Abstract
Background:
The presence of hormones in milk and dairy foods was discussed decades ago but rather more concerns attended to that with respect to finding hormones as biomarkers in milk for diseases and pregnancy diagnosis. Moreover, considerable amount of studies demonstrated that existing of hormones in humans and animals milk are essential for infants growing and immunity. During the last couple of years, increasing body of evidence are indicating another property of hormones in dairy products as possible impact on human health including the role of some estrogens and insulin-like growth factor-1 in initiation and provoking of breast, prostate and endometrial tumours.
Methods:
Data was gathered from the published articles in database such as MEDLINE, science direct, Google scholar and web of science. We put no limitation on date of published date. Moreover, our own published and conducted methods and results also are presented. In this review we concentrated on several aspects of presence of hormones in dairy foods with especial emphasize on cow’s milk as a major source of consuming milk for humans especially for children.
Results:
The collected data from other researchers and our own data are indicating that the presence of steroid hormones in dairy products could be counted as an important risk factor for various cancers in humans.
Conclusion:
Our gathered data in this review paper may suggest more sophisticate analytical detection methods for oestrogens determination and also could be considered as a remarkable concern for consumers, producers and public health authorities.
Keywords: Cancer, Dairy foods, Hormones
Introduction
Providing consumer information about the relationship between diet and health over the last decade has been raised and consequently elevated the awareness and demand for functional food ingredients. Milk or dairy products such as cheese, butter, and yoghurt are the most important components of human diet especially in the Western culture and recently in Asia. Cow’s milk is frequently consumed, although there is some other geographical variation in consumption of goat, sheep and camel milk. It is well known apart from dairy foods basic nutritional role many of them contain a number of hormones, which they have capability to mediate specific physiological and pathological functions (1). Moreover, the presence of hormones in dairy products that have the potential to disrupt the physiological function of endocrine systems has raised great concern worldwide (2). Any subtle changes in endocrine function may alter the growth, development, and reproduction in exposed animals and humans.
The most important hormones found in milk and other dairy products by using a variety of analytical methods consist of prolactin, steroids including estrogens, progesterone, corticoids, and androgens. Moreover, the existence of other hormones such as insulin-like growth factor-1 (IGF-1) (3) and local hormones including prostaglandins (PGs) (4, 5), in dairy products has been reported. It has been assumed that most of the hormones are transferred into milk by diffusion. However, evidence is available for active mechanisms like those for progesterone in goats and prolactin in cows (6, 7). PGs level in milk samples could be used as a marker of mastitis in cows (8).
The naturally occurring hormones in dairy foods have biological effects in humans and animals, which are ranging from growth promoting effects that related to sex steroids (9), to carcinogenic properties that associate to some active metabolites of oestrogens and IGF-1 (10). However, when a hormone or its metabolite is evaluated as a potential hazard for humans and animals, a few critical points should be clarified. In first step, determining of accurate amounts of free and bound forms of naturally occurring hormones in dairy foods must be conducted. Secondly, it should be clarified that how percentage of determined hormones are absorbed via gastrointestinal tract and consequently, how percentage of absorbed hormones undergo hepatic biotransformation, which at the end will provide a clear picture of bioavailability for each individual hormones. Performing in parallel some in vivo and in vitro toxicological studies to prove the potential biological effects including beneficial or detrimental effects of proposed hormones at the same levels, which are present in circulation system, will be the final step of this evaluation.
In this review, we focused on:
➢ The level of naturally occurring hormones in dairy products, the key influencing factors including physiologic conditions, diet composition, breed role, veterinary drug utilize are discussed;
➢ The pathologic conditions and any other factors, which influencing the normal values of hormones in milk;
➢ Shortly, the main origin, physiologic functions, and the known metabolism pathway(s) of each hormones of different hormones;
➢ Any implication of hormone assay as a biomarker;
➢ Possible known effects of proposed hormones on human health and
➢ The analytical methods, which are used for analysis of hormones in milk and other dairy products.
Methods
Data were collected from the published articles in database MEDLINE, Science Direct, Google scholar and web of science. We put no limitation on date of published date. Moreover, our own published and conducted methods and results also are presented.
Prolactin
Prolactin (PRL), is a polypeptide hormone, which is found in milk of several species including cows, sheep, goats, saws, rats, and humans (11). In lactation period, PRL is released from the anterior pituitary gland in response to milking stimuli and suckling. Moreover, the couplomimetic stimulus to the cervix during coitus are also classified as physiological stimuli for PRL release (12). The secretion of PRL is not limited to the anterior pituitary gland as some other organs and tissues such as hypothalamus, telencephalon, hippocampus, amygdala, septum, brain stem, and spinal cord, showed capability of PRL synthesis in animal studies (13, 14). Experimental studies demonstrated that subcutaneously injection of PRL in rat resulted in selectively accumulation in milk gland (15). In contrast to other hormones, which are endocytosed and then immediately degraded in lysosomes, PRL is transported into the mammary epithelial cells by transcytosis and consequently released in the milk either in intact or cleaved forms (16). The highest concentration of PRL were found in the milk of cows and women during the first days after parturition (6). Concentration of PRL in composite milk of Holstein × Sim-mental cows in day 2 (colostrum) and week 4 (mature milk) of lactation were found as 120 ± 16 and 15.4 ± 1 μg/L, respectively (17). In cows, there is no significant difference in PRL content of the milk between different milking. In contrast, season dose have a strong influence on PRL concentration, as the highest concentrations of PRL were found during July and the lowest levels obtained during November (6).
The best-established sites of PRL action in mammals include the mammary gland and ovaries, however, PRL binding sites have been identified in other parts of the body such as CNS, pituitary gland, heart, lung, thymus, spleen, liver, pancreas, kidney, adrenal gland, uterus, skeletal muscle and skin (18). The Lactogenesis, regulation of ovarian and testicular functions, contribution in reproductive and parental behaviours, angiogenesis, home-ostasis of the immune system and osmoregulation are the well-known functions of the PRL (19). It seems, for adults of all species, the PRL in consumed milk is more likely hydrolysed to its constituent amino acids before absorption (6). Thus, it is thought at least for adults, existing of high or low levels of PRL in consumed milk do not have biological impact. However, other studies demonstrated that milk PRL is absorbed and exert biologically effects including differentiation and maturation of neonatal neuroendocrine, regulation of the reproductive and immune systems in the neonates (20). Although there is not much available data about the influence of different diet regimen on level of milk-prolactin in cow’s milk, but a few published studies indicating a remarkable influence of diet on hormonal profile including prolactin level, as suggested that a low-caloric diet may suppress PRL release in plasma and eventually in milk of women (21). Moreover, it is indeed demonstrated there were positive correlations between PRL concentration in plasma and increasing saturated fatty acid intake and by contrast, negative relationship between PRL concentration in plasma and vitamin C level of the diet (22). Jonhansson and co-workers have shown that the level of milk prolactin was lower when cows were fed before and after than during milking (23).
Insulin-like Growth Factor- 1 (IGF-1)
IGF-1 is a 70 amino acid-linked polypeptide produced mainly by mammary gland and liver (24), but in general by all tissues and can act as an endocrine as well as paracrine and or autocrine fashion (25). This hormone is exist at higher concentrations in colostrums (103 ± 21ng/mL) than in blood, however, after parturition milk IGF-1 drops below blood levels (17, 26). The physiologic concentration of cows’ milk IGF-1 has been determined using immunoassay method (3). The authors reported that the physiologic levels of milk IGF-1 in cows are ranged from 4 ± 1 ng/ml. The broad area between minimum and maximum concentrations of IGF-1 in caws, milk could be due to differences in body weight, milk composition, diet, management practices, and other environmental factors. Since treatment of lactating cows with bovine somatotropin showed significant increase of milk IGF-1 concentration (27). No significant relationship between IGF-1 level in milk and milk production was reported. However, concentration of milk IGF-1 dramatically dropped during the lactation period as 6.3 ng/ml was detected on days 6–15 and 1.6 ng/ml was measured on days 210. As milk IGF-1 is not destroyed by milk processing especially pasteurisation, thus it will be present in shelf milk, too (3).
Data about the bioavailability of milk IGF-1 in animals and humans is lacking and it is not clear yet, how percentage of IGF-1 in consumed dairy products could be reached through the gastrointestinal tract into central compartment since it is structurally more similar to the insulin, which is rapidly degraded in gut. However, obvious beneficial effects of the milk- and more importantly colostrums IGF-1 in neonates indicates a higher absorption and less degradation of milk IGF-1 in milk-consumer neonates in comparison to the neonates, which are received milk replacer with lack of IGF-1 (28).
The physiological role of IGF-1 on glucose metabolism and growth was demonstrated early in the 1980s (29). IGF-1 like as insulin play a central role in cellular glucose metabolism, amino acid uptake, glycogen synthesis, lipogenesis, and mitogenesis (30). Physiological effects of IGF-1 mediated via binding of IGF-1 to the type-1 IGF receptor, which is a heterotetramer with α-and two β-subunits linked by disulfide bonds. IGF-1 receptor is a member of the tyrosine kinase family, which following binding to agonist compounds (IGF-1), causes autophosphorylation of tyrosine residues in the carboxyl end of the intracellular domain and eventually leads to a cellular response (31). At the same time, the anabolic signals by IGF-1 (or insulin) can promote tumour development by anti-apoptosis effect and also by stimulating cell proliferation (30). During the last years according to the epidemiological evidence accuracy of the hypothesis is promoting, which indicates the risk of the colon, pancreas, endometrium, breast and prostate tumours are associated to the high level of IGF-1, insulin, or both (32).
Prostaglandin’s
Prostaglandins (PGs) including PGE2, PGD2, PGF2, PGI2, and Tromboxan A2 are structurally related bioactive lipid molecules, which are synthesized in cyclooxygenase pathway of arachidonic acid metabolism. Despite the relatively similar structure, PGs are differently produced in various tissues and exert divers’ functions, as well. For example, PGI2 is a major prostaglandin product in gastrointestinal tract and play a crucial role in the cytoprotection of gastric mucosal surface (33). At the same time it is shown that PGI2 dose have protective property in cardiomycytes against oxidative stress, as well (34).
The excretion of PGI2 into goat milk in concentrations ranging from 32 to 99 pg/ml and possible local vasodilatory role of it in the mammary gland has been reported (35). Atroshi and co-workers (1986) demonstrated that the levels of PGE2, PGF2α, and TXB2 in milk were 2–4 times higher than blood plasma in healthy cows. Moreover, the authors found significant positive correlation between cow’s milk PGs concentrations and somatic cell count, as PGE2 concentrations raised from 2.4 in healthy cows, milk to 3.4 ng/ml in milk of cows with mastitis which the somatic cell density rose from < 100 × 103 to > 500 × 103(36). The presence of PGs in commercial milk was studied and 3 ng/ml PGE2 in whole milk, heavy cream and yoghurt could be measured, whereas 2.04 ± 0.18 ng/ml PGE2 was detected in low-fat milk. The same group showed the levels of other PGs such as thromboxan and prostacyclin were less than 500 pg/ml in commercial milk (37). Previous reports determined the concentration of PGF2α in cows, raw milk about 698 ± 27 pg/ml (38). Another study by using radio immune assay method reported the levels of PGF2α and TXB2 in healthy cows, milk 1–3 and 0.5–2 ng/ml, respectively (39). Regardless to the bioavailability of the dairy products PGs following oral consumption, due to protective effects of them in gastric ulcer, beneficial property of them in human diet in normal level positively is considerable. However, due to the rapid metabolic degradation of PGs, it is far unlikely that dairy foods PGs exert considerable systemic biological effects because these compounds are degraded in the liver by oxidation at the site of carbon 15 and producing of 15-ketoprostaglandins, which are biologically inactive metabolites (40). On the other hand, high level of PGs in dairy foods especially in milk could be considered as a marker for bacterial mastitis and other inflammations (39).
Steroid Hormones
Glucocorticoids
Glucocorticoids (GCs) are another group of hormones in dairy foods especially milk, which act synergistically with other hormones in lactogenesis. Endogenous glucocorticoids are mainly synthesised in the adrenal gland and both synthesis and release of them are controlled by the adrenocorticotrophic hormone (ACTH). Among the others, cortisol as the predominant glucocorticoid, is taken up from blood by the mammary glands of goats and cows (41, 42). The normal concentration of total GCs (0.46 to 0.65 ng/ml) has been determined by means of protein binding method in raw cow’s milk and it was found that there is no differences between the level of the GCs in processed and non-processed milk samples (43). Another study by using the same method reported the normal range of corticosteroids in cow’s milk from 8 to 18 ng/ml, which comparing to human (20 to 136 ng/ml), and rat (144 ng/ml) milks corticosteroids content revealed the low levels of cows, milk corticosteroids (44). GCs are located mainly in the non-lipid fraction of the milk (6). Table 1 shows the concentrations of main hormones in cows, milk.
Table 1:
The concentrations of hormones (ng/mL) in cow’s milk
| Hormones | Concentrations | Analytical method | Reference |
|---|---|---|---|
| Prolacin | 15.4 ± 1 | radio immunoassay | (17) |
| IGF-1 | 4 ± 1 | radio immunoassay | (17) |
| PGE2 | 2.4 ± 0.3 | radio immunoassay | (36) |
| PGF2α | 2 ± 0.5 | radio immunoassay | (39) |
| TXB2 | 1 ± 0.5 | radio immunoassay | (39) |
| Corticosteroids | 14 ± 4 | competitive protein binding(CPB) assay | (44) |
| Testosterone | 0.09 ± 0.03 | radio immunoassay | (53) |
| 5α-esteroids | 3 ± 1 | radio immunoassay | (54) |
| Progesterone | 12 ± 2 | radio immunoassay | (64) |
| Esterone | 0.13 | GC-MS | (89) |
| 17β-estradiol | 0.02 | GC-MS | (89) |
| Esteriol | 0.027 ± 0.01 | HPLC | (94) |
It is found that due to widespread utilize of dexamethasone as a synthetic glucorticoid, in veterinary medicine, for treatment of metabolic diseases in ruminants, e.g. ketosis, and of inflammatory diseases in other animals, maximum residue limits in milk and other tissues could be raised (45). Moreover, another study showed that following treatment of the lactating Holstein cows by adrenocorticotropin hormone the milk cortisol level increased four-fold and that increase was due to sustained increase in plasma corisol level (46). At the same time, stressful conditions such as transport, neuronal diseases, diet changes, environmental alteration including temperature changes and hundreds other stress producing factors might elevate the levels of GCs in milk. Despite all the progress in terms of detection and quantification of GCs in milk, there is lack of knowledge about the possible absorption and consequently biological effects of milk GCs in animals and humans.
GCs are comprehensively metabolised (oxidation, reduction, and hydroxylation) in the liver and the conjugated (glucoronidated or sulfatated) forms of the metabolites are excreted mainly via the urine and the bile depending on the species. In ruminants 21 cortisol metabolites were detected in faecal samples using HPLC/mass spectrometry (47). Some of the metabolites (5α-reduced GCs) may be reabsorbed during entrohepatic circulation and these metabolites might exert some biological effects (48).
Since two decades ago a number of different analytical methods have been developed to detect and quantify the concentrations of naturally occurring and synthetic GCs in dairy products particularly in milk. Very early studies for detection and quantification of GCs have been performed using comparative protein binding (CPB) method (43), and radioimmunoassay technique (49). Currently, advanced methods such as ELISA (50), gas chromatography – mass spectrometry (GC-MS) (51) and competitive immunoenzymatic assay (52), are used for determination of GCs in milk. Recently, liquid chromatography- mass spectrometry (LC-MS) techniques have been developed for quantitative determination of synthetic GCs in bovine milk (45).
Androgens
Despite the fact that the main source of androgens and in particular testosterone is the testes but previous studies showed the presence of testosterone (45–150 pg/ml) and other members of androgens family including 5α-androstane-3, 17-dion (1–5 ng/ml) in cows, milk (53, 54). It seems this hormone is produced either in adrenal glands or at least in part, in the mammary gland and indicating a lactogenesis role for 5α-steroids (55). Another study figured out that anderstanedion concentrations of plasma and milk were increased during pregnancy but the rate of elevation in milk was determined as twice as plasma after day 90 (56).
Until now, no more data is available on new analytical techniques, on minimum- biological-effects-producing levels, on diversity of androgens level in different dairy food, and on oral bioavailability of the androgens. However, very early studies postulated that the measured naturally occurring androgens in milk unlikely could posse the biological effects in milk consumers (6).
Progesterone
Progesterone and 20-dihydroprogestrones are largely produced in ovaries and placenta. Progesterone is one of the essential hormones in the whole processes of animals and humans reproduction from ovulation to the maintenance of the pregnancy, development of mammary glands and neurobehavioral roles associated with sexual responsiveness (57, 58). In addition of central role in reproduction system, Progesterone exert some other effects including immunosuppressive effect during pregnancy, increasing myelin-specific protein levels and γ-aminobutric acid (GABA)-induced chloride current (59, 60). Progesterone was detected and measured in milk of different species of animals but for the first time in cows, milk and due to highly non-polarity property it is mostly distributed within the fat fraction of milk (61). Progesterone presence in milk or in a correct term an increase in milk Progesterone concentration has been used as a good diagnostic tool in pregnancy confirming in veterinary medicine (62). The physiologic concentrations of Progesterone in cows, milk at different reproductive status of oestrus, day 21 of cycle, midcycle, and pregnancy were determined as 5.42, 11.36, 8.53, and 11.75 ng/ml, respectively (63). The measurable level of P in dairy foods and commercial milk products by using radioimmunoassay was 1.4, 6, 17, 12, 98, 43, and 300 ng/ml or ng/g in skim milk, butter milk, skim milk powder, drinking milk, milk powder, cream and butter, respectively. This investigation confirmed again the high lipophylicity of progesterone (64). Other studies also demonstrated the differences between progesterone concentrations in milk and milk products (Table 2).
Table 2:
The concentrations (ng/ml or ng/g) of progesterone, estrogens in milk and milk products (93)
| Hormones | Milk | Cream | Butter | Yogurt | Gouda cheese |
|---|---|---|---|---|---|
| Progesterone | 9.81 | 48.6 | 141 | 13.3 | 44.2 |
| 17β-estradiol | 0.02 | 0.03 | 0.3 | 0.02 | 0.03 |
| Estreone | 0.13 | 0.26 | 1.47 | 0.16 | 0.17 |
The kinetics studies of Progesterone transfer from blood into milk show that the pathways of trans-cellular, simple and facilitate diffusion are involved (65). It is well known that Progesterone is metabolised by CYP 450s (CYP3A, CYP2D6) in human. However, involvement of hydroxysteroid dehydrogenases, in biotransformation of the progesterone has also been demonstrated. The most important and biologically active metabolites of the Progesterone are 20α-dihydroprogesterone, 5α-dihydroprogesterone, and 3α-hydroxy-4-pregnen-20-one, which exhibit various effects (66, 67). Previous studies demonstrated that following oral administration of P in human, circulating concentration of Progesterone and its active metabolite (20α-dihydroprogesterone) was raised. Moreover, it has been also shown that this increase could exert proposed progestational response in target tissues (68). However, other studies indicate that due to rapid absorption following oral administration (equally to consumption via dairy foods) and extensive first pass effect in the hepatic biotrans-formation, the oral bioavailability of exogenous Progesterone is less than 10% (69). There is lack of knowledge about the kinetics of Progesterone and its potentially active metabolites in human following consuming of dairy foods in particular fatty foods (butter) which contain high amounts of progesterone.
The milk Progesterone concentration was lowered in cows, which fed by high level of concentrate and the possible interpretation for that reduction was a consequence of increased metabolic clearance of progesterone associated with increased energy intake. It has been found that the milk-cumulative progesterone concentration during the third luteal phase postpartum was higher for cows with low milk yields (70).
Estrogens
Estrogens play a critical role in most metabolic, behavioural and morphological requirements, which are essential in reproduction of the female vertebrates. There is no doubt that estrogens govern important activities including metabolic reactions in male, too (71). During the last decades huge amount of studies have been performed to screen the presence of exohormones in human environment, which these efforts have led to increasing concern about their impact on wildlife and human health (72). In contrast, there is lack of knowledge about possible exposure to endogenous sex steroids from food, about precise measurement methods, about the possible impact of naturally occurring steroid hormones and their metabolites on human and animal health and so on.
It is known that steroid hormones naturally occurring either in animals such as beef and veal because of their misuse as anabolic agents (73) and in non-treated cattle (74). Almost, all foodstuff of animal origin contains 17β-estradiol and its metabolites, although the levels of hormone and its metabolites vary with the kind of food, gender, animal species, age and physiological condition of the animals. Thus, estrogens are unavoidable hormones in non-vegetarian human nutrition.
Previous studies have shown that about 60–80% of estrogens come from milk and dairy products in western diets (75). Although the oral bioactivity of free 17β-estradiol and oestrone may be a bit low, but oestrogen sulphate as a main conjugate in milk, has a relatively high oral bioactivity (9). Recent epidemiological studies indicating a very strong relation between milk and dairy products high consumption and high incidence of testicular and prostate cancers (76). In the following pages of current review the estrogens and their metabolites content of human diets with especial emphasis on milk and dairy products is summarized. Moreover, the possible impact of milk and dairy products estrogens on human health along with analytical methods of estrogens quantification are discussed.
Natural occurrence of estrogens in foods with plants and animal origin
Since, nowadays use of more vegetal flours, which are known to contain high amounts of phytoestrogens including isoflavones in animal feeds, is usual, then it could create a great concern about the possible transfer of these compounds from the bovine feed to the milk and finally to human food chain (77). Phytoestrogens are capable to interact with both typical oestrogen receptors (α and β) (78), thus they may act as endocrine disruptors. Plant isoflavonoids especially in soybean products and other legumes are converted by intestinal bacteria to hormone-like compounds with estrogenic activity (79). While, the beneficial or detrimental effects of phytoestrogens are controversially a big challenge however, existence of phytoestrogens in cow’s milk and breast milk has been identified (80).
Naturally occurring of steroid hormones in animals has been frequently reported. Oestrogens especially 17-β-oestradiol and estrone are found in different parts of beef and veal including muscle, liver and fat (81, 82). Tissues from adult cattle in comparison to the calves consist of low amounts of estrogens. However the values only exceeded by pregnant cows (83). A similar steroid hormone profile as in ruminants has been reported in pork meat and estrogens indeed detected either in gilt and adult pigs meat (83). Boar tissues showed high concentrations of estrogens in comparison to the female adult but non-pregnant pigs. Despite the frequent use of steroid hormones in broiler fattening, data about the contents of estrogens in poultry tissues are rare. Estrogens are accumulated in fat and the levels of steroid precursors reach high concentrations in goose-fat and the meat of laying hens. There is very little known about the steroid hormones content of eggs, however, presence of 17-β-oestradiol and estrone in eggs has been reported (83). Oestrogen hormones contents in different parts of fish follows a vary profile than that of mammals and presence of steroid hormones in fish vary largely depend on the season and reproductive stage (84, 85).
Estrogens in milk and milk products
Since steroid hormones pass the blood-milk barrier thus there is no doubt about the existing of them in milk, as the presence of steroid hormones especially progesterone content in milk is used as a diagnostic toll of pregnancy. Very early studies showed that the main estrogen in cow’s milk is the biologically inactive 17β-estradiol, which followed by estrone and 17β-oestradiol (86). The presence of 17β-oestradiol, estrone- and estriol-sulphate in human breast cyst fluid has already been demonstrated (87, 88). The source of the estrogens synthesis and secretion is the big challenge for endocrinologists, which there are considerable attempts to show that whether or not it is mammary glands that synthesize and secret the bovine milk estrogens or some other tissues and glands and/or it is combination of them. Janwski and co-workers showed that oestrone, oestrone sulphate and 17β-oestradiol are not secreted by bovine mammary gland, however their preliminary in vitro studies indicated the synthesis of 17-β-oestradiol by mammary tissues (89).
The main source of animal-derived estrogens (60–70%) in the human diet is milk and dairy products (83). Recently, free and conjugated forms of estrogens including 17β-oestradiol, estrone and estriol (Fig. 1), have been detected and quantified (90). They found that estrone is the major estrogens with 69% participation between the detected estrogens and for the estrone, the conjugated form is the predominant (90%). The distribution of estrogens especially 17β-oestradiol in fat or non-fat parts of milk still is controversial, as Abeyawardene and co-workers reported that 52% of 17β-oestradiol content in milk is distributed in fat phase, in contrast Lopez and his team demonstrated that there is no difference in 17β-oestradiol concentrations between milk samples which processed as composite whole milk or composite defatted milk (91, 92). Hartmann and co-workers conducted a market basket survey and measured the steroid concentration in milk and milk products using GS/MS method (93). They found a maximum concentration of total estrone (sum of free and conjugated forms) in butter with 1.47 ng/g, which followed by 0.26, 0.17, 0.16, and 0.13 ng/g (or ng/ml) in cream, Gouda cheese, yoghurt, and milk, respectively. The level of 17β-oestradiol, which has been determined in that particular survey, was less than 0.02–0.03 ng/g in all milk and milk products samples (Table 2). Qin and his research team by using HPLC method could determine the free concentration of estriol in Holestain cows, milk as 27 ± 12 pg/ml (94). Indeed, they could determine both free and conjugated forms of estrogens in cows, milk (Table 3). We also analysed the occurrence of estrogens in processed and raw milks from pregnant and non-pregnant cows, using liquid chromatography-tandem mass spectrometry (95). The cumulative concentration of free and enzymatically deconjugated estrogens in the third trimester (1639 ng/L) was >27 times higher than that in milk of cows in their first trimester of pregnancy (60 ng/L) (Table 4). It is well known that the high concentrations of estrogens appear at oestrus period of non-pregnant cows and levels increase markedly after pregnancy (96, 97).
Fig. 1:
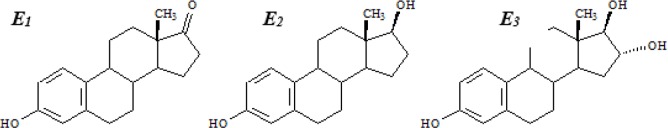
Chemical structure of main estrogens in milk
Table 3:
The concentrations of free and conjugated estrogens (ng/mL) in Holstein cows, milk (94)
| Hormones | Free | Conjugated |
|---|---|---|
| 17β-estradiol | 0.036 ± 0.005 | 0.085 ± 0.033 |
| Esterone | 0.039 ± 0.013 | 0.368 ± 0.076 |
| Esteriol | 0.027 ± 0.012 | 0.038 ± 0.013 |
Table 4:
Concentrations (ng/L) of the sum of free and conjugated E1, αE2, βE2, and E3 in bovine milk collected at different trimesters of pregnancy from lactating cows (95)
| Hormone | Trimester of gestation | ||
|---|---|---|---|
| first | second | third | |
| E1 | 7.9 ± 0.7 | 452 ± 66 | 1266 ± 38 |
| αE2 | 33 ± 7 | 84 ± 4 | 322 ± 35 |
| βE2 | 18.6 ± 0.2 | 51.4 ± 2.7 | 51.2 ± 1.5 |
| E3 | <LOD | <LOD | <LOD |
<LOD, lower than limit of detection level
Milk and milk products as risk factors for carcinogenesis
There is very controversial evidence about counting of milk as a risk factor in terms of being carcinogen.
While, some studies indicating that there is positive correlation between high consumption of milk and high incidence of prostate cancer (98–100), some other studies have opposite ideas and demonstrated that the dairy products including milk not only do not have carcinogenesis property, but they also showed by the inverse association between milk dairy foods consumption and incidence of breast cancer with Cohort studies (101, 102). A strong correlation between considerable increase of about 20-fold in milk consumption and the incidence rate of prostate cancer in Japan is increasing at the fastest rate in the world (103).
Possible health impact of milk oestrogens
In the last couple of decades it has been identified that nearly all body tissues do exert a response to estrogens (71). In this respect, esterogen receptors and the involving enzymes in estrogens synthesis and metabolism have been identified in other tissues than reproductive system including the brain, pituitary, thymus, heart, gut, bone, adipose tissue, and muscles (104). The most sensitive section of human population to estrogens are children who are passing the prepubertal period and it is well known that despite the low concentrations of estrogens in this period, they play very crucial roles such as developing and growth during childhood. At the same time possible biological effects of dietary estrogens at such low concentrations are not clear yet. Very early data showed the high potency of estradiol as a growth factor in promoting cancers. On the other hand there is increasing body of evidence, which indicating that not only estradiol but its metabolites also playing role in initiating of cancers (105). Cytochrome P450 monooxygenase oxidize the estradiol and oestrone into 2- or 4-catechol oestrogens. These two metabolites can also be methylated into methoxy-oestrogens by catechol-o methyltransferase. Another metabolic pathway of oestrogens is formation of 16α-hydroxyoestrone. Finally, the methoxyoestrogens may converted into semiquinone and quinone, which previous studies demonstrated the latest metabolites can act as endogenous tumour initiators by formation of depurinating DNA adducts (106). The metabolism profile of estrogens is depicted in Fig. 2.
Fig. 2:
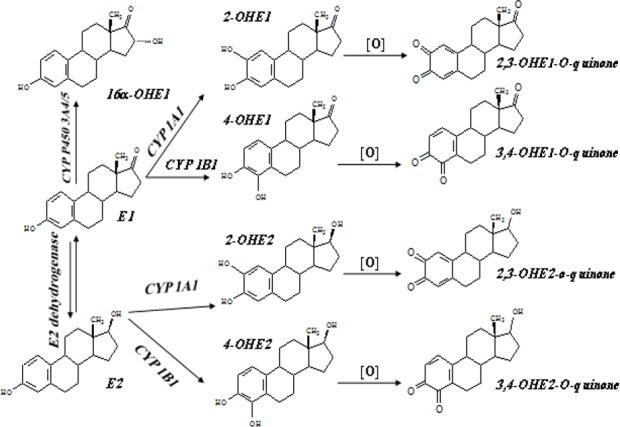
Metabolic profile of oestrogens
Mechanism(s) of action of estrogens
An increasing body of evidence made clear that steroids action not only mediated via well-recognised classical estrogen receptors (ERs), but also based upon membrane-bound responses and a complex network of interaction and cross-talk with various intracellular signalling pathways (107). ERα and ERβ classically mediate their action by ligand-dependent binding to the estrogen-response element of target gene and consequently causing their transcription regulation. Estrogen receptors are highly similar in the DNA-binding domain; however, they are different in the N-terminal domain and in the ligand-binding domain. These differences could be reason for distinct functions and biological effect which are mediated via each receptor (108, 109).
Analysis of the ERα and ERβ expression in estrogen-sensitive cancers showed an increase in ERα and ERβ mRNA and protein ratios in cancer as compared with normal tissues. The results of that analysis suggested that ERβ dose have a sort of protective effect against the mitogenic activity of estrogens (108). Despite the fact that ERα and ERβ have some overlapping tissue distribution but at the same time they display high relative tissue-specific functions. For example ERα play a neuroprotective role,
While ERβ mediate the induction of apoptosis in neuronal cells (110). The cross-talk phenomena also is a great deal in ERs actions, as forms of oestrogenic (oestradiol) and androgenic (androstanes) hormones are both binding to the ERβ It is shown that oestradiol is less potent for ERβ than ERα whilst natural ligands for ERβ may be androgens (111).
In addition of receptor-mediated estrogenic effects of estrogens including carcinogenesis property of them, there is growing body of evidence supporting a complementary mechanism that the conversion of estrogens to catecholestrogens (CEs) also involves in carcinogenesis (106). Further metabolism of CEs results in forming of CE-semioquinones and EC-quinones, which they are capable of binding to DNA and consequently to form carcinogenic stable or depurinating DNA adducts (112).
Dose-dependent effects of oestrogens
Oestrogens exert different effects upon used doses as low doses of oestrogens resulted in an important biological effect in girls with Turner syndrome. An oral administration of 100 ng ethinyloestradiol /kg (b.w.) per day for 5 weeks caused significant increase of growth velocity in girls, however, high doses exert either no or even inhibitory effect on growth rate. This dose-dependent property of estrogens indicates of biphasic relationship for epiphyseal growth by estrogens. Although there is similar picture of dose-dependency in boys too, but at the same time considerable differences are in serum level of oestradiol between boys and girls at prepubertal period (111). The differences between boys and girls serum oestrogen levels might be acceptable reason for faster maturation of girls than boys. Girls with having higher concentrations of oestrogen which cause maturation and eventually fusion of the epiphyses and termination of linear growth reach to the maturation sooner than boys (9).
Other studies with low doses of estrogens revealed in Turner girls a divers sensitivity of different tissues, as at low dose of ethynilestradiol (100 ng/kg/day corresponding to 4 μg/day in a 40 kg child) resulted in a significant increase in growth rate but no effects on vaginal maturation, breast development and onset of menses. Moreover, in healthy postmenopausal women signs of estrogenic stimulation showed specific-tissue responses as the mean plasma oestradiol concentration was found about 8 pg/ml, while the concentration in target tissues such as vagina, endometrium and myometrium were 198, 655, and 149 pg/g, respectively (113).
In vitro studies also figured out estrogens low dose effects. An enhance in specific gene expression, and cell proliferation in the MCF-7 cell lines following exposing to pico-molar oestrogen concentrations, have been reported (114, 115).
Estrogens and male reproductive system
Despite the fact that estrogens are known as female hormones but as early as 1930’s it was considered that estrogens do have some functions in male reproductive and even non-reproductive systems. It is well documented that the major part of estrogens in the male are produced in the testis by aromatase P450 (116), and the rest from other organs including adrenal glands and exogenous sources such as water, meat, egg, and dairy foods. Diet is the most important human exposure route to the estrogens as a glass of red wine contains 0.5–2 μg of oestrogen equivalents (117) and milk and dairy products may contain more than 70% of the animal-derived estrogens (118).
Since estrogens regulate most of the cell function via estrogen receptors of α and β, it has been found that males lacking ERα are completely infertile because estrogens through the ERβ are regulating the reabsorption of luminal fluid in the head of the epididymis, which disruption of this important function results in infertility. Moreover, estrogens besides being critical factor for normal male reproduction including maturation and survival of germ cells, play an important role in the control of osteoprosis and of atherosclerosis, particularly in elderly men (119).
Recently it has been clarified that feeding of mice with xenooestrogen-containing fish-derived diet caused significant decrease in epididymal weight, adult sperm concentration and sperm motility as compared to the control group, which indicating that environmental estrogens may pass through the food chain and exert some disorders in male reproductive system (120). In contrast, stimulatory role of oestrogen on sperm motility in the male golden hamster has been reported (121). It has also been demonstrated that estrogens are essential for androgens pubertal periosteal bone expansion (122).
Analytical methods of estrogens quantification in dairy products
All foodstuffs of animal origin including dairy products contain oestrogens albeit with various concentrations, which depends on kind of food, species, gender, age and physiological stage of the animals. The importance of existence of the oestrogens in dairy products are considerable with respect to the few points: (i) dairy products especially cows, milk is the second major source of infants and growing children after breast milk and in some cases even it is in first place; (ii) increasing body of the evidence indicating possible effect of dairy food’s estrogens in tumour provoking or initiation and this evidence are great concern; (iii) intensively veterinary drug especially growth promoting hormones utilize in veterinary medicine results in an increase of the residues in milk and other dairy foods. The mentioned reasons demand the development of the best and at the same time the most sensitive analytical methods for extraction, detection and quantification of the estrogens in dairy products.
Since the last couple of decades tremendous efforts have been put to develop the new and more sensitive and accurate methods for oestrogens quantification in foods. Hoffman and co-workers used phenolate extraction and gel chromatography for estrone and oestradiol separation. A few years later organic solvent extraction which followed by solid phase extraction (SPE) and radioimmunoassay has been provided (123). Meyer and co-workers developed rather sophisticated method of oestrogens extraction and quantification by using SPE and HPLC separation (124). Advance method of oestrogens detection in milk was provided by using hydrolysis reaction implementing before extraction which followed organic solvent and solid phase extractions and finally quantified by gas chromatography and mass spectrometry (93, 125).
Conclusion
We made a re-evaluation of available data about hormone content of dairy products with emphasizing on estrogens. Physiologic concentrations of present hormones, occasionally and conditionally increasing or decreasing rates were summarized. The physiologic function of each hormone was discussed and briefly the biosynthesis and metabolism pathways were clarified. More attention has been paid on steroid hormones in dairy products and consequently on their possible biological effects on animals and humans. It is thought that the great concern about dairy foods hormone content is steroid hormones especially estrogens due to current epidemiological evidence, which indicating most probable effects of them in initiating and provoking of breast and prostate cancers. At the same time, the latest progress in analytical point of view also reviewed.
In summary, it seems that steroid hormones are very potent compounds in dairy foods, which exerting profound biological effects in animals and humans. Most of the previous knowledge about the steroids is according on their physiologic and sometimes supra-physiologic concentrations of steroids but recently it is found that these compounds even at very low doses may have significant biological effects. Special concern should be paid to the effects, which may occur during certain and sensitive time points including perinatal and pubertal periods. To this end and with respect to the considerable progress in developing of analytical methods and bioassays, it is critically needed to clarify the possible and potential impact of the present hormones especially estrogens in dairy foods on consumers health situation because it is already pointed out that possible unwanted effects on human health by consumption of meat from oestrogen-treated animals cannot be excluded.
Ethical considerations
Ethical issues (Including plagiarism, informed consent, misconduct, data fabrication and/or falsification, double publication and/or submission, redundancy, etc.) have been completely observed by the authors.
Acknowledgements
The authors declare that that there is no conflict of interest.
References
- 1. Fitz-Gerald RJ, Murray BA, Walsh DJ. (2004). Hypotensive peptides from milk proteins. J Nutr, 134(4): 980S–988S. [DOI] [PubMed] [Google Scholar]
- 2. Servos MR, Bennie DT, Burnison BK, Jurkovic A, McInnis R, Neheli T, Schnell A, Seto P, Smyth SA, Ternes TA. (2005). Distribution of estrogens, 17beta-estradiol and estrone in Canadian municipal wastewater treatment plants. Sci Total Environ, 336(1–3): 155–170. [DOI] [PubMed] [Google Scholar]
- 3. Collier RJ, Miller MA, Hildebrandt JR, Torkelson AR, White TC, Madsen KS, Vicini JL, Eppard PJ, Lanza GM. (1991). Factors affecting insulin-like growth factor-I concentration in bovine milk. J Dairy Sci, 74 ( 9): 2905–2911. [DOI] [PubMed] [Google Scholar]
- 4. Atroshi F, Rizzo A, Osterman T, Parantainen J. (1989). Free fatty acids and lipid peroxidation in normal and mastitic bovine milk. Zentralbl Veterinarmed, A 36 (5): 321–330. [DOI] [PubMed] [Google Scholar]
- 5. Hansel W, Hixon J, Shemesh M, Tobey D. (1976). Concentrations and activities of prostaglandins of the F series in bovine tissue, blood and milk. J Dairy Sci, 59 ( 7): 1353–1365. [DOI] [PubMed] [Google Scholar]
- 6. Schams D, Karg H. (1986). Hormones in milk. Ann N Y Acad Sci, 464: 75–86. [DOI] [PubMed] [Google Scholar]
- 7. Foulkes JA, Cookson AD, Sauer MJ. (1982). Artificial insemination of cattle based on daily enzyme immunoassay of progesterone in whole milk. Vet Rec, 111 ( 13): 302–303. [DOI] [PubMed] [Google Scholar]
- 8. Atroshi F, Parantainen J, Kangasniemi R, Osterman T. (1987). Milk prostaglandins and electrical conductivity in bovine mastitis. Vet Res Commun, 11 ( 1): 15–22. [DOI] [PubMed] [Google Scholar]
- 9. Andersson AM, Skakkebaek NE. (1999). Exposure to exogenous estrogens in food: possible impact on human development and health. Eur J Endocrinol, 140 ( 6): 477–485. [DOI] [PubMed] [Google Scholar]
- 10. Vadgama JV, Wu Y, Datta G, Khan H, Chillar R. (1999). Plasma insulin-like growth factor-I and serum IGF-binding protein 3 can be associated with the progression of breast cancer, and predict the risk of recurrence and the probability of survival in African-American and Hispanic women. Oncology, 57 ( 4): 330–340. [DOI] [PubMed] [Google Scholar]
- 11. Yamamuro Y, Sensui N. (1980). Changes in prolactin bioactivity of milk following isolation from litter and oxytocin administration in rats. Life Sci, 60 ( 11): 809–815. [DOI] [PubMed] [Google Scholar]
- 12. Freeman ME, Banks JA. (1980). Hypothalamic sites which control the surges of prolactin secretion induced by cervical stimulation. Endocrinology, 106 ( 3): 668–673. [DOI] [PubMed] [Google Scholar]
- 13. Freeman ME, Kanyicska B, Lerant A, Nagy G. (2000). Prolactin: Structure, Function, and Regulation of Secretion. 80(4): 1523–1631 [DOI] [PubMed] [Google Scholar]
- 14. Emanuele NV, Metcalfe L, Wallock L, Tentler J, Hagen TC, Beer CT, Martinson D, Gout PW, Kirsteins L, Lawrence AM. (1987). Extrahypothalamic brain prolactin: characterization and evidence for independence from pituitary prolactin. Brain Res, 421 (1–2): 255–262. [DOI] [PubMed] [Google Scholar]
- 15. Ryszka F, Dolinska B, Suszka-Switek A. (2002). Distribution of prolactin in selected rat organs and tissues. Int J Tissue React, 24 ( 1): 33–36. [PubMed] [Google Scholar]
- 16. Sinha YN. (1995). Structural variants of prolactin occurrence and physiological significance. Endocr Rev, 16 ( 3): 354–369. [DOI] [PubMed] [Google Scholar]
- 17. Ontsouka CE, Bruckmaier RM, Blum JW. (2003). Fractionized milk composition during removal of colostrum and mature milk. J Dairy Sci, 86 ( 6): 2005–2011. [DOI] [PubMed] [Google Scholar]
- 18. Freeman ME, Kanyicska B, Lerant A, Nagy G. (2000). Prolactin: structure, function, and regulation of secretion. Physiol Rev, 80 (80): 1523–1631. [DOI] [PubMed] [Google Scholar]
- 19. Meaney AM, O'Keane V. (2002). Prolactin and schizophrenia: clinical consequences of hyperprolactinaemia. Life Sci, 71 ( 9): 979–992. [DOI] [PubMed] [Google Scholar]
- 20. Ellis LA, Mastro AM, Picciano MF. (1996). Milk-borne prolactin and neonatal development. J Mammary Gland Biol Neoplasia, 1 ( 3): 259–269. [DOI] [PubMed] [Google Scholar]
- 21. Okamura T, Takeuchi T, Nishii O, Yaginuma T, Kawana T. (1987). Effects of low-caloric diet in puerperium on prolactin, TSH estradiol and milk secretion. Nippon Sanka Fujinka Gakkai Zasshi, 39 ( 11): 2059–2065. [PubMed] [Google Scholar]
- 22. Baghurst PA, Carman JA, Syrette JA, Baghurst KI, Crocker JM. (1992). Diet, prolactin, and breast cancer. Am J Clin Nutr, 56 ( 5): 943–949. [DOI] [PubMed] [Google Scholar]
- 23. Johansson B, Uvnas-Moberg K, Knight CH, Svennersten-Sjaunja K. (1999). Effect of feeding before, during and after milking on milk production and the hormones oxytocin, prolactin, gastrin and somatostatin. J Dairy Res, 66 ( 2): 151–163. [DOI] [PubMed] [Google Scholar]
- 24. Jones LP, Stefansson S, Kim MS, Ahn SN. (2011). Comparison of Radioimmuno and carbon nano-tube field-effect transistor assays for measuring Insulin-Like Growth Factor-1 in a preclinical model of human breast cancer. J Nanobiotechnol, 9(1): 36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Fruchtman S, McVey DC, Borski RJ. (2002). Characterization of pituitary IGF-I receptors: modulation of prolactin and growth hormone. Am J Physiol Regul Integr Comp Physiol, 283 (2): R468–476. [DOI] [PubMed] [Google Scholar]
- 26. Campbell PG, Baumrucker CR. (1989). Insulin-like growth factor-I and its association with binding proteins in bovine milk. J Endocrinol, 120 ( 1): 21–29. [DOI] [PubMed] [Google Scholar]
- 27. Prosser CG, Fleet IR, Corps AN. (1989). Increased secretion of insulin-like growth factor I into milk of cows treated with recombinantly derived bovine growth hormone. J Dairy Res, 56 ( 1): 17–26. [DOI] [PubMed] [Google Scholar]
- 28. Burrin DG. (1997). Is milk-borne insulin-like growth factor-I essential for neonatal development? J Nutr , 127 ( 5 ): 975S – 979S . [DOI] [PubMed] [Google Scholar]
- 29. Guler HP, Zapf J, Froesch ER. (1987). Short-term metabolic effects of recombinant human insulin-like growth factor I in healthy adults. N Engl J Med, 317 ( 3): 137–140. [DOI] [PubMed] [Google Scholar]
- 30. Ranke MB. (2005). Insulin-like growth factor-I treatment of growth disorders, diabetes mellitus and insulin resistance. Trends Endocrinol Metab, 16 ( 4): 190–197. [DOI] [PubMed] [Google Scholar]
- 31. LeRoith D, Werner H, Beitner-Johnson D, Roberts CT., Jr (1995). Molecular and cellular aspects of the insulin-like growth factor I receptor. Endocr Rev, 16 ( 2): 143–163. [DOI] [PubMed] [Google Scholar]
- 32. Chaves J, Saif MW. (2011). IGF system in cancer: from bench to clinic. Anticancer Drugs, 22( 3): 206–212. [DOI] [PubMed] [Google Scholar]
- 33. Musumba C, Pritchard DM. (2009). Cellular and molecular mechanisms of NSAID-induced peptic ulcers. Aliment Pharmacol Ther, 30( 6): 517–531 [DOI] [PubMed] [Google Scholar]
- 34. McAdam BF, Catella-Lawson F, Mardini IA, Kapoor S, Lawson JA, FitzGerald GA. (1999). Systemic biosynthesis of prostacyclin by cyclooxygenase (COX)-2: the human pharmacology of a selective inhibitor of COX-2. Proc Natl Acad Sci U S A, 96 ( 1): 272–277. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Christensen K, Nielsen MO, Jarlov N. (1989). The excretion of prostacyclin (PGI2) in milk and its possible role as a vasodilator in the mammary gland of goats. Comp Biochem Physiol A, 93 ( 2): 477–481. [DOI] [PubMed] [Google Scholar]
- 36. Atroshi F, Parantainen J, Sankari S, Osterman T. (1986). Prostaglandins and glutathione peroxidase in bovine mastitis. Res Vet Sci, 40 ( 3): 361–366. [PubMed] [Google Scholar]
- 37. Materia A, Jaffe BM, Money SR, Rossi P, De Marco M, Basso N. (1984). Prostaglandins in commercial milk preparations. Their effect in the prevention of stress-induced gastric ulcer. Arch Surg, 119 ( 3): 290–292. [DOI] [PubMed] [Google Scholar]
- 38. Simmons KR, Moses SC, Perkins BL. (1979). Pros-taglandin in milk, days open, and estrus detection in dairy cows treated with prostaglandin F2 alpha. J Dairy Sci, 62 ( 9): 1443–1448. [DOI] [PubMed] [Google Scholar]
- 39. Anderson KL, Kindahl H, Petroni A, Smith AR, Gustafsson BK. (1985). Arachidonic acid metabolites in milk of cows during acute coliform mastitis. Am J Vet Res, 46 ( 7): 1573–1577. [PubMed] [Google Scholar]
- 40. Bygdeman M. (2003). Pharmacokinetics of prostaglandins. Best Pract Res Clin Obstet Gynaecol, 17 ( 5): 707–716. [DOI] [PubMed] [Google Scholar]
- 41. Schwalm JW, Tucker HA. (1978). Glucocorticoids in mammary secretions and blood serum during reproduction and lactation and distributions of glucocorticoids, progesterone, and estrogens in fractions of milk. J Dairy Sci, 61 ( 5): 550–560. [DOI] [PubMed] [Google Scholar]
- 42. Gorewit RC, Tucker A. (1976). Comparison of binding proteins of glucocorticoids in mammary tissue and in blood sera from lactating cows. J Dairy Sci, 59 ( 7): 1247–1253. [DOI] [PubMed] [Google Scholar]
- 43. Schwalm JW, Kirk J, Secrest S, Tucker HA. (1978). Effects of processing milk on concentrations of glucocorticoids in milk. J Dairy Sci, 61 ( 10): 1517–1518. [DOI] [PubMed] [Google Scholar]
- 44. Alexandrova M, Macho L. (1983). Glucocorticoids in human, cow and rat milk. Endocrinol Exp, 17 (3–4): 183–189. [PubMed] [Google Scholar]
- 45. Cherlet M, De Baere S, De Backer P. (2004). Quantitative determination of dexamethasone in bovine milk by liquid chromatography-atmospheric pressure chemical ionization-tandem mass spectrometry. J Chromatogr B Analyt Technol Biomed Life Sci, 805 ( 1): 57–65. [DOI] [PubMed] [Google Scholar]
- 46. Fox L, Butler WR, Everett RW, Natzke RP. (1981). Effect of adrenocorticotropin on milk and plasma cortisol and prolactin concentrations. J Dairy Sci, 64 ( 9): 1794–1803. [DOI] [PubMed] [Google Scholar]
- 47. Mostl E, Maggs JL, Schrotter G, Besenfelder U, Palme R. (2002). Measurement of cortisol metabolites in faeces of ruminants. Vet Res Commun, 26 ( 2): 127–139. [DOI] [PubMed] [Google Scholar]
- 48. McInnes KJ, Kenyon CJ, Chapman KE, Livingstone DE, Macdonald LJ, Walker BR, Andrew R. (2004). 5alpha-reduced glucocorticoids, novel endogenous activators of the glucocorticoid receptor. J Biol Chem, 279 (22): 22908–22912. [DOI] [PubMed] [Google Scholar]
- 49. Butler WR, Des Bordes CK. (1980). Radioimmuno-assay technique for measuring cortisol in milk. J Dairy Sci, 63 ( 3): 474–477. [DOI] [PubMed] [Google Scholar]
- 50. Groer M, Davis M, Casey K, Short B, Smith K, Groer S. (2005). Neuroendocrine and immune relationships in postpartum fatigue. MCN Am J Matern Child Nurs, 30 ( 2): 133–138. [DOI] [PubMed] [Google Scholar]
- 51. Delahaut P, Jacquemin P, Colemonts Y, Dubois M, De Graeve J, Deluyker H. (1997). Quantitative determination of several synthetic corticosteroids by gas chromatography-mass spectrometry after purification by immunoaffinity chromatography. J Chromatogr B Biomed Sci Appl, 696 ( 2): 203–215. [DOI] [PubMed] [Google Scholar]
- 52. Caloni F, Belloli C, Crescenzo G, Ormas P, Archimbault P. (2000). Determination of dexamethasone in milk of dairy cows by immunoenzymatic assay. Vet Hum Toxicol, 42 ( 6): 345–348. [PubMed] [Google Scholar]
- 53. Hoffmann B, Rattenberger E. (1977). Testosterone concentrations in tissue from veal calves, bulls and heifers and in milk-samples. J Anim Sci, 45 ( 3): 635–641. [DOI] [PubMed] [Google Scholar]
- 54. Darling JA, Laing AH, Harkness RA. (1974). A survey of the steroids in cows' milk. J Endocrinol, 62 ( 2): 291–297. [DOI] [PubMed] [Google Scholar]
- 55. Baratta M, Grolli S, Poletti A, Ramoni R, Motta M, Tamanini C. (2000). Role of androgens in proliferation and differentiation of mouse mammary epithelial cell line HC11. J Endocrinol, 167 ( 1): 53–60. [DOI] [PubMed] [Google Scholar]
- 56. Gaiani R, Chiesa F, Mattioli M, Nannetti G, Galeati G. (1984). Androstenedione and testosterone concentrations in plasma and milk of the cow throughout pregnancy. J Reprod Fertil, 70 ( 1): 55–59. [DOI] [PubMed] [Google Scholar]
- 57. Zalanyi S. (2001). Progesterone and ovulation. Eur J Obstet Gynecol Reprod Biol, 98 (2): 152–159. [DOI] [PubMed] [Google Scholar]
- 58. Conneely OM, Lydon JP, De Mayo F, O'Malley BW. (2000). Reproductive functions of the progesterone receptor. J Soc Gynecol Investig, 7: S25–32. [DOI] [PubMed] [Google Scholar]
- 59. Miller L, Hunt JS. (1996). Sex steroid hormones and macrophage function. Life Sci, 59 ( 1): 1–14. [DOI] [PubMed] [Google Scholar]
- 60. Jung-Testas I, Do Thi A, Koenig H, Desarnaud F, Shazand K, Schumacher M, Baulieu EE. (1999). Progesterone as a neurosteroid: synthesis and actions in rat glial cells. J Steroid Biochem Mol Biol, 69 (1–6): 97–107. [DOI] [PubMed] [Google Scholar]
- 61. Gunzler O, Korndorfer L, Lohoff H, Hamburger R, Hoffmann B. (1975). Practical experience in determining progesterone in milk for evaluation of fertility in the cow. Tierarztl Umsch, 30 ( 3): 111–118. [PubMed] [Google Scholar]
- 62. Hoffmann B, Hamburger R, Hollwich W. (1977). Determination of progesterone directly in milk fat as an improved method for fertility control in cattle. Zuchthygiene, 2 ( 1): 1–7. [PubMed] [Google Scholar]
- 63. Rioux P, Rajotte D. (2004). Progesterone in milk: a simple experiment illustrating the estrous cycle and enzyme immunoassay. Adv Physiol Educ, 28 (1–4): 64–67. [DOI] [PubMed] [Google Scholar]
- 64. Hoffmann B, Hamburger R, Karg H. (1975). Natural occurrence of progesterone in commercial milk products (author's transl). Z Lebensm Unters Forsch, 158 ( 5): 257–259. [DOI] [PubMed] [Google Scholar]
- 65. Heap RB, Fleet IR, Hamon M, Brown KD, Stanley CJ, Webb AE. (1986). Mechanisms of transfer of steroid hormones and growth factors into milk. Endocrinol Exp, 20 (2–3): 101–118. [PubMed] [Google Scholar]
- 66. Lou Z, Johnson JV, James MO. (2002). Intestinal and hepatic microsomal metabolism of testosterone and progesterone by a 3 alpha-hydroxysteroid dehydrogenase to the 3 alpha-hydroxy derivatives in the channel catfish, Ictalurus punctatus. J Steroid Biochem Mol Biol, 82 (4–5): 413–424. [DOI] [PubMed] [Google Scholar]
- 67. Suzuki T, Murry BA, Darnel AD, Sasano H. (2002). Progesterone metabolism in human leukemic monoblast U937 cells. Endocr J, 49 ( 5): 539–546. [DOI] [PubMed] [Google Scholar]
- 68. Padwick ML, Endacott J, Matson C, Whitehead MI. (1986). Absorption and metabolism of oral progesterone when administered twice daily. Fertil Contracept, 46 ( 3): 402–407. [PubMed] [Google Scholar]
- 69. Maxson WS, Hargrove JT. (1985). Bioavailability of oral micronized progesterone. Fertil Steril, 44( 5): 622–626. [PubMed] [Google Scholar]
- 70. Reksen O, Grohn YT, Havrevoll O, Bolstad T, Waldmann A, Ropstad E. (2002). Relationships among milk progesterone, concentrate allocation, energy balance, milk yield and conception rate in Norwegian cattle. Anim Reprod Sci, 73 (3–4): 169–184. [DOI] [PubMed] [Google Scholar]
- 71. Daxenberger A, Ibarreta D, Meyer HH. (2001). Possible health impact of animal oestrogens in food. Hum Reprod Update, 7 ( 3): 340–355. [DOI] [PubMed] [Google Scholar]
- 72. Kavlock RJ, Daston GP, DeRosa C, Fenner-Crisp P, Gray LE, Kaattari S, Lucier G, Luster M, Mac MJ, Maczka C, Miller R, Moore J, Rolland R, Scott G, Sheehan DM, Sinks T, Tilson HA. (1996). Research needs for the risk assessment of health and environmental effects of endocrine disruptors: a report of the U.S. EPA-sponsored workshop. Environ Health Perspect, 104 Suppl 4: 715–740. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73. Scippo ML, Degand G, Duyckaerts A, Maghuin-Rogister G, Delahaut P. (1994). Control of the illegal administration of natural steroid hormones in the plasma of bulls and heifers. Analyst, 119 ( 12): 2639–2644. [DOI] [PubMed] [Google Scholar]
- 74. Hartwig M, Hartmann S, Steinhart H. (1995). Determination of naturally occurring sex steroid hormones (androgens and progestagens) in beef. Z Lebensm Unters Forsch, 201( 6): 533–536. [DOI] [PubMed] [Google Scholar]
- 75. Remesar X, Tang V, Ferrer E, Torregrosa C, Virgili J, Masanes RM, Fernandez-Lopez JA, Alemany M. (1999). Estrone in food: a factor influencing the development of obesity? Eur J Nutr, 38 (5): 247–253. [DOI] [PubMed] [Google Scholar]
- 76. Ganmaa D, Li XM, Qin LQ, Wang PY, Takeda M, Sato A. (2003). The experience of Japan as a clue to the etiology of testicular and prostatic cancers. Med Hypotheses, 60 ( 5): 724–730. [DOI] [PubMed] [Google Scholar]
- 77. Antignac JP, Cariou R, Le Bizec B, Cravedi JP, Andre F. (2003). Identification of phytoestrogens in bovine milk using liquid chromatography/electrospray tandem mass spectrometry. Rapid Commun Mass Spectrom, 17 ( 12): 1256–1264. [DOI] [PubMed] [Google Scholar]
- 78. Leclercq G, Jacquot Y. (2014). Interactions of isoflavones and other plant derived estrogens with estrogen receptors for prevention and treatment of breast cancer-considerations concerning related efficacy and safety. J Steroid Biochem Mol Biol, 139 ( 1): 237–44. [DOI] [PubMed] [Google Scholar]
- 79. Adlercreutz CH, Goldin BR, Gorbach SL, Hockerstedt KA, Watanabe S, Hamalainen EK, Markkanen MH, Makela TH, Wahala KT, Adlercreutz T. (1995). Soybean phytoestrogen intake and cancer risk. J Nutr, 125 (3 suppl): 757S–770S. [DOI] [PubMed] [Google Scholar]
- 80. Slavin JL. (1996). Phytoestrogens in breast milk--another advantage of breast-feeding? Clin Chem, 42 (6 pt 1): 841–842. [PubMed] [Google Scholar]
- 81. Henricks DM, Gray SL, Hoover JL. (1983). Residue levels of endogenous estrogens in beef tissues. J Anim Sci, 57 ( 1): 247–255. [DOI] [PubMed] [Google Scholar]
- 82. Henricks D. (1980). Assay of naturally occuring estrogens in bovine tissues. Steroids in Animal Production International Symposium: 161–170. [Google Scholar]
- 83. Hartmann S, Lacorn M, Steinhart H. (1998). Natural occurrence of steroid hormones in food. Food Chemistry, 62 ( 1): 7–20. [Google Scholar]
- 84. Baynes SM, Scott AP. (1985). Seasonal variations in parameters of milt production and in plasma concentration of sex steroids of male rainbow trout (Salmo gairdneri). Gen Comp Endocrinol, 57 (1): 150–160. [DOI] [PubMed] [Google Scholar]
- 85. van Bohemen CG, Lambert JG. (1981). Estrogen synthesis in relation to estrone, estradiol, and vitellogenin plasma levels during the reproductive cycle of the female rainbow trout, Salmo gairdneri. Gen Comp Endocrinol, 45 (1): 105–114. [DOI] [PubMed] [Google Scholar]
- 86. Erb RE, Chew BP, Keller HF. (1977). Relative concentrations of estrogen and progesterone in milk and blood, and excretion of estrogen in urine. J Anim Sci, 45 ( 3): 617–626. [DOI] [PubMed] [Google Scholar]
- 87. Bradlow HL, Rosenfeld RS, Kream J, Fleisher M, O'Connor J, Schwartz MK. (1981). Steroid hormone accumulation in human breast cyst fluid. Cancer Res, 41 ( 1): 105–107. [PubMed] [Google Scholar]
- 88. McGarrigle HH, Lachelin GC. (1983). Oestrone, oestradiol and oestriol glucosiduronates and sulphates in human puerperal plasma and milk. J Steroid Biochem, 18 (5): 607–611. [DOI] [PubMed] [Google Scholar]
- 89. Janowski T, Zdunczyk S, Malecki-Tepicht J, Baranski W, Ras A. (2002). Mammary secretion of oestrogens in the cow. Domest Anim Endocrinol, 23 (1–2): 125–137. [DOI] [PubMed] [Google Scholar]
- 90. Ganmaa D, Qin LQ, Wang PY, Tezuka H, Teramoto S, Sato A. (2004). A two-generation reproduction study to assess the effects of cows' milk on reproductive development in male and female rats. Fertil Steril, 82( Suppl 3): 1106–1114. [DOI] [PubMed] [Google Scholar]
- 91. Abeyawardene SA, Hathorn DJ, Glencross RG. (1984). Concentrations of oestradiol-17 beta and progesterone in bovine plasma and defatted milk during the post-partum anovulatory period, during oestrous cycles and following ovariectomy. Br Vet J, 140 ( 5): 458–467. [DOI] [PubMed] [Google Scholar]
- 92. Lopez H, Bunch TD, Shipka MP. (2002). Estrogen concentrations in milk at estrus and ovulation in dairy cows. Anim Reprod Sci, 72 (1–2): 37–46. [DOI] [PubMed] [Google Scholar]
- 93. Hartmann S, Larkus M, Steinhart H. (1998). Natural occurence of steroid hormones in food. Food Chemistry, 62 ( 1): 7–20. [Google Scholar]
- 94. Qin LQ, Wang PY, Kaneko T, Hoshi K, Sato A. (2004). Estrogen: One of the risk factors in milk for prostate cancer. Med Hypotheses, 62 ( 1): 133–142. [DOI] [PubMed] [Google Scholar]
- 95. Malekinejad H., Scherpenisse P, Bergwerff A.A. (2006). Naturally occurring estrogens in processed milk and in raw milk (from gestated cows). J Agric Food Chem, 54 ( 26); 9785–9791. [DOI] [PubMed] [Google Scholar]
- 96. Wolford ST, Argoudelis CJ. (1979). Measurement of estrogens in cow's milk, human milk, and dairy products. J Dairy Sci, 62 ( 9): 1458–1463. [DOI] [PubMed] [Google Scholar]
- 97. Holdsworth RJ, Heap RB, Booth JM, Hamon M. (1982). A rapid direct radioimmunoassay for the measurement of oestrone sulphate in the milk of dairy cows and its use in pregnancy diagnosis. J Endocrinol, 95 ( 1): 7–12. [DOI] [PubMed] [Google Scholar]
- 98. Schuurman AG, van den Brandt PA, Dorant E, Goldbohm RA. (1999). Animal products, calcium and protein and prostate cancer risk in The Netherlands Cohort Study. Br J Cancer, 80 ( 7): 1107–1113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99. Talamini R, Franceschi S, La Vecchia C, Serraino D, Barra S, Negri E. (1992). Diet and prostatic cancer: a case-control study in northern Italy. Nutr Cancer, 18 ( 3): 277–286. [DOI] [PubMed] [Google Scholar]
- 100. Tzonou A, Signorello LB, Lagiou P, Wuu J, Trichopoulos D, Trichopoulou A. (1999). Diet and cancer of the prostate: a case-control study in Greece. Int J Cancer, 80 ( 5): 704–708. [DOI] [PubMed] [Google Scholar]
- 101. Knekt P, Jarvinen R, Seppanen R, Pukkala E, Aromaa A. (1996). Intake of dairy products and the risk of breast cancer. Br J Cancer, 73 ( 5): 687–691. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102. Hjartaker A, Laake P, Lund E. (2001). Childhood and adult milk consumption and risk of premenopausal breast cancer in a cohort of 48,844 women - the Norwegian women and cancer study. Int J Cancer, 93 ( 6): 888–893. [DOI] [PubMed] [Google Scholar]
- 103. Hsing AW, Tsao L, Devesa SS. (2000). International trends and patterns of prostate cancer incidence and mortality. Int J Cancer, 85 ( 1): 60–67. [DOI] [PubMed] [Google Scholar]
- 104. Sharpe RM, Turner KJ, Sumpter JP. (1998). Endocrine disruptors and testis development. Environ Health Perspect, 106 (5): A220–221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105. Service RF. (1998). New role for estrogen in cancer? Science, 279 (5357): 1631–1633. [DOI] [PubMed] [Google Scholar]
- 106. Cavalieri EL, Stack DE, Devanesan PD, Todorovic R, Dwivedy I, Higginbotham S, Johansson SL, Patil KD, Gross ML, Gooden JK, Ramanathan R, Cerny RL, Rogan EG. (1997). Molecular origin of cancer: catechol estrogen-3,4-quinones as endogenous tumor initiators. Proc Natl Acad Sci U S A, 94 ( 20): 10937–10942. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107. Castagnetta L, Granata OM, Cocciadiferro L, Saetta A, Polito L, Bronte G, Rizzo S, Campisi I, Agostara B, Carruba G. (2004). Sex steroids, carcinogenesis, and cancer progression. Ann N Y Acad Sci, 1028: 233–246. [DOI] [PubMed] [Google Scholar]
- 108. Bardin A, Boulle N, Lazennec G, Vignon F, Pujol P. (2004). Loss of ERbeta expression as a common step in estrogen-dependent tumor progression. Endocr Relat Cancer, 11 ( 3): 537–551. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109. Fink-Gremmels J, Malekinejad H. (2007). Biochemical mechanisms and clinical effects associated with exposure to the mycoestrogen Zearalenone. Animal Feed Science and Technology, 137 ( 3): 326–341. [Google Scholar]
- 110. Nilsen J, Mor G, Naftolin F. (2000). Estrogen-regulated developmental neuronal apoptosis is determined by estrogen receptor subtype and the Fas/Fas ligand system. J Neurobiol, 43 ( 1): 64–78. [DOI] [PubMed] [Google Scholar]
- 111. Jacobs MN, Lewis DF. (2002). Steroid hormone receptors and dietary ligands: a selected review. Proc Nutr Soc, 61 ( 1): 105–122. [DOI] [PubMed] [Google Scholar]
- 112. Bolton JL, Shen L. (1996). p-Quinone methides are the major decomposition products of catechol estrogen o-quinones. Carcinogenesis, 17 ( 5): 925–929. [DOI] [PubMed] [Google Scholar]
- 113. van Haaften M, Donker GH, Haspels AA, Thijssen JH. (1989). Oestrogen concentrations in plasma, endometrium, myometrium and vagina of post-menopausal women, and effects of vaginal oestriol (E3) and oestradiol (E2) applications. J Steroid Biochem, 33 (4A): 647–653. [DOI] [PubMed] [Google Scholar]
- 114. Sheehan DM, Willingham E, Gaylor D, Bergeron JM, Crews D. (1999). No threshold dose for estradiol-induced sex reversal of turtle embryos: how little is too much? Environ Health Perspect, 107: 155–159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115. Malekinejad H, Fink-Gremmels J. (2005). Bioactivation of Zearalenone by porcine hepatic biotransformation. Veterinary Research, 36 (5–6): 799–810. [DOI] [PubMed] [Google Scholar]
- 116. Li D, Han X. (2004). The effect of estrogens on male reproduction. Zhonghua Nan Ke Xue, 10 ( 3): 211–214. [PubMed] [Google Scholar]
- 117. Safe S, Connor K, Gaido K. (1998). Methods for xenoestrogen testing. Toxicol Lett, 102–103: 665–670. [DOI] [PubMed] [Google Scholar]
- 118. Ganmaa D, Wang PY, Qin LQ, Hoshi K, Sato A. (2001). Is milk responsible for male reproductive disorders? Med Hypotheses, 57 (4): 510–514. [DOI] [PubMed] [Google Scholar]
- 119. Carreau S. (2003). Estrogens--male hormones? Folia Histochem Cytobiol, 41 (3): 107–111. [PubMed] [Google Scholar]
- 120. Aravindakshan J, Paquet V, Gregory M, Dufresne J, Fournier M, Marcogliese DJ, Cyr DG. (2004). Consequences of xenoestrogen exposure on male reproductive function in spottail shiners (Notropis hudsonius). Toxicol Sci, 78 ( 1): 156–165. [DOI] [PubMed] [Google Scholar]
- 121. Jin W, Arai KY, Watanabe G, Suzuki AK, Takahashi S, Taya K. (2005). The Stimulatory Role of Estrogen on Sperm Motility in the Male Golden Hamster (Mesocricetus auratus). J Androl, 26 ( 4): 478–484. [DOI] [PubMed] [Google Scholar]
- 122. Bouillon R, Bex M, Vanderschueren D, Boonen S. (2004). Estrogens are essential for male pubertal periosteal bone expansion. J Clin Endocrinol Metab, 89 ( 12): 6025–6029. [DOI] [PubMed] [Google Scholar]
- 123. JJ C. (1983). Radioimmunoassay of anabolic residues in Argentina. In Meissionier E, Mitchell-Vigneron J, (eds) Anabiloc in anaimal production Public Health Aspacts, Analytical Methds and regulation Proceedings, Symposium held at OIE, Paris: 411–442. [Google Scholar]
- 124. Meyer HH, Landwehr M, Schopper D, Karg H. (1984). Application of Synovex-H in veal calves: steroid release and residues. Food Addit Contam, 1 ( 3): 261–275. [DOI] [PubMed] [Google Scholar]
- 125. Sahlberg BL, Axelson M. (1986). Identification and quantitation of free and conjugated steroids in milk from lactating women. J Steroid Biochem, 25 ( 3): 379–391. [DOI] [PubMed] [Google Scholar]


