Abstract
Phosphorylation of the regulatory light chain is an important mechanism for the activation of myosin in non-muscle cells. Unlike most myosin light chain kinases (MLCKs), MLCK-A from Dictyostelium is not activated by Ca2+/calmodulin. Autophosphorylation increases activity, but only to a low level, suggesting that there is an additional activation mechanism. Here, we show that MLCK-A is autophosphorylated on Thr289, which is C-terminal to the catalytic domain. Phosphorylation of MLCK-A increases in response to concanavalin A (conA) treatment of cells, which was previously shown to activate MLCK-A. However, a mutant kinase with an alanine at position 289 (T289A) is also phosphorylated in vivo, indicating that there is an additional phosphorylated residue. Based on comparisons with other protein kinases, we tested whether phosphorylation of Thr166 drives activation of MLCK-A. Our data indicate that phosphorylation of Thr289 occurs in vivo, but is not associated with conA-induced activation, whereas phosphorylation of Thr166 by some as yet unidentified kinase is associated with activation. Replacement of Thrl66 with glutamate results in a 12-fold increase in activity as compared with the wild-type enzyme, supporting the idea that phosphorylation of Thr166 increases MLCK-A activity.
Full text
PDF



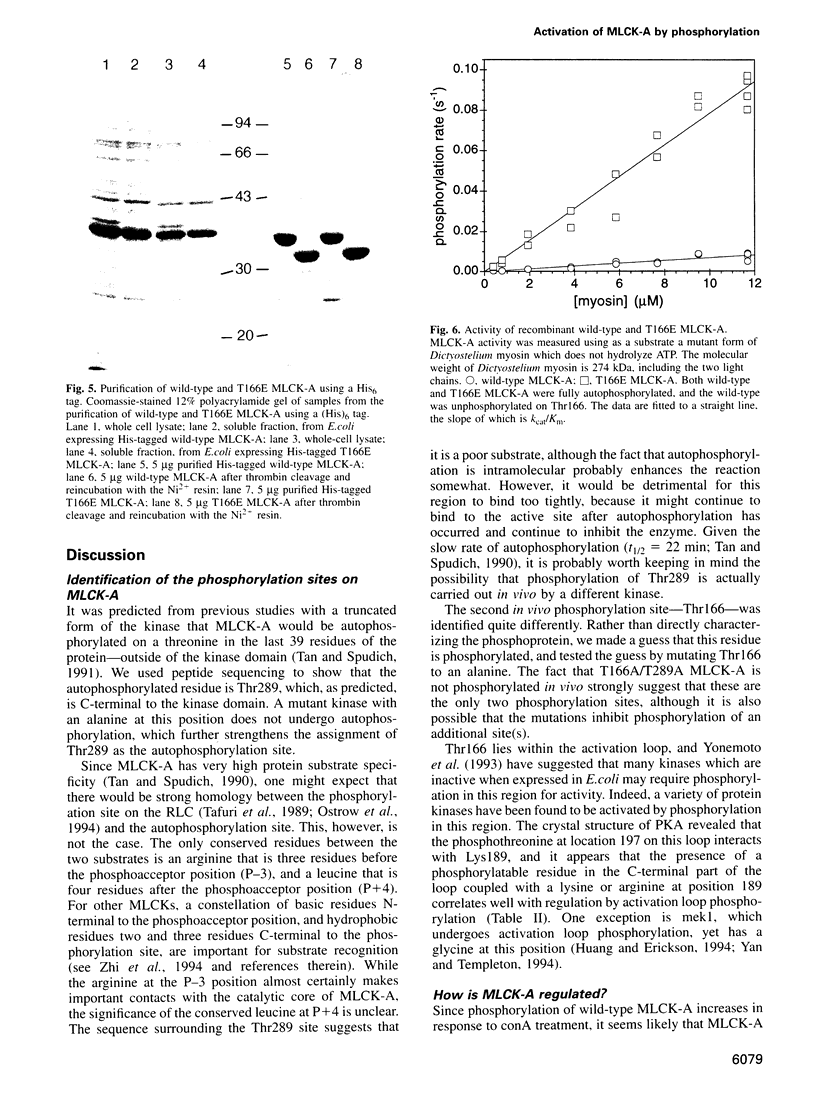




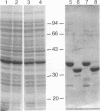
Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Ahn N. G., Seger R., Bratlien R. L., Diltz C. D., Tonks N. K., Krebs E. G. Multiple components in an epidermal growth factor-stimulated protein kinase cascade. In vitro activation of a myelin basic protein/microtubule-associated protein 2 kinase. J Biol Chem. 1991 Mar 5;266(7):4220–4227. [PubMed] [Google Scholar]
- Berlot C. H., Devreotes P. N., Spudich J. A. Chemoattractant-elicited increases in Dictyostelium myosin phosphorylation are due to changes in myosin localization and increases in kinase activity. J Biol Chem. 1987 Mar 15;262(8):3918–3926. [PubMed] [Google Scholar]
- Berlot C. H., Spudich J. A., Devreotes P. N. Chemoattractant-elicited increases in myosin phosphorylation in Dictyostelium. Cell. 1985 Nov;43(1):307–314. doi: 10.1016/0092-8674(85)90036-4. [DOI] [PubMed] [Google Scholar]
- Bossemeyer D., Engh R. A., Kinzel V., Ponstingl H., Huber R. Phosphotransferase and substrate binding mechanism of the cAMP-dependent protein kinase catalytic subunit from porcine heart as deduced from the 2.0 A structure of the complex with Mn2+ adenylyl imidodiphosphate and inhibitor peptide PKI(5-24). EMBO J. 1993 Mar;12(3):849–859. doi: 10.1002/j.1460-2075.1993.tb05725.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bradford M. M. A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal Biochem. 1976 May 7;72:248–254. doi: 10.1016/0003-2697(76)90527-3. [DOI] [PubMed] [Google Scholar]
- Chen P., Ostrow B. D., Tafuri S. R., Chisholm R. L. Targeted disruption of the Dictyostelium RMLC gene produces cells defective in cytokinesis and development. J Cell Biol. 1994 Dec;127(6 Pt 2):1933–1944. doi: 10.1083/jcb.127.6.1933. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen T. L., Kowalczyk P. A., Ho G., Chisholm R. L. Targeted disruption of the Dictyostelium myosin essential light chain gene produces cells defective in cytokinesis and morphogenesis. J Cell Sci. 1995 Oct;108(Pt 10):3207–3218. doi: 10.1242/jcs.108.10.3207. [DOI] [PubMed] [Google Scholar]
- Cohen S. M., Knecht D., Lodish H. F., Loomis W. F. DNA sequences required for expression of a Dictyostelium actin gene. EMBO J. 1986 Dec 1;5(12):3361–3366. doi: 10.1002/j.1460-2075.1986.tb04651.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cox S., Radzio-Andzelm E., Taylor S. S. Domain movements in protein kinases. Curr Opin Struct Biol. 1994 Dec;4(6):893–901. doi: 10.1016/0959-440x(94)90272-0. [DOI] [PubMed] [Google Scholar]
- De Bondt H. L., Rosenblatt J., Jancarik J., Jones H. D., Morgan D. O., Kim S. H. Crystal structure of cyclin-dependent kinase 2. Nature. 1993 Jun 17;363(6430):595–602. doi: 10.1038/363595a0. [DOI] [PubMed] [Google Scholar]
- De Lozanne A., Spudich J. A. Disruption of the Dictyostelium myosin heavy chain gene by homologous recombination. Science. 1987 May 29;236(4805):1086–1091. doi: 10.1126/science.3576222. [DOI] [PubMed] [Google Scholar]
- Ducommun B., Brambilla P., Félix M. A., Franza B. R., Jr, Karsenti E., Draetta G. cdc2 phosphorylation is required for its interaction with cyclin. EMBO J. 1991 Nov;10(11):3311–3319. doi: 10.1002/j.1460-2075.1991.tb04895.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Egelhoff T. T., Lee R. J., Spudich J. A. Dictyostelium myosin heavy chain phosphorylation sites regulate myosin filament assembly and localization in vivo. Cell. 1993 Oct 22;75(2):363–371. doi: 10.1016/0092-8674(93)80077-r. [DOI] [PubMed] [Google Scholar]
- Goldberg J., Nairn A. C., Kuriyan J. Structural basis for the autoinhibition of calcium/calmodulin-dependent protein kinase I. Cell. 1996 Mar 22;84(6):875–887. doi: 10.1016/s0092-8674(00)81066-1. [DOI] [PubMed] [Google Scholar]
- Goldsmith E. J., Cobb M. H. Protein kinases. Curr Opin Struct Biol. 1994 Dec;4(6):833–840. doi: 10.1016/0959-440x(94)90264-x. [DOI] [PubMed] [Google Scholar]
- Gould K. L., Moreno S., Owen D. J., Sazer S., Nurse P. Phosphorylation at Thr167 is required for Schizosaccharomyces pombe p34cdc2 function. EMBO J. 1991 Nov;10(11):3297–3309. doi: 10.1002/j.1460-2075.1991.tb04894.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Griffith L. M., Downs S. M., Spudich J. A. Myosin light chain kinase and myosin light chain phosphatase from Dictyostelium: effects of reversible phosphorylation on myosin structure and function. J Cell Biol. 1987 May;104(5):1309–1323. doi: 10.1083/jcb.104.5.1309. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hadwiger J. A., Firtel R. A. Analysis of G alpha 4, a G-protein subunit required for multicellular development in Dictyostelium. Genes Dev. 1992 Jan;6(1):38–49. doi: 10.1101/gad.6.1.38. [DOI] [PubMed] [Google Scholar]
- Hammer J. A., 3rd Regulation of Dictyostelium myosin II by phosphorylation: what is essential and what is important? J Cell Biol. 1994 Dec;127(6 Pt 2):1779–1782. doi: 10.1083/jcb.127.6.1779. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hu S. H., Parker M. W., Lei J. Y., Wilce M. C., Benian G. M., Kemp B. E. Insights into autoregulation from the crystal structure of twitchin kinase. Nature. 1994 Jun 16;369(6481):581–584. doi: 10.1038/369581a0. [DOI] [PubMed] [Google Scholar]
- Huang W., Erikson R. L. Constitutive activation of Mek1 by mutation of serine phosphorylation sites. Proc Natl Acad Sci U S A. 1994 Sep 13;91(19):8960–8963. doi: 10.1073/pnas.91.19.8960. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hubbard S. R., Wei L., Ellis L., Hendrickson W. A. Crystal structure of the tyrosine kinase domain of the human insulin receptor. Nature. 1994 Dec 22;372(6508):746–754. doi: 10.1038/372746a0. [DOI] [PubMed] [Google Scholar]
- Hughes K., Nikolakaki E., Plyte S. E., Totty N. F., Woodgett J. R. Modulation of the glycogen synthase kinase-3 family by tyrosine phosphorylation. EMBO J. 1993 Feb;12(2):803–808. doi: 10.1002/j.1460-2075.1993.tb05715.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johnson L. N., Noble M. E., Owen D. J. Active and inactive protein kinases: structural basis for regulation. Cell. 1996 Apr 19;85(2):149–158. doi: 10.1016/s0092-8674(00)81092-2. [DOI] [PubMed] [Google Scholar]
- Knecht D. A., Loomis W. F. Antisense RNA inactivation of myosin heavy chain gene expression in Dictyostelium discoideum. Science. 1987 May 29;236(4805):1081–1086. doi: 10.1126/science.3576221. [DOI] [PubMed] [Google Scholar]
- Knighton D. R., Bell S. M., Zheng J., Ten Eyck L. F., Xuong N. H., Taylor S. S., Sowadski J. M. 2.0 A refined crystal structure of the catalytic subunit of cAMP-dependent protein kinase complexed with a peptide inhibitor and detergent. Acta Crystallogr D Biol Crystallogr. 1993 May 1;49(Pt 3):357–361. doi: 10.1107/S0907444993000502. [DOI] [PubMed] [Google Scholar]
- Knighton D. R., Zheng J. H., Ten Eyck L. F., Xuong N. H., Taylor S. S., Sowadski J. M. Structure of a peptide inhibitor bound to the catalytic subunit of cyclic adenosine monophosphate-dependent protein kinase. Science. 1991 Jul 26;253(5018):414–420. doi: 10.1126/science.1862343. [DOI] [PubMed] [Google Scholar]
- Lee J. C., Edelman A. M. Activation of Ca(2+)-calmodulin-dependent protein kinase Ia is due to direct phosphorylation by its activator. Biochem Biophys Res Commun. 1995 May 16;210(2):631–637. doi: 10.1006/bbrc.1995.1705. [DOI] [PubMed] [Google Scholar]
- Manstein D. J., Titus M. A., De Lozanne A., Spudich J. A. Gene replacement in Dictyostelium: generation of myosin null mutants. EMBO J. 1989 Mar;8(3):923–932. doi: 10.1002/j.1460-2075.1989.tb03453.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mochizuki H., Sugita R., Ito T., Hidaka H. Phosphorylation of Ca2+/calmodulin-dependent protein kinase V and regulation of its activity. Biochem Biophys Res Commun. 1993 Dec 30;197(3):1595–1600. doi: 10.1006/bbrc.1993.2661. [DOI] [PubMed] [Google Scholar]
- Morgan D. O., De Bondt H. L. Protein kinase regulation: insights from crystal structure analysis. Curr Opin Cell Biol. 1994 Apr;6(2):239–246. doi: 10.1016/0955-0674(94)90142-2. [DOI] [PubMed] [Google Scholar]
- Noegel A. A., Luna J. E. The Dictyostelium cytoskeleton. Experientia. 1995 Dec 18;51(12):1135–1143. doi: 10.1007/BF01944731. [DOI] [PubMed] [Google Scholar]
- Orr J. W., Newton A. C. Requirement for negative charge on "activation loop" of protein kinase C. J Biol Chem. 1994 Nov 4;269(44):27715–27718. [PubMed] [Google Scholar]
- Ostrow B. D., Chen P., Chisholm R. L. Expression of a myosin regulatory light chain phosphorylation site mutant complements the cytokinesis and developmental defects of Dictyostelium RMLC null cells. J Cell Biol. 1994 Dec;127(6 Pt 2):1945–1955. doi: 10.1083/jcb.127.6.1945. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Owen D. J., Noble M. E., Garman E. F., Papageorgiou A. C., Johnson L. N. Two structures of the catalytic domain of phosphorylase kinase: an active protein kinase complexed with substrate analogue and product. Structure. 1995 May 15;3(5):467–482. doi: 10.1016/s0969-2126(01)00180-0. [DOI] [PubMed] [Google Scholar]
- Pasternak C., Spudich J. A., Elson E. L. Capping of surface receptors and concomitant cortical tension are generated by conventional myosin. Nature. 1989 Oct 12;341(6242):549–551. doi: 10.1038/341549a0. [DOI] [PubMed] [Google Scholar]
- Payne D. M., Rossomando A. J., Martino P., Erickson A. K., Her J. H., Shabanowitz J., Hunt D. F., Weber M. J., Sturgill T. W. Identification of the regulatory phosphorylation sites in pp42/mitogen-activated protein kinase (MAP kinase). EMBO J. 1991 Apr;10(4):885–892. doi: 10.1002/j.1460-2075.1991.tb08021.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Picciotto M. R., Czernik A. J., Nairn A. C. Calcium/calmodulin-dependent protein kinase I. cDNA cloning and identification of autophosphorylation site. J Biol Chem. 1993 Dec 15;268(35):26512–26521. [PubMed] [Google Scholar]
- Pollenz R. S., Chen T. L., Trivinos-Lagos L., Chisholm R. L. The Dictyostelium essential light chain is required for myosin function. Cell. 1992 Jun 12;69(6):951–962. doi: 10.1016/0092-8674(92)90614-i. [DOI] [PubMed] [Google Scholar]
- Robbins D. J., Zhen E., Owaki H., Vanderbilt C. A., Ebert D., Geppert T. D., Cobb M. H. Regulation and properties of extracellular signal-regulated protein kinases 1 and 2 in vitro. J Biol Chem. 1993 Mar 5;268(7):5097–5106. [PubMed] [Google Scholar]
- Ruppel K. M., Spudich J. A. Structure-function studies of the myosin motor domain: importance of the 50-kDa cleft. Mol Biol Cell. 1996 Jul;7(7):1123–1136. doi: 10.1091/mbc.7.7.1123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ruppel K. M., Uyeda T. Q., Spudich J. A. Role of highly conserved lysine 130 of myosin motor domain. In vivo and in vitro characterization of site specifically mutated myosin. J Biol Chem. 1994 Jul 22;269(29):18773–18780. [PubMed] [Google Scholar]
- Scopes R. K. Measurement of protein by spectrophotometry at 205 nm. Anal Biochem. 1974 May;59(1):277–282. doi: 10.1016/0003-2697(74)90034-7. [DOI] [PubMed] [Google Scholar]
- Shoji S., Ericsson L. H., Walsh K. A., Fischer E. H., Titani K. Amino acid sequence of the catalytic subunit of bovine type II adenosine cyclic 3',5'-phosphate dependent protein kinase. Biochemistry. 1983 Jul 19;22(15):3702–3709. doi: 10.1021/bi00284a025. [DOI] [PubMed] [Google Scholar]
- Solomon M. J., Lee T., Kirschner M. W. Role of phosphorylation in p34cdc2 activation: identification of an activating kinase. Mol Biol Cell. 1992 Jan;3(1):13–27. doi: 10.1091/mbc.3.1.13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Steinberg R. A., Cauthron R. D., Symcox M. M., Shuntoh H. Autoactivation of catalytic (C alpha) subunit of cyclic AMP-dependent protein kinase by phosphorylation of threonine 197. Mol Cell Biol. 1993 Apr;13(4):2332–2341. doi: 10.1128/mcb.13.4.2332. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sweeney H. L., Yang Z., Zhi G., Stull J. T., Trybus K. M. Charge replacement near the phosphorylatable serine of the myosin regulatory light chain mimics aspects of phosphorylation. Proc Natl Acad Sci U S A. 1994 Feb 15;91(4):1490–1494. doi: 10.1073/pnas.91.4.1490. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tafuri S. R., Rushforth A. M., Kuczmarski E. R., Chisholm R. L. Dictyostelium discoideum myosin: isolation and characterization of cDNAs encoding the regulatory light chain. Mol Cell Biol. 1989 Jul;9(7):3073–3080. doi: 10.1128/mcb.9.7.3073. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tan J. L., Ravid S., Spudich J. A. Control of nonmuscle myosins by phosphorylation. Annu Rev Biochem. 1992;61:721–759. doi: 10.1146/annurev.bi.61.070192.003445. [DOI] [PubMed] [Google Scholar]
- Tan J. L., Spudich J. A. Characterization and bacterial expression of the Dictyostelium myosin light chain kinase cDNA. Identification of an autoinhibitory domain. J Biol Chem. 1991 Aug 25;266(24):16044–16049. [PubMed] [Google Scholar]
- Tan J. L., Spudich J. A. Dictyostelium myosin light chain kinase. Purification and characterization. J Biol Chem. 1990 Aug 15;265(23):13818–13824. [PubMed] [Google Scholar]
- Thorsness P. E., Koshland D. E., Jr Inactivation of isocitrate dehydrogenase by phosphorylation is mediated by the negative charge of the phosphate. J Biol Chem. 1987 Aug 5;262(22):10422–10425. [PubMed] [Google Scholar]
- Wessels D., Soll D. R., Knecht D., Loomis W. F., De Lozanne A., Spudich J. Cell motility and chemotaxis in Dictyostelium amebae lacking myosin heavy chain. Dev Biol. 1988 Jul;128(1):164–177. doi: 10.1016/0012-1606(88)90279-5. [DOI] [PubMed] [Google Scholar]
- White M. F., Shoelson S. E., Keutmann H., Kahn C. R. A cascade of tyrosine autophosphorylation in the beta-subunit activates the phosphotransferase of the insulin receptor. J Biol Chem. 1988 Feb 25;263(6):2969–2980. [PubMed] [Google Scholar]
- Xu R. M., Carmel G., Sweet R. M., Kuret J., Cheng X. Crystal structure of casein kinase-1, a phosphate-directed protein kinase. EMBO J. 1995 Mar 1;14(5):1015–1023. doi: 10.1002/j.1460-2075.1995.tb07082.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yan M., Templeton D. J. Identification of 2 serine residues of MEK-1 that are differentially phosphorylated during activation by raf and MEK kinase. J Biol Chem. 1994 Jul 22;269(29):19067–19073. [PubMed] [Google Scholar]
- Yonemoto W., Garrod S. M., Bell S. M., Taylor S. S. Identification of phosphorylation sites in the recombinant catalytic subunit of cAMP-dependent protein kinase. J Biol Chem. 1993 Sep 5;268(25):18626–18632. [PubMed] [Google Scholar]
- Zhang F., Strand A., Robbins D., Cobb M. H., Goldsmith E. J. Atomic structure of the MAP kinase ERK2 at 2.3 A resolution. Nature. 1994 Feb 24;367(6465):704–711. doi: 10.1038/367704a0. [DOI] [PubMed] [Google Scholar]
- Zheng J., Knighton D. R., Xuong N. H., Taylor S. S., Sowadski J. M., Ten Eyck L. F. Crystal structures of the myristylated catalytic subunit of cAMP-dependent protein kinase reveal open and closed conformations. Protein Sci. 1993 Oct;2(10):1559–1573. doi: 10.1002/pro.5560021003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhi G., Herring B. P., Stull J. T. Structural requirements for phosphorylation of myosin regulatory light chain from smooth muscle. J Biol Chem. 1994 Oct 7;269(40):24723–24727. [PubMed] [Google Scholar]