ABSTRACT
We evaluated a genital herpes prophylactic vaccine containing herpes simplex virus 2 (HSV-2) glycoproteins C (gC2) and D (gD2) to stimulate humoral immunity and UL19 (capsid protein VP5) and UL47 (tegument protein VP13/14) as T cell immunogens. The HSV-2 gC2 and gD2 proteins were expressed in baculovirus, while the UL19 and UL47 genes were expressed from replication-defective adenovirus vectors. Adenovirus vectors containing UL19 and UL47 stimulated human and murine CD4+ and CD8+ T cell responses. Guinea pigs were either (i) mock immunized; (ii) immunized with gC2/gD2, with CpG and alum as adjuvants; (iii) immunized with the UL19/UL47 adenovirus vectors; or (iv) immunized with the combination of gC2/gD2-CpG/alum and the UL19/UL47 adenovirus vectors. Immunization with gC2/gD2 produced potent neutralizing antibodies, while UL19 and UL47 also stimulated antibody responses. After intravaginal HSV-2 challenge, the mock and UL19/UL47 adenovirus groups developed severe acute disease, while 2/8 animals in the gC2/gD2-only group and none in the combined group developed acute disease. No animals in the gC2/gD2 or combined group developed recurrent disease; however, 5/8 animals in each group had subclinical shedding of HSV-2 DNA, on 15/168 days for the gC2/gD2 group and 13/168 days for the combined group. Lumbosacral dorsal root ganglia were positive for HSV-2 DNA and latency-associated transcripts for 5/8 animals in the gC2/gD2 group and 2/8 animals in the combined group. None of the differences comparing the gC2/gD2-only group and the combined group were statistically significant. Therefore, adding the T cell immunogens UL19 and UL47 to the gC2/gD2 vaccine did not significantly reduce genital disease and vaginal HSV-2 DNA shedding compared with the excellent protection provided by gC2/gD2 in the guinea pig model.
IMPORTANCE HSV-2 infection is a common cause of genital ulcer disease and a significant public health concern. Genital herpes increases the risk of transmission and acquisition of HIV-1 infection 3- to 4-fold. A herpes vaccine that prevents genital lesions and asymptomatic genital shedding will have a substantial impact on two epidemics, i.e., both the HSV-2 and HIV-1 epidemics. We previously reported that a vaccine containing HSV-2 glycoprotein C (gC2) and glycoprotein D (gD2) reduced genital lesions and asymptomatic HSV-2 genital shedding in guinea pigs, yet the protection was not complete. We evaluated whether adding the T cell immunogens UL19 (capsid protein VP5) and UL47 (tegument protein VP13/14) would enhance the protection provided by the gC2/gD2 vaccine, which produces potent antibody responses. Here we report the efficacy of a combination vaccine containing gC2/gD2 and UL19/UL47 for prevention of genital disease, vaginal shedding of HSV-2 DNA, and latent infection of dorsal root ganglia in guinea pigs.
INTRODUCTION
Genital herpes is one of the most common sexually transmitted infections. An estimated 536 million people between the ages of 15 and 49 years are infected worldwide, with 23.6 million new infections annually (1). Herpes simplex virus 2 (HSV-2) establishes a latent infection in lumbosacral dorsal root ganglia (DRG) and undergoes frequent reactivations. In immunocompetent individuals, most primary and recurrent infections are asymptomatic; however, some individuals develop 4 or more symptomatic recurrences annually (2–4). Other manifestations include meningitis in adolescents and adults and neonatal herpes if newborns become infected during labor and delivery (2, 5, 6). Neonatal herpes may result in long-term neurologic complications or death (7). Primary and recurrent HSV-2 infections increase the risk of acquiring and transmitting HIV-1 approximately 3- to 4-fold (8–10). In immunosuppressed individuals, genital herpes recurrences are frequent and often severe (11). Daily suppressive antiviral therapy decreases symptomatic recurrences, asymptomatic genital viral shedding, and transmission to partners; however, the protection is incomplete, since antiviral therapy does not totally prevent recurrences or eradicate latency (12–15). HSV-2 is an important target for vaccine development to reduce HIV acquisition and transmission and prevent genital ulcer disease and neonatal infection.
Potent antibody and T cell responses will likely be required for an effective herpes vaccine. The importance of antibodies is supported by the results of the GlaxoSmithKline glycoprotein D2 (gD2) subunit antigen vaccine trial, which identified antibodies as a correlate of protection against HSV-1 infection and disease (16, 17). We previously demonstrated that the HSV-1 and HSV-2 gC proteins reduce the effectiveness of antibodies and complement in host defense (18–24). This observation led to studies using HSV-1 or HSV-2 gC subunit antigens as immunogens to induce antibodies that bind to gC and block its immune evasion functions (25–27). In a comparison of a bivalent gC2/gD2 subunit antigen vaccine and vaccines containing either subunit antigen alone, the bivalent vaccine provided significantly higher neutralizing antibody titers in the presence of complement and was significantly better at preventing DRG infection in mice and vaginal shedding of HSV-2 DNA during recurrent infection in guinea pigs (26). Our intent in adding gC2 to gD2 was to improve vaccine-induced humoral immunity; however, we also demonstrated that gC2 and gD2 stimulated gamma interferon (IFN-γ)- and tumor necrosis factor alpha (TNF-α)-producing CD4+ and CD8+ T cells. While the bivalent vaccine significantly reduced the number of days of vaginal HSV-2 DNA shedding compared to that with a vaccine containing gD2 alone, it did not eradicate shedding, which led to the consideration of including additional T cell immunogens in vaccine studies.
CD8+ T cells have been implicated in clearing HSV infection, and CD4+ T cells have been implicated in providing helper functions to maintain CD8+ T cell immunity and antibody class switching (28, 29). HSV-specific CD8+ T cells with IFN-γ effector functions have been isolated from human trigeminal ganglia, supporting a role for CD8+ T cells in controlling reactivation (30). CD8+ T cell infiltration correlates with the clearance of recurrent genital lesions, and HSV-2-specific CD8+ T cells are observed to accumulate adjacent to sensory nerve endings at the epidermal-dermal junction in biopsy specimens from human HSV-2-induced genital lesions (29, 31). Additionally, human studies performed on HSV-2-infected and HSV-2-exposed but antibody-seronegative individuals support the importance of CD4+ and CD8+ T cell responses that recognize multiple envelope glycoproteins and capsid, tegument, and immediate early proteins (32–38). HIV/AIDS patients with reduced numbers of CD4+ T cells are at increased risk of recurrent disease and HSV-2 genital shedding (39–41). In murine models, IFN-γ-producing CD4+ T cells provide protection against vaginal HSV-2 challenge and IFN-γ-secreting CD8+ T cells control HSV-1 reactivation in trigeminal ganglia (28, 42–44).
A bivalent subunit antigen vaccine containing gD2 and ICP4, an immediate early protein that is both a CD4+ and CD8+ T cell immunogen, was used as a therapeutic vaccine to treat recurrent genital disease in HSV-2-infected guinea pigs (38). The bivalent vaccine was better than vaccines with either antigen alone in preventing recurrent lesions and vaginal shedding of HSV-2 DNA, suggesting a role for T cells in controlling genital recurrences. In this study, we evaluated the T cell immunogens VP5 (major capsid protein) and VP13/14 (tegument protein), which are the protein products of UL19 and UL47, respectively. These proteins were selected based on their ability to elicit CD4+ and CD8+ T cell responses in peripheral blood mononuclear cells (PBMC) from HSV-2-seropositive individuals and HSV-seronegative individuals exposed to HSV (35–37, 45–47). In addition, UL47 contains HLA A*0201-restricted CD8+ T cell epitopes, and approximately 40 to 50% of persons in most ethnic groups express HLA A*0201 (48). We evaluated adenovirus (Ad) vectors containing UL19 and UL47 for stimulation of CD4+ and CD8+ T cell responses in mice and for recognition by UL19- and UL47-specific human CD4+ and CD8+ T cell clones derived from HSV-2-seropositive individuals. Furthermore, we assessed the efficacy of a bivalent gC2/gD2 subunit antigen vaccine, an adenovirus vector vaccine expressing UL19 and UL47, and a combination vaccine containing gC2/gD2 and the UL19 and UL47 adenovirus vectors for prevention of primary and recurrent genital disease, vaginal shedding of HSV-2 DNA, and latent DRG infection in guinea pigs.
MATERIALS AND METHODS
Immunogens, virus, complement, and Ad28 and Ad35 constructs.
The baculovirus-expressed gC2 protein, bac-gC2(426t), includes amino acids 27 to 426 of the protein from HSV-2 strain 333, where amino acid 27 is the first amino acid after the signal peptide (49). The baculovirus-expressed gD2 protein, bac-gD2(306t), extends from amino acids 26 to 331 of the protein from HSV-2 strain 333, where amino acid 26 is the first amino acid after the signal sequence (50, 51). HSV-2 strain MS was grown and titrated on Vero cells (26). Human complement was obtained from an HSV-1- and HSV-2-seronegative donor.
Codon-optimized sequences of UL19 and UL47 were inserted into replication-defective Ad28 and Ad35 vectors. The Ad28 and Ad35 vectors with UL19 and UL47 inserts are referred to as Ad28::UL19, Ad28::UL47, Ad35::UL19, and Ad35::UL47. The UL19 and UL47 sequences were derived from HSV-2 HG52 (GenBank accession no. Z86099). The 4,122-bp UL19 and 2,088-bp UL47 transgene cassettes were codon optimized by use of custom codon optimization software by GenArt (Life Technologies). The codon-optimized sequences were subsequently synthesized and cloned into the multiple-cloning site of pUC18. Recombinant E1E4-deleted Ad28 vectors and recombinant E1-deleted Ad35 vectors carrying the UL19 and UL47 expression cassettes were constructed from the pUC18 transgene plasmids by using the GenVec AdFAST system (52–55). The Ad28 and Ad35 vectors were propagated on 293-ORF6 cells, and the titers of vector genomes were determined (55, 56).
Evaluation of adenovirus expressing UL19 by use of a human CD4+ readout T cell clone.
CD4+ T cell clone ESL2.2 was recovered from a genital herpes lesion and is specific for an epitope within HSV-2 UL19-encoded amino acids 1079 to 1319 (34). ESL2.2 cells (104 cells/well) were coincubated with autologous irradiated PBMC (105 cells/well) and an adenovirus vector expressing UL19 in 96-well U-bottom plates (33). Mock-infected Vero cells and adenovirus vectors expressing green fluorescent protein (GFP) were used as negative controls, while UV-treated HSV-2 strain 186 was used as a positive control. After 72 h, 1 μCi/well [3H]thymidine was added, and cells were harvested 18 h later (57). The mean ± standard deviation [3H]thymidine incorporation is expressed in counts per minute (cpm) for ESL2.2 cells stimulated by the adenovirus vectors or as the cpm for UV-inactivated virus minus the cpm for mock-infected Vero cells.
Evaluation of adenovirus expressing UL47 by use of a human CD8+ readout T cell clone.
COS-7 cells were mock transfected or transfected with HLA A*0201 cDNA overnight in 96-well flat-bottom tissue culture plates in triplicate prior to the addition of an adenovirus expressing UL47 or GFP (control) (46, 58). As a positive control, a full-length UL47 expression plasmid was cotransfected with HLA A*0201. After 24 h, a CD8+ T cell clone specific for HSV-2 UL47 amino acids 551 to 559 from an HSV-2-infected individual was added (5 × 104 cells/well). Supernatant fluids were harvested 24 h later, and an IFN-γ-specific enzyme-linked immunosorbent assay (ELISA) was performed (46).
Mouse immunizations and measurements of cellular immune responses.
Laboratory animals were housed and handled according to the guidelines of the Institutional Animal Care and Use Committee (IACUC) of the University of Washington. C57BL/6 mice were immunized intramuscularly (i.m.) in the hind legs with Ad35::UL19 or Ad28::UL19, using 106 to 1010 PFU (5 mice per group), on days 0 and 28. Similarly, mice were immunized with Ad35::UL47 or Ad28::UL47 at doses ranging from 105 to 1010 PFU per immunization. Five control mice received phosphate-buffered saline (PBS) on the same schedule. Spleens were isolated on day 33, and cellular immune responses were measured by intracellular cytokine cytometry analysis.
Overlapping peptide libraries of full-length VP5 (UL19) and VP13/14 (UL47) were used to stimulate splenic lymphocytes. HSV-2 HG52 was used to design peptide arrays containing 15-amino-acid peptides with an 11-amino-acid overlap for UL19 (1,374 amino acids) and UL47 (696 amino acids) (New England Peptide). Four pools were created for UL19, containing 85 or 86 peptides each, and two pools were created for UL47, with 86 peptides each, with each peptide present at a final concentration of 2 μg/ml (59, 60).
Guinea pig immunizations and HSV-2 challenge studies.
Laboratory animals were housed and handled according to the guidelines of the IACUC of the University of Pennsylvania. Outbred Hartley strain guinea pigs weighing 275 to 350 g (Charles River) were either mock immunized i.m. with the CpG oligonucleotide TCGTCGTTGTCGTTTTGTCGTT (Trilink Inc.) and alum (Alhydrogel; Accurate Chemical and Scientific Corporation, NY) or immunized i.m. with adenovirus vectors and gC2/gD2 subunit antigens. Ten guinea pigs were assigned to the mock group and eight animals to each of the other three groups. The mock group received CpG and alum on days 0, 14, and 28, empty Ad28 vector on day 7, and empty Ad35 vector on day 35. The adenovirus vector-only group received Ad28::UL19 in one hind leg and Ad28::UL47 in the other hind leg on day 7 and Ad35::UL19 and Ad35::UL47 in the opposite hind legs on day 35. Preliminary experiments with mice determined that immunizing mice with one set of adenovirus vectors containing each of the inserts on day 1 followed by a booster on day 28 with the other set of adenovirus vectors containing the gene inserts gave the strongest IFN-γ response as measured by enzyme-linked immunosorbent spot (ELISPOT) assay (data not shown). The subunit antigen-only group received bac-gC2(426t) and bac-gD2(306t) given with CpG/alum i.m. in one hind leg on days 0, 14, and 28. The combined adenovirus vector and subunit antigen group received bac-gC2(426t) and bac-gD2(306t) with CpG/alum given i.m. in one hind leg on days 0, 14, and 28, Ad28::UL19 and Ad28::UL47 given in opposite hind legs on day 7, and Ad35::UL19 and Ad35::UL47 given in opposite hind legs on day 35. The subunit antigen vaccines contained 10 μg gC2 and 5 μg gD2 mixed with CpG at 100 μg/guinea pig and alum at 20 μg/μg protein in a total volume of 50 μl per immunization (26). Individual antigens were first mixed with CpG and alum and then combined just prior to immunization. Adenovirus vector immunizations were given in 50 μl PBS containing 1 × 1010 PFU.
Some guinea pigs were bled from a hind leg saphenous vein prior to the first immunization, and all animals were bled 14 to 17 days after the final immunization. Ten days later, animals were infected intravaginally with 5 × 105 PFU of HSV-2 strain MS (approximately 500 50% lethal doses [LD50], based on our unpublished dose-response studies of guinea pigs) and scored over 14 days for acute disease on a scale of 0 to 4, where 0 reflects no disease, 1 redness, 2 a single lesion, 3 coalesced lesions, and 4 ulcerated lesions (61). Urinary retention and hind leg weakness were also recorded. To assist some mock-immunized animals to survive the acute infection, 5 mg/ml acyclovir was placed in the drinking water of six guinea pigs for 7 days, starting at 48 h postinfection. Guinea pigs were swabbed daily for 6 days for determination of vaginal viral titers after vaginal challenge and were observed daily on 44 of 46 days from days 15 to 60 for recurrent genital disease. Vaginal swabs for HSV-2 DNA copy number determinations were obtained on 21 days, between days 28 and 49 after challenge. Swabs were stored at −80°C prior to culture or processing for HSV-2 DNA measurement by quantitative PCR (qPCR).
ELISA, neutralizing antibodies, and Western blotting.
ELISA titers to gC2 and gD2 were measured by coating plates with 200 ng of antigen and adding serial dilutions of serum, starting at a dilution of 1:100 (26). The endpoint titer was considered the highest dilution that resulted in an optical density (OD) of ≥0.1 and that was at least 2-fold higher than the OD of sera from mock-immunized animals at that dilution. A negative result was assigned a titer of 1:50. Neutralizing antibodies were measured by heating serum to 56°C for 30 min to inactivate guinea pig complement and then incubating the serum at a 1:40 dilution with 10% human serum from an HSV-1- and -2-seronegative donor as a source of complement and with 1 × 105 to 5 × 105 PFU/100 μl of HSV-2 at 37°C for 1 h. Virus titers were determined by plaque assay on Vero cells and are reported as log10 PFU/100 μl. Western blotting was performed using Vero cells transfected with Ad28 and Ad35 expressing UL19 and UL47 to confirm the expression of VP5 and VP13/14. VP5 (UL19) was detected using anti-HSV-UL19 monoclonal antibody (MAb) 3B6 (Abcam), while VP13/14 (UL47) was identified using a polyclonal antibody prepared by immunizing mice with a DNA carrying full-length UL47 (62). To evaluate whether guinea pigs immunized with adenovirus vectors expressing UL19 and UL47 produced antibodies to the corresponding viral proteins, Western blotting was performed using sucrose gradient-purified HSV-2 obtained from infected Vero cells or mock-infected Vero cells (as a control).
Determination of HSV-2 DNA copy number by real-time qPCR.
DNAs were isolated from guinea pig vaginal swabs or proteinase K-treated DRG by using a QIAamp 96 DNA blood kit (Qiagen). HSV-2 genome copies were quantitated by real-time PCR to detect the HSV-2 glycoprotein B (gB) gene (63). Each 30-μl PCR mix contained 10 μl of extracted DNA, 15 μl of 2× QuantiTect multiplex PCR master mix (Qiagen), 830 nM (each) HSV-2 gB primer, 100 nM gB probe, 2.5 × 104 copies of Exo as an internal control DNA template, 100 nM (each) Exo primer, 125 nM Exo probe, and 0.03 U of uracil N-glycosylase (UNG) to eliminate carryover of PCR products. An ABI 7900 HT machine was used for detection of DNA amplification. The negative result was accepted only if the Exo internal control was detected. To monitor for possible contamination, every 5 samples were matched with 1 negative-control sample, which was coprocessed with the experimental samples. Each PCR run also included two no-template negative controls. Vaginal swab samples that contained ≥3.0 copies of HSV-2 DNA, which is equivalent to ≥150 copies/ml, were considered positive (63).
HSV-2 LAT and DNA copies in DRG.
Total RNA was isolated from guinea pig DRG by using an RNeasy minikit (Qiagen) in conjunction with DNase I treatment. HSV-2 latency-associated transcripts (LAT) and the guinea pig glyceraldehyde-3-phosphate dehydrogenase (GAPDH) gene were detected by real-time reverse transcription-PCR (RT-PCR). Each 50-μl one-step real-time RT-PCR mixture contained 2 μl of RNA, 25 μl of 2× QuantiTect multiplex RT-PCR master mix, 830 nM (each) primer, 100 nM (each) probe, 0.05 U of UNG, 0.5 μl of QuantiTect multiplex RT mix, 2.5 × 104 copies of Exo internal control RNA template, 100 nM (each) Exo primer, and 125 nM Exo probe. As a control, reverse transcriptase was omitted from the reaction mix to verify that DNA was inactivated. The cycling conditions were 20 min at 50°C, 15 min at 95°C, and then 45 cycles of 94°C for 45 s and 60°C for 45 s. The primers and probes for HSV-2 LAT and GAPDH were as follows: HSV-2 LAT forward primer, 5′ GTC AAC ACG GAC ACA CTC TTT TT; LAT reverse primer, 5′ CGA GGC CTG TTG GTC TTT ATG; LAT probe, 5′ 6-carboxyfluorescein (FAM)-CAC CCA CCA AGA CAG GGA GCC A-6-carboxytetramethylrhodamine (TAMRA); guinea pig GAPDH forward primer, 5′ CCG CAT CGG TAT TCC TTC TTC; GAPDH reverse primer, 5′ TCC GAC CTT CAC CAT CTT GTC; and GAPDH probe, 5′ FAM-CGT GCA GTG CCA GCC GCA AC-TAMRA (55). LAT and GAPDH results were plotted as the real-time PCR cycle threshold (CT) values, and the CT was defined as the number of cycles of amplification required for the fluorescence signal to exceed the background level (threshold).
Statistics.
The significance of differences in survival was calculated using the log rank (Mantel-Cox) test. Fisher's exact test was performed to compare the numbers of guinea pigs in each group that developed disease, the numbers of recurrent lesion days, and the numbers of DNA-positive days of shedding. Analysis of variance (nonparametric test) followed by Tukey's posttest analysis was performed for all possible pairwise comparisons.
RESULTS
Expression of UL19 and UL47.
293T cells were transduced with an Ad28 vector containing HSV-2 UL19 DNA (Ad28::UL19) or an Ad35 vector containing HSV-2 UL47 DNA (Ad35::UL47). Forty-eight hours later, cells were lysed and protein expression confirmed by Western blotting. Proteins of the expected size were detected in the 293T-transduced cells expressing the protein products of UL19 (VP5) (Fig. 1A) and UL47 (VP13/14) (Fig. 1B). Sucrose gradient-purified HSV-2 was UV inactivated and used as a positive control for VP5 and VP13/14, while mock-infected Vero cells were purified on a sucrose gradient and served as an uninfected control.
FIG 1.
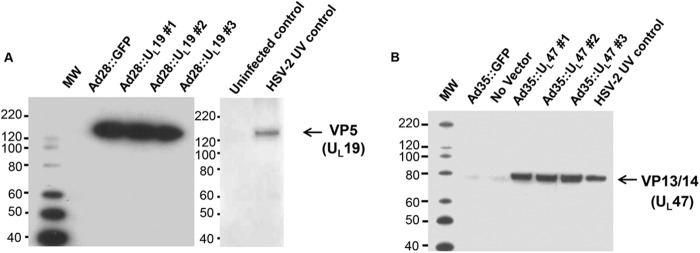
Western blots showing expression of UL19 and UL47 in adenovirus vectors. Three clones were evaluated for expression of UL19 (VP5) (A) and UL47 (VP13/14) (B). Ad28::GFP and Ad35::GFP refer to adenovirus vectors containing GFP sequences instead of HSV-2 DNA. HSV-2 UV control, sucrose gradient-purified virus prepared from HSV-2-infected Vero cells; uninfected control, sucrose gradient purification of mock-infected Vero cells. In panel B, a faint, nonspecific band is visible just below VP13/14 and in the no-vector and Ad35::GFP lanes. The positions of molecular weight (MW) markers are indicated.
HLA class II antigen processing of HSV-2 UL19 expressed in Ad28 or Ad35.
To evaluate whether HSV-2 UL19 expressed from Ad28 or Ad35 can be processed and presented to CD4+ T cells, the UL19-specific human CD4+ T cell clone ESL2.2 was incubated with autologous irradiated PBMC as antigen-presenting cells (APC) and with Ad28::UL19, Ad35::UL19, or UV-treated HSV-2 strain 186 (positive control). The responder cells were also incubated with mock-infected Vero cells and Ad28 or Ad35 expressing GFP as negative controls. Proliferative responses to these negative-control stimuli were absent (Fig. 2A). UV-inactivated HSV-2 gave a strong positive response. Both Ad35 and Ad28 expressing UL19 stimulated the proliferation of the human CD4+ T cell clone (P < 0.001 for comparing Ad35::UL19 or Ad28::UL19 to Ad35::GFP or Ad28::GFP). Ad35::UL19 stimulated significantly greater proliferation of human CD4+ T cells than Ad28::UL19 did (P < 0.001). Therefore, UL19 expressed by Ad28::UL19 and Ad35::UL19 can be processed by APC and presented to human CD4+ T cells. A similar assay for HLA class II antigen presentation of UL47 was not performed because we do not have a human CD4+ T cell clone that recognizes epitopes within UL47.
FIG 2.
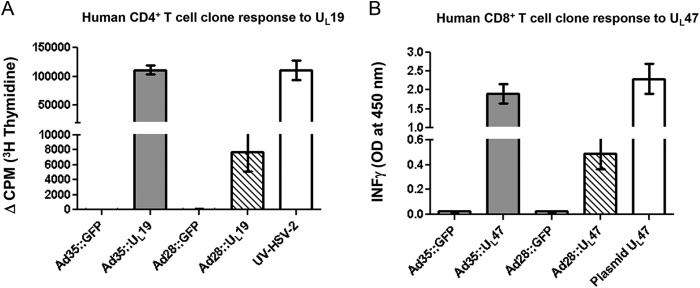
CD4+ and CD8+ T cell stimulation by adenovirus vectors expressing UL19 and UL47. (A) The human CD4+ T cell clone ESL2.2 was incubated with HLA-matched irradiated human PBMC. Ad35::UL19 or Ad28::UL19 was added as a test stimulus, while GFP-expressing adenovirus vectors were used as negative controls and UV-inactivated HSV-2 was used as a positive control. (B) HLA A*0201-transfected COS-7 cells were transduced with Ad35::UL47 or Ad28::UL47. ELISA was performed to measure IFN-γ production. The UL47 plasmid served as a positive control to stimulate a human UL47-specific CD8+ T cell clone. Ad28::GFP and Ad35::GFP were used as negative controls.
HSV-2 UL47 expressed in Ad28 or Ad35 stimulates a human CD8+ T cell clone.
HLA A*0201-transfected COS-7 cells were infected with Ad28::UL47 or Ad35::UL47 and incubated with a human CD8+ T cell clone specific for HSV-2 UL47-encoded amino acids 551 to 559. The supernatant fluids were analyzed for IFN-γ release. The negative controls, Ad35::GFP and Ad28::GFP, did not stimulate CD8+ T cells, while Ad35::UL47 and Ad28::UL47 resulted in IFN-γ release (Fig. 2B). As a positive control, a UL47 full-length plasmid was transfected into HLA A*0201-transduced COS-7 cells, which stimulated IFN-γ release. Ad28::UL47 showed a modest stimulation of CD8+ T cells, while Ad35::UL47 was a more potent inducer of IFN-γ (P < 0.001 for comparing Ad28::UL47 or Ad35::UL47 to Ad28 or Ad35 expressing GFP; P < 0.001 for comparing Ad35::UL47 to Ad28::UL47). Therefore, UL47 expressed in COS-7 cells after infection with Ad35::UL47 or Ad28::UL47 was processed and presented to human CD8+ T cells. A similar assay for HLA class I antigen presentation of UL19 was not performed because we do not have a human CD8+ T cell clone that recognizes epitopes within UL19.
CD8+ T cell responses to Ad28 or Ad35 expressing UL19 or UL47 in C57BL/6 mice.
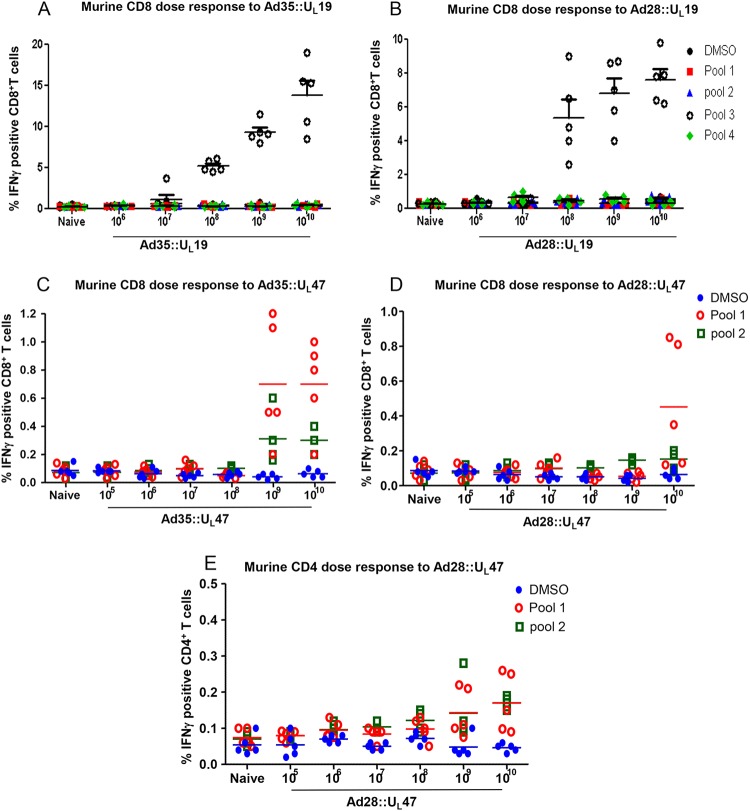
Dose determination for cellular immune responses directed toward UL19 was performed in C57BL/6 mice. Following two immunizations with a range of doses of Ad35::UL19 or Ad28::UL19, from 106 to 1010 PFU, splenocytes were isolated and stimulated with dimethyl sulfoxide (DMSO) or four pools containing 85 or 86 peptides each, spanning 1,374 amino acids (Fig. 3A and B). No induction of IFN-γ was observed for splenocytes from naive mice stimulated with any peptide pool or with DMSO. Significant stimulation of IFN-γ-positive CD8+ T cells was observed in splenocytes from mice immunized with 108, 109, or 1010 PFU of Ad35::UL19 when the cells were incubated with peptide pool 3 (Fig. 3A). Similarly, Ad28::UL19 stimulated IFN-γ positive CD8+ T cells at doses of ≥108 PFU (Fig. 3B) (P < 0.001 for comparing naive or DMSO-stimulated samples to samples obtained from mice immunized with 108 to 1010 PFU of Ad35 or Ad28 expressing UL19 and stimulated with peptide pool 3). A dose of 1010 PFU of Ad35::UL19 or Ad28::UL19 produced the largest proportion of IFN-γ-producing CD8+ T cells.
FIG 3.
Dose determination and immunogenicity in C57BL/6 mice. (A and B) Mice were immunized i.m. twice with 106 to 1010 PFU of Ad35::UL19 or Ad28::UL19. Splenocytes were stimulated with 4 peptide pools of UL19 or with DMSO. Fluorescence-activated cell sorting (FACS) was used to detect IFN-γ-positive CD8+ T cells. (C to E) Ad35::UL47 and Ad28::UL47 were evaluated at 105 to 1010 PFU, and splenocytes were stimulated with two peptide pools of UL47 or with DMSO. FACS was used to measure IFN-γ-positive CD8+ T cells (C and D) or CD4+ T cells (E). For all panels, five mice were included in each group.
Mice were immunized twice as described above to assess CD8+ T cell responses to Ad35::UL47 or Ad28::UL47. Splenocytes from naive mice exhibited no induction with DMSO or two peptide pools, each containing 86 peptides spanning 696 amino acids. Mice immunized with 109 or 1010 PFU of Ad35::UL47 had a significant increase in IFN-γ-positive CD8+ T cells when splenocytes were stimulated with peptide pool 1 (P < 0.01 for comparing naive or DMSO-stimulated splenocytes to splenocytes from mice immunized with 109 or 1010 PFU of Ad35::UL47) (Fig. 3C). A small, nonsignificant induction of IFN-γ-positive CD8+ T cells was noted with peptide pool 2. A significant increase in IFN-γ-producing CD8+ T cells was noted for splenocytes obtained from animals immunized with 1010 PFU of Ad28::UL47 and stimulated with peptide pool 1 (P < 0.05 for comparing naive or DMSO-stimulated splenocytes to splenocytes from mice immunized with 1010 PFU of Ad28::UL47) (Fig. 3D). Therefore, Ad28 and Ad35 vectors containing UL19 and UL47 stimulated murine CD8+ T cells, with higher levels of IFN-γ responses detected in mice immunized with UL19 than with UL47. An additional experiment was performed using Ad28::UL47 to stimulate murine CD4+ T cells. A modest but significant increase in IFN-γ-producing CD4+ T cells was noted at 109 and 1010 PFU for splenocytes obtained from animals immunized with Ad28::UL47 and stimulated with peptide pools 1 and 2 (Fig. 3E) (P < 0.05 for comparing naive or DMSO-stimulated splenocytes to splenocytes obtained from mice that received 109 or 1010 PFU of Ad28::UL47 and stimulated with peptide pools 1 and 2). Therefore, UL19 and UL47 both induce CD4+ and CD8+ T cell responses when evaluated with either human or murine cells.
Guinea pig immunizations with gC2/gD2 subunit antigens and Ad28 and Ad35 vectors.
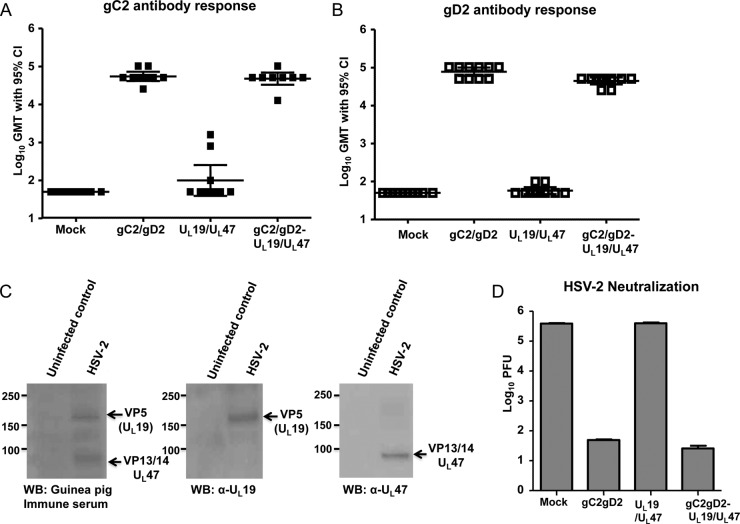
The geometric mean gC2 and gD2 antibody titers in mock-immunized and Ad28- and Ad35-immunized guinea pigs were <1:100 (Fig. 4A and B). The geometric mean gC2 antibody titers in the gC2/gD2 subunit group and the combined group were 1:64,000 and 1:51,200, respectively (Fig. 4A). Three guinea pigs immunized with UL19/UL47 had low positive titers to gC2, which likely represents nonspecific binding. The mean gD2 antibody titers in the gC2/gD2 group and the combined group were 1:72,000 and 1:51,200, respectively (Fig. 4B) (P < 0.001 for comparing gC2 and gD2 titers of the gC2/gD2 or combined group with those of the mock or adenovirus vector-only groups; the difference was not significant for comparing the gC2/gD2 and combined groups). These results indicate that immunization with Ad28 and Ad35 encoding UL19 and UL47 did not blunt the gC2 and gD2 ELISA antibody responses.
FIG 4.
Antibody responses following immunization. Antibody responses to gC2 (A) and gD2 (B) were determined by ELISA, using sera obtained after the third mock immunization or after immunizations with the adenovirus vectors expressing UL19 and UL47, the gC2/gD2 subunit antigens, or the combined subunit and adenovirus antigens. Results represent log10 geometric mean titers (GMT) with 95% confidence intervals (CI) (n = 10 for the mock group and 8 for the other groups). (C) Western blots (WB) obtained using sucrose gradient-purified HSV-2 prepared from infected Vero cells or uninfected controls obtained using sucrose gradient materials from mock-infected Vero cells. The left gel was probed with sera from guinea pigs immunized with adenovirus vectors containing UL19 and UL47, the middle gel was probed with an anti-HSV-UL19 MAb, and the right gel was probed with a mouse polyclonal antiserum to UL47. (D) Log10 titers of HSV-2 incubated with a 1:40 dilution of the same sera as in panels A and B, in the presence of 10% human complement. Results represent virus titers remaining after incubating HSV-2 with antibody and complement, expressed as mean log10 PFU/100 μl ± standard errors of the means (SEM).
Western blotting was performed to evaluate whether guinea pigs dually immunized with Ad28 or Ad35 vectors expressing UL19 and UL47 produced antibodies to VP5 and VP13/14, respectively. The guinea pig immune sera recognized proteins with molecular masses similar to those of VP5 and VP13/14 (Fig. 4C, left gel). We confirmed the presence of the VP5 and VP13/14 proteins by using an anti-HSV-VP5 MAb (Fig. 4C, middle gel) and a mouse anti-VP13/14 polyclonal antibody (Fig. 4C, right gel).
Immunizations with subunit gC2/gD2 and combined gC2/gD2-UL19/UL47 vaccines generate neutralizing antibodies against HSV-2.
Guinea pig sera were tested for neutralizing antibodies in the presence of 10% human complement obtained from an HSV-1/2-seronegative donor. Complement alone at a 10% concentration did not neutralize HSV-2 (data not shown). Mock immunization or immunization with adenovirus vectors containing UL19 and UL47 did not induce neutralizing antibodies to HSV-2 when antibodies were evaluated at a serum dilution of 1:40. Sera from guinea pigs immunized with gC2/gD2 or the combined vaccine in the presence of human complement neutralized approximately 4 log10 more HSV-2 than sera from mock-immunized or UL19/UL47-immunized animals (Fig. 4D) (P < 0.001 for comparing the gC2/gD2 or combined group with the mock or UL19/UL47 group). UL19/UL47 immunization did not interfere with the ability of gC2/gD2 to neutralize HSV-2 in the combination vaccine.
Subunit gC2/gD2 and combined gC2/gD2-UL19/UL47 immunizations protect guinea pigs from acute and recurrent genital disease.
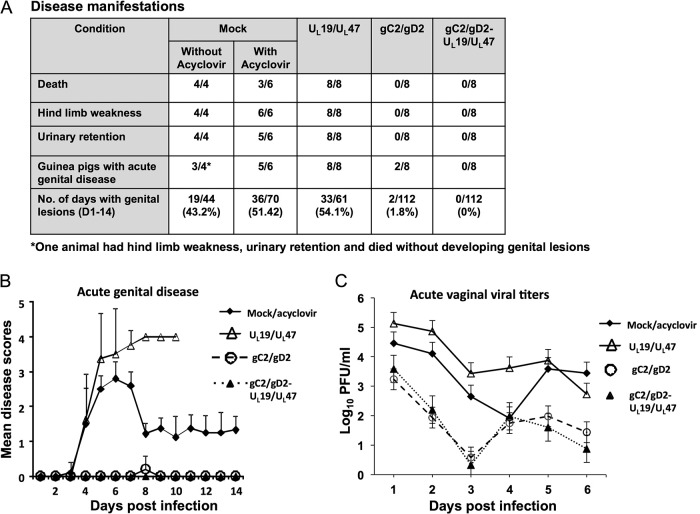
Guinea pigs were challenged intravaginally with 5 × 105 PFU of HSV-2 strain MS (≥500 LD50). Three of six acyclovir-treated mock-immunized guinea pigs survived, while 4/4 animals that were not treated with acyclovir developed severe hind limb weakness and urinary retention and were euthanized. Overall, 8/10 mock-immunized animals developed acute genital lesions (Fig. 5A). All animals immunized with adenovirus vectors containing UL19/UL47 without gC2/gD2 developed acute genital lesions, hind limb weakness, and urinary retention and were euthanized between days 8 and 10. The gC2/gD2 group and the combined group had 100% survival, with no animals developing urinary retention or hind limb weakness. Two of 8 guinea pigs in the gC2/gD2 group had mild genital disease, with each animal developing a single small lesion on day 8 that healed the next day, while the animals in the combined group had no genital disease (Fig. 5B). The mock/acyclovir and UL19/UL47 groups had significantly higher disease scores than the gC2/gD2 or combined group (P < 0.001 for comparing the gC2/gD2 or combined group to the mock or UL19/UL47 group; the difference was not significant for comparing the gC2/gD2 and combined groups). Vaginal viral titers on days 1 to 6 were significantly reduced in the gC2/gD2 and combined groups compared to the mock and UL19/UL47 groups (Fig. 5C) (P < 0.01 for comparing the mock group to the gC2/gD2 or combined group; P < 0.001 for comparing the UL19/UL47 group to the gC2/gD2 or combined group; the difference was not significant for comparing the mock and UL19/UL47 groups or the gC2/gD2 and combined groups). Therefore, immunization with adenoviruses expressing UL19/UL47 did not protect guinea pigs from acute infection, and when these adenoviruses were given with gC2/gD2, they did not have a statistically significant impact on protection against acute disease or acute vaginal viral titers.
FIG 5.
Subunit antigen gC2/gD2 immunization or combined gC2/gD2-UL19/UL47 immunization protects guinea pigs against HSV-2 vaginal challenge. Guinea pigs were mock immunized or immunized with the gC2/gD2 subunit antigens, adenovirus vectors expressing UL19 and UL47, or the combined gC2/gD2 and UL19/UL47 vaccine and challenged with 5 × 105 PFU of HSV-2 MS. (A) Table describing disease manifestations. Six of 10 mock-immunized animals were treated with acyclovir in an effort to have some animals survive the acute infection. (B) Genital disease scores of surviving animals (means ± SEM). (C) Vaginal viral titers on days 1 to 6 postinfection. Results represent log10 PFU per ml ± SEM. “Mock/acyclovir” in panels B and C represents the combination of the six acyclovir-treated and four untreated animals.
Recurrent genital disease and recurrent vaginal shedding of HSV-2 DNA.
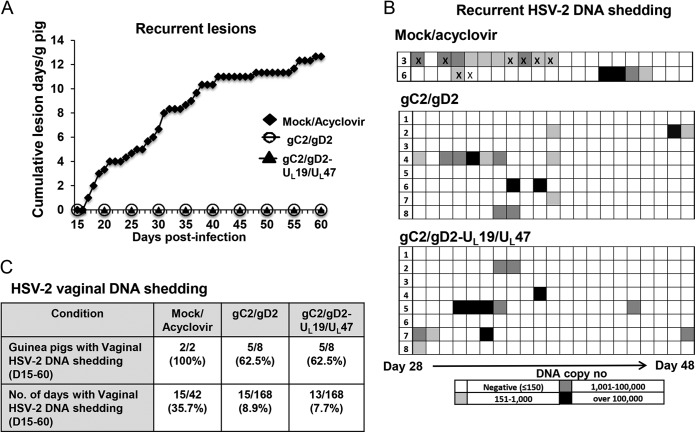
The three surviving acyclovir-treated mock-immunized animals each had recurrent disease between days 15 and 60, with lesions present on 28% of the days and a cumulative average of 12.7 lesion days per animal. None of the UL19/UL47-immunized guinea pigs survived the acute disease, and therefore these animals could not be evaluated for recurrences. The gC2/gD2 and combined groups had no recurrent genital disease (Fig. 6A). Only 2 of the 3 surviving mock-immunized/acyclovir-treated animals were evaluated for HSV-2 DNA shedding, since 1 guinea pig developed scar tissue in the vaginal canal, which prevented vaginal swabbing. HSV-2 DNA was detected on 15/42 days (Fig. 6B and C). Despite the fact that there was no recurrent genital disease, 5/8 guinea pigs in the gC2/gD2 and combined groups had subclinical vaginal shedding of HSV-2 DNA that was detected on 15/168 and 13/168 days, respectively (Fig. 6B and C) (P < 0.01 for comparing the gC2/gD2 or combined group to the mock-immunized group; the difference was not significant for comparing the gC2/gD2 and combined groups). As a control, vaginal swabs were obtained on 21 consecutive days from 6 guinea pigs that were not infected with HSV-2; no HSV-2 DNA was detected in these animals (0/126 swabs) (results not shown). On days with vaginal HSV-2 shedding, the mean HSV-2 DNA copy numbers were 3.3 log10, 3.7 log10, and 4.5 log10 for the mock, gC2/gD2, and combined groups, respectively (the differences were not significant). Therefore, the gC2/gD2 vaccine and the combined vaccine protected 100% of the guinea pigs from recurrent genital disease and reduced the frequency of vaginal HSV-2 DNA shedding but did not prevent shedding and did not lower the copy number of HSV-2 DNA on days with shedding.
FIG 6.
Subunit antigen immunization or combined gC2/gD2-UL19/UL47 immunization completely protects guinea pigs against HSV-2 recurrent genital disease. (A) The occurrence of recurrent genital disease was plotted as the average cumulative number of lesion days per animal from days 15 to 60 after HSV-2 challenge. (B) Vaginal shedding of HSV-2 DNA as measured by qPCR from days 28 to 48 postchallenge. Each block represents 1 day. X's indicate days when genital lesions were observed. (C) Table showing the incidences of vaginal HSV-2 DNA shedding and numbers of HSV-2 DNA shedding days.
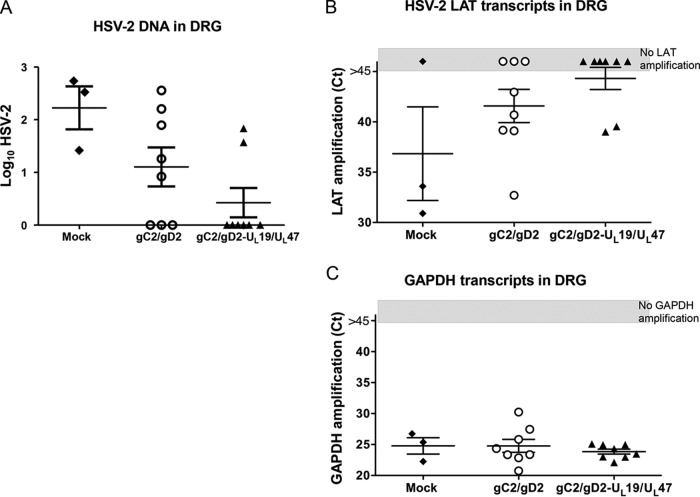
HSV-2 genomic DNA copy numbers and detection of LAT in guinea pig DRG.
Guinea pigs were euthanized at 81 days postinfection, and the lumbosacral DRG were evaluated for HSV-2 DNA copy number and the presence of LAT. The DRG of the three surviving mock-immunized/acyclovir-treated animals were positive for HSV-2 DNA, with a mean of 2.2 log10 DNA copies (Fig. 7A). The DRG from 5/8 guinea pigs in the gC2/gD2 group were positive, with a mean of 1.2 log10 DNA copies, while the combined group had 2/8 positive DRG, with a mean of 0.5 log10 DNA copies (P = 0.31 for comparing the numbers of HSV-2 DNA-positive DRG in the gC2/gD2 and combined groups; the P value was not calculated for comparing the mock-immunized/acyclovir-treated group with the other groups, since only three animals survived). Two of three DRG from mock-immunized/acyclovir-treated animals were positive for LAT, compared with 5/8 DRG from the gC2/gD2 group and 2/8 DRG from the combined group (the difference was not significant for comparing the gC2/gD2 and combined groups) (Fig. 7B). The numbers of GAPDH control transcripts were similar for all groups (Fig. 7C).
FIG 7.
HSV-2 DNA copies and detection of LAT in DRG. DRG were isolated at 81 days postchallenge and analyzed for HSV-2 DNA copies. (A) HSV-2 DNA log10 copy numbers in DRG. (B and C) LAT and GAPDH transcripts in DRG were plotted as real-time PCR CT values. The gray boxes indicate that there was no transcript amplification by 45 PCR cycles. The negative LAT samples were assigned a CT value of 46 (>45 cycles) for calculations of mean ± SEM values. CT values rather than copy numbers were used to express results because purified RNA was not available to develop standard curves.
DISCUSSION
Immunization with a gD2 subunit antigen administered with monophosphoryl lipid A and alum as adjuvants failed to meet primary endpoints in human clinical trials, although a secondary endpoint was met in one of the trials, in that the vaccine prevented genital herpes in HSV-1/2 doubly seronegative women, and in a subsequent trial, a subset analysis determined that the vaccine reduced infection with HSV-1 but not HSV-2 (64, 65). We formulated a combined subunit antigen and adenovirus vector vaccine that included gC2/gD2, UL19 (VP5), and UL47 (VP13/14), with the expectation that the vaccine would induce potent neutralizing antibodies, CD4+ T helper cells, and CD8+ cytotoxic T cells (26, 36, 46). We used the guinea pig genital infection model to determine whether the combined vaccine prevents genital lesions and eliminates HSV-2 DNA vaginal shedding, which is a useful surrogate marker of the risk of transmission (66).
Full-length HSV-2 UL19 and UL47 were expressed in replication-defective adenoviral vectors 28 and 35. These constructs were capable of stimulating human UL19-specific CD4+ and UL47-specific CD8+ T cell clones. In mice, UL19 peptide pool 3 (amino acids 681 to 1031) and UL47 peptide pool 1 (amino acids 1 to 356) induced IFN-γ-secreting CD8+ T cells, while UL47 peptide pools 1 and 2 stimulated CD4+ T cells. T cell responses were not evaluated in guinea pigs because of a lack of guinea pig-specific reagents; however, we demonstrated that antibodies to UL19 and UL47 were produced in guinea pigs. The development of IgG is usually dependent on cognate CD4+ T cell helper responses, and as shown in Fig. 4C, vaccinated animals mounted IgG responses to UL47 and UL19.
Animals immunized with the adenovirus vectors expressing UL19 and UL47 developed severe acute disease and died, indicating that these T cell immunogens did not prevent lethal infection or severe genital disease. The gC2/gD2 subunit antigen vaccine was highly efficacious in preventing acute and recurrent genital disease, leaving little room for improvement by the combined gC2/gD2 and adenovirus vector UL19/UL47 vaccine. Small differences were noted on comparing the gC2/gD2 subunit antigen and combined groups, including differences in the incidence of acute genital disease (2/8 versus 0/8 animals), number of days with acute genital disease (2/112 versus 0/112 days), number of days of vaginal HSV-2 DNA shedding (15/168 versus 13/168 days), HSV-2 DNA copy number in DRG (1.2 log10 versus 0.5 log10), number of DRG with detectable HSV-2 LAT (5/8 versus 2/8 DRG), and HSV-2 DNA log10 copy number in vaginal swabs during episodes of shedding (3.7 log10 versus 4.5 log10). Although the differences favored the combined group in all situations except the vaginal HSV-2 DNA copy number during episodes of vaginal shedding, none was statistically significant. We did a sample size calculation with the assumption, based on the results of the current study, that the gC2/gD2 vaccine protects 80% of guinea pigs from acute and recurrent disease and that the combined vaccine protects 90% of animals. Achieving 80% power to obtain a P value of ≤0.05 for comparing the subunit antigen group with the combined group will require a sample size of 398 guinea pigs for a 2-sided test of significance or 314 animals for a 1-sided test. Therefore, repeating the study by adding another 8 to 10 animals per group will not be useful. The results for acute and recurrent lesions and vaginal shedding of HSV-2 DNA obtained in the comparator-arm, gC2/gD2- and CpG/alum-immunized guinea pigs of the current study are virtually identical to our previously published results using the same antigens and adjuvants, which supports the reproducibility of the results (26). A vaccine that combines potent humoral and T cell antigens is a concept worthy of pursuit; however, our results suggest that the optimal combination of immunogens may differ from those used in this study.
The possibility exists that UL19 and UL47 may be less potent CD8+ T cell immunogens in guinea pigs than in mice and humans or that the potency of UL19 and UL47 is not sufficiently superior to that of gC2 and gD2 when given with CpG and alum for stimulating CD4+ and CD8+ T cell responses. The lack of reagents to identify T cell responses in guinea pigs makes it difficult to assess these possibilities. A prior study demonstrated that the HSV-2 immediate early protein ICP4 induces robust CD4+ and CD8+ T cell responses in mice. When used as a vaccine to treat guinea pigs with recurrent genital herpes disease, the combination of ICP4 and gD2 subunit antigens given with Matrix M-2 (MM2) adjuvant significantly reduced recurrent genital disease and vaginal shedding of HSV-2 DNA, which supports the use of a vaccine designed to stimulate neutralizing antibodies and T cell immunity for treatment of genital herpes disease (38). The same combination is in early-phase human trials and has shown encouraging results in reducing recurrent genital disease and HSV-2 DNA shedding (67). A DNA-based approach that administered gD2, UL46 (VP11/12), and UL47 with Vaxfectin as an adjuvant was efficacious in guinea pigs at preventing genital disease and as immunotherapy for treating genital disease; however, the incidence of HSV-2 DNA shedding and the titer of HSV-2 DNA shed were not significantly reduced compared to those with gD2 alone (68). The UL46 and UL47 DNA vaccine without gD2 did not reduce recurrent genital disease in HSV-2-infected guinea pigs (68). These studies suggest that immunization with a small number of T cell immunogens without including antigens that induce protective antibody responses is unlikely to be effective for prevention or treatment of genital herpes.
Vaginal shedding of HSV-2 DNA has been studied extensively in humans (69–71). The presence of vaginal HSV-2 DNA shedding in the absence of genital disease in guinea pigs is similar to observations in humans; however, HSV-2 DNA shedding is less well understood for guinea pigs, since there is no transmission model to correlate shedding with infectivity (70, 72, 73). Using mathematical modeling, a threshold for transmitting infection in humans was proposed to be 104 HSV-2 DNA copies (74). DNA shedding correlates well with virus culture results from genital swab samples; however, even at 106 HSV-2 DNA copies, only 50% of virus cultures are positive (75). Additional studies of guinea pigs are needed to address the significance of low levels of HSV-2 DNA detected in immunized animals. It will be important to determine whether immunized animals are shedding infectious virus or mostly defective viral genomes before considering HSV-2 DNA shedding to be a valid marker for vaccine failure in animal models, and perhaps human studies.
The guinea pig genital herpes infection model has been used effectively to screen for candidate vaccines. Immunogens that perform poorly in this model are unlikely to succeed in humans. Although animal models have limitations in predicting outcomes of vaccine trials in humans, the models can be helpful by (i) defining immune correlates of protection; (ii) determining the significance of seroconversion after genital challenge in vaccinated animals, since it is possible that immunized animals may seroconvert based on genital replication without developing genital lesions or establishing latency; (iii) establishing the biologic relevance of subclinical shedding of HSV-2 DNA; and (iv) evaluating whether immunization protects against both HSV-1 and HSV-2 genital challenges. In the current study, a high degree of protection was provided by the gC2/gD2-CpG/alum vaccine; however, the protection was not total. Room for improvement exists, particularly related to reducing episodes of recurrent shedding of HSV-2 DNA. Yet no significant reduction in vaginal DNA shedding was detected by adding the UL19 and UL47 T cell immunogens. Future studies that use suboptimal concentrations of gC2/gD2 may be required to demonstrate a benefit of T cell immunogens in controlling acute and recurrent lesions and subclinical shedding of HSV-2 DNA.
Several approaches are worth considering that may improve the efficacy of recombinant HSV-2 vaccines. One approach is to expand the number of B cell immunogens by including additional entry molecules, such as glycoproteins B, H, and L, or adding another immune evasion molecule, glycoprotein E (76). A second approach is to add more T cell immunogens, including UL39 (ribonucleotide reductase subunit 1), UL1 (glycoprotein L), and/or immediate early proteins ICP4 and ICP0, that are all major targets of T cell responses in HSV-2-exposed, antibody-seronegative subjects (38, 77). A third consideration is to evaluate novel adjuvants that may enhance the potency and durability of immune responses (78). A fourth possibility is that potent systemic immune responses may not be sufficient without robust local genital mucosal responses generated by mucosal immunization or chemokines applied to mucosal surfaces to attract tissue-resident memory B and T cells (79). We postulate that attaining sterilizing immunity for a prophylactic genital herpes vaccine remains a realistic goal that will require one or more of the additional approaches described above.
ACKNOWLEDGMENTS
This work was supported by NIH grants RO1 AI04854, RO1 094019, R21 AI105959, and PO1 AI030731 and a grant from the University of Washington, Seattle, WA.
We thank Gary Cohen and Roselyn Eisenberg at the University of Pennsylvania for providing bac-gC2(426t) and bac-gD2(306t).
REFERENCES
- 1.Looker KJ, Garnett GP, Schmid GP. 2008. An estimate of the global prevalence and incidence of herpes simplex virus type 2 infection. Bull World Health Organ 86:805–812. doi: 10.2471/BLT.07.046128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Corey L, Adams HG, Brown ZA, Holmes KK. 1983. Genital herpes simplex virus infections: clinical manifestations, course, and complications. Ann Intern Med 98:958–972. doi: 10.7326/0003-4819-98-6-958. [DOI] [PubMed] [Google Scholar]
- 3.Mertz GJ, Benedetti J, Ashley R, Selke SA, Corey L. 1992. Risk factors for the sexual transmission of genital herpes. Ann Intern Med 116:197–202. doi: 10.7326/0003-4819-116-3-197. [DOI] [PubMed] [Google Scholar]
- 4.Koutsky LA, Stevens CE, Holmes KK, Ashley RL, Kiviat NB, Critchlow CW, Corey L. 1992. Underdiagnosis of genital herpes by current clinical and viral-isolation procedures. N Engl J Med 326:1533–1539. doi: 10.1056/NEJM199206043262305. [DOI] [PubMed] [Google Scholar]
- 5.Brown ZA, Vontver LA, Benedetti J, Critchlow CW, Sells CJ, Berry S, Corey L. 1987. Effects on infants of a first episode of genital herpes during pregnancy. N Engl J Med 317:1246–1251. doi: 10.1056/NEJM198711123172002. [DOI] [PubMed] [Google Scholar]
- 6.Brown ZA, Selke S, Zeh J, Kopelman J, Maslow A, Ashley RL, Watts DH, Berry S, Herd M, Corey L. 1997. The acquisition of herpes simplex virus during pregnancy. N Engl J Med 337:509–515. doi: 10.1056/NEJM199708213370801. [DOI] [PubMed] [Google Scholar]
- 7.Kimberlin DW, Lin CY, Jacobs RF, Powell DA, Corey L, Gruber WC, Rathore M, Bradley JS, Diaz PS, Kumar M, Arvin AM, Gutierrez K, Shelton M, Weiner LB, Sleasman JW, de Sierra TM, Weller S, Soong SJ, Kiell J, Lakeman FD, Whitley RJ, National Institute of Allergy Infectious Diseases Collaborative Antiviral Study Group. 2001. Safety and efficacy of high-dose intravenous acyclovir in the management of neonatal herpes simplex virus infections. Pediatrics 108:230–238. doi: 10.1542/peds.108.2.230. [DOI] [PubMed] [Google Scholar]
- 8.Reynolds SJ, Risbud AR, Shepherd ME, Zenilman JM, Brookmeyer RS, Paranjape RS, Divekar AD, Gangakhedkar RR, Ghate MV, Bollinger RC, Mehendale SM. 2003. Recent herpes simplex virus type 2 infection and the risk of human immunodeficiency virus type 1 acquisition in India. J Infect Dis 187:1513–1521. doi: 10.1086/368357. [DOI] [PubMed] [Google Scholar]
- 9.Renzi C, Douglas JM Jr, Foster M, Critchlow CW, Ashley-Morrow R, Buchbinder SP, Koblin BA, McKirnan DJ, Mayer KH, Celum CL. 2003. Herpes simplex virus type 2 infection as a risk factor for human immunodeficiency virus acquisition in men who have sex with men. J Infect Dis 187:19–25. doi: 10.1086/345867. [DOI] [PubMed] [Google Scholar]
- 10.Wald A, Link K. 2002. Risk of human immunodeficiency virus infection in herpes simplex virus type 2-seropositive persons: a meta-analysis. J Infect Dis 185:45–52. doi: 10.1086/338231. [DOI] [PubMed] [Google Scholar]
- 11.Schacker T, Zeh J, Hu HL, Hill E, Corey L. 1998. Frequency of symptomatic and asymptomatic herpes simplex virus type 2 reactivations among human immunodeficiency virus-infected men. J Infect Dis 178:1616–1622. doi: 10.1086/314486. [DOI] [PubMed] [Google Scholar]
- 12.Corey L, Wald A, Patel R, Sacks SL, Tyring SK, Warren T, Douglas JM Jr, Paavonen J, Morrow RA, Beutner KR, Stratchounsky LS, Mertz G, Keene ON, Watson HA, Tait D, Vargas-Cortes M, Valacyclovir HSV Transmission Study Group . 2004. Once-daily valacyclovir to reduce the risk of transmission of genital herpes. N Engl J Med 350:11–20. doi: 10.1056/NEJMoa035144. [DOI] [PubMed] [Google Scholar]
- 13.Bryson YJ, Dillon M, Lovett M, Acuna G, Taylor S, Cherry JD, Johnson BL, Wiesmeier E, Growdon W, Creagh-Kirk T, Keeney R. 1983. Treatment of first episodes of genital herpes simplex virus infection with oral acyclovir. A randomized double-blind controlled trial in normal subjects. N Engl J Med 308:916–921. [DOI] [PubMed] [Google Scholar]
- 14.Sawtell NM, Bernstein DI, Stanberry LR. 1999. A temporal analysis of acyclovir inhibition of induced herpes simplex virus type 1 in vivo reactivation in the mouse trigeminal ganglia. J Infect Dis 180:821–823. doi: 10.1086/314958. [DOI] [PubMed] [Google Scholar]
- 15.Sawtell NM, Thompson RL, Stanberry LR, Bernstein DI. 2001. Early intervention with high-dose acyclovir treatment during primary herpes simplex virus infection reduces latency and subsequent reactivation in the nervous system in vivo. J Infect Dis 184:964–971. doi: 10.1086/323551. [DOI] [PubMed] [Google Scholar]
- 16.Belshe RB, Heineman TC, Bernstein DI, Bellamy AR, Ewell M, van der Most R, Deal CD. 2014. Correlate of immune protection against HSV-1 genital disease in vaccinated women. J Infect Dis 209:828–836. doi: 10.1093/infdis/jit651. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Awasthi S, Friedman HM. 2014. A paradigm shift: vaccine-induced antibodies as an immune correlate of protection against herpes simplex virus 1 genital herpes. J Infect Dis 209:813–815. doi: 10.1093/infdis/jit658. [DOI] [PubMed] [Google Scholar]
- 18.Seidel-Dugan C, Ponce de Leon M, Friedman HM, Fries LF, Frank MM, Cohen GH, Eisenberg RJ. 1988. C3b receptor activity on transfected cells expressing glycoprotein C of herpes simplex virus types 1 and 2. J Virol 62:4027–4036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Hung SL, Peng C, Kostavasili I, Friedman HM, Lambris JD, Eisenberg RJ, Cohen GH. 1994. The interaction of glycoprotein C of herpes simplex virus types 1 and 2 with the alternative complement pathway. Virology 203:299–312. doi: 10.1006/viro.1994.1488. [DOI] [PubMed] [Google Scholar]
- 20.Rux AH, Lou H, Lambris JD, Friedman HM, Eisenberg RJ, Cohen GH. 2002. Kinetic analysis of glycoprotein C of herpes simplex virus types 1 and 2 binding to heparin, heparan sulfate, and complement component C3b. Virology 294:324–332. doi: 10.1006/viro.2001.1326. [DOI] [PubMed] [Google Scholar]
- 21.Fries LF, Friedman HM, Cohen GH, Eisenberg RJ, Hammer CH, Frank MM. 1986. Glycoprotein C of herpes simplex virus 1 is an inhibitor of the complement cascade. J Immunol 137:1636–1641. [PubMed] [Google Scholar]
- 22.Eisenberg RJ, Ponce de Leon M, Friedman HM, Fries LF, Frank MM, Hastings JC, Cohen GH. 1987. Complement component C3b binds directly to purified glycoprotein C of herpes simplex virus types 1 and 2. Microb Pathog 3:423–435. doi: 10.1016/0882-4010(87)90012-X. [DOI] [PubMed] [Google Scholar]
- 23.Hook LM, Lubinski JM, Jiang M, Pangburn MK, Friedman HM. 2006. Herpes simplex virus type 1 and 2 glycoprotein C prevents complement-mediated neutralization induced by natural immunoglobulin M antibody. J Virol 80:4038–4046. doi: 10.1128/JVI.80.8.4038-4046.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Harris SL, Frank I, Yee A, Cohen GH, Eisenberg RJ, Friedman HM. 1990. Glycoprotein C of herpes simplex virus type 1 prevents complement-mediated cell lysis and virus neutralization. J Infect Dis 162:331–337. doi: 10.1093/infdis/162.2.331. [DOI] [PubMed] [Google Scholar]
- 25.Awasthi S, Lubinski JM, Friedman HM. 2009. Immunization with HSV-1 glycoprotein C prevents immune evasion from complement and enhances the efficacy of an HSV-1 glycoprotein D subunit vaccine. Vaccine 27:6845–6853. doi: 10.1016/j.vaccine.2009.09.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Awasthi S, Lubinski JM, Shaw CE, Barrett SM, Cai M, Wang F, Betts M, Kingsley S, Distefano DJ, Balliet JW, Flynn JA, Casimiro DR, Bryan JT, Friedman HM. 2011. Immunization with a vaccine combining herpes simplex virus 2 (HSV-2) glycoprotein C (gC) and gD subunits improves the protection of dorsal root ganglia in mice and reduces the frequency of recurrent vaginal shedding of HSV-2 DNA in guinea pigs compared to immunization with gD alone. J Virol 85:10472–10486. doi: 10.1128/JVI.00849-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Judson KA, Lubinski JM, Jiang M, Chang Y, Eisenberg RJ, Cohen GH, Friedman HM. 2003. Blocking immune evasion as a novel approach for prevention and treatment of herpes simplex virus infection. J Virol 77:12639–12645. doi: 10.1128/JVI.77.23.12639-12645.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Harandi AM, Svennerholm B, Holmgren J, Eriksson K. 2001. Differential roles of B cells and IFN-γ-secreting CD4+ T cells in innate and adaptive immune control of genital herpes simplex virus type 2 infection in mice. J Gen Virol 82:845–853. [DOI] [PubMed] [Google Scholar]
- 29.Koelle DM, Posavad CM, Barnum GR, Johnson ML, Frank JM, Corey L. 1998. Clearance of HSV-2 from recurrent genital lesions correlates with infiltration of HSV-specific cytotoxic T lymphocytes. J Clin Invest 101:1500–1508. doi: 10.1172/JCI1758. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Verjans GM, Hintzen RQ, van Dun JM, Poot A, Milikan JC, Laman JD, Langerak AW, Kinchington PR, Osterhaus AD. 2007. Selective retention of herpes simplex virus-specific T cells in latently infected human trigeminal ganglia. Proc Natl Acad Sci U S A 104:3496–3501. doi: 10.1073/pnas.0610847104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Zhu J, Koelle DM, Cao J, Vazquez J, Huang ML, Hladik F, Wald A, Corey L. 2007. Virus-specific CD8+ T cells accumulate near sensory nerve endings in genital skin during subclinical HSV-2 reactivation. J Exp Med 204:595–603. doi: 10.1084/jem.20061792. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Mikloska Z, Ruckholdt M, Ghadiminejad I, Dunckley H, Denis M, Cunningham AL. 2000. Monophosphoryl lipid A and QS21 increase CD8 T lymphocyte cytotoxicity to herpes simplex virus-2 infected cell proteins 4 and 27 through IFN-gamma and IL-12 production. J Immunol 164:5167–5176. doi: 10.4049/jimmunol.164.10.5167. [DOI] [PubMed] [Google Scholar]
- 33.Koelle DM, Corey L, Burke RL, Eisenberg RJ, Cohen GH, Pichyangkura R, Triezenberg SJ. 1994. Antigenic specificities of human CD4+ T-cell clones recovered from recurrent genital herpes simplex virus type 2 lesions. J Virol 68:2803–2810. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Koelle DM, Frank JM, Johnson ML, Kwok WW. 1998. Recognition of herpes simplex virus type 2 tegument proteins by CD4 T cells infiltrating human genital herpes lesions. J Virol 72:7476–7483. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Koelle DM, Schomogyi M, McClurkan C, Reymond SN, Chen HB. 2000. CD4 T-cell responses to herpes simplex virus type 2 major capsid protein VP5: comparison with responses to tegument and envelope glycoproteins. J Virol 74:11422–11425. doi: 10.1128/JVI.74.23.11422-11425.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Posavad CM, Wald A, Hosken N, Huang ML, Koelle DM, Ashley RL, Corey L. 2003. T cell immunity to herpes simplex viruses in seronegative subjects: silent infection or acquired immunity? J Immunol 170:4380–4388. doi: 10.4049/jimmunol.170.8.4380. [DOI] [PubMed] [Google Scholar]
- 37.Hosken N, McGowan P, Meier A, Koelle DM, Sleath P, Wagener F, Elliott M, Grabstein K, Posavad C, Corey L. 2006. Diversity of the CD8+ T-cell response to herpes simplex virus type 2 proteins among persons with genital herpes. J Virol 80:5509–5515. doi: 10.1128/JVI.02659-05. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Skoberne M, Cardin R, Lee A, Kazimirova A, Zielinski V, Garvie D, Lundberg A, Larson S, Bravo FJ, Bernstein DI, Flechtner JB, Long D. 2013. An adjuvanted herpes simplex virus 2 subunit vaccine elicits a T cell response in mice and is an effective therapeutic vaccine in guinea pigs. J Virol 87:3930–3942. doi: 10.1128/JVI.02745-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Posavad CM, Koelle DM, Shaughnessy MF, Corey L. 1997. Severe genital herpes infections in HIV-infected individuals with impaired herpes simplex virus-specific CD8+ cytotoxic T lymphocyte responses. Proc Natl Acad Sci U S A 94:10289–10294. doi: 10.1073/pnas.94.19.10289. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Posavad CM, Wald A, Kuntz S, Huang ML, Selke S, Krantz E, Corey L. 2004. Frequent reactivation of herpes simplex virus among HIV-1-infected patients treated with highly active antiretroviral therapy. J Infect Dis 190:693–696. doi: 10.1086/422755. [DOI] [PubMed] [Google Scholar]
- 41.Schacker T, Ryncarz AJ, Goddard J, Diem K, Shaughnessy M, Corey L. 1998. Frequent recovery of HIV-1 from genital herpes simplex virus lesions in HIV-1-infected men. JAMA 280:61–66. doi: 10.1001/jama.280.1.61. [DOI] [PubMed] [Google Scholar]
- 42.Khanna KM, Bonneau RH, Kinchington PR, Hendricks RL. 2003. Herpes simplex virus-specific memory CD8+ T cells are selectively activated and retained in latently infected sensory ganglia. Immunity 18:593–603. doi: 10.1016/S1074-7613(03)00112-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Liu T, Khanna KM, Chen X, Fink DJ, Hendricks RL. 2000. CD8(+) T cells can block herpes simplex virus type 1 (HSV-1) reactivation from latency in sensory neurons. J Exp Med 191:1459–1466. doi: 10.1084/jem.191.9.1459. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Liu T, Tang Q, Hendricks RL. 1996. Inflammatory infiltration of the trigeminal ganglion after herpes simplex virus type 1 corneal infection. J Virol 70:264–271. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Posavad CM, Koelle DM, Corey L. 1996. High frequency of CD8+ cytotoxic T-lymphocyte precursors specific for herpes simplex viruses in persons with genital herpes. J Virol 70:8165–8168. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Koelle DM, Chen HB, Gavin MA, Wald A, Kwok WW, Corey L. 2001. CD8 CTL from genital herpes simplex lesions: recognition of viral tegument and immediate early proteins and lysis of infected cutaneous cells. J Immunol 166:4049–4058. doi: 10.4049/jimmunol.166.6.4049. [DOI] [PubMed] [Google Scholar]
- 47.Koelle DM, Liu Z, McClurkan CM, Topp MS, Riddell SR, Pamer EG, Johnson AS, Wald A, Corey L. 2002. Expression of cutaneous lymphocyte-associated antigen by CD8(+) T cells specific for a skin-tropic virus. J Clin Invest 110:537–548. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Koelle DM, Liu Z, McClurkan CL, Cevallos RC, Vieira J, Hosken NA, Meseda CA, Snow DC, Wald A, Corey L. 2003. Immunodominance among herpes simplex virus-specific CD8 T cells expressing a tissue-specific homing receptor. Proc Natl Acad Sci U S A 100:12899–12904. doi: 10.1073/pnas.2131705100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Tal-Singer R, Peng C, Ponce De Leon M, Abrams WR, Banfield BW, Tufaro F, Cohen GH, Eisenberg RJ. 1995. Interaction of herpes simplex virus glycoprotein gC with mammalian cell surface molecules. J Virol 69:4471–4483. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Tengvall S, Lundqvist A, Eisenberg RJ, Cohen GH, Harandi AM. 2006. Mucosal administration of CpG oligodeoxynucleotide elicits strong CC and CXC chemokine responses in the vagina and serves as a potent Th1-tilting adjuvant for recombinant gD2 protein vaccination against genital herpes. J Virol 80:5283–5291. doi: 10.1128/JVI.02013-05. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Canziani G, Zhang W, Cines D, Rux A, Willis S, Cohen G, Eisenberg R, Chaiken I. 1999. Exploring biomolecular recognition using optical biosensors. Methods (Duluth) 19:253–269. doi: 10.1006/meth.1999.0855. [DOI] [PubMed] [Google Scholar]
- 52.Kahl CA, Bonnell J, Hiriyanna S, Fultz M, Nyberg-Hoffman C, Chen P, King CR, Gall JG. 2010. Potent immune responses and in vitro pro-inflammatory cytokine suppression by a novel adenovirus vaccine vector based on rare human serotype 28. Vaccine 28:5691–5702. doi: 10.1016/j.vaccine.2010.06.050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Hamilton MM, Byrnes GA, Gall JG, Brough DE, King CR, Wei LL. 2008. Alternate serotype adenovector provides long-term therapeutic gene expression in the eye. Mol Vis 14:2535–2546. [PMC free article] [PubMed] [Google Scholar]
- 54.McVey D, Zuber M, Ettyreddy D, Brough DE, Kovesdi I. 2002. Rapid construction of adenoviral vectors by lambda phage genetics. J Virol 76:3670–3677. doi: 10.1128/JVI.76.8.3670-3677.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Lemiale F, Haddada H, Nabel GJ, Brough DE, King CR, Gall JG. 2007. Novel adenovirus vaccine vectors based on the enteric-tropic serotype 41. Vaccine 25:2074–2084. doi: 10.1016/j.vaccine.2006.11.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Brough DE, Lizonova A, Hsu C, Kulesa VA, Kovesdi I. 1996. A gene transfer vector-cell line system for complete functional complementation of adenovirus early regions E1 and E4. J Virol 70:6497–6501. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Koelle DM, Reymond SN, Chen H, Kwok WW, McClurkan C, Gyaltsong T, Petersdorf EW, Rotkis W, Talley AR, Harrison DA. 2000. Tegument-specific, virus-reactive CD4 T cells localize to the cornea in herpes simplex virus interstitial keratitis in humans. J Virol 74:10930–10938. doi: 10.1128/JVI.74.23.10930-10938.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Koelle DM. 2003. Expression cloning for the discovery of viral antigens and epitopes recognized by T cells. Methods (Duluth) 29:213–226. doi: 10.1016/S1046-2023(02)00344-4. [DOI] [PubMed] [Google Scholar]
- 59.Laing KJ, Magaret AS, Mueller DE, Zhao L, Johnston C, De Rosa SC, Koelle DM, Wald A, Corey L. 2010. Diversity in CD8(+) T cell function and epitope breadth among persons with genital herpes. J Clin Immunol 30:703–722. doi: 10.1007/s10875-010-9441-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Rhee EG, Blattman JN, Kasturi SP, Kelley RP, Kaufman DR, Lynch DM, La Porte A, Simmons NL, Clark SL, Pulendran B, Greenberg PD, Barouch DH. 2011. Multiple innate immune pathways contribute to the immunogenicity of recombinant adenovirus vaccine vectors. J Virol 85:315–323. doi: 10.1128/JVI.01597-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Stanberry LR, Kern ER, Richards JT, Abbott TM, Overall JC Jr. 1982. Genital herpes in guinea pigs: pathogenesis of the primary infection and description of recurrent disease. J Infect Dis 146:397–404. doi: 10.1093/infdis/146.3.397. [DOI] [PubMed] [Google Scholar]
- 62.Muller WJ, Dong L, Vilalta A, Byrd B, Wilhelm KM, McClurkan CL, Margalith M, Liu C, Kaslow D, Sidney J, Sette A, Koelle DM. 2009. Herpes simplex virus type 2 tegument proteins contain subdominant T-cell epitopes detectable in BALB/c mice after DNA immunization and infection. J Gen Virol 90:1153–1163. doi: 10.1099/vir.0.008771-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Jerome KR, Huang ML, Wald A, Selke S, Corey L. 2002. Quantitative stability of DNA after extended storage of clinical specimens as determined by real-time PCR. J Clin Microbiol 40:2609–2611. doi: 10.1128/JCM.40.7.2609-2611.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Belshe RB, Leone PA, Bernstein DI, Wald A, Levin MJ, Stapleton JT, Gorfinkel I, Morrow RLA, Ewell MG, Stokes-Riner A, Dubin G, Heineman TC, Schulte JM, Deal CD, Herpevac Trial for Women. 2012. Efficacy results of a trial of a herpes simplex vaccine. N Engl J Med 366:34–43. doi: 10.1056/NEJMoa1103151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Stanberry LR, Spruance SL, Cunningham AL, Bernstein DI, Mindel A, Sacks S, Tyring S, Aoki FY, Slaoui M, Denis M, Vandepapeliere P, Dubin G, GlaxoSmithKline Herpes Vaccine Efficacy Study Group. 2002. Glycoprotein-D-adjuvant vaccine to prevent genital herpes. N Engl J Med 347:1652–1661. doi: 10.1056/NEJMoa011915. [DOI] [PubMed] [Google Scholar]
- 66.Mertz GJ. 2008. Asymptomatic shedding of herpes simplex virus 1 and 2: implications for prevention of transmission. J Infect Dis 198:1098–1100. doi: 10.1086/591914. [DOI] [PubMed] [Google Scholar]
- 67.Wald A, Bernstein A, Fife K, Lee P, Tyring S, Van Wagoners N, Warren T, Magaret A, Flechtner J, Hetherington S. 2013. Abstr 53rd Intersci Conf Antimicrob Agents Chemother, Denver, CO, abstr 183G. [Google Scholar]
- 68.Veselenak RL, Shlapobersky M, Pyles RB, Wei Q, Sullivan SM, Bourne N. 2012. A Vaxfectin(®)-adjuvanted HSV-2 plasmid DNA vaccine is effective for prophylactic and therapeutic use in the guinea pig model of genital herpes. Vaccine 30:7046–7051. doi: 10.1016/j.vaccine.2012.09.057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Schiffer JT, Abu-Raddad L, Mark KE, Zhu J, Selke S, Koelle DM, Wald A, Corey L. 2010. Mucosal host immune response predicts the severity and duration of herpes simplex virus-2 genital tract shedding episodes. Proc Natl Acad Sci U S A 107:18973–18978. doi: 10.1073/pnas.1006614107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Tronstein E, Johnston C, Huang M-L, Selke S, Magaret A, Warren T, Corey L, Wald A. 2011. Genital shedding of herpes simplex virus among symptomatic and asymptomatic persons with HSV-2 infection. JAMA 305:1441–1449. doi: 10.1001/jama.2011.420. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Quinn TC. 2011. Viral kinetics of genital herpes: a molecular probe into host-viral interactions. J Infect Dis 204:495–498. doi: 10.1093/infdis/jir317. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Wald A, Zeh J, Selke S, Ashley RL, Corey L. 1995. Virologic characteristics of subclinical and symptomatic genital herpes infections. N Engl J Med 333:770–775. doi: 10.1056/NEJM199509213331205. [DOI] [PubMed] [Google Scholar]
- 73.Wald A, Zeh J, Selke S, Warren T, Ryncarz AJ, Ashley R, Krieger JN, Corey L. 2000. Reactivation of genital herpes simplex virus type 2 infection in asymptomatic seropositive persons. N Engl J Med 342:844–850. doi: 10.1056/NEJM200003233421203. [DOI] [PubMed] [Google Scholar]
- 74.Schiffer JT, Mayer BT, Fong Y, Swan DA, Wald A. 2014. Herpes simplex virus-2 transmission probability estimates based on quantity of viral shedding. J R Soc Interface 11:20140160. doi: 10.1098/rsif.2014.0160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Wald A, Huang ML, Carrell D, Selke S, Corey L. 2003. Polymerase chain reaction for detection of herpes simplex virus (HSV) DNA on mucosal surfaces: comparison with HSV isolation in cell culture. J Infect Dis 188:1345–1351. doi: 10.1086/379043. [DOI] [PubMed] [Google Scholar]
- 76.Awasthi S, Shaw C, Friedman H. 2014. Improving immunogenicity and efficacy of vaccines for genital herpes containing herpes simplex virus glycoprotein D. Expert Rev Vaccines 13:1475–1488. doi: 10.1586/14760584.2014.951336. [DOI] [PubMed] [Google Scholar]
- 77.Posavad CM, Remington M, Mueller DE, Zhao L, Magaret AS, Wald A, Corey L. 2010. Detailed characterization of T cell responses to herpes simplex virus-2 in immune seronegative persons. J Immunol 184:3250–3259. doi: 10.4049/jimmunol.0900722. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Coffman RL, Sher A, Seder RA. 2010. Vaccine adjuvants: putting innate immunity to work. Immunity 33:492–503. doi: 10.1016/j.immuni.2010.10.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Shin H, Iwasaki A. 2012. A vaccine strategy that protects against genital herpes by establishing local memory T cells. Nature 491:463–467. doi: 10.1038/nature11522. [DOI] [PMC free article] [PubMed] [Google Scholar]