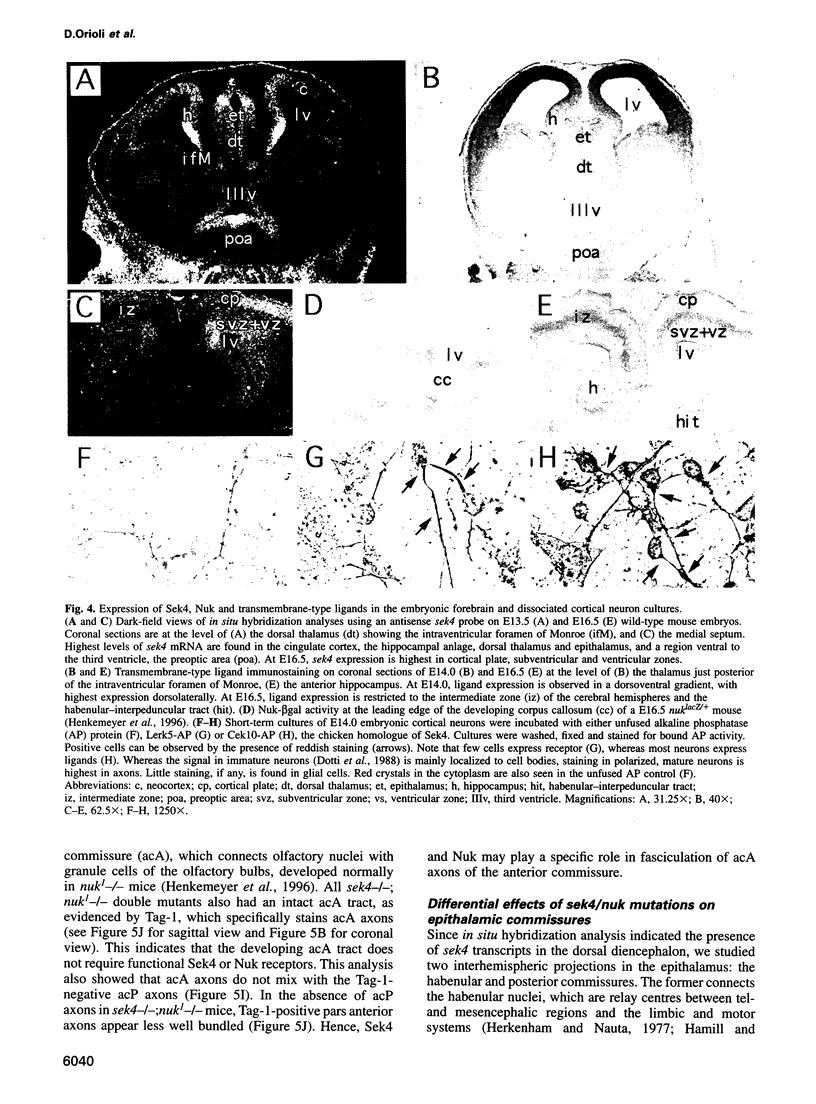
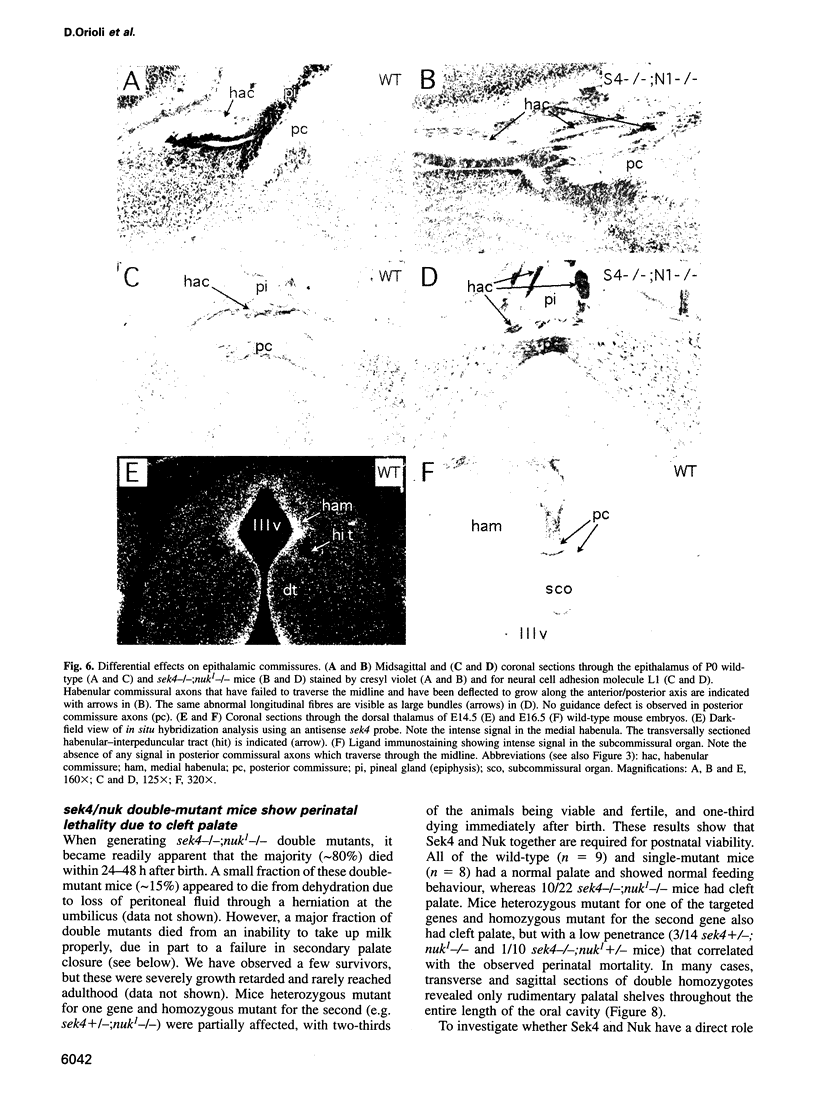
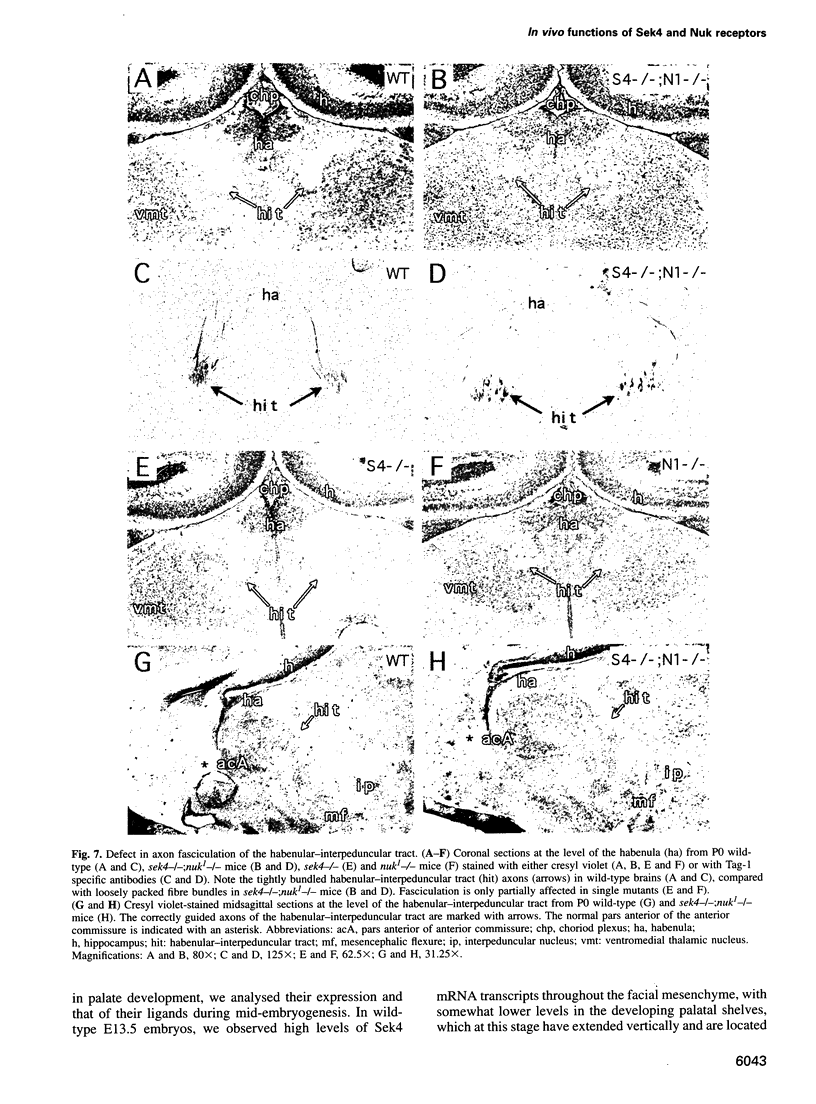
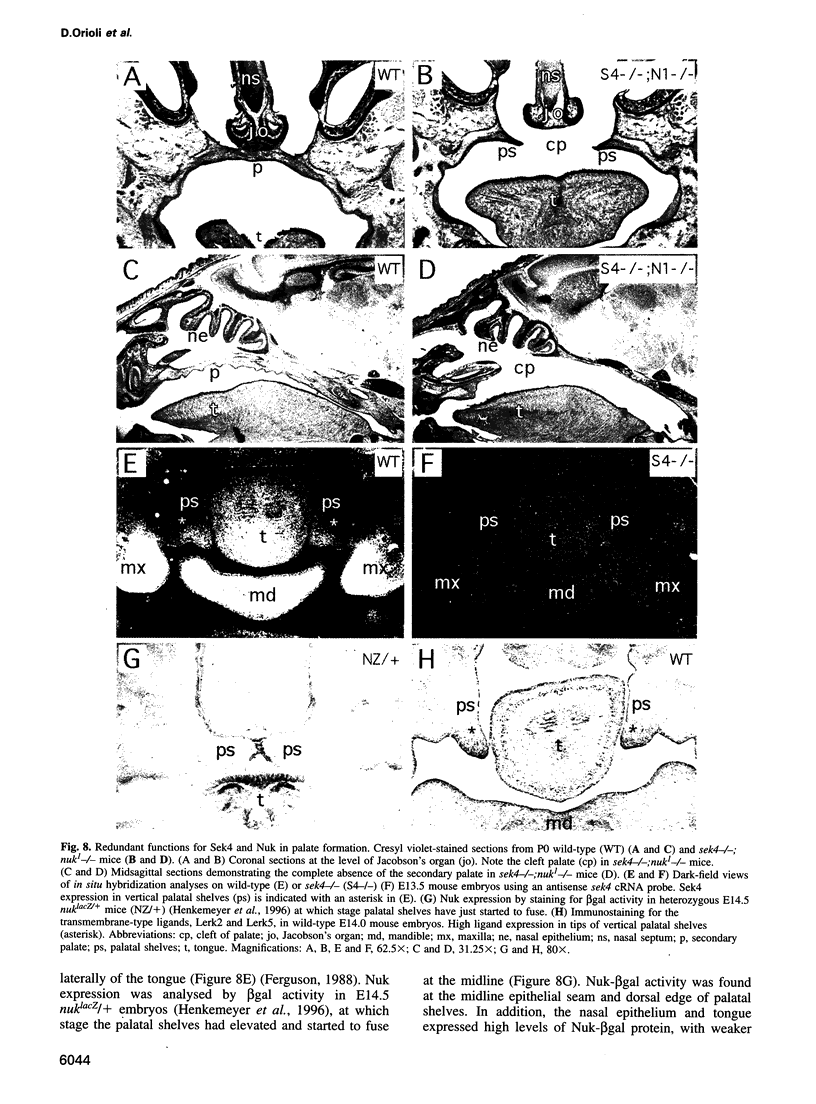
Abstract
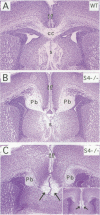
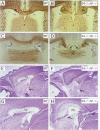
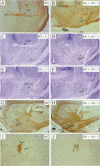
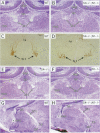
Sek4 and Nuk are members of the Eph-related family of receptor protein-tyrosine kinases. These receptors interact with a set of cell surface ligands that have recently been implicated in axon guidance and fasciculation. We now demonstrate that the formation of the corpus callosum and anterior commissure, two major commissural axon tracts that connect the two cerebral hemispheres, is critically dependent on Sek4 and Nuk. While mice deficient in Nuk exhibit defects in pathfinding of anterior commissure axons, sek4 mutants have defects in corpus callosum formation. The phenotype in both axon tracts is markedly more severe in sek4/nuk1 double mutants, indicating that the two receptors act in a partially redundant fashion. sek4/nuk1 double mutants also exhibit specific guidance and fasciculation defects of diencephalic axon tracts. Moreover, while mice singly deficient in either Sek4 or Nuk are viable, most sek4/nuk1 double mutants die immediately after birth primarily due to a cleft palate. These results demonstrate essential and cooperative functions for Sek4 and Nuk in establishing axon pathways in the developing brain, and during the development of facial structures.
Full text
PDF

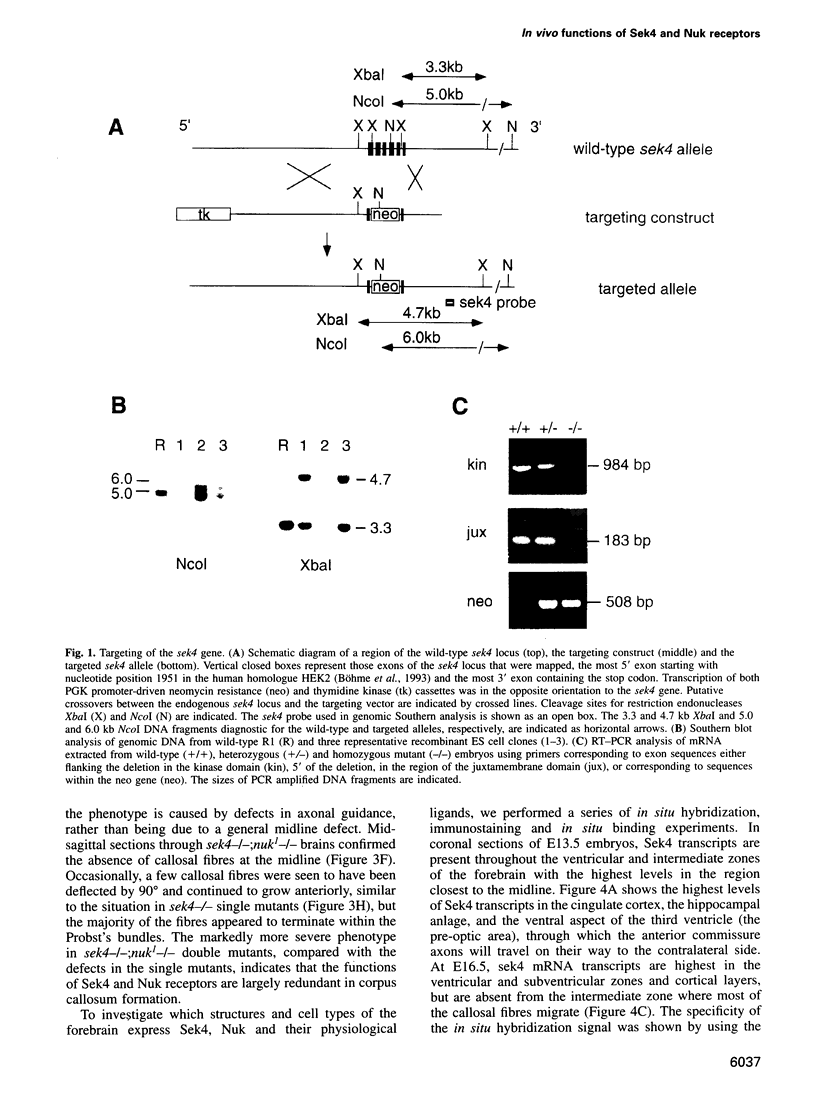
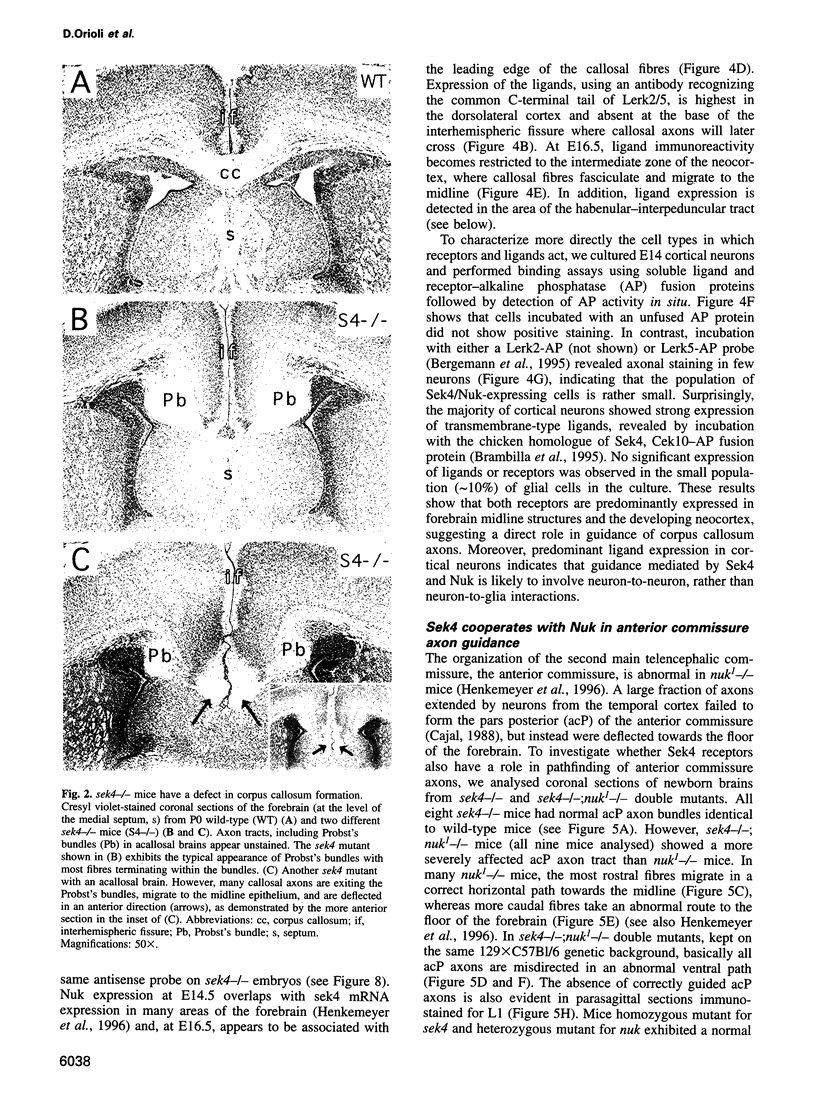
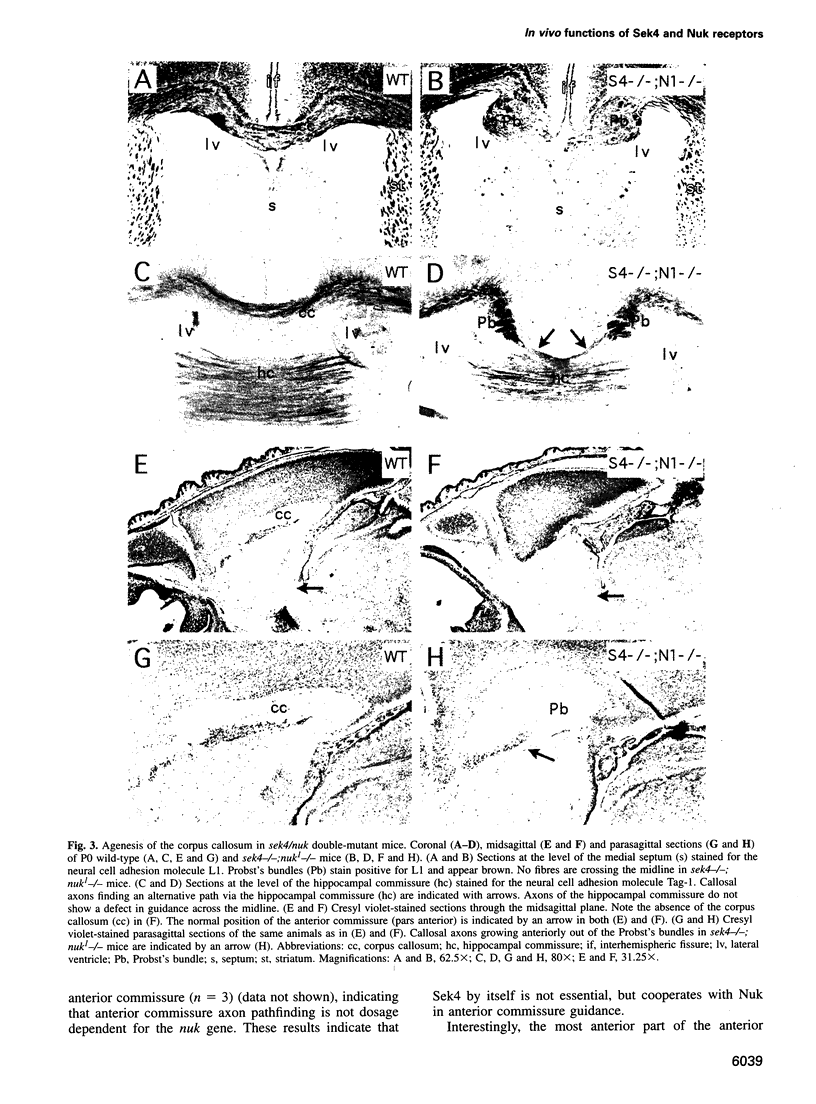






Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bartley T. D., Hunt R. W., Welcher A. A., Boyle W. J., Parker V. P., Lindberg R. A., Lu H. S., Colombero A. M., Elliott R. L., Guthrie B. A. B61 is a ligand for the ECK receptor protein-tyrosine kinase. Nature. 1994 Apr 7;368(6471):558–560. doi: 10.1038/368558a0. [DOI] [PubMed] [Google Scholar]
- Becker N., Seitanidou T., Murphy P., Mattéi M. G., Topilko P., Nieto M. A., Wilkinson D. G., Charnay P., Gilardi-Hebenstreit P. Several receptor tyrosine kinase genes of the Eph family are segmentally expressed in the developing hindbrain. Mech Dev. 1994 Jul;47(1):3–17. doi: 10.1016/0925-4773(94)90091-4. [DOI] [PubMed] [Google Scholar]
- Bergemann A. D., Cheng H. J., Brambilla R., Klein R., Flanagan J. G. ELF-2, a new member of the Eph ligand family, is segmentally expressed in mouse embryos in the region of the hindbrain and newly forming somites. Mol Cell Biol. 1995 Sep;15(9):4921–4929. doi: 10.1128/mcb.15.9.4921. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bouillet P., Oulad-Abdelghani M., Vicaire S., Garnier J. M., Schuhbaur B., Dollé P., Chambon P. Efficient cloning of cDNAs of retinoic acid-responsive genes in P19 embryonal carcinoma cells and characterization of a novel mouse gene, Stra1 (mouse LERK-2/Eplg2). Dev Biol. 1995 Aug;170(2):420–433. doi: 10.1006/dbio.1995.1226. [DOI] [PubMed] [Google Scholar]
- Brambilla R., Klein R. Telling axons where to grow: a role for Eph receptor tyrosine kinases in guidance. Mol Cell Neurosci. 1995 Dec;6(6):487–495. doi: 10.1006/mcne.1995.0001. [DOI] [PubMed] [Google Scholar]
- Brambilla R., Schnapp A., Casagranda F., Labrador J. P., Bergemann A. D., Flanagan J. G., Pasquale E. B., Klein R. Membrane-bound LERK2 ligand can signal through three different Eph-related receptor tyrosine kinases. EMBO J. 1995 Jul 3;14(13):3116–3126. doi: 10.1002/j.1460-2075.1995.tb07314.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Böhme B., Holtrich U., Wolf G., Luzius H., Grzeschik K. H., Strebhardt K., Rübsamen-Waigmann H. PCR mediated detection of a new human receptor-tyrosine-kinase, HEK 2. Oncogene. 1993 Oct;8(10):2857–2862. [PubMed] [Google Scholar]
- Carpenter M. B., Harbison J. W., Peter P. Accessory oculomotor nuclei in the monkey: projections and effects of discrete lesions. J Comp Neurol. 1970 Oct;140(2):131–154. doi: 10.1002/cne.901400202. [DOI] [PubMed] [Google Scholar]
- Cheng H. J., Flanagan J. G. Identification and cloning of ELF-1, a developmentally expressed ligand for the Mek4 and Sek receptor tyrosine kinases. Cell. 1994 Oct 7;79(1):157–168. doi: 10.1016/0092-8674(94)90408-1. [DOI] [PubMed] [Google Scholar]
- Cheng H. J., Nakamoto M., Bergemann A. D., Flanagan J. G. Complementary gradients in expression and binding of ELF-1 and Mek4 in development of the topographic retinotectal projection map. Cell. 1995 Aug 11;82(3):371–381. doi: 10.1016/0092-8674(95)90426-3. [DOI] [PubMed] [Google Scholar]
- Ciossek T., Lerch M. M., Ullrich A. Cloning, characterization, and differential expression of MDK2 and MDK5, two novel receptor tyrosine kinases of the eck/eph family. Oncogene. 1995 Nov 16;11(10):2085–2095. [PubMed] [Google Scholar]
- Davis S., Gale N. W., Aldrich T. H., Maisonpierre P. C., Lhotak V., Pawson T., Goldfarb M., Yancopoulos G. D. Ligands for EPH-related receptor tyrosine kinases that require membrane attachment or clustering for activity. Science. 1994 Nov 4;266(5186):816–819. doi: 10.1126/science.7973638. [DOI] [PubMed] [Google Scholar]
- DeMyer W. Median facial malformations and their implications for brain malformations. Birth Defects Orig Artic Ser. 1975;11(7):155–181. [PubMed] [Google Scholar]
- Delezoide A. L., Narcy F., Larroche J. C. Cerebral midline developmental anomalies: spectrum and associated features. Genet Couns. 1990;1(3-4):197–210. [PubMed] [Google Scholar]
- Dodd J., Schuchardt A. Axon guidance: a compelling case for repelling growth cones. Cell. 1995 May 19;81(4):471–474. doi: 10.1016/0092-8674(95)90066-7. [DOI] [PubMed] [Google Scholar]
- Dotti C. G., Sullivan C. A., Banker G. A. The establishment of polarity by hippocampal neurons in culture. J Neurosci. 1988 Apr;8(4):1454–1468. doi: 10.1523/JNEUROSCI.08-04-01454.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Drescher U., Kremoser C., Handwerker C., Löschinger J., Noda M., Bonhoeffer F. In vitro guidance of retinal ganglion cell axons by RAGS, a 25 kDa tectal protein related to ligands for Eph receptor tyrosine kinases. Cell. 1995 Aug 11;82(3):359–370. doi: 10.1016/0092-8674(95)90425-5. [DOI] [PubMed] [Google Scholar]
- Ellis J., Liu Q., Breitman M., Jenkins N. A., Gilbert D. J., Copeland N. G., Tempest H. V., Warren S., Muir E., Schilling H. Embryo brain kinase: a novel gene of the eph/elk receptor tyrosine kinase family. Mech Dev. 1995 Aug;52(2-3):319–341. doi: 10.1016/0925-4773(95)00411-s. [DOI] [PubMed] [Google Scholar]
- Ferguson M. W. Palate development. Development. 1988;103 (Suppl):41–60. doi: 10.1242/dev.103.Supplement.41. [DOI] [PubMed] [Google Scholar]
- Fischer M., Ryan S. B., Dobyns W. B. Mechanisms of interhemispheric transfer and patterns of cognitive function in acallosal patients of normal intelligence. Arch Neurol. 1992 Mar;49(3):271–277. doi: 10.1001/archneur.1992.00530270085023. [DOI] [PubMed] [Google Scholar]
- Gale N. W., Holland S. J., Valenzuela D. M., Flenniken A., Pan L., Ryan T. E., Henkemeyer M., Strebhardt K., Hirai H., Wilkinson D. G. Eph receptors and ligands comprise two major specificity subclasses and are reciprocally compartmentalized during embryogenesis. Neuron. 1996 Jul;17(1):9–19. doi: 10.1016/s0896-6273(00)80276-7. [DOI] [PubMed] [Google Scholar]
- Gassmann M., Casagranda F., Orioli D., Simon H., Lai C., Klein R., Lemke G. Aberrant neural and cardiac development in mice lacking the ErbB4 neuregulin receptor. Nature. 1995 Nov 23;378(6555):390–394. doi: 10.1038/378390a0. [DOI] [PubMed] [Google Scholar]
- Hamill G. S., Jacobowitz D. M. A study of afferent projections to the rat interpeduncular nucleus. Brain Res Bull. 1984 Oct;13(4):527–539. doi: 10.1016/0361-9230(84)90035-2. [DOI] [PubMed] [Google Scholar]
- Henkemeyer M., Marengere L. E., McGlade J., Olivier J. P., Conlon R. A., Holmyard D. P., Letwin K., Pawson T. Immunolocalization of the Nuk receptor tyrosine kinase suggests roles in segmental patterning of the brain and axonogenesis. Oncogene. 1994 Apr;9(4):1001–1014. [PubMed] [Google Scholar]
- Henkemeyer M., Orioli D., Henderson J. T., Saxton T. M., Roder J., Pawson T., Klein R. Nuk controls pathfinding of commissural axons in the mammalian central nervous system. Cell. 1996 Jul 12;86(1):35–46. doi: 10.1016/s0092-8674(00)80075-6. [DOI] [PubMed] [Google Scholar]
- Herkenham M., Nauta W. J. Afferent connections of the habenular nuclei in the rat. A horseradish peroxidase study, with a note on the fiber-of-passage problem. J Comp Neurol. 1977 May 1;173(1):123–146. doi: 10.1002/cne.901730107. [DOI] [PubMed] [Google Scholar]
- Hirai H., Maru Y., Hagiwara K., Nishida J., Takaku F. A novel putative tyrosine kinase receptor encoded by the eph gene. Science. 1987 Dec 18;238(4834):1717–1720. doi: 10.1126/science.2825356. [DOI] [PubMed] [Google Scholar]
- Joyner A. L., Skarnes W. C., Rossant J. Production of a mutation in mouse En-2 gene by homologous recombination in embryonic stem cells. Nature. 1989 Mar 9;338(6211):153–156. doi: 10.1038/338153a0. [DOI] [PubMed] [Google Scholar]
- Kennedy T. E., Tessier-Lavigne M. Guidance and induction of branch formation in developing axons by target-derived diffusible factors. Curr Opin Neurobiol. 1995 Feb;5(1):83–90. doi: 10.1016/0959-4388(95)80091-3. [DOI] [PubMed] [Google Scholar]
- Kessler J., Huber M., Pawlik G., Heiss W. D., Markowitsch H. J. Complex sensory cross integration deficits in a case of corpus callosum agenesis with bilateral language representation: positron-emission-tomography and neuropsychological findings. Int J Neurosci. 1991 Jun;58(3-4):275–282. doi: 10.3109/00207459108985443. [DOI] [PubMed] [Google Scholar]
- Kilpatrick T. J., Brown A., Lai C., Gassmann M., Goulding M., Lemke G. Expression of the Tyro4/Mek4/Cek4 gene specifically marks a subset of embryonic motor neurons and their muscle targets. Mol Cell Neurosci. 1996 Jan;7(1):62–74. doi: 10.1006/mcne.1996.0005. [DOI] [PubMed] [Google Scholar]
- Klein R., Parada L. F., Coulier F., Barbacid M. trkB, a novel tyrosine protein kinase receptor expressed during mouse neural development. EMBO J. 1989 Dec 1;8(12):3701–3709. doi: 10.1002/j.1460-2075.1989.tb08545.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Klein R. Role of neurotrophins in mouse neuronal development. FASEB J. 1994 Jul;8(10):738–744. doi: 10.1096/fasebj.8.10.8050673. [DOI] [PubMed] [Google Scholar]
- Kozlosky C. J., Maraskovsky E., McGrew J. T., VandenBos T., Teepe M., Lyman S. D., Srinivasan S., Fletcher F. A., Gayle R. B., 3rd, Cerretti D. P. Ligands for the receptor tyrosine kinases hek and elk: isolation of cDNAs encoding a family of proteins. Oncogene. 1995 Jan 19;10(2):299–306. [PubMed] [Google Scholar]
- Lai C., Lemke G. An extended family of protein-tyrosine kinase genes differentially expressed in the vertebrate nervous system. Neuron. 1991 May;6(5):691–704. doi: 10.1016/0896-6273(91)90167-x. [DOI] [PubMed] [Google Scholar]
- Lammer E. J., Chen D. T., Hoar R. M., Agnish N. D., Benke P. J., Braun J. T., Curry C. J., Fernhoff P. M., Grix A. W., Jr, Lott I. T. Retinoic acid embryopathy. N Engl J Med. 1985 Oct 3;313(14):837–841. doi: 10.1056/NEJM198510033131401. [DOI] [PubMed] [Google Scholar]
- Lohnes D., Mark M., Mendelsohn C., Dollé P., Dierich A., Gorry P., Gansmuller A., Chambon P. Function of the retinoic acid receptors (RARs) during development (I). Craniofacial and skeletal abnormalities in RAR double mutants. Development. 1994 Oct;120(10):2723–2748. doi: 10.1242/dev.120.10.2723. [DOI] [PubMed] [Google Scholar]
- Nagy A., Rossant J., Nagy R., Abramow-Newerly W., Roder J. C. Derivation of completely cell culture-derived mice from early-passage embryonic stem cells. Proc Natl Acad Sci U S A. 1993 Sep 15;90(18):8424–8428. doi: 10.1073/pnas.90.18.8424. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pandey A., Lindberg R. A., Dixit V. M. Cell signalling. Receptor orphans find a family. Curr Biol. 1995 Sep 1;5(9):986–989. doi: 10.1016/s0960-9822(95)00195-3. [DOI] [PubMed] [Google Scholar]
- Pasquale E. B., Connor R. J., Rocholl D., Schnürch H., Risau W. Cek5, a tyrosine kinase of the Eph subclass, is activated during neural retina differentiation. Dev Biol. 1994 Jun;163(2):491–502. doi: 10.1006/dbio.1994.1165. [DOI] [PubMed] [Google Scholar]
- Pasquale E. B. Identification of chicken embryo kinase 5, a developmentally regulated receptor-type tyrosine kinase of the Eph family. Cell Regul. 1991 Jul;2(7):523–534. doi: 10.1091/mbc.2.7.523. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rathjen F. G., Schachner M. Immunocytological and biochemical characterization of a new neuronal cell surface component (L1 antigen) which is involved in cell adhesion. EMBO J. 1984 Jan;3(1):1–10. doi: 10.1002/j.1460-2075.1984.tb01753.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- SPERRY R. W. CHEMOAFFINITY IN THE ORDERLY GROWTH OF NERVE FIBER PATTERNS AND CONNECTIONS. Proc Natl Acad Sci U S A. 1963 Oct;50:703–710. doi: 10.1073/pnas.50.4.703. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sajjadi F. G., Pasquale E. B. Five novel avian Eph-related tyrosine kinases are differentially expressed. Oncogene. 1993 Jul;8(7):1807–1813. [PubMed] [Google Scholar]
- Settleman J., Narasimhan V., Foster L. C., Weinberg R. A. Molecular cloning of cDNAs encoding the GAP-associated protein p190: implications for a signaling pathway from ras to the nucleus. Cell. 1992 May 1;69(3):539–549. doi: 10.1016/0092-8674(92)90454-k. [DOI] [PubMed] [Google Scholar]
- Shuler C. F., Halpern D. E., Guo Y., Sank A. C. Medial edge epithelium fate traced by cell lineage analysis during epithelial-mesenchymal transformation in vivo. Dev Biol. 1992 Dec;154(2):318–330. doi: 10.1016/0012-1606(92)90071-n. [DOI] [PubMed] [Google Scholar]
- Silver J., Edwards M. A., Levitt P. Immunocytochemical demonstration of early appearing astroglial structures that form boundaries and pathways along axon tracts in the fetal brain. J Comp Neurol. 1993 Feb 15;328(3):415–436. doi: 10.1002/cne.903280308. [DOI] [PubMed] [Google Scholar]
- Silver J., Lorenz S. E., Wahlsten D., Coughlin J. Axonal guidance during development of the great cerebral commissures: descriptive and experimental studies, in vivo, on the role of preformed glial pathways. J Comp Neurol. 1982 Sep 1;210(1):10–29. doi: 10.1002/cne.902100103. [DOI] [PubMed] [Google Scholar]
- Tessier-Lavigne M. Eph receptor tyrosine kinases, axon repulsion, and the development of topographic maps. Cell. 1995 Aug 11;82(3):345–348. doi: 10.1016/0092-8674(95)90421-2. [DOI] [PubMed] [Google Scholar]
- Tuzi N. L., Gullick W. J. eph, the largest known family of putative growth factor receptors. Br J Cancer. 1994 Mar;69(3):417–421. doi: 10.1038/bjc.1994.77. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tybulewicz V. L., Crawford C. E., Jackson P. K., Bronson R. T., Mulligan R. C. Neonatal lethality and lymphopenia in mice with a homozygous disruption of the c-abl proto-oncogene. Cell. 1991 Jun 28;65(7):1153–1163. doi: 10.1016/0092-8674(91)90011-m. [DOI] [PubMed] [Google Scholar]
- Valenzuela D. M., Rojas E., Griffiths J. A., Compton D. L., Gisser M., Ip N. Y., Goldfarb M., Yancopoulos G. D. Identification of full-length and truncated forms of Ehk-3, a novel member of the Eph receptor tyrosine kinase family. Oncogene. 1995 Apr 20;10(8):1573–1580. [PubMed] [Google Scholar]
- Wahlsten D., Bulman-Fleming B. Retarded growth of the medial septum: a major gene effect in acallosal mice. Brain Res Dev Brain Res. 1994 Feb 18;77(2):203–214. doi: 10.1016/0165-3806(94)90197-x. [DOI] [PubMed] [Google Scholar]
- Wahlsten D. Genetic and developmental defects of the mouse corpus callosum. Experientia. 1989 Sep 15;45(9):828–838. doi: 10.1007/BF01954057. [DOI] [PubMed] [Google Scholar]
- Winslow J. W., Moran P., Valverde J., Shih A., Yuan J. Q., Wong S. C., Tsai S. P., Goddard A., Henzel W. J., Hefti F. Cloning of AL-1, a ligand for an Eph-related tyrosine kinase receptor involved in axon bundle formation. Neuron. 1995 May;14(5):973–981. doi: 10.1016/0896-6273(95)90335-6. [DOI] [PubMed] [Google Scholar]
- Wolfer D. P., Henehan-Beatty A., Stoeckli E. T., Sonderegger P., Lipp H. P. Distribution of TAG-1/axonin-1 in fibre tracts and migratory streams of the developing mouse nervous system. J Comp Neurol. 1994 Jul 1;345(1):1–32. doi: 10.1002/cne.903450102. [DOI] [PubMed] [Google Scholar]
- Woolf N. J., Butcher L. L. Cholinergic systems in the rat brain: II. Projections to the interpeduncular nucleus. Brain Res Bull. 1985 Jan;14(1):63–83. doi: 10.1016/0361-9230(85)90178-9. [DOI] [PubMed] [Google Scholar]
- Xu Q., Alldus G., Macdonald R., Wilkinson D. G., Holder N. Function of the Eph-related kinase rtk1 in patterning of the zebrafish forebrain. Nature. 1996 May 23;381(6580):319–322. doi: 10.1038/381319a0. [DOI] [PubMed] [Google Scholar]
- Zhou R., Copeland T. D., Kromer L. F., Schulz N. T. Isolation and characterization of Bsk, a growth factor receptor-like tyrosine kinase associated with the limbic system. J Neurosci Res. 1994 Jan;37(1):129–143. doi: 10.1002/jnr.490370117. [DOI] [PubMed] [Google Scholar]