ABSTRACT
Most HIV-1 variants isolated from early-stage human infections do not use nonhuman primate versions of the CD4 receptor for cellular entry, or they do so poorly. We and others have previously shown that CD4 has experienced strong natural selection over the course of primate speciation, but it is unclear whether this selection has influenced the functional characteristics of CD4 as an HIV-1 receptor. Surprisingly, we find that selection on CD4 has been most intense in the New World monkeys, animals that have never been found to harbor lentiviruses related to HIV-1. Based on this, we sampled CD4 genetic diversity within populations of individuals from seven different species, including five species of New World monkeys. We found that some, but not all, CD4 alleles found in Spix's owl monkeys (Aotus vociferans) encode functional receptors for early-stage human HIV-1 isolates representing all of the major group M clades (A, B, C, and D). However, only some isolates of HIV-1 subtype C can use the CD4 receptor encoded by permissive Spix's owl monkey alleles. We characterized the prevalence of functional CD4 alleles in a colony of captive Spix's owl monkeys and found that 88% of surveyed individuals are homozygous for permissive CD4 alleles, which encode an asparagine at position 39 of the receptor. We found that the CD4 receptors encoded by two other species of owl monkeys (Aotus azarae and Aotus nancymaae) also serve as functional entry receptors for early-stage isolates of HIV-1.
IMPORTANCE Nonhuman primates, particularly macaques, are used for preclinical evaluation of HIV-1 vaccine candidates. However, a significant limitation of the macaque model is the fact that most circulating HIV-1 variants cannot use the macaque CD4 receptor to enter cells and have to be adapted to these species. This is particularly true for viral variants from early stages of infection, which represent the most relevant vaccine targets. In this study, we found that some individuals from captive owl monkey populations harbor CD4 alleles that are compatible with a broad collection of HIV-1 isolates, including those isolated from early in infection in highly affected populations and representing diverse subtypes.
INTRODUCTION
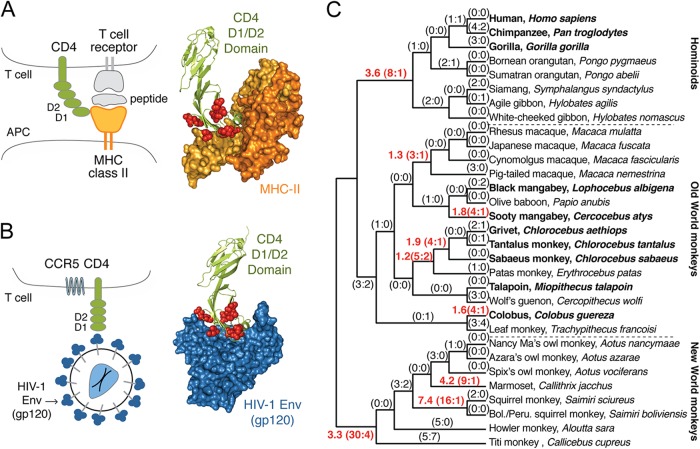
CD4 is expressed on the surface of a subset of human T cells, where it stimulates interaction between the T cell receptor and major histocompatibility complex class II (MHC-II) molecules expressed on antigen-presenting cells (Fig. 1A). While the T cell receptor interacts with the presented peptide antigen, the D1 domain of CD4 interacts with an invariant portion of the MHC class II molecule itself (1), an interaction that is expected to be preserved over evolutionary time. Despite this, we and others previously demonstrated that CD4 is evolving under diversifying selection in primates, with natural selection working in favor of new allelic forms (2–4). This is presumably because the CD4 D1 domain also interacts with the envelope (Env) surface protein of the lentiviruses human immunodeficiency virus (HIV) and simian immunodeficiency virus (SIV) (Fig. 1B) (5). This interaction is required for virus entry into cells, and over evolutionary time, primate genomes may have experienced selection for new allelic forms of CD4 that limit lentiviral entry. In turn, lentiviruses would have been selected for new allelic forms of env that permit entry using new forms of CD4. Tit-for-tat evolution such as this is referred to as an evolutionary arms race and results in accelerated evolution at the binding interface of the two interacting proteins (6). Indeed, codons in both primate CD4 and HIV-1 env that correspond to residues in this interaction interface have been previously characterized by us and others to evolve under positive natural selection (2–4, 7–9). While these evolutionary findings show that primate CD4 is genetically diverse, the functional significance of this genetic diversity has not been well characterized.
FIG 1.
Positive natural selection has shaped the CD4 D1 domain, particularly in the New World monkeys. (A) A schematic of the interaction between CD4 and MHC-II, alongside its cocrystal (PDB code: 1JL4) (49), where the sites under positive selection in CD4 (3) are represented by red spheres. APC, antigen-presenting cell. (B) Schematic of the interaction between CD4 and HIV-1 gp120, alongside its cocrystal (PDB code: 1RZJ) (50), where the sites under positive selection in CD4 (3) are represented by red spheres. (C) Evolutionary analysis of the D1 domain of CD4 showing the number of nonsynonymous and synonymous mutations (in parentheses; N:S) predicted to have occurred along each branch of a 31-species primate phylogeny. Branches with red text have a dN/dS ratio of >1, and this ratio is shown before the parentheses. This ratio cannot be calculated in cases where dS is 0. Primate species shown in bold text are known to be naturally infected with a simian or human immunodeficiency virus (51). Only one CD4 sequence per species was utilized in this analysis. Accession numbers for primate CD4 sequences can be found in Materials and Methods.
Humans, chimpanzees, and white-handed gibbons are the only mammals that are known to support HIV type 1 (HIV-1) replication, but the latter two primates are endangered and only rarely develop immunodeficiencies upon infection (10). Cells from all other nonhuman primate species are resistant to HIV-1 infection even in cell culture, in most cases due to restriction factors that they encode (11, 12). However, entry into the cell is also a major barrier to HIV-1 infection of nonhuman primate cells (13). For example, HIV-1 variants isolated directly from individuals at early stages of infection, which are most relevant to the HIV-1 pandemic, have been shown to be compatible only with human CD4 (14), whereas lab-adapted or chronic-stage isolates of HIV-1 can use the CD4 receptor encoded by multiple nonhuman primate species (15, 16). Species-specific differences at three sites in the CD4 D1 domain, N39, P48, and R59, have been shown to alter interactions with HIV-1 (14, 17). For instance, a single amino acid difference at position 39 of CD4 between human (asparagine) and pig-tailed macaque (Macaca nemestrina; isoleucine) accounts for the species-specific differences in the ability of these CD4s to function as receptors for early-stage isolates of HIV-1 (14). Studies of viral pathogenesis as well as vaccine and prevention studies typically employ viruses that are chimeras between SIV and HIV-1, called SHIVs, that infect macaques. SHIVs encode antagonists of key macaque restriction factors, and to make these viruses relevant to vaccine studies, they encode an Env derived from HIV-1. Because most Envs from circulating HIV-1 variants do not use macaque CD4 efficiently, SHIVs typically only replicate well in macaques after serial passage and adaptation in this species.
Because Env is a major antigen targeted by human antibodies, the constraints placed on Env by macaque CD4 may fundamentally compromise the SHIV/macaque model for vaccine studies. First, the process of adapting SHIVs to replicate in macaques leads to changes in Env (18), and these have antigenic consequences, changing the ability of HIV-1-specific antibodies to recognize Env (19). More recent studies have identified HIV-1 variants that can replicate in macaques without passage (20), suggesting that there are rare variants that can use the macaque CD4 receptor. Whether these represent the key antigenic features of the majority of transmitted variants remains to be determined. Second, the specific viral variants that are actually transmitted between humans best represent the molecular properties that successful vaccines and prevention approaches will need to target to limit new infections. However, most SHIVs bear Env from lab-adapted HIV-1 strains, which were typically originally isolated from chronic rather than early stages of infection. Finally, the majority of SHIVs represent subtype B Env sequences, and few are representative of subtypes A, C, and D, which are the most prevalent types in sub-Saharan Africa and account for the highest percentage of new infections and HIV-1-related mortality.
Many nonhuman primate species have previously been explored as possible animal models for HIV-1. However, like in humans, significant genetic polymorphism exists within nonhuman primate populations, and this remains largely unexplored. In this study, we analyzed small populations representing different nonhuman primate species and evaluated these individual animals for CD4 polymorphisms. Three CD4 alleles were identified in one New World monkey species, Spix's owl monkey (Aotus vociferans), that support entry mediated by Envs isolated from unpassaged or minimally passaged early isolates of HIV-1. Further, CD4 encoded by these alleles, but not another allele found in the same Spix's owl monkey population, supports entry by all of the major clades of HIV-1 group M. Interestingly, we find that only some Envs isolated from subtype C can use the CD4 receptor encoded by these permissive alleles, suggesting distinct architectures within group C Envs. In summary, some Spix's owl monkeys encode a CD4 that is broadly permissive to diverse, early isolates of HIV-1. We find that these permissive alleles are common in a captive Spix's owl monkey colony that we have surveyed. We also find that two other species of owl monkeys, Azara's (Aotus azarae) and Nancy Ma's (Aotus nancymaae) owl monkeys, also encode CD4 receptors that are functional for HIV-1 variants isolated from early-stage human infections.
MATERIALS AND METHODS
Primate samples.
Nonhuman primate samples were acquired from the Michale E. Keeling Center for Comparative Medicine and Research (KCCMR) in Bastrop, TX, or from the New England Primate Research Center (NEPRC) in Southborough, MA. For each individual sampled at KCCMR, 2.5 ml of blood was collected in PaxGene blood RNA tubes (BD; 762165). All tissue collections performed for this study were reviewed and approved through The University of Texas MD Anderson Cancer Center Institutional Animal Care and Use Committee (IACUC). For each individual housed at NEPRC, B cell lines were received and expanded in suspension culture in RPMI medium, 20% fetal bovine serum (FBS), penicillin-streptomycin (Pen-Strep), l-glutamine, HEPES, and zidovudine (AZT). Genomic DNA and RNA from blood and cell lines were isolated using the PaxGene microRNA (miRNA) kit (Qiagen; 763134) and/or the Qiagen All Prep DNA/RNA minikit (Qiagen; 80204). cDNA libraries were generated using oligo(dT) primers with isolated primate RNA and the Superscript III First-Strand Synthesis System (Invitrogen; 18080-051).
CD4 genotyping.
The CD4 coding region from rhesus macaques and owl monkeys was amplified using cDNA templates and primers that recognize the untranslated regions (NRM238 [5′-AAGCAGCGGGCAAGAAAGACG-3′] and NRM242 [5′-CAAGTTCCTGCCCTCTGTGG-3′]). PCR with cDNA templates was performed using PCR SuperMix High Fidelity (Invitrogen; 10790-020) with an annealing temperature of 58°C. Rhesus macaque CD4 amplicons were sequenced using NRM240 (5′-AGAAAGACGCAAGCCCAGAGG-3′), and owl monkey amplicons were sequenced using NRM799 (5′-GCCTGCTGGAAAGCTAGTACC-3′). The CD4 coding region from cynomolgus macaques and squirrel monkeys was amplified from genomic DNA. Two amplicons that span the D1 domain of CD4 were generated using primers that sit in introns and amplify exons 1 and 2 (NRM457 [5′-TCTTGCTTCTGCTCCTACTCATTCC-3′] and NRM459 [5′-TGGGCCACCAGCAGTTGG-3′]) or exons 3 to 6 (NRM465 [5′-GGAGTTGGTGCTCTCCAAATAAGG-3′] and NRM471 [5′-TCTCTGCCAACCACAGGAAGG-3′]). PCR with genomic DNA (gDNA) templates was performed using Phusion High Fidelity PCR master mix (NEB; F-531S) with annealing temperatures of 58°C for exons 1 and 2 and 67°C for exons 3 to 6. CD4 exons 1 and 2 were sequenced using NRM457 (5′-TCTTGCTTCTGCTCCTACTCATTCC-3′), and exon 3 was sequenced using NRM484 (5′-AGCTCAGGCTGGATTTGGTGC-3′). All newly identified single nucleotide polymorphisms (SNPs) were verified with independent PCR and sequencing reactions.
Expression constructs.
Human CD4 was amplified from RNA isolated from Jurkat T cells. Owl monkey CD4 was amplified from RNA isolated from blood samples as described above. Each CD4 was subcloned into the pCR8 Gateway entry vector using TA cloning (Invitrogen; K2500-20). An LR Clonase II reaction (Invitrogen; 11791-100) was used to move these constructs into a Gateway-converted pLPCX retroviral packaging vector (Clontech; 631511). The expression plasmid encoding rhesus macaque CD4 was described previously (22).
Envelope clones.
The following envelope clones from early HIV-1 infections were used: Q461e2 (23); QH343.21M.A10 (24); Q23ENV.17 (25); BG505.W6M.B1 (26); WITO4160.33 and TRO.11 (27); CAP210.2.00.E8, ZM53M.PB12, ZM109F.PB4, ZM197M.PB7, ZM214M.PL15, Du156.12, Du172.17, and Du422.1 (28); QC406.70M.F3 (24); and QA013.70I.H1 and QB857.110I.B3 (24). As a control, 2 subtype B HIV-1 env clones (BaL.01 and SF162) representing variants known to infect macaque cells (14) were also used. GFP reporter pseudoviruses were generated in HEK293T cells by cotransfecting 667 ng of Q23ΔEnvGFP (21) and 333 ng of the HIV-1 env clone of interest using Fugene 6 (Roche) transfection reagent at a ratio of 3 μl of Fugene 6 to 1 μg of DNA according to the manufacturer's protocol.
Generation of stable cell lines.
HEK293T and Cf2Th/syn CCR5 (29) cells were cultured in Dulbecco's modified Eagle medium (Invitrogen) with 10% FBS (Gibco), 2 mM l-glutamine (Gibco), and 1% antibiotic (Gibco) (complete medium) at 37°C and 5% CO2. Cf2Th/syn CCR5 cells, which are canine thymocytes engineered to express human CCR5 (29), were further supplemented with 400 μg/ml of Geneticin (Gibco) to maintain CCR5 expression. For generation of CD4-expressing cell lines, retroviral virus-like particles (VLPs) were generated in HEK293T cells by cotransfecting pLPCX (retroviral vector encoding the CD4 of interest), pJK3 (MLV-based packaging plasmid), and pMD.G (vesicular stomatitis virus glycoprotein [VSV-G] envelope plasmid) at a ratio of 1:1:0.5 using Fugene 6 (Roche) transfection reagent according to the manufacturer's protocol. Forty-eight hours posttransfection, the supernatants containing VLPs were collected, filtered through 0.22-micrometer filters, and concentrated using Amicon Ultracel 100K filters (Millipore). The concentrated VLPs (∼200 μl) were used immediately to transduce Cf2Th/syn CCR5 cells that had been plated 24 h prior at a density of 105 cells/well in a 6-well plate in 2 ml of drug-free complete medium. The cells were transduced in the presence of 10 μg/ml of DEAE-dextran by spinoculation at 1,200 × g for 90 min. The following day, cells were split and transferred in new T75 flasks in 10 ml of drug-free complete medium and cultured for 48 h. The cells were then passaged and maintained in complete medium supplemented with 400 μg/ml of Geneticin (to maintain CCR5 expression) and 2 μg/ml of puromycin (to select for CD4 expression). The transduced cells with high levels of CD4 expression were obtained by sorting the cells on a FACSAria II cell sorter using an allophycocyanin (APC)-conjugated CD4 monoclonal antibody (BD Biosciences; 551980) as described previously (14). Cf2Th/syn CCR5 cells stably expressing rhesus CD4 have been described previously (14).
CD4 infectivity assay.
Cf2Th/syn CCR5 cells stably expressing CD4 (2.5 × 104 cells/well in a 12-well plate in 1 ml of drug-free complete medium) were seeded 24 h prior to infection. The cells were infected with HIV-1 pseudoviruses in duplicate wells at a multiplicity of infection (MOI) of 0.5 in the presence of 10 μg/ml of DEAE-dextran by spinoculation at 1,200 × g for 90 min. After 72 h, the cells were washed once with 200 μl of 1× phosphate-buffered saline (PBS), harvested using 200 μl of 0.05% trypsin-EDTA (Gibco), and fixed in 200 μl of 2% paraformaldehyde. The fixed cells were washed twice with 500 μl of fluorescence-activated cell sorter (FACS) buffer (1× PBS buffer containing 1% FBS and 1 mM EDTA). The cells were resuspended in 400 μl of FACS buffer, filtered through a 35-μm-pore-size nylon mesh cap (BD Falcon), and analyzed for GFP expression on BD FACSCanto II flow cytometer. The data from ∼104 cells were analyzed using FlowJo version 9.7.5.
Infectivity assay for cells transiently expressing CD4 and CCR5.
HEK293T cells (2.5 × 105 cells/well in a 6-well plate in 2 ml of complete medium) were seeded 24 h prior to transfection. Cells were cotransfected with plasmids encoding the desired CD4 (0.5 μg) and human CCR5 (1 μg) using Fugene 6 (Roche) transfection reagent by following the manufacturer's protocol. Forty-eight hours posttransfection, cells were trypsinized, counted, and reseeded at a density of 8 × 104/well in a 12-well plate in 1 ml of complete medium. Six hours later, when the cells had adhered to the plate, cells were infected with HIV-1 pseudoviruses in duplicate wells at an MOI of 15 in the presence of 10 μg/ml of DEAE-dextran by spinoculation at 1,200 × g for 90 min. The percentage of green fluorescent protein (GFP)-positive cells was measured by flow cytometry as described above. Forty-eight hours posttransfection, CD4 and CCR5 expression levels were determined using flow cytometry on aliquots of transfected cells using APC-conjugated CD4 antibody (BD Biosciences; 551980) and fluorescein isothiocyanate (FITC)-conjugated CCR5 antibody (BD Biosciences; 561747) as described previously (21).
Evolutionary analysis.
An alignment of primate CD4 was analyzed using the codeml program contained in PAML 4 (30). The free-ratio model was used to estimate the ratio of nonsynonymous to synonymous evolutionary changes (dN/dS ratio) that occurred along each branch. Accession numbers for the primate CD4 sequences (all from GenBank unless otherwise indicated) are as follows: human, BC025782.1; chimpanzee, M31135.1; gorilla, KJ531711.1; Bornean orangutan, KJ531712.1; Sumatran orangutan, not applicable (assembled from the UCSC genome database [31]); siamang, KJ531713.1; agile gibbon, KJ531714.1; white-cheeked gibbon, KJ531715.1; rhesus macaque, D63347.1; Japanese macaque, D63348.1; cynomolgus macaque, D63349.1; pig-tailed macaque, D63346.1; black mangabey, KJ531719.1; olive baboon, KJ531718.1; sooty mangabey, X73327.1; grivet, AF001226; tantalus monkey, AF001221.1; sabaeus monkey, AF001225; patas monkey, X73324.1; talapoin, KJ531716.1; wolf's guenon, KJ531717.1; colobus, KJ531721.1; leaf monkey, KJ531722.1; Nancy Ma's owl monkey, KR902343; Azara's owl monkey, KR902342; Spix's owl monkey, KR902344; marmoset, AF452616.1; squirrel monkey, AF452617.1; Bolivian/Peruvian squirrel monkey, not applicable (assembled from the UCSC genome database [31]); howler monkey, KJ531724.1; and titi monkey, KJ531723.1.
Nucleotide sequence accession numbers.
Newly generated full-length owl monkey CD4 gene sequences have been deposited in GenBank (accession numbers KR902342 to KR902344).
RESULTS
The evolution of the CD4 D1 domain.
To assess which primate species should be the focus of our screen, we looked at patterns of evolution in the D1 domain across the primate phylogeny. Currently, CD4 sequences exist for 27 different primate species, 14 of which were generated by our group in a previous study (3). We sequenced CD4 from representative individuals of 4 additional species (Nancy Ma's owl monkey, Aotus nancymaae; Azara's owl monkey, Aotus azarae; Spix's owl monkey, Aotus vociferans; and Bolivian/Peruvian squirrel monkey, Saimiri boliviensis) and used the resulting 31-species CD4 data set to model the pattern of substitutions that has occurred along each branch of the primate phylogeny, using PAML (30) (Fig. 1C). On each branch of the tree, the ratios in parentheses represent the numbers of nonsynonymous and synonymous substitutions predicted to have occurred in the D1 domain. In order to assess patterns of selection, these values must be normalized to the number of opportunities that existed for each type of mutation (converting these raw counts to the rates dN and dS; reviewed in reference 32). We find that the D1 domain has experienced selection in favor of nonsynonymous mutations (dN/dS ≫ 1) along several branches of the tree (shown in red type in Fig. 1C). The species listed in bold type are African species known to harbor SIV in the wild. Surprisingly, three of the four most extreme dN/dS values are found in the New World monkey clade (dN/dS = 3.3, 4.2, and 7.4). None of these species are found in Africa, and New World monkeys have never been shown to harbor lentiviruses. The rapid evolution of CD4 in New World monkeys, along with the presence of the potent TRIM5-CypA lentivirus restriction factor found in the Aotus genus of New World monkeys (33–35), suggests that New World monkeys may have been infected by lentiviruses in their evolutionary past. In summary, the positive selection of CD4 is most acute in the New World monkey clade.
Polymorphism in the primate CD4 gene.
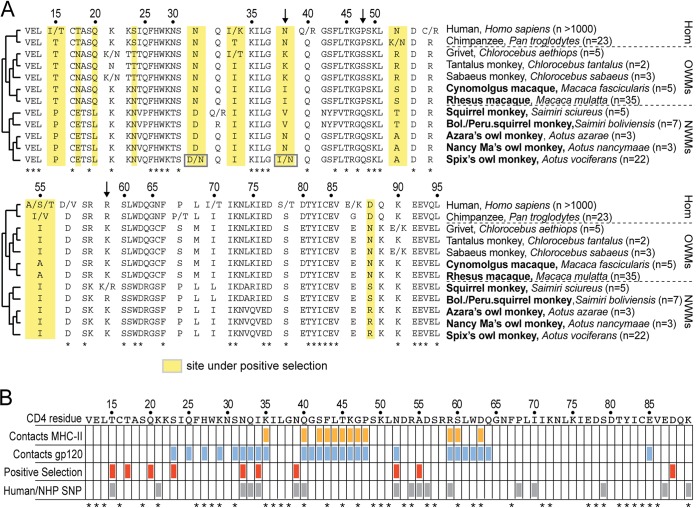
The specific residues in CD4 previously found to be targeted by positive selection are illustrated in Fig. 1A and B (3). Every round of positive selection in CD4 would have started with the appearance of a random SNP. If that SNP made CD4 resistant to virus entry while not compromising MHC binding, natural selection would have favored alleles carrying this SNP. For this reason, selection acts to enrich for the prevalence of beneficial SNPs within populations. To date, not much is known about CD4 SNPs in primates because only one representative CD4 sequence is available from most primate species. We next sequenced CD4 from small populations of animals representing seven nonhuman primate species (bold type in Fig. 2A). Because selective pressure on CD4 has been intense in New World monkeys, these included 5 species of New World monkeys: the four owl and squirrel monkey species mentioned previously, as well as the common squirrel monkey, Saimiri sciureus. These five New World monkey populations ranged in size from 3 to 22 individuals per species, as indicated in Fig. 2A. While there has been interest by others in developing New World monkeys as an HIV-1 model system (16, 36), in practice macaques have been used as the main animal model. For this reason, we also included in our study two macaque species (cynomolgus macaques, n = 4, and rhesus macaques, n = 34). Blood or cells from each individual, a total of 75 different monkeys in all, were obtained from primates housed at two different primate research centers as summarized in Materials and Methods. Nucleic acid was isolated from these samples and used to amplify and sequence the D1 domain of CD4 (see Materials and Methods for details on PCR and sequencing). Finally, we compiled information from human SNP databases and previous reports on CD4 diversity in chimpanzees and three different species of African green monkeys (grivets, tantalus monkeys, and sabaeus monkeys) (Fig. 2A) (37, 38). Additional CD4 sequences from one chimpanzee (GenBank accession number NM_001009043), one rhesus macaque (GenBank accession number D63347), one cynomolgus macaque (GenBank accession number D63349), two squirrel monkeys (GenBank accession numbers D86588 and AF452617), and one Bolivian squirrel monkey (UCSC genome database [31]) were also included in our SNP analysis.
FIG 2.
There are many nonsynonymous SNPs in the portion of CD4 encoding the D1 domain. (A) An amino acid alignment of the D1 domain from species included in the population study. Nonsynonymous SNPs identified are indicated with slashes, where the two alternately encoded amino acids are given on either side. Dotted lines separate the major clades of simian primates (Hom, hominoids; OWMs, Old World monkeys; NWMs, New World monkeys). The number of individuals analyzed from each species is shown in parentheses adjacent to the species name. Amino acid positions highlighted in yellow were previously identified to be evolving under positive selection (3). Numbering along the top is relative to the mature CD4 protein, after cleavage of the 25-amino-acid N-terminal signal peptide. Species shown in bold indicate populations that were sequenced in this study. Arrows indicate three sites that have been shown previously to affect HIV-1 entry (14, 17). (B) A table showing amino acids in the CD4 D1 domain that contact gp120 (blue) or MHC-II (orange), are under positive selection (red) (3), or are polymorphic for nonsynonymous mutations in human or nonhuman primate (NHP) populations included in the present study (gray). Asterisks along the bottom denote residue positions that are completely conserved in the 31 primate species shown in Fig. 1C. The table format is modified from reference 4.
Even in the relatively small populations surveyed, we found that 6 of the 12 primate species exhibit nonsynonymous polymorphism in the D1 domain (slashed positions in Fig. 2A). A total of 17 sites in the D1 domain contain nonsynonymous SNPs. These SNPs (gray boxes in Fig. 2B) cluster around codons previously found to be under positive selection, and there are 6 positions where SNPs exactly overlap sites under selection (T15, N32, I34, N39, N52, and A55) (Fig. 2). It was previously shown that a single amino acid difference at position 39 between human (asparagine) and pig-tailed macaque (Macaca nemestrina; isoleucine) CD4 accounts for the species-specific differences in the ability of these CD4s to function as receptors for HIV-1 (14). This site was also previously identified to be under positive selection (3), and accordingly, at this position we found high levels of genetic diversity with various nonhuman primate species encoding asparagine (N), isoleucine (I), lysine (K), or valine (V) (Fig. 2A). In this study, we found that this position is also polymorphic in the Spix's owl monkey species, where certain alleles encode the amino acid found at position 39 in humans (asparagine) and other alleles encode the amino acid found in macaque species (isoleucine) (Fig. 2A). On the basis of these findings, we hypothesized that there might be functional variation in the CD4 proteins encoded by different Spix's owl monkey individuals.
Some Spix's owl monkey CD4 alleles encode functional receptors for HIV-1 isolates from early-stage human infections.
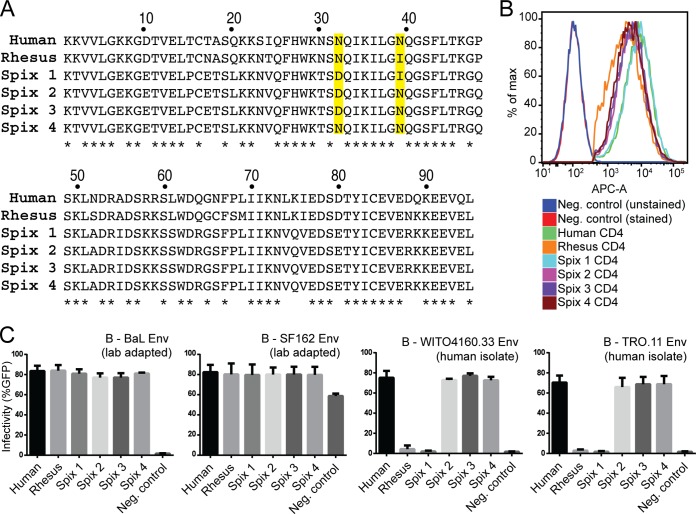
To test the ability of Spix's owl monkey CD4 to support HIV-1 infection, we cloned all four CD4 alleles from two individuals that encode nonsynonymous SNPs at residue 39, or a neighboring site, residue 32 (both boxed in Fig. 2A). These alleles encode proteins that are identical at every other amino acid position with one exception: the Spix 2 and Spix 3 (Fig. 3A) alleles encode identical proteins but differ by a T275A SNP outside the D1 domain (not shown in alignment). Cell lines stably expressing these CD4 alleles were generated in Cf2Th/syn CCR5 cells, which express human CCR5. Cells expressing human or rhesus macaque CD4 were also generated as controls. Similar expression levels of CD4 were observed across these cell lines (Fig. 3B).
FIG 3.
Some Spix's owl monkey CD4 alleles encode receptors permissive for entry by early, nonadapted HIV-1 isolates. (A) Amino acid alignment of the D1 domain from six CD4 receptors tested for virus entry. Sites that are polymorphic in Spix's owl monkeys are highlighted in yellow. (B) Expression levels of CD4s stably introduced into Cf2Th/syn CCR5 cells as measured by flow cytometry using an APC-conjugated anti-CD4 antibody. (C) Cell lines expressing different CD4 alleles (indicated along the x axis) were infected with the indicated Env-pseudotyped virions. Env variants are labeled such that the first letter represents the subtype, followed by the strain name. Infection is indicated as percent GFP-positive cells measured by flow cytometry 72 h postinfection. All pseudotyped virions were generated using the subtype A-derived Q23ΔEnvGFP proviral clone. Error bars represent the standard deviations of the means from three independent experiments conducted in duplicate.
Virions bearing Envs from two lab-adapted subtype B variants (BaL and SF162 [14]) gained entry through all CD4s tested, including those encoded by the 4 Spix's owl monkey alleles (Fig. 3C). This is consistent with the ability of Envs from lab-adapted HIV-1 to use CD4 receptors encoding either N or I at amino acid position 39 (14). We then tested Env from subtype B viruses isolated from early stages of human infections, including one that was not passaged prior to cloning (WITO4160.33) and one that was cloned after low passage (TRO.11) (27) (Table 1). As observed previously (14), human but not rhesus macaque CD4 supported entry of these viruses (Fig. 3C). Surprisingly, three out of the four Spix's owl monkey CD4 receptors tested were also functional receptors for HIV-1 pseudotyped with these Envs, and at a level similar to that of human CD4 (Fig. 3C). The three Spix's owl monkey CD4 receptors that support infection (encoded by the Spix 2, 3, and 4 alleles) all have an asparagine at position 39, while the Spix 1 allele encodes an isoleucine at this position (Fig. 3A). This amino acid substitution is solely responsible for this phenotype, because Spix 1 and Spix 3 encode identical proteins except at position 39. Therefore, Spix 2, 3, and 4 are likely variants of a single allele, originally bearing the mutation at position 39 but which have become unique as they have acquired additional mutations elsewhere in the gene. Thus, some CD4 alleles found in a Spix's owl monkey population encode functional entry receptors for nonadapted, early-stage variants of HIV-1.
TABLE 1.
Primary envelope clones used in this study
| Env clone | Subtype | Mode of transmissiona | Sourceb | GenBank accession no. | Time postinfection (wks) |
|---|---|---|---|---|---|
| Q461e2 | A | M-F | PBMC | AF407156 | 4 |
| QH343.21M.ENV.A10 | A | M-F | PBMC | FJ866119 | 3 |
| Q23.ENV.17 | A | M-F | PBMC | AF004885 | 11 |
| BG505.W6M.B1 | A | M-C | PBMC | DQ208457 | 6 |
| WITO4160.33 | B | F-M | Plasma | AY835451 | 1 |
| TRO.11 | B | M-M | ccPBMC | AY835445 | 4 |
| CAP210.2.00.E8 | C | M-F | Plasma | DQ435683 | 5 |
| QC406.70M.ENV.F3 | C | M-F | PBMC | FJ866133 | 10 |
| ZM53 M.PB12 | C | F-M | PBMC | AY423984 | <14 |
| ZM109F.PB4 | C | M-F | PBMC | AY424138 | <14 |
| ZM197M.PB7 | C | F-M | PBMC | DQ388515 | <15 |
| ZM214M.PL15 | C | F-M | Plasma | DQ388516 | <13 |
| Du156.12 | C | M-F | ccPBMC | DQ411852 | <4 |
| Du172.17 | C | M-F | ccPBMC | DQ411853 | 12 |
| Du422.1 | C | M-F | ccPBMC | DQ411854 | 8 |
| QA013.70I.Env.H1 | D | M-F | ccPBMC | FJ866134 | 10 |
| QB857.110I.Env.B3 | D | M-F | PBMC | FJ866138 | 16 |
M-F, male to female, F-M, female to male; M-M, male to male; M-C, mother to child.
PBMC, Env was cloned from uncultured PBMCs isolated directly from patient; plasma, Env was cloned from virion-associated RNA in plasma isolated directly from patient; ccPBMC, patient PBMCs (or virus from these PBMCs) underwent short-term coculture with PBMCs from HIV-1-negative donors in order to amplify virus before cloning.
Spix's owl monkey CD4 alleles encode receptors that are broadly functional for major HIV-1 subtypes.
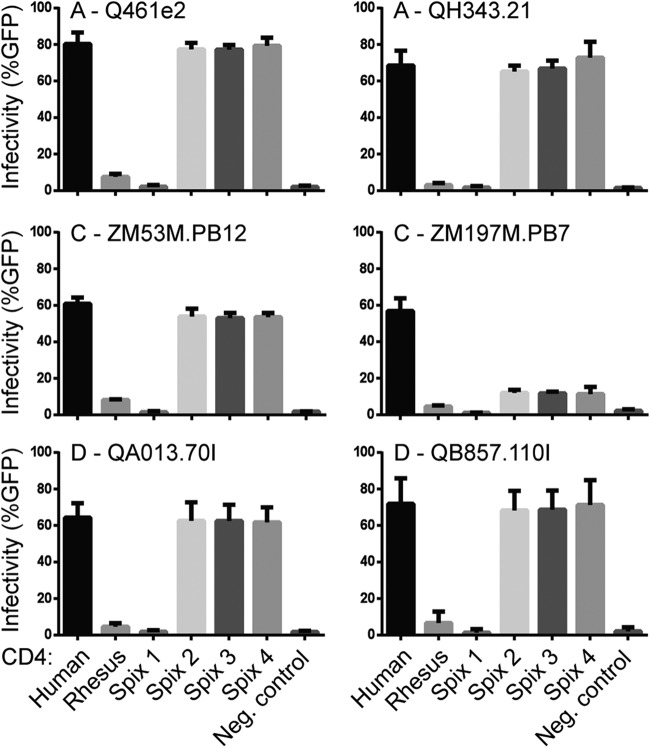
To further investigate the function of receptors encoded by these Spix's owl monkey CD4 alleles, we challenged the CD4-expressing cell lines with a panel of viruses pseudotyped with Envs from the major clades of HIV-1 group M circulating globally (Fig. 4). This panel includes R5-tropic Envs from two subtype A variants (Q461e2 and QH343.21), two subtype C variants (ZM53M.PB12 and ZM197M.PB7), and two subtype D variants (QA013.70I and QB857.110I). All of these Envs are derived from HIV-1 isolated from early stages of infection after sexual transmission and, importantly, are representative of the prevalent circulating variants of HIV-1 group M (Table 1). Human CD4 facilitates entry by all of these viruses (Fig. 4). In contrast, the rhesus macaque CD4 receptor does not facilitate efficient entry by any of them. The three permissive Spix's owl monkey CD4 alleles (Spix 2, 3, and 4) encode receptors that facilitate entry by 5 of the 6 Envs tested at levels similar to human CD4. In summary, these alleles support entry by at least one Env from each subtype tested (A, B, C, or D) (Fig. 3 and 4).
FIG 4.
Some Spix's owl monkey CD4 receptors support entry by representative viruses of the major clades of HIV-1 group M. Cf2Th/syn CCR5 cells expressing various CD4 alleles (indicated along the x axis) were infected with the indicated Env-pseudotyped virions. Env variants are labeled such that the first letter represents the subtype, followed by the strain name. Infection was measured by flow cytometry as percentage GFP-positive cells 72 h postinfection. All pseudotyped virions were generated in the Q23ΔEnvGFP background. Error bars represent the standard deviations of the means from three independent experiments conducted in duplicate.
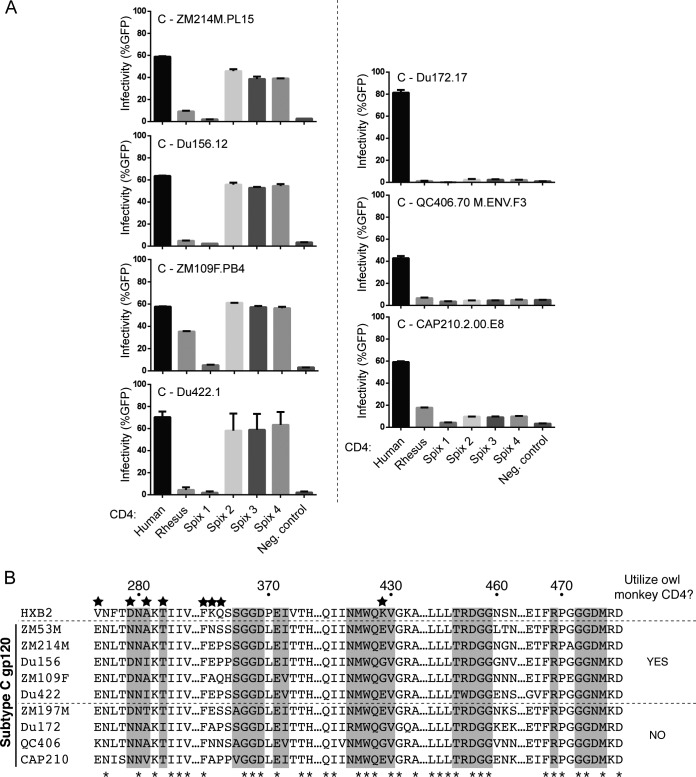
Interestingly, only one of the two subtype C Envs tested entered cells through the permissive Spix's owl monkey CD4s (Fig. 4). Subtype C Env ZM197M.PB7 is unable to efficiently use any Spix's owl monkey CD4 compared to human CD4, while subtype C Env ZM53M.PB12 readily enters through CD4 encoded by three of the Spix's owl monkey alleles. This observation motivated us to test an expanded panel of subtype C Envs (Fig. 5A). An additional seven Envs were tested (Table 1), and four of these (ZM214M.PL15, Du156.12, ZM109F.PB4, and Du422.1) could utilize the permissive owl monkey CD4s at levels similar to human CD4, whereas three of them (Du172.17, QC406.70 M.ENV.F3, and CAP210.2.00.E8) could not. In total, nine subtype C Envs were tested, and five of them were found to utilize the permissive owl monkey CD4s. These data show that while the CD4 receptors encoded by the Spix 2, 3, and 4 alleles allow entry of many vaccine-relevant HIV-1 isolates, they are not exactly equivalent to human CD4 (Fig. 5A). It appears from these data that other sites in CD4, beyond position 39, that differ between human and owl monkey CD4 are also important for the interaction between CD4 and Env in the case of some subtype C viruses. An alignment of all nine subtype C Envs used in this study was generated to compare CD4-binding sites in gp120 (Fig. 5B). While there are many polymorphic sites within these regions, including sites previously characterized to be under positive selection (stars in Fig. 5B), there is no single site that perfectly tracks with the observed entry phenotypes.
FIG 5.
Subtype C Envs differentially utilize owl monkey CD4 for cellular entry. (A) Cf2Th/syn CCR5 cells expressing various CD4 alleles (indicated along the x axis) were infected with the indicated Env-pseudotyped virions. Infection was measured by flow cytometry as percent GFP-positive cells 72 h postinfection. All pseudotyped virions were generated in the Q23ΔEnvGFP background. Error bars represent the standard deviations of the means from three independent experiments conducted in duplicate. (B) A partial amino acid alignment is shown for the nine subtype C Envs used in this study, along with the reference subtype B HXB2 strain. Numbering is relative to HXB2. Amino acids that make direct contact with CD4 (22) are highlighted in gray. Asterisks indicate positions that are completely conserved. Stars indicate amino acid residues that were identified to be evolving under positive selection in previous analyses (7–9).
Permissive CD4 alleles are common in a breeding colony of Spix's owl monkeys.
In the laboratory, mutations can be introduced into virtually any cellular receptor or restriction factor to make it compatible with HIV-1 replication. However, the creation of transgenic laboratory animals, particularly monkeys, expressing these genes has been a consistent roadblock in translating these findings. Since these permissive Spix's owl monkey CD4 alleles are already present in the genomes of living animals, we wished to know if they are common or rare. The Keeling Center for Comparative Medicine and Research (KCCMR), affiliated with the University of Texas, houses the only research-designated breeding colony of Spix's owl monkeys in the United States (Fig. 6A). Using husbandry records of animals housed at the KCCMR, we reconstructed the pedigree of the entire colony and overlaid CD4 genotypes of the 22 individuals studied, which constitute most of the living individuals in this colony (Fig. 6B). We were further able to unambiguously infer the genotypes of 4 additional individuals (inferred genotypes are shown in gray type). We found that 25 out of these 26 individuals carry at least one of the three permissive CD4 alleles and that 23 are homozygous for permissive alleles. This suggests that the HIV-1-permissive alleles are common and ancestral, at least in this captive population. It is unknown if these alleles are common, rare, or nonexistent in wild Spix's owl monkey populations. Nonetheless, living animals bearing the desirable genotype are common.
FIG 6.
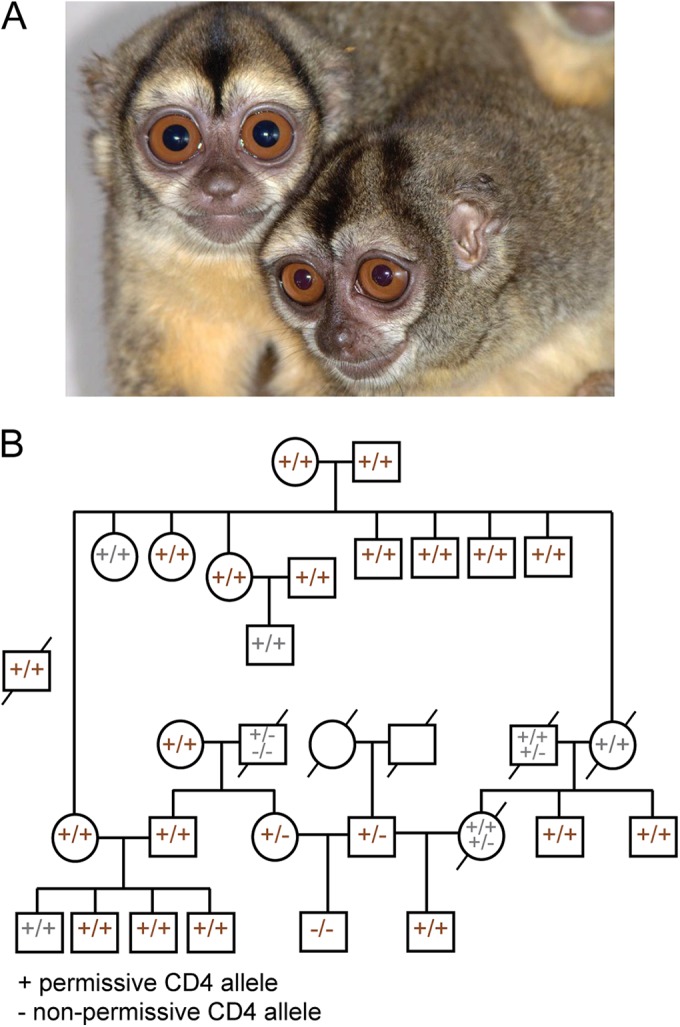
CD4 genotypes are shown for the Spix's owl monkey colony at the KCCMR. (A) Two Spix's owl monkeys from the KCCMR colony (photo credit: Julio Ruiz). (B) A pedigree illustrates the structure of the Aotus vociferans colony housed at the KCCMR. The CD4 genotype was determined for most living individuals, with a plus indicating the presence of a permissive CD4 allele and a minus indicating the presence of a nonpermissive CD4 allele. Squares indicate males, and circles indicate females. Diagonal slashes indicate deceased individuals. Genotypes in gray are deduced from progeny or parents, which in some cases yielded two possible genotypes as indicated.
Permissive CD4 alleles in other species of owl monkeys.
We noted that two other species of owl monkeys that we surveyed, Azara's owl monkey and Nancy Ma's owl monkey, also encode an asparagine at position 39 of CD4 (Fig. 2A). We cloned a CD4 allele from each of these two species into an expression plasmid and transiently transfected it into 293T cells along with a plasmid encoding human CCR5 (Fig. 7A). We found that CD4 from both species supported entry of virions bearing two subtype A HIV-1 envelopes, Q23.ENV.17 and BG505.W6M.B1 (Fig. 7B). These envelopes were isolated from infected individuals near the time of transmission. In our initial population study, only three individuals from each of these two owl monkey species were genotyped (Fig. 2A). Therefore, after getting these results, we obtained samples from more Azara's and Nancy Ma's owl monkey individuals. We genotyped amino acid position 39 in an additional 21 Azara's and 35 Nancy Ma's owl monkeys from KCCMR. All of these individuals were homozygous for an asparagine at position 39, which suggests that all, or the majority, of the owl monkeys of these two species housed at KCCMR bear CD4 alleles that are broadly compatible with early-stage HIV-1 isolates.
FIG 7.
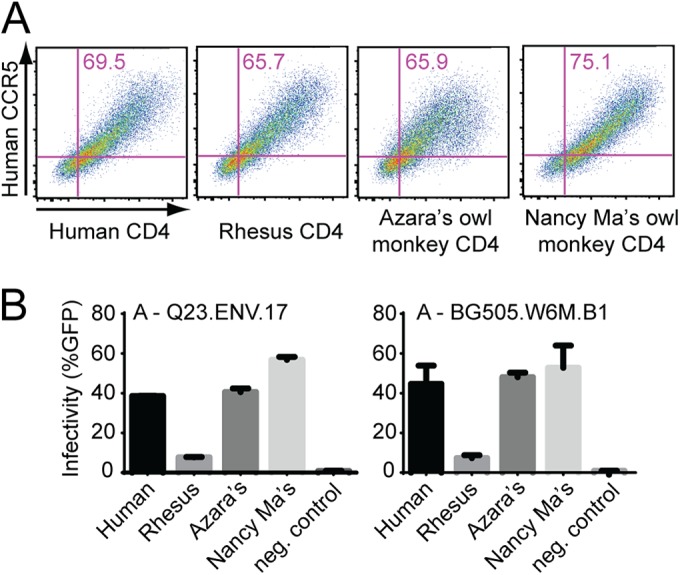
CD4 from Azara's and Nancy Ma's owl monkeys are also permissive for HIV-1 entry. (A) Flow cytometry scatter plots showing CD4 expression of various primate alleles (indicated along the x axis) and human CCR5 expression (indicated along the y axis) in 293T cells. (B) 293T cells from panel A were infected with the indicated Env-pseudotyped virions. Infection was measured by flow cytometry as percent GFP-positive cells 72 h postinfection. All pseudotyped virions were generated in the Q23ΔEnvGFP background. Error bars represent the standard deviations obtained from duplicate wells. The results are representative of three independent experiments.
DISCUSSION
A host-virus arms race appears to exist between CD4 and lentiviral env, and this has driven the positive selection of CD4 over evolutionary time, leaving behind substantial sequence and functional variation even in the CD4 alleles carried by different individuals of the same species. In this study, we have identified three nonhuman primate species, all in the Aotus genus of owl monkeys, in which some individuals encode a CD4 receptor that is functional for circulating variants of HIV-1 associated with the worldwide pandemic. We have characterized the CD4 alleles circulating in one of these species, Spix's owl monkey, in depth. Unlike macaque CD4, Spix's owl monkey CD4 functions as a receptor for most early-stage HIV-1 variants, including representatives from the main circulating subtypes, not just lab-adapted and rare HIV-1 Env variants. Although further work will be needed, Spix's owl monkeys possibly represent a novel model organism for a new class of SHIVs bearing HIV-1 Envs that better represent successful vaccine targets.
The next hurdle will be to determine whether a SHIV bearing Env from early-stage human infections can be engineered to replicate in peripheral blood mononuclear cells (PBMCs) from owl monkeys. New World monkeys became a subject of interest as potential models for HIV-1 when it was observed that their cellular blocks to infection are minimal. For instance, cells from various species of squirrel monkeys, marmosets, and tamarins are infected at high levels by VSV-G-pseudotyped HIV-1 vectors, suggesting that no restriction factor blocks exist after virus entry up to the stage of genome integration (39). Further, primary cells from common marmosets (Callithrix jacchus) and squirrel monkeys (Saimiri sciureus) were found to support each of the post entry steps of HIV-1 replication, including reverse transcription, integration, transcription, translation, assembly, and budding (17). Owl monkeys, on the other hand, have been found to harbor three restriction factors active against HIV-1. First, all species of owl monkeys, including Spix's owl monkey (data not shown), encode the TRIM5-CypA restriction factor (33–35). It is possible to bypass this restriction with a single amino acid mutation in the HIV-1 capsid protein (G89V) (33, 34). It has also been shown that the SIVmac capsid, which is used in most SHIV strains, is not susceptible to owl monkey TRIM5-CypA (40). Second, some species of owl monkeys, including Spix's owl monkey, have functional versions of the BST-2/tetherin restriction factor (16). Third, some species of owl monkeys express levels of APOBEC3G high enough to interfere with HIV-1 replication, but HIV-1 Vif can partially overcome this block (16). The use of owl monkeys, including Spix's owl monkey, as a model organism for vaccine studies is not precluded by any of these, as it is possible to bypass these restrictions through modifications of HIV or SIV that are independent of Env. Alternately, by surveying populations of individuals, it may be possible to identify functional CD4 alleles in other New World monkey species that do not have as many restriction factor blocks against HIV-1.
New World primates, including owl monkeys, are well-established models of human disease in biomedical research (41). This is in part due to the fact that most New World species are relatively small in body size and have rapid reproduction rates which facilitate the maintenance of productive breeding colonies. Owl monkeys are particularly good models for research in that they are easily acclimated to handling and do not generally require pharmaceutical sedation for routine blood collections, in contrast to most larger primate species. This helps minimize any confounding effects on research that may be associated with repeated sedations. The use of owl monkeys in research is further promoted by the fact that these species harbor no known zoonotic pathogens. One potential drawback in the use of owl monkeys in research is their reduced size, and therefore smaller attainable blood volume, compared to other primate species. However, it is noteworthy that owl monkeys are a lymphocyte-predominant species. That is, unlike macaques and most other Old World monkeys in which lymphocytes usually represent only 20 to 35% of the total blood leukocytes, in owl monkey species the lymphocytes routinely constitute between 65 and 75% of the differential leukocyte count (42).
The major circulating subtypes of HIV-1 in sub-Saharan Africa include subtypes A, C, and D. In the United States, subtype B is the most common variant. Here we show that strains from each of these subtypes can use the owl monkey CD4 protein as an entry receptor. However, variation on the side of the virus also has specific effects on the interaction with owl monkey CD4 (Fig. 5). We show that some strains of subtype C can utilize owl monkey CD4, whereas others do not. It is unclear whether this variation is specific to subtype C or is a characteristic of other subtypes as well. Further testing with a larger panel of Envs will be necessary to delineate these scenarios and provide enough information to carry out genetic mapping studies.
In summary, some Spix's owl monkey individuals encode CD4 receptors that are broadly permissive to circulating strains of HIV-1. These permissive alleles were discovered by bioprospecting within populations of monkeys, and similar future studies may continue to reveal alleles that will be useful in HIV research. The literature suggests that there is rich genetic variation to be unearthed. Primate SNPs at restriction factor loci have been shown to dramatically affect the outcome of infection experiments (43–48). Less is known about polymorphism at host loci that encode the HIV-1 receptors, but this can be elucidated through population studies such as the one described here.
ACKNOWLEDGMENTS
This work was supported by grants from the National Institutes of Health (R01-GM-093086 to S.L.S. and AI38518 to J.O.) and an Investigator in the Pathogenesis of Infectious Disease award from the Burroughs Wellcome Fund (to S.L.S.). N.R.M. is supported by a National Science Foundation graduate research fellowship.
We thank Maryska Kaczmarek, Paul Rowley, Zhao Shan, and Alex Stabell for critical readings of the manuscript. We also thank John Nahabedian and Sandra Emery for help with initial infection studies and Welkin Johnson for cell lines. We thank Christian Abee and the KCCMR for their continued support of this project.
REFERENCES
- 1.Huang J, Meyer C, Zhu C. 2012. T cell antigen recognition at the cell membrane. Mol Immunol 52:155–164. doi: 10.1016/j.molimm.2012.05.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Ortiz M, Guex N, Patin E, Martin O, Xenarios I, Ciuffi A, Quintana-Murci L, Telenti A. 2009. Evolutionary trajectories of primate genes involved in HIV pathogenesis. Mol Biol Evol 26:2865–2875. doi: 10.1093/molbev/msp197. [DOI] [PubMed] [Google Scholar]
- 3.Meyerson NR, Rowley PA, Swan CH, Le DT, Wilkerson GK, Sawyer SL. 2014. Positive selection of primate genes that promote HIV-1 replication. Virology 454-455:291–298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Zhang ZD, Weinstock G, Gerstein M. 2008. Rapid evolution by positive Darwinian selection in T-cell antigen CD4 in primates. J Mol Evol 66:446–456. doi: 10.1007/s00239-008-9097-1. [DOI] [PubMed] [Google Scholar]
- 5.Kwong PD, Wyatt R, Robinson J, Sweet RW, Sodroski J, Hendrickson WA. 1998. Structure of an HIV gp120 envelope glycoprotein in complex with the CD4 receptor and a neutralizing human antibody. Nature 393:648–659. doi: 10.1038/31405. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Meyerson NR, Sawyer SL. 2011. Two-stepping through time: mammals and viruses. 6. Trends Microbiol 19:286–294. doi: 10.1016/j.tim.2011.03.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Cenci A, D'Avenio G, Tavoschi L, Chiappi M, Becattini S, Narino MDP, Picconi O, Bernasconi D, Fanales-Belasio E, Vardas E, Sukati H, Presti Lo A, Ciccozzi M, Monini P, Ensoli B, Grigioni M, Buttò S. 2014. Molecular characterization of HIV-1 subtype C gp-120 regions potentially involved in virus adaptive mechanisms. PLoS One 9:e95183. doi: 10.1371/journal.pone.0095183. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Yamaguchi-Kabata Y, Gojobori T. 2000. Reevaluation of amino acid variability of the human immunodeficiency virus type 1 gp120 envelope glycoprotein and prediction of new discontinuous epitopes. J Virol 74:4335–4350. doi: 10.1128/JVI.74.9.4335-4350.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Yang Z. 2001. Maximum likelihood analysis of adaptive evolution in HIV-1 gp120 env gene. Pac Symp Biocomput 21:226–237. [PubMed] [Google Scholar]
- 10.Hatziioannou T, Evans DT. 2012. Animal models for HIV/AIDS research. Nat Rev Microbiol 10:852–867. doi: 10.1038/nrmicro2911. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Harris RS, Hultquist JF, Evans DT. 2012. The restriction factors of human immunodeficiency virus. J Biol Chem 287:40875–40883. doi: 10.1074/jbc.R112.416925. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Blanco-Melo D, Venkatesh S, Bieniasz PD. 2012. Intrinsic cellular defenses against human immunodeficiency viruses. Immunity 37:399–411. doi: 10.1016/j.immuni.2012.08.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Hessell AJ, Haigwood NL. 2015. Animal models in HIV-1 protection and therapy. Curr Opin HIV AIDS 10:170–176. doi: 10.1097/COH.0000000000000152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Humes D, Emery S, Laws E, Overbaugh J. 2012. A species-specific amino acid difference in the macaque CD4 receptor restricts replication by global circulating HIV-1 variants representing viruses from recent infection. J Virol 86:12472–12483. doi: 10.1128/JVI.02176-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Fomsgaard A, Johnson PR, Nielsen C, Novembre FJ, Hansen J, Goldstein S, Hirsch VM. 1995. Receptor function of CD4 structures from African green monkey and pig-tail macaque for simian immunodeficiency virus, SIVsm, SIVagm, and human immunodeficiency virus type-1. Viral Immunol 8:121–133. [DOI] [PubMed] [Google Scholar]
- 16.Wong SK, Connole M, Sullivan JS, Choe H, Carville A, Farzan M. 2009. A New World primate deficient in tetherin-mediated restriction of human immunodeficiency virus type 1. J Virol 83:8771–8780. doi: 10.1128/JVI.00112-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.LaBonte JA, Babcock GJ, Patel T, Sodroski J. 2002. Blockade of HIV-1 infection of New World monkey cells occurs primarily at the stage of virus entry. J Exp Med 196:431–445. doi: 10.1084/jem.20020468. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Karlsson GB, Halloran M, Li J, Park IW, Gomila R, Reimann KA, Axthelm MK, Iliff SA, Letvin NL, Sodroski J. 1997. Characterization of molecularly cloned simian-human immunodeficiency viruses causing rapid CD4+ lymphocyte depletion in rhesus monkeys. J Virol 71:4218–4225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Boyd DF, Peterson D, Haggarty BS, Jordan APO, Hogan MJ, Goo L, Hoxie JA, Overbaugh J. 2015. Mutations in HIV-1 envelope that enhance entry with the macaque CD4 receptor alter antibody recognition by disrupting quaternary interactions within the trimer. J Virol 89:894–907. doi: 10.1128/JVI.02680-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Del Prete GQ, Ailers B, Moldt B, Keele BF, Estes JD, Rodriguez A, Sampias M, Oswald K, Fast R, Trubey CM, Chertova E, Smedley J, LaBranche CC, Montefiori DC, Burton DR, Shaw GM, Markowitz M, Piatak M Jr, KewalRamani VN, Bieniasz PD, Lifson JD, Hatziioannou T. 2014. Selection of unadapted, pathogenic SHIVs encoding newly transmitted HIV-1 envelope proteins. Cell Host Microbe 16:412–418. doi: 10.1016/j.chom.2014.08.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Humes D, Overbaugh J. 2011. Adaptation of subtype A human immunodeficiency virus type 1 envelope to pig-tailed macaque cells. J Virol 85:4409–4420. doi: 10.1128/JVI.02244-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Puffer BA, Pohlmann S, Edinger AL, Carlin D, Sanchez MD, Reitter J, Watry DD, Fox HS, Desrosiers RC, Doms RW. 2002. CD4 independence of simian immunodeficiency virus Envs is associated with macrophage tropism, neutralization sensitivity, and attenuated pathogenicity. J Virol 76:2595–2605. doi: 10.1128/JVI.76.6.2595-2605.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Long EM, Rainwater SMJ, Lavreys L, Mandaliya K, Overbaugh J. 2002. HIV type 1 variants transmitted to women in Kenya require the CCR5 coreceptor for entry, regardless of the genetic complexity of the infecting virus. 8. AIDS Res Hum Retroviruses 18:567–576. doi: 10.1089/088922202753747914. [DOI] [PubMed] [Google Scholar]
- 24.Blish CA, Jalalian-Lechak Z, Rainwater S, Nguyen M-A, Dogan OC, Overbaugh J. 2009. Cross-subtype neutralization sensitivity despite monoclonal antibody resistance among early subtype A, C, and D envelope variants of human immunodeficiency virus type 1. J Virol 83:7783–7788. doi: 10.1128/JVI.00673-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Rainwater SMJ, Wu X, Nduati R, Nedellec R, Mosier D, John-Stewart G, Mbori-Ngacha D, Overbaugh J. 2007. Cloning and characterization of functional subtype A HIV-1 envelope variants transmitted through breastfeeding. Curr HIV Res 5:189–197. doi: 10.2174/157016207780076986. [DOI] [PubMed] [Google Scholar]
- 26.Wu X, Parast AB, Richardson BA, Nduati R, John-Stewart G, Mbori-Ngacha D, Rainwater SMJ, Overbaugh J. 2006. Neutralization escape variants of human immunodeficiency virus type 1 are transmitted from mother to infant. J Virol 80:835–844. doi: 10.1128/JVI.80.2.835-844.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Li M, Gao F, Mascola JR, Stamatatos L, Polonis VR, Koutsoukos M, Voss G, Goepfert P, Gilbert P, Greene KM, Bilska M, Kothe DL, Salazar-Gonzalez JF, Wei X, Decker JM, Hahn BH, Montefiori DC. 2005. Human immunodeficiency virus type 1 env clones from acute and early subtype B infections for standardized assessments of vaccine-elicited neutralizing antibodies. J Virol 79:10108–10125. doi: 10.1128/JVI.79.16.10108-10125.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Li M, Salazar-Gonzalez JF, Derdeyn CA, Morris L, Williamson C, Robinson JE, Decker JM, Li Y, Salazar MG, Polonis VR, Mlisana K, Karim SA, Hong K, Greene KM, Bilska M, Zhou J, Allen S, Chomba E, Mulenga J, Vwalika C, Gao F, Zhang M, Korber BTM, Hunter E, Hahn BH, Montefiori DC. 2006. Genetic and neutralization properties of subtype C human immunodeficiency virus type 1 molecular env clones from acute and early heterosexually acquired infections in Southern Africa. J Virol 80:11776–11790. doi: 10.1128/JVI.01730-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Mirzabekov T, Bannert N, Farzan M, Hofmann W, Kolchinsky P, Wu L, Wyatt R, Sodroski J. 1999. Enhanced expression, native purification, and characterization of CCR5, a principal HIV-1 coreceptor. J Biol Chem 274:28745–28750. doi: 10.1074/jbc.274.40.28745. [DOI] [PubMed] [Google Scholar]
- 30.Yang Z. 2007. PAML 4: phylogenetic analysis by maximum likelihood. Mol Biol Evol 24:1586–1591. doi: 10.1093/molbev/msm088. [DOI] [PubMed] [Google Scholar]
- 31.Karolchik D, Baertsch R, Diekhans M, Furey T, Hinrichs A, Lu Y, Roskin K, Schwartz M, Sugnet C, Thomas D, Weber R, Haussler D, Kent W. 2003. The UCSC Genome Browser Database. Nucleic Acids Res 31:51–54. doi: 10.1093/nar/gkg129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Sironi M, Cagliani R, Forni D, Clerici M. 2015. Evolutionary insights into host-pathogen interactions from mammalian sequence data. Nat Rev Genet 16:224–236. doi: 10.1038/nrg3905. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Sayah DM, Sokolskaja E, Berthoux L, Luban J. 2004. Cyclophilin A retrotransposition into TRIM5 explains owl monkey resistance to HIV-1. Nature 430:569–573. doi: 10.1038/nature02777. [DOI] [PubMed] [Google Scholar]
- 34.Nisole S, Lynch C, Stoye JP, Yap MW. 2004. A Trim5-cyclophilin A fusion protein found in owl monkey kidney cells can restrict HIV-1. Proc Natl Acad Sci U S A 101:13324–13328. doi: 10.1073/pnas.0404640101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Ribeiro IP, Menezes AN, Moreira MAM, Bonvicino CR, Seuánez HN, Soares MA. 2005. Evolution of cyclophilin A and TRIMCyp retrotransposition in New World primates. J Virol 79:14998–15003. doi: 10.1128/JVI.79.23.14998-15003.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Pacheco B, Basmaciogullari S, LaBonte JA, Xiang S-H, Sodroski J. 2008. Adaptation of the human immunodeficiency virus type 1 envelope glycoproteins to New World monkey receptors. J Virol 82:346–357. doi: 10.1128/JVI.01299-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Fomsgaard A, Müller-Trutwin MC, Diop O, Hansen J, Mathiot C, Corbet S, Barré-Sinoussi F, Allan JS. 1997. Relation between phylogeny of African green monkey CD4 genes and their respective simian immunodeficiency virus genes. J Med Primatol 26:120–128. doi: 10.1111/j.1600-0684.1997.tb00043.x. [DOI] [PubMed] [Google Scholar]
- 38.Hvilsom C, Carlsen F, Siegismund HR, Corbet S, Nerrienet E, Fomsgaard A. 2008. Genetic subspecies diversity of the chimpanzee CD4 virus-receptor gene. Genomics 92:322–328. doi: 10.1016/j.ygeno.2008.07.003. [DOI] [PubMed] [Google Scholar]
- 39.Hofmann W, Schubert D, LaBonte J, Munson L, Gibson S, Scammell J, Ferrigno P, Sodroski J. 1999. Species-specific, postentry barriers to primate immunodeficiency virus infection. J Virol 73:10020–10028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Diaz-Griffero F, Vandegraaff N, Li Y, McGee-Estrada K, Stremlau M, Welikala S, Si Z, Engelman A, Sodroski J. 2006. Requirements for capsid-binding and an effector function in TRIMCyp-mediated restriction of HIV-1. Virology 351:404–419. doi: 10.1016/j.virol.2006.03.023. [DOI] [PubMed] [Google Scholar]
- 41.Ward JM, Vallender EJ. 2012. The resurgence and genetic implications of New World primates in biomedical research. Trends Genet 28:586–591. doi: 10.1016/j.tig.2012.09.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Fox JG, Anderson LC, Loew FM, Quimby FW. 2002. Laboratory animal medicine. Academic Press, New York, NY. [Google Scholar]
- 43.Krupp A, McCarthy KR, Ooms M, Letko M, Morgan JS, Simon V, Johnson WE. 2013. APOBEC3G polymorphism as a selective barrier to cross-species transmission and emergence of pathogenic SIV and AIDS in a primate host. PLoS Pathog 9:e1003641. doi: 10.1371/journal.ppat.1003641. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Sawyer SL, Wu LI, Akey JM, Emerman M, Malik HS. 2006. High-frequency persistence of an impaired allele of the retroviral defense gene TRIM5α in humans. Curr Biol 16:95–100. doi: 10.1016/j.cub.2005.11.045. [DOI] [PubMed] [Google Scholar]
- 45.Duggal NK, Fu W, Akey JM, Emerman M. 2013. Identification and antiviral activity of common polymorphisms in the APOBEC3 locus in human populations. Virology 443:329–337. doi: 10.1016/j.virol.2013.05.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Compton AA, Hirsch VM, Emerman M. 2012. The host restriction factor APOBEC3G and retroviral Vif protein coevolve due to ongoing genetic conflict. Cell Host Microbe 11:91–98. doi: 10.1016/j.chom.2011.11.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Kirmaier A, Wu F, Newman RM, Hall LR, Morgan JS, O'Connor S, Marx PA, Meythaler M, Goldstein S, Buckler-White A, Kaur A, Hirsch VM, Johnson WE. 2010. TRIM5 suppresses cross-species transmission of a primate immunodeficiency virus and selects for emergence of resistant variants in the new species. PLoS Biol 8:e1000462. doi: 10.1371/journal.pbio.1000462. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.OhAinle M, Kerns JA, Li MMH, Malik HS, Emerman M. 2008. Antiretroelement activity of APOBEC3H was lost twice in recent human evolution. Cell Host Microbe 4:249–259. doi: 10.1016/j.chom.2008.07.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Wang JH, Meijers R, Xiong Y, Liu JH, Sakihama T, Zhang R, Joachimiak A, Reinherz EL. 2001. Crystal structure of the human CD4 N-terminal two-domain fragment complexed to a class II MHC molecule. Proc Natl Acad Sci U S A 98:10799–10804. doi: 10.1073/pnas.191124098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Huang CC, Venturi M, Majeed S, Moore MJ, Phogat S, Zhang MY, Dimitrov DS, Hendrickson WA, Robinson J, Sodroski J, Wyatt R, Choe H, Farzan M, Kwong PD. 2004. Structural basis of tyrosine sulfation and VH-gene usage in antibodies that recognize the HIV type 1 coreceptor-binding site on gp120. Proc Natl Acad Sci U S A 101:2706–2711. doi: 10.1073/pnas.0308527100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Sharp PM, Hahn BH. 2011. Origins of HIV and the AIDS pandemic. Cold Spring Harb Perspect Med 1:a006841. [DOI] [PMC free article] [PubMed] [Google Scholar]