Abstract
The objective of this study was to assess whether migraine and tension-type headache (TTH) are best viewed as discrete entities or points on a severity continuum using taxometric analysis. Historically, classification systems have conceptualized the primary headache disorders of migraine and TTH as fundamentally different disorders that are differentiated by their characteristic symptom profiles and, as such, imply differing pathophysiologies and required treatments. Despite this categorical nosology, findings continue to emerge suggesting that migraine and TTH instead reflect dimensions of severity within the same headache construct. However, few studies have assessed this issue using taxometric statistical analyses or investigated how this taxonomic structure varies as a function of age and headache frequency. We conducted a latent-mode factor analysis of headache symptomatology obtained from 3449 individuals with headache from 2 previous, large-scale cross-sectional studies of primary headache sufferers (Martin et al., 2005, and Smitherman and Kolivas, 2013). Stratified taxometric analyses suggest that the validity of a categorical vs dimensional classification varies as a function of sample characteristics. Specifically, graphical results revealed that high headache frequency (≥ 15 d/mo) and younger age (≤ 24 years old) were associated with unimodal distributions suggestive of a dimensional construct of primary headache, whereas lower headache frequency and older age were associated with bimodal distributions characteristic of discrete diagnostic entities. Conceptualizing primary headache as a severity continuum was supported for young adults and those with frequent headaches. The distinctions of a categorical classification system were supported for adults (> 24 years old) and those with infrequent headache.
Keywords: Migraine, Tension type headache, Taxometric analysis
Introduction
Multiple classification systems have been created for the diagnosis of headache disorders [1,14,15,16]. A consistent organizing principle behind these classification systems is that the “primary” headache disorders of migraine and tension-type headache (TTH) are distinct diagnostic entities. For each of the nosological classifications to date, the differential diagnosis between migraine and TTH is entirely a function of differing self-reported symptom typologies. They nevertheless presuppose fundamentally different pathophysiologies underlie each of the primary disorders that, in turn, indicate differing treatment approaches [6,41].
The conceptualization of migraine and TTH as distinct phenomena has not gone unchallenged [6,20,40,43]. An alternative perspective— the continuum severity model—holds that these headache ‘types’ instead represent points on a single continuum of severity, with migraine falling at the more severe end of the symptom spectrum [4,10,27,30,48,56] Findings from a latent class analysis of migraine have indicated a severity continuum among patients with and without aura [34]. In support of a continuum perspective, a growing number of studies have highlighted similarities between migraine and TTH in symptomatology, response to treatment, and pathophysiology. Individuals with migraine often report prototypical TTH symptoms such as muscle tension and neck pain [21], and those with TTH (particularly chronic TTH [CTTH]) often experience photophobia, phonophobia, and aggravation by activity [52]. Beyond symptom overlap, individuals with migraine and TTH endorse similar attack triggers [23,33,36,57], have elevated rates of psychiatric comorbidities [8,19,37,47], and respond well to EMG biofeedback and other behavioral interventions [17,18,35,39,42]. Merging lines of evidence also suggest indirectly that migraine and TTH may share pathophysiological underpinnings, as evidenced by the prevalence of central sensitization in chronic forms of migraine and TTH [11], efficacy of amitriptyline as a preventive treatment for both conditions [7,18,49,51], and the finding that sumatriptan is most effective for migraine when neck pain (a common feature of TTH) is present [21].
The value and consequences of any headache diagnostic system are significant. If headache disorders actually represent positions on a severity continuum instead of discrete entities, the present ICHD diagnostic system may be needlessly complicated and of limited utility. Within an individual person, attacks of varying severity could inform targeting of treatment based on the phenomenology of individual attacks. In the absence of discriminating biological markers, there is at present insufficient evidence to definitively conclude that migraine and TTH are in fact distinct headache ‘types’ versus manifestations of an underlying dimensional construct of headache severity.
The goal of the present study was to use well-established taxometric analyses to assess whether migraine and TTH are best viewed as distinct categorical versus dimensional entities. We hypothesized that historical clinical perceptions would be supported in that there would be evidence of taxometrically different ‘types’ of headaches. However, in light of data indicating less distinct phenotypes among individuals with frequent headaches [5] and those of younger age [43,53], we posited that diagnostic/symptomatic distinctions between headache types would be less prominent among the latter subgroups.
Methods
This is a reanalysis of previously published data from two data collection efforts [31,50]. All data were collected under institutional review board approval, and only the anonymized headache data were used for analysis in this study. Detailed data collection methods are available in the original papers [31,50]. The data from these two sets were pooled for this analysis.
The data described in Martin, Penzien, Houle, Andrew, and Lofland [31] included a database of adult patients from a headache clinic (N = 390), patients from a neurology practice (N = 290), college students (N = 99) who endorsed “frequent and bothersome headaches,” and community participants who responded to an advertisement offering “free headache diagnostic information” (N = 784) (Figure 1). These participants underwent the Structured Diagnostic Interview for Headache, a validated computer-based algorithm [3], and were assigned a headache diagnosis based on the 1988 ICHD-I criteria [14]. The data previously described in Smitherman and Kolivas [50] were obtained from a sample of 1886 college students, ages 18–30, who were offered the opportunity to earn extra credit in a psychology course as compensation for their participation (i.e., these were not treatment seeking individuals). These students completed an online survey battery pertaining to headache and psychiatric symptoms including a web-administered version of the Structured Diagnostic Interview for Headache [3] revised to be consistent with ICHD-II criteria [16].
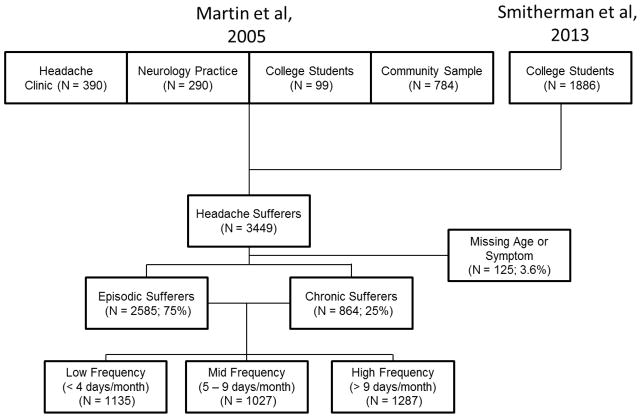
Figure 1.
Flow chart of the available sample with sample sizes for various subgroups.
The headache information gathered thus afforded characterization of headache symptoms, exclusion of secondary causes, and determination of an ICHD headache diagnosis for each participant. In instances where participants reported experiencing more than one type of headache, only the information on the ‘primary’ type was retained and used in the analysis (i.e., the one deemed by the participant to be the most problematic). However, because it is conceptually difficult for an individual to assign a monthly headache frequency to their different types of headache, the headache frequency was assigned as the total number of headaches of any kind experienced in a typical month in line with IHS criteria [15]. The Smitherman and Kolivas [50] sample did not contain participants with medication overuse headache whereas the Martin et al [31] did. No further exclusions were applied from the original studies.
Taxometric Analysis
Paul Meehl pioneered the methods of taxometric analysis developed to assist in addressing the question of whether constructs under study are categorical or dimensional in nature [32,44,45,54], and the approach increasingly is being applied to inform theory, research, and practice particularly within psychopathology. Direct taxometric analyses have been successfully applied to address the validity of categorical versus dimensional perspectives with respect to a variety of diagnostic classifications including depression [12,13], dementia [55], and other areas of inquiry [54].
Waller and Meehl reported graphical approaches to determine if a construct is best conceived as dimensional or categorical and characterized this taxometric approach as “carving nature at its joints” [54,p1], with the intended purpose of identifying latent taxa in the context of imperfect but measurable indicators used to quantify them. Although other approaches are available (e.g., mixture models, latent class models), a taxometric approach is attractive because of the use of easy-to-understand graphical outputs from the analysis. However, one potential limitation of taxometric analyses is the lack of formal statistical inferences to quantify the observed taxa. To overcome this limitation, replications across different taxometric approaches such as MAMBAC (mean above minus below a cut) [32], MAXEIG (maximum eigenvalue) [54], or L-Mode (latent mode) [54] are examined, with consistency across approaches interpreted as evidence for reliability of findings.
Due to the dichotomous nature of many indicators of migraine and TTH (symptoms present vs. absent), the present study could effectively utilize only one of Meehl’s methods, latent-mode (L-Mode) factor analysis. In L-Mode analysis, all of the indicators of a potential taxon (e.g., migraine) are entered into a factor analysis and forced to load onto a one-dimensional scale. This first principle component represents a latent construct (e.g., severity of headache, or ‘migraineness’). When plotted, the distribution can be evaluated for evidence of multiple modes indicative of multiple taxa or discrete categories (e.g., a distribution with two relatively distinct modes reflecting TTH and migraine) or a single mode indicative of one taxon with an underlying dimensional structure. Although the headache diagnoses are known (i.e., each individual has been classified), these diagnoses are not actually used to create the underlying latent dimension. Only the indicators (i.e., symptoms) are used to create the latent dimension, with the diagnoses later imposed on this dimension for the purposes of interpretation of the plots.
Indicators of Migraine or Tension-Type Headache
Table 1 lists the symptoms of migraine and TTH used in the L-Mode analysis. These symptoms are the diagnostic criteria used to establish an ICHD diagnosis of migraine (i.e., those that distinguish migraine from TTH) and are thus both necessary and sufficient to make a clinical distinction between these two headache types.
Table 1.
International Classification of Headache Disorders (1988, 2004) diagnostic criteria for migraine and coding for the taxometric analyses.
| Symptom | Coding in Analysis | |
|---|---|---|
| A. At least 5 attacks1 fulfilling criteria B–D | Not used | |
| B. Headache attacks lasting 4–72 hours (untreated or unsuccessfully treated) | Not used | |
| C. Headache has at least two of the following characteristics: | 1. unilateral location 2. aggravation by or causing avoidance of routine physical activity |
0, 1a 0, 1a |
| 3. moderate or severe pain intensity | 0 – 10 | |
| 4. pulsating quality | 0, 1a | |
| D. During headache at least one of the following: | 1. nausea and/or vomiting | 0 – 2b |
| 2. photophobia and phonophobia | 0, 1 0, 1 |
|
| E. Not attributed to another disorder | Not used | |
| Other | aura symptoms present | 0, 1 a |
0 = no, 1 = yes
0 =none, 1 = one, 2 = both
The Criterion A (number of lifetime attacks experienced), Criterion B (duration of attacks), and Criterion E (not attributable to another disorder) were not used in the L-Mode analysis because they do not afford strong discrimination between migraine and TTH. The remaining symptoms in Table 1 could be discretely coded to reflect the absence or presence of a symptom (i.e., 0 or 1), the degree of nausea and/or vomiting symptoms (0, 1, or 2), or the rating of pain intensity (0 to 10). Visual aura symptoms were also coded as present or absent, though they are not necessary for a diagnosis of migraine but are uniquely associated with the second most common presentation of migraine (i.e., migraine with aura). Monthly attack frequency was used to distinguish between “episodic” (<15 headache days/month) and “chronic” (≥15 days/month) forms of headache but was not used as a latent indicator, as both migraine and TTH have episodic and chronic subforms.
Illustration of a Taxometric Analysis
To illustrate the graphical approach to the evaluation of taxonic structure, we simulated data that were either generated from two distinct categorical groups (Figure 2A) or a single dimensional continuum (Figure 2B). To accomplish this, we used the createData function [44,46] to simulate 7 indicator variables that either distinguish group membership or are distributed along a continuum. This is analogous to using the seven headache indicator symptoms to examine for taxonic structure in headache. For this illustration, we assumed that the average indicator variable differed by d = 1.0 standard deviation units between the two groups in the taxonic structure. If these were the real headache data, this would indicate the existence of sizeable and reliable indicators of headache diagnosis (e.g., photophobia is experienced primarily by migraineurs but not tension-type headache sufferers).
Figure 2.
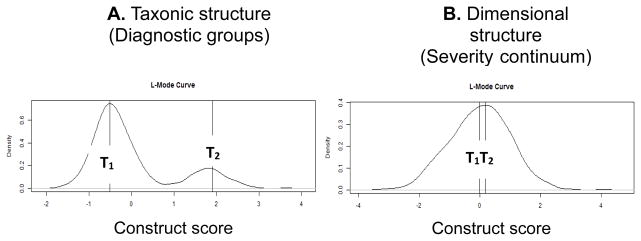
An illustration of L-Mode analysis examining whether there is a categorical or continuous difference between two groups of individuals. These data result from a hypothetical simulation where the x-axis is the degree of a latent construct (e.g., headache severity) and the y-axis represents the density of the sample for each value of the construct. Figure 2A displays two modes representing two distinct taxons (T1 and T2) that are distinct entities or groups. Figure 2B displays two groups that do not show a clear categorical distinction such that they can be viewed as points along a continuum with T2 being more severe than T1. These two plots are extreme examples of evidence that would support a categorical (2A) or continuous (2B) interpretation of an underlying construct (e.g., headache severity).
As was expected, there were clear distinctions between individuals on the latent scale in Figure 2A (i.e., two distinct modes) but not Figure 2B (i.e., one mode). The distance between the modes on the x-axis in the same plot is directly proportional to the ability of the indicator variables (i.e., symptoms) to discriminate between the two groups. If the indicator variables are distributed along a continuum of the latent dimension, there will be no clear distinction between modes and a dimensional structure will be supported (i.e., there will be virtually no distance between the modes, or there will only be one discernible mode). Thus, highly taxonic structures will more closely resemble two distributions, and continuums will resemble single distributions that may be skewed in either direction. The height of the mode(s) on the y-axis is directly proportional to that group’s representation in the sample. For example, in Figure 2A, 20% of the sample was simulated to be in the right mode (T2) and 80% in the left mode (T1) with the height of both modes being in proportion to proportion of group membership. In the present analysis, the heights of the modes are entirely related to the distributions in the sample and may not reflect underlying population distributions.
This illustration demonstrates two extremes of what might be observed in a taxometric analysis (i.e., clearly taxonic versus clearly dimensional). When reliable indicators are able to distinguish between taxons, two very clear modes appear with a distinctive valley in between the two. In reality, it is difficult to find situations with such clear-cut interpretations such as plotted here, and most analyses will find distributions somewhere between these two extremes.
Statistical Analyses
The L-Mode taxometric analyses were conducted using the Ruscio’s TaxProg.R for the R computing environment [46]. All of the plots and point estimates from the analysis were generated from the L-Mode function. Unless otherwise stated, the default settings for the function were used (e.g., N = 100 simulations for comparison). Although formal statistical inferences are not reported for the taxometric analyses (as these are graphical analyses), comparisons of symptoms across headache frequency groups were conducted using one-way ANOVA or Chi-Square as appropriate. To examine the hypotheses, we created artificial dichotomizations in several variables based either on clinical considerations or arbitrary splits based on the available data. For headache frequency, we created a dichotomy based on the clinical diagnosis of episodic (< 15 days/month) or chronic headaches (≥ 15 days/month). To examine further gradients in headache frequency we applied a tertiary split that roughly divided the sample into thirds (< 3 days/month, 4 to 8 days/month, 9 days/month). These divisions were entirely based on creating roughly equally sized groups that could support a taxometric analysis and not on any a priori clinical rationale. Finally, we dichotomized the data for the age analyses based on age cut-offs of ≤ 24 years-old and > 24 years-old. This split was again chosen based on the ideal of creating two roughly equal sized groups. All data are reported using frequency counts or mean (SD). Statistical significance was interpreted at p < 0.05.
Results
The pool of available data is displayed in Figure 1 according to original source and headache frequency characteristics. A total of N = 3449 individuals with migraine or TTH according to ICHD criteria were included in the initial sample. Of these, 125 were missing either monthly headache frequency measurements, age, or a headache symptom, leaving 3324 individuals for subsequent analyses. The analyses were conducted by including all available data for each comparison (i.e., comparisons ranged from N = 3449 to 3324).
Table 2 displays demographic information and headache symptoms of the 3449 headache sufferers as a function of three headache frequency subgroups (Low: 0 to 3 headache days/month; Middle: 4 to 9 days/month; High: 10 to 30 days/month). Age and sex were differentially associated with headache frequency, such that both age and the proportion of females increased with headache frequency (p < 0.0001). These differences should not be overly-interpreted given that in this sample individuals with more frequent headaches were recruited from the community/clinic samples, wherein they tended to be older and were more likely to be women (Table 2). The nature of the sample could also have profoundly influenced the fact that the proportion of occurrence of nearly all of the individual symptoms typically associated with migraine increased with headache frequency. Only pulsatile pain quality and unilateral location exhibited an inverse association with headache frequency. There were moderate correlations ranging from 0.034 to 0.542 among the various symptoms (i.e., indicators), as is required to assume that they are all indicators of an underlying construct that could be analyzed using a Latent Mode analysis.
Table 2.
Demographic and Headache Characteristics of the Sample by Headache Frequency Group
| Low Headache Frequency N = 1135 |
Mid Headache Frequency N = 1027 |
High Headache Frequency N = 1287 |
pa | |
|---|---|---|---|---|
| Attacks/Month | 0 to 3 | 4 to 9 | 10 to 30 | |
| Intensity (0 to 10) | 4.6 (2.6) | 5.7 (2.3) | 6.7 (2.2) | <0.0001 |
| Pulsating | 58% | 58% | 48% | <0.0001 |
| Unilateral | 45% | 46% | 41% | 0.019 |
| Aggravated | 44% | 49% | 49% | 0.019 |
| Nausea | 28% | 37% | 50% | <0.0001 |
| Vomit | 13% | 15% | 21% | <0.0001 |
| Phonophobia | 54% | 61% | 62% | <0.0001 |
| Photophobia | 48% | 60% | 59% | <0.0001 |
| Aura | 14% | 20% | 20% | <0.0001 |
| Ageb | 23.9 (10.7) | 25.5 (11.8) | 33.2 (15.5) | <0.0001 |
| Sex-Female | 63% | 75% | 79% | <0.0001 |
Data presented as column percentage, mean (SD)
Group main effect from one-way ANOVA (Intensity, Age) or Chi-squared
Missing for n = 125 (3.6% of individuals)
Headache Frequency
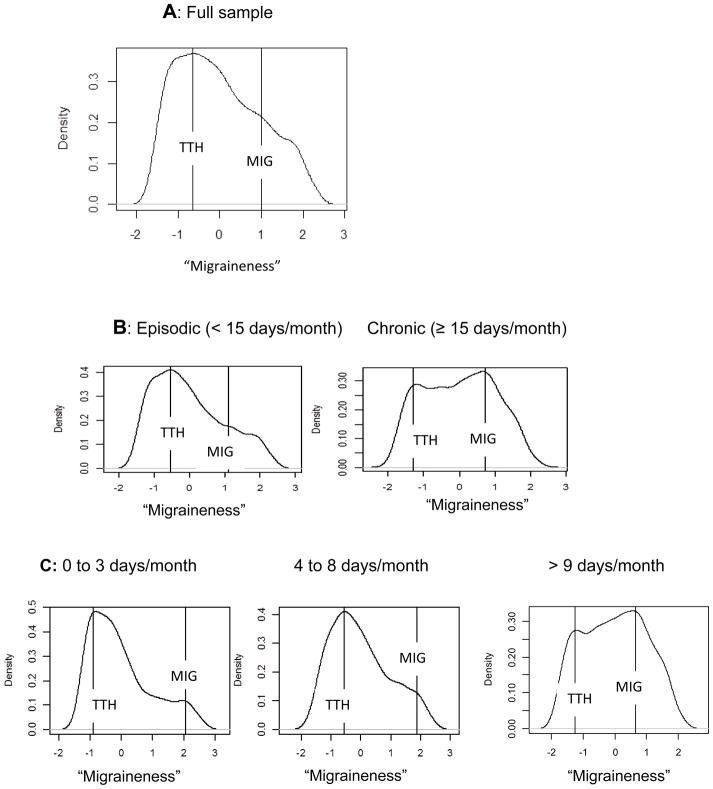
To examine if a taxonic structure exists in the sample of headache sufferers, we first examined the entire sample of headache sufferers (N = 3354) and then subdivided the sample into finer subgroups based on their reported headache frequency. Figure 3A displays the L-mode analysis of the entire sample of headache sufferers. Two lines are overlaid on the plot to indicate the closest two modes (i.e., taxons). The distribution of scores is such that no obvious visual taxons exist; the distribution of scores is skewed without clear distinctions between modes. This analysis suggests that, as a whole, the headache sufferers in the sample differed in degree of symptoms but not type (i.e., a severity continuum). When the sample was divided into episodic (< 15 days/month) versus chronic ≥15 days/month (Figure 3B) sufferers, subtle differences in the two distributions could be observed though interpretation of these differences is complicated by differences in the heights of the modes (i.e., the proportions of each subsample that are located at that mode). The episodic sufferers were distributed along a skewed distribution that contained relatively few individuals high on ‘Migraineness.’ The chronic sufferers exhibited two distinct modes (with the migraine mode actually being larger), but these modes were very close to each other with differences between the two groups not very pronounced and only a subtle valley between them. When the sample was even further divided into low-frequency (< 4 days/month), mid-frequency (4 to 9 days/month), and high-frequency (> 9 days/month; Figure 3C), differences appeared to emerge such that the distinction between the latent modes was consistently reduced with each division. For example, in the 0 to 3 headache frequency group, most of the sample was located at the low end of the continuum, with only a small mode indicative of highly migraineous headaches around a latent score of 2. The distinctions in this shape appears somewhat diminished in the 4 to 8 days/month group with a nearly unimodal distribution observed in the >9 days/month group (i.e., the imposed lines identify two discernible modes of equal size, but they are quite close to one another with only a very small valley between them). The reduced distinction among the higher headache frequency groups suggests that a categorical structure is better supported in low frequency headaches and that as headache frequency increases, the distinction (i.e., separation of symptoms) between migraineous and tension-type headaches appears to diminish.
Figure 3.
An L-Mode analysis of examining whether there is a categorical or continuous difference between those who have tension-type headache and those who have migraine headache. The x-axis represents that degree of migraine symptoms (“migraineness”) with greater scores indicating greater degree of migraine-like symptoms. The complete sample is examined in row A. The sample is split into those who exhibit episodic headache (< 15 headaches/month; N = 2585) versus those with chronic headaches (≥ 15 headaches/month; N = 864) in row B. Finally, the sample is further split into tertiles with low frequency headache (< 4 headaches/month; N = 1135), mid frequency headaches (4 to 9 headaches/month; N = 1027), and high frequency headaches (> 9 headaches/month; N = 1287) in row C. Those individuals with Tension-Type Headache (TTH) are plotted in relation to those with Migraine (MIG).
Age of Sufferer
Because younger adults do not always exhibit the same headache symptom presentation as older adults [38,40], we conducted one final analysis examining age of sufferer (and ignoring all other factors). Figure 4 displays the L-mode analysis of the sample of headache sufferers who were ≤ 24 years-old (N = 2012) or > 24 years-old (N = 1312), which approximated a median split in our sample of headache sufferers. The ≤ 24 years/old subsample exhibited a single mode and a high degree of skew. A much greater distinction can be observed in the headache characteristics of the > 24 years/old individuals, such that two modes are visually discernible (though relatively close together). The findings refute the notion of a taxonic structure in young sufferers and instead suggest that headaches in younger adults differ more in degree than type.
Figure 4.
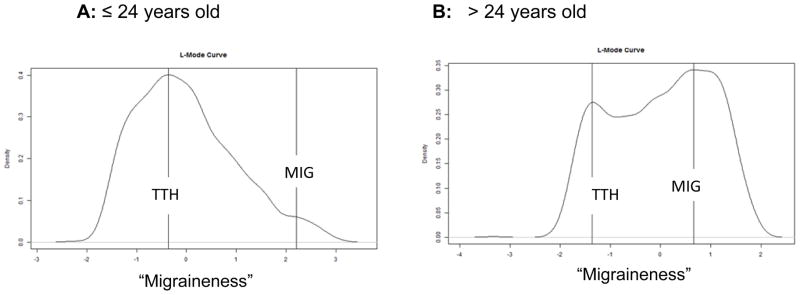
An L-Mode analysis of examining whether there is a categorical or continuous difference between those who have tension-type headache and those who have migraine headache by age groups. To isolate the effect of age, the entire sample was divided into those ≤ 24 years/old versus those > 24 years/old. Those individuals with Tension-Type Headache (TTH) are plotted in relation to those with Migraine (MIG).
Discussion
To our knowledge, this is the first study examining headache nosology using these taxometric methods. In general, our findings refute the notion that migraine and TTH represent discrete entities that differ by type rather than degree. Diagnostic distinction between these two common headache types was not readily apparent in individuals a) with chronic headaches and b) of young age. Among these specific subgroups of headache sufferers, a dimensional classification was most appropriate.
Several prior studies have found results similar to ours regarding difficulty distinguishing headache types among these particular subgroups. As migraine “chronification” occurs, protypical migraine features (i.e., nausea, photophobia, and phonophobia) can overlap with TTH symptoms as the latter become more prominent, convoluting the diagnostic process [5]. Given the well-established relation between headache frequency and medication overuse, migraineurs who overuse medications also commonly experience a progressive reduction in migraine-associated features and develop headaches that more closely resemble TTH [5,29]. Rokicki and colleagues [43] found a mixed presentation of TTH and migraine symptoms among a college-aged sample, and others have shown a similar mixed pattern of headache symptoms in children and adolescents, with unilateral distribution becoming more prominent with age [22,53].
Clinically then, the extent to which the current ICHD diagnostic system has utility in young or frequent headache sufferers, including those at risk for overusing acute medications, is limited. This conclusion is underscored by data from large-scale studies indicating that roughly 40% of migraine cases go unrecognized or misdiagnosed by physicians [9,25,28], and many individuals meet criteria for both migraine and TTH [2,9]. Among children and adolescents, 20–25% transition from migraine to TTH or from TTH to migraine within a matter of years [24], thus supporting the continuum theory of headaches in this subgroup of individuals. Not only are mixed presentations and diagnostic shifts common with younger age, but otherwise prototypical migraine attacks of less than 4 hours duration are often reported [38]. In light of the present data and findings from related studies, the application of strict diagnostic nosology to younger patients (below the age of 25) and those with frequent or refractory headache conditions (e.g., medication overuse headache, patients who present to headache specialty clinics) may have low clinical yield. For patients with “chronic” diagnostic subforms, attending to a distinction in attack severity is likely to be of greater clinical value than distinguishing precisely between CM and CTTH, insofar as the prescription of acute agents can be matched to the nature of the attacks.
Although thought provoking, our study is limited by the nature of the data used to examine the hypotheses. We analyzed two large datasets from previously published studies, both of which were collected through convenience sampling. As such, the sample is not expected to be entirely representative of the underlying population, particularly given that older individuals were more strongly represented among those seeking treatment. We identified several different associations in our data that could be due entirely to the available sample (i.e., the association of age with increased severity of headache), and not reflective of any underlying population effect. In that regard, we endeavored to sample a diverse group of individual sufferers from various settings (e.g., headache specialty clinics, community samples, undergraduate classrooms) who experienced both mild and severe headaches. The methods we use rely heavily on the covariance mixture theorem [44] and, as such, the general pattern of experienced headache symptoms is of primary interest, rather than the proportion of individuals who experience each symptom. Stated differently, we rely on the covariances between the individual symptoms and not exclusively on their base rate in the sample. Nevertheless, the extent to which this analysis reflects individuals within the larger population is unknown.
Our analysis is also limited by the nature of the available data. Taxometric methods are usually undertaken on predictors that have scale properties (i.e., interval or ratio data that are known to be related to the diagnosis under study). Headache diagnosis is not based on these scales (see Table 1) but instead on a series of typically dichotomous symptoms (present vs. absent) that are difficult to use in such an analysis. For this reason, MAMBAC and MAXEIG were not used for confirmatory analyses because these methods largely rely on measurements that have scale properties (i.e., continuous data on interval or ratio scales). We could have incorporated headache-related disability scales as others have attempted [26], but this approach is problematic given that disability levels are only weakly associated with headache diagnosis. A final potential limitation resides with our use of subgroup analyses as a function of various sample characteristics (e.g., headache frequency, age). Subgroup analyses involving multiple cuts of the same data can yield results that are less likely to be replicated [44], partly through having to re-estimate a unique latent dimension for each subgroup. Because of this limitation, it is also difficult to compare L-mode plots across subgroups given that the base rates of the symptoms can differ across these subgroups (i.e., the heights of the modes can and do change), and any differences in the latent dimensional scaling can be due at least part to sampling error. Despite these weaknesses, we opted to perform these analyses given a strong theoretical rationale existed to support a priori hypotheses about these various factors. It would also have been interesting to examine independent combinations of our subgroups (e.g., young episodic sufferers versus older chronic sufferers), but the limited sample sizes precluded this. A final limitation is the exclusive use of only one headache type in the analysis. In cases where individuals experience more than one headache type, the recorded symptom presentation might be less reliable as individuals are forced to make distinctions between their various headache types. In light of our findings and limitations, future taxometric studies should be undertaken that utilize more representative sampling strategies and thus include a higher proportion of treatment-seeking patients.
Acknowledgments
Study funding: Research reported in this publication was supported by the National Institute of Neurological Disorders and Stroke of the National Institutes of Health under award number 1R01NS06525701.
Footnotes
Conflicts of interest:
D. P. Turner: Ms. Turner receives unrestricted grant funding from Merck, Inc.
T.A. Smitherman: Dr. Smitherman receives unrestricted grant funding from Merck, Inc.
A.K. Black: Ms. Black reports no disclosures.
D. B. Penzien: Dr. Penzien receives unrestricted grant funding from Merck, Inc.
J. A. H. Porter: Dr. Porter is a member of the speaker’s bureau for Novartis, UCB Pharma, Teva, and Impax and receives research funding from Roche.
K. R. Lofland: Dr. Lofland reports no disclosures.
T. T. Houle: Dr. Houle receives unrestricted grant funding from Merck, Inc. and is a consultant for Allergan and Depomed.
Contributor Information
Todd A. Smitherman, Email: tasmithe@olemiss.edu.
Anna Katherine Black, Email: akblack@go.olemiss.edu.
Donald B. Penzien, Email: dpenzien@wakehealth.edu.
John A. H. Porter, Email: john.porter@cornerstonehealthcare.com.
Kenneth R. Lofland, Email: Lofland1@aol.com.
Timothy T. Houle, Email: thoule@wakehealth.edu.
References
- 1.Ad Hoc Committee on Classification of Headache. Classification of headache. JAMA. 1962;179:127–128. [Google Scholar]
- 2.Aeseth K, Grande RB, Lundqvist C, Russell MB. Interrelation of chronic tension-type headache with and without medication overuse and migraine in the general population: the Akershus study of chronic headache. Cephalalgia. 2008;29:331–337. doi: 10.1111/j.1468-2982.2008.01729.x. [DOI] [PubMed] [Google Scholar]
- 3.Andrew ME, Penzien DB, Rains JC, Knowlton GE, McAnulty RD. Development of a computer application for headache diagnosis: the Headache Diagnostic System. Int J Biomed Comput. 1992;31:17–24. doi: 10.1016/0020-7101(92)90050-3. [DOI] [PubMed] [Google Scholar]
- 4.Bakal DA, Demjen S, Kaganov J. The continuous nature of headache susceptibility. Soc Sci Med. 1984;19:1305–1311. doi: 10.1016/0277-9536(84)90017-0. [DOI] [PubMed] [Google Scholar]
- 5.Blumenfeld A, Schim J, Brower J. Pure tension-type headache versus tension-type headache in the migraineur. Curr Pain Headache Rep. 2010;14:465–469. doi: 10.1007/s11916-010-0147-1. [DOI] [PubMed] [Google Scholar]
- 6.Bruehl S, Lofland KR, Semenchuk EM, Rokicki LA, Penzien DB. Use of cluster analysis to validate IHS diagnostic criteria for migraine and tension-type headache. Headache. 1999;39:181–189. doi: 10.1046/j.1526-4610.1999.3903181.x. [DOI] [PubMed] [Google Scholar]
- 7.Bulut S, Berilgen MS, Baran A, Tekatas A, Atmaca M, Mungen B. Venlafaxine versus amitriptyline in the prophylactic treatment of migraine: randomized, double-blind, crossover study. Clin Neurol Neurosurg. 2004;107:44–48. doi: 10.1016/j.clineuro.2004.03.004. [DOI] [PubMed] [Google Scholar]
- 8.Cassidy EM, Tomkins E, Hardiman O, O’Keane V. Factors associated with burden of primary headache in a specialty clinic. Headache. 2003;43:638–644. doi: 10.1046/j.1526-4610.2003.03106.x. [DOI] [PubMed] [Google Scholar]
- 9.Diamond ML. The role of concomitant headache types and non-headache co-morbidities in the underdiagnosis of migraine. Neurology. 2002;58(Suppl 6):S3–S9. doi: 10.1212/wnl.58.9_suppl_6.s3. [DOI] [PubMed] [Google Scholar]
- 10.Featherstone HJ. Migraine and muscle contraction headaches: a continuum. Headache. 1985;25:194–198. doi: 10.1111/j.1526-4610.1985.hed2504194.x. [DOI] [PubMed] [Google Scholar]
- 11.Filatova E, Latysheva N, Kurenkov A. Evidence of persistent central sensitization in chronic headaches: a multi-method study. J Headache Pain. 2008;9:295–300. doi: 10.1007/s10194-008-0061-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Gibb BE, Alloy LB, Abramson LY, Beevers CG, Miller IW. Cognitive vulnerability to depression: a taxometric analysis. J Abnorm Psychol. 2004;113:81–89. doi: 10.1037/0021-843X.113.1.81. [DOI] [PubMed] [Google Scholar]
- 13.Hankin BL, Fraley RC, Lahey BB, Waldman ID. Is depression best viewed as a continuum or discrete category? A taxometric analysis of childhood and adolescent depression in a population-based sample. J Abnorm Psychol. 2005;114:96–110. doi: 10.1037/0021-843X.114.1.96. [DOI] [PubMed] [Google Scholar]
- 14.Headache Classification Committee of the International Headache Society. Classification and diagnostic criteria for headache disorders, cranial neuralgias and facial pain. Cephalalgia. 1988;8:1–96. [PubMed] [Google Scholar]
- 15.Headache Classification Committee of the International Headache Society. The International Classification of Headache Disorders, 3rd edition (beta version) Cephalalgia. 2013;33:629–808. doi: 10.1177/0333102413485658. [DOI] [PubMed] [Google Scholar]
- 16.Headache Classification Subcommittee of the International Headache Society. The International Classification of Headache Disorders. Cephalalgia. (2) 2004;24(Suppl 1):S1–S151. doi: 10.1111/j.1468-2982.2003.00824.x. [DOI] [PubMed] [Google Scholar]
- 17.Holroyd KA, Drew JB. Behavioral approaches to the treatment of migraine. Semin Neurol. 2006;26:199–207. doi: 10.1055/s-2006-939920. [DOI] [PubMed] [Google Scholar]
- 18.Holroyd KA, O’Donnell FJ, Stensland M, Lipchik GL, Cordingley GE, Carlson BW. Management of chronic tension-type headache with tricyclic antidepressant medication, stress management therapy, and their combination: a randomized controlled trial. JAMA. 2001;285:2208–2215. doi: 10.1001/jama.285.17.2208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Holroyd KA, Stensland M, Lipchik GL, Hill KR, O’Donnell FR, Cordingley G. Psychosocial correlates and impact of chronic tension-type headaches. Headache. 2000;40:3–16. doi: 10.1046/j.1526-4610.2000.00001.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Iversen HK, Langemark M, Andersson PG, Hansen PE, Olesen J. Clinical characteristics of migraine and episodic tension-type headache in relation to old and new diagnostic criteria. Headache. 1990;30:514–519. doi: 10.1111/j.1526-4610.1990.hed3008514.x. [DOI] [PubMed] [Google Scholar]
- 21.Kaniecki RB, Totten J. Cervicalgia in migraine: prevalence, clinical characteristics, and response to treatment. Cephalalgia. 2001;21:296. [Google Scholar]
- 22.Karli N, Akgoz S, Zarifoglu M, Akis N, Erer S. Clinical characteristics of tension-type headache and migraine in adolescents: a student-based study. Headache. 2006;46:399–412. doi: 10.1111/j.1526-4610.2006.00372.x. [DOI] [PubMed] [Google Scholar]
- 23.Kelman L. The triggers or precipitants of the acute migraine attack. Cephalalgia. 2007;27:394–402. doi: 10.1111/j.1468-2982.2007.01303.x. [DOI] [PubMed] [Google Scholar]
- 24.Kienbacher C, Wöber C, Zesch HE, Hafferi-Gattermayer A, Posch M, Karwautz A, Zormann A, Berger G, Zebenholzer K, Konrad A, Wöber-Bingol C. Clinical features, classification and prognosis of migraine and tension-type headache in children and adolescents: a long-term follow-up study. Cephalalgia. 2006;26:820–830. doi: 10.1111/j.1468-2982.2006.01108.x. [DOI] [PubMed] [Google Scholar]
- 25.Lipton RB, Diamond S, Reed M, Stewart WF. Migraine diagnosis and treatment: results from the American Migraine Study II. Headache. 2001;41:638–645. doi: 10.1046/j.1526-4610.2001.041007638.x. [DOI] [PubMed] [Google Scholar]
- 26.Lipton RB, Serrano D, Pavlovic JM, Manack AN, Reed ML, Turkel CC, Buse DC. Improving the classification of migraine subtypes: an empirical approach based on factor mixture models in the American Migraine Prevalance and Prevention (AMPP) study. Headache. 2014;54:830–849. doi: 10.1111/head.12332. [DOI] [PubMed] [Google Scholar]
- 27.Lipton RB, Stewart WF, Cady R, Hall C, O’Quinn S, Kuhn T, Gutterman D. 2000 Wolfe Award. Sumatriptan for the range of headaches in migraine sufferers: results of the Spectrum Study. Headache. 2000;40:783–791. doi: 10.1046/j.1526-4610.2000.00143.x. [DOI] [PubMed] [Google Scholar]
- 28.Lipton RB, Stewart WF, Simon D. Medical consultation for migraine: results from the American Migraine Study. Headache. 1998;38:87–96. doi: 10.1046/j.1526-4610.1998.3802087.x. [DOI] [PubMed] [Google Scholar]
- 29.Manzoni GC, Torelli P. Chronic migraine and chronic tension-type headache: are they the same or different? Neurol Sci. 2009;30(Suppl 1):81–84. doi: 10.1007/s10072-009-0078-y. [DOI] [PubMed] [Google Scholar]
- 30.Marcus DM. Migraine and tension-type headaches: the questionable validity of current classification systems. Clin J Pain. 1992;8:28–36. [PubMed] [Google Scholar]
- 31.Martin VT, Penzien DB, Houle TT, Andrew ME, Lofland KR. The predictive value of abbreviated migraine diagnostic criteria. Headache. 2005;45:1102–1112. doi: 10.1111/j.1526-4610.2005.00234.x. [DOI] [PubMed] [Google Scholar]
- 32.Meehl PE, Yonce LJ. Taxometric analysis: I. Detecting taxonicity with two quantitative indicators using means above and below a sliding cut (MAMBAC procedure) Psychol Rep. 1994;74:1059–1274. [Google Scholar]
- 33.Nash JM, Thebarge RW. Understanding psychological stress, its biological processes, and impact on primary headache. Headache. 2006;46:1377–1386. doi: 10.1111/j.1526-4610.2006.00580.x. [DOI] [PubMed] [Google Scholar]
- 34.Nyholt DR, Gillespie NG, Heath AC, Merikangas KR, Duffy DL, Martin NG. Latent class and genetic analysis does not support migraine with aura and migraine without aura as separate entities. Genet Epidemiol. 2004;26:231–244. doi: 10.1002/gepi.10311. [DOI] [PubMed] [Google Scholar]
- 35.Penzien DB, Rains JC, Lipchik GL, Creer TL. Behavioral interventions for tension-type headache: Overview of current therapies and recommendation for a self-management model for chronic headache. Current Pain & Headache Reports. 2004;8:489–499. doi: 10.1007/s11916-004-0072-2. [DOI] [PubMed] [Google Scholar]
- 36.Prince PB, Rapoport AM, Sheftell FD, Tepper SJ, Bigal ME. The effect of weather on headache. Headache. 2004;44:596–602. doi: 10.1111/j.1526-4610.2004.446008.x. [DOI] [PubMed] [Google Scholar]
- 37.Radat F, Swendsen J. Psychiatric comorbidity in migraine: A review. Cephalalgia. 2005;25:165–178. doi: 10.1111/j.1468-2982.2004.00839.x. [DOI] [PubMed] [Google Scholar]
- 38.Rains JC, Penzien DB, Lipchik GL, Ramadan NM. Diagnosis of migraine: Empirical analysis of a large clinical sample of atypical migraine (IHS 1. 7) patients and proposed revision of the IHS criteria. Cephalalgia. 2001;21:584–595. doi: 10.1046/j.1468-2982.2001.00210.x. [DOI] [PubMed] [Google Scholar]
- 39.Rains JC, Penzien DB, McCrory DC, Gray RN. Behavioral headache treatment: History, review of the empirical literature, and methodological critique. Headache. 2005;45:S91–S108. doi: 10.1111/j.1526-4610.2005.4502003.x. [DOI] [PubMed] [Google Scholar]
- 40.Rasmussen BK, Jensen R, Olesen J. A population-based analysis of the diagnostic criteria of the International Headache Society. Cephalalgia. 1991;11:129–134. doi: 10.1046/j.1468-2982.1991.1103129.x. [DOI] [PubMed] [Google Scholar]
- 41.Rasmussen BK, Jensen R, Schroll M, Olesen J. Interrelations between migraine and tension-type headache in the general population. Arch Neurol. 1992;49:914–918. doi: 10.1001/archneur.1992.00530330036012. [DOI] [PubMed] [Google Scholar]
- 42.Rokicki LA, Holroyd KA, France CR, Lipchik GL, France JL, Kvaal SA. Change mechanisms associated with combined relaxation/EMG biofeedback training for chronic tension headache. Appl Psychophysiol Biofeedback. 1997;22:21–41. doi: 10.1023/a:1026285608842. [DOI] [PubMed] [Google Scholar]
- 43.Rokicki LA, Semenchuk EM, Bruehl S, Lofland KR, Houle TT. An examination of the validity of the HIS classification system for migraine and tension-type headache in the college student population. Headache. 1999:39-720–727. doi: 10.1046/j.1526-4610.1999.3910720.x. [DOI] [PubMed] [Google Scholar]
- 44.Ruscio J. Taxometric analysis: an empirically grounded approach to implementing the method. Criminal Justice and Behavior. 2007;34:1588–1622. [Google Scholar]
- 45.Ruscio J, Ruscio AM, Carney LM. Performing Taxometric Analysis to Distinguish Categorical and Dimensional Variables. J Exp Psychopathol. 2011;2:170–196. doi: 10.5127/jep.010910. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Ruscio J, Ruscio AM, Meron M. Applying the bootstrap to taxometric analysis: generating empirical sampling distributions to help interpret results. Multivar Behav Res. 2007;42:349–386. doi: 10.1080/00273170701360795. [DOI] [PubMed] [Google Scholar]
- 47.Saunders K, Merikangas K, Low NCP, Von Korff M, Kessler RC. Impact of comorbidity on headache-related disability. Neurology. 2008;70:538–547. doi: 10.1212/01.wnl.0000297192.84581.21. [DOI] [PubMed] [Google Scholar]
- 48.Schade AJ. Quantitative assessment of the tension-type headache and migraine severity continuum. Headache. 1997;37:646–653. doi: 10.1046/j.1526-4610.1997.3710646.x. [DOI] [PubMed] [Google Scholar]
- 49.Silberstein SD, Holland S, Freitag F, Dodick DW, Argoff C, Ashman E. Evidence-based guideline update: pharmacologic treatment for episodic migraine prevention. Neurology. 2012;78:1337–1345. doi: 10.1212/WNL.0b013e3182535d20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Smitherman TA, Kolivas ED. Trauma exposure versus posttraumatic stress disorder: relative associations with migraine. Headache. 2013;53:775–786. doi: 10.1111/head.12063. [DOI] [PubMed] [Google Scholar]
- 51.Smitherman TA, Walters AB, Maizels M, Penzien DB. The use of antidepressants for headache prophylaxis. CNS Neuroscience & Therapeutics. 2011;17:462–469. doi: 10.1111/j.1755-5949.2010.00170.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Spierings ELH, Ranke AH, Honkoop PC. Precipitating and aggravating factors of migraine versus tension-type headache. Headache. 2001;41:554–558. doi: 10.1046/j.1526-4610.2001.041006554.x. [DOI] [PubMed] [Google Scholar]
- 53.Turkdogan D, Cagirici S, Soylemez D, Sur H, Bilge C, Turk U. Characteristic and overlapping features of migraine and tension-type headache. Headache. 2006;46:461–468. doi: 10.1111/j.1526-4610.2006.00377.x. [DOI] [PubMed] [Google Scholar]
- 54.Waller NG, Meehl PE. Multivariate taxometric procedures: distinguishing types from continua. Thousand Oaks, CA: SAGE Publications, Inc; 1998. [Google Scholar]
- 55.Walters GD. Dementia: continuum or distinct entity? Psychol Aging. 2010;25:534–544. doi: 10.1037/a0018167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Waters WE. The epidemiological enigma of migraine. Int J Epidemiol. 1973;2:189–194. doi: 10.1093/ije/2.2.189. [DOI] [PubMed] [Google Scholar]
- 57.Wöber C, Wöber-Bingöl C. Triggers of migraine and tension-type headache. Handbook of Clinical Neurology. 2010;97:161–172. doi: 10.1016/S0072-9752(10)97012-7. [DOI] [PubMed] [Google Scholar]