of related AGMs in other Chlorocebus monkey species point to the long-term presence of SIV in the African green monkey.
Here, we compare the viral sequences, including SIV, identified by deep sequencing of plasma from wild-caught AGMs from two Caribbean islands to those in AGMs from the Gambia in West Africa, close to where the slave ships departed for the Caribbean.
MATERIALS AND METHODS
Sample locations.
This study was conducted on African green monkeys (Chlorocebus sabaeus) (n = 87 total) living wild in various regions within Gambia on the African continent and on the islands of St. Kitts and Nevis, a two-island country located in the Leeward Islands, Lesser Antilles, Caribbean.
In Gambia, blood samples were collected from 43 animals from various locations both Downriver and Upriver. On St. Kitts island, 32 animals from diverse locations were sampled. Twelve animals were sampled from various sites on neighboring Nevis. The geographic coordinates of sampling sites, along with a satellite map of locations, are provided in Table S1 and Fig. S1 in the supplemental material. Mapping of sampling locations using the geographic coordinates was conducted with GPS Visualizer (http://www.gpsvisualizer.com).
Sample collection.
Plasma samples were collected between 13 January and 8 June 2011 (see Table S1 in the supplemental material). The ages of the animals were determined based on dental eruption patterns, as previously described (15), and included infants, young juveniles, juveniles, subadults, and adults, with nearly matched ages between the Gambian and Caribbean cohorts. The male-to-female ratios were 1:1.3 for Gambian and 1.9:1 for Caribbean monkeys.
Virus particle purification.
In order to reduce the background of nonviral sequences, plasma samples were subjected to virus particle enrichment. One hundred forty-microliter amounts of plasma were clarified by centrifugation at 10,000 × g for 10 min and filtered through 0.45-μm filters (Millipore, Billerica, MA, USA). The resulting 110-μl viral-particle-enriched filtrates were then incubated with 30 μl of a mixture containing 0.5 U Turbo DNase I (Ambion, Life Technologies, Grand Island, NY, USA), 0.25 U Baseline-Zero DNase I (Epicentre, Chicago, IL, USA), 0.5 U Benzonase nuclease (Novagen/Merck), and 0.25 U RNase A (Fermentas/Fisher Scientific) at 37°C for 1.5 h to reduce background nucleic acids from the host cells and bacteria (21). Nucleic acids protected from nuclease digestion were then extracted using a Maxwell 16 viral total nucleic acid purification kit (Promega, Madison, WI, USA) for robotic extraction according to the manufacturer's recommendations and eluted in 50-μl volumes of nuclease-free water.
Metagenomic sequencing.
In order to randomly amplify enriched viral nucleic acids for deep sequencing, the following method was used. Viral cDNA synthesis was performed from 5 μl of nucleic acid using a SuperScript III kit (Invitrogen, Waltham, MA, USA) and random nanomer primers. Following the reverse transcription (RT) step, the cDNA was denatured at 95°C for 1 min and then cooled to 4°C to enable reannealing of the random primers. The complementary strand was then synthesized using 5 U of Klenow fragment DNA polymerase (New England BioLabs, Ipswich, MA, USA) at 37°C for 1 h. Using this double-stranded DNA, libraries were prepared using the Nextera XT kit according to the manufacturer's protocol, except that the number of PCR cycles was increased to 15 (Illumina, San Diego, CA, USA). The amount of DNA with appropriate extremities for Illumina sequencing was quantified using the KAPA library quantification kit (Kapa Biosystems, Wilmington, MA, USA). The library samples were sequenced using the MiSeq platform (Illumina, San Diego, CA, USA) with 250-bp paired ends and dual molecular tags (barcodes) for each sample.
Deep sequencing and data analysis.
Paired-end reads were generated and debarcoded using Illumina software. In-house analysis pipelines were used for adaptor trimming of the generated reads. Reads with lengths of >100 bp were compared with the GenBank virus RefSeq protein database using BLASTx (22), and E values of <10−5 were considered significant. Contigs and singlets with best matches to annotated viruses in GenBank were then sorted by virus taxon. The software Geneious version 7.1.5. (Biomatters Limited) was used for assessing reads and contigs, as well as further genome analysis that included putative open reading frame (ORF) prediction of particular virus species.
Phylogenetic analyses and pairwise identity comparisons.
Reference viral amino acid sequences representing the Anelloviridae family, SIV, and simian pegivirus (SPgV) were obtained from GenBank. Sequence alignments were performed using MUSCLE with the default settings, and phylogenies were generated by the neighbor-joining method (p-distance model) and the maximum-likelihood method (Tamura-Nei model) integrated in the MEGA package, version 6.0 (23). The statistical significance of tree topologies was evaluated by 1,000-bootstrap resampling iterations.
A color-coded matrix of sequence identities was calculated using the Sequence Demarcation Tool for Windows (SDT version 1.2, www.cbio.uct.ac.za/SDT), using pairwise genetic identity calculations to classify the set of amino acid sequences. The same program generated the plots of the pairwise identity score frequency distribution (24).
Identification of putative recombination breakpoints.
The recombination analyses were made on multiple alignment of full-length pegivirus nucleotide sequences using SimPlot software, version 3.2 (25). Bootscan analysis was used to investigate the recombination breakpoints, based on the Kimura 2-parameter nucleotide substitution model with a window size of 400 bp and a step size of 20 bp.
Acquisition of viral genome sequences.
Total nucleic acid was extracted from plasma samples using the QIAamp DNA minikit (Qiagen). To sequence the entire genome of the AGM anelloviruses, rolling circle amplification (RCA) was first performed, using the Phix29 DNA polymerase (TempliPhi; GE Healthcare). Inverse PCR using outward-pointing PCR primers designed from the metagenomic sequences was then used on the RCA products, and the nearly genome-sized amplicons were Sanger sequenced using primer walking (26).
The PCR mixture (containing 1 μM each primer, 200 μM deoxynucleoside triphosphates, 1 U LaTaq DNA polymerase [TaKaRa], 10× LaTaq reaction buffer, and 5 μl target DNA in a 50-μl reaction mixture volume) was amplified as follows: 95°C for 2 min, 35 cycles of 95°C for 30 s, 48°C in the first cycle and a 0.2°C increase per cycle for 30 s, and 72°C for 4 min, followed by 72°C for 10 min. The cycle sequencing reactions were performed using a BigDye Terminator version 1.1 cycle sequencing kit (Applied Biosystems) according to the manufacturer's instructions. The resulting genomes of simian anellovirus were annotated using Geneious R7 (Biomatters) and deposited into GenBank.
Statistical analysis.
One-way analysis of variance (ANOVA) was applied to compare results of simian anellovirus sequences pairwise identities in GraphPad Prism version 3.10 (GraphPad Software, Inc., La Jolla, CA, USA) and used for statistical analysis. P values of <0.05 were regarded as statistically significant.
Nucleotide sequence accession numbers.
All simian pegivirus and anellovirus sequences have been deposited in GenBank, with accession numbers KP296804 to KP296860.
RESULTS
Metagenomic analysis of green monkey plasma virome.
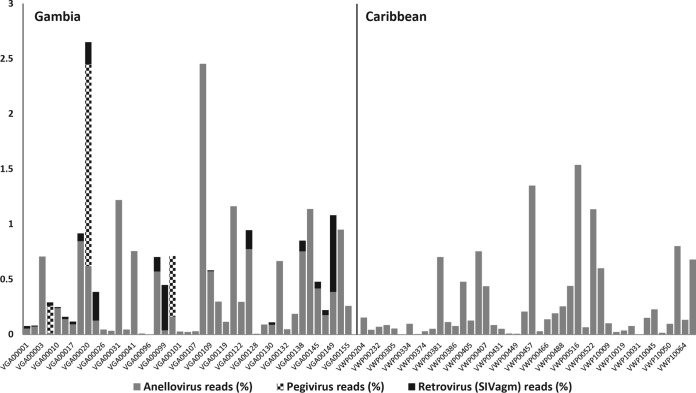
Plasma samples of African green monkeys were collected in Gambia and the Caribbean Islands of St. Kitts and Nevis (see Materials and Methods). Each of 87 samples was individually processed to enrich viral nucleic acids, which were then randomly reverse transcribed, amplified, and sequenced using the Illumina MiSeq platform (see Materials and Methods). A total of ∼46 million reads were generated, of which 83,557 sequences (including single reads and contigs) showed similarities (E values of ≤10−2) to known virus sequences present in the RefSeq virus genome database of GenBank (see Materials and Methods). These viruslike reads were compared with an in-house nonvirus-nonredundant (NVNR) universal protein database using BLASTx. Reads with higher sequence similarity (lower E scores) to nonviral proteins were removed, and 40,335 viruslike reads were found to pass this filter (see Table S2 in the supplemental material). The best hits (BLASTx E values of ≤10−5) were selected for further analysis. Because of the higher sequence diversity of this viral genus, E values of ≤10−3 were used for anelloviruses. The viral reads were assigned to 3 known virus families: Anelloviridae, Flaviviridae, and Retroviridae. The percentages of reads assigned to these 3 families in the African green monkey samples are shown in Fig. 1.
FIG 1.
Percentages of sequence reads assigned to different viruses identified in Gambian and Caribbean AGMs.
Prevalence of SIV infection in Gambian AGMs.
SIVagm was detected in 18/43 (42%) AGMs from Gambia, including 4 males (22%) and 14 (78%) females (see Table S1 in the supplemental material). None of the 16 Gambian infants or young juveniles was SIV positive. Eighteen of the 27 (66%) juvenile, young adult, and adult Gambian animals were SIV positive. Of the 44 Caribbean AGMs, including 35 juvenile, young adult, and adult animals, none were found to be viremic for SIV.
The percentages of SIV sequence reads ranged from 0.008% to 0.069% (Fig. 1). Sequence analysis revealed that the SIV sequences were 99% identical to an SIVagm sequence deposited in GenBank (accession number KJ467393) or to previously published sequences from a different set of Gambian AGMs (15). Two major Gambian sampling regions were compared. SIVagm viremia was 46% at Downriver and 35% at Upriver (see Fig. S1 in the supplemental material).
Characterization of novel simian pegivirus genome.
Pegiviruses are members of the Flaviviridae family of RNA viruses, which in humans are largely transmitted by blood and sexual contact (27). Simian pegiviruses were detected in 3/43 (7%) AGMs from Gambia but in none of the 44 Caribbean samples. A two-tailed test of proportion used to test for a significant difference in pegivirus prevalence between Gambian and Caribbean monkeys yielded a P value of 0.064. Two infected animals were subadults (male and female) and one a juvenile (female). De novo assembly of sequence reads from a Gambian AGM (animal VGA00020) with a high number of reads (1.83%) (Fig. 1) recovered a nearly complete pegivirus genome (GenBank accession no. KP296858) (28). The RNA-dependent RNA polymerase (RdRp) sequence was confirmed by RT-PCR amplification and Sanger sequencing. Partial genome sequences of the helicase gene (nonstructural protein 3 [NS3] gene) were also recovered from two other animals, VGA00004 (GenBank accession no. KP296860) and VGA00100 (GenBank accession no. KP296859).
The genome sequence recovered was 9,146 nucleotides (nt) long with a single expected ORF and partial 5′ and 3′ untranslated regions (UTRs) that were 450 and 49 nt long, respectively. Genome analysis of the entire coding region revealed 76% nucleotide identity to the genome of SPgV in red-tailed guenon (Cercopithecus ascanius) from Kibale National Park, Uganda (SPgVkrtg) (see Fig. S2 in the supplemental material). Based on its level of genomic nucleotide identity to SPgVkrtg_RT11 (GenBank accession no. KF234529) and recently adopted nomenclature (27, 29), we identified it as a novel viral species from African green monkeys and designated it simian pegivirus in African green monkey (SPgVagm).
The 1,692-nucleotide NS5B (RdRp) sequence was used for phylogenetic analysis, together with the recently reported genomes of pegiviruses from Old World monkeys, including PgVs from the olive baboon (SPgVkob), red colobus monkey (SpgVkrc), and red-tailed guenon (SPgVkrtg) (Fig. 2). The SPgVagm showed the closest relationship to red-tailed guenon isolate SPgVkrtg_RT11, detected in Kibale, Uganda (East Africa).
FIG 2.
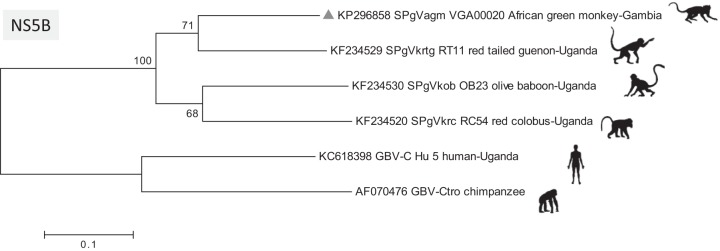
Phylogeny of simian pegiviruses based on complete NS5B (RdRp) nucleotide sequences. The maximum-likelihood tree was generated with the Tamura-Nei model of estimated distances, using 1,000-times bootstrap resampling. The scale bar indicates the average number of nucleotide substitutions per site. GBV-Ctro, GB virus C from Pan troglodytes.
Recombination events in SPgVagm.
Two possible recombination events between the SPgVagm_VGA00020 genome and closely related nonhuman primate species were detected (see Materials and Methods). Overall, SPgVagm was closest to SPgVkrtg, from red tailed guenons (Cercopithecus ascanius). One recombination with potential donor SPgVkob_OB23, from olive baboons (Papio anubis), occurred over the NS2 C-terminal and NS3 N-terminal regions (approximate positions, nt 2585 to 3104). The second possible recombination event occurred with SPgVkrc_RC54, from a red colobus monkey (Procolobus tephrosceles), involved the NS4B C-terminal half at approximately positions nt 4623 to 5772. All nucleotide positions are relative to the SPgVkrc_RC54 complete genome (see Fig. S3 in the supplemental material).
Characterization of African green monkey anellovirus genomes.
Numerous sequence reads were detected with translated amino acid similarity to viruses in the family Anelloviridae. Anellovirus reads were detected in all 43 plasma samples from Gambia, with a percentage-of-reads distribution of 0.003% to 2.5%, and in 43/44 (95.5%) plasma samples from the Caribbean, with a percentage-of-reads distribution of 0.004 to 1.5% (Fig. 1). All age groups were infected, including infants.
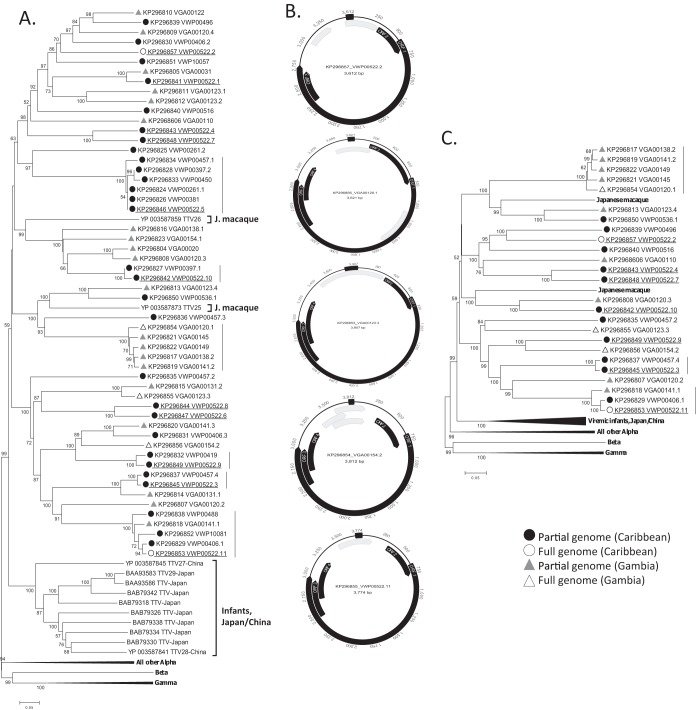
In total, 54 partial anellovirus ORF1 sequences were obtained (amino acid positions 410 to 657 of ORF1 of TTV1 human strain TA278; GenBank accession no. AB017610). Thirty-one sequences were from 14 Caribbean animals, and 23 sequences were from 12 Gambian animals. High levels of coinfection were detected, with individual animals carrying highly distinct anellovirus variants. Coinfections were detected in 5 of 14 Caribbean AGMs, with one animal (VWP00522) carrying 11 distinct variants, two animals carrying 4 variants (VWP00457) and 3 variants (VWP00406) each, and two animals (VWP00261 and VWP00397) carrying two variants each. Coinfections were also detected in 6 of 12 Gambian AGMs, with two animals (VGA00120 and VGA00123) carrying 4 variants each, another (VGA00141) carrying 3 variants, and 3 animals (VGA00131, VGA00138, and VGA00154) with two variants each. Phylogenetic relationships and sequence identity plots are shown (Fig. 3A; see also Fig. S4 and S5 in the supplemental material).
FIG 3.
Simian anelloviruses. (A) Phylogenetic analysis based on partial ORF1 sequences (247 amino acids [aa] at position 410 to 657 relative to the reference sequence with GenBank accession no. AB017610) using the neighbor-joining method (p-distance model). (B) Genomic organization of the 5 complete anellovirus genomes from AGMs. (C) Phylogenetic analysis of complete ORF1 sequences (∼715 aa), generated by the neighbor-joining method (p-distance model). Different variants from the same animal (VWP00522) are underlined. Vertical lines indicate clusters of closely related variants. Alpha, alphatorqueviruses; Beta, betatorqueviruses; Gamma, gammatorqueviruses.
Twenty-five complete ORF1 sequences from 14 animals (8 from Gambia and 6 from Caribbean) could be assembled and were also analyzed phylogenetically. ORF1 sequences were 715 amino acids long on average (range, 650 to 780 amino acids), with typical arginine-rich regions at their N termini (49 arginine residues in the first 70 amino acids). The four major phylogenetic clusters correspond to identical clusters seen using the partial ORF1 data set (Fig. 3C).
Finally, five complete circular genomes were obtained from the longest contigs using inverse PCR (see Materials and Methods). The genomes were deposited in GenBank. Three isolates were from Gambian monkeys, VGA00123.3 (GenBank accession no. KP296853; 3,907 bases), VGA00154.2 (GenBank accession no. KP296854; 3,621 bases), and VGA00120.1 (GenBank accession no. KP296856; 3,812 bases), and two from Caribbean monkeys, VWP00522.11 (GenBank accession no. KP296855; 3,774 bases) and VWP00522.2 (GenBank accession no. KP296857; 3,612 bases). The circular genomes are depicted in Fig. 3B. These showed similar genomic organization of their ORF1, -2, -3, and -4 sequences, although ORF4 could not be detected in one genome and the locations of other theoretical ORFs of unknown function differed.
The five anellovirus genomes showed 40 to 58% overall nucleotide identity to each other over the entire genome (44 to 53% in ORF1, 37 to 59% ORF2, and 40 to 47% ORF3) (see Fig. S6 in the supplemental material). The major elements required for transcription, including a TATA box (ATATAA) and Sp1 site (GGGCGGG), were all present, although minor variations were observed relative to the reference anellovirus TA278 genome (GenBank accession no. AB017610) (Table 1).
TABLE 1.
Genomic length and key elements of anelloviruses from African green monkeys
| Isolate | Genome length (nt) | Motif, position in genome (nt) ofb: |
||||||
|---|---|---|---|---|---|---|---|---|
| TATA box | TATA box Sp1 site | Cap site | Kozak, ORF1 | Coding region | Poly(A) | GC-rich region | ||
| TA278a | 3,853 | ATATAA, 85–90 | GGGCGG, 171–176 | GGGGCAATT, 209–217 | CACCATGG, 585–592 | 263–3074 | AATAAA, 3073–3078 | 3732–3853 |
| VGA00120.1 | 3,621 | ATATAA, 137–142 | GGGCGG, 230–235 | GGGGCAATT, 255–263 | GCTCATGG, 728–735 | 163–3088 | AATAAA, 3084–3089 | 3554–3621 |
| VGA00123.3 | 3,907 | ATAAGA, 132–137 | GGGCGG, 217–222 | GGGGCAATT, 268–276 | GCTCATGG, 729–736 | 316–3253 | AATAAA, 3244–3249 | 3769–3907 |
| VGA00154.2 | 3,812 | ATAAAA, 131–136 | GGGCGG, 216–221 | GGGGCAATT, 254–262 | CGCCATGG, 689–696 | 313–3235 | AATAAA, 3226–3231 | 3753–3812 |
| VWP00522.2 | 3,612 | ATAAGA, 126–131 | GGGCGG, 213–218 | GGGGCAATT, 252–260 | CAACATGC, 562–569 | 303–2761 | AATAAA, 2969–2974 | 3531–3612 |
| VWP00522.11 | 3,774 | ATATAA, 132–137 | GGGCGG, 215–220 | GGGGCCTAT, 253–261 | TGGTGTGG, 657–664 | 318–3243 | AATAAA, 3234–3239 | 3695–3774 |
The prototype TTV isolate TA278 (GenBank accession no. AB017610) was used as a reference.
Underlining indicates differences relative to canonical sequences.
Phylogenetic analyses of partial and complete ORF1 sequences showed that all of the AGM-derived sequences clustered together within the Alphatorquevirus genus. Interspersed with AGM sequences were ORF1 sequences from two Japanese macaques (Macaca fuscata fuscata) (30, 31). The next closest ORF1 alphatorquevirus sequences were derived from a group of Chinese and Japanese infants (32). All other alphatorqueviruses, from humans, chimpanzees, and other nonhuman primates (33), fell outside the AGM/Japanese macaque cluster.
Genetic diversity of simian anelloviruses from Gambia and the Caribbean.
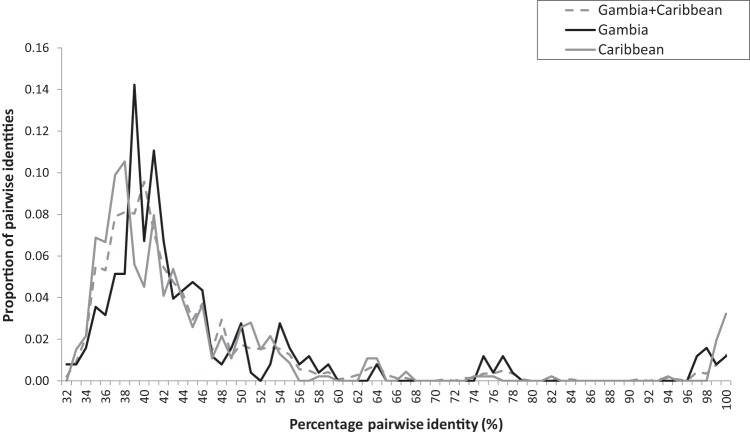
Pairwise amino acid distances were calculated for partial ORF1 sequences, and the distribution of these distances was plotted (Fig. 4; see also Fig. S4 in the supplemental material) (see Materials and Methods). The diversity of Caribbean ORF1 sequences was not reduced compared to that of Gambian ORF1 sequences, and the Caribbean-Gambian pairwise distances showed a similar distribution. These results indicate that reduced anellovirus genetic diversity was not observed in AGMs from the Caribbean relative to that in Gambian animals. Similarly, the phylogenetic analyses of partial or complete ORF1 sequences showed intermingling of the Caribbean and Gambian ORF1 taxa (Fig. 3A and C).
FIG 4.
Pairwise amino acid identity distributions of partial anellovirus ORF1 amino acid sequences (aa positions 410 to 657 relative to reference sequence with GenBank accession no. AB017610) among Gambian (n = 31) and Caribbean (n = 23) and between Gambian and Caribbean monkeys.
Tight clusters of closely related partial ORF1 sequences were also detected. The geographic sites of origin for the animal carriers were determined. One cluster of 5 Gambian animals with closely related ORF1 sequences all came from the same GPS coordinates at Upriver (see Table S1 in the supplemental material). Three pairs of closely related sequences were seen, all from St. Kitts, an island less than 20 miles long. Finally, one cluster of five sequences consisted of 4 ORF1 sequences from both St. Kitts and neighboring Nevis and one sequence from the Gambia. The Gambian sequence and the closest Caribbean sequences showed 92% nucleotide identity over their partial ORF1 sequences (see Table S3). A nucleotide distance matrix generated from complete anellovirus ORF1 sequences revealed the presence of multiple new species based on International Committee on Taxonomy of Viruses (ICTV) (34) classification criteria, in which >56% nucleotide difference defines new genera and >35% differentiates new species (see Table S4).
DISCUSSION
Human viruses resulting in chronic infections, such as HTLVs, may have been dispersed worldwide by early human colonizers in small isolated groups (3, 4, 35). Other viruses, such as smallpox and measles viruses, seem to have been absent from populations in the new world, resulting in high mortality following exposure (36, 37). Reductions in virome diversity may be the result of small founder populations lacking these viruses or host populations too small to maintain ongoing transmission.
Here, we measured the impact of a population bottleneck on the blood virome of AGMs imported into the Caribbean. Using an unbiased metagenomics approach, members of three virus families were detected. Based on current understanding of human viral homologues, these simian viruses also likely result in chronic viremia in AGMs and are acquired at different developmental stages. SIVagm results in a persistent infection, and it is mostly sexually acquired (15, 16). We detected SIV RNA in 42% of Gambian AGMs, with a 3/1 female/male ratio, and in none of the 16 infants/juveniles, as observed in prior studies (16, 38). The absence of SIVagm in the Caribbean population is not due to resistance of these animals to SIV, as these animals remain susceptible to SIVagm (39). The lack of detection of SIV RNA in St. Kitts and Nevis is supported by prior serosurveys of Caribbean AGMs that reported them to be SIVagm seronegative (40, 41). The absence of SIV in the Caribbean AGM population may therefore result from the importation of a small number of immature, still unexposed AGMs (41) and/or of an even smaller number of fortuitously uninfected adults.
GB virus C (GBV-C) was first characterized in 1995 (42) and was later renamed human pegivirus (HPgV) in the Pegivirus genus (27) of the Flaviviridae family. HPgV infects a large proportion (43) of adults, ranging from 1 to 5% of blood donors in developed countries and up to 20% of donors in developing countries (27). HPgV is thought to be transmitted sexually and by blood-blood contact (44–50). Persistent HPgV infections have not been shown to be pathogenic and may reduce mortality in HIV-coinfected subjects (43, 51). We report here the detection of a pegivirus in AGMs whose closest relatives were recently described in Old World monkeys from Uganda, although from different genera in the Cercopithecidae family (29, 52). Evidence of recombination with the pegiviruses of other Old World monkeys may reflect an ancient association between these viruses' ancestors within coinfected hosts. As is the case for sexually transmitted SIV, the lack of pegivirus detection in Caribbean AGMs may therefore also be due to importation of only a small number of uninfected infant and young juvenile AGMs into the Caribbean. The low frequency of SPgVagm detection in Gambian AGMs would also increase the likelihood that only pegivirus-negative animals would have been imported into the Caribbean.
Anelloviruses, first characterized in 1997 (53), have been reported in a high proportion of human adults (54, 55), where they show a very high level of genetic diversity (56–58). Anelloviruses are under immunological control, since induced or inborn immunodeficiency is associated with increases in viral loads (59–63). The rates of seroreactivity were shown to increase rapidly in infants (64), and infection is believed to be generally chronic. Anelloviruses have been reported in many tissues and bodily fluids, including respiratory fluids, blood, breast milk, cervical secretions, semen, urine, and feces (65–73). Anelloviruses are transmitted at a very young age, most likely from the mother or close contacts, possibly through the oral route (65, 74–79). Other anelloviruses have been characterized from diverse mammals, including rodents, pigs, and primates (31, 80–83). Coinfections with multiple anellovirus variants simultaneously present in the same individual have been described (55, 73, 84–87).
The detection of anelloviruses in 100% of Gambian AGMs likely accounts for their successful passage through the host population bottleneck to the Caribbean AGM. The high level of anellovirus genetic diversity in the Caribbean, similar to that seen in the Gambia, may also result from each of a small number of imported animals carrying distinct anelloviruses. Since 40% (11/26) of animals with partial ORF1 data were coinfected, with one Caribbean AGM carrying as many as 11 distinct variants, each imported animal could have imported several variants. As all AGM infants/juveniles from both countries were anellovirus positive by deep sequencing, viremia is established at an early age, as occurs in humans. Anelloviruses may therefore have escaped the sieving effect of the AGM genetic bottleneck that eliminated SIVagm and SPgVagm because each of the imported animals, possibly restricted to more amenable infants and young juveniles, were already infected with multiple anellovirus variants.
Small human populations appear to have successfully carried some of their largely adult-acquired chronic viruses, such as HTLVs and HPgV, to isolated territories (3–5, 88). Successful population bottlenecks, consisting of only infants and young juveniles, may therefore be rare occurrences. While the AGMs studied here may mimic events leading to highly susceptible populations with reduced viral and HLA diversity, the elimination of the adult-acquired viruses experienced by Caribbean AGMs may be more akin to that seen during the establishment of specific-pathogen-free monkey colonies by isolating and nursery raising infant baboons for later breeding (89, 90).
Supplementary Material
ACKNOWLEDGMENTS
Support was provided by BSRI and grant R01 HL105770. Sample collection for the Repository was funded by NIH grants R01 RR016300 and R01 OD010980 to N.F.
We thank Terry Ng for bioinformatics advice. The UCLA Systems Biology Sample Repository provided samples for these studies. We thank the Department of Parks & Wildlife Management, Ministry of Forestry & the Environment, and Medical Research Council (MRC) The Gambia Unit for enabling our sample collection from free-ranging monkeys. We thank MRC The Gambia Unit for making laboratory, equipment, and cold-storage space available for our project and all of the MRC staff that helped with organizing field sample collection and providing administrative support and transportation, in particular, Sanneh Mamkumba for administrative help, Ousman Secka for help with supplies and sample storage and shipment, and drivers Ousman Bah and Lamin Gibba. We gratefully acknowledge the expertise and assistance of Oliver (Pess) Morton, Ebou Jarjou, and Katherine Camfield during the field work, as well as Ben Kigbu and Toye Adegboye for veterinary care and Christopher Schmitt, Jennifer Danzy-Cramer, and Trudy Turner for their expertise and contributions to the field collection.
Footnotes
Supplemental material for this article may be found at http://dx.doi.org/10.1128/JVI.00671-15.
REFERENCES
- 1.Black FL. 1975. Infectious diseases in primitive societies. Science 187:515–518. doi: 10.1126/science.163483. [DOI] [PubMed] [Google Scholar]
- 2.Nathanson N. 2001. Epidemiology. In Knipe DM, Howley PM, Griffin DE, Lamb RA, Martin MA, Roizman B, Straus SE (ed), Fields virology, 4th ed, vol 1 Lippincott Williams & Wilkins, Philadelphia, PA. [Google Scholar]
- 3.Cassar O, Einsiedel L, Afonso PV, Gessain A. 2013. Human T-cell lymphotropic virus type 1 subtype C molecular variants among indigenous Australians: new insights into the molecular epidemiology of HTLV-1 in Australo-Melanesia. PLoS Negl Trop Dis 7:e2418. doi: 10.1371/journal.pntd.0002418. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Slattery JP, Franchini G, Gessain A. 1999. Genomic evolution, patterns of global dissemination, and interspecies transmission of human and simian T-cell leukemia/lymphotropic viruses. Genome Res 9:525–540. [PubMed] [Google Scholar]
- 5.Vandamme AM, Salemi M, Van Brussel M, Liu HF, Van Laethem K, Van Ranst M, Michels L, Desmyter J, Goubau P. 1998. African origin of human T-lymphotropic virus type 2 (HTLV-2) supported by a potential new HTLV-2d subtype in Congolese Bambuti Efe Pygmies. J Virol 72:4327–4340. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Cawthon Lang K. 3 January 2006, posting date. Primate factsheets: vervet (Chlorocebus) taxonomy, morphology, & ecology. Primate Info Net, Wisconsin Primate Research Center Library, University of Wisconsin—Madison, Madison, WI: http://pin.primate.wisc.edu/factsheets/entry/vervet Accessed 1 March 2015. [Google Scholar]
- 7.Jasinska AJ, Lin MK, Service S, Choi OW, DeYoung J, Grujic O, Kong SY, Jung Y, Jorgensen MJ, Fairbanks LA, Turner T, Cantor RM, Wasserscheid J, Dewar K, Warren W, Wilson RK, Weinstock G, Jentsch JD, Freimer NB. 2012. A non-human primate system for large-scale genetic studies of complex traits. Hum Mol Genet 21:3307–3316. doi: 10.1093/hmg/dds160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.McGuire MT. 1974. Contributions to primatology, vol 1 The St Kitts vervet. S. Karger, Basel, Switzerland. [Google Scholar]
- 9.van der Kuyl AC, Dekker JT. 1996. St. Kitts green monkeys originate from West Africa: genetic evidence from feces. Am J Primatol 40:361–364. [DOI] [PubMed] [Google Scholar]
- 10.Denham WW. 1987. Contributions to primatology, vol 24 West Indian green monkeys: problems in historical biogeography. S. Karger, Basel, Switzerland. [Google Scholar]
- 11.Aarnink A, Jacquelin B, Dauba A, Hebrard S, Moureaux E, Muller-Trutwin M, Blancher A. 2014. MHC polymorphism in Caribbean African green monkeys. Immunogenetics 66:353–360. doi: 10.1007/s00251-014-0770-9. [DOI] [PubMed] [Google Scholar]
- 12.Wiseman RW, Blanchard TJJL, Jorgensen MJ, Jasinska AJ, Freimer NB, Schmitz JE, O'Connor DH. 2011. Caribbean-origin African green monkeys exhibit remarkably limited major histocompatibility complex (MHC) diversity. J Med Primatol 40:262–262. [Google Scholar]
- 13.Broussard SR, Staprans SI, White R, Whitehead EM, Feinberg MB, Allan JS. 2001. Simian immunodeficiency virus replicates to high levels in naturally infected African green monkeys without inducing immunologic or neurologic disease. J Virol 75:2262–2275. doi: 10.1128/JVI.75.5.2262-2275.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Goldstein S, Brown CR, Ourmanov I, Pandrea I, Buckler-White A, Erb C, Nandi JS, Foster GJ, Autissier P, Schmitz JE, Hirsch VM. 2006. Comparison of simian immunodeficiency virus SIVagmVer replication and CD4+ T-cell dynamics in vervet and sabaeus African green monkeys. J Virol 80:4868–4877. doi: 10.1128/JVI.80.10.4868-4877.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Ma D, Jasinska AJ, Feyertag F, Wijewardana V, Kristoff J, He T, Raehtz K, Schmitt CA, Jung Y, Cramer JD, Dione M, Antonio M, Tracy R, Turner T, Robertson DL, Pandrea I, Freimer N, Apetrei C. 2014. Factors associated with siman immunodeficiency virus transmission in a natural African nonhuman primate host in the wild. J Virol 88:5687–5705. doi: 10.1128/JVI.03606-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Ma D, Jasinska A, Kristoff J, Grobler JP, Turner T, Jung Y, Schmitt C, Raehtz K, Feyertag F, Martinez Sosa N, Wijewardana V, Burke DS, Robertson DL, Tracy R, Pandrea I, Freimer N, Apetrei C. 2013. SIVagm infection in wild African green monkeys from South Africa: epidemiology, natural history, and evolutionary considerations. PLoS Pathog 9:e1003011. doi: 10.1371/journal.ppat.1003011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Bibollet-Ruche F, Brengues C, Galat-Luong A, Galat G, Pourrut X, Vidal N, Veas F, Durand JP, Cuny G. 1997. Genetic diversity of simian immunodeficiency viruses from West African green monkeys: evidence of multiple genotypes within populations from the same geographical locale. J Virol 71:307–313. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Johnson PR, Gravell M, Allan J, Goldstein S, Olmsted RA, Dapolito G, McGann C, London WT, Purcell RH, Hirsch VM. 1989. Genetic diversity among simian immunodeficiency virus isolates from African green monkeys. J Med Primatol 18:271–277. doi: 10.1002/ajp.1350180312. [DOI] [PubMed] [Google Scholar]
- 19.Muller MC, Saksena NK, Nerrienet E, Chappey C, Herve VM, Durand JP, Legal-Campodonico P, Lang MC, Digoutte JP, Georges AJ. 1993. Simian immunodeficiency viruses from central and western Africa: evidence for a new species-specific lentivirus in tantalus monkeys. J Virol 67:1227–1235. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Jin MJ, Hui H, Robertson DL, Muller MC, Barre-Sinoussi F, Hirsch VM, Allan JS, Shaw GM, Sharp PM, Hahn BH. 1994. Mosaic genome structure of simian immunodeficiency virus from west African green monkeys. EMBO J 13:2935–2947. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Li L, Deng X, Mee ET, Collot-Teixeira S, Anderson R, Schepelmann S, Minor PD, Delwart E. 2014. Comparing viral metagenomics methods using a highly multiplexed human viral pathogens reagent. J Virol Methods 213:139–146. doi: 10.1016/j.jviromet.2014.12.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Altschul SF, Gish W, Miller W, Myers EW, Lipman DJ. 1990. Basic local alignment search tool. J Mol Biol 215:403–410. doi: 10.1016/S0022-2836(05)80360-2. [DOI] [PubMed] [Google Scholar]
- 23.Tamura K, Stecher G, Peterson D, Filipski A, Kumar S. 2013. MEGA6: Molecular Evolutionary Genetics Analysis version 6.0. Mol Biol Evol 30:2725–2729. doi: 10.1093/molbev/mst197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Muhire BM, Varsani A, Martin DP. 2014. SDT: a virus classification tool based on pairwise sequence alignment and identity calculation. PLoS One 9:e108277. doi: 10.1371/journal.pone.0108277. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Lole KS, Bollinger RC, Paranjape RS, Gadkari D, Kulkarni SS, Novak NG, Ingersoll R, Sheppard HW, Ray SC. 1999. Full-length human immunodeficiency virus type 1 genomes from subtype C-infected seroconverters in India, with evidence of intersubtype recombination. J Virol 73:152–160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Sanger F, Coulson AR. 1975. A rapid method for determining sequences in DNA by primed synthesis with DNA polymerase. J Mol Biol 94:441–448. doi: 10.1016/0022-2836(75)90213-2. [DOI] [PubMed] [Google Scholar]
- 27.Stapleton JT, Foung S, Muerhoff AS, Bukh J, Simmonds P. 2011. The GB viruses: a review and proposed classification of GBV-A, GBV-C (HGV), and GBV-D in genus Pegivirus within the family Flaviviridae. J Gen Virol 92:233–246. doi: 10.1099/vir.0.027490-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Deng X, Naccache SN, Ng T, Federman S, Li L, Chiu CY, Delwart EL. 2015. An ensemble strategy that significantly improves de novo assembly of microbial genomes from metagenomic next-generation sequencing data. Nucleic Acids Res 43(7):e46. doi: 10.1093/nar/gkv002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Sibley SD, Lauck M, Bailey AL, Hyeroba D, Tumukunde A, Weny G, Chapman CA, O'Connor DH, Goldberg TL, Friedrich TC. 2014. Discovery and characterization of distinct simian pegiviruses in three wild African Old World monkey species. PLoS One 9:e98569. doi: 10.1371/journal.pone.0098569. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Gron K. 26 April 2007, posting date. Primate factsheets: Japanese macaque (Macaca fuscata) taxonomy, morphology, & ecology. Primate Info Net, Wisconsin Primate Research Center Library, University of Wisconsin—Madison, Madison, WI: http://pin.primate.wisc.edu/factsheets/entry/japanese_macaque Accessed 1 March 2015. [Google Scholar]
- 31.Okamoto H, Nishizawa T, Tawara A, Peng Y, Takahashi M, Kishimoto J, Tanaka T, Miyakawa Y, Mayumi M. 2000. Species-specific TT viruses in humans and nonhuman primates and their phylogenetic relatedness. Virology 277:368–378. doi: 10.1006/viro.2000.0588. [DOI] [PubMed] [Google Scholar]
- 32.Peng YH, Nishizawa T, Takahashi M, Ishikawa T, Yoshikawa A, Okamoto H. 2002. Analysis of the entire genomes of thirteen TT virus variants classifiable into the fourth and fifth genetic groups, isolated from viremic infants. Arch Virol 147:21–41. doi: 10.1007/s705-002-8301-7. [DOI] [PubMed] [Google Scholar]
- 33.Ninomiya M, Takahashi M, Hoshino Y, Ichiyama K, Simmonds P, Okamoto H. 2009. Analysis of the entire genomes of torque teno midi virus variants in chimpanzees: infrequent cross-species infection between humans and chimpanzees. J Gen Virol 90:347–358. doi: 10.1099/vir.0.007385-0. [DOI] [PubMed] [Google Scholar]
- 34.King AMQ, Adams MJ, Carstens EB, Lefkowitz EJ. 2012. Virus taxonomy: classification and nomenclature of viruses. Ninth Report of the International Committee on Taxonomy of Viruses. Elsevier Academic Press, San Diego. [Google Scholar]
- 35.Gessian A, Yanagihara R, Franchini G, Garruto RM, Jenkins CL, Ajdukiewicz AB, Gallo RC, Gajdusek DC. 1991. Highly divergent molecular variants of human T-lymphotropic virus type I from isolated populations in Papua New Guinea and the Solomon Islands. Proc Natl Acad Sci U S A 88:7694–7698. doi: 10.1073/pnas.88.17.7694. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Neel JV, Centerwall WR, Chagnon NA, Casey HL. 1970. Notes on the effect of measles and measles vaccine in a virgin-soil population of South American Indians. Am J Epidemiol 91:418–429. [DOI] [PubMed] [Google Scholar]
- 37.Patterson KB, Runge T. 2002. Smallpox and the Native American. Am J Med Sci 323:216–222. doi: 10.1097/00000441-200204000-00009. [DOI] [PubMed] [Google Scholar]
- 38.Phillips-Conroy JE, Jolly CJ, Petros B, Allan JS, Desrosiers RC. 1994. Sexual transmission of SIVagm in wild grivet monkeys. J Med Primatol 23:1–7. doi: 10.1111/j.1600-0684.1994.tb00088.x. [DOI] [PubMed] [Google Scholar]
- 39.Pandrea I, Apetrei C, Dufour J, Dillon N, Barbercheck J, Metzger M, Jacquelin B, Bohm R, Marx PA, Barre-Sinoussi F, Hirsch VM, Muller-Trutwin MC, Lackner AA, Veazey RS. 2006. Simian immunodeficiency virus SIVagm.sab infection of Caribbean African green monkeys: a new model for the study of SIV pathogenesis in natural hosts. J Virol 80:4858–4867. doi: 10.1128/JVI.80.10.4858-4867.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Daniel MD, Letvin NL, Sehgal PK, Schmidt DK, Silva DP, Solomon KR, Hodi FS Jr, Ringler DJ, Hunt RD, King NW, Desrosiers RC. 1988. Prevalence of antibodies to 3 retroviruses in a captive colony of macaque monkeys. Int J Cancer 41:601–608. doi: 10.1002/ijc.2910410421. [DOI] [PubMed] [Google Scholar]
- 41.Hendry RM, Wells MA, Phelan MA, Schneider AL, Epstein JS, Quinnan GV. 1986. Antibodies to simian immunodeficiency virus in African green monkeys in Africa in 1957-62. Lancet ii:455. [DOI] [PubMed] [Google Scholar]
- 42.Simons JN, Leary TP, Dawson GJ, Pilot-Matias TJ, Muerhoff AS, Schlauder GG, Desai SM, Mushahwar IK. 1995. Isolation of novel virus-like sequences associated with human hepatitis. Nat Med 1:564–569. [DOI] [PubMed] [Google Scholar]
- 43.Bhattarai N, Stapleton JT. 2012. GB virus C: the good boy virus? Trends Microbiol 20:124–130. doi: 10.1016/j.tim.2012.01.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Bernardin F, Operskalski E, Busch M, Delwart E. 2010. Transfusion transmission of highly prevalent commensal human viruses. Transfusion 50:2474–2483. doi: 10.1111/j.1537-2995.2010.02699.x. [DOI] [PubMed] [Google Scholar]
- 45.Bjorkman P, Naucler A, Winqvist N, Mushahwar I, Widell A. 2001. A case-control study of transmission routes for GB virus C/hepatitis G virus in Swedish blood donors lacking markers for hepatitis C virus infection. Vox Sang 81:148–153. doi: 10.1046/j.1423-0410.2001.00098.x. [DOI] [PubMed] [Google Scholar]
- 46.El-Zayadi AR, Abe K, Selim O, Naito H, Hess G, Ahdy A. 1999. Prevalence of GBV-C/hepatitis G virus viraemia among blood donors, health care personnel, chronic non-B non-C hepatitis, chronic hepatitis C and hemodialysis patients in Egypt. J Virol Methods 80:53–58. doi: 10.1016/S0166-0934(99)00036-1. [DOI] [PubMed] [Google Scholar]
- 47.Giret MT, Kallas EG. 2012. GBV-C: state of the art and future prospects. Curr HIV/AIDS Rep 9:26–33. doi: 10.1007/s11904-011-0109-1. [DOI] [PubMed] [Google Scholar]
- 48.Kleinman S. 2001. Hepatitis G virus biology, epidemiology, and clinical manifestations: implications for blood safety. Transfus Med Rev 15:201–212. doi: 10.1053/tmrv.2001.24589. [DOI] [PubMed] [Google Scholar]
- 49.Poovorawan Y, Theamboonlers A, Chongsrisawat V, Seksarn P, Jarvis L, Simmonds P. 1998. High prevalence of hepatitis G virus infection in multiply transfused children with thalassaemia. J Gastroenterol Hepatol 13:253–256. doi: 10.1111/j.1440-1746.1998.01552.x. [DOI] [PubMed] [Google Scholar]
- 50.Ribeiro-dos-Santos G, Nishiya AS, Nascimento CM, Bassit L, Chamone DF, Focaccia R, Eluf-Neto J, Sabino EC. 2002. Prevalence of GB virus C (hepatitis G virus) and risk factors for infection in Sao Paulo, Brazil. Eur J Clin Microbiol Infect Dis 21:438–443. doi: 10.1007/s10096-002-0752-y. [DOI] [PubMed] [Google Scholar]
- 51.Vahidnia F, Petersen M, Stapleton JT, Rutherford GW, Busch M, Custer B. 2012. Acquisition of GB virus type C and lower mortality in patients with advanced HIV disease. Clin Infect Dis 55:1012–1019. doi: 10.1093/cid/cis589. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Okamoto H. 2009. History of discoveries and pathogenicity of TT viruses. Curr Top Microbiol Immunol 331:1–20. [DOI] [PubMed] [Google Scholar]
- 53.Nishizawa T, Okamoto H, Konishi K, Yoshizawa H, Miyakawa Y, Mayumi M. 1997. A novel DNA virus (TTV) associated with elevated transaminase levels in posttransfusion hepatitis of unknown etiology. Biochem Biophys Res Commun 241:92–97. doi: 10.1006/bbrc.1997.7765. [DOI] [PubMed] [Google Scholar]
- 54.Beland K, Dore-Nguyen M, Gagne MJ, Patey N, Brassard J, Alvarez F, Halac U. 2014. Torque Teno virus in children who underwent orthotopic liver transplantation: new insights about a common pathogen. J Infect Dis 209:247–254. doi: 10.1093/infdis/jit423. [DOI] [PubMed] [Google Scholar]
- 55.Ninomiya M, Takahashi M, Nishizawa T, Shimosegawa T, Okamoto H. 2008. Development of PCR assays with nested primers specific for differential detection of three human anelloviruses and early acquisition of dual or triple infection during infancy. J Clin Microbiol 46:507–514. doi: 10.1128/JCM.01703-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Biagini P. 2009. Classification of TTV and related viruses (anelloviruses). Curr Top Microbiol Immunol 331:21–33. [DOI] [PubMed] [Google Scholar]
- 57.Chan PK, Chik KW, Li CK, Tang NL, Ming MS, Cheung JL, Ng KC, Yuen PM, Cheng AF. 2001. Prevalence and genotype distribution of TT virus in various specimen types from thalassaemic patients. J Viral Hepat 8:304–309. doi: 10.1046/j.1365-2893.2001.00276.x. [DOI] [PubMed] [Google Scholar]
- 58.de Villiers EM, Borkosky SS, Kimmel R, Gunst K, Fei JW. 2011. The diversity of torque teno viruses: in vitro replication leads to the formation of additional replication-competent subviral molecules. J Virol 85:7284–7295. doi: 10.1128/JVI.02472-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Beland K, Dore-Nguyen M, Gagne MJ, Patey N, Brassard J, Alvarez F, Halac U. 2014. Torque Teno virus load as a biomarker of immunosuppression? New hopes and insights. J Infect Dis 210:668–670. doi: 10.1093/infdis/jiu210. [DOI] [PubMed] [Google Scholar]
- 60.De Vlaminck I, Khush KK, Strehl C, Kohli B, Luikart H, Neff NF, Okamoto J, Snyder TM, Cornfield DN, Nicolls MR, Weill D, Bernstein D, Valantine HA, Quake SR. 2013. Temporal response of the human virome to immunosuppression and antiviral therapy. Cell 155:1178–1187. doi: 10.1016/j.cell.2013.10.034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Li L, Deng X, Linsuwanon P, Bangsberg D, Bwana MB, Hunt P, Martin JN, Deeks SG, Delwart E. 2013. AIDS alters the commensal plasma virome. J Virol 87:10912–10915. doi: 10.1128/JVI.01839-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Maggi F, Pifferi M, Michelucci A, Albani M, Sbranti S, Lanini L, Simi P, Macchia P, Pistello M, Bendinelli M. 2011. Torque teno virus viremia load size in patients with selected congenital defects of innate immunity. Clin Vaccine Immunol 18:692–694. doi: 10.1128/CVI.00466-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Young JC, Chehoud C, Bittinger K, Bailey A, Diamond JM, Cantu E, Haas AR, Abbas A, Frye L, Christie JD, Bushman FD, Collman RG. 2015. Viral metagenomics reveal blooms of anelloviruses in the respiratory tract of lung transplant recipients. Am J Transplant 15:200–209. doi: 10.1111/ajt.13031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Chen T, Vaisanen E, Mattila PS, Hedman K, Soderlund-Venermo M. 2013. Antigenic diversity and seroprevalences of Torque teno viruses in children and adults by ORF2-based immunoassays. J Gen Virol 94:409–417. doi: 10.1099/vir.0.046862-0. [DOI] [PubMed] [Google Scholar]
- 65.Chan PK, Tam WH, Yeo W, Cheung JL, Zhong S, Cheng AF. 2001. High carriage rate of TT virus in the cervices of pregnant women. Clin Infect Dis 32:1376–1377. doi: 10.1086/319983. [DOI] [PubMed] [Google Scholar]
- 66.Deng X, Terunuma H, Handema R, Sakamoto M, Kitamura T, Ito M, Akahane Y. 2000. Higher prevalence and viral load of TT virus in saliva than in the corresponding serum: another possible transmission route and replication site of TT virus. J Med Virol 62:531–537. doi:. [DOI] [PubMed] [Google Scholar]
- 67.Goto K, Sugiyama K, Ando T, Mizutani F, Terabe K, Tanaka K, Nishiyama M, Wada Y. 2000. Detection rates of TT virus DNA in serum of umbilical cord blood, breast milk and saliva. Tohoku J Exp Med 191:203–207. doi: 10.1620/tjem.191.203. [DOI] [PubMed] [Google Scholar]
- 68.Inami T, Konomi N, Arakawa Y, Abe K. 2000. High prevalence of TT virus DNA in human saliva and semen. J Clin Microbiol 38:2407–2408. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Itoh M, Shimomura H, Fujioka S, Miyake M, Tsuji H, Ikeda F, Tsuji T. 2001. High prevalence of TT virus in human bile juice samples: importance of secretion through bile into feces. Dig Dis Sci 46:457–462. doi: 10.1023/A:1005618308943. [DOI] [PubMed] [Google Scholar]
- 70.Ross RS, Viazov S, Runde V, Schaefer UW, Roggendorf M. 1999. Detection of TT virus DNA in specimens other than blood. J Clin Virol 13:181–184. doi: 10.1016/S1386-6532(99)00015-3. [DOI] [PubMed] [Google Scholar]
- 71.Schroter M, Polywka S, Zollner B, Schafer P, Laufs R, Feucht HH. 2000. Detection of TT virus DNA and GB virus type C/hepatitis G virus RNA in serum and breast milk: determination of mother-to-child transmission. J Clin Microbiol 38:745–747. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Toyoda H, Naruse M, Yokozaki S, Morita K, Nakano I, Itakura A, Okamura M, Fukuda Y, Hayakawa T. 1999. Prevalence of infection with TT virus (TTV), a novel DNA virus, in healthy Japanese subjects, newborn infants, cord blood and breast milk. J Infect 38:198–199. doi: 10.1016/S0163-4453(99)90254-2. [DOI] [PubMed] [Google Scholar]
- 73.Vasconcelos HC, Cataldo M, Niel C. 2002. Mixed infections of adults and children with multiple TTV-like mini virus isolates. J Med Virol 68:291–298. doi: 10.1002/jmv.10187. [DOI] [PubMed] [Google Scholar]
- 74.Bagaglio S, Sitia G, Prati D, Cella D, Hasson H, Novati R, Lazzarin A, Morsica G. 2002. Mother-to-child transmission of TT virus: sequence analysis of non-coding region of TT virus in infected mother-infant pairs. Arch Virol 147:803–812. doi: 10.1007/s007050200027. [DOI] [PubMed] [Google Scholar]
- 75.Gerner P, Oettinger R, Gerner W, Falbrede J, Wirth S. 2000. Mother-to-infant transmission of TT virus: prevalence, extent and mechanism of vertical transmission. Pediatr Infect Dis J 19:1074–1077. doi: 10.1097/00006454-200011000-00009. [DOI] [PubMed] [Google Scholar]
- 76.Iso K, Suzuki Y, Takayama M. 2001. Mother-to-infant transmission of TT virus in Japan. Int J Gynaecol Obstet 75:11–19. doi: 10.1016/S0020-7292(01)00450-7. [DOI] [PubMed] [Google Scholar]
- 77.Komatsu H, Inui A, Sogo T, Kuroda K, Tanaka T, Fujisawa T. 2004. TTV infection in children born to mothers infected with TTV but not with HBV, HCV, or HIV. J Med Virol 74:499–506. doi: 10.1002/jmv.20204. [DOI] [PubMed] [Google Scholar]
- 78.Lin HH, Kao JH, Lee PI, Chen DS. 2002. Early acquisition of TT virus in infants: possible minor role of maternal transmission. J Med Virol 66:285–290. doi: 10.1002/jmv.2143. [DOI] [PubMed] [Google Scholar]
- 79.Ohto H, Ujiie N, Takeuchi C, Sato A, Hayashi A, Ishiko H, Nishizawa T, Okamoto H. 2002. TT virus infection during childhood. Transfusion 42:892–898. doi: 10.1046/j.1537-2995.2002.00150.x. [DOI] [PubMed] [Google Scholar]
- 80.McKeown NE, Fenaux M, Halbur PG, Meng XJ. 2004. Molecular characterization of porcine TT virus, an orphan virus, in pigs from six different countries. Vet Microbiol 104:113–117. doi: 10.1016/j.vetmic.2004.08.013. [DOI] [PubMed] [Google Scholar]
- 81.Nishiyama S, Dutia BM, Stewart JP, Meredith AL, Shaw DJ, Simmonds P, Sharp CP. 2014. Identification of novel anelloviruses with broad diversity in United Kingdom rodents. J Gen Virol 95:1544–1553. doi: 10.1099/vir.0.065219-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Okamoto H, Takahashi M, Nishizawa T, Tawara A, Fukai K, Muramatsu U, Naito Y, Yoshikawa A. 2002. Genomic characterization of TT viruses (TTVs) in pigs, cats and dogs and their relatedness with species-specific TTVs in primates and tupaias. J Gen Virol 83:1291–1297. [DOI] [PubMed] [Google Scholar]
- 83.Thom K, Morrison C, Lewis JC, Simmonds P. 2003. Distribution of TT virus (TTV), TTV-like minivirus, and related viruses in humans and nonhuman primates. Virology 306:324–333. doi: 10.1016/S0042-6822(02)00049-1. [DOI] [PubMed] [Google Scholar]
- 84.Gallian P, Biagini P, Attoui H, Cantaloube JF, Dussol B, Berland Y, de Micco P, de Lamballerie X. 2002. High genetic diversity revealed by the study of TLMV infection in French hemodialysis patients. J Med Virol 67:630–635. doi: 10.1002/jmv.10150. [DOI] [PubMed] [Google Scholar]
- 85.Matsubara H, Michitaka K, Horiike N, Kihana T, Yano M, Mori T, Onji M. 2001. Existence of TT virus DNA and TTV-like mini virus DNA in infant cord blood: mother-to-neonatal transmission. Hepatol Res 21:280–287. doi: 10.1016/S1386-6346(01)00115-2. [DOI] [PubMed] [Google Scholar]
- 86.Okamoto H, Takahashi M, Nishizawa T, Ukita M, Fukuda M, Tsuda F, Miyakawa Y, Mayumi M. 1999. Marked genomic heterogeneity and frequent mixed infection of TT virus demonstrated by PCR with primers from coding and noncoding regions. Virology 259:428–436. doi: 10.1006/viro.1999.9770. [DOI] [PubMed] [Google Scholar]
- 87.Toyoda H, Fukuda Y, Nakano I, Katano Y, Yokozaki S, Hayashi K, Ito Y, Suzuki K, Nakano H, Saito H, Takamatsu J. 2001. TT virus genotype changes frequently in multiply transfused patients with hemophilia but rarely in patients with chronic hepatitis C and in healthy subjects. Transfusion 41:1130–1135. doi: 10.1046/j.1537-2995.2001.41091130.x. [DOI] [PubMed] [Google Scholar]
- 88.Simmonds P. 2001. Reconstructing the origins of human hepatitis viruses. Philos Trans R Soc Lond B Biol Sci 356:1013–1026. doi: 10.1098/rstb.2001.0890. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Budda ML, Ely JJ, Doan S, Chavez-Suarez M, White GL, Wolf RF. 2013. Evaluation of reproduction and raising offspring in a nursery-reared SPF baboon (Papio hamadryas anubis) colony. Am J Primatol 75:798–806. doi: 10.1002/ajp.22136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Wolf RF, Eberle R, White GL. 2010. Generation of a specific-pathogen-free baboon colony. J Am Assoc Lab Anim Sci 49:814–820. [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.