Abstract
Sublethal γ irradiation eliminates CD8+ T cell mediated memory responses. In this work, we explored how these memory responses could be rescued in the aftermath of such exposure. We utilized two models of CD8+ T cell mediated immunity: a mouse model of Listeria monocytogenes (LM) infection in which CD8+ T cells specific for LM expressed antigens (Listeriolysin O, LLO) can be tracked, and a murine skin graft model in which CD8+ T cells mediate rejection across a MHC class I (Dd) disparity. In the LM immunized mice, LL0 specific CD8+ T memory cells were lost on irradiation, preserved with rapid revaccination with an attenuated strain 1-3 days post-irradiation (PI), and these mice survived a subsequent wild type LM challenge. A genetic “signature of rescue” identified a group of immune-associated mRNA maintained or upregulated following irradiation and rescue. A number of these factors, including IL-36γ, dectin-2 (Clec4n), and mir101c are upregulated rapidly after exposure of mice to sublethal γ radiation alone and are sustained by early, but not later rescue. Such factors will be evaluated as potential therapeutics to replace individual vaccines for global rescue of CD8+ T memory cell responses following sublethal γ irradiation. The skin allograft model mirrored that of the LM model in that the accelerated Dd skin allograft rejection response was lost in mice exposed to sublethal γ radiation, but infusion of allogeneic Dd expressing bone marrow cells 1-4 days PI preserved the CD8+ T memory mediated accelerated rejection response, further suggesting that innate immune responses may not always be essential to rescue of CD8+ memory T cells following γ irradiation.
Introduction
Immune suppression is a serious and immediate concern for victims of sublethal ionizing radiation exposure, such as were individuals exposed at Hiroshima and Chernobyl, who exhibited long term alterations in the composition of peripheral lymphoid populations and life-long impairment of immune responses [1,2]. Effects of the recent earthquake damage to the Fukushima Daiichi nuclear power plant exposed numerous individuals to sublethal ionizing radiation, and there is the potential as well for exposure from medical procedures and terrorist acts. Of particular concern is the loss of memory T cell responses mediating vaccine immunity, as subsequent to sublethal γ radiation exposure, survivors reacquire only a limited level of immune competence [3,4]. The loss of CD8+ T memory responses following sublethal γ radiation exposure [5,6] could contribute substantially to immune incompetence in humans following such exposure [3,4,7,8] and raises particular concern with respect to responses to both latent virus infection (e.g., Epstein-Barr virus, herpes simplex virus, CMV), lytic virus infection (e.g. influenza, respiratory syncytial virus), bacterial agents (e.g. Listeria monocytogenes, Mycobacterium tuberculosis, Salmonella typhimurium), and several parasitic infections including Malaria and Leishmaniasis [9].
In previous studies in mice, we have documented [5] that memory CD8+ T cell responses mediating accelerated graft rejection are lost following sublethal γ irradiation. Our goals in this work were to evaluate loss of such responses in a mouse model of vaccine immunity in which CD8+ T memory responses were traceable, and the means to preserve such responses.
Here we show, in two distinct models, that though memory CD8+ T cell responses are lost following sublethal total body irradiation (SLTBI), rapid revaccination following such exposure can rescue memory CD8+ CTL and vaccine mediated memory responses and that there is a window of several days post-irradiation (PI) in which this can be accomplished. In the mouse Listeriosis model, revaccination within the window of rescue induced a gene expression “rescue signature” of cytokines, chemokines, and other factors which can be mined to evaluate novel T cell memory preserving therapeutics.
Materials and Methods
Animals and Cells
Female BALB/cByJ and CByB6F1 mice, 6-8 weeks old, were purchased from Jackson Laboratories (Bar Harbor, ME). Adult thymectomies and sham adult thymectomies were performed by Jackson Laboratories. FVB mice (H-2q) were purchased from Taconic Farms (Germantown, NY). Strain 3604 (MHC-Dd) transgenic mice were generated as described [10]. Group size was 5 mice except as indicated. All injections were intravenous and were administered via the lateral tail vein in a volume of 100 μL PBS with a 27G1/2 needle. All animal experiments were approved by the Center for Biologics Evaluation and Research (CBER) Institutional Animal Care and Use Committee. This study was carried out in strict accordance with the recommendations in the Guide for the Care and Use of Laboratory Animals of the National institutes of Health.
Skin Grafting
Mouse tail skin was engrafted on the flank of recipient mice as described [11].
Irradiation
Sublethal total body γ irradiation (550-600 cGy) was performed using a Gammacell 40 Cs-137 irradiator (MDS Nordion, Mississauga, ON) with a dose rate of 64-68 cGy/min. Irradiated mice were confined in an acrylic irradiation chamber and sham irradiated animals were confined in an identical irradiation chamber for the same amount of time required for the irradiations.
Listeria monocytogenes Vaccination and Challenge
Wild-type (WT) LM strain 10403s, and attenuated strain ΔActA DPL1942, and Lm-ΔActA -OVA were provided by Dr. Sing Sing Way (University of Cincinnati [12,13]. and propagated on Brain Heart Infusion Agar (Becton Dickenson, Franklin Lakes, NJ). Vaccinated or revaccinated mice received 106 cfu LM-ΔActA or LM-ΔActA-OVA i.v. Mice were challenged with 4 × 105 cfu of WT LM i.v. There was a 4 week interval between vaccination and irradiation and between irradiation and challenge except as noted.
Microarray processing and analysis
Samples were prepared according to Affymetrix protocols (Affymetrix, Santa Clara, CA). RNA quality and quantity was ensured using the Bioanalyzer (Agilent, Santa Clara, CA). and NanoDrop (Thermo Scientific, Waltham, MA) respectively. Per RNA labeling, 200 nanograms of total RNA was used in conjunction with the Affymetrix recommended protocol for the GeneChip 2.0 ST chips.
The hybridization cocktail containing the fragmented and labeled cDNAs was hybridized to The Affymetrix Mouse Genome 2.0 ST GeneChip. The chips were washed and stained by the Affymetrix Fluidics Station using the standard format and protocols as described by Affymetrix. The probe arrays were stained with streptavidin phycoerythrin solution (Molecular Probes, Carlsbad, CA) and enhanced by using an antibody solution containing 0.5 mg/mL of biotinylated anti-streptavidin (Vector Laboratories, Burlingame, CA). An Affymetrix Gene Chip Scanner 3000 was used to scan the probe arrays. Gene expression intensities were calculated using Affymetrix AGCC software. Partek Genomic Suite was used to RMA normalize (Robust Multichip Analysis), summarize, log2 transform the data, run ANOVA analysis and unsupervised Hierarchical clustering. The raw data is deposited in NCBI Gene Expression Omnibus (GEO): http://www.ncbi.nlm.nih.gov/geo/query/acc.cgi?token=ohybgqwodfwzxor&acc=GSE64434
Flow Cytometry
Rat anti-mouse CD8 clone 53-6.7 and isotype control was obtained from BD Pharmigen (San Jose, CA). LLO (H-2Kd GYKDGNEYI), OVA (H-2Kd SIINFEKL), and GFP (H-2Kd GYKDGNEYI) pentamers were obtained from Proimmune (Sarasota, FL) and used according to manufacturer's instructions. Flow cytometry was performed using a Becton Dickinson (Franklin Lakes, NJ) FacsCalibur, and data was analyzed using FlowJo software, Treestar (Ashland, OR).
qPCR Analysis
Total RNA was isolated from whole splenocytes after preparation of a single cell suspension with a Gentle MACS Dissociator (Miltenyi, San Diego, CA). RNA was isolated by use of a RNeasy Mini kit (Qiagen, Valencia, CA) following manufacturer's protocol. RNA was reverse-transcribed using a High Capacity complementary DNA (cDNA) Reverse Transcription Kit (Applied Biosystems, Calsbad, CA). cDNA was amplified using TaqMan Universal PCR Master Mix (Applied Biosystems) with validated gene-specific assays (Applied Biosystems) on an Applied Biosystems 7500 Fast Real-Time PCR System. The abundance of transcripts in each reaction was normalized to that of GAPDH.
Statistics
One-way ANOVA with Dunnett's Test were used as described in the figure legends. Kaplan-Meier LogRank survival analysis was used for survival studies. For 2 group comparisons, Mann-Whitney Test was used. Statistics were performed using SigmaPlot software (San Jose, CA).
Results
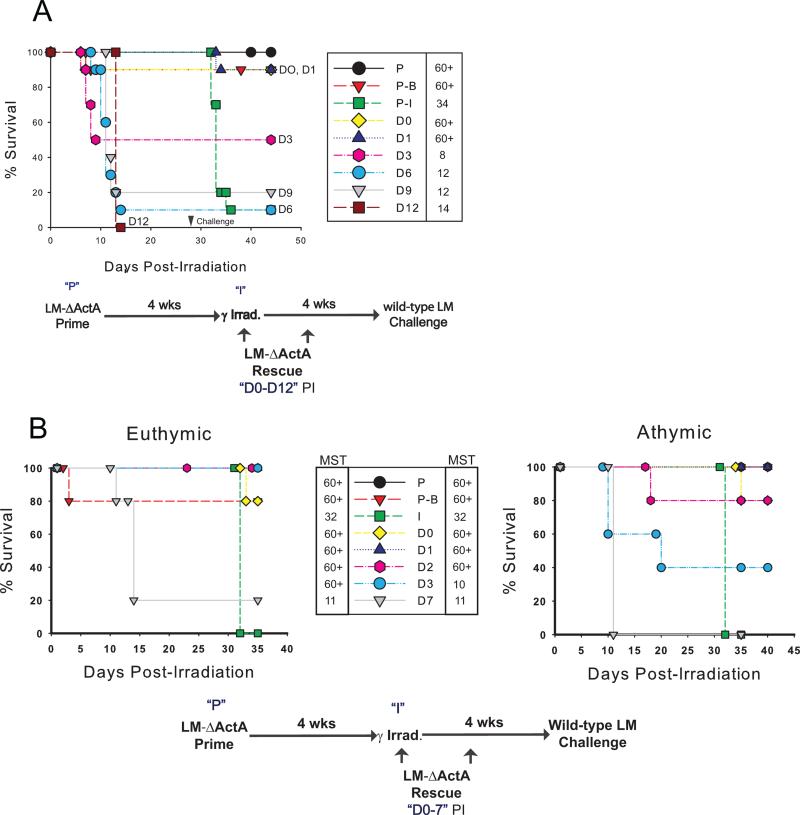
To investigate rescue of memory CD8+ T cell responses in a vaccine mediated protection model, in which we could track antigen-specific CD8+ T cells, we chose the mouse model of Listeria monocytogenes for which there is an attenuated vaccine strain lacking the virulence factor ActA (LM-ΔActA) [12], Mice were immunized with LM-ΔActA, 4 weeks later were exposed to SLTBI (600 cGy) and were revaccinated with LM-ΔActA on one of 7 days PI (DO, D1, D2, D3, D6, D9 or D12) (Fig. 1A). We found that revaccination of immunized and irradiated mice with the attenuated LM-ΔActA strain within 3 days PI, resulted in survival of all or most mice from infection with both the attenuated LM-ΔActA strain, and with wild type LM four weeks PI. The dose of wild type LM is lethal in LM-ΔActA-immunized and irradiated mice, but unirradiated LM-ΔActA-immunized mice are completely protected. In contrast to mice revaccinated within 3 days PI, mice revaccinated outside of this limited time frame usually died from reexposure to the attenuated strain, with mortality increasing with time PI (Table I), demonstrating irreversible loss of protective immunity after irradiation. We termed this preservation of the memory response by early reinfusion of antigen “rescue”. Interestingly, all mice that survived revaccination after irradiation, even if rescued beyond the 3 day limit, were able to survive challenge with the wild-type strain of LM 4 weeks PI, suggesting preservation of the vaccine memory response in rare, late rescued animals. To evaluate whether this protection was mediated by T cells freshly emerged from the thymus, we evaluated rescue in LM-ΔActA-immunized thymectomized mice and in their sham thymectomized counterparts. Results for euthymic and athymic mice were highly similar up to 3 days post-rescue where there was diminished survival in athymic compared to euthymic mice (Fig. 1B). However, Day 3 rescue has some degree of variability in effectiveness even in euthymic mice. This study indicates that rescue of the LM-specific T cell-mediated vaccine memory response is indeed mediated by a memory immune response and does not require newly emerged T cells from the thymus. To determine if rescue was associated with the survival of LM-specific CD8+ memory cells, we immunized mice with LM-ΔActA, irradiated them 4 weeks later (550 cGy), and revaccinated at D1 PI. Mice were then boosted with LM-ΔActA 3 weeks later, and spleens harvested for flow cytometry after 2 days. The fraction of CD8+ T cells specific for LLO was very low in irradiated but not rescued mice whereas the percentage of LLO-specific CD8+ T cells was significantly elevated in irradiated and rescued animals, demonstrating enhanced survival of LM specific memory CD8+ T cells by revaccination following irradiation (Fig. 2A).
Figure 1. Rapid revaccination (rescue) following sublethal total body γ irradiation preserves memory responses.
(A) Rescue of vaccine memory in the mouse Listeria monocytogenes (LM) model. BALB/cByJ mice (10 per group) were treated as indicated. MST is shown to the right of the legend. D1 revaccination is significantly different from D6 (P = 0.003), D9 (P = 0.03, and D12 (P= 0.0005) revaccination by Kaplan-Meier LogRank Survival Analysis. Similar experiments have been performed three times with comparable results. (B). New thymic output has little impact on survival from wt-LM challenge. Adult thymectomized or sham thymectomized BALB/cByJ mice were treated as described. The MST for the euthymic mice are to the left of the legend, and the MST for the athymic mice to the right. The euthymic and athymic groups are not significantly different from each other except at D3 (P = .049) by a Kaplan-Meier LogRank Survival Analysis, however D1 through D3 are significantly different from D7 for both euthymic and athymic groups (P < .05).
Table I.
Deaths following LM-ΔActA revaccination.
| Revaccination (days post-irradiation) | Deaths (days post-revaccination) |
|---|---|
| 0 | 0 |
| 2 | 0 |
| 3 | 5,5,6,6 |
| 6 | 5,5,5,6,6,6,7,8 |
| 9 | 3,3,3,3,3,3,4,4 |
| 12 | 2,2,2,2,2,2,2,2,2,2 |
Figure 2. Revaccination at D1 PI rescues antigen-specific memory CD8+ T cells following irradiation.
(A) BALB/cByJ mice were primed and irradiated as indicated. Three weeks PI, mice were boosted with 106 cfu of LM-ΔActA, and then 2 days later individual spleens were analyzed by flow cytometry for expression of CD8 and the T cell receptor specific for LLO. The percent of CD8+ T cells specific for LLO are shown. The rescue group was shown to be significantly increased over irradiation alone P = < .05 by ANOVA. Rescue is the control group for Dunnett's Test. This experiment has been repeated 3 times with similar results. (B) Antigenic specificity of rescue. Groups of 4 CByB6F1 (BALB/cByJ × C57BL/6J F1) mice were immunized with either LM-ΔActA, or LM-ΔActA-OVA. Four weeks later, the indicated groups were exposed to 600 cGy of γ radiation. One day PI, mice were revaccinated with LM-ΔActA, LM-ΔActA-OVA, or PBS, and 7 days PI, spleens were harvested for flow cytometry using anti-CD8 and pentamers specific for OVA or GFP. Significance among rescued groups was evaluated using ANOVA, P < .05 . Group 11 is the control group for Dunnett's test.
We examined the antigenic specificity of rescue by immunizing with LM-ΔActA, or LM-ΔActA expressing chicken ovalbumin (LM-ΔActA-OVA) [13], sublethally irradiating, and then rescuing with LM-ΔActA or LM-ΔActA-OVA at D1 PI. (Fig. 2B). Antigenic-specificity of rescue was demonstrated by enhanced survival of OVA-specific cells in LM-ΔActA-OVA immunized and irradiated mice only when revaccinated with LM-ΔActA-OVA (group 11), and not by LM-ΔActA alone (group 10). To investigate the mechanism of rescue, we performed genome wide expression analyses to identify whether there was a genetic “signature” of rescue. mRNA was obtained from spleens 6 hours after revaccination with LM-ΔActA at D1 (within rescue window) or D4 PI (outside of the window of rescue) for mRNA microarray analysis. Striking fold increases in a subset of immune response-associated genes were observed following D1 but not day 4 PI rescue, including IL-36γ, IRG1, mir101c, and Clec4e. Fascinatingly, these genes were highly upregulated following irradiation alone and sustained or increased by day 1 rescue (Fig 3A vs 3C), but not D4 PI rescue. The majority of the most highly upregulated mRNA following D4 PI rescue (Fig. 3B) though distinct from day 1 still pertained to immune-responses with chemokines, IFNγ, and IFNγ-induced genes predominating (Fig. 3B). These findings were confirmed and extended by quantitative PCR analyses (qPCR) (S1, Fig 4A). qPCR revealed significant differences in expression of key immune response associated factors, cytokines and chemokines at day 1 vs day 4 or day 7 rescue (Fig 4A), correlating with rescue at day 1 but not days 4 or 7. Levels of IL-36γ, IL-15, IRG1, TNFα, and CXCL10 were elevated with day 1 rescue, and lost with rescue on days 4 and 7 (Fig 4A). Figure 4B illustrates the relative roles of irradiation and rescue at D1 PI. Taken together, these data (Fig. 3,4) show clear differences in gene expression when revaccination occurs within the window of successful revaccination rescue (D1) as opposed to outside of the window of rescue (D4, D7) and suggest potential therapeutic approaches to preservation of vaccine immunity following SLTBI.
Figure 3. The impact of the day of rescue PI on splenic gene expression.
Groups of 3 BALB/cByJ mice were primed and irradiated. Spleens were harvested at 6 hours post-rescue on D1 and D4 PI and whole spleen mRNA were purified for microarray analysis. Data shown are fold increases for the top 10 immune-associated mRNA from rescued groups as compared to primed only groups. (A) shows the top 10 mRNA fold increases for D1 PI rescue. (B), The 10 most highly upregulated genes at D4 PI rescue are compared with expression by D1 PI rescue. Gene upregulation by sublethal γ radiation alone is shown at D1 PI (C) and D4 PI (D). The data shown are the top 10 mRNA fold increases of immune-associated genes at D1 and D4 PI as compared to primed only groups.
Figure 4. Factors induced by rescue as assessed by qPCR.
BALB/cByJ mice were primed, irradiated, and revaccinated on D1 or D4 or D7 PI. Spleens were harvested from individual mice for mRNA isolation at 6 hours post-rescue. Results shown are qPCR data comparing mRNA upregulation with rescue at D1 with rescue at D4 or D7 PI. mRNA with significant increases at D1 only are shown. Results were evaluated by ANOVA, P < .05 D1 rescue is the control group for Dunnett's Test. (B) Factors shown in (A) with D1 rescue are compared to mice that received sublethal irradiation alone. TNFα (P = 0.032), CXCL10 and IRG1 (P = 0.008) by Mann-Whitney Test. Additional data including controls from this experiment are presented as Supplemental figures S1A and S1B. This experiment has been repeated. D1 rescue is the control group for Dunnett's Test.
Finally, to evaluate whether such antigen stimulated rescue of CD8+ T memory cells requires innate immune responses, we turned to a mouse skin allograft model in which Dd expressing skin grafts were rejected across an MHC class I barrier solely by CD8+ T cells [5]. While naïve FVB mice (H-2q) reject MHC class I disparate Dd skin grafts (from transgenic donors expressing Dd on the FVB background, strain 3604) with a median graft survival time (MST) of 14 days [5,10], mice immunized (primed) with MHC class I disparate Dd spleen cells prior to skin grafting, reject H-2Dd skin grafts in an accelerated fashion (MST of 8 days). Primed animals skin grafted 4 weeks following SLTBI (550 cGy), not only demonstrated loss of the accelerated rejection response, but in fact, demonstrated a markedly delayed graft rejection response (MST 26 days). However, following SLTBI in primed mice, infusion of Dd bone marrow cells (BMC) within 0-4 days PI preserved the accelerated rejection response (Fig. 5). The accelerated rejection response was previously found to be totally dependent on CD8+ T cells [5], strongly suggesting that revaccination preserves or rescues CD8+ memory T cells from radiation-induced death in the absence of robust innate immune system contributions. In marked contrast, delaying infusion of Dd BMC to day 7 or 14 PI not only failed to preserve or restore accelerated rejection, but in fact, appeared to have no effect beyond irradiation alone, consistent with loss of CD8+ T memory cells and/or, to induction of regulatory T cells [5,6].
Figure 5. Rescue of memory responses in skin allograft model.
FVB mice (H-2q) were immunized with 20 × 106 H-2Dd 3604 strain allogeneic spleen cells. 4 weeks later mice were exposed to 550 cGy of total body γ radiation. D0 through D14 refers to the day PI on which the mice were revaccinated with an i.v. dose of 30 × 106 H-2Dd 3604 bone marrow cells. Two weeks following irradiation, mice were engrafted with 3604 H-2Dd tail skin. Mice were observed daily and grafts were considered rejected when <20% of the graft remained. Median survival time (MST) for the skin allografts are listed to the right of the legend. Primed alone and D0 and D1 revaccination following irradiation were significantly different from irradiation alone and D7 and D4 revaccination following irradiation by Kaplan-Meier LogRank Survival Analysis (P = 0.002 for all comparisons). This experiment was repeated 3 times with similar results. The days of revaccination PI, (D1 through D14), are indicated on the plot.
Discussion
We have demonstrated, in two very different mouse models of CD8+ T cell mediated memory responses, that such memory responses are lost following sublethal irradiation, but that a window of time exists following sublethal γ irradiation in which re-exposure to or revaccination with the vaccine antigen can preserve CD8+ T memory cells and their in vivo responses from loss by irradiation. Revaccination or preferably a therapeutic which mimics revaccination given during this privileged window has the potential to more broadly retain a panoply of CD8+ T cell-mediated memory responses to vaccine or latent virus antigens following sublethal γ radiation exposure [3,4].
The rescue of CD8+ LM specific memory T cells following irradiation may well reflect the unique lymphocyte specific phenomenon that proliferation confers resistance to ionizing radiation rather than the increased susceptibility to radiation-induced cell death observed in somatic cells [14-16]. Thus, activation in a narrow window PI may stimulate proliferation of CD8+ memory T cells and facilitate their survival. This is supported by the antigen-specificity of rescue, suggesting activation through the TCR (Fig. 2B). Our study is consistent with the finding that low dose (60-240 cGy) γ irradiation of human T cells in vitro followed by antigen-specific T cell activation, enhances a variety of T cell functions including cytotoxicity, proliferation, and cytokine production (IFNγ) [17]. These data collectively support the premise that activation of T cells promotes radioresistance [14-16] and provide a mechanism for our observation that CD8+ memory T cells are rescued following γ radiation exposure by antigen-specific stimulation. Studies to assess the expression of apoptotic markers on memory CD8+ T cells have been hampered by the scarcity of such cells in irradiated spleen. However, use of a TCR transgenic model will facilitate such studies which are underway.
Ionizing radiation has been shown to upregulate a number of cytokines including TNFα, IL-1α, IL-1β, IL-6, IFNγ, and TGFβ, among others [18]. We have presented here a “signature of rescue” in which a number of largely immune response-associated mRNAs are upregulated at D1 PI following irradiation and rescue, some of which may be involved in the mechanism of rescue. As significant immune cell death occurs within one day of irradiation, we investigated a signature of rescue at 6 hours PI, before significant immune cell death had occurred. However, time course studies evaluating factors likely involved in rescue such as cytokines and chemokines are underway. We examined whole spleen mRNA, enabling us to focus on the total response of all relevant cells of the immune system, including memory T cells, interacting at the site of infection whereas other studies have focused on peripheral blood (18) which has the advantage of practicality in real life scenarios, yet is limited in terms of numbers of relevant cell populations. Thus, we have launched studies to examine CD8+ T memory cells in PBL using a TCR transgenic model. We plan to use our “rescue signature” data to investigate and develop novel therapeutics to replace revaccination rescue, which, given the number of applicable vaccines and the lack of such vaccines for latent viruses, is truly impractical. Specifically with regard to rescue of CD8+ T cell immunity is the upregulation of IL-15 by rescue at D1 as compared to D4 or D7 (Fig. 4A). IL-15 is an activation and survival factor for CD8+ T cells [19], and thus is a candidate therapeutic. IL-36γ, an IL-1 family cytokine which enhances expression of molecules involved in antigen presentation as well as the production of numerous proinflammatory cytokines [20], is highly upregulated at D1 vs D4 and D7 PI, is the mRNA most increased by irradiation alone (Fig. 3C), and has the second highest fold increase with D1 PI rescue (Fig. 3A). Moreover, IL-36γ has also been identified in α-particle irradiated human lymphocytes [21] and perhaps could serve as a biomarker of radiation exposure. Puzzling is the upregulation of mir101c by day 1 rescue as this microRNA has been reported to inhibit DNA double strand break repair [22]. Thus, though high levels of mir101c in response to irradiation could contribute to the exquisite sensitivity of lymphocytes to ionizing radiation, the sustained expression in the face of rescue of CD8+ memory T cells and their function by day 1 rescue is enigmatic and requires further study. Also upregulated by irradiation alone and enhanced with rescue at D1 PI (Fig. 3A,C) are a group of C type lectins: Clec4n, 4e, and 4d. These lectins have been associated with pattern associated molecular patterns (PAMP) and damage-associated molecular patterns (DAMP) recognition by myeloid cells as well as innate intracellular stimulatory functions [23], and T cell costimulatory activity [24]. Agents shown to upregulate C type lectins could thus be explored as candidate rescue therapeutics. Using PBL and macrophage cell lines, others have shown that irradiation appears to “prime the pump” for immune activation in the form of reactive oxygen species production, proliferation, and cytotoxicity [17,25,26]. We have extended these findings with documentation of a robust production of mRNA for numerous cytokines, chemokines and other factors by a macrophage-like cell line (data not shown) as well as in vivo, in the spleens of mice following irradiation alone.
The several days following exposure to sublethal ionizing radiation is a privileged period in which revaccination can preserve CD8+ T cell responses. This “window of rescue” is associated with the production of numerous immune-related factors, , which are maintained or increase with D1 PI rescue, and may be useful in the creation of a therapeutic with the capacity to broadly rescue vaccine memory responses.
Supplementary Material
Highlights.
Sublethal γ radiation impairs memory CD8+ T cell responses
Revaccination during a short window after irradiation rescues vaccine memory
Memory rescue correlates with upregulation of unique immune-associated factors
Acknowledgments
We wish to thank Dr. Sing Sing Way and Dr. Karen Elkins for kindly providing the Listeria monocytogenes strains, and Weiwei Wu for microarray technical assistance. This research was supported [in part] by the Intramural Research Program of the National Human Genome Research Institute, National Institutes of Health, and the U.S. Food and Drug Administration Medical Countermeasures Initiative.
Abbreviations
- D
day
- P
primed or immunized
- I
irradiated
- C
challenged
- PI
post-irradiation
- LLO
Listeriolysin O
- OVA
chicken ovalbumin
- LM
Listeria monocytogenes
- qPCR
quantitative real-time PCR
- SLTBI
sublethal total body irradiation
- CTL
cytotoxic T lymphocyte
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Authorship
Contribution: H.I.M. and A.S.R. designed the studies and wrote the paper; H.I.M., J.P.L., J.D.B, and K.P.M. performed the experiments and analyzed the data: A.G.E. supervised and analyzed the microarray experiment.
Conflict of interest: none.
References
- 1.Kusunoki Y, Hayashi T. Long-lasting alterations of the immune system by ionizing radiation exposure: Implications for disease development among atomic bomb survivors. Int J Radiat Biol. 2008;84:1–14. doi: 10.1080/09553000701616106. [DOI] [PubMed] [Google Scholar]
- 2.Yarilin AA, Belyakov IM, Kusmenok OI, Arshinov VY, Simonova AV, Nadezhina NM, et al. Late T cell deficiency in victims of the Chernobyl radiation accident: possible mechanisms of induction. Int J Radiat Biol. 1993;63:519–528. doi: 10.1080/09553009314550681. [DOI] [PubMed] [Google Scholar]
- 3.Akiyama M, Kusunoki Y, Kyoizumi S, Ozaki K, Mizuno S, Cologne JB. Study of the titers of anti-Epstein-Barr virus antibodies in the sera of atomic bomb survivors. Radiat Res. 1993:297–03. [PubMed] [Google Scholar]
- 4.Kanemitsu M, Morita K, Finch SC, Kato H, Onishi S. Serologic response of atomic bomb survivors following Asian influenza vaccination. JPN J Med Sci Biol. 1966;19:73–84. [PubMed] [Google Scholar]
- 5.McFarland HI, Puig M, Grajkowska LT, Tsuji K, Lee JP, Mason KP, et al. Regulatory T-cells in γ irradiation-induced immune suppression. PLoS One. 2012 doi: 10.1371/journal.pone.0039092. DOI: 10.1371/journal.pone.0039092. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Grayson JM, Harrington LE, Lanier JG, Wherry EJ, Ahmed R. Differential sensitivity of naïve and memory CD8+ T-cells to apoptosis in vivo. J Immunol. 2002;169:3760–70. doi: 10.4049/jimmunol.169.7.3760. [DOI] [PubMed] [Google Scholar]
- 7.Clave E, Socie G, Cosset JM, Chaillet MP, Tartour E, Girinsky T, et al. Multicolor flow cytometry analysis of blood cell subsets in patients given total body irradiation before bone marrow transplantation. Int J Radition Oncology Biol Phys. 1995;33(4):881–6. doi: 10.1016/0360-3016(95)00213-6. [DOI] [PubMed] [Google Scholar]
- 8.Dainiak N. Hematologic consequences of exposure to ionizing radiation. Exp Hematol. 2002;30:513–28. doi: 10.1016/s0301-472x(02)00802-0. [DOI] [PubMed] [Google Scholar]
- 9.Wong P, Pamer EG. CD8 T cell responses to infectious pathogens. Ann Rev Immunol. 2003;21:29–70. doi: 10.1146/annurev.immunol.21.120601.141114. [DOI] [PubMed] [Google Scholar]
- 10.Hansal SA, Morris DI, Sechler JMG, Love PE, Rosenberg AS. Induction of antigen-specific hyporesponsiveness by transplantation of hematopoietic cells containing an MHC class I transgene regulated by a lymphocyte-specific promoter. J Immunol. 1998;161:1063–8. [PubMed] [Google Scholar]
- 11.McFarland HI, Rosenberg AS. Skin Allograft Rejection. In: Coligan JE, Kruisbeek AM, Margulies DH, Shevach EM, Strober W, editors. Current Protocols in Immunology. Vol. 84. John Wiley & Sons; New York, NY.: 2009. pp. 4.4.1–4.4.13. [DOI] [PubMed] [Google Scholar]
- 12.Brundage RA, Smith GA, Camilli A, Theriot JA, Portnoy DA. Expression and phosphorylation of the Listeria monocytogenes ActA protein in mammalian cells. Proc Natl Acad Sci USA. 1993;90:11890–4. doi: 10.1073/pnas.90.24.11890. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Kollmann TR, Reikie B, Blimkie D, Way SS, Hajjar AM, Arispe K, et al. Induction of protective immunity to Listeria monocytogenes in neonates. J Immunol. 2007;178:3695–3701. doi: 10.4049/jimmunol.178.6.3695. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Nichols WS, Troup GM, Anderson RE. Radiosensitivity of sensitized and nonsensitized human lymphocytes evaluated in vitro. Am J. Pathol. 1975;79:499–508. [PMC free article] [PubMed] [Google Scholar]
- 15.Sprent J, Anderson RE, Miller JFAP. Radiosensitivity of T and B lymphocytes. II. Effect of irradiation on response of T-cells to alloantigens. Eur J Immunol. 1974;4:204–10. doi: 10.1002/eji.1830040310. [DOI] [PubMed] [Google Scholar]
- 16.Carloni M, Meschini R, Ovidi L, Palitti PHA-induced cell proliferation rescues human peripheral blood lymphocytes from X-ray-induced apoptosis. Mutagenesis. 2001;16(2):115–20. doi: 10.1093/mutage/16.2.115. [DOI] [PubMed] [Google Scholar]
- 17.Spary LK, Al-Taei S, Salimu J, Cook AD, Ager A, Watson HA, et al. Enhancement of T cell responses as a result of synergy between lower doses of radiation and T cell stimulation. J Immunol. 2014:3101–10. doi: 10.4049/jimmunol.1302736. [DOI] [PubMed] [Google Scholar]
- 18.Schaue D, Kachikwu EL, McBride WH. Cytokines in radiobiological responses: A review. Radiat Res. 2012;178:505–23. doi: 10.1667/RR3031.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Schluns KS, Lefrancois L. Cytokine control of memory T-cell development and survival. Nat Rev Immunol. 2003;3:269–79. doi: 10.1038/nri1052. [DOI] [PubMed] [Google Scholar]
- 20.Vigne S, Palmer G, Lamacchia C, Martin P, Talabot-Ayer D, Rodriguez E, et al. IL-36R ligands are potent regulators of dendritic and T-cells. Blood. 2011;118:8513–23. doi: 10.1182/blood-2011-05-356873. [DOI] [PubMed] [Google Scholar]
- 21.Turtoi A, Brown I, Schlager M, Schneeweiss FHA. Gene expression profile of human lymphocytes exposed to 211At α particles. Radiat Res. 2010;174:125–36. doi: 10.1667/RR1659.1. [DOI] [PubMed] [Google Scholar]
- 22.Yan D, Ng WL, Zhang X, Wang P, Zhang Z, Mo Y-Y, et al. Targeting DNA-PKcs and ATM with miR-101 sensitizes tumors to radiation. PLoS One. 2013;5(7):e11397. doi: 10.1371/journal.pone.0011397. doi:10.1371/journal.pone.0011397. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Drummond RA, Brown GD. Signalling C-type lectins in antimicrobial immunity. PLoS Pathog. 2013;9(7):e1003417. doi: 10.1371/journal.ppat.1003417. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Aragane Y, Maeda A, Schwarz A, Tezuka T, Ariizumi K, Schwarz T. Involvement of Dectin-2 in ultraviolet radiation-induced tolerance. J Immunol. 2014;171:3801–7. doi: 10.4049/jimmunol.171.7.3801. [DOI] [PubMed] [Google Scholar]
- 25.McKinney LC, Aquila EM, Coffin D, Wink DA, Vodovotz Y. Ionizing radiation potentiates the induction of IFNγ and/or LPS in murine macrophage cell lines: role of TNFα. Leuk Biol. 1998;64:459–66. doi: 10.1002/jlb.64.4.459. [DOI] [PubMed] [Google Scholar]
- 26.Gallin EK, Green SW, Sheehy PA. Enhanced activity of the macrophage-like cell line J774.1 following exposure to gamma radiation. J Leuk Biol. 1985;38:369–81. doi: 10.1002/jlb.38.3.369. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.