Abstract
The intuitive association between self-focused rumination in major depressive disorder (MDD) and the self-referential operations performed by the brain’s default-mode network (DMN) has prompted interest in examining the role of the DMN in MDD. In this paper we present meta-analytic findings showing reliably increased functional connectivity between the DMN and subgenual prefrontal cortex (sgPFC)—connectivity that often predicts levels of depressive rumination. We also present meta-analytic findings that, while there is reliably increased regional cerebral blood flow in sgPFC in MDD, no such abnormality has been reliably observed in nodes of the DMN. We then detail a model that integrates the body of research presented. In this model, we propose that increased functional connectivity between sgPFC and the DMN in MDD represents an integration of the self-referential processes supported by the DMN with the affectively laden, behavioral withdrawal processes associated with sgPFC—an integration that produces a functional neural ensemble well suited for depressive rumination and that, in MDD, abnormally taxes only sgPFC and not the DMN. This synthesis explains a broad array of existing data concerning the neural substrates of depressive rumination and provides an explicit account of functional abnormalities in sgPFC in MDD.
Keywords: default-mode network, major depressive disorder, rumination, subgenual prefrontal cortex, intrinsic functional connectivity, medial-dorsal thalamus
Introduction
Ruminative responding in major depressive disorder (MDD) is defined as a recurrent, self-reflective, and uncontrollable focus on depressed mood and its causes and consequences (1–3). Higher levels of rumination have been found to predict both more severe depressive symptoms in depressed individuals (4) and the onset of depressive symptomatology in non-depressed people (5). Although ruminative responding is not considered a criterion symptom of depression in DSM-5 or ICD-10, measures of rumination nonetheless consistently (and often, perfectly, e.g., 6) differentiate depressed from never-depressed individuals. Indeed, theorists have posited that rumination is a central aspect of the phenomenology of MDD (7).
Over the past decade, investigators have elucidated the intrinsic functional connectivity (IFC) of the brain, an endeavor that has proven useful in understanding brain functioning at a systems level (see the supplement for a brief history of work on the brain’s IFC). Combining findings from the brain mapping literature and correlations reported between network activity and measures of overt behavior, researchers have identified at least seven networks with distinct patterns of connectivity and functions (8). The perspective afforded by emerging knowledge of the IFC of the brain has provided clinical neuroscientists with a means of conceptualizing the neural substrates of psychopathology (9–11). Among the identified neural networks, the default-mode network (DMN) has received the most attention in the context of the clinical neuroscience of depression, largely because the self-referent processes attributed to the DMN (12) provide an intuitive basis for the neural conceptualization of rumination in MDD. In the following review, we describe the current status of the growing but enigmatic neuroimaging literature involving DMN functioning in MDD—both with respect to where DMN abnormalities are found, and where they are not. We then present a neural model of rumination in MDD that integrates and explains this literature.
Properties of the DMN
In working to elucidate the role of the DMN in supporting ruminative processes in MDD, it is important to understand the nature of the operations carried out within ventromedial prefrontal cortex (vmPFC) and posterior cingulate cortex (PCC), two regions implicated most reliably in this network. Investigators have documented activation in vmPFC during valuation of goal-directed choices (13), while individuals are forming preference judgments (14), and as people are determining the financial value of a transaction (15). These findings, in addition to data documenting impairment in the ability to form preferences following damage to vmPFC (16), indicate that this structure plays a vital role in the valuation of appetitive goals. Further, investigators have found that vmPFC activates more strongly when individuals receive a stimulus they believe is of higher value than when they receive a stimulus of lower value (e.g., expensive vs. cheap wine, 17), and when stimuli are presented in more versus less appealing ways (e.g., cheese odor vs. foot odor, 18). Considered collectively, these findings indicate that the vmPFC is involved broadly in assigning abstract properties of reward value to stimuli.
A growing functional neuroimaging literature is also elucidating the properties of PCC. Sestieri and colleagues (19) documented early activation of PCC, but not of vmPFC, in a task that required episodic memory retrieval and elaboration, suggesting that PCC plays a special role in autobiographical search and retrieval processes, as opposed to elaborating on stimuli. In addition, two meta-analyses found that PCC is reliably involved in spatial navigation from a self-centered reference frame (20), and in knowledge of the sensory attributes of concrete objects, but not in verbal knowledge (21). Finally, in a graph theoretic study incorporating both structural and functional connectivity analyses, Hagmann and colleagues (22) found that PCC was among a small number of regions with hub-like properties that integrated information across the cerebral cortex. Together, these studies suggest that the broad role of PCC is in integrating self-relational information within a spatial-temporal context.
Although we are acquiring a more comprehensive understanding of the unique functions of vmPFC and PCC, their combined function in the context of the DMN is not as well understood. Given that vmPFC activation tracks consistently with assigning reward labels to stimuli, and that PCC functions to add layers of egocentric spatio-temporal context to stimuli, we propose that the function of the DMN complements that of another intrinsic functional network, the salience network. This latter network comprises the dorsal anterior cingulate cortex (ACC), fronto-insular cortex, and amygdala (23), and plays a pivotal role in determining the biological significance of external stimuli. Thus, and as other investigators have (24), we posit that, in contrast to assessing the significance of external stimuli, the DMN assigns valence to internally represented stimuli and, to an extent consistent with the intensity of the assigned valence, elaborates on these stimuli from an egocentric perspective. Findings that attenuation of the deactivation characteristic of the DMN during task performance is associated with internal mentation at both state and trait levels (25) are consistent with this formulation. For readers interested in additional perspectives on functioning of the DMN, we recommend (20, 26–29).
The DMN in MDD
Resting-state fMRI
There are now a number of studies that have examined resting-state functional magnetic resonance imaging (fMRI) connectivity of the DMN in MDD. To identify the most robust findings in this literature, we conducted for this review a systematic meta-analysis of these studies. Briefly, we searched the Web of Science for articles with titles or topics matching the search phrase [depress* AND (fMRI OR “functional MRI” OR “functional magnetic”) AND default]. Among the articles that met these search criteria, we kept for subsequent meta-analysis those that compared DMN connectivity in currently and never depressed individuals across the whole brain using either seed-based functional connectivity or independent components analysis. In addition, we retained only those articles in which coordinates for regions showing between-groups differences in DMN connectivity were provided in MNI or Talairach space. Six studies (30–35) met criteria for inclusion in our meta-analysis (see Table 1 for characteristics of included studies); to add to this, we conducted an additional analysis of DMN functional connectivity in MDD (using the method presented in 36) in a sample of 17 unmedicated unipolar depressed and 17 matched healthy control participants from our laboratory.
Table 1.
Demographic, clinical, and analytic data for studies meeting inclusion criteria for the meta-analysis.
| Study | Number of participants per group |
Characteristics of MDD samples |
Current comorbidities in MDD samples |
Technique used to estimate DMN connectivity |
|||
|---|---|---|---|---|---|---|---|
| Major Depressive Disorder |
Healthy Control |
Medicated (%) |
Female (%) |
Mean Age |
|||
| Alexopoulos et al (30) | 16 | 10 | 0 | NR | 69.0 | None | Seed region |
| Berman et al (32) | 15 | 15 | 40 | 66 | 25.7 | Anxiety (2) | Seed region |
| Gaffrey et al (33) | 21 | 18 | 0 | 57 | 9.5 | Internalizing disorder (3), Internalizing and Externalizing Disorders (12) | Seed region |
| Greicius et al (34) | 28 | 29 | 71 | 57 | 38.5 | Psychosis (11) | ICA |
| Sambataro et al (31) | 20 | 20 | 70 | 75 | 33.6 | None | ICA |
| Zhu et al (35) | 32 | 33 | 0 | 56 | 20.5 | None | ICA |
| Hamilton et al (novel sample) | 17 | 17 | 0 | 65 | 32.1 | None | Seed region |
Note: DMN = default-mode network; ICA = independent components analysis; MDD = major depressive disorder; NR = not reported
To the coordinates of between-groups differences in DMN connectivity reported in the seven studies meeting our inclusion criteria, we applied a multilevel kernel density analysis approach (37–39) to meta-analysis in which we first converted reported coordinates from each of N studies into sample-size-weighted binary indicator maps. Next, we summed the indicator maps at each voxel (v) to obtain the meta-analytic statistic (P̂v) such that:
where wn is the square root of the number of subjects in the full study group (depressed plus comparison subjects) of the nth of N studies. Finally, we thresholded this map voxelwise using Monte Carlo simulation and clusterwise using 3dClustSim in AFNI (40) so that familywise error was held at p = .05 (voxelwise p = .0001; cluster threshold = 271 mm3). See previous meta-analyses (37, 39) for more details about this approach.
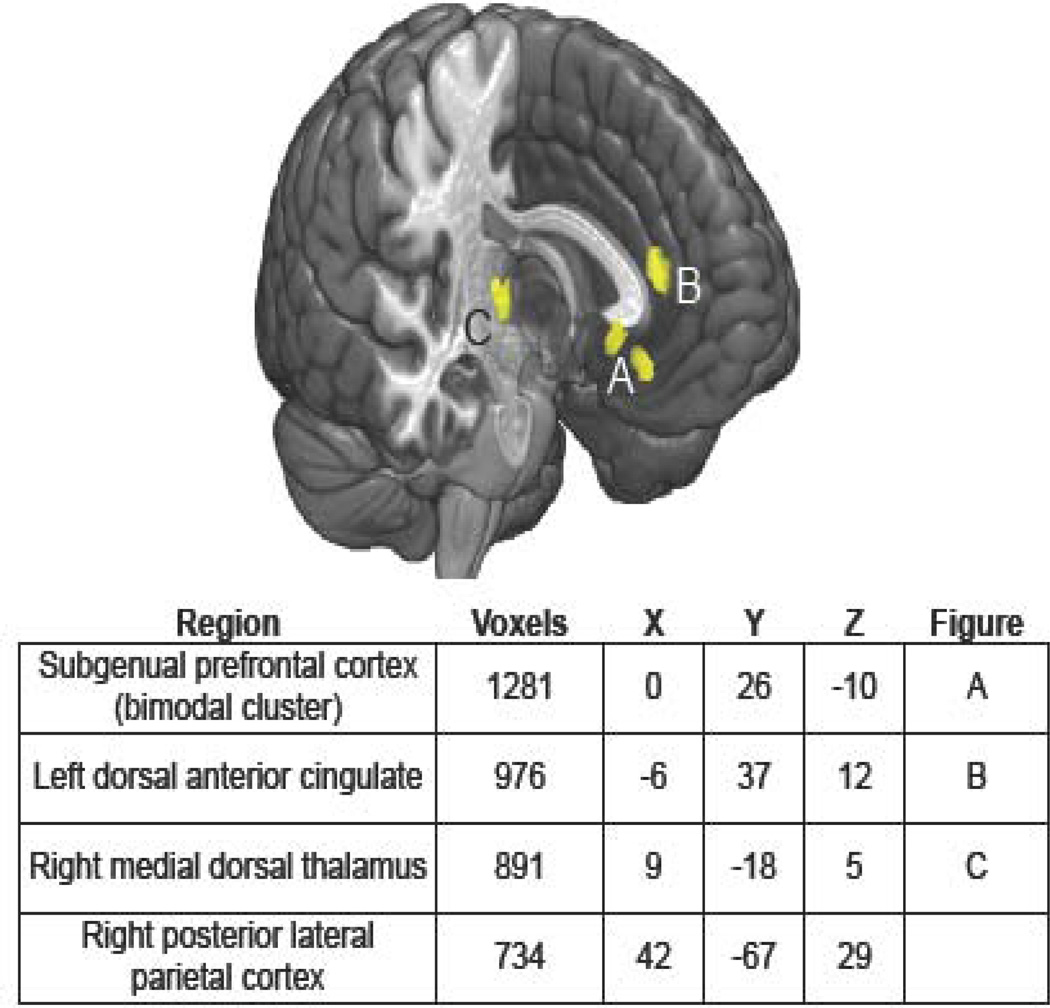
We found highly reliable increases in MDD in functional connectivity between the DMN and the subgenual prefrontal cortex (sgPFC) as well as the medial-dorsal thalamus (MDT), dorsal ACC, and posterior lateral parietal cortex (see Figure 1). Importantly, Bermann et al. (32) and Zhu and colleagues (35) reported that stronger connectivity between sgPFC and the DMN predicted higher levels of ruminative responding in MDD. Further confirming these findings, we conducted effective connectivity analysis using sgPFC as a seed region and found evidence of mutually propagating activation between sgPFC and vmPFC in MDD that, itself, predicted higher levels of rumination about depressive symptomatology (36).
Figure 1.
Regions showing reliably increased connectivity with the default-mode network in major depressive disorder.
Resting regional cerebral blood flow
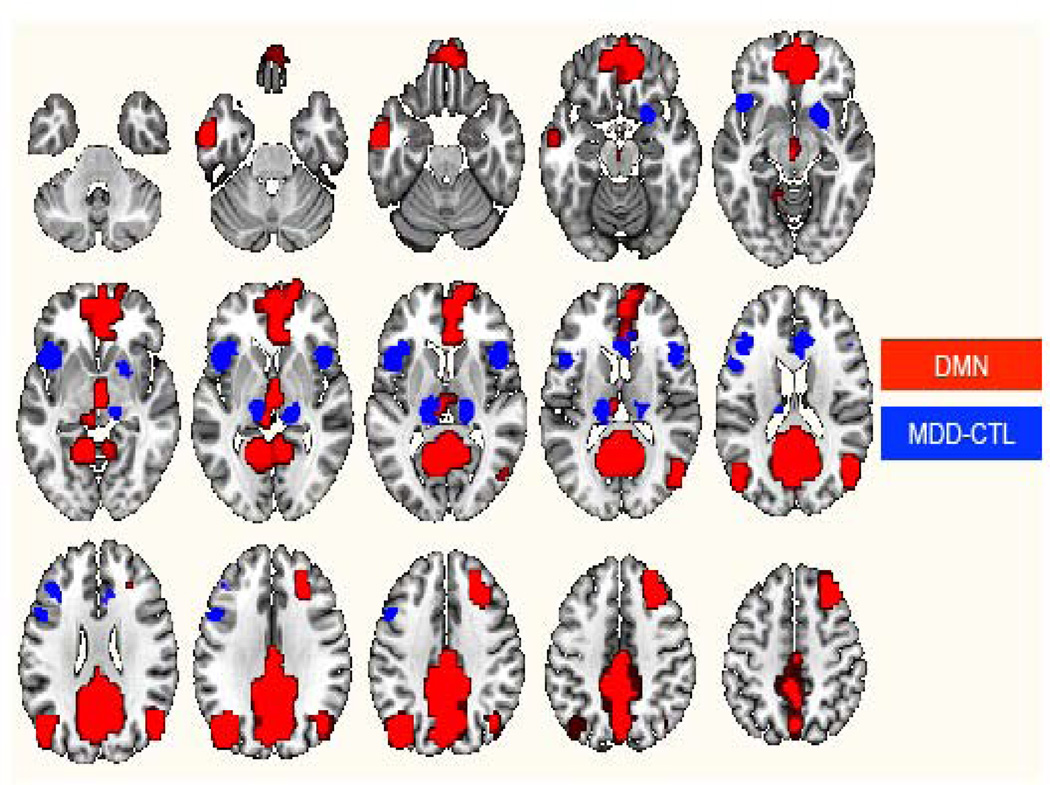
Given both the purported role of the DMN in the self-relational aspects of rumination and the high prevalence of rumination in depression, we might expect to see abnormally increased activation in nodes of the DMN in MDD in studies using techniques like PET and single photon emission computed tomography (SPECT), both of which assess resting-state regional cerebral blood flow (rCBF). For purposes of this review, we updated a recent meta-analysis of resting rCBF abnormalities in MDD (four studies added to 39; see Supplementary Table 1 for details on the 18 studies included) and compared the resulting meta-analytic map to a map of the DMN as defined by Greicius and colleagues (41). Notably, we did not find statistically reliable, or even trend-level, differences between depressed and control samples in rCBF in any region of the DMN; see Figure 2 for a rendering of both MDD-versus-control rCBF maps and the DMN in the same standard space. We did find in both our original (39) and updated meta-analyses, however, statistically reliable increases in sgPFC rCBF in MDD—a finding that converges with findings from region-of-interest approaches investigating rCBF abnormalities in MDD (42–44). While the region of the sgPFC in which we found increased baseline activation (39) is lateral to regions that we found show increased DMN connectivity in MDD, it is important to consider that subregions of the sgPFC have reliably shown similar response profiles and are thought to comprise a functionally homogenous region (45–50).
Figure 2.
Maps of the default-mode network (DMN; red) and (in blue) meta-analytically reliable regional cerebral blood flow differences between samples with major depressive disorder (MDD) and healthy control (CTL) samples.
DMN dominance and depressive rumination
In a recent investigation, we computed an index of the degree to which DMN activity exceeded activity in the task positive network—a network that shows an anti-correlated relation with the DMN and is involved in effortful tasks requiring attention (12)—over the course of a resting-state fMRI scan in depressed and non-depressed individuals (6). In both of these groups, we then computed correlations between DMN dominance and three types of rumination: reflection, brooding, and rumination about depressive symptoms. Reflective rumination has been construed as a process that entails agency and adaptive focus, and has been found to predict lower levels of depressive symptoms (51); in contrast, other forms of rumination are considered to be maladaptive and have been found to be associated with attentional bias to negative stimuli in MDD (52). Although the depressed and non-depressed groups did not differ with respect to DMN dominance, we did find only in the group with MDD that higher DMN dominance was associated with higher levels of maladaptive rumination about depressive symptoms and with lower levels of more adaptive reflective rumination (6).
Interim summary
Above, we note several neural functional and behavioral regularities in MDD. First, a hallmark feature of depression is negative, self-focused rumination. Second, across 18 whole-brain rCBF investigations of MDD there are no reliable findings suggesting that components of the DMN are overactive in depression—a finding that stands in apparent contrast to our data showing that DMN dominance predicts levels of rumination in MDD. Further, increased sgPFC rCBF in MDD has been reliably observed but, as we detail below, is not well understood in terms of its contributions to the pathophysiology of depression. Next, from the functional connectivity literature, we showed that depressed individuals are characterized by reliably increased connectivity of the sgPFC and the MDT with the DMN, where levels of connectivity between the sgPFC and DMN in MDD often predict levels of rumination in depression. Below, we present a model in which we explain the enigmatic rCBF findings in MDD in terms of the DMN-related functional connectivity findings in depression. In doing this we integrate a broad and paradoxical body of data and provide a more explicit account of the role of functional abnormalities of sgPFC in MDD.
A Neural Model of Rumination in MDD
The function of sgPFC
Most work examining the functioning of sgPFC has been conducted in the context of clinical depression and sad mood. Specifically, researchers frequently report elevated sgPFC activity associated with a state of depression (42–44) or with response to negative affective challenge in MDD (46) or, in non-depressed persons, with sad mood induced either through elaboration of sad autobiographical scripts (43) or through inflammation challenge (53). Although these are important findings, they beg questions concerning the role of sgPFC functioning in depression and sadness. In addressing these questions, it is informative to consider the results of studies examining sgPFC activity in the context of normal affective processing. Early formulations of affective processing in the ACC and adjacent pericingulate regions proposed opposing and complementary functions for the dorsal and ventral aspects of this region (49). Specifically, researchers posited that dorsal ACC and sgPFC functioning are complementary with respect to a continuum of autonomic tone, with dorsal ACC involved in active, energy-expending functions (54) and sgPFC subserving behavioral withdrawal, resource conservation, and the promotion of safety behaviors (55, 56). Subsequent work showing that increased sgPFC response is associated with feelings of guilt resulting from behaviors that run counter to social values (57) indicates that the behavioral withdrawal supported by sgPFC also involves affective appraisal. Further confirming this formulation, investigators have documented increased sgPFC activity in response to peer rejection during fMRI scanning, activity that predicts the subsequent development of depressive symptomatology (58).
Integrating the data
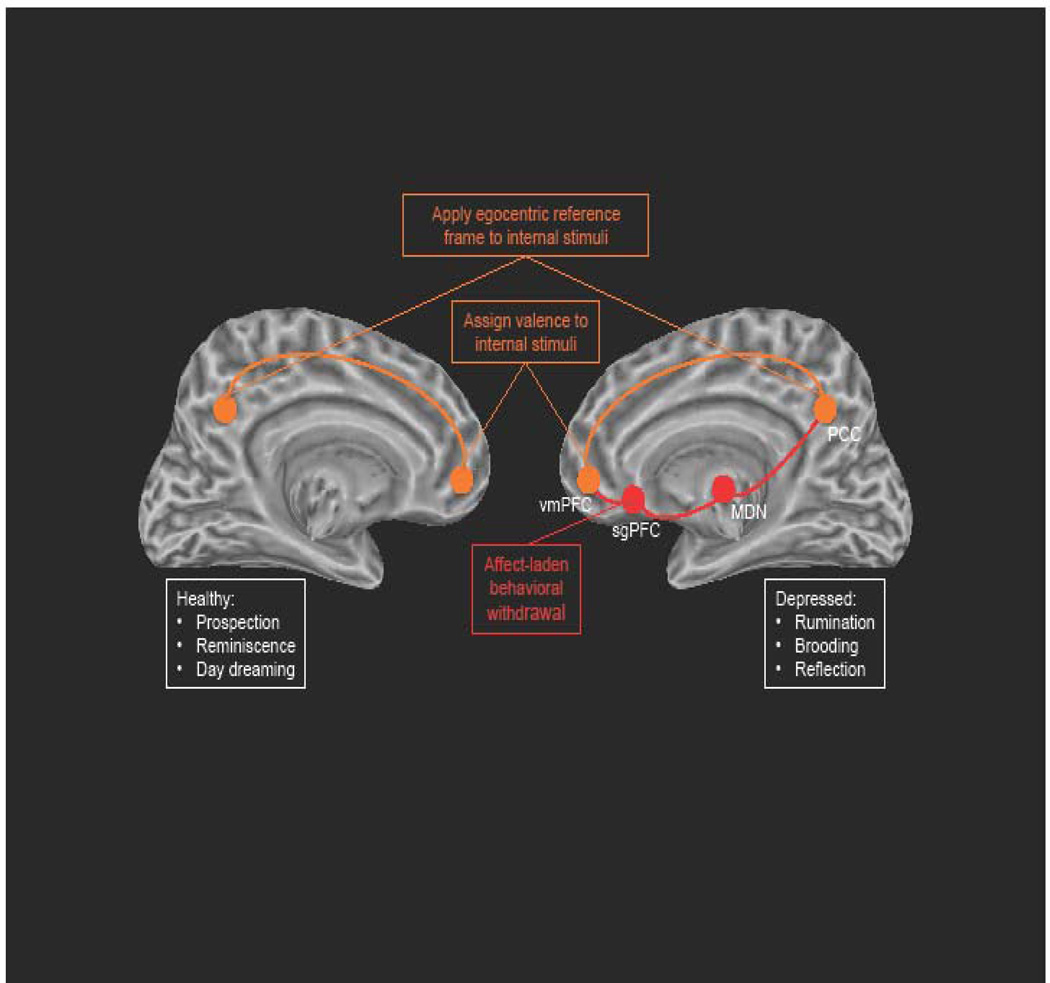
To synthesize and reconcile the bodies of work presented above, we posit that findings of increased functional connectivity between the DMN and sgPFC in MDD constitute a neural-level rendering of depressive rumination. Specifically, we propose that increased functional connectivity between the DMN and sgPFC in MDD reflects a functional integration of properties of the sgPFC and DMN. Thus, we propose that in MDD the DMN-supported processes of imbuing internal stimuli with valence and an egocentric frame of reference are united with sgPFC-related processes that support affectively laden behavioral withdrawal to produce a ruminative state that is self-focused, valenced, and withdrawn. See Figure 3 for a visual rendering of this model.
Figure 3.
Graphical rendering of our DMN-sgPFC functional integration model of depressive rumination. Orange nodes and connections represent normal functionality; red nodes and connections represent depressotypic functioning. Note: We present our model using left- and right-cerebral hemisphere underlays not to indicate inter-hemispheric anomalies associated with MDD, but to juxtapose DMN functioning in depressed and non-depressed individuals.
Reliably increased connectivity between sgPFC and the DMN in MDD helps to explain the enigmatic literature described above concerning DMN function in depression. Specifically, our formulation explains why investigators using measures of rCBF have not found over-activation of DMN nodes in MDD (39) even though depressed persons to a much greater extent than non-depressed individuals report engaging in self-reflective, ruminative thought considered to be supported by the DMN (6). Specifically, in proposing that functional integration of sgPFC and the DMN subserves depressive rumination, our model does not imply that additional demands are made of the DMN in MDD, only that the normal functions of this network are united to an abnormal degree with sgPFC functions—an integration of functions which underlies a maladaptive pattern of thought.
An additional advantage of our sgPFC-DMN model of rumination in MDD is that it provides an explicit functional account of increased DMN-MDT functional connectivity in depression (see Figure 1, above). Given that communication among cortical sites can be either direct, or indirect and routed through the thalamus, and given that tractography studies of sgPFC have failed to reveal direct connectivity between sgPFC and nodes of the DMN either in humans (59) or in non-human primates (60), it is likely that the increased correlation in activity between sgPFC and the DMN observed in MDD is mediated through the MDT, the part of the thalamus that receives information from ACC and the amygdala before routing that information back to the cortex (61). Because connectivity between sgPFC and the DMN is abnormally elevated in MDD, and is likely mediated by the MDT, we posit that the additional burden on the MDT in MDD of mediating communication between the sgPFC and DMN accounts for increased functional connectivity between the MDT and DMN in depression (see Figure 3).
An analogy to the current model
To further explain our model, we make an analogy with bonding of atoms into molecules. Elemental sodium can be joined with chlorine to form the ionic compound sodium chloride, or table salt. Elemental sodium can also be combined with water, however, to form the caustic compound sodium hydroxide. In contributing to a desirable, as opposed to an undesirable, outcome nothing needs to change about sodium, only the elements or compounds with which it is combined. Further, natural variations in a given sample of sodium — such as its purity — can influence the compound it helps to produce, whether healthy or caustic. We propose that the relation between sodium and water is analogous to that between the DMN and sgPFC in depression. Thus, we posit that the primary dysfunction in the DMN in MDD involves its relation with sgPFC: the DMN need not be overactive, only functionally united with sgPFC to contribute to depressive rumination. Our formulation still allows that natural variation in DMN activity and in the egocentric attributions it subserves — which, we argue, are necessary but not sufficient conditions for depressive rumination — can influence levels of rumination in MDD, thereby providing an account of our recent finding that while DMN dominance is not abnormal in depression, it still predicts levels of depressive rumination (6). This phenomenon of a network that contributes to depression but does not show overt dysfunction in terms of anomalies in rCBF or fMRI activation motivates the title of this paper. We propose, further, that, provided they show anomalous functional connectivity with specific structures in MDD (62), normal variation in activity in other intrinsic networks can constitute similar “dark matter” in the clinical neuroscience of depression that can be observed only through their capacity to predict patterns of depressive thought and affect. Thus, in continuing to develop more comprehensive neural models of MDD, we believe that it will be necessary to examine patterns of correlations between neural activity and measures of depressive thought and affect, even if these neural measures do not differentiate depressed from non-depressed individuals.
Limitations
It is important to acknowledge that, while the current synthesis presents a plausible and integrative neural account of depressive rumination, it would benefit from elaboration in two ways. First, our model does not specify a mechanism underlying increased functional connectivity between sgPFC and the DMN in MDD. In this context, given findings that GABA deficits are prevalent in MDD (63) and that GABA concentrations in pericingulate cortex may control fluctuations of activity in the DMN (64), future work might explore more explicitly the relation between the DMN and the pericingulate GABA-ergic system in MDD. Second, the model we present relies strongly on functional connectivity findings from neuroimaging investigations of depression. In this context, it is important to consider that the nature of the neural computations associated with fMRI functional connectivity is still not well understood. Thus, while increased functional connectivity has been observed across a variety of cognitive and perceptual domains requiring distinct contributions of functionally specialized regions—from autobiographical planning tasks in normal participants (65) to cross-modal perceptual integration in grapheme-color synesthesia (66)—investigators have remained non-committal about the computational significance of fMRI functional connectivity, typically ascribing to it, as we do here, ‘integrative’ or ‘uniting’ properties (67).
Summary and Future Directions
In this review we discussed findings suggesting that increased functional connectivity between sgPFC and the DMN in MDD is a neural substrate of depressive rumination. We demonstrated how this neural conceptualization of rumination in MDD can account both for the involvement of the DMN in ruminative responding in depression and for the lack of DMN rCBF abnormalities documented in MDD. We also described how our neural model of ruminative responding in MDD provides an explicit and intuitive formulation of the involvement of sgPFC and the MDT in the pathophysiology of depression.
The model presented in this paper generates at least two novel and testable predictions. First, recently developed techniques for inhibiting DMN activity via repetitive transcranial magnetic stimulation of the fronto-parietal central executive network (68) should have the simultaneous effects of changing DMN connectivity with sgPFC and MDT in depression and selectively ameliorating depressive rumination. The second prediction from our model is that specialized therapies focused on reducing depressive rumination, such as rumination-focused cognitive behavior therapy (RFCBT; 69), should selectively reduce the hyper-connectivity between the DMN and both sgPFC and the MDT in depression. Moreover, we predict further that successful RFCBT should selectively reduce rCBF in sgPFC in MDD.
Supplementary Material
Acknowledgments
Preparation of this article was facilitated by NIMH Grant MH74849 to Ian Gotlib. We thank Marc Berman for performing additional analyses on his functional neuroimaging data and sharing his findings with us. We thank Wayne Drevets for his insightful comments on an earlier version of this manuscript. We dedicate this article to the memory of Susan Nolen-Hoeksema and her pioneering work on rumination in depression.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Financial Disclosure
Dr. Hamilton, Ms. Farmer, Ms. Fogelman, and Dr. Gotlib have no biomedical financial interests or potential conflicts of interest to report.
Contributor Information
J. Paul Hamilton, Laureate Institute for Brain Research and College of Health Sciences, University of Tulsa
Madison Farmer, Laureate Institute for Brain Research and College of Health Sciences, University of Tulsa
Phoebe Fogelman, University of Tennessee.
Ian H. Gotlib, Department of Psychology, Stanford University
References
- 1.Morrow J, Nolen-Hoeksema S. Effects of responses to depression on the remediation of depressive affect. Journal of Personality and Social Psychology. 1990;58:519–527. doi: 10.1037//0022-3514.58.3.519. [DOI] [PubMed] [Google Scholar]
- 2.Nolenhoeksema S. RESPONSES TO DEPRESSION AND THEIR EFFECTS ON THE DURATION OF DEPRESSIVE EPISODES. Journal of Abnormal Psychology. 1991;100:569–582. doi: 10.1037//0021-843x.100.4.569. [DOI] [PubMed] [Google Scholar]
- 3.Whitmer AJ, Gotlib IH. An Attentional Scope Model of Rumination. Psychological Bulletin. doi: 10.1037/a0030923. (in press) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Kuehner C, Weber I. Responses to depression in unipolar depressed patients: an investigation of Nolen-Hoeksema's response styles theory. Psychological Medicine. 1999;29:1323–1333. doi: 10.1017/s0033291799001282. [DOI] [PubMed] [Google Scholar]
- 5.Nolen-Hoeksema S, Wisco BE, Lyubomirsky S. Rethinking Rumination. Perspectives on Psychological Science. 2008;3:400–424. doi: 10.1111/j.1745-6924.2008.00088.x. [DOI] [PubMed] [Google Scholar]
- 6.Hamilton JP, Furman DJ, Chang C, Thomason ME, Dennis E, Gotlib IH. Default-mode and task-positive network activity in Major Depressive Disorder: Implications for adaptive and maladaptive rumination. Biological Psychiatry. 2011;70:327–733. doi: 10.1016/j.biopsych.2011.02.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Lyubomirsky S, Tucker KL, Caldwell ND, Berg K. Why ruminators are poor problem solvers: Clues from the phenomenology of dysphoric rumination. Journal of Personality and Social Psychology. 1999;77:1041–1060. doi: 10.1037//0022-3514.77.5.1041. [DOI] [PubMed] [Google Scholar]
- 8.Yeo BTT, Krienen FM, Sepulcre J, Sabuncu MR, Lashkari D, Hollinshead M, et al. The organization of the human cerebral cortex estimated by intrinsic functional connectivity. Journal of Neurophysiology. 2011;106:1125–1165. doi: 10.1152/jn.00338.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Anticevic A, Cole MW, Murray JD, Corlett PR, Wang XJ, Krystal JH. The role of default network deactivation in cognition and disease. Trends in Cognitive Sciences. 2012;16:584–592. doi: 10.1016/j.tics.2012.10.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Whitfield-Gabrieli S, Ford JM. Default Mode Network Activity and Connectivity in Psychopathology. In: NolenHoeksema S, editor. Annual Review of Clinical Psychology. Vol. 8. 2012. p. 49-+. [DOI] [PubMed] [Google Scholar]
- 11.Menon V. Large-scale brain networks and psychopathology: a unifying triple network model. Trends in Cognitive Sciences. 2011;15:483–506. doi: 10.1016/j.tics.2011.08.003. [DOI] [PubMed] [Google Scholar]
- 12.Fox MD, Snyder AZ, Vincent JL, Corbetta M, Van Essen DC, Raichle ME. The human brain is intrinsically organized into dynamic, anticorrelated functional networks. Proceedings of the National Academy of Sciences of the United States of America. 2005;102:9673–9678. doi: 10.1073/pnas.0504136102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Tom SM, Fox CR, Trepel C, Poldrack RA. The neural basis of loss aversion in decision-making under risk. Science. 2007;315:515–518. doi: 10.1126/science.1134239. [DOI] [PubMed] [Google Scholar]
- 14.Paulus MP, Frank LR. Ventromedial prefrontal cortex activation is critical for preference judgments. Neuroreport. 2003;14:1311–1315. doi: 10.1097/01.wnr.0000078543.07662.02. [DOI] [PubMed] [Google Scholar]
- 15.Plassmann H, O'Doherty J, Rangel A. Orbitofrontal cortex encodes willingness to pay in everyday economic transactions. Journal of Neuroscience. 2007;27:9984–9988. doi: 10.1523/JNEUROSCI.2131-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Fellows LK, Farah MJ. The role of ventromedial prefrontal cortex in decision making: Judgment under uncertainty or judgment per se? Cerebral Cortex. 2007;17:2669–2674. doi: 10.1093/cercor/bhl176. [DOI] [PubMed] [Google Scholar]
- 17.Plassmann H, O'Doherty J, Shiv B, Rangel A. Marketing actions can modulate neural representations of experienced pleasantness. Proceedings of the National Academy of Sciences of the United States of America. 2008;105:1050–1054. doi: 10.1073/pnas.0706929105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.de Araujo IE, Rolls ET, Velazco MI, Margot C, Cayeux I. Cognitive modulation of olfactory processing. Neuron. 2005;46:671–679. doi: 10.1016/j.neuron.2005.04.021. [DOI] [PubMed] [Google Scholar]
- 19.Sestieri C, Corbetta M, Romani GL, Shulman GL. Episodic Memory Retrieval, Parietal Cortex, and the Default Mode Network: Functional and Topographic Analyses. Journal of Neuroscience. 2011;31:4407–4420. doi: 10.1523/JNEUROSCI.3335-10.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Spreng RN, Mar RA, Kim ASN. The Common Neural Basis of Autobiographical Memory, Prospection, Navigation, Theory of Mind, and the Default Mode: A Quantitative Meta-analysis. Journal of Cognitive Neuroscience. 2009;21:489–510. doi: 10.1162/jocn.2008.21029. [DOI] [PubMed] [Google Scholar]
- 21.Binder JR, Desai RH, Graves WW, Conant LL. Where Is the Semantic System? A Critical Review and Meta-Analysis of 120 Functional Neuroimaging Studies. Cerebral Cortex. 2009;19:2767–2796. doi: 10.1093/cercor/bhp055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Hagmann P, Cammoun L, Gigandet X, Meuli R, Honey CJ, Wedeen V, et al. Mapping the structural core of human cerebral cortex. Plos Biology. 2008;6:1479–1493. doi: 10.1371/journal.pbio.0060159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Seeley WW, Menon V, Schatzberg AF, Keller J, Glover GH, Kenna H, et al. Dissociable intrinsic connectivity networks for salience processing and executive control. Journal of Neuroscience. 2007;27:2349–2356. doi: 10.1523/JNEUROSCI.5587-06.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Northoff G, Bermpohl F. Cortical midline structures and the self. Trends in Cognitive Sciences. 2004;8:102–107. doi: 10.1016/j.tics.2004.01.004. [DOI] [PubMed] [Google Scholar]
- 25.Mason MF, Norton MI, Van Horn JD, Wegner DM, Grafton ST, Macrae CN. Wandering minds: The default network and stimulus-independent thought. Science. 2007;315:393–395. doi: 10.1126/science.1131295. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Ralchle ME, Snyder AZ. A default mode of brain function: A brief history of an evolving idea. Neuroimage. 2007;37:1083–1090. doi: 10.1016/j.neuroimage.2007.02.041. [DOI] [PubMed] [Google Scholar]
- 27.Harrison BJ, Pujol J, Lopez-Sola M, Hernandez-Ribas R, Deus J, Ortiz H, et al. Consistency and functional specialization in the default mode brain network. Proceedings of the National Academy of Sciences of the United States of America. 2008;105:9781–9786. doi: 10.1073/pnas.0711791105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Laird AR, Eickhoff SB, Li K, Robin DA, Glahn DC, Fox PT. Investigating the Functional Heterogeneity of the Default Mode Network Using Coordinate-Based Meta-Analytic Modeling. Journal of Neuroscience. 2009;29:14496–14505. doi: 10.1523/JNEUROSCI.4004-09.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Uddin LQ, Kelly AMC, Biswal BB, Castellanos FX, Milham MP. Functional Connectivity of Default Mode Network Components: Correlation, Anticorrelation, and Causality. Human Brain Mapping. 2009;30:625–637. doi: 10.1002/hbm.20531. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Alexopoulos GS, Hoptman MJ, Kanellopoulos D, Murphy CF, Lim KO, Gunning FM. Functional connectivity in the cognitive control network and the default mode network in late-life depression. Journal of Affective Disorders. 2012;139:56–65. doi: 10.1016/j.jad.2011.12.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Sambataro F, Wolf ND, Pennuto M, Vasic N, Wolf RC. Revisiting default mode network function in major depression: evidence for disrupted subsystem connectivity. Psychological Medicine. 2014;44:2041–2051. doi: 10.1017/S0033291713002596. [DOI] [PubMed] [Google Scholar]
- 32.Berman MG, Peltier S, Nee DE, Kross E, Deldin PJ, Jonides J. Depression, rumination and the default network. Social Cognitive and Affective Neuroscience. 2011;6:548–555. doi: 10.1093/scan/nsq080. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Gaffrey MS, Luby JL, Botteron K, Repovs G, Barch DM. Default mode network connectivity in children with a history of preschool onset depression. Journal of Child Psychology and Psychiatry. 2012;53:964–972. doi: 10.1111/j.1469-7610.2012.02552.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Greicius MD, Flores BH, Menon V, Glover GH, Solvason HB, Kenna H, et al. Resting-state functional connectivity in major depression: Abnormally increased contributions from subgenual cingulate cortex and thalamus. Biological Psychiatry. 2007;62:429–437. doi: 10.1016/j.biopsych.2006.09.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Zhu XL, Wang X, Xiao J, Liao J, Zhong MT, Wang W, et al. Evidence of a Dissociation Pattern in Resting-State Default Mode Network Connectivity in First-Episode, Treatment-Naive Major Depression Patients. Biological Psychiatry. 2012;71:611–617. doi: 10.1016/j.biopsych.2011.10.035. [DOI] [PubMed] [Google Scholar]
- 36.Hamilton JP, Chen G, Thomason ME, Johnson RF, Gotlib IH. Investigating neural primacy in Major Depressive Disorder: Multivariate granger causality analysis of resting-state fMRI time-series data. Molecular Psychiatry. 2011;16:763–772. doi: 10.1038/mp.2010.46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Etkin A, Wager TD. Functional Neuroimaging of Anxiety: A Meta-Analysis of Emotional Processing in PTSD, Social Anxiety Disorder, and Specific Phobia. Am J Psychiatry. 2007;164:1476–1488. doi: 10.1176/appi.ajp.2007.07030504. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Wager TD, Phan KL, Liberzon I, Taylor SF. Valence, gender, and lateralization of functional brain anatomy in emotion: a meta-analysis of findings from neuroimaging. Neuroimage. 2003;19:513–531. doi: 10.1016/s1053-8119(03)00078-8. [DOI] [PubMed] [Google Scholar]
- 39.Hamilton JP, Etkin A, Furman DJ, Lemus MG, Johnson RF, Gotlib IH. Functional neuroimaging of Major Depressive Disorder: A meta-analysis and new integration of baseline activation and neural response data. American Journal of Psychiatry. 2012:693–703. doi: 10.1176/appi.ajp.2012.11071105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Cox RW. AFNI: Software for analysis and visualization of functional magnetic resonance neuroimages. Computers and Biomedical Research. 1996;29:162–173. doi: 10.1006/cbmr.1996.0014. [DOI] [PubMed] [Google Scholar]
- 41.Greicius MD, Krasnow B, Reiss AL, Menon V. Functional connectivity in the resting brain: A network analysis of the default mode hypothesis. Proceedings of the National Academy of Sciences of the United States of America. 2003;100:253–258. doi: 10.1073/pnas.0135058100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Drevets WC, Price JL, Simpson JR, Todd RD, Reich T, Vannier M, et al. Subgenual prefrontal cortex abnormalities in mood disorders. Nature. 1997;386:824–827. doi: 10.1038/386824a0. [DOI] [PubMed] [Google Scholar]
- 43.Mayberg HS, Liotti M, Brannan SK, McGinnis S, Mahurin RK, Jerabek PA, et al. Reciprocal limbic-cortical function and negative mood: Converging PET findings in depression and normal sadness. American Journal of Psychiatry. 1999;156:675–682. doi: 10.1176/ajp.156.5.675. [DOI] [PubMed] [Google Scholar]
- 44.Mayberg HS, Lozano AM, Voon V, McNeely HE, Seminowicz D, Hamani C, et al. Deep brain stimulation for treatment-resistant depression. Neuron. 2005;45:651–660. doi: 10.1016/j.neuron.2005.02.014. [DOI] [PubMed] [Google Scholar]
- 45.Hamani C, Mayberg H, Snyder B, Giacobbe P, Kennedy S, Lozano AM. Deep brain stimulation of the subcallosal cingulate gyrus for depression: anatomical location of active contacts in clinical responders and a suggested guideline for targeting Clinical article. Journal of Neurosurgery. 2009;111:1209–1215. doi: 10.3171/2008.10.JNS08763. [DOI] [PubMed] [Google Scholar]
- 46.Laxton AW, Neimat JS, Davis KD, Womelsdorf T, Hutchison WD, Dostrovsky JO, et al. Neuronal Coding of Implicit Emotion Categories in the Subcallosal Cortex in Patients with Depression. Biological Psychiatry. 2013;74:714–719. doi: 10.1016/j.biopsych.2013.03.029. [DOI] [PubMed] [Google Scholar]
- 47.Murray EA, Wise SP, Drevets WC. Localization of Dysfunction in Major Depressive Disorder: Prefrontal Cortex and Amygdala. Biological Psychiatry. 2011;69:E43–E54. doi: 10.1016/j.biopsych.2010.09.041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Price JL, Drevets WC. Neural circuits underlying the pathophysiology of mood disorders. Trends in Cognitive Sciences. 2012;16:61–71. doi: 10.1016/j.tics.2011.12.011. [DOI] [PubMed] [Google Scholar]
- 49.Bush G, Luu P, Posner MI. Cognitive and emotional influences in anterior cingulate cortex. Trends in Cognitive Sciences. 2000;4:215–222. doi: 10.1016/s1364-6613(00)01483-2. [DOI] [PubMed] [Google Scholar]
- 50.Etkin A, Egner T, Kalisch R. Emotional processing in anterior cingulate and medial prefrontal cortex. Trends in Cognitive Sciences. 2011;15:85–93. doi: 10.1016/j.tics.2010.11.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Treynor W, Gonzalez R, Nolen-Hoeksema S. Rumination reconsidered: A psychometric analysis. Cognitive Therapy and Research. 2003;27:247–259. [Google Scholar]
- 52.Joormann J, Dkane M, Gotlib IH. Adaptive and maladaptive components of rumination? Diagnostic specificity and relation to depressive biases. Behavior Therapy. 2006;37:269–280. doi: 10.1016/j.beth.2006.01.002. [DOI] [PubMed] [Google Scholar]
- 53.Harrison NA, Brydon L, Walker C, Gray MA, Steptoe A, Critchley HD. Inflammation Causes Mood Changes Through Alterations in Subgenual Cingulate Activity and Mesolimbic Connectivity. Biological Psychiatry. 2009;66:407–414. doi: 10.1016/j.biopsych.2009.03.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Critchley HD, Mathias CJ, Josephs O, O'Doherty J, Zanini S, Dewar BK, et al. Human cingulate cortex and autonomic control: converging neuroimaging and clinical evidence. Brain. 2003;126:2139–2152. doi: 10.1093/brain/awg216. [DOI] [PubMed] [Google Scholar]
- 55.Matthews SC, Simmons AN, Arce E, Paulus MP. Dissociation of inhibition from error processing using a parametric inhibitory task during functional magnetic resonance imaging. Neuroreport. 2005;16:755–760. doi: 10.1097/00001756-200505120-00020. [DOI] [PubMed] [Google Scholar]
- 56.Yang TT, Simmons AN, Matthews SC, Tapert SF, Frank GK, Bischoff-Grethe A, et al. Adolescent subgenual anterior cingulate activity is related to harm avoidance. Neuroreport. 2009;20:19–23. doi: 10.1097/WNR.0b013e328317f3cb. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Zahn R, Moll J, Paiva M, Garrido G, Krueger F, Huey ED, et al. The Neural Basis of Human Social Values: Evidence from Functional MRI. Cerebral Cortex. 2009;19:276–283. doi: 10.1093/cercor/bhn080. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Masten CL, Esenberger NI, Borofsky LA, McNealy K, Pfeifer JH, Dapretto M. Subgenual anterior cingulate responses to peer rejection: A marker of adolescents' risk for depression. Development and Psychopathology. 2011;23:283–292. doi: 10.1017/S0954579410000799. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Johansen-Berg H, Gutman DA, Behrens TEJ, Matthews PM, Rushworth MFS, Katz E, et al. Anatomical connectivity of the subgenual cingulate region targeted with deep brain stimulation for treatment-resistant depression. Cerebral Cortex. 2008;18:1374–1383. doi: 10.1093/cercor/bhm167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Freedman LJ, Insel TR, Smith Y. Subcortical projections of area 25 (subgenual cortex) of the macaque monkey. Journal of Comparative Neurology. 2000;421:172–188. [PubMed] [Google Scholar]
- 61.Alexander GE, Delong MR, Strick PL. Parallel Organization of Functionally Segregated Circuits Linking Basal Ganglia and Cortex. Annual Review of Neuroscience. 1986;9:357–381. doi: 10.1146/annurev.ne.09.030186.002041. [DOI] [PubMed] [Google Scholar]
- 62.Sheline YI, Price JL, Yan ZZ, Mintun MA. Resting-state functional MRI in depression unmasks increased connectivity between networks via the dorsal nexus. Proceedings of the National Academy of Sciences of the United States of America. 2010;107:11020–11025. doi: 10.1073/pnas.1000446107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Luscher B, Shen Q, Sahir N. The GABAergic deficit hypothesis of major depressive disorder. Molecular Psychiatry. 2011;16:383–406. doi: 10.1038/mp.2010.120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Northoff G, Walter M, Schulte RF, Beck J, Dydak U, Henning A, et al. GABA concentrations in the human anterior cingulate cortex predict negative BOLD responses in fMRI. Nature Neuroscience. 2007;10:1515–1517. doi: 10.1038/nn2001. [DOI] [PubMed] [Google Scholar]
- 65.Spreng RN, Stevens WD, Chamberlain JP, Gilmore AW, Schacter DL. Default network activity, coupled with the frontoparietal control network, supports goal-directed cognition. Neuroimage. 2010;53:303–317. doi: 10.1016/j.neuroimage.2010.06.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.van Leeuwen TM, den Ouden HEM, Hagoort P. Effective Connectivity Determines the Nature of Subjective Experience in Grapheme-Color Synesthesia. Journal of Neuroscience. 2011;31:9879–9884. doi: 10.1523/JNEUROSCI.0569-11.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Rubinov M, Sporns O. Complex network measures of brain connectivity: Uses and interpretations. Neuroimage. 2010;52:1059–1069. doi: 10.1016/j.neuroimage.2009.10.003. [DOI] [PubMed] [Google Scholar]
- 68.Chen AC, Oathes DJ, Chang C, Bradley T, Zhou Z-W, Williams LM, et al. Causal interactions between fronto-parietal central executive and default-mode networks in humans. Proceedings of the National Academy of Sciences of the United States of America. 2013;110:19944–19949. doi: 10.1073/pnas.1311772110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Watkins E, Scott J, Wingrove J, Rimes K, Bathurst N, Steiner H, et al. Rumination-focused cognitive behaviour therapy for residual depression: A case series. Behaviour Research and Therapy. 2007;45:2144–2154. doi: 10.1016/j.brat.2006.09.018. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.