Abstract
Objective
To determine the impact of patient weight on frequency of surgical staging lymphadenectomy and pelvic radiation. Adverse effects, disease relapse, and survival outcomes were investigated.
Study Design
Records of 766 women undergoing surgery for presumed corpus-confined endometrial cancer were reviewed. Body mass index (BMI) was calculated categorizing women as obese (BMI ≥ 30) or nonobese (BMI < 30). Radiation-related toxicity was retrospectively scored. Median duration of follow-up was 38 months. Chi-square, logistic regression, correlation, Kaplan-Meier and Cox multivariate proportional hazards were used for analysis.
Results
Lymphadenectomy was completed as often in nonobese as obese women (p=0.24). Adjuvant pelvic radiation treatment was administered more often in nonobese women (p=0.01). Among 681 women with endometrioid histopathology, four-year cancer-related survival in obese women was 10 percent higher than all cause mortality, compared to 6 percent in nonobese women.
Conclusion
Obesity was not a barrier to lymphadenectomy, but did influence adjuvant pelvic radiation use.
Keywords: obesity, endometrial cancer, lymphadenectomy, radiation
INTRODUCTION
Obesity directly influences endometrial carcinoma incidence with nearly 40%1 of the 40,000 newly diagnosed cases annually related to excess body weight. As compared to matched controls, women whose weight is between 9 and 22 kilograms above their ideal body weight have a three-fold increased risk of developing endometrial carcinoma.2 Risk increases to nine-fold for weight more than 22 kilograms above ideal body weight.2 Obesity also influences completeness of surgical lymph node staging during surgical laparotomy3,4 and on radiation-related adverse sequelae.5,6
Despite recommendations for comprehensive surgical staging by gynecologic oncologists,7,8 obese women may not undergo surgical staging routinely. Comprehensive staging is difficult and may be hazardous in obese women where adequate exposure of the uterus and pelvic and para-aortic lymph nodes are suboptimal. Operative time, blood loss, and wound breakdown are more common in obese women.3,4 In addition, obese women are more likely to have confounding medical co-morbidities that may preclude adequate staging.3,4 However, despite possible increased surgical morbidity in obese women, comprehensive staging demonstrating that no disease exists outside the uterus may limit use of potentially morbid adjuvant radiation. In some centers, motivation to lessen radiation-related toxicity after surgery has led to the controversial practice of more stringent pelvic radiation use, favoring intravaginal brachytherapy alone to lessen pelvic relapse rate. In order to address this important clinical issue, a more clear understanding of the association between patient weight, lymphadenectomy, and adjuvant radiation use is needed.
The purpose of our analysis was to determine the relative influence of patient weight on frequency of surgical staging lymphadenectomy and pelvic radiation use. Radiation-related adverse effects, disease relapse, and survival outcomes as related to obesity were also investigated.
MATERIAL AND METHODS
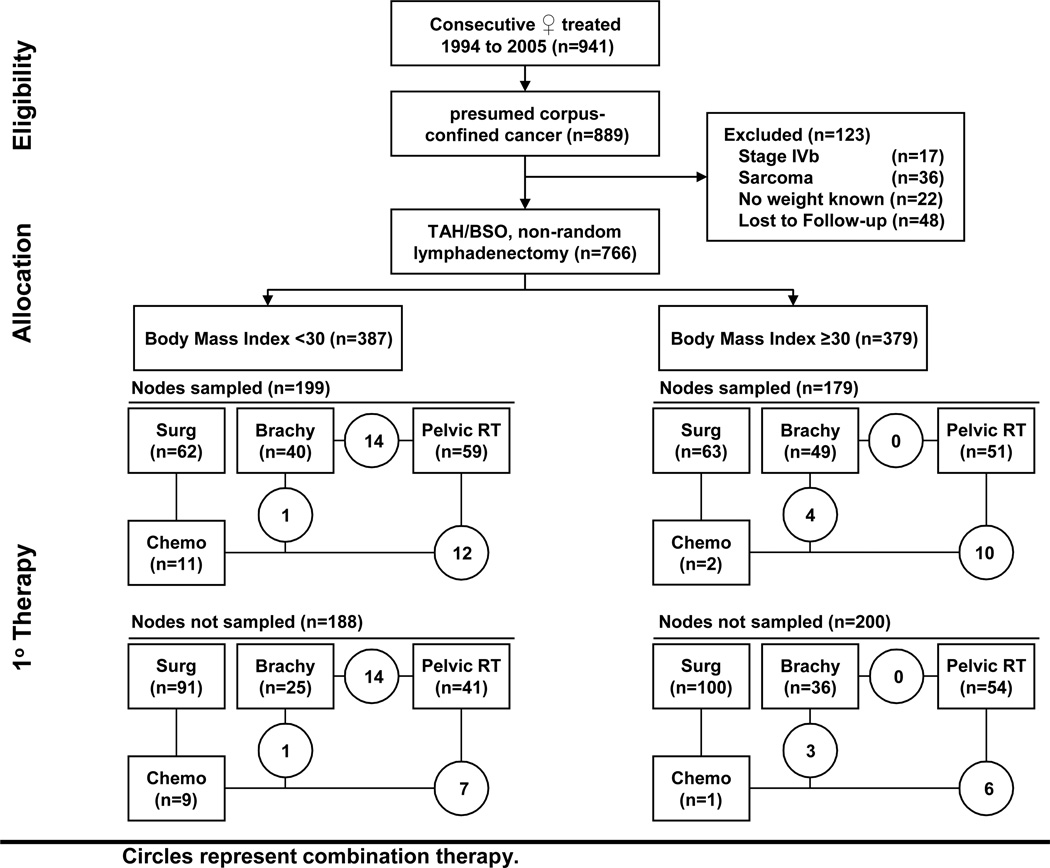
Women were included in our retrospective study if they had clinically-suspected corpus-confined endometrial carcinoma prior to surgical laparotomy and post-surgical follow-up data (Figure 1). Women found to have stage IVB (TanyNanyM1) endometrial cancer, sarcoma within the pathological specimen, or without a recorded hospital admission or weight were excluded. Comparisons were conducted among women whose body mass index (BMI) classified them as nonobese (<30) or obese (≥ 30) as advocated by the Centers for Disease Control.9 BMI (kg/m2) was calculated using admission or office charted height and weight.10 Institutional review board approval was obtained prior to our review.
Figure 1.
Flow diagram of study enrollment and treatment
Surgery
Between March 1994 and March 2005, 766 consecutive women underwent hysterectomy and bilateral salpingo-oophorectomy either at University of Turin (Turin, Italy, n=306) or University Hospitals Case Medical Center (UHC, Cleveland, Ohio, USA, n=460). Surgical staging retroperitoneal lymphadenectomy was performed non-randomly in 378 (49%) women, with 278 (74%) performed at UHC (Table 1). Fellowship-trained gynecologic oncologists (UHC) or experienced, specialized gynecologic surgeons (Turin) performed surgical staging lymphadenectomies according to institutional standards. Histopathology, grade, myometrial invasion, and lymphvascular invasion were assessed at each cancer center independently and abstracted from hospital records.
TABLE 1.
Patient Characteristics.
| Parameters | Body Mass Index < 30 (N = 387) (n, %) |
Body Mass Index ≥ 30 (N = 379) (n, %) |
p Value * |
|---|---|---|---|
| Cancer Center | |||
| University of Turin, Italy | 176 (46) | 130 (34) | 0.002 |
| University Hospitals of Cleveland, USA | 211 (55) | 249 (66) | |
| Age at presentation | |||
| < 60 years | 111 (29) | 125 (33) | 0.21 |
| ≥ 60 years | 276 (71) | 254 (67) | |
| Race | |||
| Caucasian | 373 (96) | 347 (92) | 0.001 |
| African American | 14 (4) | 32 (8) | |
| Staging Lymphadenectomy | |||
| Yes | 199 (51) | 179 (47) | 0.24 |
| No | 188 (49) | 200 (53) | |
| Final surgicopathologic stage | |||
| IA | 36 (9) | 29 (8) | <0.001 |
| IB | 139 (36) | 189 (50) | |
| IC | 72 (19) | 80 (21) | |
| IIA/B | 63 (16) | 44 (12) | |
| IIIA/B | 48 (12) | 16 (4) | |
| IIIC | 24 (6) | 17 (4) | |
| IVA | 5 (1) | 4 (1) |
Radiation Therapy
Indications for pelvic radiation were similar at both cancer centers and included high-risk histopathological factors (moderate to poor tumor grade, outer-half myometrial invasion, or presence of lymphvascular invasion). Parallel-opposed anteroposterior (AP) with or without lateral pelvic external-beam treatment portals were used. AP treatment fields encompassed the L4–L5 disk interspace superiorly to the ischial tuberosities inferiorly with the lateral margin one-centimeter greater than the widest pelvic diameter. If used, lateral treatment fields enclosed the anterior pubic symphysis, posterior one-half of the sacrum, and matched to the superior and inferior AP field borders. Median dose was 50.4 Gy (range: 45 to 50.4 Gy) delivered by 6-MV to 18-MV photons.
Intravaginal brachytherapy recommendations were not uniform over the retrospective study period. Intravaginal brachytherapy was performed non-randomly in 187 (24%) women, with 184 (98%) performed at UHC. A 2.5 to 3.5 cm cylindrical afterloaded applicator was placed to the proximal vagina in three weekly treatments. Dose was prescribed to a depth of 0.5cm from the vaginal surface, treating at least the proximal 4cm of the vagina with attention to normal tissue tolerance of the underlying urethra, bladder, and rectum. A dose per fraction for brachytherapy alone was 7 Gy (total dose 21 Gy). If brachytherapy was done after pelvic radiation, a dose of 5 Gy per fraction was used (total dose 15 Gy).
Follow-up
Assessments for acute toxicity and physical examinations were completed four to nine weeks after completing surgery and radiation. Follow-up medical history and physical examination were conducted generally every three months for the first two years, semiannually for two years, then annually thereafter. Diagnostic images were obtained to assess for relapse as clinically indicated.
Statistical Analysis
Women were grouped according to BMI (<30, ≥ 30) and lymphadenectomy (sampled/not sampled). If lymphadenectomy was attempted and nodal tissue retrieved, it was scored as a lymphadenectomy regardless of lymph node yield. Our study achieves 80 percent power at a significance level of 0.05 to detect a difference in outcomes of 10 percent or greater between BMI and lymphadenectomy groups. Primary study endpoints were frequency of lymphadenectomy and number of women undergoing pelvic radiation as related to BMI. Fisher’s exact tests and binary logistic regression analyses (p < 0.05) were computed for categorical variables and dichotomous outcomes.
Radiation-related gastrointestinal, genitourinary, and cutaneous toxicities were retrospectively scored only in patients treated at UHC using Common Terminology Criteria for Adverse Events, version 3.0. Association of BMI with toxicity was evaluated by rank correlation using BMI as a continuous variable. All toxicities (grade 1–4) were included in analyses, although clinically significant toxicities are often only grade 3 or 4.
Tumor relapses at the proximal vagina or confined to the anatomic pelvis were scored as pelvic relapses. Tumor relapses beyond the pelvis were designated as distant relapses. Time-dependent progression-free, endometrial cancer-related, and overall survival were measured from date of definitive surgery. Four-year endpoints were arbitrary, and used to contrast with prior randomized clinical trials.11,12 Progression-free survival was measured to the date of disease progression or death, or to date of last contact for patients alive and progression-free. Endometrial cancer-related survival was measured to the date of death associated with documented endometrial disease progression. Overall survival was measured to the date of death from any cause. Kaplan-Meier estimates with log-rank significance tests (p < 0.05) were determined for time-dependent endpoints with surgical nodal staging or pelvic radiation as dichotomous variables. Factors contributing to tumor recurrence (age, BMI, surgical stage, histopathology, tumor grade, myometrial invasion, or radiation) were included in Cox multivariate proportional hazards analyses. If factors were found to have independent prognostic value (p < 0.05) by multivariate analysis, a hazard ratio (HR) with a 95% confidence interval (95% CI) (p ≤ 0.05) were determined for time-dependent endpoints with BMI as a dichotomous variable (< 30, ≥ 30). Statistics were computed using statistical software (SPSS 12.0, Chicago, IL).
RESULTS
Characteristics of Patients
Mean BMI was 30.8 (SD=8.3) for these 766 women with 38% categorized as obese (BMI 30–39.9) and 12% labeled as morbidly obese (BMI ≥ 40). Characteristics of the two BMI groups are summarized in Table 1. More obese women were treated at UHC (p=0.002). Number of women with occult cervical stromal invasion (stage IIB) or occult adnexal or serosal invasion or malignant pelvic washing cytology (stage IIIA) was higher in the nonobese cohort (Table 1).
There were significant differences in depth of myometrial invasion, cervical invasion, and positive peritoneal cytology among BMI groups (Table 2). Poor prognostic cell types (papillary serous, clear cell, mixed non-sarcoma carcinomas) were higher in nonobese women. Ninety-two percent of obese women had endometrioid histopathology.
TABLE 2.
Tumor Characteristics.
| Parameters | Body Mass Index < 30 (N = 387) (n, %) |
Body Mass Index ≥ 30 (N = 379) (n, %) |
p Value* |
|---|---|---|---|
| Histopathology | |||
| Endometrioid | 333 (86) | 348 (92) | 0.03 |
| Papillary Serous | 32 (8) | 15 (4) | |
| Clear Cell | 8 (2) | 8 (2) | |
| Other (not sarcoma) | 14 (4) | 7 (2) | |
| Tumor grade | |||
| Grade 1 | 100 (26) | 119 (31) | 0.09 |
| Grade 2 or 3 | 287 (74) | 260 (69) | |
| Depth of myometrial invasion | |||
| Inner one-half | 219 (57) | 254 (67) | 0.004 |
| Outer one-half | 168 (43) | 125 (33) | |
| Cervical invasion | |||
| Negative | 286 (74) | 313 (83) | 0.004 |
| Positive | 101 (26) | 66 (17) | |
| Lymphvascular invasion | |||
| Negative | 323 (84) | 319 (84) | 0.85 |
| Positive | 64 (16) | 60 (16) | |
| Lower uterine segment involvement | |||
| Missing | 176 (46) | 130 (34) | 0.22 |
| Negative | 118 (30) | 154 (41) | |
| Positive | 93 (24) | 95 (25) | |
| Peritoneal Cytology | |||
| Missing | 96 (25) | 90 (24) | <0.001 |
| Negative | 249 (64) | 277 (73) | |
| Positive | 42 (11) | 12 (3) |
Treatments and Staging
Surgical staging lymphadenectomy, denoted by at least a lymph node sampling, was performed in 378 (49%) of the 766 women (Table 1). Nodal sampling procedures were performed as commonly among nonobese (< 30) as obese (≥ 30) women (p=0.24). Significantly fewer women underwent nodal sampling at the University of Turin (33% v 60% [UHC], p<0.001). Median total lymph node yield was 13 lymph nodes. Median nodal yield was significantly different among BMI groups (15 [< 30] v 10 [≥ 30], p=0.02) but not among cancer centers (11 [Turin] v 15 [UHC], p=0.12). After nodal staging, nodal metastases were detected in 24 (6%) of 387 nonobese women (BMI<30) and 17 (5%) obese women (BMI ≥ 30). Malignant nodal metastases were positively correlated with outer one-half myometrial invasion (p<0.001) and moderate to poorly-differentiated (Grade 2 or 3) tumor (p=0.02), but not the number of nodes sampled (p=0.95). Among the 681 women with endometrioid histopathology, the positive correlation of outer one-half myometrial invasion (p<0.001) and Grade 2 or 3 tumor (p=0.03) persisted.
Adjuvant pelvic radiation was administered more often in nonobese women (BMI<30; 148/387 [38%]) as compared to obese women (BMI ≥ 30; 112/379 [30%], p=0.01). Pelvic radiation use was similar among women treated at the two cancer centers (38% [Turin] v 31% [UHC], p=0.06). Among 333 women who had a negative staging lymphadenectomy, 120 (36%) received adjuvant pelvic radiation. In this group, more nonobese women received pelvic radiation (75/171 [44%]) as compared to obese women (45/162 [28%], p=0.003). In the 171 nonobese women, adjuvant pelvic radiation was administered more often when outer one-half myometrial invasion (46/76 [61%], p<0.001) or when Grade 2 or 3 tumor (65/137 [47%], p=0.08) were identified. In the 162 obese women, Grade 2 or 3 tumor (42/125 [34%], p=0.002) and outer one-half myometrial invasion (20/56 [36%], p=0.14) were associated with adjuvant radiation treatment.
Multivariate-adjusted analyses showed that lymphadenectomy yield and BMI significantly impacted adjuvant radiation use. For every one-node increase in lymphadenectomy yield, there was a three percent decrease in odds of undergoing pelvic radiation (p=0.02; 95% CI: 0.4% to 5%). The odds of undergoing pelvic radiation decreased five percent for increases in calculated BMI (p=0.002; 95% CI: 2% to 8%).
Radiation Toxicity
Among the 268 women who received pelvic radiation, grade 1 to 4 gastrointestinal, genitourinary, and cutaneous toxicity was correlated with BMI. Increasing BMI was correlated with more gastrointestinal and more cutaneous toxicity (Table 3), but these correlations did not reach statistical significance.
TABLE 3.
Correlation of body mass index with adverse event.
| Adverse Event | Correlation | p value* |
|---|---|---|
| Gastrointestinal | 0.141 | 0.11 |
| Genitourinary | 0.008 | 0.93 |
| Cutaneous | 0.094 | 0.29 |
pearson product-moment correlation
Outcomes
Median follow-up was 38 months, with 25 percent for more than 65 months. As of October 2006, 124 women had recurred and 120 women had died. Nonobese and obese women had similar frequencies of proximal vagina and pelvic relapse (Table 4). After controlling for factors contributing to tumor recurrence, lower uterine segment/cervical invasion was not associated with an increased frequency of pelvic relapse (p=0.91). Among women whose nodes were sampled, distant relapses were more numerous in nonobese women who did not receive pelvic radiation and in obese women who did receive pelvic radiation. Comparing all 766 women, nine more endometrial cancer-related deaths were observed in nonobese women (29/49, 59%), but this did not reach statistical significance (p=0.19, Table 5). For non cancer-related deaths, 46 percent were in nonobese and 53 percent were in obese women. In the 681 women having endometrioid histopathology, 11 more endometrial cancer-related deaths were observed in nonobese women (25/333 [8%, BMI <30] v 14/348 [4%, BMI ≥ 30], p=0.07). In these 681 women, observed deaths from all causes were similar among nonobese and obese women (52/333 [16%, BMI <30] v 51/348 [15%, BMI ≥ 30], p=0.75).
TABLE 4.
Local and systemic relapse by lymphadenectomy.*
| BMI < 30 | BMI ≥ 30 | |||||
|---|---|---|---|---|---|---|
| Sampled n (%) Relapse |
Not Sampled n (%) Relapse |
p Value |
Sampled n (%) Relapse |
Not Sampled n (%) Relapse |
p Value † |
|
| Pelvic Radiation | ||||||
| Vaginal Vault | 6 (7) | 3 (5) | 0.74 | 4 (7) | 2 (4) | 0.68 |
| Within Pelvis (includes vault) | 11 (12) | 7 (16) | 0.62 | 9 (16) | 8 (14) | 0.79 |
| Systemic (outside pelvis) | 10 (11) | 5 (9) | 0.78 | 14 (25) | 3 (5) | 0.01 |
| No Pelvic Radiation | ||||||
| Vaginal Vault | 6 (6) | 15 (12) | 0.17 | 4 (3) | 8 (6) | 0.40 |
| Within Pelvis (includes vault) | 8 (7) | 17 (13) | 0.20 | 5 (4) | 9 (6) | 0.58 |
| Systemic (outside pelvis) | 10 (9) | 2 (2) | 0.01 | 7 (6) | 3 (2) | 0.20 |
All histologies included.
chi-square test for proportions
TABLE 5.
Cause of death by body mass index group.*
| BMI < 30 | BMI ≥ 30 | |||||
|---|---|---|---|---|---|---|
| Cause of Death | Sampled n (%) event |
Not Sampled n (%) event |
p Value |
Sampled n (%) event |
Not Sampled n (%) event |
p Value † |
| Endometrial cancer-related | 20 (51) | 9 (39) | 0.43 | 15 (42) | 5 (23) | 0.17 |
| Other cause | 19 (49) | 14 (61) | 21 (58) | 17 (77) | ||
All histopathological subtypes included.
chi-square test for proportions
Four-year recurrence-free, endometrial cancer-related, and overall survival for BMI, lymphadenectomy, and pelvic radiation groups are reported in Table 6. Among the 378 women in whom nodes were sampled, the difference in recurrence-free survival was 3% (76% BMI<30 v. 79% BMI ≥ 30, p=0.64). The difference in endometrial cancer-related survival was 1% (88% BMI<30 v. 89% BMI ≥ 30, p=0.69). Difference in overall survival was 2% (79% BMI<30 v. 81% BMI ≥ 30, p=0.62). In the 388 women whose nodes were not sampled, differences in recurrence free survival were 7% (78% BMI<30 v. 85% BMI ≥ 30, p=0.03), in endometrial cancer-related survival 4% (93% BMI<30 v. 97% BMI ≥ 30, p=0.22), and in overall survival 1% (86% BMI<30 v. 87% BMI ≥ 30, p=0.65). Multivariate proportional hazard models identified age, surgicopathologic stage, histopathologic tumor grade, BMI, and pelvic radiation as significant covariates for four-year mortality (Table 7). In the subset of 681 women with endometrioid histopathology, there was a trend for significantly improved four-year endometrial cancer-related survival in obese women (95% [95% CI: 92 to 98%] v 90% [BMI < 30, 95% CI: 86 to 94%], p=0.08) while overall survival was similar among BMI groups (85% in obese v 84% in nonobese, p=0.87).
TABLE 6.
Estimated survival rates at four years, according to stratification variables
| Variable | 4-yr recurrence- free survival (%, 95% CI) |
4-yr cancer-related survival (%, 95% CI) |
4-yr overall survival (%, 95% CI) |
|---|---|---|---|
| Body Mass Index < 30 | |||
| Lymph Nodes Sampled (n=199) | |||
| Pelvic Radiation | 71 (61–81) | 86 (78–94) | 76 (66–86) |
| No Pelvic Radiation | 82 (74–90) | 89 (81–97) | 83 (74–92) |
| p-value | 0.07 | 0.28 | 0.11 |
| Lymph Nodes NOT Sampled (n=188) | |||
| Pelvic Radiation | 65 (52–78) | 90 (64–92) | 78 (65–91) |
| No Pelvic Radiation | 84 (76–92) | 98 (95–100) | 88 (82–94) |
| p-value | 0.04 | <0.001 | 0.08 |
| Body Mass Index ≥ 30 | |||
| Lymph Nodes Sampled (n=179) | |||
| Pelvic Radiation | 67 (54–80) | 80 (69–91) | 69 (56–82) |
| No Pelvic Radiation | 86 (78–94) | 94 (88–100) | 86 (78–94) |
| p-value | 0.01 | 0.01 | 0.004 |
| Lymph Nodes NOT Sampled (n=200) | |||
| Pelvic Radiation | 78 (66–90) | 90 (82–100) | 74 (61–87) |
| No Pelvic Radiation | 89 (81–97) | 99 (97–100) | 93 (87–99) |
| p-value | 0.03 | 0.01 | <0.001 |
Kaplan Meier survival analysis
TABLE 7.
Hazard ratio for Mortality at four years
| Variable | Hazard Ratio |
95% confidence interval |
p value* |
|---|---|---|---|
| Age | 1.06 | 1.04 – 1.08 | <0.001 |
| Surgicopathologic Stage | 1.38 | 1.26 – 1.51 | <0.001 |
| Body Mass Index | 1.04 | 1.01 – 1.07 | <0.001 |
| Histopathology | 0.95 | 0.71 – 1.27 | 0.73 |
| Tumor Grade | 0.49 | 0.30 – 0.81 | 0.005 |
| Depth of myometrial invasion | 0.76 | 0.51 – 1.16 | 0.20 |
| Pelvic Radiation | 0.66 | 0.45 – 0.97 | 0.04 |
Cox proportional hazards model
COMMENT
Obesity is associated with elevated endometrial cancer risk secondary to longstanding estrogen stimulation of the endometrium resulting from adipocyte conversion of androstenedione to estrone.13–15 Following the Gynecologic Oncology Group (GOG) 0033 surgicopathologic study, gynecologic oncologists have encouraged comprehensive surgical staging including retroperitoneal lymphadenectomy at the time of laparotomy to assess for extrauterine disease.16,17 However, surgical excision of lymph node-bearing tissue in obese women is often difficult but without excessive surgical morbidity.3,4 Despite elevated morbidity risk, lymphadenectomy remains an integral component of surgical staging as this procedure guides radiation therapy recommendations and treatment.
The prospect that pathological node-negative lymphadenectomy would reduce frequency of pelvic radiation is reasonable as radiation-targeted lymph node bearing tissues are surgically assessed for occult extrauterine disease. It appears that the prognostic significance of surgical nodal sampling has become increasingly important as a guide to treatment recommendations. In one study,18 five-year relapse-free survival for women free of nodal metastases was 90 percent, as compared to 54 percent when nodal metastases were found. In women with adverse prognostic histopathological risk factors (moderate to high tumor grade, deep myometrial invasion, lymphovascular invasion), vaginal or pelvic relapse risk at five years approaches 25 percent. Randomized clinical trials show a significant and substantial reduction in pelvic relapse with radiation as compared to observation.11,12 However, since pelvic irradiation does increase the risk of complications, the controversial suggestion that pelvic radiation can be omitted in favor of local intravaginal brachytherapy has been made. Often, the argument for intravaginal brachytherapy substituting for pelvic radiation is predicated on nodal yield at laparotomy and pathological confirmation of no occult nodal disease. In our study, we observed that for every one-node increase in lymphadenectomy yield, there was a significant three percent decrease in odds of adjuvant pelvic radiation. Moreover, across groups as BMI increased there was a significant five percent decrease in odds of adjuvant pelvic radiation. When controlling for lymphadenectomy yield, women found to have histopathological grade 2 or 3 cancers or deep myometrial invasion still underwent pelvic radiation given the high risk of pelvic relapse. This seems reasonable in that grade 2 or 3 histopathology and outer one-third myometrial invasion confer 25 to 40 percent relapse risk.16,17 Data suggests that women whose tumors are moderate to high grade or invade the outer one-half of the myometrium are at sufficient risk for relapse and likely to benefit from radiation.11, 12, 19 Whether the same radiotherapeutic benefit can be realized with pelvic radiation or intravaginal brachytherapy remains controversial.20
Whether obese women who often have low grade, early stage endometrial cancer need to undergo surgical staging lymphadenectomy remains controversial. While lymphadenectomy may have a therapeutic benefit, this procedure has been associated with longer operative times and more blood loss in obese women.3 And yet, the rate of intraoperative and postoperative complications are similar.3 Advocates for staging lymphadenectomy argue that pelvic nodal staging removing occult nodal metastases seems to have a survival benefit in low- and high-risk groups while retroperitoneal para-aortic lymphadenectomy may also confer a relapse-free survival benefit in high-risk women.21,22 Weight related co-morbidities may be a barrier to safely performing this surgical procedure. In a recent study BMI serving as a surrogate for weight related co-morbidities has been shown to significantly confound overall survival.6
Our findings corroborate the GOG’s findings6 that obese women have superior endometrial cancer-related survival when compared to non-obese women. Among women with endometrioid histopathology, there was a 10 percent higher four-year cancer related (95%) survival in obese women, as compared to overall all-cause mortality (85%). In nonobese women, survival estimates differed by 5 percent. Perhaps disparity in observed attributable mortality in obese women is influenced by overall health status in as much as pathological or treatment-related variables. This postulate is reasonable given that obese women are more likely to have other comorbid conditions and cancers related to obesity. A commonly cited deterrent to pelvic radiation for gynecologic cancer is radiation-related gastrointestinal, genitourinary, and cutaneous toxicity. A recently reported GOG analysis of prospectively collected graded toxicity data concluded that obese women had higher radiation-related cutaneous and lower gastrointestinal toxicity.6 Our findings fail to corroborate these observations. However, our results must be viewed as subject to underreporting in the medical record and to observer bias.
Strengths of our study include a mostly contemporary study population drawn from two independent, international cancer centers. Also, surgical staging lymphadenectomies were performed by fellowship-trained gynecologic oncologists (UHC) or experienced, specialized gynecologic surgeons (Turin). Nearly 50 percent of women in each BMI group underwent lymphadenectomy, allowing an albeit biased retrospective comparison of outcomes based on staging lymphadenectomy.
Weaknesses include patient selection bias resulting from endometrioid histology criterion, non-random assignment of lymphadenectomy and adjuvant radiation. The two institutions differed in regards to obesity and lymphadenectomy rates. UHC had a much higher obesity and lymphadenectomy rate as compared to Turin. In our study, only half of obese and nonobese women underwent surgical staging, as omission of lymphadenectomy or peritoneal cytology was attributable to less surgical staging procedures performed in Turin. Invariable differences in surgical aggressiveness, pathologic processing of nodal tissue, and lack of central histopathological and surgical morbidity review are recognized. Despite a probable selection bias for lymphadenectomy in women deemed pre-operatively at high clinical risk for extrauterine disease, the proportion of women undergoing surgical staging lymphadenectomy were similar among nonobese and obese women. Our median nodal yield of 13 nodes may at first seem low, but is comparable to the 11 node criterion suggested by Cragun et al.23 in their evaluation of selective lymphadenectomy in endometrial cancer. Recommendations for pelvic radiation were uniform at both cancer centers and by treating radiation oncologists, but may not have been equally applied biasing treatment outcomes. Our proportion of women receiving adjuvant pelvic radiation (35%) was higher than other contemporary studies (~20%)7,8 because GOG protocol 009911 and the Post-Operative Radiation Therapy in Endometrial Carcinoma (PORTEC)12 results were unpublished when many women in this retrospective study underwent treatment..
Obesity was not a barrier to lymphadenectomy as similar numbers of procedures were performed in obese and nonobese women. Obese women underwent less adjuvant pelvic radiation most often due to absence of adverse histopathological risk factors. Among women who did receive pelvic radiation, obesity was not associated with increased radiation-related toxicity. We observed a trend for significantly higher endometrial cancer-related but not overall survival in obese women as compared to nonobese women. Whether adjuvant treatments coordinated with an overall health intervention may confer improved clinical survival outcomes remains unsettled. Given the preponderance of available randomized data and that of this study, obesity should be considered in future clinical studies reporting endometrial cancer treatment outcomes.
Acknowledgments
SOURCES OF SUPPORT: Supported in part by a grant (K12 CA076917) to Dr. Kunos from the National Institutes of Health and the CASE Comprehensive Cancer Center.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Presented as a poster at the Society of Gynecologic Oncologists Annual Meeting, March 22–26, 2006, Palm Springs, CA
Each author certifies that he or she has no commercial associations (e.g., consultancies, stock ownership, equity interest, patent/licensing arrangements, etc.) that might pose a conflict of interest in connection with the submitted article.
Each author certifies that his or her institution has approved this retrospective investigation and that all investigations were conducted in conformity with ethical principles of research.
REFERENCES
- 1.Parazzini F, La Vecchia C, Bocciolone L, Franceschi S. The epidemiology of endometrial cancer. Gynecol Oncol. 1991;41:1–16. doi: 10.1016/0090-8258(91)90246-2. [DOI] [PubMed] [Google Scholar]
- 2.Kaaks R, Lukanova A, Kurzer MS. Obesity, endogenous hormones, and endometrial cancer risk: a synthetic review. Cancer Epidemiol, Biomarkers Prev. 2002;11:1531–1543. [PubMed] [Google Scholar]
- 3.Everett E, Tamimi H, Greer B, Swisher E, Paley P, Mandel L, et al. The effect of body mass index on clinical/pathologic features, surgical morbidity, and outcome in patients with endometrial cancer. Gynecol Oncol. 2003;90:150–157. doi: 10.1016/s0090-8258(03)00232-4. [DOI] [PubMed] [Google Scholar]
- 4.Pavelka JC, Ben-Shachar I, Fowler JM, Ramirez NC, Copeland LJ, Eaton LA, et al. Morbid obesity and endometrial cancer: surgical, clinical, and pathologic outcomes in surgically managed patients. Gynecol Oncol. 2004;95:588–592. doi: 10.1016/j.ygyno.2004.07.047. [DOI] [PubMed] [Google Scholar]
- 5.Eifel PJ, Jhingran A, Bodurka DC, Levenback C, Thames H. Correlation of smoking history and other patient characteristics with major complications of pelvic radiation therapy for cervical cancer. J Clin Oncol. 2002;20(17):3651–3657. doi: 10.1200/JCO.2002.10.128. [DOI] [PubMed] [Google Scholar]
- 6.von Gruenigen VE, Tian C, Frasure H, Waggoner S, Keys H, Barakat RR. Treatment effects, disease recurrence, and survival as related to obesity in women with early endometrial carcinoma: a Gynecologic Oncology Group study. Cancer. 2006;107:2786–2791. doi: 10.1002/cncr.22351. [DOI] [PubMed] [Google Scholar]
- 7.Goudge C, Bernhard S, Cloven NG, Morris P. The impact of complete surgical staging on adjuvant treatment decisions in endometrial cancer. Gynecol Oncol. 2004;93:536–539. doi: 10.1016/j.ygyno.2004.02.020. [DOI] [PubMed] [Google Scholar]
- 8.Roland PY, Kelly FJ, Kulwicki CY, Blitzer P, Curcio M, Orr JW., Jr The benefits of a gynecologic oncologist: a pattern of care study for endometrial cancer treatment. Gynecol Oncol. 2004;93:125–130. doi: 10.1016/j.ygyno.2003.12.018. [DOI] [PubMed] [Google Scholar]
- 9.Garrow J, Webster J. Quetelet's index (W/H2) as a measure of fatness. Int J Obes. 1985;9:147–153. [PubMed] [Google Scholar]
- 10.Executive summary of the clinical guidelines on the identification, evaluation, and treatment of overweight and obesity in adults. Arch Intern Med. 1998;158:1855–1867. doi: 10.1001/archinte.158.17.1855. [DOI] [PubMed] [Google Scholar]
- 11.Keys HM, Roberts JA, Brunetto VL, Zaino RJ, Spirtos NM, Bloss JD, et al. A phase III trial of surgery with or without adjunctive external pelvic radiation therapy in intermediate risk endometrial adenocarcinoma: a Gynecologic Oncology Group study. Gynecol Oncol. 2004;92:744–751. doi: 10.1016/j.ygyno.2003.11.048. [DOI] [PubMed] [Google Scholar]
- 12.Creutzberg CL, van Putten WL, Koper PC, Lybeert ML, Jobsen JJ, Warlam-Rodenhuis CC, et al. Surgery and postoperative radiotherapy versus surgery alone for patients with stage-1 endometrial carcinoma: multicentre randomised trial. PORTEC Study Group. Post Operative Radiation Therapy in Endometrial Carcinoma. Lancet. 2000;355:1404–1411. doi: 10.1016/s0140-6736(00)02139-5. [DOI] [PubMed] [Google Scholar]
- 13.Grodin JM, Siiteri PK, MacDonald PC. Source of estrogen production in postmenopausal women. J Clin Endocrinol Metab. 1973;36:207–214. doi: 10.1210/jcem-36-2-207. [DOI] [PubMed] [Google Scholar]
- 14.Edman CD, MacDonald PC. Effect of obesity on conversion of plasma androstenedione to estrone in ovulatory and anovulatory young women. Amer J Obstet Gynecol. 1978;130:456–461. doi: 10.1016/0002-9378(78)90288-0. [DOI] [PubMed] [Google Scholar]
- 15.Meseguer A, Puche C, Cabero A. Sex steroid biosynthesis in white adipose tissue. Horm Metab Res. 2002;34:731–736. doi: 10.1055/s-2002-38249. [DOI] [PubMed] [Google Scholar]
- 16.Creasman WT, Morrow CP, Bundy BN, Homesley HD, Graham JE, Heller PB. Surgical pathologic spread patterns of endometrial cancer. a Gynecologic Oncology Group Study. Cancer. 1987;60:2035–2041. doi: 10.1002/1097-0142(19901015)60:8+<2035::aid-cncr2820601515>3.0.co;2-8. [DOI] [PubMed] [Google Scholar]
- 17.Morrow CP, Bundy BN, Kurman RJ, Creasman WT, Heller P, Homesley HD, et al. Relationship between surgical-pathological risk factors and outcome in clinical stage I and II carcinoma of the endometrium: a Gynecologic Oncology Group study. Gynecol Oncol. 1991;40:55–65. doi: 10.1016/0090-8258(91)90086-k. [DOI] [PubMed] [Google Scholar]
- 18.Lurain JR, Rice BL, Rademaker AW, Poggensee LE, Schink JC, Miller DS. Prognostic factors associated with recurrence in clinical stage I adenocarcinoma of the endometrium. Obstet Gynecol. 1991;78:63–69. [PubMed] [Google Scholar]
- 19.Lee CM, Szabo A, Shrieve DC, Macdonald OK, Gaffney DK. Frequency and effect of adjuvant radiation therapy among women with stage I endometrial adenocarcinoma. JAMA. 2006;295:389–397. doi: 10.1001/jama.295.4.389. [DOI] [PubMed] [Google Scholar]
- 20.Small W, Lurain JR. Current management of endometrial cancer: surgical staging and postoperative radiotherapy. Amer J Oncol Rev. 2006;5:165–174. [Google Scholar]
- 21.Kilgore LC, Partridge EE, Alvarez RD, Austin JM, Shingleton HM, Noojin F, 3rd, et al. Adenocarcinoma of the endometrium: survival comparisons of patients with and without pelvic nodal sampling. Gynecol Oncol. 1995;56:29–33. doi: 10.1006/gyno.1995.1005. [DOI] [PubMed] [Google Scholar]
- 22.Mariani A, Webb MJ, Galli L, Podratz KC. Potential therapeutic role of para-aortic lymphadenectomy in node-positive endometrial cancer. Gynecol Oncol. 2000;76:348–356. doi: 10.1006/gyno.1999.5688. [DOI] [PubMed] [Google Scholar]
- 23.Cragun JM, Havrilesky LJ, Calingaert B, Synan I, Secord AA, Soper JT, et al. Retrospective analysis of selective lymphadenectomy in apparent early-stage endometrial cancer. J Clin Oncol. 2005;23:3668–3675. doi: 10.1200/JCO.2005.04.144. [DOI] [PubMed] [Google Scholar]