Abstract
Molecular signature of advanced and metastatic thyroid carcinoma involves deregulation of multiple fundamental pathways activated in the tumor microenvironment. They include BRAFV600E and AKT that affect tumor initiation, progression and metastasis. Human thyroid cancer orthotopic mouse models are based on human cell lines that generally harbor genetic alterations found in human thyroid cancers. They can reproduce in vivo and in situ (into the thyroid) many features of aggressive and refractory human advanced thyroid carcinomas, including local invasion and metastasis. Humanized orthotopic mouse models seem to be ideal and commonly used for preclinical and translational studies of compounds and therapies not only because they may mimic key aspects of human diseases (e.g. metastasis), but also for their reproducibility. In addition, they might provide the possibility to evaluate systemic effects of treatments. So far, human thyroid cancer in vivo models were mainly used to test single compounds, non selective and selective. Despite the greater antitumor activity and lower toxicity obtained with different selective drugs in respect to non-selective ones, most of them are only able to delay disease progression, which ultimately could restart with similar aggressive behavior. Aggressive thyroid tumors (for example, anaplastic or poorly differentiated thyroid carcinoma) carry several complex genetic alterations that are likely cooperating to promote disease progression and might confer resistance to single-compound approaches. Orthotopic models of human thyroid cancer also hold the potential to be good models for testing novel combinatorial therapies. In this article, we will summarize results on preclinical testing of selective and nonselective single compounds in orthotopic mouse models based on validated human thyroid cancer cell lines harboring the BRAFV600E mutation or with wild-type BRAF. Furthermore, we will discuss the potential use of this model also for combinatorial approaches, which are expected to take place in the upcoming human thyroid cancer basic and clinical research.
Keywords: metastatic human thyroid cancer, orthotopic, BRAFV600E, preclinical and translational model, vemurafenib, tyrosine kinase inhibitors
INTRODUCTION
Thyroid carcinoma is the most common endocrine malignancy, with an incidence rapidly increasing worldwide.1,2 The most common type of thyroid malignancies that originate from epithelial follicular cells is papillary thyroid cancer (PTC), which accounts for 80% and follicular thyroid cancer (FTC) accounts for 10% of all thyroid cancer cases PTC and FTC generally present well-differentiated characteristics, such as thyroid hormone production and iodine uptake, and are therefore classified as differentiated thyroid cancers (DTCs). DTCs are mainly treated with a combination of surgery, thyroid-stimulating hormone suppression and radioactive iodine (131I) therapy, resulting in a good prognosis.3,4 Nevertheless, patients with DTC could have recurrence/persistent disease5–9 and progress to poorly differentiated (PDTC) and anaplastic thyroid cancers (ATC). In addition, other rare forms of thyroid cancer, such as medullary thyroid cancer arising from para-follicular C cells and Hurthle cell carcinoma, present a more aggressive phenotype at the time of diagnosis and are typically associated with a poorer survival rate.10–15 Altogether, aggressive thyroid cancers (that is, PDTC and ATC) have very little response to current therapies, high lethality and represent a therapeutic challenge.
The genetic changes involved in DTC initiation have been explored,16 however, much less is known about mechanisms of tumor progression toward PDTC or ATC, metastasis and recurrence. Numerous tumor models have demonstrated that overexpression of single activating mutations such as RET/PTC, BRAFV600E and oncogenic RAS are sufficient to promote thyroid tumorigenesis. In PTCs there are common point mutations in the BRAF gene (for example, exon 15: V600E and T1799A), or RET/PTC rearrangements, whereas in FTCs there are frequent single point mutations in the RAS gene, or the PPARγ/Pax-8 translocation.16–18 However, tumorigenesis in genetically engineered mouse (GEM) models is not induced when these mutations are expressed at endogenous levels, indicating that additional co-occurring alterations are required.19–21 In general, more aggressive tumors usually have mutations affecting different pathways. For example, in ATC, coexistence of two or more genetic alterations in receptor tyrosine kinases (RTKs), phosphatidylinositol 3-kinase (PI3K)/AKT and MAPK (for example, ERK1/2) pathways has been reported in 77.1% of the analyzed samples.22 In addition, many RTKs, including vascular endothelial growth factor receptor (VEGFR), platelet-derived growth factor receptor, epidermal growth factor receptor (EGFR), c-KIT and c-MET are able to activate the AKT pathway,23–25 indicating that RTK-AKT pathways have an important role in thyroid cancer. Activating mutations of AKT or PIK3CA (that encodes the activating subunit of the phosphatidylinositol 3(OH) kinase) could occur in several types of carcinomas26,27 and lead to an aggressive tumor phenotype. In DTC, mutations in PIK3CA are less common, but in ATC are found in about 23–28% of cases.22,28 In addition, copy number gain of PIK3CA has been found in about 24% of FTCs and 38% of ATCs.22 Recently, a remarkable proportion (21%) of aggressive thyroid tumors (PDTC and ATC) were also shown to harbor point mutations in BRAF and PIK3CA or BRAF and AKT1 genes.27 Importantly, although BRAF mutations were found both in the primary tumor and in the matched metastasis, activating mutations of the AKT pathway were found only in metastasis indicating a role for tumor progression but not initiation. Overall, metastatic advanced thyroid tumors could harbor different genetic alterations (as exemplified by the frequent co-activation of MAPK and AKT pathway in ATC) that affect different pathways, each of which likely cooperates to promote disease progression and ultimately patient death.29–31 Biomedical research needs appropriate models to recapitulate the genetic complexity of aggressive and advanced thyroid cancers (that is, PDTC and ATC) in order to test single compounds but also combinatorial treatments, which are expected to be more effective in treating those types of thyroid carcinomas. In addition, the perspective of patient stratification based on high-throughput molecular profiling rather than histopathological features will increase the number of disease ‘classes’ and hence the options of possible treatment regimens according to the type of specific genetic alterations (for example, point mutations, translocations, copy number variation and so on) and activated pathways in cancer.32 Seeing this trend, it becomes evident that biomedical research will increase the demand of rapid and cost-effective model systems for preclinical testing.
GEM models harbor single or multiple genetic alterations, can recapitulate DTC features and can provide great advances in the understanding of mechanisms of tumor initiation and progression33–38 (Table 1). However, application of GEM models in preclinical studies could be limited by the length of time needed to develop tumors, the high costs of model establishment and maintenance and relatively few recapitulate the features of advanced human cancer, including high frequency metastasis.39–43
Table 1.
Properties of genetically engineered mouse (GEM), subcutaneous and orthotopic mouse models of thyroid cancer
| GEM | Subcutaneous | Orthotopic | |
|---|---|---|---|
| Model type | Genetically defined | Human cell line based | Human cell lines based |
| Model purposes | Define the role of specific mutations in tumor initiation and progression | Define the role of specific genes in local tumor growth and drug testing | Define the role of specific genes in in situ tumor growth, invasion, metastasis and drug testing |
| Establishment | Difficult (genetic engineering and animal crossing) | Very easy (under skin inoculation) | Easy (surgical intervention for in situ inoculation) |
| Costs | Very high | Low | Low |
| Genetic alterations | Defined, simple | Multiple (or sometime single) aberrations, extremely complex | Multiple (or sometime single) aberrations and extremely complex |
| Timing for tumor development | Slow | Fast or slow | Fast or slow |
| Tumor growth monitoring | Requiring specific equipment (that is, PET and MRI) | By caliper | Requiring specific equipment (that is, PET, MRI, fluorescence and luciferase) |
| Tumor growth reproducibility | Low, variable between GEM models | Very high | High |
| Local invasion reproducibility/target tissues | Low/neck mesenchyme and neck muscles, | High/skin mesenchyme and vessels | High/neck mesenchyme, neck muscles and lymph nodes, Frequent to lymph nodes, lungs and so on |
| Reproducible distant metastasis | Rare | Rare | |
| Tissue microenvironment/immune system | In situ/wild type | Ectopic/immunocompromised | In situ/immunocompromised |
| Preclinical drug testing for tumor growth and metastasis inhibition | Limited by costs/design/time | Suited for local growth inhibition | Suited for in situ growth and metastasis inhibition |
Abbreviations: GEM, genetically modified models; PET, positron emission tomography; MRI, magnetic resonance imaging.
Both subcutaneous and orthotopic xenograft models (Table 1) reduce the lag of time needed to obtain tumors in vivo; these models could recapitulate histopathological features of aggressive and advanced thyroid carcinoma, are easy to establish, reproducible and are cost effective. However, only orthotopic models could recapitulate metastatic behavior with sufficient penetrance and reproducibility.44–46 Subcutaneous approaches can robustly model in vivo tumor growth and also local invasion toward skin mesenchyme, but poorly reproduce metastasis likely because of ectopic anatomical context.46 Also, orthotopic mouse models compared with subcutaneous xenografts models could have a better predictive value of disease, specifically when taking into account clinically relevant drug dosages.44,45,47–49
Xenograft models are based on cancer cell lines directly derived from human thyroid carcinomas, and therefore they carry the full panel of complex genetic alterations present in the tumor tissue of origin.50 For preclinical studies, this is a major advantage, as it is important to recapitulate the complex genetic scenario of human thyroid cancers rather than the simplified, although theoretically more tractable, genetic controlled situation of GEMs. In addition, most thyroid cancer cell lines closely resemble ATC and therefore are more likely suitable for studying pathologic aspects of advanced disease, such as intravascular invasion and metastasis. Orthotopic xenografts of ATC or PDTC, although more complex to establish than subcutaneous xenograft models, as they require a surgical intervention, have a significantly higher tumorigenicity and are unique in their ability to reproduce lymph node metastasis and lung micrometastasis.51 Cancer cells are injected directly into the thyroid of severe combined immunodeficienct mice mimicking better the anatomical and tissue context of human thyroid cancers (Figure 1). Using immunocompromised hosts, tissue microenvironment, which includes not only stromal cells but also immune cells (adult severe combined immunodeficient mice are genetically ‘leaky’ and generate few clones of functional B and T cells) is partially recapitulated; nonetheless, all the other elements of the microenvironment, such as stromal cells, lymphatic/hematic vessels and the innate immune system are present.52,53 Hence, orthotopic xenografts could be suited for testing compounds specifically targeting these components. On the other hand, GEMs have an ‘intact’ microenvironment and might be more suited to test therapies that would target (or might be affected by) the acquired immune system. Overall, although no animal model is perfectly recapitulating human thyroid cancers, orthotopic models offer advantages of reproducing in situ growth in a fast and reliable manner, local invasion and distant metastasis.51,54–56 Importantly, orthotopic mouse models allow direct in vivo testing of compounds for anti-ATC capacity without disregarding systemic effects;54,57,58 in addition, genetic manipulation can be made in vitro on the tumor cells and tested in vivo to provide genetic proofs for the mechanism of the compound’s action (that is, the effects of a compound can be demonstrated by reproducing its effects by genetic targeting of the putative targets). Orthotopic mouse models of human thyroid cancer (for example, PDTC and ATC) offer a good compromise of genetic complexity, human disease mimicking and experimental tractability, making them relevant and widely used models for preclinical and translational studies of novel compounds and therapies.59 In Table 1 we provide a resume of the different approaches (GEM, orthotopic xenograft and subcutaneous xenograft) to model human thyroid cancer in vivo and their properties.
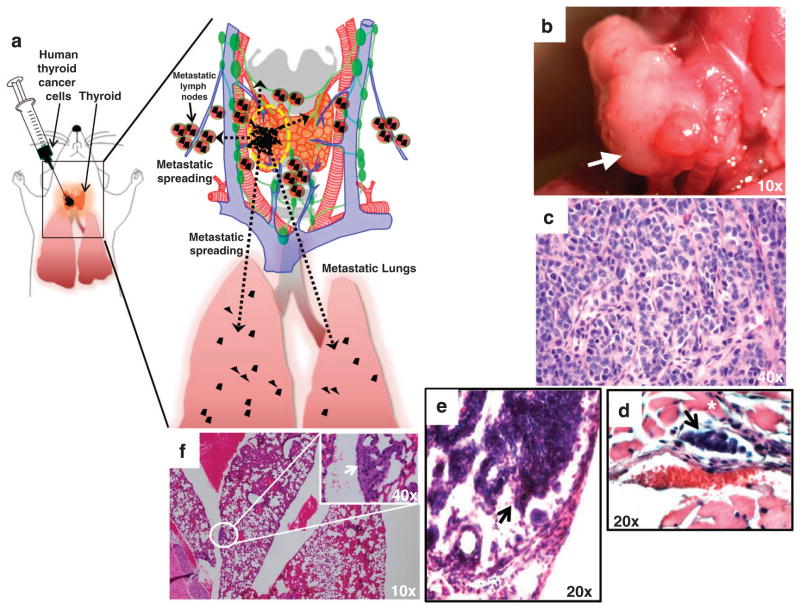
Figure 1.
Orthotopic mouse models of human thyroid carcinoma growth and metastasis. (a) Cartoon of an orthotopic mouse model of human anaplastic thyroid carcinoma (ATC) recapitulating local invasion and distant metastasis. Human thyroid carcinoma cell lines (black) are injected into the right thyroid. Inset shows in detail the mouse thyroid gland, blood vessels (arteries, red; veins, blue), lymph nodes (green) and lungs (pink). After few days, tumors invade locally and metastasize to the lymph nodes (micrometastasis, black) and lungs (micrometastasis, black). (b) Gross image of an orthotopic human ATC mass with tracheal impingement, 35 days post tumor implantation in the right thyroid of an SCID mouse. (c) Histological section (hematoxylin and eosin) of an orthotopic human ATC mass showing high-grade features and marked cellular pleomorphism. (d) Histological section (hematoxylin and eosin) of ATC cells intravascular invasion (arrow) from an orthotopic mouse model of BRAFV600E-human ATC (asterisk highlights skeleton muscle tissue). (e) Histological section (hematoxylin and eosin) of neck lymph node ATC cell micrometastasis (arrow) from an orthotopic mouse model of BRAFV600E-human ATC. (f) Histological section (hematoxylin and eosin) of lungs micrometastasis (arrow) from an orthotopic mouse model of BRAFV600E-human ATC.
In this article we will focus on the preclinical applications of orthotopic mouse models (Figure 1) of human thyroid cancers and will summarize studies that have been used to test single compounds (Table 2), also including some examples of a combinatorial approach that used surgery and a selective drug treatment as a neo-adjuvant. We are considering only studies that used validated cell lines of thyroid origin.50 Finally, we will discuss the possible use of thyroid cancer orthotopic models for combinatorial approaches, which are expected to take place in the thyroid cancer research.
Table 2.
Compounds and treatments tested in mouse orthotopic thyroid cancer models based on STR-validated cell lines
| Drug | Cell lines (type) | Molecular targets | Study (author, year) | Response |
|---|---|---|---|---|
| Calcitriol | WRO (FTC) | Unknown | Dackiw et al.65 | 38% reduction of tumor growth, block of metastatic growth and restoration of differentiated phenotype |
| Vandetanib (ZD6474) | Hth83 (ATC) and 8505c (ATC) | EGFR, VEGFR2 and RET | Gule et al.72 | 60–70% reduction of tumor growth, reduction of vascular permeability and angiogenensis and metastasis not evaluated |
| Dasatinib (BMS-354825) | BCPAP (poorly differentiated PTC) and 8505c (ATC) | Src, ABL, DDR2, and PDGF kinases | Chan et al.55 | 90% growth blockage for BCPAP, no effect for 8505C. Reduction of metastatic growth in preventive- and late-intervention protocols. Extended survival. Tumor reactivation. |
| Sorafenib (Nexavar, BAY 43-9006) | TPC1 (RET/PTC1) (PTC) | BRAF, c-RAF, RET, c-kit, PDGFR and VEGFR | Henderson et al.86 | Over 90% block of tumor growth and metastasis for TPC1 via cytostasis. |
| Thiostrepton | 8505c (ATC) | FoxM1 | Bellelli et al.59 | Inhibition of tumor growth and metastasis blockage. |
| PLX4720 | 8505c (ATC) | BRAFV600E | Nucera et al.54 Nucera et al.91 Nehs et al.58 |
Inhibition of tumor growth and metastasis with early- and late-intervention protocols. Extended survival. Reversal of cachexia. No evident toxicity. |
| PLX4720 and thyroidectomy | 8505c (ATC) | BRAFV600E | Nehs et al.58 | Reduction of tumor growth and reduction of metastasis by PLX4720 alone, no detectable tumor and synergic reduction of metastasis when in combination with thyroidectomy. Extended survival. Reversal of cachexia. No evident toxicity. Tumor reactivation when treatment is stopped. |
ORTHOTOPIC INJECTIONS OF HUMAN THYROID CARCINOMA CELLS INTO THE THYROID OF IMMUNOCOMPROMISED MICE
Female 6–10-week-old severe combined immunodeficient mice are anesthetized using ketamine and xylazine, or isoflurane. For each mouse, the neck is shaved and prepared with betadine. The skin and subcutaneous tissues are incised with scissors, and the salivary glands are reflected superiorly. The central component of the neck is exposed, and the overlying strap muscles are dissected away from the right thyroid lobe using forceps. Once the right thyroid is exposed, human thyroid cancer cells resuspended in 10 μl of serum-free growth medium are injected into the thyroid using a Hamilton syringe (Fisher Scientific, Pittsburgh, PA, USA) attached to a 27-gauge needle (Figure 1). After the injection, the salivary glands will be repositioned, and the incision will be closed using nylon sutures. Antibiotic ointment will be applied to the wound, and the mice will be placed under a warming lamp while they recover from anesthesia. There will not be surgical or anesthetic complications.51,57,58
Preclinical and translational therapeutic models
Calcitriol is the active form of vitamin D (1,25-dihydroxyvitamin D3—vitD3). Calcitriol modulates in vitro and in vivo cell proliferation and differentiation of different cancer cell lines60–62 including different thyroid carcinoma cell lines, leading to the accumulation of the tumor suppressor p27 in vitro.64 In an orthotopic mouse model of FTC, (WRO cell line) animals were treated intraperitoneally three times a week for 21 days with 0.75 μg/kg of calcitriol.65 Calcitriol administration restored nuclear p27, enhanced cellular differentiation with restoration of thyroglobulin staining, reduced tumor volume by 38% and prevented metastatic growth. Tumors from control animals presented morphological features of epithelial malignancies with characteristics of insular carcinoma and multiple metastases to the lungs. These results indicate that calcitriol could reduce tumor growth and prevent metastatic growth, but the mechanism of action remains unknown. The evaluation of a panel of validated thyroid cancer cell lines for VitD receptor expression and response to calcitriol or the noncalcemic analog DP006 have shown that not all cell lines respond to VitD, and the response did not correlate with receptor expression levels suggesting resistance via other mechanisms.66 In summary, VitD receptor targeting in PDTC represents a treatment strategy, but mechanisms of action are still unclear and markers to predict sensitivity are lacking.
Vandetanib (ZD6474) is a non-selective small-molecule tyrosine kinase inhibitor able to target EGFR, VEGFR2 and RET. Several studies have correlated RET with PTC development67 and EGFR and VEGF (the activating ligand of VEGFR) with tumor progression and disease aggressiveness.68–71 The ability of vandetanib to inhibit EGFR and VEGFR activation was investigated in seven STR-validated ATC cell lines in vitro. Also, its ability to inhibit tumor growth (of two of the seven lines, Hth83 and 8505c) was studied in vivo in an orthotopic mouse model.72 In vivo tumor growth was precisely evaluated by luminescence using cell lines transfected with luciferase. Tumor volume of Hth83 or 8505c xenografts was inhibited by 69.3% and 66.6% after 3 and 4 weeks of daily treatment with vandetanib, respectively. In addition, in vivo vascular permeability and vascular volume fraction, evaluated by dynamic contrast enhanced magnetic resonance imaging (DCE-MRI), significantly diminished after 1 week of treatment in both cell line xenografts, indicating that vandetanib was able to disrupt tumor angiogenesis.72 In a clinical trial for metastatic medullary thyroid cancer, vandetanib improved progression-free survival from 19.3–30.5 months compared with a placebo. However, adverse effects were frequent, patients were not made disease free and exact mechanisms of drug action on tumor and normal tissue remain to be determined.73
Dasatinib (BMS-354825) has demonstrated non-selective inhibitory effects on Src, BCR-ABL, DDR2 and PDGF and others.74,75 Src family of protein tyrosine kinases (SFKs) regulate processes of growth, survival, migration and invasion.76 SFKs are overexpressed or activated in several tumor types77 and can activate different downstream effectors, including MAPK, PI3K and focal adhesion kinase.78 SFKs are activated in PTC and ATC cell lines, and phosphorylated focal adhesion kinase is detected in the invasive edge of human PTCs.79 Altogether this data made SFKs of interest for invasive thyroid cancer treatment. In a recent study,55 the effects of dasatinib were evaluated in vitro in a number of authenticated cell lines representing PTC and ATC (that is, C643, TPC1, SW1736, BCPAP, K1, 8505c, HTh74 and HTh7). Therapeutic potential was also evaluated in vivo using an orthotopic and a bone metastasis model. In vitro, dasatinib treatment inhibited SFK signaling, decreased growth and induced cell-cycle arrest and apoptosis in a subset of thyroid cell lines, although 8505C, HTh7 and HTh74 were found to be resistant. A sensitive (BCPAP) and an insensitive cell line (8505c) stably expressing a luciferase-IRES-GFP reporter were chosen to check dasatinib effects in vivo in an orthotopic model. Daily treatment (50 mg/kg) initiated on day 10 after cell implantation resulted in tumor growth inhibition by more than 90% for BCPAP cells (PTC-derived cell line). Conversely, xenografts of 8505c (ATC-derived cell line) were found resistant to treatment in vivo, similarly to in vitro data. Dasatinib was further tested for its ability to inhibit metastasis formation in a preventive manner or block metastasis growth of already established metastasis. Animals pretreated with 50 mg/kg by daily oral gavage 48 h before intracardiac injection of BCPAP cells showed total inhibition of bone metastasis formation respective to controls. To test its efficacy on established metastasis, mice were similarly treated, but on day 11 after intracardiac cells inoculation. Treated animals showed significant slowdown of metastatic growth. This data provided the first evidence that Src has a role in thyroid cancer growth and metastasis in PTC, indicating that Src inhibitors can be used as both antitumor and antimetastatic agents.55 Importantly, in the pre-treatment paradigm, although overall survival was improved (63 versus 123 days of mean), after treatment suspension in dasatinib versus vehicle, animals were not made disease free, designating dasatinib as an adjuvant. Accordingly, in another work,80 exclusive inhibition of the SFKs signaling was effective in reducing PTC tumor volume in an orthotopic model (TPC1 and K2 thyroid cancer cell lines). In addition to the MEK/ERK signaling transduction pathway, Src may have an important role in regulating growth in PTC cells. Inhibition of the Src signaling transduction pathway alone was effective in reducing PTC tumor volume but did not improve survival. Combined therapy targeting other intracellular signaling transduction pathways may be crucial to improve overall survival.
Sorafenib (Nexavar, BAY 43-9006) is a multikinase inhibitor that has been shown to inhibit CRAF, VEGFRs, BRAF and others.81–84 Studies using subcutaneous models have shown that sorafenib can inhibit in vivo tumor growth of cancer cell lines of different origin, including thyroid cell lines.83–85 The antitumor effects of sorafenib were evaluated in vitro and in vivo using an orthotopic mouse model of PTC carrying the RET/PTC1 rearrangement (TPC1).86 For in vivo experiments, animals were treated, on orthotopic tumor establishment (2 or 3 weeks after cell inoculation), with 40 mg/kg twice daily by oral gavage for 5 consecutive days/week for 3 weeks. In mice inoculated with cells carrying the RET/PTC1 rearrangement, sorafenib treatment lead to a 94% tumor volume reduction or no tumor growth compared with controls. In summary, PTC cells carrying the RET/PTC1 rearrangement were arrested in vitro and in vivo in G1 by sorafenib, indicating that tumor volume reduction was via cytostasis. These results support the clinical use of this drug for patients with RET/PTC1-positive PTC. However, they indicate that there is no cancer cell clearance and after treatment suspension disease might progress.
Thiostrepton is a thiazole antibiotic that has been identified as an anticancer agent in a cell-based screening to identify molecules targeting the oncogenic transcription factor forkhead box M1 (FoxM1).87 FoxM1 is upregulated in several different carcinomas,88 including ATC,59 and regulates genes that critically control cell proliferation.89 Bellelli et al.59 showed that thiostrepton could reduce, in a dose-dependent manner, FOXM1 transcriptional activity as well as its protein levels in vitro. Cell viability was reduced and cells were arrested in G2/M. Also cell invasion and motility were reduced. In an orthotopic mouse model of ATC (8505c cell line), thiostrepton treatment (twice weekly with 500 mg thiostrepton/kg per day for 21 days) resulted in an 80% tumor burden reduction and lung metastasis inhibition. This study showed in an early-intervention model (7 days after tumor implantation) that FOXM1 could represent a potential therapeutic target for ATC.
PLX4720 is a potent and highly selective inhibitor of BRAFV600E kinase able to act selectively in BRAFV600E-positive human cancer cells.90 In a xenograft model of BRAFV600E melanoma, PLX4720 treatment delayed or induced regression of tumor growth, without evident toxicities.90 Similar results were obtained in an orthotopic model of BRAFV600E ATC (8505c cell line).54 Animals were treated by oral gavage (30 mg/kg per day for 21 days) 1 week after cell implantation, resulting in a 90% tumor volume reduction and a more than 80% decrease in the number of lung metastases compared with control mice, with no evident toxicity. Another study, comparing the PLX4720 effects on the BRAFV600E-positive 8505c ATC cell line and the RET/PTC1-positive and BRAF wild-type TPC1 PTC cell line, confirmed that PLX4720 treatment in a BRAFV600E 8505c ATC tumor model can greatly reduce tumor volume, reduce tumor aggressiveness and virtually inhibit metastatic spread to the lungs without any obvious toxicity.91 A subsequent work, taking advantage of the same orthotopic model, tested PLX4720 in a late-intervention model.58 Animals were treated with PLX4720 or vehicle by oral gavage 28 days after 8505c ATC cell implantation, when tumors are already growing around the trachea with invasion into the esophagus and the non-injected thyroid lobe. Non treated animals died due to widespread metastatic lung disease and neck compressive symptoms by day 35 and were severely cachectic. PLX4720-treated mice showed a significant decrease in tumor volume and lung metastases, cachexia was reverted and survival was extended to 49 days.
PLX4032 (RG7204, vemurafenib) is a small-molecule inhibitor of BRAFV600E kinase activity that displays high selectivity, similarly to PLX4720, and is able to reduce tumor growth and improve survival in preclinical models of melanoma, without major toxicities.92–96 Despite auspicious results for the phase I–II clinical trials, melanoma patients were not rendered disease free and the duration of the response ranged from 2–18 months, with 4/16 patients still having partial or complete response at the data cutoff date.96 Indeed, melanomas can develop resistance to PLX4032. Potential mechanisms could include gatekeeper mutations in BRAF, overexpression of wild-type RAF isoforms98 or activating mutations in parallel signaling pathways or downstream effectors, such as RAS or MEK.99–101 PLX4032 has also been confirmed as an effective BRAFV600E mutation-selective therapeutic agent in PDTC cell lines102 but no studies using thyroid cancer orthotopic models have been performed so far. Nevertheless, owing to its high selectivity (similar to PLX4720) and its promising results for melanoma, it has been tested in a three-patient phase I clinical trial for thyroid cancer.103 In this study, two patients had stable disease and relatively long progression time, and the other had partial response for 7.6 months with 31% reduction of pulmonary metastasis before the disease progressed. PLX4032 has been also used in a patient with ATC, with extensive lungs metastatization. On 28 days of treatment, the patient, which presented the BRAFV600E mutation, had nearly complete clearing of metastatic disease.104
PLX4720 and thyroidectomy
Using the same orthotopic ATC model based on 8505c, PLX4720 was tested as an adjuvant to thyroidectomy.57 PLX4720 or vehicle administration started 7 days after tumor implantation and thyroidectomy or sham surgery was performed at day 14. Mice were kept under treatment until day 21. All animals treated with PLX4720 were alive and well-appearing at day 50, whereas controls had large tumors and cachexia. Mice receiving only PLX4720 presented small tumors and rescue of cachexia, whereas three out of six animals receiving PLX4720 and thyroidectomy didn’t present tumor at day 35; two mice were still disease free at day 50. In addition, PLX4720 reduced 2.7-fold the number of lung metastasis at 50 days and 18-fold when in combination with thyroidectomy. Importantly, despite inhibition of tumor progression in the PLX4720-treated groups, there was reactivation of tumor growth after the drug was stopped at day 21, indicating that PLX4720 must be continued for lasting effects.
These results highlight the importance of the use of combinatorial therapies, which can be tested in orthotopic models of human thyroid cancers.
CONCLUSION
Current therapies for metastatic, recurrent or inoperable/unresectable thyroid cancers are not curative and compounds tested in clinical trials are still showing partial responses.105,106 Preclinical data based on orthotopic animal models indicate that single compounds are mainly able to retard disease progression through reduction of invasive properties, reduction of angiogenesis, cytostatic effects or even inducing tumor cell death54,55,57,59,72,86,91 (Table 2). However, drugs are not able to reach total clearance of cancer cells, leading inexorably to resistance and recurrence.
Recent research has focused on the development of selective compounds (that is, PLX4032 or PLX4720) that have effectively decreased side effects and toxicities, which were a major problem of non-selective multi-target drugs, however, strategies to overcome resistance and recurrence are lacking. Single-compound approaches might fail in treating aggressive thyroid tumors because, similarly to other types of tumors, they can depend on more than one altered pathway (and/or rapidly develop resistance via feedback mechanisms.107–109 Inhibition of MAPK (for example MEK1/2-ERK1-2) signaling by PLX4032 has been shown to be transient in thyroid cancer cell lines that can quickly develop resistance via a complex feedback mechanism involving an autocrine activation of the ErbB/HER pathway. In vitro, the combined use of HER kinase inhibitor lapatinib and MAP-ERK kinase inhibitors prevented MAPK rebound and sensitizes BRAF-mutant thyroid cancer cells.108 In vitro, although sensitivity to MAPK inhibitors were not predicted by coexisting mutations in PIK3CA or by PTEN status,110 similar synergistic effects were observed in others studies targeting MAPK and AKT pathways in thyroid cancer cell lines harboring both the activating BRAFV600E and PIK3CA mutations.111,112 Thus, most likely similar to other cancers, combinatorial therapies are expected to be more successful in treating aggressive thyroid cancers and preliminary studies in vitro on thyroid cancer cell lines support this perspective. In addition, many inhibitors have significant toxicity at their effective dosage (that is, AKT inhibitors)113 but lower doses of individual drugs in combination are expected to be less harmful, giving similar or better therapeutic efficacy than single compounds.
The use of orthotopic models of human thyroid cancer to test novel compounds and combinatorial therapies will increase proportionally to the discovery of novel targets, the identification of co-occurring genetic alterations and the understanding of drug-resistance mechanisms. Development of high-throughput oncogene mutation profiling in human cancer is providing a more comprehensive view of the oncogenic abnormalities, leading to a more precise patient stratification.32 In this perspective, it is likely that the number of possible treatment regimens will increase, as treatment will be adjusted according to the type of mutations and pathways deregulated in the tumor (personalized medicine).
Human thyroid cancer orthotopic mouse models have demonstrated to be effective in modeling human disease in an affordable and tractable manner and be valuable tools for single-compound testing in different setups, that is, in early- or late-intervention models55,58,91 (Table 1), but also in combination with surgical intervention.57 Combinatorial use of compounds will be a central matter of future preclinical/translational and clinical trials on thyroid cancers, and it becomes evident that biomedical research will increase the demand of rapid and cost-effective preclinical/coclinical model systems such as orthotopic thyroid cancer models. Collectively, orthotopic mouse models seem to be ideal for preclinical and translational studies of compounds and therapies not only because they may mimic key aspects of complex genetic alterations of human cancer, but also for their reproducibility. In addition, they might provide the possibility to evaluate systemic effects of treatments.
Acknowledgments
This work was supported by the National Institutes of Health Grants NIHR21CA165039-01A1 and the American Thyroid Association funds for Thyroid Cancer Research to Carmelo Nucera (Principal Investigator: Human Thyroid Cancers Preclinical and Translational Research). Carmelo Nucera was also a recipient of the Guido Berlucchi research award (Brescia, Italy). Zeus A Antonello was a Masters Student at the BIDMC and is currently a PhD Student at the Instituto de Neurociencias (Alicante, Spain). We thank Mark Duquette and Neal Smith (BIDMC, Harvard Medical School) for critical reading of our manuscript. We thank those authors who we have neglected to cite owing to limitation on the number of references.
Footnotes
CONFLICT OF INTEREST
The authors declare no conflict of interest.
References
- 1.Davies L, Welch HG. Increasing incidence of thyroid cancer in the United States, 1973–2002. JAMA. 2006;295:2164–2167. doi: 10.1001/jama.295.18.2164. [DOI] [PubMed] [Google Scholar]
- 2.Ito Y, Nikiforov YE, Schlumberger M, Vigneri R. Increasing incidence of thyroid cancer: controversies explored. Nat Rev Endocrinol. 2013;9:178–184. doi: 10.1038/nrendo.2012.257. [DOI] [PubMed] [Google Scholar]
- 3.Maxon HR. Detection of residual and recurrent thyroid cancer by radionuclide imaging. Thyroid. 1999;9:443–446. doi: 10.1089/thy.1999.9.443. [DOI] [PubMed] [Google Scholar]
- 4.American Thyroid Association Guidelines Taskforce on Thyroid N, Differentiated Thyroid C. Cooper DS, Doherty GM, Haugen BR, Kloos RT, et al. Revised American Thyroid Association management guidelines for patients with thyroid nodules and differentiated thyroid cancer. Thyroid. 2009;19:1167–1214. doi: 10.1089/thy.2009.0110. [DOI] [PubMed] [Google Scholar]
- 5.Brown RL, de Souza JA, Cohen EE. Thyroid cancer: burden of illness and management of disease. J Cancer. 2011;2:193–199. doi: 10.7150/jca.2.193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Mazzaferri EL, Jhiang SM. Long-term impact of initial surgical and medical therapy on papillary and follicular thyroid cancer. Am J Med. 1994;97:418–428. doi: 10.1016/0002-9343(94)90321-2. [DOI] [PubMed] [Google Scholar]
- 7.Durante C, Haddy N, Baudin E, Leboulleux S, Hartl D, Travagli JP, et al. Long-term outcome of 444 patients with distant metastases from papillary and follicular thyroid carcinoma: benefits and limits of radioiodine therapy. J Clin Endocrinol Metab. 2006;91:2892–2899. doi: 10.1210/jc.2005-2838. [DOI] [PubMed] [Google Scholar]
- 8.Brassard M, Borget I, Edet-Sanson A, Giraudet AL, Mundler O, Toubeau M, et al. Long-term follow-up of patients with papillary and follicular thyroid cancer: a prospective study on 715 patients. J Clin Endocrinol Metab. 2011;96:1352–1359. doi: 10.1210/jc.2010-2708. [DOI] [PubMed] [Google Scholar]
- 9.Tisset H, Kamar N, Faugeron I, Roy P, Pouteil-Noble C, Klein M, et al. Is thyroid cancer recurrence risk increased after transplantation? J Clin Endocrinol Metab. 2013;98:3981–3988. doi: 10.1210/jc.2013-1357. [DOI] [PubMed] [Google Scholar]
- 10.McDonald MP, Sanders LE, Silverman ML, Chan HS, Buyske J. Hurthle cell carcinoma of the thyroid gland: prognostic factors and results of surgical treatment. Surgery. 1996;120:1000–1004. doi: 10.1016/s0039-6060(96)80046-8. discussion 4–5. [DOI] [PubMed] [Google Scholar]
- 11.Sanders LE, Silverman M. Follicular and Hurthle cell carcinoma: predicting outcome and directing therapy. Surgery. 1998;124:967–974. [PubMed] [Google Scholar]
- 12.Shaha AR, Loree TR, Shah JP. Prognostic factors and risk group analysis in follicular carcinoma of the thyroid. Surgery. 1995;118:1131–1136. doi: 10.1016/s0039-6060(05)80124-2. discussion 6–8. [DOI] [PubMed] [Google Scholar]
- 13.Yutan E, Clark OH. Hurthle cell carcinoma. Curr Treat Options Oncol. 2001;2:331–335. doi: 10.1007/s11864-001-0026-4. [DOI] [PubMed] [Google Scholar]
- 14.Girelli ME, Nacamulli D, Pelizzo MR, De Vido D, Mian C, Piccolo M, et al. Medullary thyroid carcinoma: clinical features and long-term follow-up of seventy-eight patients treated between 1969 and 1986. Thyroid. 1998;8:517–523. doi: 10.1089/thy.1998.8.517. [DOI] [PubMed] [Google Scholar]
- 15.Modigliani E, Cohen R, Campos JM, Conte-Devolx B, Maes B, Boneu A, et al. Prognostic factors for survival and for biochemical cure in medullary thyroid carcinoma: results in 899 patients. The GETC Study Group. Groupe d’etude des tumeurs a calcitonine. Clin Endocrinol. 1998;48:265–273. doi: 10.1046/j.1365-2265.1998.00392.x. [DOI] [PubMed] [Google Scholar]
- 16.Xing M. Molecular pathogenesis and mechanisms of thyroid cancer. Nat Rev Cancer. 2013;13:184–199. doi: 10.1038/nrc3431. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Knauf JA, Fagin JA. Role of MAPK pathway oncoproteins in thyroid cancer pathogenesis and as drug targets. Curr Opin Cell Biol. 2009;21:296–303. doi: 10.1016/j.ceb.2009.01.013. [DOI] [PubMed] [Google Scholar]
- 18.Nucera C, Goldfarb M, Hodin R, Parangi S. Role of B-Raf(V600E) in differentiated thyroid cancer and preclinical validation of compounds against B-Raf(V600E) Biochim Biophys Acta. 2009;1795:152–161. doi: 10.1016/j.bbcan.2009.01.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Franco AT, Malaguarnera R, Refetoff S, Liao XH, Lundsmith E, Kimura S, et al. Thyrotrophin receptor signaling dependence of Braf-induced thyroid tumor initiation in mice. Proc Natl Acad Sci USA. 2011;108:1615–1620. doi: 10.1073/pnas.1015557108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Orim F, Bychkov A, Shimamura M, Nakashima M, Ito M, Matsuse M, et al. Thyrotropin signaling confers more aggressive features with higher genomic instability on BRAFV600E-induced thyroid tumors in a mouse model. Thyroid. 2013;7:7. doi: 10.1089/thy.2013.0038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Shimamura M, Nakahara M, Orim F, Kurashige T, Mitsutake N, Nakashima M, et al. Postnatal Expression of BRAFV600E Does Not Induce Thyroid Cancer in Mouse Models of Thyroid Papillary Carcinoma. Endocrinology. 2013;154:4423–4430. doi: 10.1210/en.2013-1174. [DOI] [PubMed] [Google Scholar]
- 22.Liu Z, Hou P, Ji M, Guan H, Studeman K, Jensen K, et al. Highly prevalent genetic alterations in receptor tyrosine kinases and phosphatidylinositol 3-kinase/akt and mitogen-activated protein kinase pathways in anaplastic and follicular thyroid cancers. J Clin Endocrinol Metab. 2008;93:3106–3116. doi: 10.1210/jc.2008-0273. [DOI] [PubMed] [Google Scholar]
- 23.Saji M, Ringel MD. The PI3K-Akt-mTOR pathway in initiation and progression of thyroid tumors. Mol Cell Endocrinol. 2010;321:20–28. doi: 10.1016/j.mce.2009.10.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Carlomagno F, Santoro M. Thyroid cancer in 2010: a roadmap for targeted therapies. Nat Rev Endocrinol. 2011;7:65–67. doi: 10.1038/nrendo.2010.232. [DOI] [PubMed] [Google Scholar]
- 25.Xing M. Genetic alterations in the phosphatidylinositol-3 kinase/Akt pathway in thyroid cancer. Thyroid. 2010;20:697–706. doi: 10.1089/thy.2010.1646. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Samuels Y, Wang Z, Bardelli A, Silliman N, Ptak J, Szabo S, et al. High frequency of mutations of the PIK3CA gene in human cancers. Science. 2004;304:554. doi: 10.1126/science.1096502. [DOI] [PubMed] [Google Scholar]
- 27.Ricarte-Filho JC, Ryder M, Chitale DA, Rivera M, Heguy A, Ladanyi M, et al. Mutational profile of advanced primary and metastatic radioactive iodine-refractory thyroid cancers reveals distinct pathogenetic roles for BRAF, PIK3CA, and AKT1. Cancer Res. 2009;69:4885–4893. doi: 10.1158/0008-5472.CAN-09-0727. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Garcia-Rostan G, Costa AM, Pereira-Castro I, Salvatore G, Hernandez R, Hermsem MJ, et al. Mutation of the PIK3CA gene in anaplastic thyroid cancer. Cancer Res. 2005;65:10199–10207. doi: 10.1158/0008-5472.CAN-04-4259. [DOI] [PubMed] [Google Scholar]
- 29.Deshpande HA, Roman S, Sosa JA. New targeted therapies and other advances in the management of anaplastic thyroid cancer. Curr Opin Oncol. 2013;25:44–49. doi: 10.1097/CCO.0b013e32835a448c. [DOI] [PubMed] [Google Scholar]
- 30.Lee J, Hwang JA, Lee EK. Recent progress of genome study for anaplastic thyroid cancer. Genomics Inform. 2013;11:68–75. doi: 10.5808/GI.2013.11.2.68. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Smallridge RC, Ain KB, Asa SL, Bible KC, Brierley JD, Burman KD, et al. American Thyroid Association guidelines for management of patients with anaplastic thyroid cancer. Thyroid. 2012;22:1104–1139. doi: 10.1089/thy.2012.0302. [DOI] [PubMed] [Google Scholar]
- 32.Thomas RK, Baker AC, Debiasi RM, Winckler W, Laframboise T, Lin WM, et al. High-throughput oncogene mutation profiling in human cancer. Nat Genet. 2007;39:347–351. doi: 10.1038/ng1975. [DOI] [PubMed] [Google Scholar]
- 33.Miller KA, Yeager N, Baker K, Liao XH, Refetoff S, Di Cristofano A. Oncogenic Kras requires simultaneous PI3K signaling to induce ERK activation and transform thyroid epithelial cells in vivo. Cancer Res. 2009;69:3689–3694. doi: 10.1158/0008-5472.CAN-09-0024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Chakravarty D, Santos E, Ryder M, Knauf JA, Liao XH, West BL, et al. Small-molecule MAPK inhibitors restore radioiodine incorporation in mouse thyroid cancers with conditional BRAF activation. J Clin Invest. 2011;121:4700–4711. doi: 10.1172/JCI46382. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Charles RP, Iezza G, Amendola E, Dankort D, McMahon M. Mutationally activated BRAF(V600E) elicits papillary thyroid cancer in the adult mouse. Cancer Res. 2011;71:3863–3871. doi: 10.1158/0008-5472.CAN-10-4463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Lu C, Zhu X, Willingham MC, Cheng SY. Activation of tumor cell proliferation by thyroid hormone in a mouse model of follicular thyroid carcinoma. Oncogene. 2012;31:2007–2016. doi: 10.1038/onc.2011.390. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Saji M, Narahara K, McCarty SK, Vasko VV, La Perle KM, Porter K, et al. Akt1 deficiency delays tumor progression, vascular invasion, and distant metastasis in a murine model of thyroid cancer. Oncogene. 2011;30:4307–4315. doi: 10.1038/onc.2011.136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Abate-Shen C, Pandolfi PP. Effective utilization and appropriate selection of genetically engineered mouse models for translational integration of mouse and human trials. Cold Spring Harb Protoc. doi: 10.1101/pdb.top078774. (e-pub ahead of print 1 Novenber 2013) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Knauf JA, Ma X, Smith EP, Zhang L, Mitsutake N, Liao XH, et al. Targeted expression of BRAFV600E in thyroid cells of transgenic mice results in papillary thyroid cancers that undergo dedifferentiation. Cancer Res. 2005;65:4238–4245. doi: 10.1158/0008-5472.CAN-05-0047. [DOI] [PubMed] [Google Scholar]
- 40.Bos PD, Nguyen DX, Massague J. Modeling metastasis in the mouse. Curr Opin Pharmacol. 2010;10:571–577. doi: 10.1016/j.coph.2010.06.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Khanna C, Hunter K. Modeling metastasis in vivo. Carcinogenesis. 2005;26:513–523. doi: 10.1093/carcin/bgh261. [DOI] [PubMed] [Google Scholar]
- 42.McClatchey AI. Modeling metastasis in the mouse. Oncogene. 1999;18:5334–5339. doi: 10.1038/sj.onc.1203086. [DOI] [PubMed] [Google Scholar]
- 43.Van Dyke T, Jacks T. Cancer modeling in the modern era: progress and challenges. Cell. 2002;108:135–144. doi: 10.1016/s0092-8674(02)00621-9. [DOI] [PubMed] [Google Scholar]
- 44.Kerbel RS. Human tumor xenografts as predictive preclinical models for anticancer drug activity in humans: better than commonly perceived-but they can be improved. Cancer Biol Ther. 2003;2(Suppl 1):S134–S139. [PubMed] [Google Scholar]
- 45.Killion JJ, Radinsky R, Fidler IJ. Orthotopic models are necessary to predict therapy of transplantable tumors in mice. Cancer Metastasis Rev. 1998;17:279–284. doi: 10.1023/a:1006140513233. [DOI] [PubMed] [Google Scholar]
- 46.Sharkey FE, Fogh J. Metastasis of human tumors in athymic nude mice. Int J Cancer. 1979;24:733–738. doi: 10.1002/ijc.2910240605. [DOI] [PubMed] [Google Scholar]
- 47.Johnson JI, Decker S, Zaharevitz D, Rubinstein LV, Venditti JM, Schepartz S, et al. Relationships between drug activity in NCI preclinical in vitro and in vivo models and early clinical trials. Br J Cancer. 2001;84:1424–1431. doi: 10.1054/bjoc.2001.1796. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Scholz CC, Berger DP, Winterhalter BR, Henss H, Fiebig HH. Correlation of drug response in patients and in the clonogenic assay with solid human tumour xenografts. Eur J Cancer. 1990;26:901–905. doi: 10.1016/0277-5379(90)90196-z. [DOI] [PubMed] [Google Scholar]
- 49.Priolo C, Agostini M, Vena N, Ligon AH, Fiorentino M, Shin E, et al. Establishment and genomic characterization of mouse xenografts of human primary prostate tumors. Am J Pathol. 2010;176:1901–1913. doi: 10.2353/ajpath.2010.090873. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Schweppe RE, Klopper JP, Korch C, Pugazhenthi U, Benezra M, Knauf JA, et al. Deoxyribonucleic acid profiling analysis of 40 human thyroid cancer cell lines reveals cross-contamination resulting in cell line redundancy and misidentification. J Clin Endocrinol Metab. 2008;93:4331–4341. doi: 10.1210/jc.2008-1102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Nucera C, Nehs MA, Mekel M, Zhang X, Hodin R, Lawler J, et al. A novel orthotopic mouse model of human anaplastic thyroid carcinoma. Thyroid. 2009;19:1077–1084. doi: 10.1089/thy.2009.0055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Bosma MJ, Carroll AM. The SCID mouse mutant: definition, characterization, and potential uses. Annu Rev Immunol. 1991;9:323–350. doi: 10.1146/annurev.iy.09.040191.001543. [DOI] [PubMed] [Google Scholar]
- 53.Bancroft GJ, Kelly JP. Macrophage activation and innate resistance to infection in SCID mice. Immunobiology. 1994;191:424–431. doi: 10.1016/S0171-2985(11)80448-1. [DOI] [PubMed] [Google Scholar]
- 54.Nucera C, Porrello A, Antonello ZA, Mekel M, Nehs MA, Giordano TJ, et al. B-Raf(V600E) and thrombospondin-1 promote thyroid cancer progression. Proc Natl Acad Sci USA. 2010;107:10649–10654. doi: 10.1073/pnas.1004934107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Chan CM, Jing X, Pike LA, Zhou Q, Lim DJ, Sams SB, et al. Targeted inhibition of Src kinase with dasatinib blocks thyroid cancer growth and metastasis. Clin Cancer Res. 2012;18:3580–3591. doi: 10.1158/1078-0432.CCR-11-3359. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Liu W, Cheng S, Asa SL, Ezzat S. The melanoma-associated antigen A3 mediates fibronectin-controlled cancer progression and metastasis. Cancer Res. 2008;68:8104–8112. doi: 10.1158/0008-5472.CAN-08-2132. [DOI] [PubMed] [Google Scholar]
- 57.Nehs MA, Nagarkatti S, Nucera C, Hodin RA, Parangi S. Thyroidectomy with neoadjuvant PLX4720 extends survival and decreases tumor burden in an orthotopic mouse model of anaplastic thyroid cancer. Surgery. 2010;148:1154–1162. doi: 10.1016/j.surg.2010.09.001. discussion 62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Nehs MA, Nucera C, Nagarkatti SS, Sadow PM, Morales-Garcia D, Hodin RA, et al. Late intervention with anti-BRAF(V600E) therapy induces tumor regression in an orthotopic mouse model of human anaplastic thyroid cancer. Endocrinology. 2012;153:985–994. doi: 10.1210/en.2011-1519. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Bellelli R, Castellone MD, Garcia-Rostan G, Ugolini C, Nucera C, Sadow PM, et al. FOXM1 is a molecular determinant of the mitogenic and invasive phenotype of anaplastic thyroid carcinoma. Endocr Relat Cancer. 2012;19:695–710. doi: 10.1530/ERC-12-0031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Colston KW, James SY, Ofori-Kuragu EA, Binderup L, Grant AG. Vitamin D receptors and anti-proliferative effects of vitamin D derivatives in human pancreatic carcinoma cells in vivo and in vitro. Br J Cancer. 1997;76:1017–1020. doi: 10.1038/bjc.1997.501. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Hershberger PA, Modzelewski RA, Shurin ZR, Rueger RM, Trump DL, Johnson CS. 1,25-Dihydroxycholecalciferol (1,25-D3) inhibits the growth of squamous cell carcinoma and down-modulates p21(Waf1/Cip1) in vitro and in vivo. Cancer Res. 1999;59:2644–2649. [PubMed] [Google Scholar]
- 62.Mathiasen IS, Sergeev IN, Bastholm L, Elling F, Norman AW, Jaattela M. Calcium and calpain as key mediators of apoptosis-like death induced by vitamin D compounds in breast cancer cells. J Biol Chem. 2002;277:30738–30745. doi: 10.1074/jbc.M201558200. [DOI] [PubMed] [Google Scholar]
- 63.Palmer HG, Sanchez-Carbayo M, Ordonez-Moran P, Larriba MJ, Cordon-Cardo C, Munoz A. Genetic signatures of differentiation induced by 1alpha,25-dihydroxyvitamin D3 in human colon cancer cells. Cancer Res. 2003;63:7799–7806. [PubMed] [Google Scholar]
- 64.Liu W, Asa SL, Fantus IG, Walfish PG, Ezzat S. Vitamin D arrests thyroid carcinoma cell growth and induces p27 dephosphorylation and accumulation through PTEN/akt-dependent and -independent pathways. Am J Pathol. 2002;160:511–519. doi: 10.1016/S0002-9440(10)64870-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Dackiw AP, Ezzat S, Huang P, Liu W, Asa SL. Vitamin D3 administration induces nuclear p27 accumulation, restores differentiation, and reduces tumor burden in a mouse model of metastatic follicular thyroid cancer. Endocrinology. 2004;145:5840–5846. doi: 10.1210/en.2004-0785. [DOI] [PubMed] [Google Scholar]
- 66.Sharma V, Fretwell D, Crees Z, Kerege A, Klopper JP. Thyroid cancer resistance to vitamin D receptor activation is associated with 24-hydroxylase levels but not the ff FokI polymorphism. Thyroid. 2010;20:1103–1111. doi: 10.1089/thy.2010.0096. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Ciampi R, Nikiforov YE. RET/PTC rearrangements and BRAF mutations in thyroid tumorigenesis. Endocrinology. 2007;148:936–941. doi: 10.1210/en.2006-0921. [DOI] [PubMed] [Google Scholar]
- 68.Ensinger C, Spizzo G, Moser P, Tschoerner I, Prommegger R, Gabriel M, et al. Epidermal growth factor receptor as a novel therapeutic target in anaplastic thyroid carcinomas. Ann N Y Acad Sci. 2004;1030:69–77. doi: 10.1196/annals.1329.009. [DOI] [PubMed] [Google Scholar]
- 69.Viglietto G, Maglione D, Rambaldi M, Cerutti J, Romano A, Trapasso F, et al. Upregulation of vascular endothelial growth factor (VEGF) and downregulation of placenta growth factor (PlGF) associated with malignancy in human thyroid tumors and cell lines. Oncogene. 1995;11:1569–1579. [PubMed] [Google Scholar]
- 70.Dhar DK, Kubota H, Kotoh T, Tabara H, Watanabe R, Tachibana M, et al. Tumor vascularity predicts recurrence in differentiated thyroid carcinoma. Am J Surgery. 1998;176:442–447. doi: 10.1016/s0002-9610(98)00238-4. [DOI] [PubMed] [Google Scholar]
- 71.Fenton C, Patel A, Dinauer C, Robie DK, Tuttle RM, Francis GL. The expression of vascular endothelial growth factor and the type 1 vascular endothelial growth factor receptor correlate with the size of papillary thyroid carcinoma in children and young adults. Thyroid. 2000;10:349–357. doi: 10.1089/thy.2000.10.349. [DOI] [PubMed] [Google Scholar]
- 72.Gule MK, Chen Y, Sano D, Frederick MJ, Zhou G, Zhao M, et al. Targeted therapy of VEGFR2 and EGFR significantly inhibits growth of anaplastic thyroid cancer in an orthotopic murine model. Clin Cancer Res. 2011;17:2281–2291. doi: 10.1158/1078-0432.CCR-10-2762. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Sherman SI. Lessons learned and questions unanswered from use of multitargeted kinase inhibitors in medullary thyroid cancer. Oral Oncol. 2013;49:707–710. doi: 10.1016/j.oraloncology.2013.03.442. [DOI] [PubMed] [Google Scholar]
- 74.Araujo J, Logothetis C. Dasatinib: a potent SRC inhibitor in clinical development for the treatment of solid tumors. Cancer Treat Rev. 2010;36:492–500. doi: 10.1016/j.ctrv.2010.02.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Day E, Waters B, Spiegel K, Alnadaf T, Manley PW, Buchdunger E, et al. Inhibition of collagen-induced discoidin domain receptor 1 and 2 activation by imatinib, nilotinib and dasatinib. Eur J Pharmacol. 2008;599:44–53. doi: 10.1016/j.ejphar.2008.10.014. [DOI] [PubMed] [Google Scholar]
- 76.Kopetz S, Shah AN, Gallick GE. Src continues aging: current and future clinical directions. Clin Cancer Res. 2007;13:7232–7236. doi: 10.1158/1078-0432.CCR-07-1902. [DOI] [PubMed] [Google Scholar]
- 77.Irby RB, Yeatman TJ. Role of Src expression and activation in human cancer. Oncogene. 2000;19:5636–5642. doi: 10.1038/sj.onc.1203912. [DOI] [PubMed] [Google Scholar]
- 78.Fizazi K. The role of Src in prostate cancer. Ann Oncol. 2007;18:1765–1773. doi: 10.1093/annonc/mdm086. [DOI] [PubMed] [Google Scholar]
- 79.Schweppe RE, Kerege AA, French JD, Sharma V, Grzywa RL, Haugen BR. Inhibition of Src with AZD0530 reveals the Src-Focal Adhesion kinase complex as a novel therapeutic target in papillary and anaplastic thyroid cancer. J Clin Endocrinol Metab. 2009;94:2199–2203. doi: 10.1210/jc.2008-2511. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Henderson YC, Toro-Serra R, Chen Y, Ryu J, Frederick MJ, Zhou G, et al. Src inhibitors in suppression of papillary thyroid carcinoma growth. Head Neck. doi: 10.1002/hed.23316. (e-pub ahead of print 1 June 2013) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Lyons JF, Wilhelm S, Hibner B, Bollag G. Discovery of a novel Raf kinase inhibitor. Endocr Relat Cancer. 2001;8:219–225. doi: 10.1677/erc.0.0080219. [DOI] [PubMed] [Google Scholar]
- 82.Wilhelm S, Chien DS. BAY 43-9006: preclinical data. Curr Pharm Des. 2002;8:2255–2257. doi: 10.2174/1381612023393026. [DOI] [PubMed] [Google Scholar]
- 83.Wilhelm SM, Carter C, Tang L, Wilkie D, McNabola A, Rong H, et al. BAY 43-9006 exhibits broad spectrum oral antitumor activity and targets the RAF/MEK/ERK pathway and receptor tyrosine kinases involved in tumor progression and angiogenesis. Cancer Res. 2004;64:7099–7109. doi: 10.1158/0008-5472.CAN-04-1443. [DOI] [PubMed] [Google Scholar]
- 84.Carlomagno F, Anaganti S, Guida T, Salvatore G, Troncone G, Wilhelm SM, et al. BAY 43-9006 inhibition of oncogenic RET mutants. J Natl Cancer Inst. 2006;98:326–334. doi: 10.1093/jnci/djj069. [DOI] [PubMed] [Google Scholar]
- 85.Salvatore G, De Falco V, Salerno P, Nappi TC, Pepe S, Troncone G, et al. BRAF Is a Therapeutic Target in Aggressive Thyroid Carcinoma. Clin Cancer Res. 2006;12:1623–1629. doi: 10.1158/1078-0432.CCR-05-2378. [DOI] [PubMed] [Google Scholar]
- 86.Henderson YC, Ahn SH, Kang Y, Clayman GL. Sorafenib potently inhibits papillary thyroid carcinomas harboring RET/PTC1 rearrangement. Clin Cancer Res. 2008;14:4908–4914. doi: 10.1158/1078-0432.CCR-07-1772. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Radhakrishnan SK, Bhat UG, Hughes DE, Wang IC, Costa RH, Gartel AL. Identification of a chemical inhibitor of the oncogenic transcription factor forkhead box M1. Cancer Res. 2006;66:9731–9735. doi: 10.1158/0008-5472.CAN-06-1576. [DOI] [PubMed] [Google Scholar]
- 88.Pilarsky C, Wenzig M, Specht T, Saeger HD, Grutzmann R. Identification and validation of commonly overexpressed genes in solid tumors by comparison of microarray data. Neoplasia. 2004;6:744–750. doi: 10.1593/neo.04277. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Wierstra I, Alves J. FOXM1, a typical proliferation-associated transcription factor. Biol Chem. 2007;388:1257–1274. doi: 10.1515/BC.2007.159. [DOI] [PubMed] [Google Scholar]
- 90.Tsai J, Lee JT, Wang W, Zhang J, Cho H, Mamo S, et al. Discovery of a selective inhibitor of oncogenic B-Raf kinase with potent antimelanoma activity. Proc Natl Acad Sci USA. 2008;105:3041–3046. doi: 10.1073/pnas.0711741105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Nucera C, Nehs MA, Nagarkatti SS, Sadow PM, Mekel M, Fischer AH, et al. Targeting BRAFV600E with PLX4720 displays potent antimigratory and anti-invasive activity in preclinical models of human thyroid cancer. Oncologist. 2011;16:296–309. doi: 10.1634/theoncologist.2010-0317. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Bollag G, Hirth P, Tsai J, Zhang J, Ibrahim PN, Cho H, et al. Clinical efficacy of a RAF inhibitor needs broad target blockade in BRAF-mutant melanoma. Nature. 2010;467:596–599. doi: 10.1038/nature09454. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Joseph EW, Pratilas CA, Poulikakos PI, Tadi M, Wang W, Taylor BS, et al. The RAF inhibitor PLX4032 inhibits ERK signaling and tumor cell proliferation in a V600E BRAF-selective manner. Proc Natl Acad Sci USA. 2010;107:14903–14908. doi: 10.1073/pnas.1008990107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Yang H, Higgins B, Kolinsky K, Packman K, Go Z, Iyer R, et al. RG7204 (PLX4032), a selective BRAFV600E inhibitor, displays potent antitumor activity in preclinical melanoma models. Cancer Res. 2010;70:5518–5527. doi: 10.1158/0008-5472.CAN-10-0646. [DOI] [PubMed] [Google Scholar]
- 95.Chapman PB, Hauschild A, Robert C, Haanen JB, Ascierto P, Larkin J, et al. Improved survival with vemurafenib in melanoma with BRAF V600E mutation. N Engl J Med. 2011;364:2507–2516. doi: 10.1056/NEJMoa1103782. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Sosman JA, Kim KB, Schuchter L, Gonzalez R, Pavlick AC, Weber JS, et al. Survival in BRAF V600-mutant advanced melanoma treated with vemurafenib. N Engl J Med. 2012;366:707–714. doi: 10.1056/NEJMoa1112302. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Flaherty KT, Puzanov I, Kim KB, Ribas A, McArthur GA, Sosman JA, et al. Inhibition of Mutated, Activated BRAF in Metastatic Melanoma. N Engl J Med. 2010;363:809–819. doi: 10.1056/NEJMoa1002011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Montagut C, Sharma SV, Shioda T, McDermott U, Ulman M, Ulkus LE, et al. Elevated CRAF as a potential mechanism of acquired resistance to BRAF inhibition in melanoma. Cancer Res. 2008;68:4853–4861. doi: 10.1158/0008-5472.CAN-07-6787. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Poulikakos PI, Zhang C, Bollag G, Shokat KM, Rosen N. RAF inhibitors transactivate RAF dimers and ERK signalling in cells with wild-type BRAF. Nature. 2010;464:427–430. doi: 10.1038/nature08902. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Su F, Bradley WD, Wang Q, Yang H, Xu L, Higgins B, et al. Resistance to selective BRAF inhibition can be mediated by modest upstream pathway activation. Cancer Res. 2012;72:969–978. doi: 10.1158/0008-5472.CAN-11-1875. [DOI] [PubMed] [Google Scholar]
- 101.Trunzer K, Pavlick AC, Schuchter L, Gonzalez R, McArthur GA, Hutson TE, et al. Pharmacodynamic effects and mechanisms of resistance to vemurafenib in patients with metastatic melanoma. J Clin Oncol. 2013;31:1767–1774. doi: 10.1200/JCO.2012.44.7888. [DOI] [PubMed] [Google Scholar]
- 102.Xing J, Liu R, Xing M, Trink B. The BRAFT1799A mutation confers sensitivity of thyroid cancer cells to the BRAFV600E inhibitor PLX4032 (RG7204) Biochem Biophys Res Commun. 2011;404:958–962. doi: 10.1016/j.bbrc.2010.12.088. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Kim K, Cabanillas M, Lazar AJ, Williams MD, Sanders DL, Ilagan JL, et al. Clinical responses to vemurafenib in patients with metastatic papillary thyroid cancer harboring V600EBRAF mutation. Thyroid. 2013;23:1277–1283. doi: 10.1089/thy.2013.0057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Rosove MH, Peddi PF, Glaspy JA. BRAF V600E inhibition in anaplastic thyroid cancer. N Engl J Med. 2013;368:684–685. doi: 10.1056/NEJMc1215697. [DOI] [PubMed] [Google Scholar]
- 105.Kim KB, Cabanillas ME, Lazar AJ, Williams MD, Sanders DL, Ilagan JL, et al. Clinical responses to vemurafenib in patients with metastatic papillary thyroid cancer harboring BRAF mutation. Thyroid. 2013;17:17. doi: 10.1089/thy.2013.0057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Tsimberidou AM, Vaklavas C, Wen S, Hong D, Wheler J, Ng C, et al. Phase I clinical trials in 56 patients with thyroid cancer: the M. D. Anderson Cancer Center experience. J Clin Endocrinol Metab. 2009;94:4423–4432. doi: 10.1210/jc.2009-0743. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Corcoran RB, Ebi H, Turke AB, Coffee EM, Nishino M, Cogdill AP, et al. EGFR-mediated re-activation of MAPK signaling contributes to insensitivity of BRAF mutant colorectal cancers to RAF inhibition with vemurafenib. Cancer Discov. 2012;2:227–235. doi: 10.1158/2159-8290.CD-11-0341. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Montero-Conde C, Ruiz-Llorente S, Dominguez JM, Knauf JA, Viale A, Sherman EJ, et al. Relief of feedback inhibition of HER3 transcription by RAF and MEK inhibitors attenuates their antitumor effects in BRAF-mutant thyroid carcinomas. Cancer Discov. 2013;3:520–533. doi: 10.1158/2159-8290.CD-12-0531. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Prahallad A, Sun C, Huang S, Di Nicolantonio F, Salazar R, Zecchin D, et al. Unresponsiveness of colon cancer to BRAF(V600E) inhibition through feedback activation of EGFR. Nature. 2012;483:100–103. doi: 10.1038/nature10868. [DOI] [PubMed] [Google Scholar]
- 110.Leboeuf R, Baumgartner JE, Benezra M, Malaguarnera R, Solit D, Pratilas CA, et al. BRAFV600E mutation is associated with preferential sensitivity to mitogen-activated protein kinase kinase inhibition in thyroid cancer cell lines. J Clin Endocrinol Metab. 2008;93:2194–2201. doi: 10.1210/jc.2007-2825. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Kandil E, Tsumagari K, Ma J, Abd Elmageed ZY, Li X, Slakey D, et al. Synergistic inhibition of thyroid cancer by suppressing MAPK/PI3K/AKT pathways. J Surg Res. 2013;184:898–906. doi: 10.1016/j.jss.2013.03.052. [DOI] [PubMed] [Google Scholar]
- 112.Liu D, Xing J, Trink B, Xing M. BRAF mutation-selective inhibition of thyroid cancer cells by the novel MEK inhibitor RDEA119 and genetic-potentiated synergism with the mTOR inhibitor temsirolimus. Int J Cancer. 2010;127:2965–2973. doi: 10.1002/ijc.25304. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Pal SK, Reckamp K, Yu H, Figlin RA. Akt inhibitors in clinical development for the treatment of cancer. Expert Opin Investigat Drugs. 2010;19:1355–1366. doi: 10.1517/13543784.2010.520701. [DOI] [PMC free article] [PubMed] [Google Scholar]